Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is responsible for an increasing number of serious nosocomial and community-acquired infections. Phenotypic heterogeneous drug resistance (heteroresistance) to antistaphylococcal beta-lactams affects the results of susceptibility testing. The present study compared the MRSA-Screen latex agglutination test (Denka Seiken Co., Ltd., Tokyo, Japan) for detection of PBP 2a with agar dilution, the VITEK-1 and VITEK-2 systems (bioMérieux, St. Louis, Mo.), and the oxacillin agar screen test for detection of MRSA, with PCR for the mecA gene used as the “gold standard” assay. Analysis of 107 methicillin-susceptible S. aureus (MSSA) isolates and 203 MRSA isolates revealed that the MRSA-Screen latex agglutination test is superior to any single phenotype-based susceptibility testing method, with a sensitivity of 100% and a specificity of 99.1%. Only one isolate that lacked mecA was weakly positive by the MRSA-Screen latex agglutination test. This isolate was phenotypically susceptible to oxacillin and did not contain the mecA gene by Southern blot hybridization. The oxacillin agar screen test, the VITEK-1 system, the VITEK-2 system, and agar dilution showed sensitivities of 99.0, 99.0, 99.5, and 99%, respectively, and specificities of 98.1, 100, 97.2, and 100%, respectively. The differences in sensitivity or specificity were not statistically significant. Oxacillin bactericidal assays showed that mecA- and PBP 2a-positive S. aureus isolates that are susceptible to antistaphylococcal beta-lactams by conventional methods are functionally resistant to oxacillin. We conclude that the accuracy of the MRSA-Screen latex agglutination method for detection of PBP 2a approaches the accuracy of PCR and is more accurate than any susceptibility testing method used alone for the detection of MRSA.
Of the 2 million annual nosocomial infections in the United States, approximately 260,000 are due to Staphylococcus aureus. The percentage of nosocomial S. aureus isolates that are methicillin resistant rose from 14.3% in 1987 to 39.7% in 1997 (23). Methicillin-resistant S. aureus (MRSA) has become established outside the hospital environment and is now appearing in community populations without identifiable risk factors (8).
While a few clinical isolates of MRSA express homogeneous oxacillin resistance (i.e., ≥1 in 102 cells express high-level resistance), the majority of isolates have heterogeneous drug resistance (heteroresistance) (5, 6, 13). Phenotypic expression of resistance can vary depending on the growth conditions (e.g., the temperature or osmolarity of the medium), making susceptibility testing of MRSA by standard microbiological methods potentially problematic (5). The mechanism of heteroresistance in S. aureus is poorly understood but is believed to involve the interaction of PBP 2a and various gene products such as those encoded by fem (factor essential for methicillin resistance) genes that are involved in cell wall peptidoglycan synthesis (5, 13).
Despite the standardized recommendations for susceptibility testing of MRSA given by the National Committee for Clinical Laboratory Standards (NCCLS) (17), a small percentage of isolates that carry mecA are phenotypically susceptible to methicillin. These isolates represent extremely heteroresistant isolates in which less than 1 in 108 of the population is highly resistant to methicillin (2, 3, 13, 15, 20, 22, 25).
It is known that the heterogeneous resistance phenotype of mecA-positive MRSA strains progresses to homogeneous resistance upon incubation with methicillin (5). Furthermore, since mecA-positive, phenotypically methicillin-susceptible S. aureus strains likely represent strains with an extremely heteroresistant methicillin resistance phenotype, one would suspect that the use of beta-lactams would select for highly resistant bacteria in the population, ultimately leading to the failure of therapy. In vitro studies have shown that exposure of several mecA-positive, phenotypically methicillin-susceptible S. aureus isolates to beta-lactams resulted in an increase in the MIC of oxacillin well above the established breakpoint for resistance (oxacillin MIC, ≥4 μg/ml), even though initial susceptibility testing had revealed that these isolates were susceptible (15, 24).
We performed a head-to-head comparison of several susceptibility testing methods for MRSA. Using PCR for mecA as the “gold standard” assay, we evaluated the MRSA-Screen latex agglutination test for detection of PBP 2a, an oxacillin agar screen test, agar dilution, and the VITEK-1 GPS-106 card and the VITEK-2 AST-GP55 card.
MATERIALS AND METHODS
Bacterial isolates.
We studied 203 MRSA isolates recovered from 203 different patients at the Beth Israel Deaconess Medical Center from May 1998 to October 2000 and 107 methicillin-susceptible S. aureus (MSSA) isolates recovered from 107 different patients from April 2000 to September 2000. These isolates were recovered from blood or other sterile body fluids, surgical specimens, wounds, and sputa. Isolates were characterized as MRSA or MSSA by PCR for mecA as described below.
We studied the performance of the MRSA-Screen latex agglutination assay for PBP 2a with six strains from our research laboratory that were mecA negative and for which the oxacillin MIC was 1 to 4 μg/ml by agar dilution (borderline oxacillin-resistant S. aureus [BORSA] isolates). We also tested the MRSA-Screen test with eight isolates of S. aureus with reduced susceptibility to vancomycin (MICs, 4 to 8 μg/ml). Reference strains included MRSA ATCC 33591, MSSA ATCC 25923, and MSSA ATCC 29213. Saved isolates were removed from freezer storage (−70°C), subcultured on sheep blood agar plates, and incubated at 35°C for 24 h prior to further testing.
MRSA-Screen latex agglutination test.
The MRSA-Screen latex agglutination test (Denka Seiken Co., Ltd., Tokyo, Japan) was performed according to the manufacturer's instructions. For each strain, a 5-μl loopful of S. aureus colonies was obtained from a fresh subculture and was suspended in 200 μl of extraction reagent 1 (0.1 M NaOH) by using a 1.5-ml microcentrifuge tube. The suspension was boiled for 3 min, and 50 μl (1 drop) of extraction reagent 2 (0.5 M KH2PO4) was added. The mixture was centrifuged at 1,500 × g for 5 min at room temperature, and 50 μl of the supernatant was placed on a slide and mixed with 25 μl (1 drop) of anti-PBP 2a monoclonal antibody-sensitized latex. As a negative control, 50 μl of the supernatant was placed on the slide and mixed with 1 drop (25 μl) of negative-control latex. After the contents of the slide were mixed on a shaker for 3 min, agglutination was visualized and was scored as positive, negative, or weakly positive. Weakly positive reactions were interpreted as positive, but the isolates were subjected to coagulase gene confirmation by PCR since the test has been reported to yield less consistent results with coagulase-negative staphylococci.
Detection of mecA by PCR.
A single bacterial colony was obtained from a fresh subculture and was resuspended in 100 μl of sterile water. One microliter of the suspension was added to each PCR mixture. The PCR mixture consisted of 30 μl of a mixture of 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.5 mM MgCl2, 0.1% Triton X-100, each nucleotide (Promega, Madison, Wis.) at a concentration of 0.2 mM, 10 pmol of each primer (Life Technologies, Rockville, Md.), and 0.2 U of Taq polymerase (Promega). A 10× Mg-free buffer and 25 mM MgCl2 were supplied by the manufacturer. The PCR program consisted of a bacterial lysis and DNA denaturation step of 5 min at 95°C; 30 cycles with a 30-s denaturation step at 94°C, a 30-s annealing step at 42°C, and a 30-s extension at 72°C; and a final 10-min extension step at 72°C. The primer pair used (5′-CTCAGGTACTGCTATCCACC-3′ and 5′-CACTTGGTATATCTTCACC-3′; Life Technologies, Rockville, Md.), as described by Ryffel et al. (20), yielded a 448-bp DNA fragment that was detected by 1% agarose gel electrophoresis with ethidium bromide staining and UV light.
Susceptibility testing.
Agar dilution testing of susceptibility to oxacillin (monohydrate sodium salt) and vancomycin hydrochloride (Sigma Chemical Co., St. Louis, Mo.) was performed in Mueller-Hinton agar (Becton Dickinson and Co., Cockeysville, Md.) according to the recommendations of NCCLS (17). Oxacillin-containing agar plates were supplemented with 2% NaCl. The plates were incubated at 35°C and read at 24 h after inoculation. The lowest concentration of antibiotic at which all bacterial growth was inhibited was determined to be the MIC. The oxacillin agar screen was performed by inoculating 104 CFU of the organism on Mueller-Hinton agar supplemented with 4% NaCl and oxacillin at 6 μg/ml. Any growth after incubation for 24 h at 35°C was interpreted as a positive oxacillin agar screen result for MRSA . Automated testing with the VITEK-1 GPS-106 card and the VITEK-2 AST-GP55 card (bioMérieux, St. Louis, Mo.) was performed according to the manufacturer's instructions.
Coagulase gene PCR.
The presence of the coagulase gene was determined for isolates that were highly resistant to oxacillin (MICs, ≥128 mg/ml), demonstrated the presence of the mecA gene by PCR, and gave weak latex agglutination test results. The PCR method was that described by van Griethuysen et al. (27), with minor modifications. The reaction mixture was prepared as described above for the mecA PCR. The PCR program consisted of a bacterial lysis and DNA denaturation step of 5 min at 95°C; 30 cycles with a 1-min denaturation step at 94°C, a 1-min annealing step at 55°C, and a 1-min extension at 72°C; and a final 10-min extension step at 72°C. The primer pair used (5′-CTGGTACAGGTATCCGTGAATA-3′ and 5′-TTGTATTGACTGTATGTCTTTGGA-3′; Life Technologies) (27) yielded a 200- to 600-bp DNA fragment that was detected by 1% agarose gel electrophoresis with ethidium bromide staining and UV light.
Molecular typing and mecA Southern blotting.
Molecular typing of selected isolates was performed by pulsed-field gel electrophoresis (PFGE) of SmaI-macrorestricted DNA. To demonstrate the presence or absence of the mecA gene, DNA from the PFGE gel was transferred to a nylon membrane (Micron Separations, Inc., Westborough, Mass.) and was probed with the 448-bp mecA PCR fragment labeled with digoxigenin according to the manufacturer's instructions (Boehringer Mannheim Corp., Indianapolis, Ind.). Hybridization was performed for 12 to 18 h at 65°C after 4 h of prehybridization in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.02% (wt/vol) sodium dodecyl sulfate–0.1% (wt/vol) N-lauroylsarcosine, sodium salt–0.5% (wt/vol) blocking agent (Boehringer Mannheim Corp.).
Oxacillin bactericidal assays.
Overnight cultures were diluted 1:500 in Mueller-Hinton broth to obtain a starting culture of 106 to 107 CFU/ml in 20 μg of oxacillin per ml. Samples were obtained at 0, 4, 24, and 48 h and serially diluted by a factor of 10 to 107. Twenty-five microliters of each dilution was plated in duplicate on blood agar plates. The numbers of CFU were counted at 24 and 48 h.
Statistical analysis.
Differences in susceptibility methods were evaluated by McNemar's test with software available on the Institute of Phonetic Sciences website (http://www.fon.hum.uva.nl/Service/Statistics/McNemars_test.html).
RESULTS
We studied 310 clinical S. aureus isolates, 6 BORSA isolates, and 8 S. aureus isolates with reduced vancomycin susceptibility, for a total of 324 isolates. Of the clinical isolates tested, 203 were determined to be MRSA and 107 were determined to be MSSA by mecA PCR. The six BORSA strains tested negative by the MRSA-Screen latex agglutination test. All eight S. aureus strains with reduced susceptibility to vancomycin contained the mecA gene by PCR and were positive by the MRSA-Screen latex agglutination test. The results of oxacillin resistance testing of the clinical isolates are shown in Table 1.
TABLE 1.
Phenotypic and genotypic oxacillin susceptibility testing of S. aureus
| mecA PCR result | No. of isolates tested | No. of strains with result indicated
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latex agglutination test result for PBP 2a was:
|
Oxacillin screen test result was:
|
VITEK-1 system MIC (μg/ml) was:
|
VITEK-2 system MIC (μg/ml) was:
|
Agar dilution MIC (μg/ml) was:
|
|||||||
| Positive | Negative | Positive | Negative | ≤2 | ≥4 | ≤2 | ≥4 | ≤2 | ≥4 | ||
| Positive | 203 | 203 | 0 | 201 | 2 | 2 | 201 | 1 | 202 | 2 | 201 |
| Negative | 107 | 1 | 106 | 2 | 105 | 107 | 0 | 104 | 3 | 107 | 0 |
The results of the MRSA-Screen latex agglutination test for PBP 2a agreed with those of the mecA PCR for 309 of 310 (99.7%) clinical isolates tested. By taking PCR as the gold standard method, the MRSA-Screen latex agglutination test demonstrated 100% sensitivity and 99.1% specificity. Only one isolate that was negative for mecA by PCR was weakly positive for PBP 2a by latex agglutination. This isolate was coagulase gene positive and phenotypically susceptible, and the oxacillin MIC for this isolate was 0.5 μg/ml by agar dilution. DNA from this isolate with discordant results also failed to hybridize to a mecA-specific probe. Five mecA-positive isolates yielded weakly positive latex agglutination reactions. Oxacillin MICs were ≥128 μg/ml for three isolates, 64 μg/ml for one isolate, and 16 μg/ml for one isolate. The presence of the coagulase gene in all five isolates was confirmed by PCR.
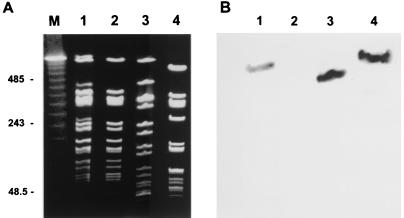
It is notable that three isolates (isolates SI285, SI299, and SI301) that were initially phenotypically resistant to oxacillin and that were positive for PBP 2a by the MRSA-Screen latex agglutination test initially tested negative for mecA. Subculture of these isolates to obtain single colonies yielded oxacillin-susceptible isolates that demonstrated a negative result for mecA by PCR and a negative MRSA-Screen test result. Analysis of SI285 DNA by PFGE before and after isolation of a single colony demonstrated that the initial sample contained a mixed population. The initial sample contained four faint bands (Fig. 1A, lane 1) that were not present after the culture was purified (Fig. 1A, lane 2). Southern blotting with a mecA-specific probe demonstrated faint hybridization in the initial mixed population characterized as MRSA (Fig. 1B, lane 1) but not in the subsequent pure MSSA isolate for which the oxacillin MIC was 1 μg/ml (Fig. 1B, lane 2). We did not attempt isolation of both MRSA and MSSA strains from among the initial mixture of isolates because our goal was to purify the cultures by picking a single colony.
FIG. 1.
PFGE (A) and mecA Southern blotting (B). (A) PFGE of isolate SI285 before (lane 1) and after (lane 2) propagation of a single colony. The initial culture was positive for PBP 2a by the MRSA-Screen latex agglutination test but was negative for mecA by single-colony-lysis PCR. Isolation of a single colony revealed that the predominant isolate was an MSSA isolate that was negative for both mecA and PBP 2a. Isolates SA110 (lane 3) and SI367 (lane 4) were unrelated isolates that were mecA and PBP 2a positive and for which oxacillin MICs were 0.25 to 1.0 and 2 μg/ml, respectively, by agar dilution. Lane M, ladder marker, with fragment sizes in multiples of 48.5 kb. Numbers on the left are in kilobases. (B) Southern blotting of the same gel shown in panel A probed for mecA.
The oxacillin agar screen identified 201 of 203 mecA-positive isolates, corresponding to a sensitivity of 99%. It yielded 2 false-positive results for 107 MSSA isolates tested for a specificity of 98.1%. The VITEK-1 GPS-106 card had a sensitivity and a specificity of 99.0 and 100%, respectively, and the VITEK-2 AST-GP55 card had a sensitivity and a specificity of 99.5 and 97.2%, respectively. Agar dilution showed a sensitivity and a specificity of 99 and 100%, respectively. No differences in sensitivity or specificity achieved statistical significance with this sample size.
The characteristics of all seven isolates that showed one or more discrepant results by the six methods used in the present study are given in Table 2. All of these isolates were coagulase gene positive and tube coagulase test positive. Our analysis yielded two isolates (isolates SA110 and SI367) that contained the mecA gene by PCR and that expressed PBP 2a as determined by latex agglutination but that were oxacillin susceptible and for which the oxacillin MICs were 0.25 and 2 μg/ml, respectively, by agar dilution. Repeated agar dilution testing of these two isolates showed that the oxacillin MIC for SI367 was 2 μg/ml by all tests and that the oxacillin MIC for SA110 ranged from 0.25 to 1.0 μg/ml.
TABLE 2.
Phenotypes and genotypes of isolates showing discrepant results by one or more testsa
| Isolate | Source | mecA PCR result | Result of MRSA-Screen test for PBP 2a | Oxacillin screen test result | MIC (μg/ml [susceptibility])
|
||
|---|---|---|---|---|---|---|---|
| Agar Dilution | VITEK-1 | VITEK-2 | |||||
| SI367 | Blood | + | + | − | 2.0 (S) | 0.5 (S) | 2 (S) |
| SA110 | Sputum | + | + | − | 0.25 (S) | 0.5 (S) | ≥4 (R) |
| SA109 | Wound | − | + (weak) | − | 0.5 (S) | 0.5 (S) | 0.5 (S) |
| SA51 | Wound | − | − | − | 1.0 (S) | 0.5 (S) | ≥4 (R) |
| SI340 | Blood | − | − | − | 2.0 (S) | 0.5 (S) | ≥4 (R) |
| SI285 | Eye | − | − | + | 1 (S) | 0.5 (S) | ≥4 (R) |
| SI929 | Wound | − | − | + | 0.5 (S) | 0.5 (S) | 0.5 (S) |
+, positive; −, negative; S, susceptible; R, resistant.
Isolates SA110 and SI367 were genotypically distinct by PFGE (Fig. 1A, lanes 3 and 4) and hybridized to mecA (Fig. 1B, lanes 3 and 4). SA110 was identified as methicillin resistant with the VITEK-2 AST-GP55 card and by the MRSA-Screen test. SI367 was identified as methicillin resistant only by the MRSA-Screen test.
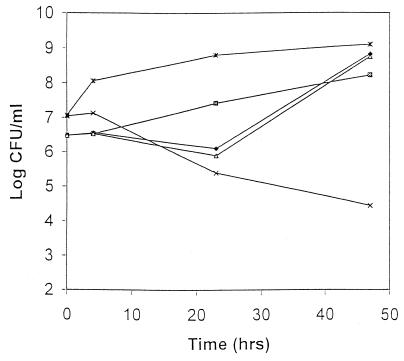
To determine the functional significance of the presence of mecA and PBP 2a in isolates SI367 and SA110 that were susceptible to oxacillin by most conventional methods, we tested the bactericidal activity of oxacillin against these isolates. Oxacillin demonstrated no activity against isolates SI367 and SA110 with and without the presence of clavulanic acid, suggesting that the lack of bactericidal activity of oxacillin was independent of beta-lactamase production. The data for SI367 are shown in Fig. 2. The lack of bactericidal activity was emphasized with the addition of NaCl to the medium, which is recommended by NCCLS in the susceptibility testing of S. aureus against beta-lactamase-resistant penicillins. In this assay, mecA-negative, oxacillin-susceptible S. aureus isolates demonstrated a 2- to 3-log10 decline in the numbers of CFU at 48 h.
FIG. 2.
Oxacillin bactericidal assay of isolate SI367 in Mueller-Hinton broth with oxacillin at 20 μg/ml (⧫), oxacillin at 20 μg/ml and clavulanic acid at 20 μg/ml (▵), and Mueller-Hinton broth supplemented with 2% NaCl and oxacillin 20 at μg/ml (▨). Included for comparison are randomly selected clinical isolates of MRSA (∗; oxacillin MICs, >128 μg/ml; mecA positive) and MSSA (×; oxacillin MICs, 1 μg/ml; mecA negative).
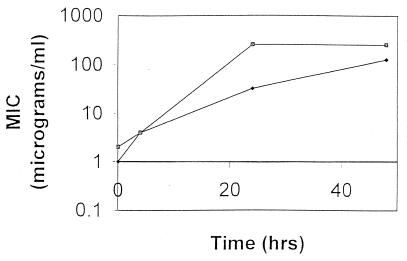
We formally tested the susceptibilities of isolates SI367 and SA110 to oxacillin over time in the oxacillin bactericidal assay. Figure 3 demonstrates the increase in the oxacillin MIC for SI367 from 2 to 256 μg/ml and that for SA110 from 1 to 128 μg/ml. These isolates remained resistant to oxacillin after six serial passages on antibiotic-free blood agar plates.
FIG. 3.
MIC versus time of exposure to oxacillin. The increase in the MIC of oxacillin was determined for isolates SI367 (▨) and SA110 (⧫) over time during exposure to oxacillin by using oxacillin at 20 μg/ml in Mueller-Hinton broth.
DISCUSSION
Susceptibility testing of methicillin resistance in S. aureus may be problematic because of the heterogeneous resistance displayed by many clinical isolates. While methods of susceptibility testing are standardized (17), the few isolates that have been found to contain mecA yet that appear to be phenotypically susceptible have the potential to become highly resistant if exposed to antistaphylococcal penicillins. Furthermore, standard susceptibility testing requires an additional 24-h incubation period compared to the time required for assays for mecA or PBP 2a.
The MRSA-Screen latex agglutination test for PBP 2a was easy to perform, gave results rapidly (15 to 20 min), was amenable to the processing of large numbers of samples, and approached the accuracy of PCR for mecA with respect to sensitivity (100%) and specificity (99.1%). Our results are comparable to those of other studies that have evaluated the MRSA-Screen latex agglutination test, which showed sensitivities without beta-lactam induction ranging from 93.5 to 100% and specificities ranging from 96.9 to 100% (4, 10, 12, 14, 16, 19, 22, 26–29, 30; M. Cavassini, A. Wenger, K. Jaton, J. Bille, and D. S. Blanc, Letter, J. Clin. Microbiol. 37:3784, 1999; J. Vuopio-Varkila, J. Swenson, G. Killgore, B. Hill, S. McAllister, and F. C. Tenover, Abstr. 39th Intersci Conf. Antimicrob. Agents Chemother., abstr. 874, 1999; B. M. Willey, B. Tennant, T. C. Moore, L. Pearce, A. McGeer, D. E. Low, and M. Skulnick, Abstr. 39th Intersci Conf. Antimicrob. Agents Chemother., abstr. 871, 1999). While PCR is considered the gold standard assay for the detection of MRSA, it remains too time-consuming and expensive to be practical in the clinical microbiology laboratory.
Noteworthy is the recent study that reported the lowest sensitivity (93.5%) of the MRSA-Screen latex agglutination test without beta-lactam induction (19). That study used MRSA isolates that showed delayed agglutination (longer than 3 min) by the MRSA-Screen latex agglutination test without induction. A significant portion of these MRSA isolates was obtained from an outbreak in Zurich, Switzerland, in 1999 caused by a clone that demonstrated low-level methicillin resistance. In that setting, the sensitivity of the MRSA-Screen test was increased to 100% with induction of the isolates with cefoxitin (19).
Induction of PBP 2a by beta-lactams seems to increase the sensitivity of the MRSA-Screen latex agglutination test, especially with coagulase-negative staphylococci (9). This added step may not be necessary in the analysis of most S. aureus isolates except when low-level methicillin resistance is prevalent, as was the case in Zurich in 1999. One of the benefits of latex agglutination methods is the rapid turnaround time from the isolation of an organism to provision of a susceptibility report to clinicians. The drawback of the additional 24 h required for induction may outweigh the very small increase in sensitivity in the detection of MRSA isolates that are uncommon in most settings. However, the findings of the present study support the idea that susceptibility testing methods based on the identification of mecA or PBP 2a should complement rather than replace conventional phenotypic susceptibility testing. Furthermore, the manufacturer of the MRSA-Screen test will modify the package insert to recommend induction with oxacillin or ceftizoxime for S. aureus isolates showing delayed agglutination after 3 min (19).
The MRSA-Screen latex agglutination test showed 100% correlation with PCR in the evaluation of six BORSA strains and eight S. aureus strains with reduced susceptibility to vancomycin. None of the BORSA isolates demonstrated mecA or PBP 2a. We specifically chose to evaluate S. aureus isolates with reduced susceptibility to vancomycin because the changes that have been described in the cell walls of these isolates could have hindered the ability of the latex agglutination antibody to bind to PBP 2a and thus potentially decrease the sensitivity of the assay. However, all eight S. aureus isolates for which vancomycin MICs were 4 to 8 μg/ml that we evaluated demonstrated both mecA and PBP 2a.
The present study demonstrated that the microdilution method with the VITEK-2 AST-GP55 card may be more sensitive yet less specific than that with the VITEK-1 GPS-106 card in detecting MRSA. With a sensitivity of 99.5%, it was the most sensitive conventional susceptibility testing method for the detection of MRSA, surpassing the agar dilution method as well as the widely applied oxacillin agar screen method. The specificity of method with the VITEK-2 card was 97.2%.
We deliberately chose not to include coagulase-negative staphylococci in our analysis, given that others have shown that the MRSA-Screen latex agglutination test for PBP 2a is less reliable for the testing of these isolates (9). While the test's performance improves with incubation of coagulase-negative staphylococci in the presence of a beta-lactam to induce expression of mecA (9), the MRSA-Screen test is to be routinely used only against S. aureus in the clinical microbiology laboratory, according to the manufacturer. Because of this, we genotypically and phenotypically confirmed the presence of the coagulase gene in all five MRSA isolates that showed only weakly positive MRSA-Screen test results and in one MSSA isolate falsely positive by the MRSA-Screen test.
Three isolates gave positive latex agglutination test results and were phenotypically resistant yet were negative for mecA by PCR. This was explained by our discovery that the initial cultures of these isolates were a mixture of MRSA and MSSA. The latex agglutination test uses a loopful of bacteria and therefore is much more likely to pick up both MRSA and MSSA isolates from a culture with a mixture of isolates. The colony lysis PCR method uses a single colony and may give inconsistent results when working with mixed cultures. One may circumvent this problem by picking multiple colonies and resuspending them in a larger volume in preparation for use in the PCR template. We also point out recent reports that indicate the potential instability of the mecA gene in S. aureus, with the loss of mecA resulting in an oxacillin-susceptible subpopulation (7, 11, 29). Therefore, discrepant results between susceptibility methods should alert microbiologists to this possibility as well.
Two of the 203 isolates tested carried mecA yet were phenotypically susceptible to oxacillin. One of these two isolates was reported on previously (21). Isolate SI367 is a blood isolate from a patient with recurrent S. aureus bacteremia; the patient was initially infected with an MRSA strain and was subsequently infected with SI367. The patient had a prosthetic aortic valve and a mycotic ascending aortic aneurysm. The oxacillin MIC for this isolate was 2 μg/ml, and the isolate was susceptible to oxacillin by all conventional susceptibility tests in the present study. Isolate SA110, from a patient's leg wound, was shown to be susceptible to oxacillin (MIC range, 0.25 to 1.0 μg/ml). This patient also had a history of infection with an MRSA isolate. Two other previously described S. aureus isolates that are phenotypically susceptible to oxacillin and that contain mecA (15, 21) were not formally included in the present study but tested positive for PBP 2a by the MRSA-Screen test.
Isolate SA110 was identified as oxacillin resistant only with the VITEK-2 system, whereas all other susceptibility testing methods used in the present study identified it as susceptible. Isolate SI367 was identified as oxacillin susceptible by all phenotypic susceptibility testing methods. In our study, this represented a 0.5 to 1% rate of failure to detect MRSA by the susceptibility testing methods used in the clinical microbiology laboratory. In light of the tens of thousands of MRSA infections in the United States each year, even this low error rate could translate into the misclassification of several hundred cases of MRSA infections as MSSA infections.
No data dictating the optimal therapy for infections with S. aureus isolates that are phenotypically susceptible to oxacillin but that carry mecA are available. In vitro data demonstrate that such isolates are very heteroresistant, with only 1 in 108 or fewer cells expressing high-level resistance (5, 15, 24). Because the inoculum sizes used in standard susceptibility testing are orders of magnitude lower than the numbers of isolates with high-level oxacillin resistance, such isolates may not be detected as methicillin resistant. Incubation of these heteroresistant isolates in gradually higher levels of a beta-lactam can yield highly resistant subclones (15, 24). In a focus of infection, these highly resistant cells might be selected and lead to treatment failure. Our oxacillin bactericidal assay confirmed that phenotypically oxacillin-susceptible S. aureus isolates that contain mecA and that express PBP 2a as determined by the latex agglutination test are functionally oxacillin resistant.
An additional benefit of the mecA PCR and the MRSA-Screen test is the potential to generate a susceptibility report 24 h earlier than the time of generation of results of conventional susceptibility testing methods. While this may translate into improved clinical outcomes, especially in those in whom MRSA is undetected and who have been treated empirically with a beta-lactam antibiotic, small studies have failed to demonstrate the benefit of early appropriate therapy (1, 18).
In summary, we demonstrated that the rapid MRSA-Screen latex agglutination test for PBP 2a is comparable to the mecA PCR with respect to sensitivity and specificity for the detection of MRSA. With the MRSA-Screen test, it is feasible to rapidly process a large number of specimens in a busy clinical microbiology laboratory. Molecular susceptibility testing methods can be used to complement conventional susceptibility methods in order to increase the sensitivity and the specificity of MRSA detection, particularly in serious infections in which phenotypically methicillin-susceptible S. aureus is isolated from a patient with a prior history of MRSA infection (21). While not achieving statistical significance, our findings suggest a higher sensitivity and a lower specificity of the VITEK-2 system compared to that of the VITEK-1 system for MRSA detection. S. aureus isolates that contain mecA and PBP 2a should be considered resistant to antistaphylococcal beta-lactams, regardless of the results of conventional susceptibility testing.
ACKNOWLEDGMENT
We thank Denka Seiken Co., Ltd., for kindly supplying us with the MRSA-Screen kit for the present analysis.
REFERENCES
- 1.Allaouchiche B, Jaumain H, Zambardi G, Chassard D, Freney J. Clinical impact of rapid oxacillin susceptibility testing using a PCR assay in Staphylococcus aureus bacteremia. J Infect. 1999;39:198–204. doi: 10.1016/s0163-4453(99)90049-x. [DOI] [PubMed] [Google Scholar]
- 2.Araj G F, Talhouk R S, Simaan C J, Maasad M J. Discrepancies between mecA PCR and conventional tests used for the detection of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1999;11:47–52. doi: 10.1016/s0924-8579(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 3.Bignardi G E, Woodford N, Chapman A, Johnson A P, Speller D C. Detection of the mecA gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother. 1996;37:53–63. doi: 10.1093/jac/37.1.53. [DOI] [PubMed] [Google Scholar]
- 4.Cavassini M, Wenger A, Jaton K, Blanc D S, Bille J. Evaluation of MRSA-Screen, a simple anti-PBP-2a slide latex agglutination kit, for rapid detection of methicillin resistance in Staphylococcus aureus. J Clin Microbiol. 1999;37:1591–1594. doi: 10.1128/jcm.37.5.1591-1594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers H F, Sachdeva M. Binding of β-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 7.Deplano A, Tassios P T, Glupczynski Y, Godfroid E, Struelens M J. In vivo deletion of the methicillin resistance mec region from the chromosome of Staphylococcus aureus strains. J Antimicrob Chemother. 2000;46:617–620. doi: 10.1093/jac/46.4.617. [DOI] [PubMed] [Google Scholar]
- 8.Herold B C, Immergluck L C, Maranan M C, Lauderdale D S, Gaskin R E, Boyle-Vavra S, Leitch C D, Daum R S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 9.Hussain Z, Stoakes L, Garrow S, Longo S, Fitzgerald V, Lannigan R. Rapid detection of mecA-positive and mecA-negative coagulase-negative staphylococci by an anti-penicillin binding protein 2a slide test. J Clin Microbiol. 2000;38:2051–2054. doi: 10.1128/jcm.38.6.2051-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafri A K, Reisner B S, Woods G L. Evaluation of a latex agglutination assay for rapid detection of oxacillin resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2000;36:57–59. doi: 10.1016/s0732-8893(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence C, Cosseron M, Durand P, Costa Y, Leclerq R. Consecutive isolation of homologous strains of methicillin-resistant and methicillin-susceptible Staphylococcus aureus from a hospitalized child. J Hosp Infect. 1996;33:49–53. doi: 10.1016/s0195-6701(96)90028-6. [DOI] [PubMed] [Google Scholar]
- 12.Louie L, Matsumura S O, Choi E, Louie M, Simor A E. Evaluation of three rapid methods for detection of methicillin resistance in Staphylococcus aureus. J Clin Microbiol. 2000;38:2170–2173. doi: 10.1128/jcm.38.6.2170-2173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maranan M C, Moreira B, Boyle-Vavra S, Daum R S. Antimicrobial resistance in staphylococci. Infect Dis Clin. 1997;11:813–849. doi: 10.1016/s0891-5520(05)70392-5. [DOI] [PubMed] [Google Scholar]
- 14.Marriott D J, Karagiannis T, Harkness J L. Further evaluation of the MRSA-Screen kit for rapid detection of methicillin resistance. J Clin Microbiol. 1999;37:3783–3784. doi: 10.1128/jcm.37.11.3783-3784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martineau F, Picard F J, Lansac N, Menard C, Roy P H, Ouellette M, Bergeron M G. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:231–238. doi: 10.1128/aac.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakatomi Y, Sugiyama J. A rapid latex agglutination assay for the detection of penicillin-binding protein 2′. Microbiol Immunol. 1998;42:739–743. doi: 10.1111/j.1348-0421.1998.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically. 5th ed. 2000. Approved standard M7–A5. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 18.Roghmann M C. Predicting methicillin resistance and the effect of inadequate empirical therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med. 2000;160:1001–1004. doi: 10.1001/archinte.160.7.1001. [DOI] [PubMed] [Google Scholar]
- 19.Rohrer S, Tschierske M, Zbinden R, Berger-Bachi B. Improved methods for detection of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2001;20:267–270. doi: 10.1007/pl00011263. [DOI] [PubMed] [Google Scholar]
- 20.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bachi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 21.Sakoulas G, DeGirolami P C, Gold H S. Methicillin-susceptible Staphylococcus aureus: believe it or not. Arch Intern Med. 2001;161:1237–1238. doi: 10.1001/archinte.161.9.1237. [DOI] [PubMed] [Google Scholar]
- 22.Smyth R W, Kahlmeter G, Olsson Liljequist B, Hoffman B. Methods for identifying methicillin-resistance in Staphylococcus aureus. J Hosp Infect. 2001;48:103–107. doi: 10.1053/jhin.2001.0933. [DOI] [PubMed] [Google Scholar]
- 23.Tenover F C, Gaynes R F. The epidemiology of Staphylococcus aureus. In: Fischett V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: American Society for Microbiology; 2000. pp. 414–421. [Google Scholar]
- 24.Tokue Y, Shoji S, Satoh K, Watanabe A, Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasz A, Drugeon H B, de Lencastre H M, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989;33:1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udo E E, Mokadas E M, Al-Haddad A, Mathew B, Jacob L E, Sanyal S C. Rapid detection of methicillin resistance in staphylococci using a slide latex agglutination kit. Int J Antimicrob Agents. 2000;15:19–24. doi: 10.1016/s0924-8579(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 27.Van Griethuysen A, Pouw M, van Leeuwen N, Heck M, Willemse P, Buiting A, Kluytmans J. Rapid slide latex agglutination test for detection of methicillin resistance in Staphylococcus aureus. J Clin Microbiol. 1999;37:2789–2792. doi: 10.1128/jcm.37.9.2789-2792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Leeuwen W B, van Pelt C, Luijendijk A, Verbrugh H A, Goessens W H. Rapid detection of methicillin resistance in Staphylococcus aureus isolates by the MRSA-Screen latex agglutination test. J Clin Microbiol. 1999;37:3029–3030. doi: 10.1128/jcm.37.9.3029-3030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagenvoort J H T, Toenbreker H M J, Heck M E O C, van Leeuwen W J, Wannet W J B. Hospital outbreak of methicillin-resistant Staphylococcus aureus followed by an in vivo change to a mecA-negative mutant with loss of epidemicity. Eur J Clin Microbiol Infect Dis. 2000;19:976–977. doi: 10.1007/s100960000404. [DOI] [PubMed] [Google Scholar]
- 30.Yamazumi T, Marshall S A, Wilke W W, Diekema D J, Pfaller M A, Jones R N. Comparison of the VITEK Gram-positive susceptibility 106 card and the MRSA-Screen latex agglutination test for determining oxacillin resistance in clinical bloodstream isolates of Staphylococcus aureus. J Clin Microbiol. 2001;39:53–56. doi: 10.1128/JCM.39.1.53-56.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]