ABSTRACT
We analyzed risk factors for mortality in febrile neutropenic patients with bloodstream infections (BSI) presenting with septic shock and assessed the impact of empirical antibiotic regimens. A multicenter retrospective study (2010 to 2019) of two prospective cohorts compared BSI episodes in patients with or without septic shock. Multivariate analysis was performed to identify independent risk factors for mortality in episodes with septic shock. Of 1,563 patients with BSI, 257 (16%) presented with septic shock. Those patients with septic shock had higher mortality than those without septic shock (55% versus 15%, P < 0.001). Gram-negative bacilli caused 81% of episodes with septic shock, Gram-positive cocci caused 22%, and Candida species caused 5%. Inappropriate empirical antibiotic treatment (IEAT) was administered in 17.5% of septic shock episodes. Empirical β-lactam combined with other active antibiotics was associated with the lowest mortality observed. When amikacin was the only active antibiotic, mortality was 90%. Addition of empirical specific Gram-positive coverage had no impact on mortality. Mortality was higher when IEAT was administered (76% versus 51%, P = 0.002). Age of >70 years (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.2 to 4.7), IEAT for Candida spp. or Gram-negative bacilli (OR, 3.8; 95% CI, 1.3 to 11.1), acute kidney injury (OR, 2.6; 95% CI, 1.4 to 4.9), and amikacin as the only active antibiotic (OR, 15.2; 95% CI, 1.7 to 134.5) were independent risk factors for mortality, while the combination of β-lactam and amikacin was protective (OR, 0.32; 95% CI, 0.18 to 0.57). Septic shock in febrile neutropenic patients with BSI is associated with extremely high mortality, especially when IEAT is administered. Combination therapy including an active β-lactam and amikacin results in the best outcomes.
KEYWORDS: bacteremia, empirical treatment, mortality, neutropenia, shock
INTRODUCTION
Bloodstream infections (BSI) are the most frequent infection in febrile neutropenic, onco-hematological patients, with incidence rates spanning from 10% to 38% (1–3). Septic shock is the most severe clinical presentation form of such infection. However, there is scarce information on incidence, characteristics, and outcomes of neutropenic patients who present with this complication (4–6). This information, nonetheless, is now more important than ever. Rates of Gram-negative bacilli (GNB) in onco-hematological patients are progressively increasing (7, 8), which could impact a greater percentage of patients presenting with septic shock (9). Additionally, empirical antibiotic therapy is challenging in the era of emerging multidrug-resistant (MDR) GNB. Indeed, inappropriate empirical antibiotic therapy (IEAT) has been associated with increased mortality in patients with febrile neutropenia and BSI (8, 10, 11).
For many years, international guidelines have recommended broadening the antimicrobial spectrum to cover drug-resistant Gram-negative and Gram-positive microorganisms in neutropenic patients with septic shock. This includes considering the addition of aminoglycosides, quinolones, vancomycin, and/or anti-Candida coverage to classic antipseudomonal β-lactams (cefepime, ceftazidime, piperacillin-tazobactam, or meropenem) (12). However, based on the current epidemiological context, we hypothesized that such antibiotic recommendations should be reassessed.
Therefore, we aimed to describe the frequency, clinical characteristics, and etiology of BSI in febrile neutropenic patients with septic shock and analyze risk factors for mortality. We also assessed the impact of different empirical antibiotic regimens on mortality.
RESULTS
Characteristics of and outcomes in patients with and without shock.
Over the study period, 1,563 BSI episodes were identified in onco-hematological patients with febrile neutropenia. Of these, 257 (16%) presented with septic shock. Table 1 and also Table S2 in the supplemental material describe the main clinical and demographic characteristics in patients with and without shock. Overall, 30-day mortality was 22%, being higher in patients with septic shock (55% versus 15%, P < 0.001).
TABLE 1.
Clinical and demographic characteristics of patients with BSI episodes presenting with and without septic shockc
| Characteristic | All episodes (n = 1,563) | No septic shock (n = 1,306) | Septic shock (n = 257) | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, median (IQR), yr | 59 (48–67) | 61 (51–69) | 59 (47–66) | 0.616 |
| Male sex | 918 (59) | 768 (59) | 150 (58) | 0.896 |
| Underlying disease | ||||
| Hematological malignancy | 1,348 (86) | 1,168 (89) | 180 (70) | <0.001 |
| Solid neoplasma | 238 (15) | 157 (12) | 81 (32) | <0.001 |
| Hematopoietic stem cell transplant | 400 (26) | 355 (27) | 45 (18) | 0.001 |
| Allogenic/autologous | 249/151 (62/38)b | 215/140 (61/39)b | 34/11 (76/24)b | 0.051 |
| Any comorbidity | 456 (29) | 366 (28) | 90 (35) | 0.024 |
| Corticosteroid therapy | 588 (38) | 461 (35) | 127 (49) | <0.001 |
| Nosocomial BSI (vs health care or community acquired) | 999 (64) | 883 (68) | 116 (45) | <0.001 |
| Source of BSI | ||||
| Endogenous/unknown | 763 (49) | 650 (50) | 113 (44) | 0.089 |
| Catheter related | 333 (21) | 309 (24) | 24 (9) | <0.001 |
| Abdominal | 102 (7) | 72 (6) | 30 (12) | <0.001 |
| Pulmonary | 97 (6) | 49 (4) | 48 (19) | <0.001 |
| Urinary | 83 (5) | 62 (5) | 21 (8) | 0.025 |
| Inappropriate empirical antibiotic therapy | 471 (30) | 426 (32.6) | 45 (17.5) | <0.001 |
| For Gram-positive cocci | 290 (18.6) | 277 (21.2) | 13 (5.1) | <0.001 |
| For Gram-negative bacilli | 146 (9.3) | 121 (9.3) | 25 (9.7) | 0.816 |
| Outcome | ||||
| Mechanical ventilation requirement | 100 (6.6) | 29 (2.3) | 71 (27.6) | <0.001 |
| 30-day mortality | 342 (21.9) | 201 (15.4) | 141 (54.9) | <0.001 |
There were 25 patients who had both a hematological malignancy and a solid neoplasm.
Percentage among hematopoietic stem cell transplant recipients.
Abbreviations: IQR, interquartile range; BSI, bloodstream infection. All values except age are shown as no. (%).
Table 2 displays the etiological microorganisms involved in episodes presenting with or without shock. Overall, GNB caused 56% of bacteremia episodes, with 13% of all episodes being caused by MDR-GNB. Gram-positive cocci (GPC) caused 42% of all BSI episodes, and Candida spp. caused 3%. Of all bacteremia episodes, 12% were polymicrobial. In patients with septic shock, specifically, 81% of the episodes were caused by GNB, 22% by GPC, and 5% by Candida spp.
TABLE 2.
Etiological microorganisms in BSI episodes presenting with and without septic shocka
| Microorganism | All episodes (n = 1,563) | No septic shock (n = 1,306) | Septic shock (n = 257) | P value |
|---|---|---|---|---|
| Gram-positive cocci | 656 (42) | 600 (46) | 56 (22) | <0.001 |
| Coagulase-negative staphylococci | 291 (19) | 280 (21) | 11 (4) | <0.001 |
| S. aureus | 72 (5) | 65 (5) | 7 (3) | 0.142 |
| MRSA | 12 (17)* | 8 (12)* | 4 (57)* | 0.120 |
| Enterococcus spp. | 222 (14) | 205 (16) | 17 (7) | <0.001 |
| E. faecalis | 66 (30)* | 56 (27)* | 10 (59)* | 0.772 |
| E. faecium | 148 (67)* | 142 (69)* | 6 (35)* | <0.001 |
| E. faecium, vancomycin resistant | 10 (5)* | 10 (5)* | 0 | 0.383 |
| Streptococcus spp. | 98 (6) | 76 (6) | 22 (9) | 0.098 |
| S. pneumoniae | 17 (17)* | 10 (13)* | 7 (32)* | 0.006 |
| Gram-negative bacilli | 881 (56) | 674 (52) | 207 (81) | <0.001 |
| E. coli | 376 (24) | 284 (22) | 92 (36) | <0.001 |
| ESBL | 80 (21)* | 67 (24)* | 13 (14)* | 0.962 |
| Carbapenemase | 2 (1)* | 2 (1)* | 0 | 1.000 |
| Klebsiella spp. | 137 (9) | 103 (8) | 34 (13) | 0.006 |
| ESBL | 32 (23)* | 27 (26)* | 5 (15)* | 0.900 |
| Carbapenemase | 3 (2)* | 3 (3)* | 0 | 1.000 |
| P. aeruginosa | 234 (15) | 162 (12) | 72 (28) | <0.001 |
| MDR | 59 (25)* | 46 (28)* | 13 (18)* | 0.238 |
| XDR | 38 (16)* | 30 (19)* | 8 (11)* | 0.438 |
| Enterobacter spp. | 56 (4) | 49 (4) | 7 (3) | 0.418 |
| AmpC-hyperproducer strain | 9 (16)* | 8 (16)* | 1 (14)* | 1.000 |
| S. maltophilia | 35 (2) | 33 (3) | 2 (1) | 0.104 |
| MDR-GNB | 210 (13) | 171 (13) | 39 (15) | 0.371 |
| Candidemia | 50 (3) | 37 (3) | 13 (5) | 0.064 |
| C. albicans | 11 (22)* | 6 (16)* | 5 (38)* | 0.009 |
| Non-albicans candidemia | 39 (78)* | 31 (84)* | 8 (62)* | 0.487 |
| Polymicrobial | 180 (12) | 146 (11) | 34 (13) | 0.347 |
Abbreviations: MRSA, methicillin-resistant S. aureus; ESBL, extended-spectrum β-lactamase; MDR, multidrug resistant; XDR, extensively drug resistant; GNB, Gram-negative bacilli. *, asterisk indicates percentage among its species.
Figure S1 details the most significant differences in baseline characteristics, epidemiology, and outcomes between patients with and without septic shock.
Antibiotic therapy and outcomes in patients with septic shock.
Table S3 shows the comparison of antibiotic therapy in patients with and without septic shock. Among those episodes presenting with septic shock, 17.5% received IEAT, mainly in BSI caused by Pseudomonas aeruginosa and Candida spp. Table S4 summarizes the set of etiological agents of episodes with septic shock receiving IEAT. Acute kidney injury (AKI) presented in 71.5% of patients with septic shock.
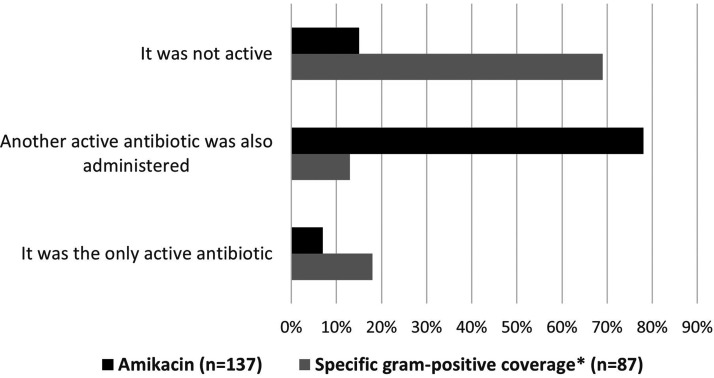
Figure 1 shows additional values for specific Gram-positive coverage and amikacin, respectively, in patients with septic shock. Both Gram-positive coverage and amikacin were administered almost exclusively in combination with other antibiotics. Specific Gram-positive coverage (including vancomycin, teicoplanin, daptomycin, and linezolid) was used in 87 of 257 (34%) cases. In 27 of 87 (31%) cases, the isolated microorganism was susceptible to specific Gram-positive coverage, and Gram-positive coverage was the only active antibiotic in 16 of 87 (18%) cases. Amikacin was used in 137 (53%) episodes. In 126 (85%) of these 137 cases, the isolated microorganism was susceptible to this antibiotic, and in 10 (7%) of these 137 cases, amikacin was the only active antibiotic.
FIG 1.
Additional value for empirical combination treatment with specific Gram-positive coverage and amikacin in patients with septic shock regarding antimicrobial susceptibility of the isolated microorganism. For specific Gram-positive coverage, (i) isolated microorganism was resistant to Gram-positive coverage in 69% of cases; (ii) isolated microorganism was susceptible to Gram-positive coverage, but another active antibiotic was also administered in 13% of cases; and (iii) Gram-positive coverage was the only active antibiotic in 18% of cases. For amikacin, (i) isolated microorganism was resistant to amikacin in 15% of cases; (ii) isolated microorganism was susceptible to amikacin, but another active antibiotic was also administered in 78% of cases; and (iii) amikacin was the only active antibiotic in 7% of cases. *, including glycopeptides (vancomycin and teicoplanin), daptomycin, and linezolid.
Risk factors for mortality in patients with septic shock.
Overall, 141 (55%) patients with septic shock died within 30 days of BSI onset. In the univariate analysis, patients with BSI and septic shock who had chronic leukemia (91% versus 53%, P = 0.014), pulmonary source of BSI (69% versus 52%, P = 0.032), BSI caused by MDR P. aeruginosa (85% versus 53%, P = 0.042), and episodes presenting with AKI (62% versus 38%, P = 0.001) and requiring mechanical ventilation (85% versus 44%, P < 0.001) experienced higher mortality. Conversely, an endogenous source was associated with lower mortality (48% versus 60%, P = 0.043).
Empirical β-lactam (53% versus 79%, P = 0.032) was associated with lower mortality in patients with septic shock compared to those who did not receive empirical β-lactam. Empirical combination therapy with a β-lactam plus amikacin was associated with lower mortality compared to those receiving β-lactam without amikacin (41% versus 70%, respectively; P < 0.001). Combination therapy including Gram-positive coverage (namely, vancomycin, teicoplanin, daptomycin, or linezolid) had no significant impact on mortality (48.8% in episodes receiving specific Gram-positive coverage versus 57.9% in those not receiving Gram-positive coverage, P = 0.169).
Mortality in episodes receiving IEAT was 76%, contrasting with the 51% reported in patients receiving appropriate empirical treatment (P = 0.002). Specifically, in those episodes caused by GNB presenting with shock, mortality was 83% in cases of IEAT and 51% in cases of appropriate empirical therapy (P = 0.003). When analyzing BSI episodes due to GPC, we did not observe any significant differences in mortality regarding appropriateness of empirical treatment (54% versus 52%, P = 0.926).
Table 3 shows mortality rates according to active empirical antibiotic coverage administered to patients with GNB BSI presenting with septic shock. In those cases, in which amikacin was the only active antibiotic administered (mainly because the isolated microorganism was resistant to the combined β-lactam), the mortality rate was 90%. Conversely, when only a β-lactam was active, mortality was 66%. The lowest mortality reported was when patients received an active combination of β-lactam and either amikacin (39%) or quinolone (33%). When selecting those episodes caused by microorganisms susceptible to both β-lactam and amikacin, mortality was 65.6% (40/61) in patients receiving monotherapy with a β-lactam versus 39.6% (42/106) in those receiving combination therapy with amikacin.
TABLE 3.
Mortality according to active empirical antibiotic coverage administered in Gram-negative bloodstream infection with septic shocka
| Active antibiotic(s) | Survival, n (%) | Death, n (%) |
|---|---|---|
| Only 1 β-lactam was active (n = 64) | 22 (34) | 42 (66) |
| Only amikacin was active (n = 10) | 1 (10) | 9 (90) |
| Combined β-lactam and amikacin were both active (n = 101) | 62 (61) | 39 (39) |
| Combined β-lactam, quinolone, and amikacin were all active (n = 4) | 2 (50) | 2 (50) |
| Combined β-lactam and quinolone were both active (n = 6) | 4 (67) | 2 (33) |
| No active empirical antibiotic was administered (n = 22) | 3 (14) | 19 (86) |
P value for all data is <0.001.
Table 4 shows the univariate and multivariate analyses of risk factors for mortality in patients with shock. In the multivariate analysis, age of >70 years (odds ratio [OR], 2.4; 95% confidence interval [CI], 1.2 to 4.7), IEAT for GNB or Candida spp. (OR, 3.8; 95% CI, 1.3 to 11.1), episodes presenting with AKI (OR, 2.6; 95% CI, 1.4 to 4.9), and the reception of amikacin as the only active antibiotic (OR, 15.2; 95% CI, 1.7 to 134.5) were independent risk factors for increased mortality, while combination therapy with a β-lactam and amikacin was protective (OR, 0.3; 95% CI, 0.2 to 0.6). The Hosmer-Lemeshow test was 0.232, and the area under the receiver operating characteristic (ROC) curve was 0.74 (95% CI, 0.68 to 0.80), showing a moderate ability to predict mortality. The same variables, with minor changes in OR values, were independently associated with mortality when the propensity score for receiving IEAT in BSI caused by GNB or Candida spp. was incorporated into the model (ROC curve, 0.77; 95% CI, 0.70 to 0.83). The Durbin-Watson test was 2.161 and variance inflation factor was <2 for all variables, suggesting the lack of collinearity among variables used in the multivariable analysis.
TABLE 4.
Risk factors for overall mortality, by univariate and multivariate analysisa
| Risk factor | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value |
|---|---|---|---|---|
| Male sex | 0.78 (0.48–1.30) | 0.346 | ||
| Age ≥70 yr | 2.23 (1.20–4.15) | 0.010 | 2.36 (1.19–4.68) | 0.014 |
| Acute leukemia | 0.65 (0.38–1.13) | 0.125 | ||
| Non-Hodgkin lymphoma | 0.94 (0.52–1.72) | 0.847 | ||
| Multiple myeloma | 0.90 (0.37–2.19) | 0.811 | ||
| Chronic leukemia | 8.78 (1.10–69.63) | 0.014 | 5.02 (0.60–42.22) | 0.138 |
| Solid neoplasia | 0.96 (0.57–1.64) | 0.906 | ||
| Hematopoietic stem cell transplantation | 1.29 (0.67–2.48) | 0.446 | ||
| Any comorbidity | 1.04 (0.62–1.75) | 0.870 | ||
| Corticosteroid therapy | 1.16 (0.71–1.89) | 0.560 | ||
| Nosocomial acquisition | 1.41 (0.86–2.31) | 0.177 | ||
| Pulmonary source | 2.06 (1.06–4.01) | 0.032 | 1.35 (0.58–3.18) | 0.486 |
| Endogenous/unknown source | 0.60 (0.37–0.98) | 0.043 | 0.69 (0.39–1.23) | 0.211 |
| Catheter-related BSI | 0.81 (0.35–1.87) | 0.615 | ||
| Acute kidney injury | 2.48 (1.41–4.37) | 0.001 | 2.60 (1.39–4.90) | 0.003 |
| Empirical β-lactam | 0.26 (0.73–0.94) | 0.037 | 0.41 (0.08–2.16) | 0.294 |
| Empirical carbapenem | 0.94 (0.58–1.55) | 0.819 | ||
| Empirical β-lactam plus aminoglycoside | 0.30 (0.18–0.50) | <0.001 | 0.32 (0.18–0.57) | <0.001 |
| Empirical β-lactam plus specific Gram-positive coverage | 0.69 (0.41–1.17) | 0.169 | ||
| Amikacin as the only active antibiotic | 7.84 (0.98–62.83) | 0.025 | 15.24 (1.73–134.45) | 0.014 |
| β-Lactam as the only active antibiotic | 1.81 (1.01–3.26) | 0.046 | 1.66 (0.72–3.82) | 0.236 |
| Coagulase-negative staphylococci | 0.34 (0.09–1.34) | 0.193 | ||
| Staphylococcus aureus | 2.10 (0.40–11.01) | 0.462 | ||
| Enterococcus spp. | 1.19 (0.44–3.23) | 0.734 | ||
| Streptococcus spp. | 1.08 (0.45–2.55) | 0.867 | ||
| E. coli | 0.97 (0.58–1.62) | 0.901 | ||
| Klebsiella spp. | 0.80 (0.39–1.64) | 0.541 | ||
| Pseudomonas aeruginosa | 1.32 (0.76–2.29) | 0.329 | ||
| MDR P. aeruginosa | 3.19 (0.87–11.71) | 0.096 | ||
| MDR-GNB | 1.57 (0.77–3.18) | 0.208 | ||
| Candidemia | 4.82 (1.05–22.22) | 0.042 | 2.18 (0.34–13.94) | 0.411 |
| Polymicrobial | 1.86 (0.86–3.99) | 0.108 | ||
| Inappropriate empirical antibiotic therapy for GNB or Candida spp. | 5.74 (2.14–15.38) | <0.001 | 3.81 (1.31–11.11) | 0.014 |
Abbreviations: ESBL, extended-spectrum β-lactamase; MDR, multidrug resistant; GNB, Gram-negative bacilli. Boldface indicates statistically significant values (P value < 0.05).
DISCUSSION
The current study showed that neutropenic patients with BSI frequently presented with septic shock (16%), especially those with solid neoplasms or who had received corticosteroids. The fact that oncologic patients with BSI present with septic shock more frequently than hematological patients has been previously reported (13–15) and may be related to the higher incidence of pulmonary, abdominal, and urinary source in this population. Another potential explanation is the higher presence of coagulase-negative staphylococcal BSI in hematological patients, which are less likely associated with septic shock.
Remarkably, BSI with septic shock is essentially caused by GNB, predominantly by Escherichia coli, P. aeruginosa, and Klebsiella spp. GNB and E. coli, especially, have been previously associated with the presence of septic shock in neutropenic patients (9, 16, 17). In fact, in the current study, these three pathogens together caused over three-quarters of all BSI episodes in patients with septic shock. Most importantly, and similar to a recent Korean study (16), almost 20% of GNB isolates producing septic shock were MDR. Conversely, GPC caused only 20% of BSI episodes with septic shock, and approximately one-third of those were polymicrobial episodes along with GNB. This was not unexpected, as most Gram-positive bacteria in this cohort were low-virulence pathogens like coagulase-negative staphylococci and Enterococcus spp. causing catheter-related episodes. Additionally, the overall prevalence of Staphylococcus aureus and Streptococcus spp. was relatively low.
Mortality in neutropenic patients with BSI and septic shock was extremely high (55%). Most series of patients with septic shock and BSI including nonneutropenic patients report remarkably lower mortalities ranging from 35% to 45% (9, 18, 19). This highlights the pivotal role of granulocytes in controlling infection (20), emphasizing the importance of early and effective treatment to diminish this devastating mortality in neutropenic patients.
The Infectious Diseases Society of America (IDSA) febrile neutropenic guidelines recommend adding specific Gram-positive coverage in patients with hemodynamic instability (12), even though common empirical regimens offer pertinent coverage for most GPC, excluding methicillin-resistant staphylococci and Enterococcus spp. However, in our cohort, we did not find that empirical, specific Gram-positive coverage had a significant impact on mortality. Several studies have demonstrated toxicity due to vancomycin in neutropenic patients (21), and new drugs like daptomycin could be associated with increased costs. In view of these results, specific Gram-positive coverage is questionable in patients without proven infection, suspicion of catheter-related BSI, or methicillin-resistant S. aureus (MRSA) colonization.
In this study, combination therapy with a β-lactam plus amikacin was an independent factor for lower mortality in patients with septic shock compared to those not receiving amikacin combination. A prior meta-analysis failed to demonstrate any benefit of β-lactam–aminoglycoside combination in neutropenic patients with cancer (22). However, this is still a controversial issue. For example, Martinez et al. showed that aminoglycoside combination was able to reduce mortality, but only in those episodes presenting with septic shock or neutropenia (23). It has been hypothesized that a potential benefit of combination therapy may be the broadening of the initial antimicrobial spectrum and a resulting decreased likelihood of IEAT (23). However, in this study, amikacin as the only active antibiotic (basically when the isolated microorganism is resistant to empirical β-lactam) was independently associated with increased mortality, suggesting that any potential benefit is mainly due to a synergistic effect (24, 25). Similar results were recently found in the AMINOLACTAM study, in which our hospitals participated (26), analyzing only BSI episodes receiving adequate empirical antibiotic therapy. In that study, focusing on hematologic neutropenic patients, empirical short-course aminoglycoside combined with a β-lactam significantly improved the 7-day case fatality rate, without significantly impairing renal function. However, as in the present study, mortality increased when the aminoglycoside was the only active antibiotic, suggesting again that the benefit of combination is mainly driven by an enhanced bactericidal effect.
AKI is commonly associated with increased mortality in patients with shock. In this sense, some may speculate that the protective role of amikacin in this cohort was due to less administration of the drug in those patients with AKI. However, in the multivariate analysis performed, both AKI and combination therapy with amikacin were independently associated with mortality.
Our study reinforces the relevance of β-lactam therapy adequacy to improve patient outcomes. Approximately 20% of patients with septic shock received IEAT, and this was mainly due to P. aeruginosa and Candida spp. Most importantly, mortality in episodes caused by GNB and Candida spp. presenting with septic shock and receiving IEAT was extremely high (83% and 90%, respectively). Considering that amikacin “monotherapy” is associated with increased mortality, warranting an active β-lactam is essential to improve the prognosis of this highly lethal entity. In this sense, new β-lactams such as ceftazidime-avibactam and ceftolozane-tazobactam should be considered empirical therapeutic options for patients with a higher risk of MDR-GNB, even in a potentially synergistic association with carbapenems (27). Following this broad and aggressive approach, early antibiotic deescalation is safe (28, 29) and must be a priority. Finally, identifying patients at risk of candidemia is critical when considering coverage for this relatively uncommon yet fatal complication (30).
The strengths of this study are the large number of patients included, prospective data collection, and thorough evaluation by an infectious disease expert. Additionally, a propensity score analysis was included to make the groups receiving appropriate versus inappropriate empirical antibiotic treatment comparable. Some limitations should, however, be acknowledged. (i) External validity of this study could be limited since it was performed in two centers from the same geographical area. Global epidemiology and prevalence of MDR pathogens may vary in other centers. (ii) Different definitions for septic shock have existed throughout the study period (31, 32). As we do not have the lactate values available for older episodes, some of them may not have met criteria for septic shock according to the current guidelines. (iii) Exact time to initiation of antibiotic, which impacts the outcome of these patients, was not available. (iv) The broad confidence interval ranges in some of the multivariate analysis variables suggest there may exist some sparse data bias. (v) Finally, as this is not a randomized clinical trial, there is no way to completely exclude confounding variables driving the observed associations.
Conclusion.
Septic shock in febrile neutropenic patients with BSI is essentially caused by GNB and is associated with extremely high mortality, especially in patients receiving IEAT. Empirical Gram-positive coverage had no impact on mortality. Empirical combination of a β-lactam plus amikacin was associated with lower mortality compared to those patients who did not receive amikacin. However, when amikacin was the only active antibiotic (mainly because the isolated microorganism was resistant to the empirical β-lactam), mortality was 90%. Administering an active β-lactam in combination with amikacin should be strongly considered in neutropenic patients with septic shock.
MATERIALS AND METHODS
Setting, study population, and design.
This descriptive, retrospective study was conducted in two university hospitals in Barcelona, Spain: Hospital Clinic and Hospital de Bellvitge (700-bed centers). This study was approved by the Ethics Committee Board (Comité de Ética de la Investigación con medicamentos) of our institution (HCB/2020/0509). Informed consent was waived due to the retrospective nature of the study. A specific database prospectively collected all BSI episodes occurring in onco-hematological patients. For this study, we retrospectively analyzed all episodes of BSI in adult (>18 years) neutropenic patients, identified between 2010 and 2019. The following data had been gathered in specific prospectively collected databases in both centers: age and gender, comorbidities and baseline disease, source of BSI, causative agents and their antibiotic susceptibility profiles, empirical antibiotic treatments, septic shock at onset, mechanical ventilation requirement, and 30-day mortality. For this study, both databases underwent unification. Episodes presenting with septic shock were compared with those without septic shock.
Definitions.
Patients with febrile neutropenia were defined as those who had either a single temperature measurement of >38.3°C or that of >38.0°C sustained over a 1-h period, and an absolute neutrophil count of ≤500 cells/mm3. Per hospital protocols, patients with expected grade IV neutropenia over 10 days received prophylaxis with levofloxacin at Hospital Clinic but not at Hospital Bellvitge. Septic shock was defined as sepsis episodes requiring the use of vasopressors due to persistent hypotension despite fluid therapy (31), with a causal and temporal relationship with the BSI episode. Acute kidney injury (AKI) was defined as a rise in serum creatinine by >0.3 mg/dL within 2 days of BSI (33). Prior antibiotic therapy was defined as the usage of any antimicrobial agent for ≥3 days throughout the month before the BSI episode, including quinolone prophylaxis. In this paper, we refer to active antibiotic according to its susceptibility testing. In this sense, “β-lactam as the only active antibiotic” or “amikacin as the only active antibiotic” refers to the fact that although other antibiotics may have been administered, these were the only active antibiotics according to susceptibility testing.
Definitions of comorbidities and site of infection have been previously provided (7, 34, 35). Catheter-related BSI was defined as the presence of one positive peripheral blood culture and a positive semiquantitative or quantitative catheter tip culture that grew the same microorganism found in the peripheral blood culture. In the absence of a positive catheter tip culture, patients were diagnosed with catheter-related BSI when local signs of phlebitis were present and there was an absence of other evident, infectious foci (36). Specifically, in the case of BSI due to coagulase-negative staphylococci without an available catheter tip culture, at least two sets of positive blood cultures drawn from different venipuncture localizations were required to make a diagnosis of BSI. The BSI was considered to be from an unknown or endogenous source when no other source was identified. Nosocomial infection was defined when clinical manifestations appeared 48 h after hospital admission. Healthcare-associated infection was defined when the subject met at least one of the following criteria: recent hospitalization (within the last 30 days), admission from a long-term-care facility, and being on either chronic hemodialysis or intravenous treatment in the previous month. The remaining patients were classified as having community-acquired BSI. IEAT was considered as such when empirical therapy did not include at least one in vitro active antibiotic against the isolated microorganism. GNB were classified as MDR per prior consolidated definitions (37). Mortality was defined as death by any cause within the first 30 days of BSI onset.
Microbiological methods.
Blood samples were processed using either the Bactec 9240 system or Bactec FX system (Becton, Dickinson Microbiology Systems), with an incubation period of 5 days. Isolates were identified by standard techniques. Antimicrobial susceptibility testing was performed with a microdilution system (MicroScan WalkAway [Dade Behring, West Sacramento, CA] or Phoenix system [Becton, Dickinson, Franklin Lakes, NJ]) or the Etest (AB Biodisk, Solna, Sweden/bioMérieux, Marcy l’Etoile, France). Current CLSI or EUCAST breakpoints for each year were used to define susceptibility or resistance to these antimicrobial agents, and intermediate susceptibility was considered resistance. Blood culture systems and antimicrobial susceptibility methods were equivalents between the two hospitals.
Statistical analysis.
Categorical variables were described by counts and percentages, whereas continuous variables were expressed as means and standard deviations (SDs) or medians and interquartile ranges (IQRs). Pearson’s chi-squared test and either the Mann-Whitney U test or Student t test were used to compare the distributions of categorical and continuous variables, respectively. Factors associated with mortality were evaluated with univariate and multivariate analyses; the multivariate analysis included all significant variables (P < 0.05) from the univariate analysis. Given the lack of randomization that could cause differences in the likelihood of receiving IEAT, a propensity score for IEAT was estimated using a backward stepwise logistic regression model that included variables related to IEAT with P values of ≤0.05 in the univariate analysis. For the propensity score analysis, only those episodes caused by GNB or Candida spp. were considered, because mortality is much lower in episodes caused by coagulase-negative staphylococci. The following variables were included based on their presence (yes/no) during the BSI episode: nosocomial BSI; previous antibiotic therapy, empirical β-lactam plus aminoglycoside combination, BSI caused by E. coli, BSI caused by Klebsiella spp., BSI caused by MDR P. aeruginosa, and candidemia. Univariate and multivariate analyses for receiving IEAT are detailed in the supplemental material (Table S1). The goodness of fit of the propensity score was assessed with the Hosmer-Lemeshow test (P = 0.844). The discriminatory power of the model, as evaluated by the area under the receiver operating characteristic (ROC) curve, was 0.87 (95% CI, 0.81 to 0.94), showing a strong ability to predict IEAT in episodes due to either GNB or Candida spp. Finally, a multivariate regression model (step-forward procedure) was used to identify independent risk factors for mortality in patients with BSI episodes presenting with shock. The propensity score for IEAT was used as a covariate in the multivariate analysis to adjust for potential confounding factors. The goodness of fit of the final multivariate model was assessed again with the Hosmer-Lemeshow test and the area under the ROC curve. The Durbin-Watson test and the variance inflation factor were used to evaluate for collinearity. The threshold for statistical significance was defined as a two-tailed P of <0.05. All analyses were performed with SPSS software (version 25.0; SPSS, Inc., Chicago, IL).
Data availability.
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ACKNOWLEDGMENTS
We thank Anthony Armenta for providing medical editing assistance for the manuscript at hand.
C.G.-V. has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, and Lilly as well as a grant from Gilead Science and MSD. A.S. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini, as well as grant support from Pfizer. P.C. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Gilead, and Alexion. J.M. has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini.
This research forms part of an activity that has received funding from EIT Health. EIT Health is supported by the European Institute of Innovation and Technology (EIT), a body of the European Union that receives support from the European Union’s Horizon 2020 Research and Innovation Program. This study has been cofunded by the European Regional Development Fund (EDRD). P.P.-A. (JR20/00012), N.G.-P. (FI19/00133), and C.G.-V. (FIS PI18/01061) have received research grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III. Our group is recognized by the AGAUR (Project 2017SGR1432) of the Catalan Health Agency. MSD provided financial support for medical writing assistance of this paper. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions: writing – original draft: M.C., P.P.-A., and C.G.-V.; writing – review & editing: all authors; conceptualization: C.G.-V. and P.P.-A.; investigation: M.C., P.P.-A., N.G.-P., C.G., J.L.-A., and C.G.-V.; methodology: P.P.-A., C.G., J.A.M., A.S., J.C., and C.G.-V.; formal analysis: M.C., P.P.-A., C.G., and C.G.-V.; project administration: C.G., C.G.-V., and A.S.; funding acquisition: P.P.-A., C.G., A.S., and C.G.-V.
Footnotes
Supplemental material is available online only.
Contributor Information
Pedro Puerta-Alcalde, Email: pedro.puerta84@gmail.com.
Carolina Garcia-Vidal, Email: cgarciav@clinic.cat.
REFERENCES
- 1.Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K, Paesmans M. 2007. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 30(Suppl 1):S51–S59. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. 2003. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis 36:1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 3.Feld R. 2008. Bloodstream infections in cancer patients with febrile neutropenia. Int J Antimicrob Agents 32(Suppl 1):30–33. doi: 10.1016/j.ijantimicag.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Jeddi R, Achour M, Amor R, Aissaoui L, Bouterâa W, Kacem K, Lakhal R, Ben Abid H, BelHadjAli Z, Turki A, Meddeb B. 2010. Factors associated with severe sepsis: prospective study of 94 neutropenic febrile episodes. Hematology 15:28–32. doi: 10.1179/102453310X12583347009577. [DOI] [PubMed] [Google Scholar]
- 5.Soares M, Caruso P, Silva E, Teles JMM, Lobo SMA, Friedman G, Dal Pizzol F, Mello PVC, Bozza FA, Silva UVA, Torelly AP, Knibel MF, Rezende E, Netto JJ, Piras C, Castro A, Ferreira BS, Réa-Neto Á, Olmedo PB, Salluh JIF, Brazilian Research in Intensive Care Network (BRICNet). 2010. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med 38:9–15. doi: 10.1097/CCM.0b013e3181c0349e. [DOI] [PubMed] [Google Scholar]
- 6.Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Laisne LM, Rabbat A, Lebert C, Perez P, Chaize M, Renault A, Meert AP, Benoit D, Hamidfar R, Jourdain M, Darmon M, Schlemmer B, Chevret S, Lemiale V. 2013. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium-A groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 31:2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 7.Puerta-Alcalde P, Cardozo C, Marco F, Suárez-Lledó M, Moreno E, Morata L, Fernández-Avilés F, Gutiérrez-Garcia G, Chumbita M, Rosiñol L, Martínez JAJA, Martínez C, Mensa J, Urbano Á, Rovira M, Soriano A, Garcia-Vidal C. 2020. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant 55:603–612. doi: 10.1038/s41409-019-0701-3. [DOI] [PubMed] [Google Scholar]
- 8.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I, Duarte R, Calvo M, Carratalà J. 2011. Bacteraemia due to multidrug-resistant gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother 66:657–663. doi: 10.1093/jac/dkq494. [DOI] [PubMed] [Google Scholar]
- 9.Rosa RG, Goldani LZ. 2014. Aetiology of bacteraemia as a risk factor for septic shock at the onset of febrile neutropaenia in adult cancer patients. Biomed Res Int 2014:561020. doi: 10.1155/2014/561020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosa RG, Goldani LZ. 2014. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother 58:3799–3803. doi: 10.1128/AAC.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F, Laporte-Amargós J, Moreno-García E, Domingo-Doménech E, Chumbita M, Martínez JA, Soriano A, Carratalà J, Garcia-Vidal C. 2020. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis 70:1068–1074. doi: 10.1093/cid/ciz319. [DOI] [PubMed] [Google Scholar]
- 12.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-A, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 13.Lemiale V, Pons S, Mirouse A, Tudesq JJ, Hourmant Y, Mokart D, Pène F, Kouatchet A, Mayaux J, Nyunga M, Bruneel F, Meert AP, Borcoman E, Bisbal M, Legrand M, Benoit D, Azoulay E, Darmon M, Zafrani L. 2020. Sepsis and septic shock in patients with malignancies: a Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. Crit Care Med 48:822–829. doi: 10.1097/CCM.0000000000004322. [DOI] [PubMed] [Google Scholar]
- 14.Georges Q, Azoulay E, Mokart D, Soares M, Jeon K, Oeyen S, Rhee CK, Gruber P, Ostermann M, Hill QA, Depuydt P, Ferra C, Toffart AC, Schellongowski P, Müller A, Lemiale V, Tinquaut F, Bourmaud A, Darmon M. 2018. Influence of neutropenia on mortality of critically ill cancer patients: results of a meta-analysis on individual data. Crit Care 22:326. doi: 10.1186/s13054-018-2076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolsma V, Schwebel C, Azoulay E, Darmon M, Souweine B, Vesin A, Goldgran-Toledano D, Lugosi M, Jamali S, Cheval C, Adrie C, Kallel H, Descorps-Declere A, Garrouste-Orgeas M, Bouadma L, Timsit JF. 2014. Sepsis severe or septic shock: outcome according to immune status and immunodeficiency profile. Chest 146:1205–1213. doi: 10.1378/chest.13-2618. [DOI] [PubMed] [Google Scholar]
- 16.Jung SM, Kim YJ, Ryoo SM, Sohn CH, Seo DW, Lim KS, Kim WY. 2019. Cancer patients with neutropenic septic shock: etiology and antimicrobial resistance. Korean J Intern Med 35:979–987. doi: 10.3904/kjim.2018.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarana M, Nucci M, Nouér SA. 2019. Shock and early death in hematologic patients with febrile neutropenia. Antimicrob Agents Chemother 63:e01250-19. doi: 10.1128/AAC.01250-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laupland KB, Davies HD, Church DL, Louie TJ, Dool JS, Zygun DA, Doig CJ. 2004. Bloodstream infection-associated sepsis and septic shock in critically ill adults: a population-based study. Infection 32:59–64. doi: 10.1007/s15010-004-3064-6. [DOI] [PubMed] [Google Scholar]
- 19.Artero A, Zaragoza R, Camarena JJ, Sancho S, González R, Nogueira JM. 2010. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care 25:276–281. doi: 10.1016/j.jcrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Bodey GP, Buckley M, Sathe YS, Freireich EJ. 1966. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 21.Paul M, Borok S, Fraser A, Vidal L, Leibovici L. 2005. Empirical antibiotics against Gram-positive infections for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 55:436–444. doi: 10.1093/jac/dki028. [DOI] [PubMed] [Google Scholar]
- 22.Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. 2013. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev 2013:CD003038. doi: 10.1002/14651858.CD003038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez JA, Cobos-Trigueros N, Soriano A, Almela M, Ortega M, Marco F, Pitart C, Sterzik H, Lopez J, Mensa J. 2010. Influence of empiric therapy with a beta-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother 54:3590–3596. doi: 10.1128/AAC.00115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safdar N, Handelsman J, Maki DG. 2004. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 4:519–527. doi: 10.1016/S1473-3099(04)01108-9. [DOI] [PubMed] [Google Scholar]
- 25.Chamot E, El Amari EB, Rohner P, Van Delden C. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 47:2756–2764. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albasanz-Puig A, Gudiol C, Puerta-Alcalde P, Ayaz CM, Machado M, Herrera F, Martín-Dávila P, Laporte-Amargós J, Cardozo C, Akova M, Álvarez-Uría A, Torres D, Fortún J, García-Vidal C, Muñoz P, Bergas A, Pomares H, Mercadal S, Durà-Miralles X, García-Lerma E, Pallarès N, Carratalà J. 2021. Impact of the inclusion of an aminoglycoside to the initial empirical antibiotic therapy for Gram-negative bloodstream infections in hematological neutropenic patients: a propensity-matched cohort study (AMINOLACTAM study). Antimicrob Agents Chemother 65:e0004521. doi: 10.1128/AAC.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montero M, VanScoy BD, López-Causapé C, Conde H, Adams J, Segura C, Zamorano L, Oliver A, Horcajada JP, Ambrose PG. 2018. Evaluation of ceftolozane-tazobactam in combination with meropenem against Pseudomonas aeruginosa sequence type 175 in a hollow-fiber infection model. Antimicrob Agents Chemother 62:e00026-18. doi: 10.1128/AAC.00026-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puerta-Alcalde P, Cardozo C, Suárez-Lledó M, Rodríguez-Núñez O, Morata L, Fehér C, Marco F, Del Río A, Martínez JA, Mensa J, Rovira M, Esteve J, Soriano A, Garcia-Vidal C. 2019. Current time-to-positivity of blood cultures in febrile neutropenia: a tool to be used in stewardship de-escalation strategies. Clin Microbiol Infect 25:447–453. doi: 10.1016/j.cmi.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Guisado M, Espigado I, Martín-Peña A, Gudiol C, Royo-Cebrecos C, Falantes J, Vázquez-López L, Montero MI, Rosso-Fernández C, de la Luz Martino M, Parody R, González-Campos J, Garzón-López S, Calderón-Cabrera C, Barba P, Rodríguez N, Rovira M, Montero-Mateos E, Carratalá J, Pérez-Simón JA, Cisneros JM. 2017. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol 4:e573–e583. doi: 10.1016/S2352-3026(17)30211-9. [DOI] [PubMed] [Google Scholar]
- 30.Delaloye J, Calandra T. 2014. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer M, Deutschman CS, Seymour C, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, Der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. 2017. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, Inker LA, Levin A, Mehrotra R, Palevsky PM, Perazella MA, Tong A, Allison SJ, Bockenhauer D, Briggs JP, Bromberg JS, Davenport A, Feldman HI, Fouque D, Gansevoort RT, Gill JS, Greene EL, Hemmelgarn BR, Kretzler M, Lambie M, Lane PH, Laycock J, Leventhal SE, Mittelman M, Morrissey P, Ostermann M, Rees L, Ronco P, Schaefer F, St Clair Russell J, Vinck C, Walsh SB, Weiner DE, Cheung M, Jadoul M, Winkelmayer WC. 2020. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 97:1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 34.De la Calle C, Morata L, Cobos-Trigueros N, Martinez JA, Cardozo C, Mensa J, Soriano A. 2016. Staphylococcus aureus bacteremic pneumonia. Eur J Clin Microbiol Infect Dis 35:497–502. doi: 10.1007/s10096-015-2566-8. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Vidal C, Cardozo-Espinola C, Puerta-Alcalde P, Marco F, Tellez A, Agüero D, Romero-Santana F, Díaz-Beyá M, Giné E, Morata L, Rodríguez-Núñez O, Martinez JA, Mensa J, Esteve J, Soriano A. 2018. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS One 13:e0199531. doi: 10.1371/journal.pone.0199531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01744-21-s0001.pdf, PDF file, 0.4 MB (446.5KB, pdf)
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.