ABSTRACT
Wound infections are caused by bacteria and/or fungi. The presence of fungal biofilms in wound beds presents a unique challenge, as fungal biofilms may be difficult to eradicate. The goal of this work was to assess the in vitro antibiofilm activity of an H2O2-producing electrochemical bandage (e-bandage) against 15 yeast isolates representing commonly encountered species. Time-dependent decreases in viable biofilm CFU counts of all isolates tested were observed, resulting in no visible colonies with 48 h of exposure by plate culture. Fluorescence microscopic analysis showed extensive cell membrane damage of biofilm cells after e-bandage treatment. Reductions in intracellular ATP levels of yeast biofilm cells were recorded post e-bandage treatment. These results suggest that exposure to H2O2-producing e-bandages reduces in vitro viable cell counts of yeast biofilms, making this a potential new topical treatment approach for fungal wound infections.
KEYWORDS: electrochemical bandage, hydrogen peroxide, yeast biofilm, Candida
INTRODUCTION
It has been estimated that in the United States alone, ∼6.5 million patients are affected by chronic wounds annually, with projected treatment costs exceeding ∼$25 billion per year (1). Chronic wounds frequently harbor bacteria and/or yeast (2–4). The presence of drug-resistant microorganisms in chronic wounds represents a complex public health challenge, which needs attention. Most wound infections are linked with microorganisms growing as biofilms. The presence of biofilms in wounds is often linked to reduced efficacy of clinical antimicrobial strategies (5, 6). Within a biofilm environment, microorganisms are encased in a secreted layer of extracellular polymeric substance (EPS), which provides protection against stressful environmental conditions, including that of many traditional antimicrobial agents (7, 8). The presence of biofilms in wound beds results in delayed wound healing, necrosis, and excessive inflammation (9–11). Hence, new treatment strategies, which do not rely on traditional antimicrobials, are needed to treat chronic wound infections associated with biofilms.
Some commonly employed approaches to treat chronic wound infections clinically include the use of topical antimicrobials, antiseptics, and biocides, such as formaldehyde, chlorhexidine gluconate, honey, sodium hypochlorite, povidone iodine, phenols, hypochlorous acid (HOCl), and hydrogen peroxide (H2O2) (12, 13). These agents are used for wound cleaning, debridement, and reducing inflammation. The antimicrobial activity of these agents is reduced in the presence of biofilms. H2O2 and HOCl are naturally found in low concentrations in wound beds (14, 15). Numerous studies have shown that the presence of low amounts of H2O2 can improve wound healing by promoting migration of keratinocytes and fibroblasts to the wound site (16, 17). Since H2O2 is not chemically stable and decomposes over time, it is not an ideal wound dressing. For effective wound dressing applications, continuous production of low amounts of H2O2 is a necessary prerequisite, and thus new technologies, which could effectively do this, may provide potential avenues for treating chronic wound infections.
Along with bacteria, the presence of fungi plays an important role in chronic wound infections (2, 4, 18). However, most of the research related to chronic wound infections is focused on bacterial biofilms. Given that human skin is also the host of a variety of yeast species, it is not surprising that yeasts are frequently found with bacteria in polymicrobial biofilm wound infections. Yeasts are most commonly found in wounds, such as diabetic foot ulcers, nonhealing surgical wounds, pressure ulcers, and venous leg ulcers (19). Many yeasts present on human skin are opportunistic pathogens and, given appropriate conditions, can survive in a biofilm mode. According to a study involving chronic wounds, almost 23% of wounds harbor fungi (20). Among the different yeasts found in chronic wounds, Candida species are most encountered; Candida albicans, Candida tropicalis, Candida pseudotropicalis, Candida parapsilosis, Candida auris, and Pichia kudriavzevii (formerly Candida krusei) have been associated with wound infections (2, 19, 21, 22). In earlier work, we developed and assessed in vitro antibiofilm activity of a novel proof-of-concept electrochemical bandage system (e-bandage) composed of carbon fabric with a 3-electrode system controlled by a wearable potentiostat, which continuously produced controlled amounts of H2O2 against monospecies and dual-species bacterial biofilms (23, 24). Given that chronic wounds can be infected with yeast, here, we assessed the in vitro antibiofilm activity of the H2O2-producing e-bandage against 15 clinical yeast isolates. Further, we investigated yeast biofilm viability through fluorescence microscopy and measured ATP levels after e-bandage treatment.
The e-bandage and wearable potentiostat development are based on our previous work (23). The e-bandage has three electrodes encased in a bandage-like structured mesh network: the working electrode and counter electrode are composed of a circular conductive carbon fabric patch having an area of 1.77 cm2 (Panex 30 PW-06; Zoltek Companies Inc., St. Louis, MO). The reference electrode is made of silver/silver chloride (Ag/AgCl) wire and acts as a quasireference electrode (QRE). Working electrode potential is controlled by a wearable potentiostat; maximum generation of H2O2 is achieved at −0.6 V Ag/AgCl (polarization potential) on the working electrode by oxygen reduction. Additional details describing construction of the e-bandage are found in reference 23. Prior to use, e-bandages were autoclaved at 121°C for 20 min.
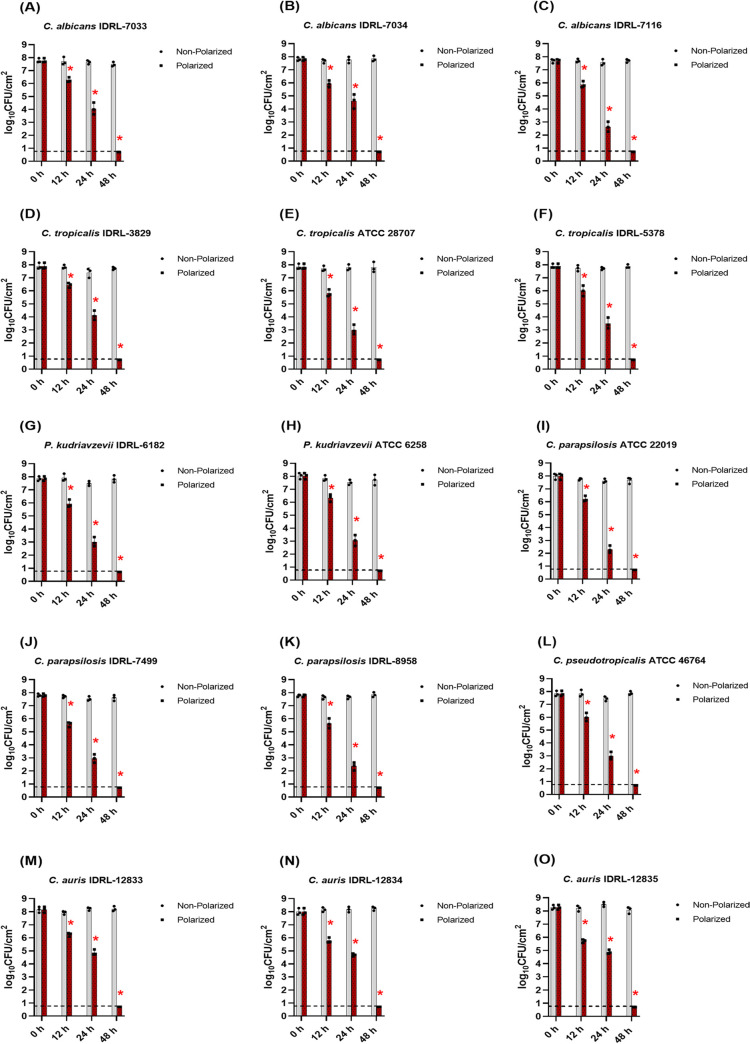
To evaluate antibiofilm activity against fungal biofilms, 15 yeast isolates (see Table S1 in the supplemental material) were tested. Yeast isolates were grown as biofilms on polycarbonate membranes on sheep blood agar plates for 48 h (see supplemental material), after which membrane biofilms were transferred to new sterile tryptic soy agar plates. Before starting e-bandage treatment, sterile e-bandages were soaked in sterile 1× phosphate buffer saline (1× PBS) in a Petri dish for 15 min. Next, 100 μl of sterile hydrogel (1.8% [wt/vol] xanthan gum in 1× PBS) was placed atop membrane biofilms between the fabric layers of e-bandage and also atop the e-bandage. Sterile Tegaderm covered the e-bandage surface. Electrode wires were connected to a wearable potentiostat, and a 3-V battery was inserted into the pocket of the potentiostat to start treatment (polarized treatment group). Yeast biofilms were exposed to polarized e-bandages that continuously produce H2O2 for 12, 24, and 48 h. The control group was composed of yeast biofilms exposed to nonpolarized e-bandages (i.e., e-bandages connected to potentiostats lacking batteries). To confirm e-bandage polarization potential, the potential of the working electrode relative to QRE was measured at the start and end of each experiment using a voltmeter. Batteries were replaced every 24 h. After e-bandage treatment, Tegaderm along with e-bandages were removed and e-bandages and membrane biofilms were combined and quantitively cultured, with results reported as log10 CFU/cm2 of membrane biofilm. Figure 1 shows the overall reduction in viable CFU counts of yeast biofilms after e-bandage treatment for 12, 24, and 48 h. All isolates treated with polarized e-bandage showed significant, time-dependent reductions in CFU counts, with 48 h exposure to polarized e-bandages resulting in no viable colony counts on agar plates (i.e., below the limit of quantification of 0.87 log10 CFU/cm2). Moreover, no growth was observed in broth cultures after 48 h of e-bandage treatment (i.e., below the limit of detection of 0.71 log10 CFU/cm2). After 12 h of e-bandage treatment, the mean CFU reduction was 1.84 log10 ± 0.32 CFU/cm2. Twenty-four hours of exposure to e-bandages resulted in a mean CFU reduction of 4.20 log10 ± 0.73 CFU/cm2. Based on the results of Fig. 1, no significant differences in biofilm reductions were observed in the nonpolarized groups among the isolates studied at all time points. Planktonic MICs were determined for one yeast isolate of each species using broth microdilution to determine susceptibilities against traditional antifungal agents (25). As seen in Table S2 in the supplemental material, no tested yeast isolates were resistant to anidulafungin, micafungin, or caspofungin, with varying susceptibilities to azole antifungal agents. As topical antifungal creams may be used to treat fungal skin infections, we tested 2% miconazole cream against selected yeast membrane biofilms. Interestingly, no significant CFU reductions were observed with miconazole treatment (see Fig. S1 in the supplemental material). These results were in contrast to those with the e-bandage system.
FIG 1.
E-bandage treatment of yeast biofilms at 12, 24, and 48 h. Data points represent means, and error bars represent standard deviations (n = 3). Comparisons across experimental groups were first performed using the Kruskal-Wallis test. Additional comparisons between experimental groups were performed using the Wilcoxon rank sum test in a pairwise manner. Data showing statistical significance (P value < 0.05) are denoted by an asterisk (*). Gray color bars represent the nonpolarized (control) group, and brown color bars represent the polarized (active treatment) group.
H2O2 is routinely used for wound debridement and disinfection in clinical settings. The U.S. Food and Drug Administration has approved the usage of up to a 3% H2O2 solution as a disinfectant and antiseptic (26). However, several studies have shown that when fungal cells are in a biofilm state, the H2O2 activity is reduced (27–29). Thus, high working concentrations of H2O2 are typically recommended for eliminating fungal biofilms. In one study, the authors observed that when grown in planktonic culture, yeast were susceptible to the recommended concentrations of H2O2. But, when the same isolates were grown as biofilms, much higher concentrations (i.e., above recommended concentrations) of H2O2 were required to remove the biofilms (30). In another study, it was reported that yeast biofilms had a significant reduction in susceptibility to H2O2, ethanol, and sodium dodecyl sulfate compared to that when they were grown in planktonic form, and that combination treatment with fluconazole increased activity of the above-mentioned biocides against biofilms (28). Using higher-than-recommended levels of biocides can result in mammalian cell toxicity, as H2O2 can result in oxidative damage via reactive oxygen species (ROS) generation (31, 32). It is in this context that the described e-bandage system is unique in that it can continuously produce low concentrations of H2O2, which can kill yeast in biofilms at concentrations that minimize mammalian toxicity (33).
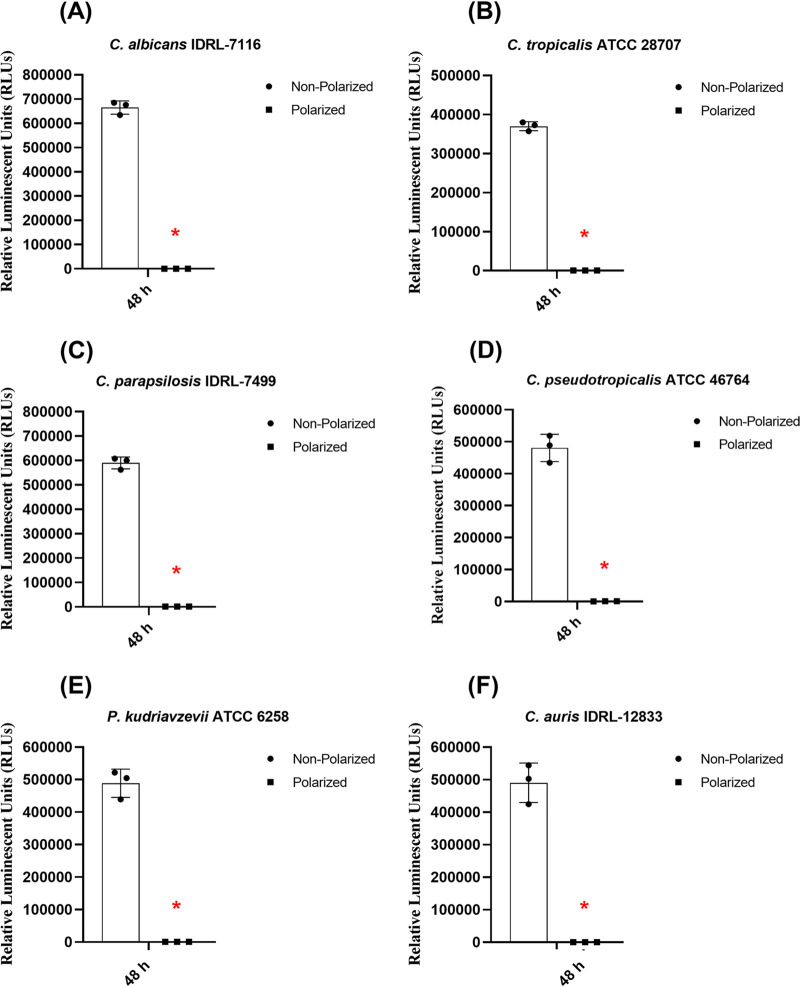
Maintaining intracellular ATP levels inside cells is critical in determining the metabolic health of cells. In the presence of external stimuli, the metabolic state of cells may be altered. Factors that alter ATP levels in fungal cells include changes in growth conditions, sudden changes in temperature, and the presence of toxic chemicals, including biocides and antifungal drugs. Some new investigative antifungal drugs specifically target components of the electron transport chain (34). Given that only metabolically viable cells continuously produce ATP, we performed a luciferin-based assay as a biofilm viability marker. In the presence of several biocidal agents, including H2O2, fungal cells are under extreme oxidative stress, which negatively impacts their metabolic state. Thus, we assessed overall intracellular levels of ATP in fungal cells after 48 h of continuous e-bandage exposure. The assay was performed according to the manufacturer's instructions with minor modifications (see the supplemental material). One yeast isolate from each species group was selected for this assay. Yeast biofilms were exposed to polarized e-bandages for 48 h, after which intracellular ATP levels were measured. As evident from Fig. 2, there was a decrease in the ATP levels of cells after e-bandage treatment. All isolates tested had similar reductions in the overall ATP levels post e-bandage treatment. Biofilms exposed to control (i.e., nonpolarized) e-bandages did not show a decrease in ATP levels. In a recent study, it was observed that in the presence of cannabidiol, overall ATP levels of cells in C. albicans biofilm are reduced (35). The authors attributed this decrease to elevated intracellular ROS generation. In another study, when C. albicans cells were exposed to H2O2, cells went into “programmed cell death” due to increased caspase activity and decreased intracellular ATP levels (36). In a study conducted by Qin et al., the authors provide evidence that excessive ROS generation caused due to H2O2 exposure results in oxidative damage of mitochondrial proteins, respiratory chain complexes I and III, and mitochondrial phosphate carrier protein. All of these significantly affected the working mechanism of ATP synthase by inhibiting its activity and eventually leading to collapse of mitochondrial membrane potential (37). In the presence of a polarized e-bandage, yeast cells are under constant oxidative stress, which could result in a decrease of cell membrane potential and membrane depolarization and blocking of regular working mechanisms of the electron transport chain, affecting ATP synthesis and eventually leading to cell death. Further studies are necessary to evaluate how the presence of H2O2 affects various parts of the electron transport system, such as transition metal/heme-like structures located in the inner mitochondrial membrane.
FIG 2.
Intracellular ATP measurement of yeast biofilm cells after 48 h of e-bandage treatment. Data points represent means, and error bars represent standard deviations (n = 3). Comparisons across experimental groups were first performed using the Kruskal-Wallis test. Additional comparisons between experimental groups were performed using the Wilcoxon rank sum test in a pairwise manner. Data showing statistical significance (P value < 0.05) are denoted by an asterisk (*) in the graphs.
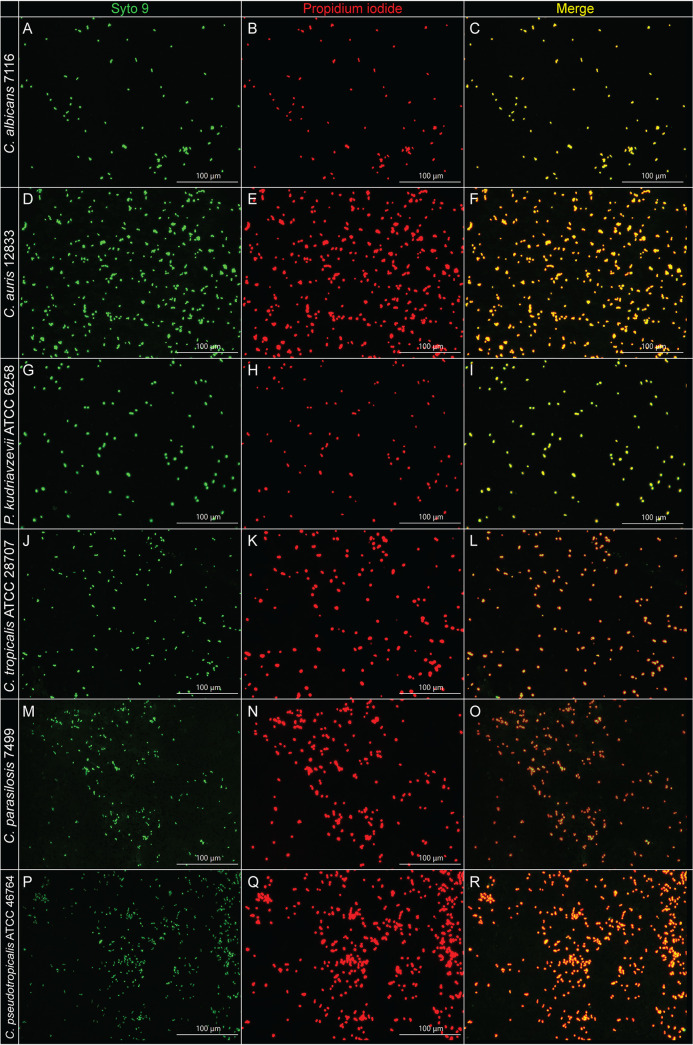
Biocides like H2O2 increase oxidative stress in cells. H2O2 is a member of the ROS family, which also includes hydroxyl radicals and superoxide radicals (31, 32). These oxygen species interact with cell membrane components, DNA, RNA, and other intracellular cellular components (31, 38). Exposure to high levels of H2O2 is associated with cell membrane damage. Thus, to assess if exposure of yeast biofilm cells to polarized e-bandages results in cell membrane damage, a live/dead cell membrane staining assay was performed. One yeast isolate of each species was selected for this assay. Yeast biofilms grown on membranes were subjected to 48 h of treatment in the presence of polarized e-bandages, following which cells were stained with a mixture of SYTO 9 and propidium iodide (see the supplemental material). Stained cells were observed under a fluorescence microscope. SYTO 9, a green-fluorescent dye, stains both live and dead cells green, whereas propidium iodide, a red-fluorescent dye, stains membrane-damaged cells/dead cells red. Figure 3 shows fluorescent images of yeast cells stained with the above-mentioned dyes after 48 h of polarized e-bandage exposure. All cells that were green in the panels turned red after e-bandage treatment, suggesting that following treatment, yeast cells are dead and have extensive cell membrane damage due to exposure to the H2O2-producing e-bandages. In contrast, cells in the control group (i.e., nonpolarized e-bandage) did not show significant cell membrane damage (see Fig. S2 in the supplemental material). In addition to live/dead imaging, we performed scanning electron microscopy (SEM) imaging on C. albicans IDRL-7116 after exposure to H2O2-producing e-bandages (see the supplemental material). As evident from Fig. S3 in the supplemental material, biofilms exposed to both nonpolarized and polarized e-bandages showed yeast cells along with hyphal networks, with no apparent differences in cell/hyphal morphology between nonpolarized and polarized groups, suggesting that H2O2 produced by e-bandages may not result in morphological changes in the biofilm structure. Several antifungal drugs (e.g., ergosterol) used in clinical settings target fungal cell membrane components (39, 40). In one study, novel heterocyclic compounds based on thiazolidinedione and succinimide derivatives were active antifungal agents with significant antibiofilm activity against C. albicans biofilm (41). The authors performed live/dead staining on Candida cells exposed to heterocyclic compounds and observed significant cell membrane damage and cell death. Results of live/dead staining and SEM imaging suggest that H2O2-producing e-bandages might (i) impair homeostasis of regulated ergosterol production which could result in membrane destabilization, (ii) result in lipid peroxidation of cell membranes via production of hydroxyl radicals which alter activity of transition metals and enzymes, thereby damaging cell membrane integrity, (iii) disrupt and inactivate the ATP synthase pump of the mitochondrial electron transport system, and/or (iv) damage fungal nucleic acids because of excessive production of ROS.
FIG 3.
Live/dead staining of yeast biofilms after 48 h of e-bandage treatment. Biofilm cells were stained with a mixture of SYTO 9 and propidium iodide dyes (live and dead cells appear green with STYO 9 dye while only dead cells appear red with propidium iodide dye). Total magnification, ×400.
In summary, results obtained in this work demonstrate that an H2O2-producing e-bandage is active against in vitro yeast biofilms and that continuous exposure to e-bandages significantly reduces overall biofilm CFU counts, reduces overall intracellular ATP levels, and causes cell membrane damage to yeast biofilm cells. These results suggest that H2O2-producing e-bandages have the potential to be used as a new wound dressing to treat yeast biofilm infections, though in vivo studies are clearly required.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under grant number R01 AI091594. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors acknowledge the technical assistance provided by Mayo Clinic Microscopy and Cell Analysis Core (special mention to Scott Gamb) in performing SEM imaging.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. 2009. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17:763–771. 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend EM, Sherry L, Kean R, Hansom D, Mackay WG, Williams C, Butcher J, Ramage G. 2017. Implications of antimicrobial combinations in complex wound biofilms containing fungi. Antimicrob Agents Chemother 61:e00672-17. 10.1128/AAC.00672-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harriott MM, Noverr MC. 2011. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol 19:557–563. 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalan L, Grice EA. 2018. Fungi in the wound microbiome. Adv Wound Care (New Rochelle) 7:247–255. 10.1089/wound.2017.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wound Repair Regen 16:37–44. 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 6.Douglas LJ. 2003. Candida biofilms and their role in infection. Trends Microbiol 11:30–36. 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 7.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 8.O'Toole GA, Stewart PS. 2005. Biofilms strike back. Nat Biotechnol 23:1378–1379. 10.1038/nbt1105-1378. [DOI] [PubMed] [Google Scholar]
- 9.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baranoski S, Ayello EA. 2008. Wound care essentials: practice principles. Lippincott Williams & Wilkins, Hagerstown, MD. [Google Scholar]
- 11.Velnar T, Bailey T, Smrkolj V. 2009. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37:1528–1542. 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 12.Lipsky BA, Hoey C. 2009. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 49:1541–1549. 10.1086/644732. [DOI] [PubMed] [Google Scholar]
- 13.Jones CE, Kennedy JP. 2012. Treatment options to manage wound biofilm. Adv Wound Care (New Rochelle) 1:120–126. 10.1089/wound.2011.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atiyeh BS, Dibo SA, Hayek SN. 2009. Wound cleansing, topical antiseptics and wound healing. Int Wound J 6:420–430. 10.1111/j.1742-481X.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. 2006. Dermal wound healing is subject to redox control. Mol Ther 13:211–220. 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drosou A, Falabella A, Kirsner RS. 2003. Antiseptics on wounds: an area of controversy. Wounds 15:149–166. [Google Scholar]
- 17.Zhu G, Wang Q, Lu S, Niu Y. 2017. Hydrogen peroxide: a potential wound therapeutic target. Med Princ Pract 26:301–308. 10.1159/000475501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malone M, Dickson HG. 2016. Understanding the role of fungi in chronic wounds. mBio 7:e01898-16. 10.1128/mBio.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M. 2017. Candida species biofilms’ antifungal resistance. J Fungi 3:8. 10.3390/jof3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowd S, Delton Hanson J, Rees E, Wolcott R, Zischau A, Sun Y, White J, Smith D, Kennedy J, Jones C. 2011. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J Wound Care 20:40–47. 10.12968/jowc.2011.20.1.40. [DOI] [PubMed] [Google Scholar]
- 21.Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 299:1–8. 10.1111/j.1574-6968.2009.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley SF. 2019. Candida auris Infection. JAMA 322:1526. 10.1001/jama.2019.13857. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed A, Anoy MMI, Tibbits G, Raval YS, Flurin L, Greenwood‐Quaintance KE, Patel R, Beyenal H. 2021. Hydrogen peroxide‐producing electrochemical bandage controlled by a wearable potentiostat for treatment of wound infections. Biotechnol Bioeng 118:2815–2821. 10.1002/bit.27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raval YS, Mohamed A, Flurin L, Mandrekar JN, Quaintance KEG, Beyenal H, Patel R. 2021. Hydrogen-peroxide generating electrochemical bandage is active in vitro against mono- and dual-species biofilms. Biofilm 3:100055. 10.1016/j.bioflm.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—4th ed. CLSI M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Linley E, Denyer SP, McDonnell G, Simons C, Maillard J-Y. 2012. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother 67:1589–1596. 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 27.Kean R, McKloud E, Townsend EM, Sherry L, Delaney C, Jones BL, Williams C, Ramage G. 2018. The comparative efficacy of antiseptics against Candida auris biofilms. Int J Antimicrob Agents 52:673–677. 10.1016/j.ijantimicag.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Nett JE, Guite KM, Ringeisen A, Holoyda KA, Andes DR. 2008. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob Agents Chemother 52:3411–3413. 10.1128/AAC.01656-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theraud M, Bedouin Y, Guiguen C, Gangneux J-P. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J Med Microbiol 53:1013–1018. 10.1099/jmm.0.05474-0. [DOI] [PubMed] [Google Scholar]
- 30.Leung C, Chan Y, Samaranayake L, Seneviratne C. 2012. Biocide resistance of Candida and Escherichia coli biofilms is associated with higher antioxidative capacities. J Hosp Infect 81:79–86. 10.1016/j.jhin.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 32.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR, Beyenal H. 2015. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Sci Rep 5:14908. 10.1038/srep14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvenage L, Munro CA, Gourlay CW. 2019. The potential of respiration inhibition as a new approach to combat human fungal pathogens. Curr Genet 65:1347–1353. 10.1007/s00294-019-01001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman M, Sionov RV, Mechoulam R, Steinberg D. 2021. Anti-biofilm activity of cannabidiol against Candida albicans. Microorganisms 9:441. 10.3390/microorganisms9020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Huang S, Dai B, Zhu Z, Lu H, Dong L, Cao Y, Wang Y, Gao P, Chai Y, Jiang Y. 2009. Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress-induced death and change in energy metabolism. Fungal Genet Biol 46:183–189. 10.1016/j.fgb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Qin G, Liu J, Cao B, Li B, Tian S. 2011. Hydrogen peroxide acts on sensitive mitochondrial proteins to induce death of a fungal pathogen revealed by proteomic analysis. PLoS One 6:e21945. 10.1371/journal.pone.0021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seaver LC, Imlay JA. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol 183:7182–7189. 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odds FC, Brown AJ, Gow NA. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 41.Kagan S, Jabbour A, Sionov E, Alquntar AA, Steinberg D, Srebnik M, Nir-Paz R, Weiss A, Polacheck I. 2014. Anti-Candida albicans biofilm effect of novel heterocyclic compounds. J Antimicrob Chemother 69:416–427. 10.1093/jac/dkt365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01792-21-s0001.pdf, PDF file, 0.6 MB (647.9KB, pdf)