Abstract
Hypoxia decreases core body temperature in animals and humans during cold exposure. In addition, hypoxia increases skin blood flow in thermoneutral conditions, but the impact of hypoxic vasodilation on vasoconstriction during cold exposure is unknown. In this study, skin blood flow was assessed using laser-Doppler flowmetry, and cutaneous vascular conductance (CVC) was calculated as red blood cell flux/mean arterial pressure and normalized to baseline (n = 7). Subjects were exposed to four different conditions in the steady state (normoxia and poikilocapnic, isocapnic, and hypercapnic hypoxia) and were cooled for 10 min using a water-perfused suit in each condition. CVC increased during all three hypoxic exposures (all P < 0.05 vs. baseline), and the magnitude of these steady-state responses was not affected by changes in end-tidal CO2 levels. During poikilocapnic and hypercapnic hypoxia, cold exposure reduced CVC to the same levels observed during normoxic cooling (P > 0.05 vs. normoxia), whereas CVC remained elevated throughout cold exposure during isocapnic hypoxia (P < 0.05 vs. normoxia). The magnitude of vasoconstriction during cold stress was similar in all conditions (P > 0.05). Thus the magnitude of cutaneous vasodilation during steady-state hypoxia is not affected by CO2 responses. In addition, the magnitude of reflex vasoconstriction is not altered by hypoxia, such that the upward shift in skin blood flow (hypoxic vasodilation) is maintained during whole body cooling.
Keywords: hypoxia, skin
it is well established that low oxygen levels exert a relaxing effect on peripheral blood vessels in the human limb circulation (1, 15, 17, 19, 20, 25). In the skeletal muscle circulation and likely the circulation to the hand, locally and humorally mediated hypoxic vasodilation is opposed by increases in sympathetic vasoconstrictor nerve activity (14, 18, 25). However, we have recently shown that hypoxic vasodilation in the nonacral skin of the forearm is not restrained by neurally mediated sympathetic vasoconstriction (20). It was suggested that this “unchecked” cutaneous vasodilation may have important thermoregulatory consequences (e.g., during cold exposure) inasmuch as relatively small changes in resting skin blood flow may have a large impact on heat loss from the body core (12).
During whole body cold stress, skin blood flow is reduced to decrease heat transfer from the core to the periphery, thereby promoting heat conservation (10). This thermoregulatory adjustment is vital to the prevention of hypothermia in cold environments. However, when cold is encountered during simulated high-altitude exposure, the rate of core cooling appears to be accelerated, and lower core temperatures are reached compared with sea-level conditions (5, 13). While core temperature falls faster at simulated high altitude, skin temperature remains higher throughout cold exposure compared with sea-level conditions (4, 5). Taken together, these results indicate greater core-to-skin heat transfer during cold exposure at altitude. Importantly, we are aware of no studies that have measured skin blood flow during combined hypoxia and whole body cold stress.
With the above information as a background, this study was designed around two main goals. First, we sought to determine whether hypoxic vasodilation persists during whole body cooling. We hypothesized that skin blood flow would be shifted to higher values by hypoxia throughout the duration of a brief whole body cold exposure (lasting 10 min). The second study aim was to test the effect of changes in CO2 levels on the cutaneous vascular response to hypoxia. Hypoxemia can present with a concomitant increase or decrease in systemic CO2 levels depending on the circumstance or pathological state (e.g., pulmonary disease vs. altitude exposure). However, it is unclear how superimposing hypocapnia or hypercapnia might affect the cutaneous vascular response to hypoxia. Because we have previously reported that hypercapnia causes modest increases in skin blood flow (20), we hypothesized that development of hypercapnia would increase, and hypocapnia would decrease, the magnitude of cutaneous vasodilation during acute hypoxia.
METHODS
This study was approved by the institutional review board of the University of Oregon, and each subject gave written informed consent before participation.
Subjects.
Seven healthy, nonsmoking, normotensive male subjects, age 25 ± 4 yr, completed this study [height 185 ± 9 (SD) cm, weight 84.9 ± 18.1 kg, body mass index 24.6 ± 3.8 kg/m2]. Subjects were taking no medications, and none had been to altitude (>1,500 m) within 5 mo.
Experimental protocol.
Experiments were performed in thermoneutral conditions with the subject supine, wearing a whole body suit perfused with 34°C water, except during cold stress. The water-perfused suit covered the entire body surface except for the head, hands, feet, and forearm where measurement of vascular responses was performed. After donning the water perfused suit, subjects were instrumented for the measurement of heart rate via electrocardiography (Cardiocap/5, Datex-Ohmeda, Madison, WI), ventilation via turbine pneumotach (VMM-400, Interface Associates, Laguna Niguel, CA), arterial pressure via brachial artery oscillometry (Cardiocap/5) and photoplethysmography (Finometer, Finapres Medical Systems BV, Arnhem, the Netherlands), estimated arterial O2 saturation via earlobe pulse oximetry (Cardiocap/5), and end-tidal Po2 and Pco2 via mass spectrometry (Marquette MGA 1100, MA Tech Services, St. Louis, MO). Isocapnia/eucapnia was defined as the mean end-tidal Pco2 (nasal cannula) during a 5-min period of quiet breathing that followed subject instrumentation and 15 min of quiet rest. To obtain an index of skin blood flow, cutaneous red blood cell flux was measured on the ventral forearm by laser-Doppler flowmetry (DRT4, Moor Instruments, Devon, UK) with integrated laser-Doppler probes fixed to the skin with adhesive tape. Skin blood flows were expressed as cutaneous vascular conductance (red blood cell flux/mean arterial pressure) and normalized to baseline values (20, 23, 24).
After instrumentation, subjects were familiarized with a custom-built breathing circuit equipped with scuba mouthpiece and nose clip as described below. When subjects were comfortable breathing from the mouthpiece, an initial cold stress was performed while subjects breathed normoxic gas and isocapnia was maintained. To induce cold stress, 5 min of baseline data was recorded, and then the water circulating through the whole body suit was cooled at a rate of 2°C/min from 34°C to 14°C during a 10-min period. Subsequently subjects were removed from the breathing apparatus, rewarmed, and rested for 20 min following this initial cold stress. Pilot data were collected in three subjects to ensure that the cutaneous vascular response to 10 min of aggressive whole body cooling is reproducible after 20 min. During these pilot studies, cutaneous vascular conductance decreased to 66 ± 5.7% baseline and 69.5 ± 1.7% baseline before and after 20 min of supine rest (P = 0.563 for pre vs. postsupine rest).
Following the first cold stress and 20-min resting period, subjects were exposed to systemic hypoxia three different times in random order, and each exposure was separated by another 20 min of quiet supine rest. Pilot data were also collected to ensure that the cutaneous vascular response to hypoxia is reproducible after 20 min. During these pilot studies, arterial oxygen saturation decreased to 80.5 ± 0.9% and 82.7 ± 0.2% and cutaneous vascular conductance increased to 127.5 ± 1.7% baseline and 129.3 ± 2.1% baseline before and after 20 min of supine rest (n = 2).
The three hypoxic exposures were identical to one another with the exception of how CO2 levels were controlled. Initially, 5 min of baseline data was collected, and then hypoxia was induced by decreasing the fraction of inspired oxygen (FiO2) progressively over a 10-min period. The rate of decrease in FiO2 was designed to produce a fall in arterial O2 saturation of ∼2%/min down to 80%. After the target level of hypoxia was reached, which approximates an altitude of 16,000 ft (2, 26), 5 min of steady-state data was recorded before a cold stress lasting 10 min was performed as described above. Subjects were then rewarmed and allowed to breathe room air after whole body cooling was complete. This hypoxic exposure (with cold stress) was performed once while end-tidal Pco2 was allowed to fall during hypoxia (poikilocapnia), once while end-tidal Pco2 was clamped at resting levels (isocapnia), and once while end-tidal Pco2 was elevated by 3 mmHg (hypercapnia). All values were recorded continuously before and throughout each simulated environmental stress. The exception was brachial artery blood pressure which was recorded every 5 min.
Control of breathing mixture.
To achieve control of tidal gases, subjects breathed from a custom-built breathing circuit using a scuba mouthpiece and nose clip. The inspiratory mixture provided at the mouthpiece originated from three gas tanks—air (20.93% O2), nitrogen (100% N2), and carbon dioxide (100% CO2)—connected to the inspiratory line by flow regulators. The FiO2 and fraction of inspired CO2 (FiCO2) were varied by adjusting the flow rate from each of the gas tanks, and the inspired air was mixed as it filled a 6-liter tube, which served as an inspiratory reservoir. The 6-liter tube connected to the breathing circuit proximal to the mouthpiece, and its distal end was open to room air. Total flow rate through the breathing circuit was always greater than the subjects' minute ventilation, and thus excess gas delivered through the inspiratory line escaped to the room through the 6-liter tube. The mouthpiece consisted of two one-way valves to ensure inspiration from the breathing circuit and expiration to room air.
Data acquisition and analysis.
Data were digitized with signal-processing software (WinDaq, Dataq Instruments, Akron, OH) and analyzed offline. Statistical analyses were completed with SAS statistical software using PROC MIXED (SAS v9.1.3, SAS Institute, Cary, NC). Data were analyzed using a two-way repeated-measures ANOVA (condition × time) with a priori contrasts of specific condition-time combinations. As such, we did not employ a multiple-comparisons adjustment. Differences were considered statistically significant when P < 0.05. All values are presented as means ± SE unless otherwise indicated.
Because changes in baseline skin blood flow can affect interpretation of cutaneous vascular responses during cold stress (11), we expressed decreases in cutaneous vascular conductance during cold exposure both in terms of normoxic and hypoxic baseline values. In this way, any effect of a baseline shift in cutaneous vascular conductance (during hypoxia) on the interpretation of vasoconstrictor responses during cold stress should be apparent in our results.
RESULTS
Figure 1 shows cutaneous vascular and cardiorespiratory responses to the three hypoxic conditions. As planned, oxyhemoglobin saturation decreased to ∼80% with only slight differences in the kinetics of this response between conditions (P < 0.001 for condition × time interaction). End-tidal Pco2 rose by ∼2 mmHg during hypercapnic hypoxia and fell by ∼5 mmHg during poikilocapnic hypoxia, with only minimal changes from preexposure baseline during isocapnic hypoxia (P < 0.001 for condition × time interaction). Importantly, small decreases in end-tidal Pco2 during isocapnic hypoxia do not represent a deviation from eucapnic values, but rather from slightly elevated values at baseline during mouthpiece breathing. Minute ventilation and heart rate both increased the most during hypercapnic hypoxia, while minute ventilation did not change during poikilocapnic hypoxia (condition × time interaction: P < 0.001 for both minute ventilation and heart rate). Cutaneous vascular conductance increased in all three conditions during steady-state hypoxia, although this effect was transient in the poikilocapnic condition (P = 0.045 for condition main effect, P = 0.063 for condition × time interaction). Steady-state mean arterial pressure was increased by hypercapnic (88.6 ± 4.5 vs. 95.3 ± 4.8 mmHg; P = 0.020) but unchanged by poikilocapnic (87.4 ± 3.9 vs. 90.9 ± 4.0 mmHg; P = 0.150) and isocapnic hypoxia (89.2 ± 3.3 vs. 89.6 ± 4.3 mmHg; P = 0.857; P = 0.012 for condition × time interaction).
Fig. 1.
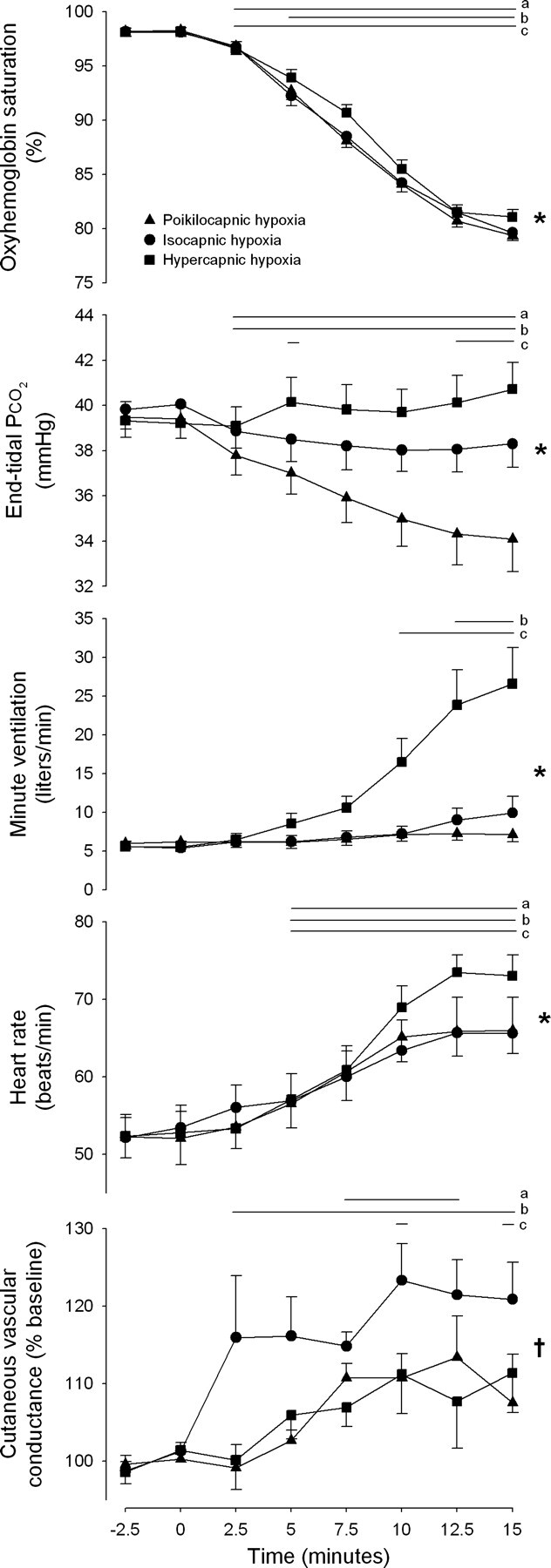
Cutaneous vascular and cardiorespiratory responses to progressive hypoxia in thermoneutral conditions. Progressive hypoxia began immediately after time point zero. Values are means ± SE; n = 7. Horizontal lines indicate time points significantly different from time 0 (P < 0.05 for apoikilocapnic, bisocapnic, and chypercapnic hypoxia). †P < 0.05 for condition main effect. *P < 0.05 for condition × time interaction effect.
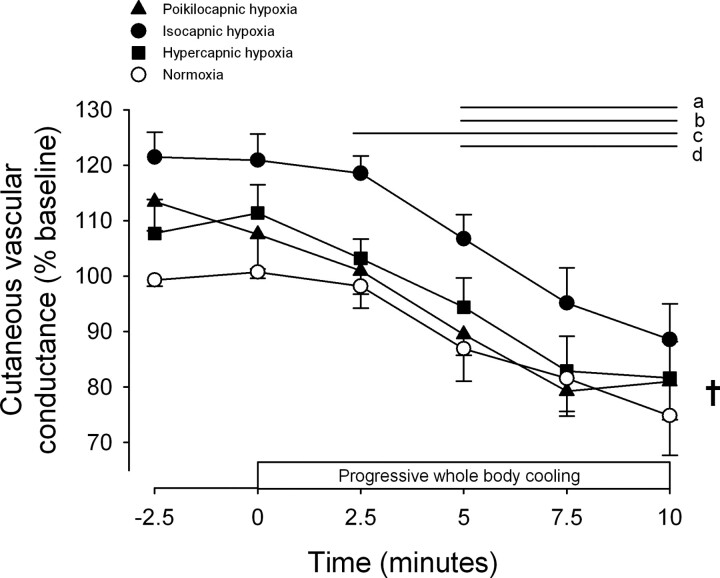
Figure 2 shows cutaneous vascular responses to whole body cooling during normoxia and poikilocapnic, isocapnic, and hypercapnic hypoxia. The decline in cutaneous vascular conductance during whole body cooling was shifted upward across all time points in the isocapnic hypoxia condition (P < 0.05 vs. normoxia at all time points; P = 0.018 for condition main effect). However, during both poikilocapnic and hypercapnic hypoxia, cutaneous vascular conductance was not significantly different vs. normoxia at any point during the cold stress (all P > 0.05).
Fig. 2.
Cutaneous vascular responses to whole body cooling during normoxia and poikilocapnic, isocapnic, and hypercapnic hypoxia. Values are means ± SE; n = 7. Horizontal lines indicate time points significantly different from time 0 (P < 0.05 for apoikilocapnic hypoxia, bisocapnic hypoxia, chypercapnic hypoxia, and dnormoxia). †P < 0.05 for condition main effect.
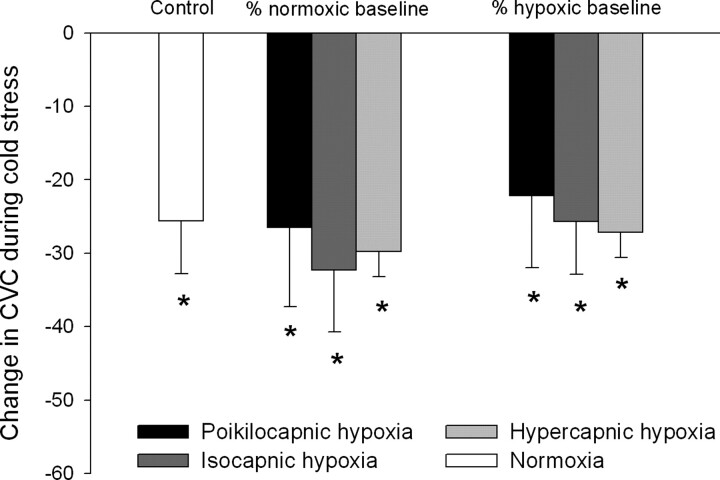
Figure 3 displays the reduction in cutaneous vascular conductance in response to cold stress during normoxic and hypoxic conditions. Responses during hypoxia are contrasted with those during normoxia (i.e., “control”) and are displayed as percent changes with respect to both normoxic and hypoxic baseline values. Regardless of how responses are expressed, cutaneous vascular conductance was reduced by ∼25–35% during cold stress with no differences between conditions (all P > 0.05). Cardiorespiratory responses during whole body cooling in each condition are displayed in Table 1.
Fig. 3.
Vasoconstrictor responses to whole body cooling during hypoxia expressed as a function of 2 different baselines. Values are means ± SE; n = 7. *Significant reduction in cutaneous vascular conductance (CVC) during whole body cooling (P < 0.05).
Table 1.
Cardiorespiratory responses to whole body cooling during normoxia and poikilocapnic, isocapnic, and hypercapnic hypoxia
| Normoxia |
Poikilocapnic Hypoxia |
Isocapnic Hypoxia |
Hypercapnic Hypoxia |
|||||
|---|---|---|---|---|---|---|---|---|
| Thermoneutral | Cold | Thermoneutral | Cold | Thermoneutral | Cold | Thermoneutral | Cold | |
| End-tidal Po2, mmHg | 101.3 ± 1.8 | 108.7 ± 3.3* | 44.5 ± 1.1† | 46.8 ± 1.5*† | 45.6 ± 0.7† | 46.8 ± 0.8† | 47.1 ± 0.8†‡ | 46.7 ± 0.4† |
| End-tidal Pco2, mmHg | 39.8 ± 0.8 | 39.2 ± 0.8 | 34.1 ± 1.4† | 31.1 ± 2.7† | 38.3 ± 1.0†‡ | 38.4 ± 1.0‡ | 40.7 ± 1.2‡§ | 41.1 ± 1.0†‡§ |
| Oxyhemoglobin saturation, % | 98.2 ± 0.5 | 98.6 ± 0.4 | 79.4 ± 0.4† | 82.3 ± 1.7*† | 79.6 ± 0.7† | 80.7 ± 0.5† | 81.1 ± 0.7† | 80.6 ± 1.4† |
| Ventilation, l/min | 5.08 ± 0.64 | 6.38 ± 0.67 | 7.11 ± 0.89 | 11.47 ± 3.35† | 9.90 ± 2.17† | 11.89 ± 2.72† | 26.57 ± 4.70†‡§ | 30.94 ± 4.86†‡§ |
| Heart rate, beats/min | 58.2 ± 3.3 | 54.3 ± 3.4* | 66.0 ± 3.0 | 68.8 ± 2.7† | 65.6 ± 4.7† | 64.2 ± 3.4† | 73.1 ± 2.7† | 74.5 ± 3.8†§ |
| Mean arterial pressure, mmHg | 86.3 ± 4.4 | 89.4 ± 5.1 | 90.9 ± 4.0† | 97.4 ± 6.4† | 89.6 ± 4.3 | 95.3 ± 6.1† | 95.3 ± 4.8†‡ | 102.0 ± 6.5†‡§ |
Values are means ± SE (n = 7).
P < 0.05 vs. thermoneutral within condition.
P < 0.05 vs. normoxia within time point.
P < 0.05 vs. poikilocapnic hypoxia within time point.
P < 0.05 vs. isocapnic hypoxia within time point.
DISCUSSION
This study yielded two new findings. First, a change in systemic CO2 does not impact the magnitude of cutaneous vasodilation during acute, steady-state hypoxia in thermoneutral conditions. Second, the magnitude of vasoconstriction elicited by brief whole body cooling is not altered by hypoxia per se. In effect, cold-induced vasoconstriction is superimposed on hypoxia-induced elevations in skin blood flow, such that skin blood flow remains elevated under hypoxic conditions relative to normoxic conditions during brief cold stress.
CO2 and hypoxic vasodilation in skin.
During acute hypoxia exposure, cardiac output is increased while mean arterial pressure is typically increased modestly or remains unchanged (16). In the peripheral circulation, we have previously demonstrated that isocapnic hypoxia causes vasodilation, which is not restrained by sympathetically mediated vasoconstriction in nonacral skin (20). In that study we also demonstrated that cutaneous vasodilation during hypoxia is not caused by the hyperpnea associated with hypoxic exposure. Thus hypoxemia per se causes cutaneous vasodilation, which is neither mediated nor opposed by sympathetic vasoconstrictor nerve activity. However, hypoxemia is rarely normocapnic in presentation and is often accompanied by hypocapnia (e.g., during altitude exposure) or hypercapnia (e.g., obstructive pulmonary disease). Therefore, in the present study we sought to test the effect of changes in systemic Pco2 on the magnitude of cutaneous vasodilation during hypoxia. We found that cutaneous vascular responses to steady-state hypoxia were not altered by changes in CO2 levels, although the response during poikilocapnia diminished toward the end of the 5-min steady-state exposure. Thus cutaneous vasodilation during poikilocapnic hypoxia is similar in magnitude to the isocapnic hypoxic response but may be either transient or biphasic in nature. Because cold stress was initiated after 5 min of steady-state hypoxia in each condition, the present data set is not suited to reveal the temporal characteristics of these hypoxic responses.
The present findings contribute to a growing body of evidence suggesting that the human cutaneous circulation is regulated differently than the skeletal muscle circulation during acute hypoxic exposure. Whereas the development of hypocapnia had little impact on the magnitude of cutaneous vasodilation during hypoxia, Black and Roddie (3) previously demonstrated that much of the forearm hypoxic vasodilation could be abolished by preventing the fall in Pco2. The basal flow values reported by Black and Roddie (3) indicate that ∼75% of forearm flow was likely directed to skeletal muscle in those experiments (6). In addition, increases in sympathetic nerve activity directed to skeletal muscle cause vasoconstriction during acute hypoxia (8, 9, 18, 21, 25), whereas this response is functionally absent in the cutaneous circulation (20). That is, blocking sympathetic neural transmission in the whole forearm circulation unmasks greater vasodilation during hypoxia (25), while similar experiments focusing on the cutaneous circulation reveal no effect (20). Thus the cutaneous vasculature is regulated independently and differently than the skeletal muscle circulation during acute hypoxia in healthy humans.
At first glance it seems that the failure of hypercapnia to potentiate hypoxic vasodilation is at odds with previous research demonstrating the vasodilator actions of CO2 in the skin circulation (7, 20, 22). However, it is important to view these data in the context of the degree of hypercapnia imposed (2 mmHg above eucapnia), which was limited by the capacity of our breathing apparatus. This hypercapnic stimulus was sufficient to increase ventilation 17 l/min beyond that achieved during isocapnic hypoxia, likely due to the synergistic effect of combined hypoxia and hypercapnia on peripheral chemoreflex activation (21). However, a greater stimulus is probably necessary to produce cutaneous vasodilation. Previous studies which demonstrated a local effect of CO2 on skin blood flow used either water baths at high CO2 concentrations (4 g of CO2/l) or subcutaneous injections of 12.5–100% CO2 gas (7, 22). In addition, our prior work (20), in which 5- and 9-mmHg elevations of end-tidal Pco2 values above eucapnia were associated with only modest vasodilation, suggested that the cutaneous vasodilation in response to such mild hypercapnia is not a local vascular response but is dependent on sympathetic neural responses (i.e., sympathetic withdrawal). Taken together, these data indicate that the lack of effect of hypercapnia on hypoxic cutaneous vasodilation in the present study should not be viewed as evidence against the impact of CO2 on skin blood flow but likely resulted from the use of a hypercapnic stimulus too weak to exert peripheral vascular effects in the cutaneous circulation.
Reflex cutaneous vasoconstriction during hypoxia.
During whole body cold stress, we found that although the pattern and magnitude of vasoconstriction was unaltered by either hypoxia or changes in CO2, vasoconstriction started at an elevated baseline due to hypoxia-induced vasodilation. As a result, skin blood flow during cooling was consistently higher compared with normoxic conditions, resulting in a relative state of dilation that persisted through the entire cooling period. However, the magnitude of this shift associated with hypoxia was somewhat diminished by any change in CO2 levels (hypercapnia or hypocapnia). Nevertheless, the data indicate that hypoxia per se is responsible for an upward shift in skin blood flow during cold stress, similar to the effect of hypoxia in thermoneutral conditions (20). The present findings agree with earlier studies demonstrating that skin temperature is elevated in hypoxic conditions and remains elevated (relative to normoxia) throughout a period of cold stress (4, 5). In one study, elevated skin temperature measured during combined hypoxia and cold stress was associated with a faster fall in core temperature compared with normoxia (5). Thus persistent cutaneous vasodilation may be unfavorable at high altitude, acting to maintain higher core-to-skin heat transfer and ultimately greater core heat loss. However, this hypothesis has not been systematically tested to date, and it is unclear how prolonged exposure to high altitude (e.g., during a mountaineering expedition) would modify cutaneous vasodilation or vasoconstriction in this setting.
Experimental considerations: baseline skin blood flow during hypoxia.
Exposure to hypoxia alters resting skin blood flow and therefore has the potential to confound interpretation of cutaneous vasoconstrictor responses when expressed relative to baseline values (11). To overcome this potential limitation, we measured cutaneous vascular conductance continuously throughout the transition between normoxic, hypoxic, and whole body cooling conditions. Therefore, we were able to express vasoconstrictor responses measured during hypoxia relative to both the normoxic and hypoxic baseline data. Using this approach, we showed that the baseline shift in cutaneous vascular conductance during hypoxia (i.e., hypoxic vasodilation) does not affect the interpretation of results from this study.
Conclusions.
In summary, we have shown that the magnitude of cutaneous vasodilation during steady-state hypoxia is not affected by CO2 responses. In addition, the magnitude of reflex vasoconstriction was not altered by hypoxia, such that an upward shift in skin blood flow was observed throughout whole body cooling during isocapnic hypoxia. It is likely that this has implications for thermoregulation against cold exposure at altitude or in other hypoxic settings.
GRANTS
This research was supported by doctoral student grants from the American College of Sports Medicine Foundation and the Eugene and Clarissa Evonuk Memorial Fund.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We express gratitude to all of the subjects who participated in this series of studies and to Robin High for statistical expertise. This study was conducted by Grant H. Simmons in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Oregon.
G. H. Simmons is currently a postdoctoral fellow in the Dept. of Biomedical Sciences at the Univ. of Missouri, and Sarah M. Fieger is currently a graduate student in the Dept. of Kinesiology at Kansas State Univ.
REFERENCES
- 1. Anderson D , Nagasawa G , Norfleet W , Olszowka A , Lundgren C. O2 pressures between 0.12 and 25 atm abs, circulatory function, and N2 elimination. Undersea Biomed Res 18: 279–292, 1991. [PubMed] [Google Scholar]
- 2. Anholm JD , Powles AC , Downey R , Houston CS , Sutton JR , Bonnet MH , Cymerman A. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis 145: 817–826, 1992. [DOI] [PubMed] [Google Scholar]
- 3. Black JE , Roddie IC. The mechanism of the changes in forearm vascular resistance during hypoxia. J Physiol 143: 226–235, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blatteis CM , Lutherer LO. Effect of altitude exposure on thermoregulatory response of man to cold. J Appl Physiol 41: 848–858, 1976. [DOI] [PubMed] [Google Scholar]
- 5. Cipriano LF , Goldman RF. Thermal responses of unclothed men exposed to both cold temperatures and high altitudes. J Appl Physiol 39: 796–800, 1975. [DOI] [PubMed] [Google Scholar]
- 6. Cooper KE , Edholm OG , Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol 128: 258–267, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diji A , Greenfield ADM. The local effect of carbon dioxide on human blood vessels. Am Heart J 60: 907–914, 1960. [DOI] [PubMed] [Google Scholar]
- 8. Halliwill JR , Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol 93: 857–864, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Halliwill JR , Morgan BJ , Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol 552: 295–302, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamlet MP. Human cold injuries. In: Human Performance Physiology and Environmental Medicine at Terrestrial Extremes, edited by , Pandolf KB , Sawka MN , Gonzalez RR. Traverse City, MI: Cooper Group, 1988, p. 435–466. [Google Scholar]
- 11. Hodges GJ , Kosiba WA , Zhao K , Alvarez GE , Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Johnson JM , Brengelmann GL , Hales JR , Vanhoutte PM , Wenger CB. Regulation of the cutaneous circulation. Fed Proc 45: 2841–2850, 1986. [PubMed] [Google Scholar]
- 13. Johnston CE , White MD , Wu M , Bristow GK , Giesbrecht GG. Eucapnic hypoxia lowers human cold thermoregulatory response thresholds and accelerates core cooling. J Appl Physiol 80: 422–429, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Kollai M. Responses in cutaneous vascular tone to transient hypoxia in man. J Auton Nerv Syst 9: 497–512, 1983. [DOI] [PubMed] [Google Scholar]
- 15. Leuenberger U , Gleeson K , Wroblewski K , Prophet S , Zelis R , Zwillich C , Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol Heart Circ Physiol 261: H1659–H1664, 1991. [DOI] [PubMed] [Google Scholar]
- 16. Rowell Human Circulation LB. Regulation During Physical Stress. New York: Oxford Univ. Press, 1986. [Google Scholar]
- 17. Sagawa S , Shiraki K , Konda N. Cutaneous vascular responses to heat simulated at a high altitude of 5,600 m. J Appl Physiol 60: 1150–1154, 1986. [DOI] [PubMed] [Google Scholar]
- 18. Saito M , Mano T , Iwase S , Koga K , Abe H , Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65: 1548–1552, 1988. [DOI] [PubMed] [Google Scholar]
- 19. Schneider EC , Sisco DL. The circulation of the blood in man at high altitudes. II. The rate of blood flow and the influence of oxygen on the pulse rate and blood flow. Am J Physiol 34: 29–47, 1914. [Google Scholar]
- 20. Simmons GH , Minson CT , Cracowski JL , Halliwill JR. Systemic hypoxia causes cutaneous vasodilation in healthy humans. J Appl Physiol 103: 608–615, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Somers VK , Mark AL , Zavala DC , Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67: 2101–2106, 1989. [DOI] [PubMed] [Google Scholar]
- 22. Stein ID , Weinstein I. The value of carbon dioxide baths in the treatment of peripheral vascular disease and allied conditions. Am Heart J 23: 349–361, 1942. [Google Scholar]
- 23. Stephens DP , Aoki K , Kosiba WA , Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Thompson CS , Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weisbrod CJ , Minson CT , Joyner MJ , Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. West JB , Lahiri S , Maret KH , Peters RM , Pizzo CJ. Barometric pressures at extreme altitudes on Mt Everest: physiological significance. J Appl Physiol 54: 1188–1194, 1983. [DOI] [PubMed] [Google Scholar]