Abstract
Background and study aims Patients with head and neck squamous cell carcinoma (HNSCC) are at risk of a second primary tumor in the gastrointestinal tract, most commonly in the esophagus. Screening these patients for esophageal carcinoma may help detect asymptomatic dysplasia and early cancer, thus allowing curative treatment and more prolonged survival, but the impact of endoscopic screening remains uncertain. Here we aimed to describe the long-term results of an esophageal SCC screening program in patients with head and neck cancer in terms of prevalence, associated risk factors, and survival.
Patients and methods We performed an observational study of a prospectively collected database including patients with HNSCC who had undergone high-definition endoscopy with chromoscopy between 2010 and 2018 at a Brazilian tertiary academic center.
Results The study included 1,888 patients. The esophageal SCC prevalence was 7.9 %, with the majority (77.8 %) being superficial lesions. Significant risk factors for esophageal high-grade dysplasia (HGD) and invasive cancer included tumors of the oral cavity and oropharynx and the presence of low-grade dysplasia (LGD). Overall survival (OS) was significantly shorter among patients in whom esophageal cancer was diagnosed at an advanced stage ( P < .001). OS did not significantly differ between patients with HGD and early esophageal cancer versus those without esophageal cancer ( P = .210)
Conclusions Endoscopic screening for superficial esophageal neoplasia in patients with HNSCC improves esophageal cancer detection. Screening could potentially benefit patients with primary cancer located at the oropharynx or oral cavity. In addition, the detection of esophageal LGD indicates a need for endoscopic surveillance.
Introduction
Among patients with head and neck squamous cell carcinoma (HNSCC), esophageal SCC is the most common secondary tumor in the gastrointestinal tract, with a reported average incidence of 5 % to 15 %, and poor prognosis due to late diagnosis in 90 % of cases 1 . Notably, when ESCC is diagnosed at an early stage, the expected 5-year survival rate is 85 % to 100 % 2 3 , highlighting the importance of early detection. However, despite this evidence, there is no consistent data regarding the effectiveness of screening programs in terms of improving survival and prognosis.
Several centers have conducted routine endoscopic screening of patients with HNSCC, reporting varying levels of success 4 . In endemic areas of China, a 10-year follow-up study revealed a 33 % reduction of ESCC-related cumulative mortality in villages where adults of 50 to 69 years old were screened by Lugol chromoendoscopy 5 . In addition, a case-control study revealed a higher prevalence of secondary ESCC in patients who underwent routine endoscopies compared to those who did not participate (4.5 % vs 3.0 %; P = .04), with diagnosis at earlier stages ( P = .03) 6 . Retrospective studies have yielded similar results, with endoscopic screening revealing 3 % to 30 % prevalence rates of esophageal high-grade dysplasia (HGD) or invasive carcinoma in high-risk populations, demonstrating that screening can be cost-effective 7 8 9 10 .
To verify the benefits of early detection, it is extremely important to clearly define risk factors and to investigate long-term survival, which can provide evidence to guide screening policy. In the present study, we aimed to describe the long-term results of an esophageal SCC screening program in patients with head and neck cancer, in terms of prevalence, associated risk factors, and survival.
Patients and methods
Study design
We performed an observational study of a prospective database containing records of individuals with HNSCC who underwent an endoscopic screening program for ESCC at a tertiary hospital in Sao Paulo, from January 2010 through December 2018. The inclusion criteria were new diagnosis of HNSCC, with or without any type of treatment with curative intent. The exclusion criteria were patient age < 18 years, histopathologic type other than SCC, head and neck tumors located outside the upper aerodigestive tract (salivary glands, parotids, thyroid, or paranasal sinuses), head and neck cancer with esophageal commitment by contiguity, failure of primary cancer treatment, previous history of esophagectomy or advanced esophageal cancer, insufficient data, and inability to perform esophagogastroduodenoscopy (EGD).
For survival comparison, the included patients were divided into five groups: 1) normal chromoendoscopy with no lesion; 2) at least one EGD with Lugol-voiding lesion that was not dysplastic or neoplastic (eg, esophagitis or acanthosis); 3) at least one EGD with a low-grade dysplasia (LGD) lesion; 4) at least one EGD with superficial cancer (high-grade dysplasia, carcinoma in situ, or invasive carcinoma); and 5) at least one EGD with advanced esophageal cancer. For general analysis purposes, normal biopsies, esophagitis, and LGD were considered negative for ESCC; while HGD, carcinoma in situ, and invasive carcinoma were considered positive for ESCC. Patients with lesions classified as LGD, but who also had another more aggressive lesion (high-grade dysplasia or carcinoma) were allocated to the ESCC group according to the worst histology.
The research proposal was reviewed by the local Insitutional Review Board, under register number N1492/19. The study protocol conforms to the 1975 Declaration of Helsinki, and all participants gave their written informed consent before entering the screening program.
Study procedure and definitions
The ESCC screening program consisted of an index EGD performed as part of the initial evaluation of a patient with a recently diagnosed HNSCC. EGD was performed annually.
First, the patients were subjected to conventional white light EGD, with evaluation of the oropharynx and hypopharynx, under conscious sedation. The esophageal lumen was washed, followed by observation for slight color changes, loss of normal vascular pattern, or surface irregularities. Second, the patients underwent narrow band imaging. Third, Lugol’s staining was performed by spraying 20 cc of a 2 % Lugol’s solution on the esophageal mucosa. After this staining, white-colored areas were suspected to be neoplasia, in contrast with brown or brownish “normal” areas. In all phases of the examination, the operator obtained biopsies of every detected suspicious lesion.
A superficial lesion was defined by involvement up to submucosal layer regardless of lymph node or distant organ metastasis (T1NxMx) and advanced lesion when invasion extended to the muscularis propria or beyond (≥ T2).
The term dysplasia was used to indicate the presence of a preformed neoplastic epithelial lesion without evident invasion of the lamina propria or beyond.
Overall survival (OS) was defined as the time from the primary HN tumor diagnosis to the patient’s death. Patients who were lost to follow-up at the end of the study were excluded from analysis.
Patients with missing data were contacted by phone. Subjects with unknown vital status were censored at the time when they were last known to be alive. The vital status of study subjects was analyzed in December, 2020.
Second primary tumor (SPT) definition was based on the Warren and Gates criteria – in which the primary tumor must be malignant and histologically confirmed, tumors must be separated by normal mucosa, and the possibility of the second tumor being metastatic is excluded 11 . ESCC was considered synchronous when detected during the first screening endoscopy, or within 6 months after primary tumor diagnosis. In cases involving multiple lesions, group allocation was based on the worst histology. Cancer stage was assessed using the American Joint Committee on Cancer (AJCC) TNM scoring system, and histopathology according to the Vienna classification 12 13 . Histological stage was performed in surgically or endoscopically resected specimens. In patients whose resection was not possible, tumor staging was based on computed tomography and endoscopic ultrasound findings.
When patients presented with metastatic tumor in a neck node and no primary site could be identified after appropriate investigation, the tumor was defined as an occult or unknown primary cancer.
Statistical analysis
Patient characteristics are summarized using descriptive statistics, and quantitative data presented as mean and significant deviation. Categorical variables were compared using the chi-squared test, or Fisher’s exact test for comparisons of small samples. To compare mean values between two groups, we used Student’s t -test for independent samples if the assumption of normal data distribution was verified using the Kolmogorov-Smirnov test, or the Mann-Whitney non-parametric test if this assumption was violated.
Univariable and multivariable logistic regression models were used to investigate how the demographic and clinical characteristics of the primary cancer influenced the occurrence of esophageal cancer during the study period. We used the Kaplan-Meier method to estimate the probability of overall survival stratified by groups. Survival curves were compared using the log rank test (Mantel-Cox). Univariable and multivariable Cox proportional hazard models were applied to simultaneously assess the effects of all predictor variables on survival time.
P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 20.0 and STATA 12 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, Texas, United States: StataCorp LP).
Results
Between January 2010 and December 2018, 4814 patients with HNSCC were admitted to the Sao Paulo Cancer Institute (ICESP). Of these patients, 2613 were excluded, 1554 with advanced disease, 480 non-HNSCC tumors, nine because previous esophagectomy, 23 with iodine allergy or refusal to sign informed consent, and 547 because of insufficient data. The remaining 2201 were screened within the program, nevertheless, 313 were also excluded because of insufficient data or lost to follow-up (n = 309) and previous esophageal cancer (n = 4). A total of 1888 subjects met the eligibility criteria and were included in the final analysis, as shown in Fig. 1 .
Fig. 1.
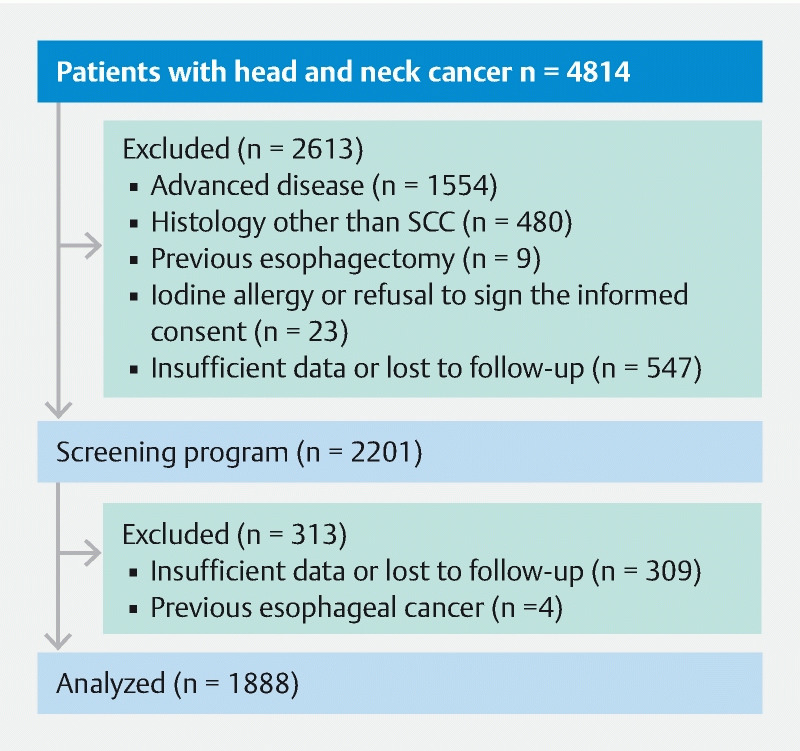
Flowchart of the study.
General characteristics
The mean age at the time of primary tumor diagnosis was 59 years (range, 18–91 years) and the male:female ratio was 5.8:1. The patients underwent a mean of 2.3 upper gastrointestinal endoscopy exams. Regarding the localization of the head and neck cancer, 34 % were in the larynx, 30 % oropharynx, 24 % oral cavity, 7 % hypopharynx, 2 % nasopharynx, and 1 % occult primary tumor. Over half of the patients exhibited an advanced stage of HNSCC. Surgical resection was the only treatment in 15 % of patients, while 84 % underwent chemotherapy or radiotherapy with or without an associated surgical procedure. The remaining patients underwent no treatment for the primary tumor. The prevalence of a SPT except head/neck and esophagus was 11 %, the most common locations were lung (30.5 %), prostate (22.6 %) and stomach (11.1 %). Table 1 shows the patients’ baseline characteristics.
Table 1. Baseline characteristics of patients.
| Without ESCC (n = 1.739) | With ESCC (n = 149) | Missed values | P | |
| Age (years) | 59.1 ± 10.0 | 57.3 ± 9.4 | 0 | 0.035 1 |
| Sex | 1.739 (100.0) | 149 (100.0) | 0 | 0.033 |
|
1.475 (84.8) | 136 (91.3) | ||
|
264 (15.2) | 13 (8.7) | ||
| Location of HNSCC | 1.739 (100.0) | 149 (100.0) | 0 | 0.001 |
|
408 (23.5) | 48 (32.4) | ||
|
523 (30.1) | 57 (38.5) | ||
|
42 (2.4) | 0 (0.0) | ||
|
125 (7.2) | 13 (8.8) | ||
|
620 (35.6) | 30 (20.1) | ||
|
21 (1.2) | 1 (0.7) | ||
| Stage of HNSCC | 1.610 (100.0) | 123 (100.0) | 129/26 | < 0.001 |
|
14 (0.9) | 3 (2.4) | ||
|
172 (10.7) | 9 (7.3) | ||
|
138 (8.6) | 10 (8.1) | ||
|
270 (16.8) | 19 (15.4) | ||
|
774 (48.1) | 56 (45.5) | ||
|
237 (14.7) | 21 (17.1) | ||
|
5 (0.3) | 5 (4.1) | ||
| Treatment of HNSCC | 1.731 (100.0) | 147 (100.0) | 8/2 | < 0.001 |
|
749 (43.3) | 95 (64.6) | ||
|
286 (16.5) | 10 (6.8) | ||
|
9 (0.5) | 1 (0.7) | ||
|
257 (14.8) | 9 (6.1) | ||
|
395 (22.8) | 27 (18.4) | ||
|
35 (2.0) | 5 (3.4) | ||
| ECOG scale | 1.646 (100.0) | 139 (100.0) | 83/10 | < 0.001 |
|
548 (33.3) | 22 (15.8) | ||
|
851 (51.7) | 89 (64.0) | ||
|
161 (9.8) | 23 (16.5) | ||
|
72 (4.4) | 5 (3.6) | ||
|
14 (0.9) | 0 (0.0) | ||
| Dysplasia | 1.739 (100.0) | 149 (100.0) | 0 | < 0.001 2 |
|
1707 (98.2) | 130 (87.2) | ||
|
32 (1.8) | 19 (12.8) | ||
| Other second primary tumor (except esophagus) | 1.737 (100.0) | 149 (100.0) | 2/0 | 0.906 |
|
1.545 (88.9) | 133 (89.3) | ||
|
192 (11.1) | 16 (10.7) |
HNSCC, head and neck squamous cell carcinoma; ECOG, Eastern Cooperative Oncology Group.
Student’s t -test.
Chi-square or Fisher test.
Screening program results
Of the 1888 patients included in our analysis, 378 (20 %) had non-dysplastic (inflammatory) lesions, and 181 (9.5 %) had dysplastic or neoplastic lesions. Among those with dysplastic lesions, 32 patients with LGD were allocated to the non-ESCC group. ESCC was detected in a total of 149 patients, constituting a prevalence of 7.9 %. Of these, 116 were superficial (6.1 %) and 33 were advanced cancer (1.7 %) ( Fig. 2 ).
Fig. 2.
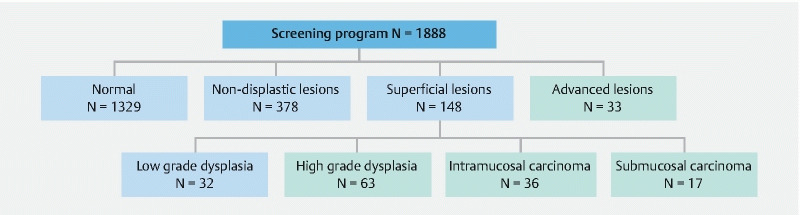
Screening program results. Blue − non-ESCC group; green − ESCC group.
Risk factors
Table 1 presents a comparison between the ESCC and non-ESCC groups. These groups showed different distributions of all characteristics, except for other second primary tumors ( P = .906). The ESCC group exhibited higher frequencies of male gender (91.3 % vs 84.8 %, P = .033), HNSCC localization in the oral cavity (32.4 % vs 23.5 %, P < .001) and oropharynx (38.5 % vs 30.1 %, P < .001), and presence of LGD (12.8 % vs 1.8 %, P < .001). In the non-ESCC group, the HNSCC localization was more frequently in the larynx (35.6 % vs 20.1 %, P < .001).
Logistic regression analysis was performed to investigate the risk factors for esophageal tumor development. The univariate regression model revealed that all tested variables were statistically significant, except other secondary tumors. For multivariable analysis, the final model included HNSCC location ( P = .001), staging ( P = .005), treatment ( P = .001), and dysplasia ( P = .007). A greater chance of ESCC development was associated with HNSCC localization in the oral cavity (odds ratio [OR], 2.43; 95 % confidence interval [CI], 1.51–3.90; P < .001) and oropharynx (OR, 2.25; 95 % CI, 1.42–3.55, P = .001), and with the presence of LGD (OR, 7.80; 95 % CI, 4.30–14.14; P < .001) ( Table 2 ).
Table 2. Univariate and multivariate analysis of risk factors for ESCC.
| Initial Model | Final Model | |||
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age (years) | 0.98 (0.96 – 1.00) | 0.112 | – | – |
| Sex female (ref. = male) | 0.56 (0.28 – 1.12) | 0.103 | – | –8 |
| Location of HNSCC (ref. = larynx/nasopharynx/occult primary tumor) | 0.001 | 0.001 | ||
|
3.16 (1.80 – 5.55) | 0.000 | 3.21 (1.83 – 5.61) | < 0.001 |
|
1.76 (1.02 – 3.04) | 0.041 | 1.84 (1.07 – 3.15) | 0.027 |
|
1.25 (0.54 – 2.87) | 0.606 | 1.29 (0.56 – 2.98) | 0.545 |
| Stage of HNSCC (ref. = Stage IVa) | 0.005 | 0.005 | ||
|
6.47 (1.30 – 32.18) | 0.023 | 6.33 (1.33 – 30.14) | 0.020 |
|
1.59 (0.65 – 3.84) | 0.308 | 1.51 (0.63 – 3.63) | 0.355 |
|
1.59 (0.75 – 3.39) | 0.226 | 1.47 (0.70 – 3.10) | 0.314 |
|
1.48 (0.81 – 2.71) | 0.203 | 1.41 (0.78 – 2.58) | 0.257 |
|
0.88 (0.49 – 1.55) | 0.652 | 0.91 (0.51 – 1.60) | 0.740 |
|
10.75 (2.71 – 42.63) | 0.001 | 11.54 (2.92 – 45.57) | < 0.001 |
| Dysplasia (ref. = no) | 6.48 (2.97 – 14.14) | < 0.001 | 6.50 (3.00 – 14.09) | < 0.001 |
| Other second primary tumor (ref. = no) | 1.00 (0.51 – 1.95) | 0.998 | – | – |
OR, odds ratio; CI, confidence interval; HNSCC, head and neck squamous cell carcinoma.
Superficial ESCC
During the study period, a total of 229 endoscopic lesions were diagnosed in 181 patients. Among these lesions, 143 had a superficial appearance at endoscopy, including carcinoma in situ/HGD (83/58 %), intramucosal carcinoma (42/29 %), and submucosal carcinoma (18/13 %).
The average lesion size was 25.5 mm (SD, 20 mm; median, 20 mm; range, 5–140 mm). Nineteen patients had two or more lesions. Of them, 70 % were in the middle third of the esophagus and 16.7 % in the lower third. The most frequently involved wall was the posterior one (29 %). Regarding the percentage of involvement of the esophageal circumference: < 50 % (80/56 %), 50 % to 75 % (29/20 %), > 75 % (30/21 %) and the entire circumference (4/2,8 %). According to the Paris Classification, 110 (76.9 %) were flat type (0-IIb), 27 (18.8 %) predominantly elevated type (0-IIa) and 6 (4.2 %), 0-IIc type.
Therapeutic strategies for these lesions were based on both the endoscopic aspect, the patient’s clinical conditions, and therapeutic strategy for the primary HN tumor. Among the 143 cases of superficial ESCC, 78 (54.5 %) were endoscopically treated (25 by mucosectomy and 53 by endoscopic submucosal dissection), 12 were surgically treated, 17 were treated exclusively with chemotherapy or radiotherapy (RT), and 36 received no treatment. One patient required adjuvant chemoradiotherapy (CRT) after ESD, and another patient required adjuvant CRT after esophagectomy. Of the 18 submucosal carcinomas, eight presented as SM1, five had deep submucosal invasion, and five were evaluated by endoscopic ultrasound and did not have pathological specimen.
In 32 patients, after a multidisciplinary evaluation, conservative treatment of the second primary esophageal tumor was chosen due to non-control of the primary tumor (n = 24), low performance status (n = 3), scheduled resection of the HNSCC after CRT completion (n = 4), and recent diagnosis of lung adenocarcinoma (n = 1).
Advanced ESCC
During the study period, 33 patients were diagnosed with advanced esophageal tumor. Among these patients, 96.6 % were male and the mean age was 58.5 years. The most common HNSCC locations in these cases were oropharynx (57.5 %) and oral cavity (21.2 %). The therapeutic strategy was chemoradiotherapy in 63 %, esophagectomy in 18 %, and conservative treatment in 18 % of these cases.
Synchronous versus metachronous lesions
Of 1888 HNSCC patients that underwent esophageal screening, 66 (3.5 %) had synchronous lesions, 41 of these (62 %) were superficial ESCC. Twenty-five patients had HNSCC and advanced ESCC diagnosed within 6 months, which changed the treatment approach (most underwent chemoradiotherapy for both tumors). Regarding advanced metachronous lesions (n = 8), most of these patients had abandoned the screening program and returned to the Institute with dysphagia.
Among superficial ESCC, 64.6 % were metachronous lesions and the median time between HNSCC and ESCC diagnoses was 21 months. Twenty-eight patients had more than one superficial ESCC during the follow-up period.
The OS rate comparison between synchronous versus metachronous ESCC showed that patients with metachronous cancers had a slight survival advantage ( P = .04. HR 1.75 95 %CI 1.01–3.02).
Survival analysis
Patients were observed for a median of 43 months (3.5 years) after their first endoscopy examination, with a follow-up rate of 61.1 %. We calculated the OS ratios for groups stratified by histology during follow-up: normal (no lesion, non-dysplastic lesions, and LGD), superficial carcinoma (intramucosal and submucosal lesions), and advanced carcinoma. These groups were then subdivided into no lesion, non-dysplastic lesions, LGD, HGD/carcinoma in situ, submucosal carcinoma, intramucosal carcinoma, and advanced carcinoma.
Table 3 presents the probabilities of 1-, 3-, and 5-year survival and Fig. 3 presents the Kaplan-Meier survival curve. Patients with advanced ESCC had shorter survival (only 11.4 % at 5 years) compared with the other groups. Survival rates did not differ between the groups with superficial ESCC vs without ESCC ( P = .210). Survival rates also did not differ among the subgroups of intramucosal ESCC, submucosal ESCC, HGD, LGD, non-dysplastic lesion, and no lesion ( Fig. 3 ).
Table 3. Overall survival rates.
| Overall survival rates | P | |||
| 1 year | 3 years | 5 years | ||
| Total | 87.29 ± 0.78 | 68.16 ± 1.15 | 58.59 ± 1.29 | – |
| group | < 0.001 | |||
|
60.19 ± 8.59 | 25.34 ± 7.72 | 11.40 ± 5.90 | |
|
90.75 ± 2.45 | 67.44 ± 4.12 | 54.75 ± 4.61 | |
|
87.53 ± 0.82 | 69.10 ± 1.20 | 60.05 ± 1.35 | |
| Subgroup | < 0.001 | |||
|
60.19 ± 8.59 | 25.34 ± 7.72 | 11.40 ± 5.90 | |
|
91.49 ± 4.70 | 63.90 ± 8.37 | 51.68 ± 9.33 | |
|
94.12 ± 5.71 | 74.12 ± 11.41 | 74.12 ± 11.41 | |
|
89.96 ± 3.89 | 66.95 ± 6.48 | 52.21 ± 7.34 | |
|
89.66 ± 5.66 | 68.14 ± 8.78 | 53.16 ± 9.53 | |
|
90.44 ± 1.54 | 72.64 ± 2.42 | 64.24 ± 2.71 | |
|
86.69 ± 0.95 | 68.10 ± 1.38 | 58.81 ± 1.55 | |
P , log rank test; ESCC, esophageal squamous cell carcinoma.
Advanced ESCC versus superficial ESCC p < 0.001. Advanced ESCC versus no ESCC P < 0.001.
Superficial ESCC versus no ESCC P = 0.210
Advanced ESCC versus all others groups P < 0.001.
Fig. 3.
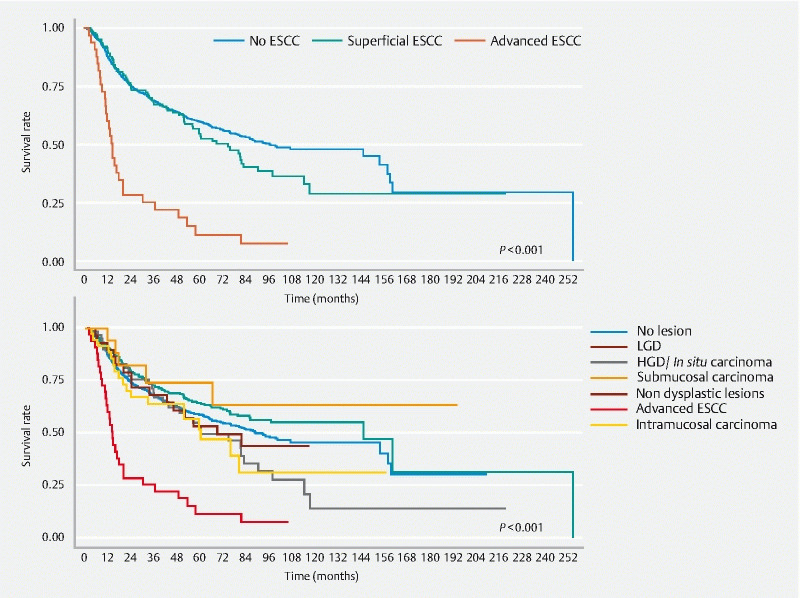
Kaplan-Meier overall survival by subgroups. Log rank test P = .001.
Discussion
In the present study, we evaluated patients diagnosed with HNSCC from 2010 to 2018. Although over 4000 patients were admitted to the Head and Neck Cancer Unit during this period, more than 30 % were not screened for esophageal cancer because they were considered clinically unfit at the time of diagnosis. The fact that this is a referral cancer hospital likely accounts for the high rate of more advanced-stage disease. On the other hand, most of the patients included in the screening program had moderate functional capacity and oncological staging of III and IV, reflecting the late diagnosis in this population, and explaining the overall HNSCC-related mortality rate of 40.3 %.
The occurrence of a SPT in patients with HNSCC illustrates the concept of field cancerization, in which carcinogen exposure leads to a process of chronic mucosal inflammation and the subsequent development of premalignant and malignant conditions. The ability to accurately differentiate between metastasis and second primary malignancy should contribute to a better treatment due to differences in prognosis and outcomes, as shown by a genomic study 14 . In our series, 357 patients (18.9 %) were diagnosed with a SPT outside of the head and neck region, with the majority located in the esophagus (149 patients; 41 %) and lung (63 patients; 17.6 %). These results reinforce the theory that these patients’ aerodigestive tracts have undergone changes that increase the risk of synchronous and metachronous tumors.
Prospective studies have reported ESCC prevalence rates of 5.1 % to 12.5 % among patients with HNSCC, with differences attributed to both geographical variation and the location of the primary head and neck tumor 6 15 16 17 18 19 . These rates are higher than the estimated prevalence of 0.74 % in the general population 20 . In our study, we found a 7.9 % prevalence of second primary esophageal tumors in a cohort of HNSCC patients, which is within the range of data reported in the literature.
The identification of predictive factors for cancer occurrence may improve the results of screening programs. Head and neck neoplasms are heterogeneous, and variables related to both the patient and the tumor can influence the incidence of esophageal cancer. Chow et al. 21 evaluated predictive factors associated with the incidence of synchronic esophageal tumors among 118 patients with HNSCC. Their multivariate analysis showed that tumor localization in the oral cavity was associated with lower risk of a second tumor, which differs from our present findings. Gong et al. 18 performed a prospective study of 458 patients with HNSCC, of whom 24 patients (5.2 %) had synchronic esophageal cancer, and found that only piriform sinus involvement was an independent risk factor for synchronic esophageal cancer (OR, 171.2; 95 % CI, 22.25–1317.23; P < .001). In a similar analysis, Wang et al. 22 reported that alcohol use (OR, 3.792; P = .0035), oropharyngeal cancer (OR, 3.618; P = .0045), and hypopharyngeal cancer (OR, 2.627; P = .0029) were independent risk factors for synchronic esophageal cancer. Tseng et al. 23 also showed that patients with hypopharynx or oropharynx cancers had a higher cumulative incidence rate of metachronous ESCC. In the present series, we found that patients with tumors of the oral cavity and oropharynx had a higher risk of esophageal cancer. These results suggest that the primary tumor location was associated with the later esophageal neoplasia development, which may tend to follow the digestive axis, probably due to contiguity. León et al. 24 and Panosetti et al. 25 reported that an index tumor located in the hypopharynx was most frequently followed by a secondary tumor in the esophagus, and that an index tumor in the larynx was most frequently followed by a secondary tumor in the lungs.
The natural history of early esophageal ESCC remains unclear, but several population studies show that dysplasia can be a precursor lesion, similar to observations in adenocarcinoma. The risk of progression to carcinoma can be stratified according to the degrees of dysplasia 26 27 . However, dysplasia does not always progress and some cases may remain stable or even regress over time 28 .
According to the literature, HGD has a high malignant potential and should be resected, while the treatment of LGD remains controversial. Recent guidelines have shown that LGD has a real potential for malignancy and visible lesions on biopsy may in fact already be malignant lesions 29 . Accordingly, endoscopic resection should be considered for lesions that are associated with a high risk of progression during follow-up, and when an upgraded histology is suspected after resection 30 .
In the present study, LGD was classified as non-neoplastic. However, 18 patients with lesions classified as LGD also had other synchronic lesions classified as carcinoma at different locations. In addition, one patient with a superficial LGD was lost to follow-up and had progressed to advanced cancer 4 years later. In fact, multivariate analysis revealed that patients with LGD had a 6.5-fold greater risk of developing carcinoma. This data suggest that LGD lesions should be carefully evaluated, and that treatment of them is always indicated when others risk factors are present.
In patients with HNSCC, development of an SPT is associated with poor prognosis. Murakami et al. 31 demonstrated that patients with SPT had a greater 5-year OS rate when ESCC was diagnosed during periodic screening, although the rate of mortality due to the first primary carcinoma was similar. Lim et al. 32 evaluated the effectiveness of esophageal screening among high-risk individuals and found that the 3-year survival rates were 71.2 % in patients with HNSCC and 48.2 % in those with HNSCC and ESCC ( P < .0001). Moreover, 2-year survival was also significantly higher in patients with early versus advanced ESCC (77.7 % vs 21.7 %; P = .01). In our series, the overall 1-, 3-, and 5-year survival rates were 87.2 %, 68.1 %, and 58.59%, respectively. In a 10-year endoscopic surveillance cohort, HNSCC patients without dysplasia had the best survival rate compared to LGD and HGD or SCC (72.3 % vs 54.9 % vs 32.4 %, P < .0001) 19 . Notably, in the present series, the survival curves were similar between the patients without ESCC versus those with ESCC detected at an early stage, while the patients with advanced ESCC had a significantly worse prognosis, with an estimated 5-year survival probability of only 11.4 %. In a nationwide population-based study that enrolled 68,131 HNSCC patients, the 5-1 and 10-year cumulative incidence rates of metachronous ESCC were 1.4 % and 2.7 %, respectively, and this rate continued to rise even after a 10-year follow-up period. This finding suggests that esophageal screening should be performed for a minimum of 10 years, especially in high-risk patients 23 .
Overall, the available data suggest that early detection through a screening program improves the prognosis of patients with HNSCC. In this study, it was necessary to perform 40 screening endoscopies per patient to diagnose one superficial cancer. However, it is important to highlight the possible effect of the selection and lead time biases, in which screening anticipates the diagnosis of cancer, but does not necessarily cause the real increase in survival, because the analysis does not account for the asymptomatic period of the natural history of early esophageal cancer. One way to overcome these limitations would be to compare mortality in the screened population versus among those not screened. However, a randomized clinical trial with a large enough sample to ensure control of potential confounding factors would be time-consuming, with high cost and potential ethical problems. Moreover, comparisons in relation to the introduction of screening (before and after) would affect mortality rates by changes in diagnosis and treatment over time. Some other limitations must be addressed: survival analysis was done with intention-to-treat concept. Therefore, heterogeneity was present and interpretation of treatment efficacy on the mortality have become difficult. Another limitation was the sample size of hypopharyngeal cancer, which could increase the likelihood of a type II error. In addition, we could not include the entire cohort of 4814 patients, and that we lost 586 patients (12 %) to follow-up, despite active search, which could have impaired reliability of survival data.
Conclusions
Endoscopic screening for superficial esophageal neoplasia in patients with HNSCC improves esophageal cancer detection. Screening could potentially benefit patients with primary cancer located in the oropharynx or oral cavity. In addition, the detection of esophageal LGD indicates a need for endoscopic surveillance.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Fukuhara T, Hiyama T, Tanaka S et al. Characteristics of Esophageal squamous cell carcinomas and Lugol-voiding lesions in patients with head and neck squamous cell carcinoma. J Clin Gastroenterol. 2010;44:e27–e33. doi: 10.1097/MCG.0b013e3181b31325. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Ogino I, Inayama Y et al. Impact of the early detection of esophageal neoplasms in hypopharyngeal cancer patients treated with concurrent chemoradiotherapy. Asia Pac J Clin Oncol. 2017;13:e3–e10. doi: 10.1111/ajco.12274. [DOI] [PubMed] [Google Scholar]
- 3.Kandiah K, Chedgy F JQ, Subramaniam S et al. Early squamous neoplasia of the esophagus: The endoscopic approach to diagnosis and management. Saudi J Gastroenterol. 2017;23:75–81. doi: 10.4103/1319-3767.203366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Codipilly D C, Qin Y, Dawsey S M et al. Effectiveness evaluation of organized screening for esophageal cancer: a case-control study in Linzhou city, China. Sci Rep. 2018;12:4–10. doi: 10.1038/srep35707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei W Q, Chen Z F, He Y T et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y Y, Chen W C, Chuang H C et al. Effect of routine esophageal screening in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:350–354. doi: 10.1001/jamaoto.2013.46. [DOI] [PubMed] [Google Scholar]
- 7.Chung C-S, Liao L-J, Lo W-C et al. Risk factors for second primary neoplasia of esophagus in newly diagnosed head and neck cancer patients: A case-control study. BMC Gastroenterol. 2013;13:1. doi: 10.1186/1471-230X-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng X, Mao X, Xu K et al. Massive endoscopic screening for esophageal and gastric cancers in a high- risk area of China. PLoS One. 2015;10:1–10. doi: 10.1371/journal.pone.0145097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Zhao Y, Zhao X et al. Clinical Outcomes of endoscopic submucosal dissection for early esophageal squamous cell neoplasms: a retrospective single-center study in China. Gastroenterol Res Pract. 2016;2016:3.741456E6. doi: 10.1155/2016/3741456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Wei W Q, Niu J et al. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18:2493–24501. doi: 10.3748/wjg.v18.i20.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren S, Gates O. Multiple primary malignant tumors: A survey of the literature and statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 12.Edge S B, Compton C C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 13.Schlemper R J, Riddell R H, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunpaweravong S, Bunbanjerdsuk S, Pongrujikorn T et al. Clonal relationship of synchronous head and neck cancer and esophageal cancer assessed by single nucleotide polymorphism-based loss of heterozygosity analysis. BMC Cancer. 2019;19:1174. doi: 10.1186/s12885-019-6394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuc J, Winnock M, Barbier J P et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: A prospective study conducted in 62 french endoscopy centers. Endoscopy. 2006;38:690–695. doi: 10.1055/s-2006-925255. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama A, Ohmori T, Makuuchi H et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928–934. doi: 10.1002/1097-0142(19950915)76:6<928::aid-cncr2820760604>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y C, Wang C P, Chen C C et al. Transnasal endoscopy with narrow-band imaging and Lugol staining to screen patients with head and neck cancer whose condition limits oral intubation with standard endoscope (with video) Gastrointest Endosc. 2009;69:408–417. doi: 10.1016/j.gie.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Gong E J, Kim D H, Ahn J Y et al. Routine endoscopic screening for synchronous esophageal neoplasm in patients with head and neck squamous cell carcinoma: a prospective study. Dis Esophagus. 2016;29:752–759. doi: 10.1111/dote.12404. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y H, Wang Y K, Chuang Y S et al. Endoscopic surveillance for metachronous esophageal squamous cell neoplasms among head and neck cancer patients. Cancers. 2020;12:3832. doi: 10.3390/cancers12123832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang H, Fan J H, Qiao Y L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33–41. doi: 10.20892/j.issn.2095-3941.2016.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow T L, Lee D TY, Choi C Y et al. Prediction of simultaneous esophageal lesions in head and neck squamous cell carcinoma: A multivariate analysis. Arch Otolaryngol Head Neck Surg. 2009;135:882–885. doi: 10.1001/archoto.2009.105. [DOI] [PubMed] [Google Scholar]
- 22.Wang W L, Lee C T, Lee Y C et al. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck. 2011;33:77–81. doi: 10.1002/hed.21397. [DOI] [PubMed] [Google Scholar]
- 23.Tseng C M, Wang H H, Lee C T et al. A nationwide population-based study to access the risk of metachronous esophageal cancers in head and neck cancer survivors. Sci Rep. 2020;10:884. doi: 10.1038/s41598-020-57630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.León X, Ferlito A, Myer C M et al. Second primary tumors in head and neck cancer patients. Acta Otolaryngol. 2002;122:765–778. [PubMed] [Google Scholar]
- 25.Panosetti E, Luboinski B, Mamelle G et al. Multiple synchronous and metachronous cancers of the upper aerodigestive tract: a nine-year study. Laryngoscope. 1989;99:1267–12773. doi: 10.1288/00005537-198912000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Wang G Q, Abnet C C, Shen Q et al. Histological precursors of oesophageal squamous cell carcinoma: Results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor P R, Abnet C C, Dawsey S M. Squamous dysplasia-The precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540–552. doi: 10.1158/1055-9965.EPI-12-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J W, Guan C T, Wang L L. Natural history analysis of 101 severe dysplasia and esophageal carcinoma cases by endoscopy. Gastroenterol Res Pract. 2017 doi: 10.1155/2017/9612854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pimentel-Nunes P, Libânio D, Marcos-Pinto R et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 30.Oyama T, Inoue H, Arima M et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami S, Hashimoto T, Noguchi T et al. The utility of endoscopic screening for patients with esophageal or head and neck cancer. Dis Esophagus. 1999;12:186–190. doi: 10.1046/j.1442-2050.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- 32.Lim H, Kim D H, Jung H-Y Y et al. Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver. 2015;9:159–166. doi: 10.5009/gnl13401. [DOI] [PMC free article] [PubMed] [Google Scholar]


