ABSTRACT
The objective of this study was to investigate the efficacy and safety of early treatment with sarilumab, added to standard of care (SOC), in hospitalized adults with COVID-19. Methods included phase II, open-label, randomized, controlled clinical trial of hospitalized patients with COVID-19 pneumonia and interleukin (IL)-6 levels ≥ 40 pg/mL and/or d-dimer > 1,500 ng/mL. Participants were randomized (1:1:1) to receive SOC (control group), SOC plus a single subcutaneous dose of sarilumab 200 mg (sarilumab-200 group), or SOC plus a single subcutaneous dose of sarilumab 400 mg (sarilumab-400 group). The primary outcome variable was the development of acute respiratory distress syndrome (ARDS) requiring high-flow nasal oxygenation (HFNO), non-invasive mechanical ventilation (NIMV) or invasive mechanical ventilation (IMV) at day 28. One-hundred and 15 participants (control group, n = 39; sarilumab-200, n = 37; sarilumab-400, n = 39) were included. At randomization, 104 (90%) patients had supplemental oxygen and 103 (90%) received corticosteroids. Eleven (28%) patients in the control group, 10 (27%) in sarilumab-200, and five (13%) in sarilumab-400 developed the primary outcome (hazard ratio [95% CI] of sarilumab-400 vs control group: 0.41 [0.14, 1.18]; P = 0.09). Seven (6%) patients died: three in the control group and four in sarilumab-200. There were no deaths in sarilumab-400 (P = 0.079, log-rank test for comparisons with the control group). In patients recently hospitalized with COVID-19 pneumonia and features of systemic inflammation, early IL-6 blockade with a single dose of sarilumab 400 mg was safe and associated with a trend for better outcomes. (This study has been registered at ClinicalTrials.gov under identifier NCT04357860.)
KEYWORDS: SARS-CoV-2. COVID-19, sarilumab, tocilizumab, interleukin 6
INTRODUCTION
Systemic inflammation seems to play a key role in the progression of COVID-19 to acute respiratory distress syndrome (ARDS) (1, 2). A disordered inflammatory host immune response ultimately leading to diffuse alveolar damage, endothelial injury, and microvascular thrombosis is thought to be a crucial step in the pathogenesis of severe COVID-19 (3, 4). Reinforcing this, adjuvant treatment with dexamethasone has been associated with better outcomes in patients with COVID-19 requiring respiratory support (5).
Interleukin (IL)-6 blockade has been proposed as an attractive immunomodulatory approach to treat systemic inflammatory response in COVID-19. Although initial retrospective studies using tocilizumab (6–8), a membrane-bound monoclonal anti-IL-6 antibody, reported a survival benefit of this strategy, results from clinical trials have been conflicting (9–14). A large meta-analysis including clinical trials assessing the efficacy of IL-6 antagonists in patients hospitalized for COVID-19 released in July 2021 has concluded that the use of IL-6 antagonists is associated with lower mortality (15).
Sarilumab, a human anti-IL-6 soluble receptor monoclonal antibody licensed for the treatment of rheumatoid arthritis, is an alternative option for IL-6 blockade. In April 2020 the SARICOR trial was conceived with the aim to investigate the efficacy and safety of early treatment with sarilumab added to standard of care (SOC) in hospitalized adults with COVID-19 pneumonia and features of systemic inflammation to prevent progression to severe pulmonary forms of COVID-19 requiring high-flow nasal oxygenation (HFNO) devices, non-invasive mechanical ventilation (NIMV), and/or invasive mechanical ventilation (IMV).
RESULTS
Features of the study population.
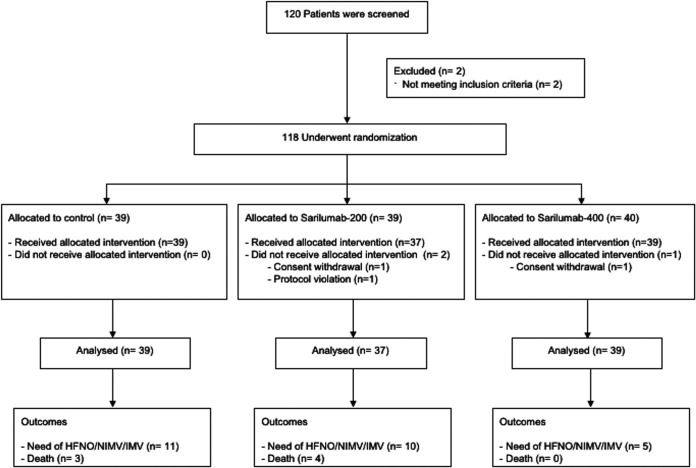
One-hundred and 20 patients were enrolled between July 13, 2020 and March 5, 2021. Two patients were initially enrolled but were not randomized due to screening failure (history of failure to complete treatment for tuberculosis and age out of inclusion criteria, respectively). Additionally, one patient initially enrolled and randomized to group 1 was excluded from the trial before receiving the intervention due to screening failure (platelet count > 100 × 103/μL at screening but < 100 × 103/μL at randomization). Two patients (one in sarilumab-200 and one in sarilumab-400) withdrew their informed consent after randomization and before receiving treatment. Therefore, 39 patients assigned to the control group, 37 to the sarilumab-200, and 39 to the sarilumab-400 were finally included and comprised the mITT study population. Fig. 1 summarizes the disposition of patient during the trial. The main characteristics of the participants are summarized in Table 1. Patients were enrolled in the trial after a median (Q1 to Q3) of 9 (7 to 11) days from symptom onset and 1 (1 to 2) days from hospital admission. One-hundred and four (90%) patients were receiving oxygen supplementation at randomization, 16 (14%) of them > 15 liters per minute.
FIG 1.
Disposition of patients in the trial.
TABLE 1.
Features of study population (N = 115)
| All (N = 115) |
Control (n = 39) |
Sarilumab 200 mg (n = 37) |
Sarilumab 400 mg (n = 39) |
P value | |
|---|---|---|---|---|---|
| Age (yrs)a <65 65 to 79 ≥80 |
59 (51-70)b 70 (61) 40 (35) 5 (4) |
57 (51-71) 23 (59) 12 (31) 4 (10) |
65 (53-72) 18 (49) 18 (49) 1 (2) |
57 (49-67) 29 (74) 10 (26) 0 (0) |
0.15 0.05 |
| Male gender, no. (%) | 78 (68) | 26 (66) | 23 (62) | 29 (74) | 0.51 |
| BMIa,c BMI ≥ 30 |
31 (26-33) 39 (56) |
32 (29-35) 16 (66) |
29 (25-31) 10 (45) |
31 (26-33) 13 (56) |
0.05 0.47 |
| Comorbidities, no. (%) | |||||
| Hypertension Diabetes mellitus Chronic heart disease Chronic respiratory disease Liver disease Chronic renal disease Charlson comorbidity indexa |
47 (41) 17 (15) 4 (4) 15 (13) 3 (3) 2 (2) 0 (0-1) |
13 (33) 6 (15) 2 (5) 6 (15) 1 (3) 0 (0) 0 (0-1) |
17 (46) 9 (24) 1 (3) 4 (11) 2 (5) 2 (5) 0 (0-1) |
17 (44) 2 (5) 1 (3) 5 (13) 0 (0) 0 (0) 0 (0-0) |
0.53 0.06 0.78 0.83 0.31 0.10 1.0 |
| Clinical characteristics and severity at baseline | |||||
| Days from symptom onset to randomization Days from hospitalization to randomizationa SOFAa Ordinal scale score, no. (%) 3 4 5 |
9 (7-11) 1 (1-2) 1 (1-2) 7 (6) 86 (75) 22 (19) |
9 (7-11) 1 (1-3) 2 (1-2) 2 (6) 29 (74) 8 (20) |
9 (7-12) 1 (1-2) 2 (1-2) 4 (11) 27 (73) 6 (16) |
9 (8-11) 1 (1-3) 1 (0-2) 1 (3) 30 (77) 8 (20) |
0.96 0.89 0.28 0.48 |
| Respiratory parameters at baseline | |||||
| Respiratory frequencya Oxygen saturation while breathing room aira <90% Fraction of inspired oxygen (FiO2)a Oxygen saturation/FiO2 ratioa Supplemental oxygen, no. (%) No Nasal cannula Non-rebreather face mask Rebreather face mask Oxygen flow (liter per min)a,d <6 L/min 6 to 14 L/min ≥15 L/min |
20 (18-24) 92 (89-95) 31 (27) 36 (21-43) 261 (205-401) 11 (10) 79 (69) 7 (6) 18 (15) 4 (3-7) 67 (58) 21 (18) 16 (14) |
20 (18-23) 93 (90-95) 8 (20) 36 (28-40) 261 (226-330) 3 (8) 28 (72) 2 (5) 6 (15) 4 (3-7) 23 (59) 8 (20) 5 (13) |
20 (17-24) 92 (89-96) 10 (27) 36 (21-55) 250 (171-428) 6 (16) 22 (60) 3 (8) 6 (16) 5 (4-8) 16 (43) 10 (27) 5 (13) |
20 (18-25) 92 (89-95) 13 (33) 32 (21-49) 290 (186-423) 2 (5) 29 (75) 2 (5) 6 (15) 4 (2-5) 28 (72) 3 (8) 6 (15) |
0.98 0.81 0.20 0.61 0.78 0.95 0.06 0.78 |
| Laboratory parameters at baseline | |||||
| Absolute lymphocyte count (109/L)a Platelets (103/microL) Lactate dehydrogenase (U/L)a Ferritin (ng/mL)a D dimer (ng/mL)a C-reactive protein (mg/L)a Procalcitonin (ng/mL)a Interleukin-6a,e |
0.91 (0.61-1.27) 217 (179-273) 354 (293-494) 720 (412-1,420) 925 (480-1,825) 79 (41-127) 0.1 (0.06-0.16) 56 (41-89) |
0.9 (0.6-1.27) 226 (179-281) 354 (296-433) 635 (301-1,207) 731 (464-1,510) 96 (30-127) 0.08 (0.04-0.13) 48 (38-80) |
1.1 (0.71-1.37) 218 (176-290) 340 (290-421) 593 (332-1,441) 686 (436-1,670) 67 (42-136) 0. 11 (0.07-0.18) 59 (43-88) |
0.81 (0.49-1.21) 212 (178-252) 424 (293-561) 890 (510-1,815) 1,090 (495-2,880) 80 (45-127) 0.1 (0.05-0.19) 70 (43-127) |
0.15 0.94 0.43 0.13 0.36 0.96 0.06 0.35 |
| Concomitant therapies, no. (%) | |||||
| Corticosteroids at randomization Dexamethasone ≥ 6 mg/day Methylprednisolone 40 to 125 mg/day Methylprednisolone 125 to 250 mg/day Methylprednisolone > 250 mg/day Remdesivir Low mol wt heparin Prophylactic dose Intermediate dose Full dose |
103 (90) 60 (52) 13 (12) 10 (9) 20 (17) 14 (12) 100 (100) 51 (44) 38 (33) 26 (23) |
34 (88) 21 (54) 5 (13) 3 (8) 5 (13) 4 (10) 39 (100) 19 (49) 11 (28) 9 (23) |
33 (89) 17 (46) 5 (13) 3 (8) 8 (22) 3 (8) 37 (100) 14 (38) 12 (32) 11 (30) |
36 (92) 22 (56) 3 (8) 4 (10) 7 (18) 7 (18) 39 (100) 18 (46) 15 (39) 6 (15) |
0.76 0.45 0.57 |
Median (Q1-Q3).
Range 28 to 82 years.
Available in 69 patients.
In 104 patients receiving supplemental oxygen at randomization.
Available in 100 patients.
BMI, body mass index; SOFA, sequential organ failure assessment.
Concomitant medications.
One-hundred and three (90%) patients were receiving corticosteroids at randomization (Table 1). In 13 (11%) patients (four [10%] in control group; four [11%] in sarilumab-200; five [13%] in sarilumab-400), dexamethasone was changed to high and/or pulse doses of methylprednisolone during follow-up. Ten patients received an immunomodulator agent other than sarilumab during the study. In four of them, immunomodulators were used after achieving the primary outcome, whereas in the remaining patients these agents were started before progression to HFNO, NIMV, or IMV. A brief description of these 10 cases is provided in Table S1.
Primary outcome.
Eleven (28%) patients in the control group, 10 (27%) in sarilumab-200, and five (13%) in sarilumab-400 had a clinical progression requiring HFNO devices, NIMV, or IMV during the first 28 days after randomization (P = 0.1, chi-square for linear trend) (Table 2). The rate ratio (95% CI) for the primary outcome of the sarilumab-400 group compared with the control group was 0.374 (0.116 to 1.205; P = 0.09). Figure 2A shows the probability of progression to HFNO, NIMV, or IMV at 28 days according to the study group. When compared with the control group, the hazard ratios (HR) for the primary outcome for sarilumab-400 was 0.412 (0.143 to 1.186; P = 0.09) (Table 2). One-hundred and three patients received corticosteroids at randomization. Of these, 10 (29%) patients in the control group, nine (27%) in sarilumab-200, and five (14%) in sarilumab-400 developed the primary outcome (hazard ratio [95% CI] of sarilumab-400 vs control group: 0.48 [0.16 to 1.41]; P = 0.18).
TABLE 2.
Primary and secondary outcomes in the study population (N = 115)
| Outcome | No. of patients with event within 28 days | % of patients with event |
Median (95% CI) days to event | Hazard ratio (95% CI) | Log-rank P value |
||
|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 28 | |||||
| Measures of worsening | |||||||
| Primary outcome: progression to HFNO, NIMV or IMV | 0.21 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
11 10 5 |
26% 27% 13% |
28% 27% 13% |
28% 27% 13% |
NR NR NR |
Reference group 0.87 (0.37-2.06) 0.41 (0.14-1.18) |
0.76 0.09 |
| Secondary outcome: Need for MV | 0.48 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
4 6 3 |
3% 11% 8% |
10% 16% 8% |
10% 16% 8% |
NR NR NR |
Reference group 1.68 (0.47-5.98) 0.78 (0.17-3.48) |
0.41 0.74 |
| Secondary outcome: Death | 0.13 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
3 4 0 |
0% 0% 0% |
0% 3% 0% |
8% 11% 0% |
NR NR NR |
Reference group 1.41 (0.31-6.31) 0.01 (0.00-160.68) |
0.64 0.07 |
| Measures of improvement | |||||||
| Secondary outcome: Clinical improvement on ordinal scale | 0.41 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
32 30 36 |
23% 30% 36% |
73% 74% 75% |
88% 84% 94% |
10 (7-12) 9 (7-10) 8 (6-10) |
Reference group 0.96 (0.58-1.59) 1.28 (0.79-2.06) |
0.91 0.28 |
| Secondary outcome: Discontinuation of supplemental oxygen in patients receiving it at baselinea | 0.16 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
30 24 34 |
28% 42% 46% |
71% 68% 79% |
83% 80% 94% |
9 (7-11) 8 (3-12) 7 (4-10) |
Reference group 0.89 (0.52-1.53) 1.39 (0.85-2.28) |
0.69 0.18 |
| Secondary outcome: Discharge alive from hospital | 0.55 | ||||||
| Control Sarilumab 200 mg Sarilumab 400 mg |
34 30 36 |
21% 24% 31% |
64% 71% 72% |
84% 84% 92% |
10 (7-12) 9 (7-10) 9 (6-11) |
Reference group 0.98 (0.60-1.60) 1.23 (0.76-1.96) |
0.94 0.38 |
CI, confidence interval; HFNO, high-flow nasal oxygen; NIMV, non-invasive mechanical ventilation; IMV, invasive mechanical ventilation; NR, not reached.
Analysis restricted to patients receiving supplemental oxygen at baseline (control group, n = 36; sarilumab 200 mg, n = 31; sarilumab 400 mg, n = 37).
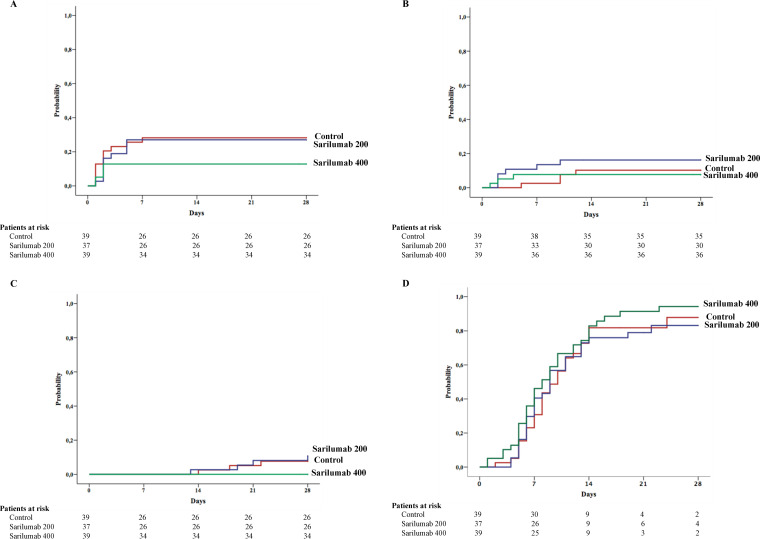
FIG 2.
Probability of developing the study outcomes in the first 28 days after randomization according to study groups in the modified intention-to-treat population (N = 115). (A) Clinical progression requiring high-flow nasal oxygen (HFNO) devices, non-invasive mechanical ventilation (NIMV) or invasive mechanical ventilation (IMV). (B) Need for IMV. (C) Overall mortality. (D) Clinical improvement. Clinical improvement was defined as a 2-point rise in a seven-category ordinal scale or hospital discharge, whichever occurred first.
Three patients in the control group received tocilizumab due to clinical deterioration but prior to achieving the primary outcome. Moreover, one patient in the sarilumab-200 group received anakinra due to progressive respiratory failure before achieving the primary outcome. Only one of these four patients progressed to the primary outcome. Analyses after excluding these four patients are summarized in Table S2. The primary outcome occurred in 28%, 28%, and 13% of the patients in the control, sarilumab-200, and sarilumab-400 groups, respectively. Tocilizumab was used in two patients in the control group due to severe respiratory failure before initiating HFNO, NIMV, or IMV but after escalating to oxygen support > 15 L per minute. This was also the case for the patient in the sarilumab-200 group receiving anakinra after randomization. Due to this we performed a post hoc analysis including these three patients, which were considered as failures. The probability of clinical progression during follow-up with this approach was 32%, 30%, and 13% in the control, sarilumab-200, and sarilumab-400 groups, respectively. The HR of receiving sarilumab 400 mg versus the control group to prevent the need for HFNO, NIMV, or IMV was 0.370 (0.130 to 1.050; P = 0.062) in this analysis.
Secondary outcomes.
The analyses of secondary outcomes in the mITT population are summarized in Table 2. Thirteen (11.3%) patients underwent IMV during the first 28 days after randomization. Figure 2B shows the probability of requiring IMV by study group. Seven (6%) patients died during the study, three in the control group and four in the sarilumab-200 group. There were no deaths in the sarilumab-400 group. Figure 2C shows the probability of death during the study by treatment groups.
The probability of clinical improvement by day 28 on the basis of the ordinal scale was 88%, 84%, and 94% in the control, sarilumab-200, and sarilumab-400 groups, respectively (Table 2 and Fig. 2D). The rate ratio (95% CI) for clinical improvement with sarilumab-400 compared with the control group was 2.625 (0.626 to 11.012; P = 0.176). The proportion of patients treated with sarilumab-400 who discontinued oxygen support or were discharged during the study was numerically higher than that observed in the control group and the sarilumab-200 group (Table 2).
Analyses were also performed of secondary outcomes after excluding 10 patients who received anti-IL-1 or anti-IL-6 other than sarilumab at any time after randomization and before day 28 (see Table S2). Three (9.1%) patients in the control group, three (8.4%) in the sarilumab-200 group, and zero (0%) in the sarilumab-400 group died (P = 0.094, chi square for linear trend; P = 0.059, log-rank test for the comparison of sarilumab-400 vs the control group) (see Table S2).
Post hoc desirability of outcome ranking analysis.
Table S3 shows the classification of patients in the three desirability of outcome ranking (DOOR) categories. The proportion of cases treated with sarilumab-200 with better DOOR than those treated in the control group was 54.6% (95% CI, 51.9% to 57.3%). In a second DOOR analysis comparing sarilumab-400 versus the control, the proportion of cases treated with sarilumab-400 with better DOOR than those treated in the control group was 63.2% (95% CI, 60.6% to 65.7%). A detailed description of the DOOR analyses is provided in Table S4.
Safety.
Adverse events are shown in Table 3. No new safety signal for sarilumab emerged. Overall, 10 (8.7%) patients had a secondary nosocomial infection during the study period.
TABLE 3.
Adverse events
| Event | All (N = 115) |
Control (n = 39) |
Sarilumab 200 mg (n = 37) |
Sarilumab 400 mg (n = 39) |
|---|---|---|---|---|
| Mortality | 7 (6) | 3 (8) | 4 (11) | 0 (0) |
| AST and/or ALT elevations grade ≥ 3 | 2 (2) | 1 (3) | 0 (0) | 1 (3) |
| Nosocomial infection Bacteremia Urinary tract infection Ventilator-associated pneumonia |
10 (9) 3 (3) 2 (2) 10 (9) |
3 (8) 1 (3) 0 (0) 2 (5) |
5 (13) 1 (3) 2 (5) 2 (5) |
2 (5) 1 (3) 0 (0) 1 (3) |
| Pulmonary embolism | 1 (1) | 0 | 0 | 1 (3) |
| Tachyarrhythmia | 3 (3) | 2 (5) | 0 | 1 (3) |
| Syncope | 1 (1) | 0 (0) | 0 (0) | 1 (3) |
| Acute confusional syndrome | 1 (1) | 1 (3) | 0 | 0 |
| Erythroderma | 1 (1) | 0 | 0 | 1 (3) |
| Thrombocytosis | 2 (2) | 0 | 1 (3) | 1 (3) |
| Arthralgias | 1 (1) | 1 (3) | 0 | 0 |
Data are expressed as number (%) of patients. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DISCUSSION
In this phase II trial, we found that in patients recently admitted to hospital with COVID-19 pneumonia and features of systemic inflammation, early IL-6 blockade with a single dose of sarilumab 400 mg was safe and associated with a trend for better outcomes than the current SOC. These benefit signals were observed for primary and secondary outcomes and across different sensitivity and secondary analyses, providing a rationale for continuing with larger, confirmatory phase III trials.
This was a phase II trial that aimed to explore if early blockade with two different doses of the sarilumab human anti-IL-6 soluble receptor monoclonal antibody prevents disease progression in COVID-19. As the study was conducted during the earlier phases of the pandemic, sample size calculations were made on the basis of ICU admissions and mortality rates at that time. Fortunately, mortality has evolved and fallen since the beginning of the epidemic, which has notably affected the power of this trial to detect differences between groups. In spite of this, our results suggest that adding sarilumab 400 mg to SOC provides clinical benefit. Thus, when compared with the control group, a 15% lower rate of need for HFNO, NIMV, or IMV with sarilumab 400 mg was observed. Notably, there were no deaths among those receiving sarilumab 400 mg. On the other hand, recovery tended to occur more frequently and faster with sarilumab 400 mg, which could help to lessen the burden of COVID-19 in an overloaded health care system. Finally, post hoc analyses using DOOR methodology were consistent with a clinically beneficial effect of this treatment. While this trial cannot definitely conclude that sarilumab 400 mg reduces clinical progression or mortality due to its lack of potency, these results support the continuation of the clinical evaluation of this strategy in further trials.
In addition to a relative low sample size, this trial has other limitations. First, it was nonblinded, which has the potential for ascertainment bias. Besides, awareness of the intervention assignment could affect management in the control group. In fact, three patients in the control group received tocilizumab due to clinical deterioration but prior to achieving the primary outcome, probably due to the perceived clinical benefit of tocilizumab by many clinicians at that time during the trial. However, sensitivity analyses excluding these patients showed similar results and other secondary outcomes such as mortality are very unlikely to be influenced by the open design. Second, recruitment extended over an 8-month period, which could potentially affect background care during the trial. Apart from the difficulties of conducting an academic clinical trial during a pandemic, the wide use of IL-6 and IL-1 blockers by clinicians, even before of any evidence of its efficacy, has been a constant in many parts of the world and hindered recruitment in a trial including a control group, thus explaining the relatively long recruitment period. However, results from the unique interventions that have showed some clinical benefit, such as remdesivir or corticosteroids, were available before enrollment of the first patient. Indeed, almost all patients in the trial received concomitant corticosteroids at baseline and overall mortality was 6%, reflecting that our trial population is highly representative of the current clinical picture of hospitalized COVID-19 patients in high-income countries.
Previous information on the use of sarilumab for the treatment of COVID-19 was scarce and inconclusive. Apart from 48 patients included in the REMAP-CAP trial (9) and two small observational studies (16, 17), information from larger randomized clinical trials was limited to that announced by press releases (18, 19). A randomized double-blind, placebo-controlled clinical trial including patients with COVID-19 pneumonia requiring oxygen or critical care not showing efficacy of intravenous sarilumab 200 mg or 400 mg compared with placebo has recently been published (20). Drawbacks of this previous trial were that only 20% of patients received corticosteroids and more than 75% had normal IL-6. Thus, suboptimal backbone therapy and inadequate selection criteria for evaluating immunomodulatory therapy might explain the negative findings. By contrast, more than 90% of patients in our study were on oxygen support and receiving corticosteroids at baseline and patients were enrolled on the basis of exhibiting features of systemic inflammation. In such a population, our results are in line with recent findings of the RECOVERY trial (21) with the use of tocilizumab and the WHO REACT meta-analysis (15), suggesting a role of IL-6 blockade if combined with steroids and in patients on oxygen support with high inflammatory markers.
Our study has several strengths. First, it was performed in a context of universal use of corticosteroids and rates of 11% for IMV use and 6% overall mortality, which is a more representative scenario of current COVID-19 care. Thus, interventions in this trial were proven in a more exigent context than other larger clinical trials assessing treatment interventions where mortality rates in the control group exceeded 30% (21). Besides, unlike other large platform COVID-19 trials, our trial included a contemporaneous control group, which is the best way to guarantee that between-group differences in outcomes can be attributed to interventions and to avoid unnoticed bias, as has been recently noted (22). Finally, we have included a post hoc DOOR analysis, a methodology designed to address several challenges in clinical trials that allows evaluating the clinically relevant question of superiority of a new strategy based on the consideration of all consequences (23). With this approach, our analyses suggest that receiving sarilumab 400 mg promotes a clinical benefit when compared to the control group.
Like other trials using tocilizumab, treatment with sarilumab was safe and was not associated with serious adverse events. Notably, we did not observe an increased risk of serious infections when compared with patients in the control group, in spite of having used higher doses than commonly used for rheumatoid arthritis in one of the arms of the study. As with other interventions, long-term safety, including assessing the risk of secondary infections beyond 28 days should be confirmed.
The role of IL-6 blockade in the treatment of COVID-19 is being clarified by recent studies. REMAP-CAP (9) and RECOVERY (21) trials have recently provided evidence of a survival benefit of tocilizumab in critically ill patients and in patients with hypoxia and evidence of inflammation receiving systemic corticosteroids, respectively. A reduction in all-cause mortality has been also found in a large meta-analysis (15). Our results suggest that combining sarilumab at high doses with corticosteroids early on in the course of COVID-19 pneumonia reduces clinical progression and shortens the time to hospital discharge. Whether similar clinical benefits can be expected with tocilizumab or sarilumab is not known, as comparative clinical trials are lacking. In the meantime, it is reasonable to prioritize the use of tocilizumab as the cumulative safety and efficacy with such a drug is larger than for sarilumab and to consider the use of sarilumab as an alternative option based on our results. The increasing demands of tocilizumab have led to shortage of the drug during the pandemic, which can occur again due to its widespread use. Our study provides evidence for the use of sarilumab as an alternative option if access to tocilizumab is limited. As our study has not included patients with critical COVID, the use of tocilizumab should be prioritized in this specific scenario.
In summary, patients hospitalized with COVID-19 pneumonia requiring oxygen support and markers of systemic inflammation receiving corticosteroids might benefit from early blockade of IL-6 with sarilumab 400 mg during the first 48 h of admission. The efficacy of this strategy should be confirmed in a well-powered randomized clinical trial.
MATERIALS AND METHODS
Trial design and oversight.
SARICOR was a phase II, open-label, randomized, multicenter, controlled clinical trial conducted in 10 hospitals in Andalusia, Southern Spain. SARICOR was an academic trial funded by the COVID-19 Research Program of the Regional Government of Andalusia (project code COVID-0013-2020). SANOFI-AVENTIS had no role in the trial. The trial was approved by the Committee for Biomedical Research Ethics of the Reina Sofía University Hospital and was conducted in accordance with the International Conference on Harmonization E6 Guideline for Good Clinical Practice and ethical principles of the Declaration of Helsinki. Authorization was also obtained from the Spanish Agency of Medicines and Medical Products (AEMPS, 20-0262). The trial is registered in accessible public databases such as the Spanish Clinical Studies Registry (REec), EUDRACT (2020-001531-27), and ClinicalTrials.gov (NCT04357860). A detailed description of the study protocol is also available (24).
Patients.
Patients were eligible for enrollment if they met the following inclusion criteria: (i) age ≥18 years; (ii) hospitalization due to COVID-19 with SARS-CoV-2 infection confirmed by a positive antigen detection test or a PCR assay; (iii) interstitial pneumonia confirmed by the presence of infiltrates on chest radiograph or a computer tomography scan; and (iv) IL-6 levels ≥40 pg/mL and/or d-dimer >1,500 ng/mL or ≥1,000 if progressive increments were documented in at least two determinations after admission. Key exclusion criteria were the presence of ARDS requiring HFNO or mechanical ventilation at randomization (or expected to be started in the first 24 h after randomization as deemed by decision of the investigator) and patients in which the decision was made to not progress to mechanical ventilation in the event of clinical deterioration. The full list of exclusion criteria is provided in the full version of the protocol (see supplementary material). Patients' informed consent was obtained before inclusion. Written informed consent was preferable but initial oral consent before a witness documented in the clinical record and ratified later in writing was also an option, which was in accordance with Spanish Agency of Medicines and Medical Products exceptional measures applicable to clinical trials to manage problems arising from the COVID-19 emergency (https://www.aemps.gob.es/informa-en/exceptional-measures-applicable-to-clinical-trials-to-manage-problems-arising-from-the-covid-19-emergency/?lang=en).
Randomization and treatment.
Patients were randomized in a 1:1:1 ratio to receive SOC alone (control group); SOC plus a single subcutaneous dose of 200 mg sarilumab (sarilumab-200 group); or SOC plus a single subcutaneous dose of 400 mg sarilumab (sarilumab-400 group). Concealed randomization was carried out by means of electronic case report forms after obtaining informed consent and stratified according to the presence of an oxygen saturation (SatO2) <90% while breathing room air and/or a partial pressure of arterial oxygen (PaO2) <60 mm Hg. Sarilumab was administered the same day of trial inclusion, generally within 3 h after informed consent was obtained.
Patients received SOC according to local practice, which included any individual drug or combination of drugs listed in the protocol of the Spanish Ministry of Health (https://www.mscbs.gob.es) and the Spanish Agency of Medicines and Medical Products (www.aemps.gob.es) during the study period. Dexamethasone was the preferred backbone therapy since the press release of the RECOVERY trial, but high and/or pulse doses (>1 mg methylprednisolone or equivalent per kilogram of body weight) of corticosteroids were also permitted. Once the primary endpoint was achieved, the use of immunomodulator agents other than sarilumab was allowed at the discretion of the investigator at each site. Otherwise, the use of tocilizumab and/or other immunomodulator agents before reaching the primary endpoint was considered a protocol deviation.
Outcomes.
The primary outcome variable was the development of ARDS requiring HFNO, NIMV, or IMV during the first 28 days after randomization. Secondary outcome variables were all-cause mortality, need for ICU admission and/or IMV, time to clinical improvement (as defined on the basis of an ordinal scale), time until oxygenation improvement, and duration of hospitalization. Clinical improvement was defined as a 2-point increase on a seven-category ordinal scale or hospital discharge, whichever occurred first (24). Oxygenation improvement was defined as discontinuation of supplemental oxygen for at least 48 h in patients requiring it at baseline. Clinical status was recorded at baseline and every day during hospitalization for a total of 28 days after randomization. Patients discharged before day 28 had a clinical and/or telephone visit at day 28 to assess their clinical and vital status and as a safety follow-up visit.
Statistical analysis.
We calculated that a sample size of 120 patients, 40 for each arm, would provide a power of 80% to detect a 30% difference in the primary outcome between treatment groups, with an alpha error of 0.05 and assuming that the modified intention-to-treat population would be 90% of randomized patients. Efficacy analyses of the primary outcome were performed both in the modified intention-to-treat (ITT) population, which included all randomized patients receiving treatment, and in the per protocol (PP) population, where patients in the control group receiving tocilizumab and/or other immunomodulator agents before achieving the primary endpoint were excluded. Secondary analyses were performed in both the mITT population and in the PP population. For secondary analyses, patients receiving tocilizumab or immunomodulator agents other than sarilumab at any time after randomization were excluded in the PP population. The safety population included all patients who underwent randomization and received therapy. Outcome variables were firstly assessed by means of a time-to-event approach. Time was computed for each specific outcome event as the days elapsed from baseline, considered as the day of randomization, to the date of each specific event or censoring date (day 28). Survival curves were compared according to the Kaplan-Meier method using the log-rank test. The differences between the treatment groups were estimated as HR with 95% confidence intervals (CI) from stratified Cox proportional hazards models. Additionally, the proportion of patients developing the different outcomes of the study in each arm of the study were compared using the chi square test and the relative risk (RR) with 95% CI was estimated. Sensitivity analyses of the time to hospital discharge and time to clinical improvement adjusting for death as a competing risk were also performed.
To provide additional information on the different components of the primary endpoint, a post hoc analysis with DOOR was performed. Patients were classified in three mutually exclusive hierarchical levels according to the following ordinal system (descending order of desirability): (i) category 1: no death at day 28 and no development of ARDS requiring HFNO/NIMV/IMV; (ii) category 2: no death at day 28 but development of ARDS requiring HFNO/NIMV/IMV; and (iii) category 3: death at day 28. For those who survived and did not develop ARDS requiring HFNO/NIMV/IMV in the first 28 days (category 1), the time to clinical improvement was considered to tiebreak (the shorter the time until improvement, the better the DOOR). The probability of a better ranking among patients treated in each sarilumab arm than among the patients in the control group was calculated where the lower bound of the 95% CI of the probability should be >50% to suggest superiority of sarilumab.
All analyses were performed using SPSS statistical software package release 24.0 (IBM Corporation, Somers, NY, USA) and STATA software. The incidence and severity of adverse events were evaluated. These events were determined and classified according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Data availability.
Deidentified participant data is available upon request and after specific approval by the Regional Government of Andalusia and the Spanish Agency of Medicines and Medical Products.
ACKNOWLEDGMENTS
We thank the following for their collaboration in the study: Rocío Herrero del Río, Jesús Machuca, Estefanía García Sánchez, Antonio Lesmes Serrano, Concepción Ferrete Morales, Jesús M. Gómez Mateos (Hospital Universitario de Valme), Patricia Jiménez-Aguilar, María Luisa Fernández-Ávila, Blanca Anaya-Baz, Rosario Castilla-Ortiz (Hospital Universitario Puerto Real), María Macías-Barrera, Rocío Gálvez- Cordero (Hospital Universitario Virgen Macarena), María Esther Guisado-Espartero Justo Sánchez-Gil, María Lorena Montero-Rivas, Raimundo Tirado-Miranda, José Pablo Mazuelas-Teatino, Juan Hidalgo-Cabrera (Hospital Infanta Margarita), Jorge Rodríguez-Gómez, María Purificación Carmona-Sánchez, José Manuel Vaquero-Barrios, Roberto Martin de León, Laura Limia-Pérez, Antonio Miguel Luque-Pineda (Hospital Universitario Reina Sofía), Alberto Benavente-Fernández, María del Mar Díaz-Alcázar, Emilio Guirao-Arrabal, Naya Faro-Mínguez (Hospital Universitario Clínico San Cecilio), María Concepción López-Robles, Judit Constan Rodríguez, Coral García Vallecillos, Carlos García de los Ríos, Miguel Ángel López Zúñiga, Ramiro Cañaveral Vaccari, Carmen Hidalgo Tenorio, María Rosario Javier Martínez, Juan Pasquau Liaño, Miguel Ángel López-Ruz (Hospital Universitario Virgen de las Nieves), Antonio Plata Ciézar, Beatriz Sobrino Díaz, María Victoria Arijo-García, Óscar Porras-Perales, José María Reguera-Iglesias, Rocío Asensi Díez, Ana Fernández Sánchez, Ignacio Márquez Gómez, Juan Diego Ruíz-Mesa, Lucía Valiente de Santis (Hospital Regional Universitario de Málaga), Carmen Sánchez Cano, Iris El Attar Acedo, María Isabel Rodríguez Higuera (Hospital Universitario Torrecárdenas), María Franco Huerta, Alicia Hidalgo Jiménez, Elena Concejo Martínez, Mercedes de Sousa Baena, Álvaro Sánchez Alcázar del Río, Francisco Javier Carrasco Sánchez, Alberto Tenorio Abreu, Laura Corpa Almazán, Raquel Sánchez del Moral, Pilar Villar Santos, Olalla Montero Pérez, and Álvaro Gragera Martínez (Hospital Universitario Juan Ramón Jiménez).
This study has been supported by the Consejeria de Salud y Familias, Junta de Andalucia, Spain (COVID-19 Research Program. Project code COVID-0013-2020). B.G.G. and J.T.C. are supported by General Sub-Directorate of Networks and Cooperative Research Centers, Ministry of Science and Innovation, Spanish Network for Research in Infectious Diseases [REIPI RD16/0016/0001, RD16/0016/0008]—co-financed by the European Regional Development Fund “A Way to Achieve Europe, Operational Program Smart Growth 2014–2020.” J.C.G. is supported by SCReN (Spanish Clinical Research Network) funded by the ISCIII-Sub-Directorate General for Research Assessment and Promotion through project PT17/0017/0032 and PT20/0039. R.L.L., C.D.L.F., J.T.-C., and B.G.-G. are supported by the Center of Biomedical Investigation Network for Infectious Diseases (CIBERINFEC) funded by ISCIII through projects CB21/13/00049 and CB21/13/00012.
Funding sources had no role in the study design; in the collection, analysis, and interpretation of data; and in the writing of the report or the decision to submit the paper for publication.
Dr. Merchante and Dr. Torre-Cisneros had full access and to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Torre-Cisneros.
Acquisition, analysis, or interpretation of data: All authors.
Statistical analysis: Merchante, Cárcel, Gutiérrez-Gutiérrez and Torre-Cisneros.
Drafting of the manuscript: Merchante.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Torre-Cisneros.
Study supervision: Merchante and Torre-Cisneros.
The authors have no conflict of interest or financial relationships relevant to the submitted work to disclose. No form of payment was given to anyone to produce the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Nicolás Merchante, Email: nicolasmerchante@gmail.com.
Julián Torre-Cisneros, Email: julian.torre.sspa@juntadeandalucia.es.
REFERENCES
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26:1636–1643. 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. 2020. Pulmonary vascular endotheliaitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 383:120–128. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, Millar T, Lerpiniere CEB, Tagliavini G, Hartley CS, Randle NP, Gachanja NN, Potey PMD, Dong X, Anderson AM, Campbell VL, Duguid AJ, Al Qsous W, BouHaidar R, Baillie JK, Dhaliwal K, Wallace WA, Bellamy COC, Prost S, Smith C, Hiscox JA, Harrison DJ, Lucas CD. 2021. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med 203:192–201. 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, Arribas JR, Berenguer J, Aznar Muñoz E, Gil Divasson P, González Muñiz P, Muñoz Aguirre C, Díaz Menéndez M, de la Calle Prieto F, Arsuaga Vicente M, Trigo Esteban E, Pérez Valero I, de Miguel Buckley R, Cadiñaños Loidi J, Diaz Pollan B, Martín Carbonero L, Ramos JC, Loeches Yagüe B, Montejano Sánchez R, González García J, García Rodríguez J, Berenguer J, Ramírez M, Gutiérrez I, Tejerina F, Aldámiz-Echevarría T, Díez C, Fanciulli C, Pérez-Latorre L, Pinilla B, López JC, Such Diaz A, Álvaro Alonso E, Torres Macho J, Cuevas Tascon G, Jiménez González de Buitrago E, Brañas Baztán F, Valencia de la Rosa J, Pérez Butragueño M, Fernández Jiménez I, Muñiz Nicolás G, Sepúlveda Berrocal A, Gato Díez A, Toledano Sierra MP, García Butenegro MP, et al. 2021. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicenter cohort study (SAM-COVID-19). Clin Microbiol Infect 27:244–252. 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Sanz J, Muriel A, Ron R, Herrera S, Pérez-Molina JA, Moreno S, Serrano-Villar S. 2021. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. Clin Microbiol Infect 27:238–243. 10.1016/j.cmi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Finkel D, Green A, Mallappallil M, Faugno AJ, Zhang J, Velez JCQ, Shaefi S, Parikh CR, Charytan DM, Athavale AM, Friedman AN, Redfern RE, Short SAP, Correa S, Pokharel KK, Admon AJ, Donnelly JP, Gershengorn HB, Douin DJ, Semler MW, Hernán MA, Leaf DE, Walther CP, Anumudu SJ, Arunthamakun J, Kopecky KF, Milligan GP, McCullough PA, Nguyen T-D, Shaefi S, Krajewski ML, Shankar S, Pannu A, Valencia JD, Waikar SS, Kibbelaar ZA, STOP-COVID Investigators, et al. 2021. Association between early treatment with tocilizumab and mortality among critically ill patients with Covid-19. JAMA Intern Med 181:41–51. 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, REMAP-CAP Investigators, et al. 2021. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen Y-D, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK, BACC Bay Tocilizumab Trial Investigators. 2020. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med 383:2333–2344. 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, Cameron ML, Garcia-Diaz J, Chávez V, Mekebeb-Reuter M, Lima de Menezes F, Shah R, González-Lara MF, Assman B, Freedman J, Mohan SV. 2021. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 384:20–30. 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. 2021. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 384:1503–1516. 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P, CORIMUNO-19 Collaborative Group. 2021. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Intern Med 181:32–40. 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C, Ballerini PF, Sciascia R, Zammarchi L, Para O, Scotton PG, Inojosa WO, Ravagnani V, Salerno ND, Sainaghi PP, Brignone A, Codeluppi M, Teopompi E, Milesi M, Bertomoro P, Claudio N, Salio M, Falcone M, Cenderello G, Donghi L, Del Bono V, Colombelli PL, Angheben A, Passaro A, Secondo G, Pascale R, Piazza I, Facciolongo N, Costantini M, RCT-TCZ-COVID-19 Study Group. 2021. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial. JAMA Intern Med 181:24–31. 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgings JPT, Spiga F, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, et al. 2021. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A Meta-analysis. JAMA 326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gremese E, Cingolani A, Bosello SL, Alivernini S, Tolusso B, Perniola S, Landi F, Pompili M, Murri R, Santoliquido A, Garcovich M, Sali M, De Pascale G, Gabrielli M, Biscetti F, Montalto M, Tosoni A, Gambassi G, Rapaccini GL, Iaconelli A, Zileri Del Verme L, Petricca L, Fedele AL, Lizzio MM, Tamburrini E, Natalello G, Gigante L, Bruno D, Verardi L, Taddei E, Calabrese A, Lombardi F, Bernabei R, Cauda R, Franceschi F, Landolfi R, Richeldi L, Sanguinetti M, Fantoni M, Antonelli M, Gasbarrini A, GEMELLI AGAINST COVID-19 Group. 2020. Sarilumab use in severe SARS-CoV2 pneumonia. EClinicalMedicine 27:100553. 10.1016/j.eclinm.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della-Torre E, Campochiaro C, Cavalli G, De Luca G, Napolitano A, La Marca S, Boffini N, Da Prat V, Di Terlizzi G, Lanzillotta M, Rovere Querini P, Ruggeri A, Landoni G, Tresoldi M, Ciceri F, Zangrillo A, De Cobelli F, Dagna L. 2020. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hypeinflammation: an open-label cohort study. Ann Rheum Dis 79:1277–1285. 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanofi provides update on Kezvara (sarilumab) phase trial in severe and critically ill COVID-19 patients outside the U.S. Press release. September 1, 2020. (https://www.sanofi.com/en/media-room/press-releases/2020/2020-09-01-07-00-00).
- 19.Sanofi and Regeneron provide update on Kezvara (sarilumab) phase 3 U.S. trial in COVID-19 patients. Press release. July 2, 2020. (https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00).
- 20.Lescure F-X, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O, Sarilumab COVID-19 Global Study Group. 2021. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 9:522–532. 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RECOVERY Collaborative Group. 2021. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. Lancet 397:1637–1645. 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd LE, Freidlin B, Korn EL. 2021. Platform trials-beware of the noncomparable control group. N Engl J Med 384:1572–1573. 10.1056/NEJMc2102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, Schoenfeld D, Chuang-Stein C, Cosgrove SE, Fowler VG, Lautenbach E, Chambers HF. 2015. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 61:800–806. 10.1093/cid/civ495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.León López R, Fernández SC, Limia Pérez L, Romero Palacios A, Fernández-Roldán MC, Aguilar Alonso E, Pérez Camacho I, Rodriguez-Baño J, Merchante N, Olalla J, Esteban-Moreno MÁ, Santos M, Luque-Pineda A, Torre-Cisneros J. 2020. Efficacy and safety of early treatment with sarilumab in hospitalised adults with COVID-19 presenting cytokine release syndrome (SARICOR STUDY): protocol of a phase II, open-label, randomised, multicentre, controlled clinical trial. BMJ Open 10:e039951. 10.1136/bmjopen-2020-039951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.02107-21-s0001.pdf, PDF file, 0.2 MB (161.8KB, pdf)
Data Availability Statement
Deidentified participant data is available upon request and after specific approval by the Regional Government of Andalusia and the Spanish Agency of Medicines and Medical Products.