Abstract
Because the maintenance of glycemia is essential during prolonged exercise, we examined the effects of endurance training, exercise intensity, and plasma lactate concentration ([lactate]) on gluconeogenesis (GNG) and hepatic glycogenolysis (GLY) in fasted men exercising at, and just below, the lactate threshold (LT), where GNG precursor lactate availability is high. Twelve healthy men (6 untrained, 6 trained) completed 60 min of constant-load exercise at power outputs corresponding to their individual LT. Trained subjects completed two additional 60-min sessions of constant-load exercise: one at 10% below the LT workload (LT-10%), and the other with a lactate clamp (LT-10%+LC) to match the [lactate] of the LT trial. Flux rates were determined by primed continuous infusion of [6,6-2H2]glucose, [3-13C]lactate, and [13C]bicarbonate tracers during 90 min of rest and 60 min of cycling. Exercise at LT corresponded to 67.6 ± 1.3 and 74.8 ± 1.7% peak O2 consumption in the untrained and trained subjects, respectively (P < 0.05). Relative exercise intensity was matched between the untrained group at LT and the trained group at LT-10%, and [lactate] during exercise was matched in the LT and LT-10%+LC trials via exogenous lactate infusion. Glucose kinetics (rate of appearance, rate of disposal, and metabolic clearance rate) were augmented with the lactate clamp. GNG was decreased in the trained subjects exercising at LT and LT-10% compared with the untrained subjects, but increasing [lactate] in the LT-10%+LC trial significantly increased GNG (4.4 ± 0.9 mg·kg−1·min−1) compared with its corresponding control (1.7 ± 0.4 mg·kg−1·min−1, P < 0.05). Hepatic GLY was higher in the trained than untrained subjects, but not significantly different across conditions. We conclude that GNG plays an essential role in maintaining total glucose production during exercise in fasted men, regardless of training state. However, endurance training increases the ability to achieve a higher relative exercise intensity and absolute power output at the LT without a significant decrease in GNG. Furthermore, raising systemic precursor substrate availability increases GNG during exercise, but not at rest.
Keywords: glucose, lactate, exercise, gluconeogenesis, training, exertion, glucose homeostasis, stable isotope tracers
blood glucose homeostasis is essential during prolonged periods of endurance exercise, when large changes occur in tissue oxygen delivery and use, metabolic rate, carbohydrate (CHO) oxidation, blood glucose disposal, and hepatic plus renal glucose production. However, compared with the extensive literature on cardiovascular regulation during exercise, the regulation of glucose production for the maintenance of glycemia during exercise in fasted humans has been minimally studied.
Following an overnight fast, gluconeogenesis (GNG) provides 25–50% of total glucose production in resting humans (4, 12, 13, 31, 52), while the remainder is supported by hepatic glycogenolysis (GLY). This relative partitioning of glucose rate of appearance (Ra) relies more on GNG as the fast progresses and hepatic glycogen stores become depleted (1, 49, 54). Staehr et al. (47) studied glucose metabolism in resting humans after fasting for 40 h, and GNG accounted for nearly 90% of total glucose production. During submaximal exercise, energy demand requires muscle glucose utilization and, consequently, increases blood glucose disposal (9). Therefore, a consistent glucose delivery into the circulation becomes paramount for preventing hypoglycemia. In contrast to rest, the relative contribution of GNG to total glucose production has been shown to decrease during hard exercise (52); for instance, %GNG decreases at power outputs (PO) approaching the lactate threshold (LT). Because hepatic glycogen reserves are partially depleted during the course of an overnight fast (40), the combination of limited hepatic GLY and attenuated GNG has been shown to result in falling blood glucose levels in postabsorptive men after 75–90 min of hard [65% maximum O2 consumption (V̇o2)] exercise (52).
Because lactate is the primary precursor for GNG in the liver and to a lesser extent the renal cortex (2, 16, 37), we hypothesized that increasing lactate availability would increase the rate of GNG, and possibly also the glucose Ra in postabsorptive men during exercise near the LT. For the purpose of testing these hypotheses, we utilized stable isotope tracers and a cross-sectional design to study the maintenance of glycemia in overnight-fasted untrained (UT) and trained (T) men at rest and during 60 min of constant-load exercise at or just below the PO eliciting the LT. This workload was selected as the target exercise intensity because the LT is widely used in exercise science and sports medicine as a measure of athletic prowess, and, as well, because exercise at the LT results in an elevated lactate (gluconeogenic precursor) supply. Additionally, beyond conducting studies at and just below the LT, to evaluate the effect of precursor concentration on GNG and total glucose production, we also incorporated a lactate clamp (LC) component in the experimental design.
METHODS
This paper is part of a larger investigation of lactate and glucose metabolism during exercise at the LT. Some of the data are to be reported elsewhere (Messonnier LA, Emhoff CW, Horning MA, Fattor JA, Carlson TJ, and Brooks GA, unpublished observations), but for the convenience of the readers, methods are repeated here.
Subjects.
Twelve healthy male subjects (6 UT and 6 trained), aged 19–33 yr, were recruited from the University of California Berkeley campus and the surrounding community by posted notices, word of mouth, and e-mail. The UT subjects were recreationally active and considered untrained if their peak oxygen consumption (V̇o2peak) was <50 ml·kg−1·min−1. Trained subjects were members of competitive cycling or triathlon teams, currently in the race phase of their training season, and considered well-trained if they had a V̇o2peak of >55 ml·kg−1·min−1. Subjects were included in the study if they had a body mass index of ≥18 and <26 kg/m2, were nonsmokers, were diet and weight stable, had a 1-s forced expiratory volume of >70% of vital capacity, and were injury/disease free, as determined by physical examination by a physician. This study was approved by the University of California Berkeley Committee for the Protection of Human Subjects (CPHS 2010-4-1300) and conformed to the standards set by the Declaration of Helsinki. All subjects gave written, informed consent before participation in the study.
Preliminary testing.
Exercise tests were performed on an electronically braked leg cycle ergometer (Monark Ergometric 839E, Vansbro, Sweden) and were conducted at least 1 wk apart. Following interviews and screening, subjects performed two graded exercise tests to determine V̇o2peak and LT. To determine V̇o2peak, as per American College of Sports Medicine guidelines (7th Ed.), exercise PO started at 75 or 120 W and was increased by 25 or 30 W for the UT and trained subjects, respectively, every 3 min until volitional fatigue. Expired respiratory gases were continuously monitored throughout the test via an open-circuit automated indirect calorimetry system (ParvoMedics TrueOne Metabolic System, Salt Lake City, UT) that was calibrated using room air and a certified calibration gas. Finger pricks drawing 10 μl of blood were conducted at the end of each stage to measure lactate concentration ([lactate]) via portable lactate analyzers (Nova Lactate Plus, Waltham, MA) and to approximate the PO eliciting the LT.
To determine LT, a second graded exercise test started at 50 W below the approximated LT PO and increased by 10 W every 3 min until volitional exhaustion. At the end of every stage, 1 ml of blood was drawn from an arterialized hand vein for enzymatic analysis of blood [lactate], which increased linearly with exercise work rate until a certain PO. After this point, a rapid acceleration in blood lactate accumulation occurred. The LT was considered to be the last stage of the slow linear increase in [lactate] before the rapid acceleration in lactate accumulation. This definition of the LT is termed by some as the second lactate turn point (27) and approximates the maximal lactate steady state (6). For both graded exercise tests, heart rate was monitored continuously using a heart rate monitor (Polar, Gay Mills, WI) and electrocardiography (Quinton 759 ECG, Seattle, WA); rating of perceived exertion (RPE) was recorded according to the Borg scale (7); and blood pressure was measured at the middle of every stage by manual auscultation.
Following graded exercise tests, subjects performed 60 min of continuous exercise at the PO corresponding to their LT to ensure stabilization of blood [lactate] over the entire duration of the exercise test. Every 10 min, finger pricks drawing 10 μl of blood were conducted to measure [lactate], and heart rate and RPE were monitored.
Experimental design.
The study design consisted of four conditions using stable isotope tracers: one condition within the UT group, and three conditions within the trained group. UT subjects completed one isotope infusion trial, consisting of a 90-min rest period followed by 60 min of continuous cycling at the LT. Trained subjects completed three isotope infusion trials, each consisting of a 90-min rest period, followed by 60 min of continuous leg ergometer cycling under one of the following conditions: 1) PO eliciting the LT; 2) PO 10% below that eliciting the LT (LT-10%); and 3) PO 10% below that eliciting the LT, but with blood [lactate] raised to the LT level via exogenous lactate infusion, i.e., clamp (LT-10%+LC). The order of the last two conditions was randomized, and all exercise trials were conducted at least 1 wk apart.
To evaluate the effects of training on metabolic responses, we compared UT and trained groups exercising at the same [lactate], but different absolute and relative intensities (UT vs. T); at the same relative intensity, but different [lactate] (UT vs. LT-10%); and at the same relative intensity and [lactate] (UT vs. LT-10%+LC). Within the trained subjects, we investigated: the effects of exercise intensity given the same [lactate] (LT vs. LT-10%+LC); the effects of [lactate] given the same exercise intensity (LT-10% vs. LT-10%+LC); and the combined effects of [lactate] and exercise intensity (LT vs. LT-10%).
Dietary controls.
Three-day diet records were collected before the study to record subjects' caloric intake and macronutrient composition (DietAnalysis Plus, version 6.1 ESHA Research, Salem, OR). Standardized diets (∼50% CHO, 30% fat, 20% protein) consisting of an average of 2,400 and 3,200 kcal for the UT and trained subjects, respectively, were given the day before each exercise trial, including an evening snack as the last meal. Subjects came to the laboratory overnight fasted, and exercise commenced 12 h after the evening snack was consumed.
Isotope tracer protocol.
Subjects reported to the laboratory on the morning of each tracer trial, and expired respiratory gases were sampled for a measurement of background 13CO2 enrichment. A catheter was placed into a warmed hand vein for “arterialized” blood sampling, and a background sample was collected. After a second catheter was placed in the antecubital vein of the contralateral arm for infusion of stable isotope tracer solutions, subjects received a primed continuous infusion of [6,6-2H2]glucose (i.e., D2-glucose) and [3-13C]lactate (Sigma-Aldrich, St. Louis, MO) while resting semisupine for 90 min. Isotopes were diluted in 0.9% sterile saline and were tested for pyrogenicity and sterility (School of Pharmacy, University of California, San Francisco, CA) and passed through a 0.2-μm Millipore filter (Nalgene, Rochester, NY) before infusion. Priming boluses were the same across trials and contained 250 mg D2-glucose, 57.5 mg [3-13C]lactate, and 136 mg [13C]bicarbonate (Isotec, Sigma-Aldrich). Tracers D2-glucose and [3-13C]lactate were then continuously infused for the 90-min rest period via a pump (Baxter Travenol 6300) at 2.0 mg/min for glucose and 2.5 mg/min for lactate in all trials, with the exception of the LT-10%+LC trial that had a resting infusion rate of 7.5 mg/min for lactate. The LT-10%+LC trial also included infusion of a lactic acid-sodium hydroxide mixture (see LC procedure) to begin raising blood [lactate] during the rest period to the level seen in exercise at the PO eliciting the LT. At the start of all exercise trials, tracer infusion rates were increased to 8 mg/min for glucose and 11.25 mg/min for lactate for the UT subjects and 10 mg/min for glucose and 15 mg/min for lactate for the trained subjects and continued for 60 min of exercise. These infusion rates were selected based on prior experience to maintain stable isotopic enrichments (IE) during rest and exercise.
LC procedure.
In the LT-10%+LC trial, a LC procedure was performed, as previously described (39). An unlabeled “cold lactate” cocktail was prepared by mixing a 30% lactic acid solution (Sigma-Aldrich) in 2 N sodium hydroxide to a pH of 4.8 and subsequently tested for pyrogenicity and sterility at the University of California San Francisco School of Pharmacy in the same manner as the isotope solutions. During the rest period, cold lactate infusion began at 2.6 mg·kg−1·min−1 to raise blood [lactate] to the one obtained individually during the LT trial. Infusion rates were increased or decreased during rest and exercise to maintain the target concentration, as determined by a portable lactate analyzer.
Blood and respiratory gas sampling.
Arterialized blood was drawn from a warmed hand vein for metabolite, IE, and hormonal analyses at 0 (background), 60, 75, and 90 min of rest and 10, 20, 30, 40, 50, and 60 min of exercise. Hematocrit was also measured at each time point using a circular microcapillary tube reader (no. 2201, International Equipment, Needham Heights, MA). Blood for glucose and [lactate] and IE determinations was immediately deproteinized with 8% perchloric acid, shaken, and placed on ice. Blood for glycerol and nonesterified fatty acid concentration was collected in EDTA tubes, blood for hormones was collected with aprotinin, and blood for catecholamines was collected with glutathione/EGTA. Samples were centrifuged at 3,000 g for 18 min, and the supernatant was transferred to storage tubes and frozen at −80°C until analysis.
Respiratory gases were analyzed continuously via indirect calorimetry for 5 min before, and coincident with, blood sampling. These measurements were used for calculation of V̇o2, carbon dioxide production, respiratory exchange ratio, and minute ventilation. Duplicate samples of expired air were collected in 10-ml evacuated containers for 13CO2 IE determinations. Careful attention was placed on flushing the line and sampling expired CO2 at the same time as blood was drawn for glucose and lactate IE. Heart rate, blood pressure, and RPE measurements were also recorded at the same frequency as blood and breath sampling.
Hormone analyses.
Catecholamines were extracted from the plasma using acid-washed WA-4 Alumina (Sigma-Aldrich, St. Louis, MO) and 1.5 Tris buffer containing 2% EGTA at a pH of 8.6. Perchloric acid (0.1 M) was used to elute the catecholamines. Finally, 100 μl of this eluent were injected in the HPLC system (Electrochemistry Separations Analysis, ESA, model LC/EC, 5200A; Coulochem, Chelmsford, MA). The mobile phase was Cataphase 2 (ESA, Cambridge, MA), and the electrodes were set at +350, +50, and −350 mV. Standard catecholamine solutions were purchased from ESA. Chromatographs were analyzed using an ESA 501 Data Chromatography System. Insulin and glucagon were measured with commercially available radioimmunoassay kits (Coat-A-Count, DPC, Los Angeles, CA).
Metabolite concentration and IE analyses.
Known amounts of uniformly labeled internal standards [U-13C]glucose, and [U-13C]lactate were added to the supernatant samples collected in 8% perchloric acid. Samples were then neutralized with 2 N KOH and transferred to ion exchange columns that were previously washed with double deionized water (ddH2O) through a cation resin (Analytical Grade 50W-X8, 50–100 mesh H+ resin, Bio-Rad Laboratories, Hercules, CA) and with ddH2O followed by 2 N formic acid through an anion resin (Analytical Grade 1-X8, 100–200 mesh formate resin). Glucose was eluted first with ddH2O, followed by elution of lactate through the anion column with 2 N formic acid. Subsequent lactate analyses and kinetics data are reported separately (Messonnier LA, et al., unpublished observations).
The glucose effluent was lyophilized and derivatized as previously described (39). Briefly, glucose IE was determined by gas chromatography/mass spectrometry (GC model 6890 series and MS model 5973N, Agilent Technologies) of the penta-acetate derivative, where methane was used for selected ion monitoring of mass-to-charge ratios 331 (nonlabeled glucose), 332 (M+1 isotopomer, [1-13C]glucose), 333 (M+2 isotopomer, D2-glucose), and 337 (M+6 isotopomer, [U-13C]glucose internal standard). Whole blood glucose concentration ([glucose]) was determined by abundance ratios of 331/337. Selected ion abundances were compared against external standard curves for calculation of concentration and IE. Breath samples were analyzed by use of isotope ratio mass spectrometry by Metabolic Solutions (Nashua, NH).
Calculations.
All calculations for concentrations and flux rates used the last 30 min of rest (60, 75, and 90 min) and the last 20 min of steady-rate exercise (40, 50, and 60 min). Glucose turnover [Ra and rate of disposal (Rd)] and metabolic clearance rate (MCR) were calculated using the non-steady-state equations of Steele modified for use with stable isotopes (56):
| (1) |
| (2) |
| (3) |
where Ra and Rd are measured in mg·kg−1·min−1; MCR is measured in ml·kg−1·min−1; F is the isotope infusion rate in mg·kg−1·min−1; V is the volume of distribution (180 ml/kg); C1 and C2 are concentrations at sampling times t1 and t2, respectively; and IE1 and IE2 are the excess IE at sampling times t1 and t2, respectively. The percentage of glucose Ra from lactate-derived GNG (%GNG) was calculated as previously described (28) in our laboratory. This approach was derived from that of Zilversmit et al. (58):
| (4) |
where glucose M+1 IE is the IE of the M+1 glucose isotopomer, and H is the Hetenyi factor to correct for loss of label in the tricarboxylic acid cycle during GNG (1.45 at rest and 1.0 during exercise). The absolute rate of GNG in mg·kg−1·min−1 was calculated as previously described (28):
| (5) |
The absolute rate of hepatic GLY was calculated as the difference between total glucose production and GNG:
| (6) |
Statistical analyses.
Significance of differences in subject characteristics between UT and trained groups were analyzed using an unpaired Student's t-test. Differences in metabolic parameters between the UT and the three trained conditions within rest and exercise were analyzed using a one-way analysis of variance. Analyses within the trained conditions were done with repeated measures. Post hoc analyses were made using Fisher's least significant difference multiple-comparisons test. Differences from rest to exercise were analyzed using a paired Student's t-test. Statistical significance was set at α = 0.05, and values are represented as means ± SE, unless otherwise noted.
RESULTS
Subject characteristics.
Anthropometric data and work capacities of subjects are reported in Table 1. Compared with UT subjects, trained subjects had a 33% greater V̇o2peak (P < 0.05), a 44% greater peak PO (P < 0.05), and a 47% greater total daily energy intake (P < 0.05). Subjects were weight stable throughout the study, as measured on each day of an exercise trial. Additionally, total energy and macronutrient composition of the subjects' diets did not change throughout the study (∼52% CHO, 30% fat, 18% protein).
Table 1.
Subject characteristics for untrained and trained groups
| Variable | Untrained | Trained |
|---|---|---|
| Age, yr | 25 ± 1 | 24 ± 2 |
| Body mass index, kg/m2 | 26.3 ± 0.7 | 23.9 ± 0.6* |
| Body fat, % | 13.0 ± 1.0 | 10.0 ± 1.3 |
| FEV1/FVC, % | 88.7 ± 2.6 | 79.6 ± 2.9* |
| Absolute V̇o2peak, l/min | 3.7 ± 0.1 | 5.0 ± 0.3* |
| Relative V̇o2peak, ml·kg−1·min−1 | 46.1 ± 1.5 | 66.6 ± 2.6* |
| Peak power output, W | 248 ± 7 | 357 ± 12* |
| 3-Day diet records | ||
| Energy, kcal/day | 2,363 ± 256 | 3,465 ± 149* |
| Carbohydrate, % | 53 ± 3 | 52 ± 4 |
| Fat, % | 28 ± 2 | 32 ± 4 |
| Protein, % | 19 ± 1 | 16 ± 1 |
Values are means ± SE; n = 6 for untrained and trained groups. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; V̇o2peak, peak oxygen consumption.
Significantly different from untrained group (P < 0.05).
Work and cardiovascular parameters.
Exercise trial workloads, exogenous lactate infusion rates, and cardiorespiratory and hemodynamic parameters are reported in Table 2. During exercise, trained subjects reached their LT at an 11% greater V̇o2peak (P < 0.05) and a 62% greater absolute PO (P < 0.05). V̇o2, carbon dioxide production, minute ventilation, heart rate, and hematocrit were increased in all conditions from rest to exercise. A significant increase in mean arterial pressure was observed in the UT group only.
Table 2.
Workload, exogenous unlabeled (cold) lactate infusion rate, and cardiorespiratory and hemodynamic parameters during rest and exercise
| Rest |
Exercise |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Untrained | Trained LT | Trained LT-10% | Trained LT-10%+LC | Untrained | Trained LT | Trained LT-10% | Trained LT-10%+LC |
| Power output, W | 161 ± 4 | 259 ± 10* | 234 ± 9*‡ | 234 ± 9*‡ | ||||
| V̇o2peak, % | 67.6 ± 1.3 | 74.8 ± 1.7* | 66.6 ± 1.4‡ | 67.9 ± 1.5‡ | ||||
| Exogenous lactate infusion rate, mg·kg−1·min−1 | 3.91 ± 0.26 | 3.16 ± 0.95 | ||||||
| V̇o2, l/min | 0.31 ± 0.01 | 0.31 ± 0.02 | 0.31 ± 0.02 | 0.33 ± 0.02 | 2.52 ± 0.06§ | 3.71 ± 0.19*§ | 3.30 ± 0.16*‡§ | 3.36 ± 0.15*‡§ |
| V̇co2, l/min | 0.25 ± 0.00 | 0.28 ± 0.01 | 0.30 ± 0.03 | 0.28 ± 0.03 | 2.33 ± 0.08§ | 3.51 ± 0.18*§ | 3.05 ± 0.15*‡§ | 3.05 ± 0.13§*‡ |
| RER | 0.80 ± 0.01 | 0.85 ± 0.02* | 0.87 ± 0.02* | 0.77 ± 0.01†‡ | 0.93 ± 0.01§ | 0.95 ± 0.00§ | 0.93 ± 0.01§ | 0.91 ± 0.00‡§ |
| V̇e, l/min | 7.7 ± 0.3 | 9.1 ± 0.6 | 10.2 ± 1.6 | 9.4 ± 1.2 | 56.2 ± 3.2§ | 84.2 ± 5.8*§ | 68.1 ± 4.2§*‡ | 64.5 ± 2.7*‡§ |
| fH, beats/min | 62.3 ± 1.8 | 60.8 ± 2.2 | 56.8 ± 2.3 | 58.9 ± 2.3 | 164.8 ± 5.4§ | 172.4 ± 2.2§ | 158.6 ± 4.5‡§ | 155.7 ± 2.6‡§ |
| MAP, mmHg | 92.0 ± 1.6 | 91.6 ± 2.6 | 93.9 ± 2.9 | 92.2 ± 5.5 | 101.4 ± 2.9§ | 99.0 ± 3.8 | 98.4 ± 3.4 | 92.5 ± 2.4 |
| Hematocrit, % | 43.4 ± 1.1 | 45.0 ± 0.8 | 44.3 ± 1.0 | 39.8 ± 1.1*†‡ | 46.1 ± 0.8§ | 47.7 ± 0.8§ | 47.2 ± 1.1§ | 42.5 ± 0.9†‡§ |
| RPE | 15.9 ± 1.2 | 14.9 ± 1.4 | 12.1 ± 0.8* | 13.3 ± 1.2 | ||||
Values are means ± SE; n = 6 for untrained and trained groups. LT, lactate threshold; LT-10%, 10% below the LT workload; LT-10%+LC, 10% below the LT workload with a lactate clamp; V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e, minute ventilation; fH, heart rate; MAP, mean arterial pressure; RPE, rating of perceived exertion.
Significantly different from rest within condition (P < 0.05).
Significantly different from untrained (P < 0.05).
Significantly different from trained LT (P < 0.05).
Significantly different from trained LT-10% (P < 0.05).
Lactate clamp.
Exogenous lactate infusion began during the rest period to raise blood [lactate] to ∼4.3 mmol/l, which was significantly higher than all other resting conditions (Table 3). During exercise, blood [lactate] was significantly elevated in the LT-10%+LC condition compared with its corresponding control (LT-10%) (4.3 ± 0.3 vs. 2.5 ± 0.5 mmol/l, P < 0.05), and it was not different from that during exercise in the LT condition (4.3 ± 0.2 mmol/l). Average lactate infusion rates were 3.91 ± 0.26 and 3.16 ± 0.95 mg·kg−1·min−1 for rest and exercise, respectively (Table 2). Exogenous lactate infusion caused mild hemodilution during rest and exercise, but no significant differences were observed in gas exchange variables, ventilation, heart rate, or RPE due to the LC, with the exception of a decreased respiratory exchange ratio.
Table 3.
Metabolite and hormonal concentrations during rest and exercise
| Rest |
Exercise |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Untrained | Trained LT | Trained LT-10% | Trained LT-10%+LC | Untrained | Trained LT | Trained LT-10% | Trained LT-10%+LC |
| Lactate, mmol/l | 0.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 4.4 ± 0.4*†‡ | 3.7 ± 0.4§ | 4.3 ± 0.2§ | 2.5 ± 0.5*‡§ | 4.3 ± 0.3† |
| FFA, μmol/l | 360 ± 50 | 276 ± 27 | 200 ± 25* | 259 ± 24* | 491 ± 67 | 262 ± 47* | 220 ± 21* | 358 ± 37† |
| Glycerol, μmol/l | 46 ± 10 | 28 ± 3 | 36 ± 4 | 36 ± 7 | 218 ± 36§ | 270 ± 39§ | 215 ± 18§ | 248 ± 23§ |
| Epinephrine, pg/ml | 39 ± 7 | 78 ± 17 | 65 ± 13 | 44 ± 16 | 303 ± 16§ | 321 ± 49§ | 216 ± 51*‡§ | 144 ± 22§*‡ |
| Norepinephrine, pg/ml | 214 ± 48 | 303 ± 83 | 272 ± 69 | 254 ± 39 | 1,929 ± 260§ | 4,011 ± 750*§ | 2,946 ± 594*‡§ | 1,512 ± 206†‡§ |
| Insulin, pg/ml | 358 ± 29 | 303 ± 22 | 307 ± 17 | 267 ± 5* | 193 ± 28§ | 187 ± 13§ | 221 ± 21§ | 226 ± 13§ |
| Glucagon, pg/ml | 63 ± 8 | 45 ± 3* | 46 ± 4* | 48 ± 4 | 85 ± 7§ | 55 ± 8* | 46 ± 8* | 71 ± 11†§ |
Values are means ± SE; n = 6 for untrained and trained groups. FFA, free fatty acids.
Significantly different from rest within condition (P < 0.05).
Significantly different from untrained (P < 0.05).
Significantly different from trained LT (P < 0.05).
Significantly different from trained LT-10% (P < 0.05).
Metabolite and hormone concentrations.
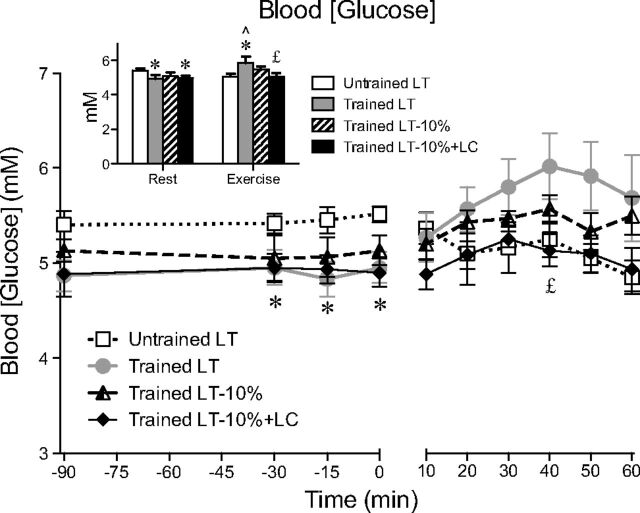
Blood [glucose] during rest and exercise are shown in Fig. 1. Resting blood [glucose] was elevated in UT subjects (5.4 ± 0.1 mmol/l) compared with trained subjects, particularly during the LT (4.9 ± 0.2 mmol/l, P < 0.05) and LT-10%+LC (4.9 ± 0.1 mmol/l, P < 0.05) trials. During exercise at LT, blood [glucose] was significantly lower in the UT subjects (5.1 ± 0.2 vs. 5.9 ± 0.4 mmol/l, P < 0.05). In the trained subjects, blood [glucose] was not different between LT-10% and LT-10%+ LC (Fig. 1), while an increase in glucagon was observed with the LC (71 ± 11 vs. 46 ± 8 pg/ml, P < 0.05). Mean concentrations for lactate, free fatty acids, glycerol, and hormones during rest and exercise are reported in Table 3. Blood [lactate] significantly increased from rest to exercise, except in the LT-10%+LC condition, where [lactate] was clamped. Plasma free fatty acid, glycerol, and catecholamines increased from rest to exercise. The LC procedure suppressed catecholamines and increased glucagon concentration. Insulin decreased during exercise, but was not different between the four trials.
Fig. 1.
Blood glucose concentration across time. Values are means ± SE; n = 6 for untrained and trained groups. Inset shows steady-state rest and exercise glucose concentrations for each condition. LT, lactate threshold; LT-10%, 10% below the LT workload; LT-10%+LC, 10% below the LT workload with a lactate clamp. ∧Significantly different from rest within condition (P < 0.05). *Significantly different from untrained (P < 0.05). £Significantly different from trained LT (P < 0.05).
Glucose kinetics.
Arterial enrichments of the glucose M+2 isotopomer (i.e., the D2-glucose tracer) are presented over time in Fig. 2A. Glucose Ra and Rd, calculated from the M+2 isotopomer, were similar during rest across all conditions and proportional to relative workload during exercise, with the exception of the LT-10%+LC condition in trained subjects. Glucose turnover (Ra and Rd) was augmented by LC during exercise at 67% V̇o2peak (Fig. 3, A and B), although this increase did not achieve significance. MCR was also increased during exercise in the LT-10%+LC condition (Fig. 3C) compared with its corresponding control (LT-10%). Rates of glucose turnover were higher in the UT compared with the trained subjects at rest, indicating greater reliance on blood glucose. This elevation in resting glucose turnover achieved significance compared with the LT-10%+LC condition.
Fig. 2.
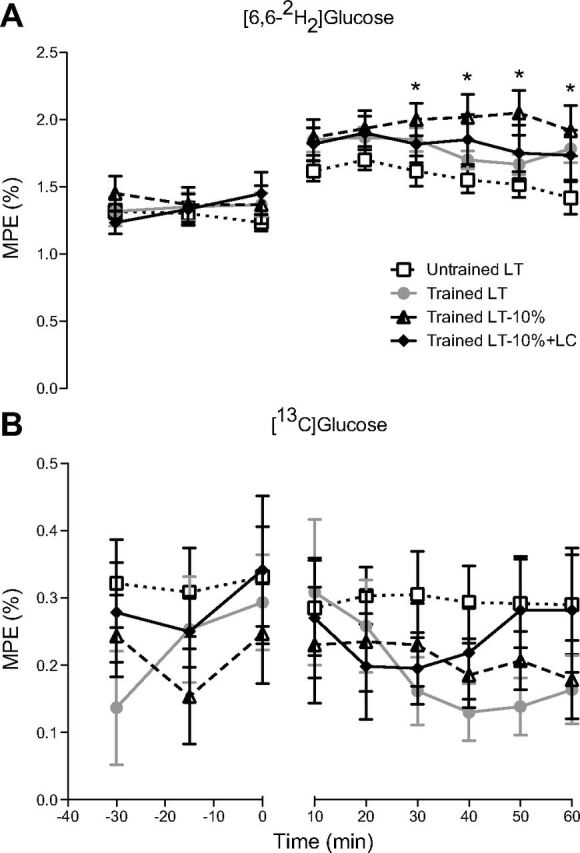
Mole percent excess (MPE) of glucose isotopomers M+2 (A) and M+1 (B) across time. Values are means ± SE; n = 6 for untrained and trained groups. *Significantly different from untrained (P < 0.05).
Fig. 3.
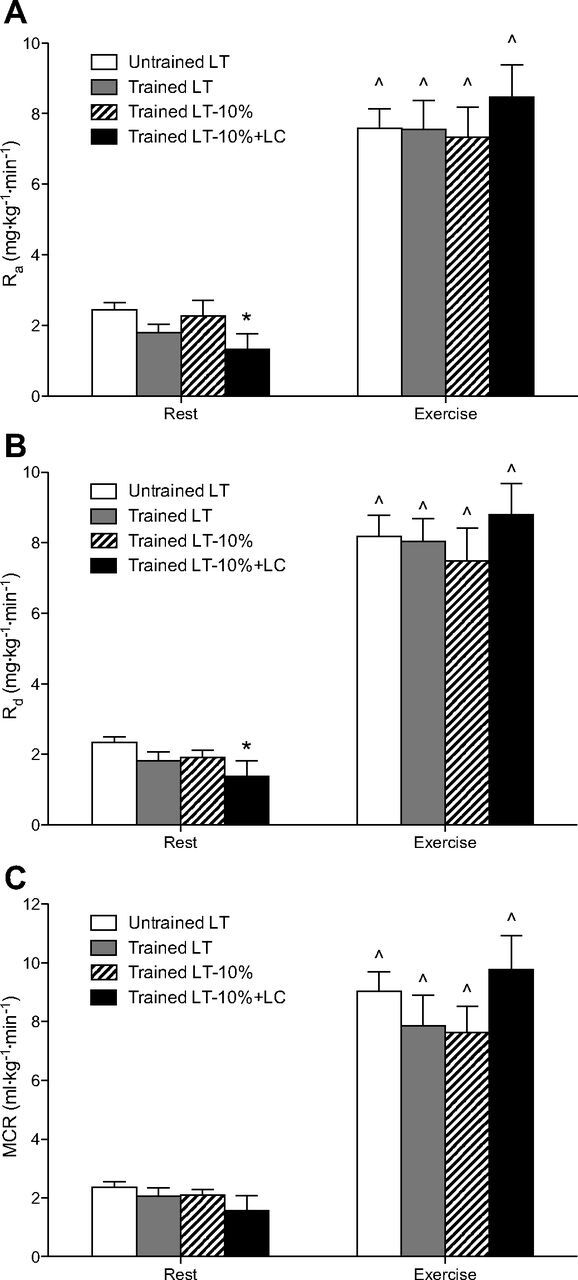
Steady-state glucose rates of appearance (Ra; A) and disposal (Rd; B), and metabolic clearance (MCR; C) during rest and exercise. Values are means ± SE; n = 6 for untrained and trained groups. ∧Significantly different from rest within condition (P < 0.05). *Significantly different from untrained (P < 0.05).
Hepatic GNG and GLY.
Arterial enrichments of the glucose M+1 isotopomer from incorporation of 13C from the lactate tracer were stable over time throughout rest and exercise (Fig. 2B), but varied due to exercise intensity and lactate tracer infusion rate between subjects. At rest, absolute rates of GNG were 50% higher, although insignificant, in the UT subjects compared with the trained subjects (Fig. 4A). From rest to exercise at LT, GNG rose approximately threefold for both UT and trained subjects. From rest to exercise at LT-10%, GNG more than doubled, but then increased nearly sevenfold from rest to exercise in the LT-10%+LC condition.
Fig. 4.
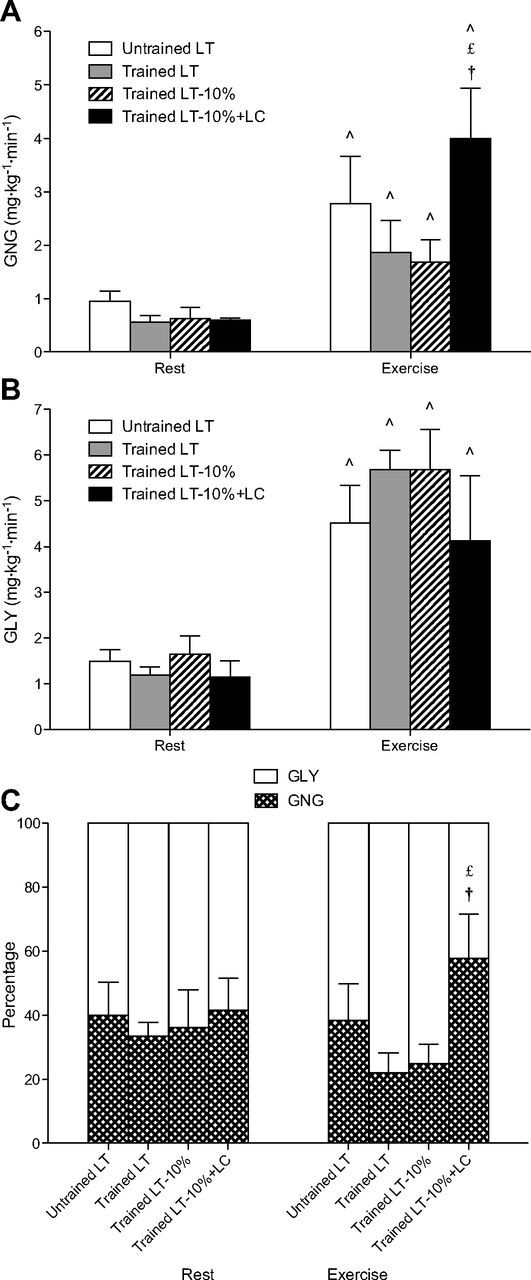
Steady-state rates of gluconeogenesis (GNG; A) and hepatic glycogenolysis (GLY; B), and partitioning of total glucose production in relative terms (C). Values are means ± SE; n = 6 for untrained and trained groups. ∧Significantly different from rest within condition (P < 0.05). £Significantly different from trained LT (P < 0.05). †Significantly different from trained LT-10% (P < 0.05).
Absolute rates of hepatic GLY (Fig. 4B), determined as the difference between total glucose production (Ra) and GNG, were similar across conditions at rest. During exercise, GLY increased by approximately threefold in all conditions.
Relative partitioning of total glucose production into hepatic GNG and GLY (Fig. 4C) showed that ∼40% of glucose production came from GNG during rest in both UT and trained groups. During exercise, this percentage dropped to ∼25% in the trained subjects exercising at LT and LT-10%. However, these percentages of glucose Ra from GNG were not statistically different from those in the UT subjects, showing that trained individuals achieved higher exercise workloads without a significant decrease in GNG. The contribution of GNG during exercise persisted at 40% in the UT subjects and increased to nearly 60% during the LT-10%+LC condition of the trained subjects, with hepatic GLY accounting for the remainder. This increase in GNG as a percentage of total glucose production was significant compared with the LT and LT-10% trials in the trained group (P < 0.05).
DISCUSSION
We examined the individual and combined effects of endurance training, relative exercise intensity, PO, and blood [lactate] on glucose kinetics and hepatic GNG and GLY during exercise at, and just below, the LT. Our main findings were that trained subjects achieved a significantly higher exercise workload at the LT without experiencing a significant reduction in GNG compared with their UT counterparts who exercised at a 40% lower PO. Additionally, when [lactate] were matched at the same relative exercise intensity using a LC, a greater gluconeogenic capacity was revealed in the trained compared with UT subjects. During the LC trial, GNG exceeded GLY in glucose production partitioning, becoming the major contributor (>50%) of glucose production. In other words, providing additional precursor lactate increases GNG during exercise.
During fasting or prolonged exercise, GNG plays a crucial role in the maintenance of blood glucose homeostasis. Rates of GNG increase with duration of fast, as shown by studies utilizing dideuterated water and mass isotopomer distribution analysis techniques (12, 23, 31, 52). In our present study, subjects were overnight fasted and likely had reduced capacity for hepatic glucose production from GLY. Thus the higher rates of GNG observed, as well as higher relative contribution of GNG to glucose Ra during rest and exercise, were consistent with results of other studies employing a 12- to 14-h fast (12, 33).
Effects of exercise intensity.
Rates of GNG depend in part on precursor supply, determined by hepatic blood flow and precursor concentration. Because hepatic blood flow decreases in direct proportion to relative exercise intensity (1, 42), limitations in precursor delivery to gluconeogenic organs may explain results obtained in this and previous studies conducted on subjects exercising at intensities >50% of maximum V̇o2, in which rates of GNG decrease as workload increases (33). Accordingly, it is reasonable to conclude that differences in GNG observed in the trained subjects between the LT and LT-10%+LC conditions may be partially explained by the reduced hepatic blood flow at the higher exercise PO. However, given the same hepatic blood flow, which can be assumed for the two exercise conditions at LT-10% (i.e., with and without exogenous lactate infusion), increasing precursor [lactate] increased GNG. Between the LT and LT-10% conditions, opposing factors of higher hepatic blood flow and lower [lactate] at the LT-10% intensity may have canceled out and did not significantly alter rates of GNG.
Effects of precursor [lactate].
Exogenous lactate infusion during the 90 min of rest before exercise during the LT-10%+LC condition likely resulted in hepatic glycogen synthesis as a fate of lactate disposal. However, this possibility requires further analysis of lactate flux and oxidation rates to be verified. Despite the presumption that subjects were equipped with greater hepatic glycogen stores for exercise during the LT-10%+LC condition, GNG rates were still significantly higher compared with its corresponding PO-matched control (LT-10%). Accordingly, it is reasonable to conclude that increased GNG during the LT-10%+LC trial was due to the increased blood lactate availability.
Previous studies have reported that exogenous lactate infusion decreased glucose oxidation in humans during rest and moderate-intensity exercise at 55% V̇o2peak (38) and increased glucose Ra and Rd with no change in oxidation during exercise at 65% V̇o2peak (39). In the present study, glucose disposal, but not oxidation, was quantified. However, based on results of previous determinations on men before and after training (19), either resting (25% glucose Rd via oxidation) or exercising at 65% V̇o2peak (80% glucose Rd via oxidation), current results can be interpreted to mean that glucose disposal via oxidation was decreased at rest and increased by lactate infusion during exercise at 67% V̇o2peak.
Consistent with results of previous studies (18), we found a reduction in catecholamine concentration during exercise with the LC. This finding is supported by evidence that high [lactate] may be sensed as a sufficiency in fuel supply by the ventromedial hypothalamus (8), suppressing the counterregulatory release of catecholamines, but not glucagon (35). Indeed, lactate infusion failed to affect pancreatic fuel sensing during hypoglycemic and euglycemic clamps in healthy men (44). As such, in our present study, we observed an increase in glucagon during exercise at LT-10%+LC compared with the LT-10% trial. Alterations in catecholamines and glucagon may affect glucose metabolism by exerting changes in GNG and GLY. Specifically, epinephrine and norepinephrine have been shown to stimulate hepatic GLY directly (15, 46), whereas the augmenting effects of catecholamines on GNG are through increased GLY in muscle and lipolysis in adipose tissue to release major gluconeogenic precursors, such as lactate, glycerol and alanine (14, 43). Others have also observed an association between plasma glucagon level and GNG (32, 50), and that the role of catecholamines in the stimulation of hepatic glucose production during exercise is probably secondary to the effects of glucagon (11, 26, 30). Therefore, our observations of a suppressed sympathetic response coupled with higher glucagon concentrations in the LT-10%+LC trial may further explain the increase in GNG and reciprocal decrease in GLY during exogenous lactate infusion.
Effects of endurance training.
The effect of endurance training on GNG in humans is not well understood, as previous studies have observed decreased, similar, or increased levels of GNG after training (4, 19, 33). Friedlander et al. (19) estimated GNG from three-carbon precursors by determining glucose recycling rate in healthy men before and after 10 wk of endurance training. Rates of GNG were lower after training, as measured for a given exercise intensity as well as [lactate]. MacRae et al. (33) reported no change in absolute rates of GNG after 9 wk of endurance training for a given metabolic rate during a progressive exercise test to exhaustion. Specifically, GNG from lactate decreased after training, while increased oxidation of lactate was simultaneously observed. Conversely, Bergman et al. (4) demonstrated that 9 wk of endurance training resulted in an increase in GNG at given absolute and relative exercise intensities (65% of pre- and posttraining V̇o2peak). These training effects on GNG were apparent in both absolute GNG rates and relative contributions to total glucose production. Subjects in this latter study were 5-h postprandial, in contrast to the former studies where subjects were 3-h postprandial in the study by Friedlander et al., and 12-h overnight-fasted in the study by MacRae et al. Furthermore, the controls in dietary composition may have varied subtly across studies. Therefore, apparent discrepancies in the effects of endurance training on GNG are judged to be due to factors such as dietary status affecting the independent dynamics of GNG and GLY as they support total glucose production.
Our methodology of controlling dietary status in determining lactate-derived glucose production begs the question of whether CHO-restricted individuals would exhibit gluconeogenic responses different from those observed in this investigation. To our knowledge, neither lactate nor GNG flux rates have been measured in individuals subjected to high-fat diets; such experiments remain to be done. Our calculations of GNG rates were based on measuring incorporation of carbon-3 from infused [3-13C]lactate into the blood glucose pool. This method of determining GNG would also include contributions of metabolites in equilibrium with lactate, such as pyruvate (via lactate dehydrogenase) and alanine (via alanine aminotransferase). However, the contributions of other gluconeogenic precursors, such as glycerol, would not be included. Hence, in these experiments, we provide minimal estimates of GNG. It is reasonable to assume that fat-adapted individuals would be equally or more dependent on GNG to maintain glycemia than in those adapted to a high-CHO diet. Compared with those who are CHO fed, fat-adapted individuals exhibit higher plasma glycerol concentration during prolonged exercise (10). In such a case, increased precursor supply via glycerol might be expected to augment GNG. However, we (51) and others (29) have shown that glycerol is a poor gluconeogenic precursor, suggesting that fat-adapted individuals with low circulating lactate levels would experience compromised capacity for GNG during exercise, unless adaptation to a high-fat diet increases the capacity of GNG from glycerol. Issues surrounding mechanisms of maintaining glycemia in highly trained individuals adapted to a high-fat diet and the role of glycerol warrant further investigation.
Results of the present investigation showed no significant effect of training on partitioning of glucose production at rest, and, furthermore, rates of GNG and GLY were not different in resting subjects across conditions following an overnight fast. However, during exercise at LT, trained subjects cycled at a substantially higher relative exercise intensity (75 vs. 67% V̇o2peak) and absolute PO (259 vs. 161 W) without a significant reduction in GNG compared with the UT subjects. Still, while not statistically different, the average relative contribution of GNG in glucose production was ∼40% lower in trained compared with UT subjects, while hepatic GLY was ∼25% greater during exercise at LT. By way of explanation, we note that trained subjects have larger stores of liver glycogen (21), which can be a determinant of hepatic GLY, particularly in states of low glycogen content (22, 45, 55). However, compared with the LT-10% trial, trained subjects exhibited higher rates of GNG during exercise in the LT-10%+LC trial, despite following a 90-min rest period of exogenous lactate infusion, which presumably preloaded hepatic glycogen reserves before exercise. Comparing the trained subjects in the LT-10%+LC trial to the UT subjects exercising at LT, both groups were cycling at the same relative exercise intensity (67% V̇o2peak) and [lactate] (∼4 mmol/l), thereby characterizing the individual effects of training. We observed higher rates of GNG, although statistically nonsignificant, in the trained subjects, suggesting that endurance training may increase gluconeogenic capacity during prolonged exercise following an overnight fast.
In terms of energy substrate partitioning, endurance training causes a small, but significant, shift in substrate utilization away from CHO and toward fatty acids (9). As well, training reduces blood glucose disposal for a given absolute PO after training (3, 19, 39, 41, 53, 57). In the present study, when UT and trained subjects exercised at a relative intensity of 67% V̇o2peak (UT at LT and trained at LT-10%), we found no significant differences in glucose Ra, Rd, or MCR between groups. Glucose turnover in the trained subjects exercising at LT was also not different from the other conditions, but blood [lactate] was significantly decreased during exercise in the LT-10% trial. This finding is consistent with studies reporting that lactate clearance is increased via oxidation at both given absolute POs and relative exercise intensities after training (5, 17, 33, 34, 48). Therefore, endurance trained individuals have increased capacities to use lactate as both an oxidative energy source and a gluconeogenic precursor. When the LC raised blood [lactate] to match the LT trial, GNG in the trained subjects increased, again showing that providing additional precursor lactate increases GNG during exercise.
Training-induced adaptations in hepatic glucose metabolism are also affected by an attenuated neuroendocrine response during exercise at a given absolute PO (36, 41). In the present study, at a matched relative exercise intensity of 67% V̇o2peak, we found that the alterations in hormone milieu (i.e., higher insulin, and significantly lower glucagon and epinephrine) persisted in trained compared with UT subjects (Table 3). Still, UT subjects were not able to regulate blood glucose as well during exercise and, therefore, experienced a greater counterregulatory response, as seen with higher glucagon concentrations, even if matched for relative exercise intensity.
Limitations.
Lactate enters the GNG pathway via pyruvate, where tracer enrichment may be reduced when dilution occurs with oxaloacetate from the tricarboxylic acid cycle. Accordingly, we used a correction (Hetenyi, H) factor of 1.45 during rest to account for this reduction in signal (24). Corrections for isotopic dilution may vary according to species, nutritional state, and training state differences (25), yet we assumed the same relative proportion of tracer dilution in both UT and trained groups. For exercise, we did not apply a correction factor (i.e., H = 1.0) to the data, because hepatic glucose metabolism through oxaloacetate is predominantly anabolic.
While lactate is widely recognized to be the major gluconeogenic precursor, the rates of GNG we calculated for healthy men may have been underestimated because we did not include contributions from glycerol, and amino acids other than alanine (2). In turn, hepatic GLY may have been overestimated. However, possible underestimation of GNG rates for lack of consideration of the roles of other precursors may have been minimized in the LT-10%+LC condition in which a surfeit of lactate limited the contributions of other gluconeogenic precursors because of the presence of autoregulatory control (29, 50, 51). Furthermore, our findings may have been different in fat-adapted individuals, who have been shown to exhibit altered patterns in substrate utilization during exercise compared with individuals who consume a larger portion of CHO (10).
Throughout the paper, we describe glucose production as appearing from the liver, although changes may have occurred in the kidneys as well. Because our measurements reflect whole body metabolism, we do not exclude the renal cortex as a contributor to GNG and GLY in our results. Importantly, because glycogen storage is low in the kidneys (20), renal GLY is unlikely to have made a significant contribution to blood glucose appearance.
Summary and conclusions.
We utilized stable isotope tracers to measure glucose metabolism in UT and trained subjects during steady-state exercise at and just below the LT. We also incorporated a LC component to study the effect of precursor concentration on GNG. From these experiments, it is concluded that lactate-derived GNG plays an essential role in hepatic and renal glucose production during exercise in the fasted state, regardless of training history. Endurance training increases the work capacity to achieve a higher relative exercise intensity and absolute PO before reaching the LT and without experiencing a significant decrease in GNG. Additionally, GNG can be augmented during exercise when blood lactate is increased by endogenous or exogenous supply, suggesting that the contribution of GNG to total glucose production may be limited by delivery of gluconeogenic precursors.
GRANTS
This research was supported by a gift from CytoSport, Inc. of Benicia, CA. L. A. Messonnier is a Fulbright fellow and supported by the France-Berkeley Fund.
DISCLOSURES
G. A. Brooks has a financial interest in CytoSport; otherwise, the authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Author contributions: L. A. M. and J. A. F. performed experiments; M.A.H. was responsible for gas chromatography and mass spectrometry (GCMS); C.W.E. was responsible for enzymatic, GCMS, and statistical analyses; J.A.F. was responsible for enzymatic and hormonal analyses; T.J.C. and G.A.B. edited and revised manuscript; T.J.C., M.A.H., and G.A.B. approved final version of manuscript; G.A.B. and L. A. M. conception and design of research; G.A.B. analyzed data; G.A.B. interpreted results of experiments; G.A.B. and C. W. E. drafted manuscript.
ACKNOWLEDGMENTS
We sincerely thank the subjects for dedication and participation in this study. We also gratefully acknowledge the technical assistance of Yeon Park and S. B. Marett.
REFERENCES
- 1. Ahlborg G , Felig P. Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J Clin Invest 69: 45–54, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahlborg G , Felig P , Hagenfeldt L , Hendler R , Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest 53: 1080–1090, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergman BC , Butterfield GE , Wolfel EE , Lopaschuk GD , Casazza GA , Horning MA , Brooks GA. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol Endocrinol Metab 277: E81–E92, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Bergman BC , Horning MA , Casazza GA , Wolfel EE , Butterfield GE , Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278: E244–E251, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Bergman BC , Wolfel EE , Butterfield GE , Lopaschuk GD , Casazza GA , Horning MA , Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87: 1684–1696, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Billat VL , Sirvent P , Py G , Koralsztein JP , Mercier J. The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med 33: 407–426, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 8. Borg MA , Tamborlane WV , Shulman GI , Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52: 663–666, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Brooks GA , Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76: 2253–2261, 1994. [DOI] [PubMed] [Google Scholar]
- 10. Burke LM , Angus DJ , Cox GR , Cummings NK , Febbraio MA , Gawthorn K , Hawley JA , Minehan M , Martin DT , Hargreaves M. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J Appl Physiol 89: 2413–2421, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Carlson KI , Marker JC , Arnall DA , Terry ML , Yang HT , Lindsay LG , Bracken ME , Winder WW. Epinephrine is unessential for stimulation of liver glycogenolysis during exercise. J Appl Physiol 58: 544–548, 1985. [DOI] [PubMed] [Google Scholar]
- 12. Chandramouli V , Ekberg K , Schumann WC , Kalhan SC , Wahren J , Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol Endocrinol Metab 273: E1209–E1215, 1997. [DOI] [PubMed] [Google Scholar]
- 13. Chen X , Iqbal N , Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest 103: 365–372, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu CA , Sindelar DK , Neal DW , Allen EJ , Donahue EP , Cherrington AD. Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest 99: 1044–1056, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu CA , Sindelar DK , Neal DW , Cherrington AD. Direct effects of catecholamines on hepatic glucose production in conscious dog are due to glycogenolysis. Am J Physiol Endocrinol Metab 271: E127–E137, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Consoli A , Nurjhan N , Reilly JJ , Bier DM , Gerich JE. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am J Physiol Endocrinol Metab 259: E677–E684, 1990. [DOI] [PubMed] [Google Scholar]
- 17. Donovan CM , Pagliassotti MJ. Enhanced efficiency of lactate removal after endurance training. J Appl Physiol 68: 1053–1058, 1990. [DOI] [PubMed] [Google Scholar]
- 18. Fattor JA , Miller BF , Jacobs KA , Brooks GA. Catecholamine response is attenuated during moderate-intensity exercise in response to the “lactate clamp”. Am J Physiol Endocrinol Metab 288: E143–E147, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Friedlander AL , Casazza GA , Horning MA , Huie MJ , Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol 82: 1360–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 20. Gaesser GA , Brooks GA. Glycogen repletion following continuous and intermittent exercise to exhaustion. J Appl Physiol 49: 722–728, 1980. [DOI] [PubMed] [Google Scholar]
- 21. Galbo H , Holst JJ , Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol 38: 70–76, 1975. [DOI] [PubMed] [Google Scholar]
- 22. Galbo H , Saugmann P , Richter EA. Increased hepatic glycogen synthetase and decreased phosphorylase in trained rats. Acta Physiol Scand 107: 269–272, 1979. [DOI] [PubMed] [Google Scholar]
- 23. Hellerstein MK , Neese RA , Linfoot P , Christiansen M , Turner S , Letscher A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J Clin Invest 100: 1305–1319, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetenyi G. Correction for the metabolic exchange of 14C for 12C atoms in the pathway of gluconeogenesis in vivo. Fed Proc 41: 104–109, 1982. [PubMed] [Google Scholar]
- 25. Hetenyi G , Ferrarotto C. Correction for metabolic exchange in the calculation of the rate of gluconeogenesis in rats. Biochem Med 29: 372–378, 1983. [DOI] [PubMed] [Google Scholar]
- 26. Hoelzer DR , Dalsky GP , Schwartz NS , Clutter WE , Shah SD , Holloszy JO , Cryer PE. Epinephrine is not critical to prevention of hypoglycemia during exercise in humans. Am J Physiol Endocrinol Metab 251: E104–E110, 1986. [DOI] [PubMed] [Google Scholar]
- 27. Hofmann P , Pokan R , von Duvillard SP , Seibert FJ , Zweiker R , Schmid P. Heart rate performance curve during incremental cycle ergometer exercise in healthy young male subjects. Med Sci Sports Exerc 29: 762–768, 1997. [DOI] [PubMed] [Google Scholar]
- 28. Huie MJ , Casazza GA , Horning MA , Brooks GA. Smoking increases conversion of lactate to glucose during submaximal exercise. J Appl Physiol 80: 1554–1559, 1996. [DOI] [PubMed] [Google Scholar]
- 29. Jahoor F , Peters EJ , Wolfe RR. The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol Endocrinol Metab 258: E288–E296, 1990. [DOI] [PubMed] [Google Scholar]
- 30. Kjaer M , Engfred K , Fernandes A , Secher NH , Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. Am J Physiol Endocrinol Metab 265: E275–E283, 1993. [DOI] [PubMed] [Google Scholar]
- 31. Landau BR , Wahren J , Chandramouli V , Schumann WC , Ekberg K , Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lavoie C , Ducros F , Bourque J , Langelier H , Chiasson JL. Glucose metabolism during exercise in man: the role of insulin and glucagon in the regulation of hepatic glucose production and gluconeogenesis. Can J Physiol Pharmacol 75: 26–35, 1997. [DOI] [PubMed] [Google Scholar]
- 33. MacRae HH , Noakes TD , Dennis SC. Effects of endurance training on lactate removal by oxidation and gluconeogenesis during exercise. Pflügers Arch 430: 964–970, 1995. [DOI] [PubMed] [Google Scholar]
- 34. MacRae HS , Dennis SC , Bosch AN , Noakes TD. Effects of training on lactate production and removal during progressive exercise in humans. J Appl Physiol 72: 1649–1656, 1992. [DOI] [PubMed] [Google Scholar]
- 35. Maran A , Cranston I , Lomas J , Macdonald I , Amiel SA. Protection by lactate of cerebral function during hypoglycaemia. Lancet 343: 16–20, 1994. [DOI] [PubMed] [Google Scholar]
- 36. Mendenhall LA , Swanson SC , Habash DL , Coggan AR. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am J Physiol Endocrinol Metab 266: E136–E143, 1994. [DOI] [PubMed] [Google Scholar]
- 37. Meyer C , Stumvoll M , Dostou J , Welle S , Haymond M , Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab 282: E428–E434, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Miller BF , Fattor JA , Jacobs KA , Horning MA , Navazio F , Lindinger MI , Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544: 963–975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller BF , Fattor JA , Jacobs KA , Horning MA , Suh SH , Navazio F , Brooks GA. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Metab 283: E889–E898, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Nilsson LH , Hultman E. Liver glycogen in man–the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest 32: 325–330, 1973. [DOI] [PubMed] [Google Scholar]
- 41. Phillips SM , Green HJ , Tarnopolsky MA , Heigenhauser GF , Hill RE , Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol 81: 2182–2191, 1996. [DOI] [PubMed] [Google Scholar]
- 42. Rowell LB , Blackmon JR , Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest 43: 1677–1690, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saccà L , Vigorito C , Cicala M , Corso G , Sherwin RS. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol Endocrinol Metab 245: E294–E302, 1983. [DOI] [PubMed] [Google Scholar]
- 44. Schmid SM , Jauch-Chara K , Hallschmid M , Oltmanns KM , Peters A , Born J , Schultes B. Lactate overrides central nervous but not beta-cell glucose sensing in humans. Metabolism 57: 1733–1739, 2008. [DOI] [PubMed] [Google Scholar]
- 45. Sonne B , Mikines KJ , Galbo H. Glucose turnover in 48-hour-fasted running rats. Am J Physiol Regul Integr Comp Physiol 252: R587–R593, 1987. [DOI] [PubMed] [Google Scholar]
- 46. Sonne B , Mikines KJ , Richter EA , Christensen NJ , Galbo H. Role of liver nerves and adrenal medulla in glucose turnover of running rats. J Appl Physiol 59: 1640–1646, 1985. [DOI] [PubMed] [Google Scholar]
- 47. Staehr P , Hother-Nielsen O , Beck-Nielsen H , Roden M , Stingl H , Holst JJ , Jones PK , Chandramouli V , Landau BR. Hepatic autoregulation: response of glucose production and gluconeogenesis to increased glycogenolysis. Am J Physiol Endocrinol Metab 292: E1265–E1269, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Stallknecht B , Vissing J , Galbo H. Lactate production and clearance in exercise. Effects of training. A mini-review. Scand J Med Sci Sports 8: 127–131, 1998. [DOI] [PubMed] [Google Scholar]
- 49. Suh SH , Paik IY , Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells 23: 272–279, 2007. [PubMed] [Google Scholar]
- 50. Toft I , Gerich JE , Jenssen T. Autoregulation of endogenous glucose production during hyperglucagonemia. Metabolism 51: 1128–1134, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Trimmer JK , Casazza GA , Horning MA , Brooks GA. Autoregulation of glucose production in men with a glycerol load during rest and exercise. Am J Physiol Endocrinol Metab 280: E657–E668, 2001. [DOI] [PubMed] [Google Scholar]
- 52. Trimmer JK , Schwarz JM , Casazza GA , Horning MA , Rodriguez N , Brooks GA. Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J Appl Physiol 93: 233–241, 2002. [DOI] [PubMed] [Google Scholar]
- 53. Turcotte LP , Richter EA , Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol Endocrinol Metab 262: E791–E799, 1992. [DOI] [PubMed] [Google Scholar]
- 54. Turcotte LP , Rovner AS , Roark RR , Brooks GA. Glucose kinetics in gluconeogenesis-inhibited rats during rest and exercise. Am J Physiol Endocrinol Metab 258: E203–E211, 1990. [DOI] [PubMed] [Google Scholar]
- 55. Vissing J , Wallace JL , Galbo H. Effect of liver glycogen content on glucose production in running rats. J Appl Physiol 66: 318–322, 1989. [DOI] [PubMed] [Google Scholar]
- 56. Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992. [Google Scholar]
- 57. Zarins ZA , Johnson ML , Faghihnia N , Horning MA , Wallis GA , Fattor JA , Brooks GA. Training improves the response in glucose flux to exercise in postmenopausal women. J Appl Physiol 107: 90–97, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zilversmit DB , Entenman C , Fishler MC. On the calculation of “turnover time” and “turnover rate” from experiments involving the use of labeling agents. J Gen Physiol 26: 325–331, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]