Abstract
STUDY QUESTION
What information and support should be offered to donors, intended parents and donor-conceived people, in general and in consideration of the availability of direct-to-consumer genetic testing and matching services?
SUMMARY ANSWER
For donors, intended parents and donor-conceived offspring, recommendations are made that cover information needs and informed consent, psychosocial implications and disclosure.
WHAT IS KNOWN ALREADY
Trends indicate that the use of donor-assisted conception is growing and guidance is needed to help these recipients/intended parents, the donors and offspring, navigate the rapidly changing environment in which donor-assisted conception takes place.
STUDY DESIGN, SIZE, DURATION
A working group (WG) collaborated on writing recommendations based, where available, on evidence collected from a literature search and expert opinion. Draft recommendations were published for stakeholder review and adapted where relevant based on the comments received.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Papers retrieved from PUBMED were included from 1 January 2014 up to 31 August 2020, focusing on studies published since direct-to-consumer genetic testing has become more widespread and accessible. The current paper is limited to reproductive donation performed in medically assisted reproduction (MAR) centres (and gamete banks): donation outside the medical context was not considered.
MAIN RESULTS AND THE ROLE OF CHANCE
In total, 32 recommendations were made for information provision and support to donors, 32 for intended parents and 27 for donor-conceived offspring requesting information/support.
LIMITATIONS, REASONS FOR CAUTION
The available evidence in the area of reproductive donation is limited and diverse with regards to the context and types of donation. General conclusions and recommendations are largely based on expert opinion and may need to be adapted in light of future research.
WIDER IMPLICATIONS OF THE FINDINGS
These recommendations provide guidance to MAR centres and gamete banks on good practice in information provision and support but should also be considered by regulatory bodies and policymakers at a national and international level to guide regulatory and legislative efforts towards the protection of donors and donor-conceived offspring.
STUDY FUNDING/COMPETING INTEREST(S)
The development of this good practice paper was funded by European Society of Human Reproduction and Embryology (ESHRE), covering expenses associated with the WG meetings, the literature searches and dissemination. The WG members did not receive any payment. The authors have no conflicts of interest to declare.
DISCLAIMER
This document represents the views of ESHRE, which are the result of consensus between the relevant ESHRE stakeholders and where relevant based on the scientific evidence available at the time of preparation.
The recommendations should be used for informational and educational purposes. They should not be interpreted as setting a standard of care, or be deemed inclusive of all proper methods of care nor exclusive of other methods of care reasonably directed to obtaining the same results. They do not replace the need for application of clinical judgement to each individual presentation, nor variations based on locality and facility type.
†ESHRE pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Keywords: oocyte/egg donation, sperm donation, gamete sharing, information, counselling, donor-conception, disclosure, direct-to-consumer genetic testing
WHAT DOES THIS MEAN FOR PATIENTS?
Conception with the use of donated sperm, eggs or embryos is a common procedure in assisted reproduction. While donors and intended parents are usually well informed about the medical aspects and risks, information provision on psychosocial issues is still limited. With increasing use of direct-to-consumer genetic testing (including ancestry databases), social media and donor registries, there is even more need for clear information and support for donors, parents and donor-conceived offspring.
This paper makes recommendations aimed at fertility centres and gamete banks on what information to provide and what support to offer.
Introduction
The scope of the current paper is to provide good practice recommendations for information provision to all parties involved in reproduction with donated gametes or embryos. The paper covers information needs and informed consent, what basic information should be provided, the psychosocial implications for donors, recipients (the intended parent[s]) and donor-conceived offspring, and includes recommendations on disclosure of information about donor conception to donor offspring. The recommendations also highlight a recent and important development; the increased availability and use of direct-to-consumer genetic testing and matching services, and the implications of this for those involved in donor-assisted conception.
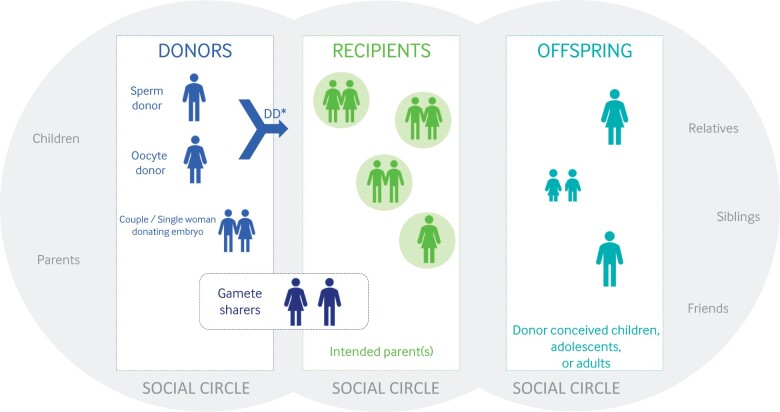
Donation of sperm, oocytes and embryos to assist others in having a family is one of the most impactful actions people can take. Donor-assisted conception is an option for heterosexual couples who cannot conceive with their own gametes, or for same-sex couples and single women who want to achieve parenthood (Fig. 1). Donor-assisted conception includes gamete (sperm or oocyte) and embryo donation. Embryos can be donated by a couple (mainly surplus embryos from couples who have completed their family via ART—‘surplus embryos’ being the commonly used term, even if the working group (WG) recognizes it is emotive and contested), or created from donated sperm and donated oocytes: the latter is more correctly described as ‘double donation’ rather than embryo donation. Gamete sharing is another pathway in donor-assisted conception.
Figure 1.
Pictorial overview of the parties directly involved in donor-assisted conception; donors, recipients, offspring and the social circle of each of them. DD, double donation is the situation of donated sperm and donated oocytes being used for creation of an embryo. This is different from ‘Embryo Donation’ where an embryo is donated by couples who have undergone ART and completed their family.
Trends indicate that the use of donor-assisted conception is growing and guidance is needed to help the recipients/intended parents, donors and offspring navigate the rapidly changing environment in which donor-assisted conception takes place.
The information in the current paper applies to all types of donation unless specified for a specific subgroup of donors, recipients, or offspring. Surrogacy is covered as far as a donor is involved, but other additional considerations specific to surrogacy were outside the scope of the current paper. We have considered gamete sharing (oocyte/sperm sharing) as gamete donation, though this is usually performed to facilitate access for the sharer to otherwise inaccessible treatment and could have additional consequences such as commodification and psychosocial implications (Cohen, 1996; Bracewell-Milnes et al., 2018).
These good practice recommendations are aimed at professionals in the field of assisted reproduction but should also be considered by relevant professional bodies and policy makers. The recommendations are written for reproductive donation in a medical context, i.e. within a medically assisted reproduction (MAR) centre. We acknowledge the growing number of people donating and conceiving outside of MAR centres/gamete banks, but the recommendations are not directed at this type of donation as there is no professional caretaker involved that has a professional responsibility to provide information. Still, parties involved in donation outside the medical system should be aware of the legal ramifications of donating gametes or becoming a parent by gamete donation. It is advised that all parties seek trustworthy information and consider the option of accessing donation services through a MAR centre/gamete bank.
All recommendations provided in this document should be applied in line with local and national guidance and legislation.
Trends in the use of gamete/embryo donation
The International Federation of Fertility Societies’ (IFFS) 2019 surveillance report, Global Trends in Reproductive Policy and Practice, reported that gamete donation is increasing, and is now permitted in the majority of countries worldwide (International Federation of Fertility Societies, 2019). Up to 60% of the countries in the IFFS survey reported use of either sperm or oocyte donation or both. Sperm donation is allowed in 48 of 71 countries, and oocyte donation in 43 of 69. Double-donation of sperm and oocytes to produce an embryo (‘de novo’) is allowed in 21 of 50 countries; embryo donation in 31 countries out of 53. In Europe, the ESHRE European IVF Monitoring Consortium (EIM) reported 50 467 donor sperm IUI cycles, and 73 927 ART treatments with donated oocytes, resulting in, respectively, 6249 and 22 497 births in Europe in 2016 (The European IVF-monitoring Consortium for ESHRE et al., 2020). Furthermore, there were 7186 ART treatments with donated embryos, resulting in 2026 births, and 24 945 ART treatments with sperm donation (outcome data not reported) (personal communication from the EIM Consortium). There is currently no data as to how often double donation is practiced in those countries where both sperm and oocyte donation is possible, nor does EIM report data on surrogacy.
The use of donated gametes is increasing steadily (The European IVF-monitoring Consortium for ESHRE et al., 2020). Implementation of the vitrification technique for oocyte cryopreservation has resulted in an improvement in oocyte survival rates, enabling the successful banking of oocytes. This facilitates improved logistics of treatment for the MAR centres but it also enables the distribution of cryopreserved oocytes. Sperm donation on the other hand is increasing owing to easier access to MAR for single women and lesbian couples.
Secrecy or non-openness in donor-assisted conception
The use of donor gametes in family formation has a long history, but its frequent use as a form of medical intervention is more recent (Richards, 2014). Donor insemination was first fully documented in clinical practice in the early 1900s (Hard, 1909; Nachtigall, 1993). Donor insemination, and later oocyte and embryo donation, was shrouded in secrecy, which included anonymizing the donor’s identity, but also recipients keeping the fact of donor-assisted conception a secret from both their child and their social circle (Frith et al., 2018). These practices, advocated by professionals, were a response to the stigma of (male) infertility, the uncertain social acceptability of donor-assisted conception, the lack of clarity on the legal status of the donor, and fears for the integrity of family relationships (Novaes, 1998). Parents of children conceived from donated gametes were less likely to tell their children how they were conceived than those who underwent fertility treatment using their own gametes (Tallandini et al., 2016).
Gradual questioning of secrecy and non-disclosure emerged in the 1980s. Research into parental disclosure behaviour conducted in the 1980s and 1990s indicated that very few challenged the prevailing orthodoxy of secrecy. Over time, however, both professional views and parental practice have changed considerably (Blyth and Frith, 2015). For example, both the American Society for Reproductive Medicine (ASRM) and the Australian National Health and Medical Research Council openly advocate parental disclosure and a 2008 amendment to the UK’s Human Fertilisation and Embryology Act (HFEA) of 1990 provided legislative endorsement for early parental disclosure (Section 13(6C)) (Human Fertilisation and Embryology Act, 1990). The Nuffield Council of Bioethics’ Report (2013) on donor conception also presented evidence on the benefits of early disclosure (Nuffield Council on Bioethics, 2013). It is important to distinguish secrecy or non-openness from donor-anonymity. Secrecy refers to the parents not disclosing the donor-assisted reproduction to the offspring or social circle, while donor anonymity refers to the identity of the donor not being known.
Direct-to-consumer genetic testing
In many healthcare systems, information about donors is still considered strictly confidential and kept in a secure manner where only a few designated people will have access. However, nowadays, personal identifiable characteristics are no longer only available in regulated systems that MAR centres, doctors, professional bodies and/or governments can control. The proliferation of medical tests, direct-to-consumer genetic testing and other diagnostic tools available on the internet make it possible for people to gain access to a wealth of medical, personal data or information on genetic relatives without going through ‘official’ channels that require navigating gatekeepers and regulatory structures.
Millions of people use ancestry databases for genetic testing, uploading their DNA details, and finding information about their origins (https://www.focusonreproduction.eu/article/News-in-Reproduction-Gamete-donation) (Janzen et al., 2018). These direct-to-consumer genetic testing services are mostly used by people doing ancestry research as a hobby rather than those doubting or looking for genetic kinship. Customers send in DNA swabs, which are analysed through DNA tests and used to examine the degree of relationship with other DNA profiles in the database (Klotz, 2016). The direct-to-consumer testing databases are mostly privately funded commercial databases, and some of them now also have a donor-conception section, specifically aimed at finding donors, genetic siblings or family.
There are two types of DNA tests available online: the detection of multiple unlinked diploid markers on autosomal DNA (atDNA) that are inherited from both parents; and ‘lineage analysis’ finding genetic links through maternal and paternal lines of descent. Lineage analysis is based on mitochondrial DNA (mtDNA) and Y chromosome analysis for female-to-female and male-to-male transmitted lineage, respectively (Moray et al., 2017). Y chromosome testing has been available commercially since the early 2000s and now most companies use atDNA testing. Autosomal tests can identify matches up to second cousins and beyond, with decreasing degrees of certainty.
Genetic testing within the context of ancestry databases or registries may give rise to different issues to the various parties involved in donor-assisted conception. More specifically, to donors who donated under the assumption that their donation was completely anonymous, to recipients who were under the impression that they could keep donor conception a secret and to donor offspring who were unaware of their donor-assisted conception (Crawshaw, 2018). Direct-to-consumer genetic testing can: reveal the lack of a genetic link between the social parent and the offspring; reveal that a family member has donated gametes/embryos; or identify genetic relatives (a donor, siblings, family). Donor-conceived offspring and donors can connect through genetic information stored in these databases and offspring can be identified even before reaching the age set by national legislation. Even donors and donor-conceived offspring that are not registered on the ancestry databases themselves may be traced through relatives who use genetic testing for family tree research (Klotz, 2016), especially in combination with social media. Direct-to-consumer genetic testing can reveal matches, which may translate to a half-sibling, an aunt/uncle, a cousin, or a grandparent. By establishing contact with such genetic matches, they may be able to gain access to information about their donor or other same-donor offspring. With millions of people profiled in ancestry databases, it is highly possible that anonymous donors or donor-conceived offspring could be traced via genetic relatives that have used such databases. It is clear that the anonymity of donors and also their relatives is no longer guaranteed (Harper et al., 2016). It is important to acknowledge these consequences, adapt to this new reality (McGovern and Schlaff, 2018) and move to supportive management of this potential new kinship knowledge (Klotz, 2016).
Donor-conceived offspring and donors can themselves share their genetic information online. A study by Klipstein et al. including 118 responses from American anonymous sperm donors showed 40.6% had sent their genetic material to a direct-to-consumer DNA database because they were willing to share their genetic material and genetic identity. In this study, 68.4% of the respondents stated that they were not concerned that they would be identified as someone’s biological parent by a direct-to-consumer genetic test (Klipstein et al., 2020). Still, contact with (presumed) genetic relatives may still have unexpected implications for both donors, donor-conceived offspring and their relatives, and currently it is unknown how this affects the well-being of everyone involved (Pennings, 2019).
In addition to ancestry databases, there are registries founded by donor-conceived offspring or their parents (e.g. Donor Sibling Registry, USA), or publicly-funded (e.g. Donor Conceived Register, 2004 UK; Fiom KID-DNA Database, the Netherlands) that assist donors and donor-conceived offspring who are seeking to make mutually desired contact with genetic relatives. When looking at databases run by governmental bodies, these are sometimes linked to a supportive network for both donors and donor-conceived offspring when contact between them is desired. These DNA databases are the results of governmental initiatives, mostly when laws were changed concerning anonymous and identifiable donation. In the Netherlands, the voluntary Fiom KID-DNA Database allows donor-conceived offspring and donors who donated prior to June 2004 to register and submit their DNA. The centralized database was founded in 2010 and is hosted by one organization, which mediates contact between donors and donor-conceived offspring and allows them to receive counselling. There was a public appeal in 2017 from the government asking anonymous donors to voluntarily register in the Fiom KID-DNA Database. In 2018, 534 donors were registered in the database, of which 20% appeared after the call of the government (Bolt et al., 2021). Through an online questionnaire, these donors were asked why they registered on the DNA database. The top three replies were that the donor-conceived offspring should: be able to ask me questions; get information on physical and social details about me; or be entitled to contact me. The reason ‘for passing on medical information’ constituted <1% of reasons for joining. Donors also indicated they wanted to know the number of donor-conceived offspring they had and how they were doing. Most donors were over 50 years old (∼90%) and had children who they raised and for whom they have legal responsibility (∼80%) (Bolt et al., 2021). In the UK, the UK DonorLink (UKDL) database was established in 2004 and transferred to the Donor-Conceived Register (DCR) in 2013. In 2018, 91 donors were registered in the DCR. The motivations of donors to be registered on the DCR were, similar to the study in the Netherlands, multifaceted (Blyth et al., 2017). Most donors wanted their offspring to be able to get information on their biogenetic and biographical heritage. Donors were also interested in the outcome of their donations and wanted to satisfy their own needs for information on the lives of their offspring.
Legislation, anonymity and donor quota
It is important to be aware that legislation and regulation of donor conception differs across different countries (Calhaz-Jorge et al., 2020). At the same time, it should be acknowledged that legislation may not take into consideration the emerging direct-to-consumer genetic testing, and the consequences for all affected.
European perspective
In Europe, the use of gamete and embryos in MAR fits within a wider regulatory framework. The European Tissue and Cell Directives (EUTCDs) define the safety and quality standards for using and storing tissues and cells. Still, each member state has to determine its national legislation, including the legislation on donor anonymity, and hence legislation can be very different across the European continent.
In almost half of European countries, legislation grants donors anonymity (e.g. Spain, Czech Republic), and offspring have no right to access the identity of the donor or ancestry information. In other countries, offspring have the legal right to access the donor’s identity at any age or at a certain age (e.g. 18 years in Finland and UK, Portugal; 16 years in Austria; ‘at mature age’ in Sweden) (Calhaz-Jorge et al., 2020). In a third category of countries (e.g. Denmark), donors can choose if they wish to be identifiable or not to offspring. In some of these countries, recipients and donors have the possibility to get to know each other before or during medical treatment (e.g. Germany), while in others, this is not possible as the offspring is the only person who can access the identity of the donor (e.g. Finland and Sweden).
Another aspect are quotas for the number of patients that can be treated with a single donor or the maximum number of children born (or families created) from a single donor. Restrictions may be based on legislation (e.g. UK, HFEA guidance) or relevant professional guidelines. In some countries, MAR centres/gamete banks themselves define a maximum number of offspring. Some jurisdictions also allow donors to regulate the number of offspring, as long as they remain beneath the levels set in national guidelines (e.g. UK). With regards to such quotas, there is a lack of evidence on what the upper limit for the amount of offspring or families should be, and suggestions vary from 5 to 10 children or families per donor (Millbank, 2014; Janssens et al., 2015; Sydsjo et al., 2015). Based on genetic considerations, up to 200 offspring per donor—except in isolated communities—may easily be acceptable, while based on psychosocial considerations in donors and offspring, a maximum of 10 families seems appropriate (Janssens et al., 2015). The international distribution of donor gametes, and the travelling of donors and intended parents complicates the calculation of quotas (Janssens et al., 2015). Some countries have opted for national registers within their legal framework (e.g. HFEA in the UK). Most countries, however, have less regulations and no national registry, making it hard to track numbers of donor-conceived offspring per genetic parent and enforce restrictions. Regulation is further complicated by incomplete information on numbers born from donor-conception.
Global perspectives
More than half the countries in the IFFS survey reported having legislation that covers gamete donation; 40 out of 73 for oocyte donation and 43 of 74 for sperm donation. This is an increase of 29% since the last survey in 2016. Many countries also have regulations regarding embryo donation, 29 of 69, or double donation, 26 of 68 (International Federation of Fertility Societies, 2019).
A majority of countries in the survey also reported having regulations that address the anonymity of gamete donors. Only 10 out of 78 countries had no regulation at all while the rest had either national or regional laws or recommendations. Further variation was reported in the kind of data that are shared with the offspring, with some countries allowing only non-identifying donor data to be shared, while others also allow identifying data to be provided (International Federation of Fertility Societies, 2019).
Allowing data concerning the offspring to be disclosed to the donor is less common, and even when allowed is only rarely practiced (International Federation of Fertility Societies, 2019). Some systems, for example the UK, allow donors who donated after 1 August 1991 to know the number, sex and year of birth for any offspring.
Increasing numbers of people are travelling across international borders to access treatment and services. This may be due to cheaper treatments, better quality of treatments, shorter waiting times and/or an attempt to circumvent legal and ethical restrictions (Shenfield et al., 2010). Specifically, for donor-assisted treatment, reproductive travelling is motivated by the prohibition and/or lack of availability of donor treatment and/or certain types of donors/donation in some countries (Glennon, 2016) and by the possibility to maintain secrecy about the origin of donor gametes (Inhorn and Gurtin, 2011; Laruelle et al., 2011; Van Hoof and Pennings, 2011). Countries that allow anonymous donation are visited by patients from countries where non-anonymous donation is the standard (Hertz et al., 2016). This is not limited to European or European Union (EU) countries. In the IFFS survey, 34 countries out of 72 reported that people came to their country for treatment using oocyte donation, while 46 responded that people travelled from their country to another country for treatment using oocyte donation. Regarding sperm donation treatments, it was reported that 36 countries had people travelling to their country, and 36 countries had people travelling to another country (International Federation of Fertility Societies, 2019).
Legislative changes with prospective and retrospective impact
A small number of countries have recently changed their legislation regarding donor anonymity (e.g. the UK in 2005, Finland in 2007, Victoria/Australia in 2016, Portugal in 2018) (Calhaz-Jorge et al., 2020), some of these inspired by the United Nations Convention on the Rights of the Child (Blyth and Farrand, 2004; Frith, 2015). Changes include both prospective (e.g. UK) and retrospective (e.g. Victoria/Australia) lifting of donor anonymity. A small survey of Australian sperm donors (n = 42) reported that many men who had donated under an anonymous regime accepted such changes and support identifiability, suggesting that the removal of anonymity does not compromise donor insemination services (Adams et al., 2016). In a review of 62 studies, a significant proportion of both oocyte donors and sharers seemed willing to donate in an identifiable donor system (Bracewell-Milnes et al., 2016) and some considered meeting with offspring to be important to very important (Bolt et al., 2021). Many donors seemed to support disclosure to children conceived by their gametes and the majority (65% of oocyte donors, 70% of sperm donors) were positive towards being contacted by offspring (Isaksson et al., 2014; Lampic et al., 2014). However, there are also reports of the opposite. A Danish study found that between 51% and 67% of all sperm donors would stop donating if anonymity was lifted, but between 15% and 22% would allow the offspring to contact them (Bay et al., 2014). A Belgian study showed that only one in five of current donors would continue to donate when they would become identifiable (Mahieu et al., 2019).
It is worth noting that these studies do not consider whether a different population would come forward in the event of identifiable donation, or the effect of local legislation on individuals’ opinions. Besides differences in legislation, international direct-to-consumer genetic testing and cross-border reproductive care make it possible to reveal information about donor conception without regard for national legislation.
Information provision for reproductive donation
Recipients, donors and their care providers need to be aware of how current technologies have changed reproductive donation. Genetic identity is now more easily accessed and accessibility is something over which the individual may have little control, i.e. a relative may access and submit their own DNA to an ancestry database. The ramifications and potential psychosocial consequences for all involved should be made clear at the first consultation for both donors and recipients and subsequently in the donation/treatment process.
Information provision in MAR centres and gamete banks is considered essential. A wide variety of information, such as patient leaflets and online information, is available to donors and prospective recipients. However, such information provided in MAR centres/gamete banks usually focuses on technical and medical aspects of the procedures and treatment (European Directorate for the Quality of Medicines & HealthCare (EDQM), 2018). There is a lack of international guidance on what information should be provided to prospective donors, recipients and donor-conceived offspring about the wider ethical and psychosocial consequences of reproductive donation and these recommendations aim to fill this gap.
Materials and methods
The current document was developed according to the ESHRE manual for the development of recommendations for good practice, which outlines a 9-step methodology (Supplementary Data S1). A glossary of terms used in the current paper is provided in Supplementary Data S2.
To support the WG in formulating recommendations, PUBMED was searched for papers on the topics of donor conception, ancestry, parenthood, disclosure and anonymity. Papers were included from 1 January 2014 up to 31 August 2020, focusing on studies published since direct-to-consumer genetic testing has become more widespread and accessible.
References were screened and considered against the general inclusion criteria (English language, donor conception following assisted reproduction). References focusing on clinical aspects of donor conception, and fertility preservation, surrogacy and preimplantation genetic testing (outside of the context of donor ART) were deemed irrelevant for the current paper. For references considered relevant, full-text papers were collected, assessed, divided per topic and summarized by the appointed WG member. Further information from guidelines and regulatory papers was added based on the experience and research of the WG members.
After the finalization of a first draft within the WG, the draft was published for consultation and relevant stakeholders were contacted to provide feedback. In total, 518 comments were received from 20 reviewers, representing professionals and donor-conceived offspring organizations. The received feedback and resulting actions are summarized in the review report published on www.eshre.eu/guidelines. The stakeholders are listed in Supplementary Data S3.
Recommendations for information provision and support for donors, recipients and donor-conceived offspring
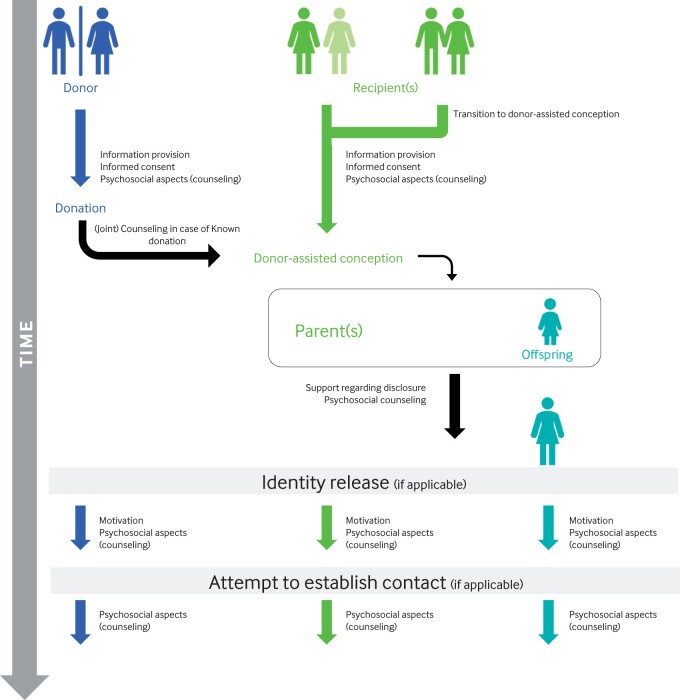
The recommendations are divided into information provision for donors, for intended parent(s) and for donor-conceived offspring. Information for each group should be provided at different timepoints in the process of donor-assisted reproduction (Fig. 2). MAR centres and gamete banks should have in place resources and training to ensure the relevant staff groups are available and can provide informed support to donors, recipient(s)/intended parent(s) and donor-conceived offspring requesting support. A summary of recommendations on information provision and checklists are available in Supplementary Data S4.
Figure 2.
The timeline of donor-assisted conception.
Information provision for donors
Donating sperm, oocytes or embryos to individuals or couples so that they can build a family is associated with short- and long-term implications for donors and their current and/or future family. Donors should be fully informed about these implications so that their agreement to donate is based on informed consent. The information on the implications for donors should explore the current legal framework, the medical investigations and procedures, the context of direct-to-consumer genetic testing and the psychosocial aspects. This should also include the wider societal context in which donation occurs and the notion that this context will evolve and is unlikely to remain fixed.
Donor recruitment and compensation
Financial issues
Many studies suggest that a mix of altruism and financial interest leads men and women to donate gametes (Van den Broeck et al., 2013; Cordier et al., 2020; Platts et al., 2021). Payment is often considered inappropriate, while compensation for expenses is acceptable. Motivation and views on compensation seem to depend on the type of donation, and the culture and values of the country in which the donors are recruited (Platts et al., 2021): for example in Denmark and the UK payment for oocyte donors is secondary and donors are mainly motivated by altruism (Graham et al., 2016; Borgstrom et al., 2019), while in a study of oocyte donors in Cyprus, 70% only donated for financial gain (Tulay and Atilan, 2019). On the other hand, Danish sperm donors expected a minimum of €38 per donation (Bay et al., 2014). A single-centre study amongst sperm donors in Belgium showed that most of them (86.6%) were motivated by altruism; in one in four donors, altruism was the sole motivation, while others were also motivated to some extent by the financial compensation or access to blood and fertility tests (Mahieu et al., 2019).
One argument for financial payment is to ensure sufficient numbers of donors (Shapiro, 2018). Some reports suggest that in countries with altruistic donation, there is a shortage of donors and long waiting lists, although this does not seem to be the case for all countries (Kool et al., 2018; Shapiro, 2018). There is inconsistency in data on whether the complete or partial withdrawal of financial payment results in a lower number of donors. Furthermore, there is controversy regarding the ethics of payment. Payment can—without necessarily compromising an altruistic attitude—compensate personal and financial sacrifices, and thereby encourage donors and increase the numbers, but payment can also result in the commodification of bodily material, exploitation of donors or incentivize donors to falsify information.
The literature seems to suggest ‘reasonable compensation’ for gamete donors is appropriate (Kool et al., 2018). Some research suggests that identifiable donors require higher compensation (Cohen et al., 2016) and this may also drive differences in commercial pricing. In contrast, identity-release donors in the UK reported that donating ‘for the money’ is problematic, and they frame their donations as other-oriented acts of giving because they were aware that they may one day be telling these donation stories to offspring (Gilman, 2018). Most recipients are in favour of compensating donors as it serves as a symbolic acknowledgement of the donor’s contribution and helps secure the type of relationship they expect from their donor (Ravelingien et al., 2015b). For donor-conceived offspring, learning about the motivation to donate is one of the reasons for searching their donor (Zadeh et al., 2018).
To proceed in a morally acceptable way, the Nuffield Council suggested a ‘ladder strategy’ to encourage donation, starting with (more) public awareness, offering compensation and then offering payment to ensure enough donors (Nuffield Council on Bioethics, 2013). In the EU, the financial gain from donation is prohibited but reimbursement of expenses or compensation (for example for loss of earnings) is permitted.
Recommendation.
Donors should be informed that donating gametes and embryos is voluntary.
Donors should be informed about any reimbursement or compensation that is allowed locally and its basis.
Information needs and informed consent
Informed consent is a vital precondition for donating, and various key elements of such informed consent have been suggested (e.g. in oocyte donation, see Cattapan, 2016).
It has been suggested that the informed consent process should be performed by an independent professional, to avoid any conflict of interest (Bass and Gregorio, 2014). Practically, if the professional providing the information follows all details of this ESHRE document, fully informed consent is achievable.
Specific considerations regarding key information that needs to be given to ensure informed consent are outlined below.
Legislation on anonymity and identifiability
All donors need to be aware of the legal context of gamete donation in the country where they donate. There are diverse legal regulations regarding anonymity and data storage to be considered. In some countries, the legislation grants donors anonymity, while in others, offspring have the legal right to access the donor’s identity, or donors can choose to be identifiable. It may even be possible for donors and recipients to get to know each other before or after donation (see ‘Legislation, anonymity and donor quota’ in the Introduction for more details). As there is increasing pan global use of gametes, the legislation of the country/countries where the donated gametes will be used in MAR treatment, which can differ from the country where the donation took place, is equally important for donors (Pennings and Gürtin, 2012). In this context, it is considered good practice for both the gamete bank and the MAR centre to share responsibility for the donors, to verify if gametes were obtained according to ethical standards, and to ensure adherence to good practice guidelines (Deech, 1998; Kool et al., 2018; Pennings et al., 2007).
In addition to being informed about current legislation, donors should be aware that legislation may change. In case of changing legislation, it is often unclear who has the duty to inform previous donors—especially in the case of retrospective changes. It should be agreed whether the MAR centre/gamete bank that recruited the donor or the relevant regulatory authority takes responsibility for informing donors on legislative changes that could impact on their identifiability and on the potential possibility of joining a voluntary register.
Irrespective of legislation, donors need to be informed that recent developments in direct-to-consumer genetic testing and commercial ancestry databases may allow donor offspring and their families to find genetic (donor) relatives.
Recommendations.
Donors need to be informed about current national legislation governing their donation and be made aware that this may change, both prospectively and retrospectively, in the future.
Donors need to be informed that the legislation regarding identifiability and anonymity may vary between countries, and that it may be different in the countries where the gametes will be used, or where recipients and offspring reside. Global differences in legislation and the absence of legislation in certain countries should be discussed with a focus on the possible impact for the donor.
Donors should receive information on what personal and medical data are recorded and what is provided to recipients and/or offspring. They should be informed by whom and when their data can be accessed.
In identifiable systems, donors should be informed at what age offspring can access their identity.
Donors should be informed about the implications of any donor registries available in their country and whether these are voluntary or mandatory.
Donors should be informed about the implications of direct-to-consumer genetic testing in combination with social media and online information. They need to be fully aware that their genetic identity could be revealed at any point through DNA testing by themselves or one of their relatives, even if they were granted anonymity by the legislation of their home/donating country.
Donors should be offered independent counselling beyond medical information provision by the MAR centre/gamete bank. Informed consent does not remove the requirement for such independent counselling.
Number of offspring
In general, the number of donations from sperm donors is higher compared to oocyte donors, as the latter involves a medical intervention and therefore medical risks. Consequently, the actual and potential number of children conceived by oocyte donation is smaller and this also applies to embryo donation.
As mentioned before, some countries restrict the number of offspring per donor, either by limiting the total number of offspring or the number of families. Some jurisdictions allow donors to define the preferred number of offspring, within the limits defined in national legislation or guidelines.
There is contradictory information as to whether donors, as well as potential donors, prefer limiting the number of offspring (Nelson et al., 2016; Thijssen et al., 2017), and there does not seem to be any data on the preferences of embryo donors. Many donors seem to want to know the number of children conceived with their donation (46.5%), and a proportion wanted more personal information about the recipient family (21%), or about the children conceived (27%) (Thijssen et al., 2017). Borgstrom et al. (2019) reported that donors tend to misjudge and assume a much higher number of offspring and concluded that information, such as the actual number, should be provided.
Whether limits are defined in legislation or by donors, it has been suggested that it is preferable to set a limit in relation to the number of families receiving the donation rather than the number of offspring. A family-based quota would ensure that once a couple had a child, a genetic sibling pregnancy would not be precluded, which in turn would be preferable to minimize the levels of non-genetic relationships within the family, ensuring full-genetic siblings and facilitate any subsequent contact with the donor (Janssens et al., 2015).
Recommendations.
Donors should be informed about the national legislation/regulation with regards to the maximum number of offspring or families that can be created from their donation and the rationale behind these limitations.
Donors should be informed about whether they themselves can set a lower limit on the number of families created and, if possible, how to incorporate these restrictions/conditions in their informed consent.
Where possible, donors should be encouraged to set limits based on the number of families created rather than the number of offspring.
Donors should be informed about the possible extent of use of their donation nationally and internationally, and the implications thereof. The lack of (inter)national rules and quotas, potentially resulting in a large number of offspring born from the donation, should be emphasized.
Donors should ideally be allowed at any point in time to request information on the number of pregnancies, live births and families resulting from their donation.
Legislation on storage of donated gametes and data
Donated gametes can be used immediately (i.e. following screening and quarantine), but they can also be stored and used at later time points. Some countries have legislative restrictions on the maximum duration of storage of donated gametes. Where no such limit is in place, gametes can be used indefinitely, possibly creating a large age difference between donor and offspring and between donor-siblings.
In addition to the donated gametes, data from the donor are stored and shared with the MAR centre. Donor data may be stored centrally (e.g. UK, Germany) or locally by the centres (e.g. Finland). The length of documentation in Europe varies between 30 years (European Parliament and Council of the European Union, 2004) and 110 years (SaRegG 2018 in Germany).
Recommendations.
Donors should be informed about the duration of storage and use of the donated gametes and the policy once the storage period has ended.
Donors should be informed whether they are allowed to further reduce the maximum duration for use of their donation and, if possible, how to incorporate these restrictions/conditions in their informed consent.
In countries that place no restriction on the duration of storage, donors and recipients should be informed that this could lead to offspring with major age differences and a theoretical risk of inter-generational consanguinity.
Donors should also be informed about what personal data are recorded, how it is stored and the retention period, and how the donor can update this information.
Donors should be encouraged to update their information held by the MAR centre/gamete bank should relevant medical information come to light concerning the donor or his/her family.
Donors should be informed about the possibility that they could be contacted by the MAR centre/gamete bank for additional medical information, such as unexpected diseases in the offspring.
Donors should be aware that the General Data Protection Regulations (GDPR) allows them to request information on the data stored about them.
Choice of a recipient
In some legal contexts, donors can fetter their informed consent to exclude some possible recipients from using their gametes. Such restrictions can be based on sexual preference, marital status, religious affiliation, phenotype/physical resemblance, attitude regarding disclosure and contact, nationality or geographical closeness/distance (Millbank et al., 2017). In many countries, the donor is not granted any rights to exclude recipients, or the MAR centres/gamete banks do not facilitate recipient selection/exclusion.
In the specific context where recipients and donors are known to each other prior to the start of treatment (i.e. known donation), some embryo donors and recipients may prefer to share common characteristics with each other to build a relationship and be open to the exchange of information and possible ongoing contact (Frith et al., 2011; Goedeke and Daniels, 2018). Some donors preferred recipients to be similar to themselves, for example regarding sexual preferences and religious beliefs, and they preferred some geographical distance to avoid too close contact. Furthermore, a number of embryo donors also viewed the children resulting from their donation as ‘their’ children and used family terms (such as aunt and uncle) to refer to themselves in relation to the children born from their donation (Goedeke et al., 2015).
Recommendation.
Donors should be informed that whether or not they can restrict access to their donation for certain recipients (i.e. fetter their informed consent) will depend on the relevant national legislation.
Withdrawal of consent
There are contradictory attitudes regarding the ownership of donated gametes. Some MAR centres/gamete banks (or countries) consider themselves to be the owner once gametes have been donated, while other centres/banks consider themselves to have custody, but not ownership. A third group considers that donors keep ownership and, as such, are entitled to withdraw their consent (Kool et al., 2018).
With regards to the donation of tissue and cells in general, the EDQM guide states that donors must be informed that they may withdraw consent at any time (European Directorate for the Quality of Medicines & HealthCare (EDQM), 2019). Although not discussed in the EDQM guidelines, for the donation of reproductive cells, this withdrawal of consent is likely to be any point in time before ‘use’, which may be defined as insemination and/or fertilization.
Recommendation.
Donors should be informed about the relevant regulations with regards to withdrawal of consent, the timeframe within which this is possible in relation to the use of the donation (insemination, fertilization, transfer etc.), and how, if relevant, they can review or change their consent provisions.
Post-mortem use of stored gametes
There should be agreement as to whether gametes can be used after the MAR centre/gamete bank becomes aware of the death of a donor and who may be entitled to decide on behalf of the deceased donor. It has been suggested that donors should make their preferences explicit during the donation process (Deech, 1998; Burrell, 2012; Dillon and Fiester, 2012; Kool et al., 2018). Others prefer donors to provide consent as an ongoing process as they may change their preferences over time (Baylis and Widdows, 2015; Stroud and O'Doherty, 2015; Kool et al., 2018). It should be clarified that in some contexts it may not be possible to re-contact the donor, while in others there is a legal obligation to check before use of the donation.
Recommendations.
Donors should be informed of the legal regulations regarding post-mortem use of their gametes.
Donors should make their preference regarding post-mortem use explicit, these should be incorporated—as far as in line with legislation—into the informed consent documentation, explained to potential recipients, and respected.
Donors should be encouraged to tell their next of kin what their wishes would be in relation to post-mortem use of their gametes, and how they can carry these out.
Health examinations, medical screening and medical risks
Donors must undergo health examinations to ensure their fertility and to avoid transmission of infectious or hereditary diseases (European Commission, 2006). Several professional organizations have developed guidelines for medical screening and these, in general, include a medical history of the donor, his/her family history, and genetic screening for specific conditions (e.g. karyotyping or carrier status) (Dondorp et al., 2014; European Directorate for the Quality of Medicines & HealthCare (EDQM), 2019). Amor et al. (2018) showed that donors (as well as recipients) are hesitant about the process of genetic screening and there are concerns about how this information may be used, and the ethics of selectivity.
Medical and/or genetic screening of the donor could result in an unanticipated diagnosis of infertility, and/or an infectious or heritable disease.
Sperm donation is generally not associated with any health risk to the donor. On the other hand, oocyte donation is associated with all the typical risks of an ART procedure, such as risks associated with hormonal stimulations and oocyte retrieval. However, the medical procedures seem to be well tolerated, and there is excellent post-donation satisfaction among oocyte donors (Bracewell-Milnes et al., 2016; Gonzalo et al., 2019). A review of 428 women who had donated on average 11 years previously found the following complications of the procedure: 7.2% had ovarian hyperstimulation syndrome (OHSS) or immediate bleeding, 11.5% experienced unsuccessful attempts to become pregnant following donation, and 4.9% were diagnosed with gynaecological conditions. The same review showed that all donors were satisfied to very satisfied and 95% would recommend oocyte donating to peers (Soderstrom-Anttila et al., 2016). More recent data for ART in general showed much lower complication rates (OHSS incidence of 0.21% in 2016) (The European IVF-monitoring Consortium for ESHRE et al., 2020). However, it is concerning that one recent study from Cyprus analysed whether oocyte donors are aware of medical risks, and according to this study, although 80% of donors had donated multiple times and 7% in different centres, only 38% were described as being fully aware of all the medical risks; the authors concluded that donors were not well informed about risks of anaesthesia, infection, bleeding and bruising (Tulay and Atilan, 2019).
Women donating oocytes resulting from their own treatment (so-called ‘oocyte sharers’) do not experience any additional medical risks as long as their hormonal stimulation is not targeted towards producing a very high number of oocytes (Ahuja et al., 1998; Bracewell-Milnes et al., 2018). Similarly, the donation of ‘surplus’ embryos or surplus stored gametes is not associated with any medical risks beyond those of their own treatment.
Recommendations.
Donors should be informed about unanticipated results of medical (and where applicable genetic) screening and, where required, offered a follow-up consultation to explain the results and potential implications.
Oocyte donors should be informed of all health risks associated with hormonal stimulation and oocyte retrieval. A follow-up consultation with oocyte donors shortly after donation is recommended.
Psychosocial aspects and psychosocial counselling
Psychosocial counselling is mandatory in some countries and offered in others (at least in the public sector). In a survey of oocyte donors in several different countries, 95% of all donors stated they had received medical counselling, but only 60–80% received psychosocial counselling (Pennings et al., 2014).
Several studies suggest that donors find it important to talk about the possible short- and long-term emotional consequences of their donation so that informed consent can be given (Deech, 1998; Söderström-Anttila et al., 2001; Ethics Committee of the American Society for Reproductive Medicine, 2004, 2009; Black, 2010). These include the decision if the donor would like to remain anonymous or meet the recipient(s) prior to treatment, during pregnancy or after the birth of the child (in those jurisdictions where this is possible) (Frith et al., 2017), disclosure to family and friends, future contact with donor offspring (Isaksson et al., 2014; Visser et al., 2016b), the emotional impact of knowledge about the donor offspring, especially if the donor her/himself remains childless or becomes infertile, learning about unanticipated high numbers of offspring (Kool et al., 2018) or offspring voicing an interest in meeting the donor’s own children (half-siblings or, in the case of embryo donation, full siblings). However, when donors were asked 14 months after donation, only a few donors reported the need for counselling (Lampic et al., 2014). Many donors do request counselling and support should offspring request contact with them (Crawshaw et al., 2007; Kirkman et al., 2014).
Many papers mention/discuss counselling donors but there does not seem to be any agreement as to what this counselling should include. As a minimum, counselling should explore the short- and long-term implications of donation, such as:
the meaning women and men attribute to their donation;
the role they associate with being a donor;
potential negative and/or unanticipated results from medical and genetic screening (see Health examinations, medical screening and medical risks);
their intention (or not) to disclose to their partner, children, family and friends that they have donated;
whether they wish to be informed about the type of recipient (if allowed);
their desire regarding accessing information about pregnancies and births achieved with their donation;
whether they are open to contact with recipients/offspring before, during, or after pregnancy (where allowed);
information that legislation regarding anonymity/identifiability may change and also may change retrospectively (see legal aspects);
information on the maximum number of offspring (families) per donor in that country and the potential impact of this number on future contact;
the possibility of leaving written/video information for offspring;
their contact preferences once offspring requests contact; and
information on the consequence of direct-to-consumer genetic testing and disclosure of identity even if according to legislation donation is anonymous (Motluk, 2005).
Recommendations.
Donors should be informed about the advisability of having psychosocial counselling and how this differs from medical information provision.
Counselling should be available before, during and after donating gametes.
Donors should consider the potential future impact of the donation on their close family and friends.
Known donation
If donors and recipients are known to each other (intra-familial donation, known or personal donor) counselling should cover clarifying everyone’s potential roles and discussing the boundaries between the donor and the recipient family (Haskovic et al., 2018). In intra-familial donation, counselling should also explore whether donors (and recipients) are aware of the implications of cross-generational donation. In all cases, all the parties involved need to decide unanimously on the stance taken to disclosure and take a decision that fits with their religious and cultural beliefs (Acharya et al., 2017). An (legal) agreement between the donor and recipient(s) clarifying everyone’s roles and responsibilities could be considered, the status of such an agreement would depend on the national legislation.
Recommendation.
Donors who are known to the recipients (i.e. known donation) should have access to counselling on their own and with recipients in order to clarify everyone’s different roles and relationships, disclosure plans and preferences.
Gamete sharing
In general, sharing oocytes is described as a positive experience with only few women regretting their donation, as they regret losing oocytes (Bracewell-Milnes et al., 2016). A slight majority are interested in knowing about the outcome (Bracewell-Milnes et al., 2016). There are no clear findings on whether oocyte sharers prefer remaining anonymous towards the recipient woman or couple, or prefer to get to know her/them prior to or after having donated (Bracewell-Milnes et al., 2016, 2018). Between 74% and 94% of all female donors tell family and friends and 66% plan to tell their own children that they have donated oocytes (Bracewell-Milnes et al., 2018). From a psychosocial perspective, oocyte sharers are subject to emotional risks. It may be difficult for them to decide against sharing oocytes if this is the only option available to enable them to afford their own treatment and this may impair the element of voluntariness in informed consent (Bracewell-Milnes et al., 2016). Oocyte sharers could find themselves in the emotionally challenging situation that there are offspring resulting from their donation, but their own treatment remained unsuccessful (Bracewell-Milnes et al., 2018). Recently, sperm sharing also has become more prevalent. Although not yet studied, the considerations and consequences could be similar to those associated with oocyte sharing.
Recommendations.
Women donating oocytes from their own treatment (i.e. oocyte sharing) should be informed of the potential repercussions for their own chances of having a child.
Gamete sharers should be encouraged to consider and discuss how they may feel if their fertility treatment is unsuccessful and the recipients’ treatment results in children.
Counselling with regard to contact
A survey of donors registered on the UKDL showed that 67% of sperm and oocyte donors were curious about offspring and wanted to find out what had happened in their lives. Most (68% of sperm donors and 75% of oocyte donors) wanted direct contact with offspring to exchange information but at the same time 60% of sperm and 42% of oocyte donors were unsure if there might be negative consequences of this (van den Akker et al., 2015). A certain degree of ambiguity was also reported by Kirkman et al. (2014); men and women who had donated anonymously feared contact, yet thought that donor offspring may be curious and accepted that offspring may have a need to know the donor.
In identifiable donor systems, donors seem to support disclosure to children conceived by their gametes and the majority (65% of oocyte donors, 70% of sperm donors) were positive towards being contacted by offspring (Isaksson et al., 2014; Lampic et al., 2014). Isaksson et al. (2014) also reported that donors wanted to be notified if offspring requested information about them, most likely so that they could prepare for contact.
Support, information and comprehensive guidance seem especially important. Miettinen et al. (2019) found that 74% of Finnish oocyte donors felt positive about contact with offspring but they were uncertain as to what an appropriate relationship with the offspring and their family might be and felt ambivalent about contact. In this study, most donors also planned to inform their own children about their donor offspring. Daniels et al. (2012) propose that men’s donation has an impact on their social networks and recommends that this needs to be considered prior to donation, as it may impact on parents and siblings of the donor, not just on his immediate family.
Recommendation.
Donors and their family should be able to access counselling before, during and after contact with offspring occurs.
Information for recipients
Transitioning from MAR with their own gametes to MAR with donated reproductive material is often very challenging for couples. When moving to MAR with donor gametes, other options should be discussed including fostering, adoption, or opting out of further treatments: the latter should include being offered counselling that addresses the possibility of life without children.
Moving to donor-assisted conception
The majority of recipients who had relied on a preparatory group session for donor-assisted (or third-party) reproduction found the contact with the following type of people helpful: the group facilitator, who had been a patient; psychosocial specialists; medical specialists; and patients currently in the same situation (Crawshaw and Montuschi, 2014).
Potential parents who received medical or psychosocial counselling at their centre prior to sperm donation shared that they felt they were being screened for eligibility rather than guided and therefore did not dare to bring up their worries (Visser et al., 2016a). Regarding disclosure, parents did not like being questioned about their own perspectives and would have preferred to be given advice. Also, they missed practical advice about secrecy and disclosure from counsellors and experienced parents (Visser et al., 2016a). Counsellors often reassured parents that the parent with no genetic link would still have a good relationship with the child by distinguishing ‘nature’ from ‘nurture’ (Visser et al., 2016a).
Recommendations.
When moving from MAR with own gametes to MAR with donor gametes, pathways to parenthood should be discussed, which include other options such as fostering or adoption.
Counsellors should discuss the implications of using donated gametes and having a child who is not genetically related to one or both of the couple by addressing the distinction between nature and nurture.
Recipients should be signposted to available resources, this may include: literature (books); websites; peer support groups; dedicated counsellors and/or organizations.
Recipients should be supported and informed on how they can talk age-appropriately with their offspring about their conception with donated gamete(s).
Information needs and informed consent
Informed consent for MAR with donor gametes should be specific and include the following key elements (based on requirements for informed consent for donors): the benefits and risks of using donated gametes, including obstetric risks; the privacy of donors and their anonymity, where applicable; the rights of the donor with regards to withdrawal of consent and the possible consequences of this; the availability of counselling; financial issues; and the possibility of treatment not resulting in the birth of a child (Cattapan, 2016).
Legislation on anonymity, identifiability and donor quota
For recipients using donor gametes/embryos, information should be given about local legislation and regulations regarding anonymity of donors and the maximum number of offspring/families that can originate from the donor they are using. The lack of international quotas for the maximum number of offspring/families is relevant to recipients as it affects the number of genetic half-siblings their child may have. They should be informed on current developments and availability of direct-to-consumer genetic testing and ancestry databases, which potentially enable people to find out if they are donor-conceived and possibly trace donors, donor-siblings and wider relatives.
Recommendations.
Recipients should be informed about the legal regulations regarding anonymity and identifiability in their country and the country of the donor.
Recipients should be informed that legislation allowing donor anonymity may change prospectively and retrospectively.
Recipients should be informed about the national limits on the number of offspring or families per donor and whether donors are allowed to set a limit on the number of offspring/families by putting certain restrictions in their informed consent. Recipients should be informed that such limits can only be adhered to if they provide feedback to their centre after the birth of their child/ren.
Recipients should be informed about the lack of (inter)national rules and quotas for the maximum number of offspring/families, and possible consequences of this.
Recipients should be informed if and at what age their children can access identifiable information about the donor. They should also be provided with information about how any medically relevant updates and details on numbers of donor-siblings will or could be given to them.
Recipients should be informed about the implications of direct-to-consumer genetic testing in combination with social media and online information with regards to their ability to not disclose donor-conception to their child and to the possibility that the donor, the offspring and/or extended family may find each other through this route.
Recipients should be informed about any donor registries in their country and how these function.
Legislation on storage and control over gametes and embryos
This section should be read in conjunction with the section above on Withdrawal of consent. Recipients should be informed of the rights of the donor with regards to consent for the donation, and the legislation with regards to the duration of storage. Especially when aiming at having later (genetic) siblings for a donor-conceived child, the right of the donor to withdraw consent (and if so, at what time), and disposal of gametes owing to storage duration limits must be taken into consideration.
Recommendations.
Recipients should be informed about the rights of the donor with regards to withdrawal of consent for the donation, and of the legislation with regards to the duration of storage (of gametes and/or personal information) and the policy once the storage period has expired.
In countries that do not restrict the duration of cryopreservation, donors and recipients should be informed that this could lead to offspring with large age ranges and a theoretical risk of inter-generational consanguinity.
Regarding post-mortem use of donated gametes, recipients should be informed that the local legal regulations and the donor’s preferences should be respected.
Choice of a donor
A review on oocyte donation reported that most recipients prefer anonymous donation, which allows them to mark explicit boundaries between the two parties involved, or known donation mainly by a sister or close friend that has a genetic link with or physical resemblance to the intended mother (Bracewell-Milnes et al., 2016). Lesbian parents who conceived via anonymous sperm donation did so to protect the family from (future) donor involvement, to protect the non-biological mother as a parent, to protect the child from disappointment in the donor, and to release the donor from any obligation (Somers et al., 2017). The same lesbian parents who had conceived via anonymous sperm donation did name three advantages of known donation: medical reasons, for example access to the donor if organ transplantation was needed; the donor could function as an extra caregiver; and the child would have the possibility to contact and have a relationship with the donor (Somers et al., 2017).
When selecting an oocyte donor, recipients most commonly wanted information about the donor’s health in order to have a healthy child, and information about the donor’s physical resemblance to the family to be able to pass as a genetically related family or as part of the process of creating kinship and connection with their child (Greenfeld, 2015; Rubin et al., 2015; Bracewell-Milnes et al., 2016). The oocyte donor’s resemblance to the intended mother is often more important to the intended mother than to the intended father (Greenfeld, 2015).
Women selecting an oocyte donor valued being offered a choice, which made them feel in control and allowed them to build a fantasy of the donor and see signs that the donor was right for them (Rubin et al., 2015). Being offered a choice, however, also led to dissatisfaction with their options and the obligation to select based on the given information (Rubin et al., 2015). In a legal context that did not allow much choice, lesbian recipients stated the selection of their sperm donor was not a major concern and they trusted the hospital to make that decision (Ravelingien et al., 2015a).
Other aspects that recipients wanted to know about were race, lifestyle (e.g. smoking), intelligence and/or education, and personality (Bracewell-Milnes et al., 2016). Lesbian recipients, who were offered little choice, were mainly interested in traits that would facilitate normal child development and increase family coherence (Ravelingien et al., 2015a).
Finding a donor online blurs the distinction between categories of ‘known’, ‘anonymous’ and ‘identity-release’ donation. Lesbian and single women mainly searched for sperm donors online as they value meeting and getting along with the donor rather than valuing the ability of the child to meet the donor (Jadva et al., 2018).
In the choice of the donor, the results of medical and, where relevant, genetic screening (and the limitations thereof) are also to be considered but this is part of medical donor selection rather than the choice of the recipients.
Recommendations.
Recipients should consider the possibility of multiple genetic (half-)siblings when choosing a donor.
Recipients should be informed about the different forms of donation available to them, and—if there is a choice—the (legal) implications of ‘known’, ‘anonymous’ and ‘identity-release’ donation.
Recipients should be informed that their child may want to contact the donor.
Recipients should be encouraged to reflect on their choice criteria and what these will communicate to their child.
Health and medical risk
Couples deciding to use donor gametes should be informed of the benefits and risks, including medical risks and obstetric risks. The benefits include the significantly higher odds of a live birth in donor ART cycles compared to autologous cycles (odds ratio: 1.26, 95% CI: 1.18–1.33, adjusting for patient age, number of oocytes retrieved and number of embryos transferred) (Yeh et al., 2014). Recipients should be informed of their estimated chances of a live birth (based on published data; e.g. a 40-year old woman’s chances would be 23% and 55% with her own eggs and donor oocytes, respectively (Luke et al., 2014)). Similar data are available for sperm donation (Berntsen et al., 2019).
Medical risks in donor-assisted conception are those associated with the MAR procedures generally, with the addition of the risks of transmission of infectious diseases from the donor to the recipient, and the possible transmission of genetic hereditable conditions. Recipients should be informed on how these risks are mitigated: transmission of infectious diseases through testing of samples as prescribed in the European Tissue and Cells Directives (European Commission, 2006); and (known) heritable conditions through a thorough medical and family history of the donor and genetic testing during the donor screening process. Still, recipients should be informed that while these risks are minimized, they are not zero. Discussion is ongoing on the relevance of more extended genetic screening for donors (as expanded carrier screening) (Dondorp et al., 2014). It should be noted that if donor-conceived offspring are unaware of their genetic origin, they may fail to benefit from any updates on genetic risks available from the MAR centre/gamete bank. Regarding genetic screening of the recipients, Mertes et al. (2018) argued that there should be no distinction between parents who reproduce without MAR and those using donor gametes.
With regards to obstetric risks, women pregnant after oocyte donation should be informed that, compared to women pregnant using their own gametes, they may have a higher risk of pre-eclampsia, hypertensive disorders of pregnancy, preterm birth, low birthweight and caesarean section (Mascarenhas et al., 2017; Storgaard et al., 2017).
Recommendation.
Recipients should be informed about medical risks and obstetric risks, in general and specifically for pregnancies using donor oocytes.
Disclosure
There is no agreement regarding the benefits of disclosure or the age at which children should be informed of their donor conception (Golombok, 2017; Pennings, 2017). There is no definitive evidence on the long-term benefits or harms of disclosing/telling the child they are donor-conceived and, following on from that, what levels of information about the gamete donor should be provided. Such information can range from non-identifying details to information that could identify the donor (full name, last known address). This lack of conclusive evidence is reflected in the common advice in professional recommendations, that disclosure is a personal decision and that different parties may have different values (Nuffield Council on Bioethics, 2013; Ethics Committee of the American Society for Reproductive Medicine, 2018).
Arguments in favour of openness and hence disclosure/telling can be divided into two main types: consequentialist arguments, that a lack of knowledge about one’s donor origins harms the donor-conceived person; and a principled argument, that knowing is a basic human right (Ravitsky, 2017). In terms of harms created by not knowing, openness is held to promote at least three vital interests of donor-conceived people: flourishing family relationships, health and the forging of a strong sense of self-identity (de Melo-Martin, 2016).
Effects of disclosure on family relationships
A longitudinal study comparing family relationships in disclosing, non-disclosing families, and those formed by natural conception reported no overall differences between disclosing families and other families. Within the disclosing families, there were more positive family relationships and higher levels of wellbeing for adolescents who had been told about their biological origins before age 7 years (Ilioi et al., 2017). Another study found little difference between those children who knew and those who did not in terms of family functioning and child wellbeing (Kovacs et al., 2015).
Many experts advocate planned parental disclosure before adolescence—and preferably in early childhood—and we found no studies identifying early disclosure as problematic. A commonly reported response among those told young was curiosity (Jadva et al., 2009), and early disclosure was associated with a neutral to positive impact on parent–child relationships (Frith et al., 2018). While some parents adopted the seed planting strategy of very early disclosure, caution was expressed that very young children may show little understanding of it, thus emphasizing the importance of disclosure as an ongoing process rather than a one-off event. The age of becoming aware of their origins appeared to affect its impact—the younger the age of finding out, the less ‘disruptive’ the effects appeared to be (Ilioi et al., 2017). However, even some of those told during childhood could still find the knowledge profoundly hard to come to terms with, challenging the idea that knowledge at a relatively young age always renders it unproblematic (Frith et al., 2018).
Conversely, participants told later in life or who discovered through routes other than planned parental disclosure often reported the information coming as an unwelcome shock and stated that the inherent secrecy and deception had generated anger, mistrust and adverse impact on family relationships. Indeed, these participants were frequently more concerned about prior parental deception than about their parents’ use of donor conception (Frith et al., 2018). Adverse relationships with mothers—often attributed to the withholding information or lying about donor conception—were more often reported than adverse relationships with fathers, although this conclusion is drawn largely from sperm donor insemination studies. Those who learned later in life were more likely to report a lack of genetic continuity, and difficulty in assimilating to their new identity as being donor-conceived (Turner and Coyle, 2000).
Increasing uptake of direct-to-consumer genetic testing combined with social media may increase the chance of offspring discovering that they were donor-conceived other than through planned parental disclosure.
Processes and patterns of disclosure
In addition to the effects on the child, studies have explored broader influences and effects of disclosing the use of donor-assisted conception in wider social circles and the types of demands this placed on parents (Indekeu and Lampic, 2021), how parents negotiated disclosure decisions and effects of disagreement (Gebhardt et al., 2017), and the process of information sharing after identity-release sperm donation (Isaksson et al., 2016).
Some caveats need to be borne in mind concerning research on parental disclosure. First, a tendency to conflate expressed intentions to disclose with actual disclosure. A small study reported that of three families who had planned to tell the child about being conceived by oocyte donation, only one had done so 12 years later (Hershberger et al., 2021). Another study explored this potential discrepancy and found that 43% of their study population had disclosed to their offspring as intended and 39% had delayed disclosure because of uncertainty about how and when to disclose (Applegarth et al., 2016).
Second, disclosing parents may be more likely than non-disclosing parents to participate in research. Nevertheless, numerous studies are indicative of increased parental disclosure over time (Blyth et al., 2012). A study on French recipients of donor sperm reported that 71% intended to tell their child (Lassalzede et al., 2017; Kalampalikis et al., 2018). In other jurisdictions, there is less desire to disclose. A Czech study found that most parents were against disclosure whereas those from abroad receiving treatment in the Czech Republic stated they were more likely to disclose (Rumpikova et al., 2018). A study in Iran found most of their participants (who had used donor oocytes, donor embryos and surrogacy) had decided not to disclose to their social circle mainly to ensure the child did not find out (Hadizadeh-Talasaz et al., 2015).
There are also variable disclosure patterns according to the type of donation and family structure, with the highest levels of reporting being recorded for oocyte donation and among families headed by same-sex couples and single parents (Freeman et al., 2016). There are interesting findings of partial disclosure amongst those who have used a surrogate, with disclosure of the use of a surrogate but not of an oocyte donor (Jadva et al., 2012; Blake et al., 2016).
Finally, it is worth noting that parents’ views and intentions are likely to change over time as well as children’s and, later, adults’, views (Freeman, 2015; Frith et al., 2017). Therefore, studies often only capture a slice in time.
Support in the process of disclosure
Dutch recipients reported a desire for psychosocial counselling to address openness and disclosure (Schrijvers et al., 2020). Disclosure of the child’s donor origins to the child was more often associated with optimal levels of parental psychosocial adjustment, but not always for fathers (Blake et al., 2014; Imrie and Golombok, 2018). A longitudinal qualitative study in donor-conceived families reported that parents who intended to disclose at first feel anxious but later feel more confident, while those who do not intend to disclose bear the burden of keeping a secret and remain anxious that the lack of physical resemblance will lead to people suspecting the child was donor-conceived and that they have fertility problems (Indekeu et al., 2014).
Recommendations.
Disclosing to offspring that they were conceived using donated gametes is advised. In line with other guidance documents, disclosing is preferably done when children are young.
Disclosure should be an ongoing process, rather than a one-off event.
Recipients should be encouraged to reflect on informing those immediately around and close to their child about disclosing to the child.
Support and guidance should be offered to families with regards to disclosure. Such support should be tailored to different family types and cultural settings.
Families may be assisted throughout the disclosure process by signposting to resources such as literature, peer support groups, dedicated counsellors and organizations with this focus.
Psychosocial aspects and psychosocial counselling
In a Canadian study, women’s grief about having to rely on donor oocytes was attributable to the challenge of re-thinking motherhood as not requiring a genetic link and to their concern about other people’s perception of the atypical path to motherhood (Hammond, 2018). Belgian and Swedish (intended) parents who discussed their donor-assisted conception with others reported, besides the mainly positive reaction, also awkward and inhibiting reactions such as considering heterosexual two-parent households as the norm and genes as the determinants for parenthood (Indekeu and Lampic, 2021). Belgian couples, who conceived with donor sperm, actively made efforts to be considered a normal family with a sole father by the outside world (Wyverkens et al., 2017). (Intended) parents shared that sensitization/education of the general public about donor conception would be helpful (Indekeu and Lampic, 2021). Accounts of (intended) parents who had relied on donor sperm or oocytes showed that wanting to comply with ‘normality’ resulted, for some, in minimizing the role of or relationship with the donor (Wyverkens et al., 2017; Hammond, 2018).
A recent study in the Netherlands interviewing intended parents reported that coping with questions from family and friends should be included in psychosocial counselling. In addition, and where relevant, the following topics should also be covered: the decision to opt for donor treatment, choosing a donor, non-genetic parenthood, single motherhood, openness and disclosure, and future contact between the child and half-siblings (Schrijvers et al., 2020).
Intended parents generally favoured genetic over non-genetic parenthood, even more so for their partner than for themselves (Hendriks et al., 2017). The couples’ main reasons for valuing genetic parenthood were the wish to experience a natural process, to ensure sovereignty, and to protect their relationship (Hendriks et al., 2017). A recent discrete choice experiment among couples confronted with severe infertility showed that they would be willing to switch to treatment with donated gametes in return for a child health risk reduction of 3.6%, a cost reduction of €3500, an OHSS risk reduction of 4.6%, a maternal cancer risk reduction of 2.7% or a pregnancy rate increase of 18% (Hendriks et al., 2019). Intended parents who rely on third-party reproduction often initially grieve the loss of the prospect of having genetic ties with their children (Goedeke et al., 2015; Hertz and Nelson, 2016; Hammond, 2018). This loss, however, does not result—for most parents—in doubting their ability to bond with the child prior to birth and does not limit the joy of parenthood after birth (Hertz and Nelson, 2016). Data suggest that some mothers of children conceived through oocyte donation required some time (up to 1 year) to bond (Imrie et al., 2020). Some couples who had used donor embryos did continue to attach importance to the genetic make-up of the child and believed that genetics would play a role in the child’s medical and/or psychosocial characteristics (Goedeke et al., 2015).
In known donation, recipients should be informed that a (legal) agreement between the donor and recipient(s) can be considered and the status of such an agreement would depend on the national legislation.
Recommendations.
Recipients should be informed about the advisability of psychosocial counselling and how this differs from medical information provision.
Counselling should be available before, during and after using donated gametes.
Counselling should include exploration of how to handle questions from family, friends and others about their atypical road to parenthood.
Counselling can help couples decide whether to use donor gametes by discussing the implications of non-genetic parenthood and the disadvantages and advantages, such as increased success rates.
Counselling should address the decision of whether or not to disclose to the child.
In known donation, recipients should have access to counselling on their own and together with the donor in order to clarify roles and relationships, boundaries and disclosure.
How recipients see their donor
How people think about their donor and the meaning attached to both the person and the act of donation can raise difficult issues for some recipients, whether these are heterosexual couples, same-sex couples or single parents. The majority of heterosexual intended parents undergoing donor sperm conception, considered their sperm donor as a ‘generous and unselfish man’ but avoid personalization by considering him as ‘nobody in particular’ and describing him as a ‘gamete donor only’ rather than any type of father (Kalampalikis et al., 2018). Interviews among heterosexual parents showed that limiting the space taken up by the donor was a strategy to protect the position of the ‘social’ father and thereby the family relationships (Wyverkens et al., 2017). Depersonalization of the donor was also often observed among lesbian mothers of sperm donor-conceived children who saw the donors as instruments—or more specifically an ‘entity’—who they tried to erase and deny, or as a ‘gene giver’ who was part of the medical process (Wyverkens et al., 2014; Lingiardi et al., 2016). Some single women similarly thought of the sperm donor as an unknown absence, who had been part of the process but was out of sight and mind (Zadeh et al., 2016). Other heterosexual parents, and lesbian and single mothers considered the donor as providing an esteemed gift and a giving person who they were curious about and/or who was in some distant way part of the family (Lingiardi et al., 2016; Zadeh et al., 2016; Kalampalikis et al., 2018). As time evolved, lesbian and single mothers became more likely to consider the sperm donor as a person as they wondered about physical resemblances, became more confident about their bond with the child and were preparing to disclose the donor conception (Wyverkens et al., 2014; Lingiardi et al., 2016; Zadeh et al., 2016).
Some couples and single parents who turned to embryo donation had a very different way of thinking about their donors as they drew parallels with open adoption and used extended family constructs (e.g. aunt and uncle, godparents) to describe the donor (Goedeke et al., 2015).
The psychosocial wellbeing of parents of donor-conceived offspring
A literature review reported that the process of deciding to have donor-assisted conception is characterized by experiencing the following four intense stages: acknowledging the desire for parenthood; coming to terms with using donor gametes; navigating all the decisions related to relying on donor gametes (e.g. which donor, disclosure); and living with the lasting legacy of having relied on donor gametes (Greenfeld, 2015). A review of longitudinal studies showed that parents of donor oocyte-conceived children, aged 1–12 years are psychologically well adjusted in terms of their level of depression, anxiety, parenting stress and couple relationship quality (Blake et al., 2014; Imrie and Golombok, 2018). Similarly, parents of sperm donor-conceived children did not differ from MAR parents who used their own gametes in terms of psychosocial functioning (Blake et al., 2014; Imrie and Golombok, 2018).
The relationship between parents and their donor-conceived offspring
Two literature reviews of studies observing parents and their children and/or surveying parents and comparing family types reported overall harmonious relationships between parents and their offspring at different ages of the child (Bracewell-Milnes et al., 2016; Imrie and Golombok, 2018). During infancy, parent–infant relationship quality did not differ between oocyte donor-conceived and other types of families, especially when families with twins were excluded and maternal age was controlled for (Imrie and Golombok, 2018). In families with very young children, parents of donor-conceived children report more positive experiences (i.e. enjoyment, warmth, pleasure, emotional involvement), positive maternal feelings and closer family connections (Hertz and Nelson, 2016; Imrie and Golombok, 2018). From observing and interviewing parents and their 7-year-olds, it was concluded that mother-child interaction quality was less optimal in oocyte donor-conceived families while this was not observed for the father–child interaction quality (Imrie and Golombok, 2018). Same-sex mothers of donor-conceived children aged 1–9 years were concerned about their children’s present and future acceptance of their choice for donor conception and had invested in creating a donor conception narrative that was satisfactory for all members of their family (Van Parys et al., 2016). Mothers of oocyte donor-conceived 12-year-olds expressed equal amounts of warmth towards their child and they all responded in an average or above-average way to their child’s needs (Imrie and Golombok, 2018). In adolescence, questionnaires taken from mothers indicated poorer relationship quality in oocyte donation families as compared to sperm donation families although no difference in adolescent–mother interaction was observed (Golombok et al., 2017).
Parents and contact with the donor and/or same-donor offspring
Evidence is limited on recipients’ motivations for seeking contact with the donor and/or same-donor offspring. Whether contact is desired varies between individuals. Reasons given by some lesbian and single parents of donor sperm-conceived children for seeking contact with families with same-donor offspring were to obtain: support for their children (feel less alone, or even expand their family, have siblings); support for themselves (connect with others with donor offspring, discuss donor choice and ways to talk with the child about the donor); information about shared traits (physical, personality) and medical problems (e.g. dyslexia); and to prevent their child from having a romantic relationship with someone related (Goldberg and Scheib, 2015). The quality of the encounters varied from uncomfortable to the creation of a special bond both between parents and children, and not all families had the same desire for contact (Goldberg and Scheib, 2015).
Parents relying on embryo donation often draw strong parallels with (open) adoption (Goedeke et al., 2015; Frith et al., 2017). Qualitative research showed that some parents kept in contact with embryo donors and families with same-donor offspring as they considered openness and honesty to be in the best interests of their child (Frith et al., 2017). The intensity of contact and whether young children or only adults were involved differed, but strong ongoing relationships were built (Goedeke et al., 2015; Frith et al., 2017). The contact with the donors was, however, sometimes associated with a degree of anxiety for parents (Goedeke et al., 2015; Frith et al., 2017).
Recommendation.
Recipients should be informed about the possibilities of donor-conceived offspring connecting through direct-to-consumer genetic testing with their donor or other genetic relatives and the importance of support and counselling for all the parties involved.
Recipients and donor-conceived offspring with anonymous donors should be encouraged to consider that the donor may not expect to be found and will not have had counselling to prepare them.
Information for donor-conceived offspring
Until recently, reproductive care in assisted conception for donors and recipients ended with a confirmed pregnancy or at childbirth. In the last decade, with affordable and easy access to direct-to-consumer genetic testing and a considerable and growing number of donor-conceived children being born after the removal of anonymity in some countries, MAR centres are being confronted with new demands such as requests for information from donor-conceived offspring about their donors (Beeson et al., 2011). Donor-conceived offspring have to navigate their way through new challenges related to their identity formation (Zweifel, 2015). Therefore, assisted reproduction healthcare professionals have a responsibility to provide information regarding the psychosocial issues that donor-conceived offspring can encounter.
The majority of studies including donor-conceived offspring as participants refer mainly to sperm donation. The situation will likely change in the next decade as oocyte donation use has increased.
For all recommendations in this section, we use the term donor-conceived offspring, including both children, adolescents and adults. Counselling and information provision to children and adolescents ideally should involve the social parents. As recommended above, recipients should be provided with support and information on how they can talk age-appropriately with their offspring about their conception.
Recommendation.
Counselling should be offered for donor-conceived offspring who want to know more about what it means to be donor-conceived.
Information provision
Legislation on anonymity and donor identifiability
As discussed earlier, legislation varies across countries with regards to anonymity, identifiability of donors and restrictions on the number of offspring from the same donor. On restrictions on offspring number, there have been several news items as well as documented reports of large groups of same-donor offspring (Jadva et al., 2010).
Recommendations.
Donor-conceived offspring requesting information should be informed about what information they are able to access from the authorities and/or MAR centre/gamete bank in their jurisdiction.
Donor-conceived offspring should be informed about any donor registries in their country and how these function.
Donor-conceived offspring should be informed that legislation allowing donor anonymity may change prospectively and retrospectively.
Donor-conceived offspring born after the lifting of anonymity should be informed from an early age about the type and content of information which they can receive and at what age they are able to access it (this will vary across jurisdictions see above).
Besides being informed about the relevant regulations/legislation on the maximum number of same-donor offspring/families that they could be genetically related to, donor-conceived offspring should be informed that there is no guarantee that this number will not be surpassed.
Psychosocial aspects and psychosocial counselling
The psychosocial health of donor-conceived offspring
From early childhood to adolescence, donor-conceived children do not differ from children conceived naturally or from ART with own-gametes, as measured by several socio-emotional development indicators such as emotional symptoms, conduct problems, hyperactivity, prosocial behaviour and quality of peer relationships (Ilioi and Golombok, 2015; Golombok et al., 2017; Ilioi et al., 2017).
Being a donor-conceived offspring does not seem to pose particular obstacles in everyday life. Most donor-conceived offspring feel that third-party reproduction is an acceptable way of creating a family, particularly if the donor is identifiable and early disclosure is made by parents (Blyth et al., 2013). Zadeh et al. (2018) reported that even during adolescence donor-conceived children express no distress or concerns about the way they were conceived, but the results might have been different if most of these adolescents had not been told about their conception before the age of 7 years. Distress seems to be correlated with age of disclosure, as growing evidence suggests that donor-conceived children told about their conception in early childhood report more curiosity or indifference, less stress and higher adjustment compared with those whose families disclose later (Blyth et al., 2013; Blake et al., 2014; Ilioi et al., 2017). Low distress about being donor-conceived is also positively correlated with psychosocial wellbeing and high donor-conceived child–mother relationship quality (Golombok et al., 2017; Ilioi et al., 2017). Qualitative findings have indicated that one of the sources of distress in adolescents is caused by having to answer questions about donor conception from their peers (Van Parys et al., 2016; Zadeh et al., 2017, 2018).
Bos and Gartrell (2011) analysed differences, both in childhood and adolescence, between having a known and unknown donor in the psychosocial wellbeing of donor-conceived children of lesbian mothers over 7 years and found none.
Recommendations.
Counsellors should discuss with donor-conceived offspring, who requested counselling, the implications and consequences of revealing to others (e.g. peers) that they are donor-conceived. This should include how to deal with unwanted questioning, potential judgements others may make, difficulties others may find with discussing this in appropriate language, and that once a decision has been made to reveal this, it is not something that can be reversed.
Donor-conceived offspring should be signposted to available resources, which may include literature (books), websites, peer support groups, dedicated counsellors and/or organizations.
Perception of the relationship with parents
Using the Assisted Reproduction Families Study sample at ages 7 and 10 years, Blake et al. (2014) analysed the perceptions of donor-conceived offspring regarding their relationship with their parents. They reported no significant differences, compared with naturally-conceived children, in how the parents were represented in terms of caretaking, affection, harshness and anger. They also reported no significant differences regarding the amount of interests and activities shared with both their mothers and fathers (Blake et al., 2014). Similarly, no differences were found in adolescents’ perception of child–parent relationships (Ilioi and Golombok, 2015).
Disclosure
Early disclosure of conception is associated with a higher relationship quality with parents (Blake et al., 2014; Schrijvers et al., 2019), and donor-conceived offspring expressed the view that prospective parents should have counselling before treatment to prepare for disclosure so that parents could talk more openly about donor conception (Schrijvers et al., 2019). More information on disclosure is described above (Information for recipients).
Searching for and contacting genetically related people
Donor-conceived offspring can search for donors and same-donor offspring through the MAR centre/gamete bank, direct-to-consumer genetic testing websites, or through registries set up for this purpose. The existence of these organizations seems to be beneficial for some donor-conceived offspring, with positive feedback on searching and knowing that there are others in a similar situation to exchange experiences with (van den Akker et al., 2015; Schrijvers et al., 2019). Others (estimated at 30%) did not want to have peer contact (Schrijvers et al., 2019). About two-thirds (64%) of donor-conceived offspring registered to a US-based donor sibling registry indicated that they had searched for both their donor and same-donor offspring, with others replying they searched specifically for their donor (15%), same-donor offspring (13%), or neither (Jadva et al., 2010).
In a recent study, all of the donor-conceived offspring reported a wish to know where to find a specialist counsellor in case of need, particularly to offer assistance with how to find information about the donor and how to search for their donor and same-donor offspring (Schrijvers et al., 2019).
Reasons for searching the donor
Curiosity about physical appearance and trait resemblances with the donor have been the most common reasons given by donor-conceived offspring for searching for their donor (Beeson et al., 2011; van den Akker et al., 2015; Slutsky et al., 2016). Curiosity seems to increase with age in general and is higher in those who have learned about their donor conception at an older age (Blyth et al., 2012; Hertz et al., 2013). Other reasons for searching for the donor included wanting to learn about their medical history and ancestry and wanting to establish a relationship with the donor. The reasons for searching for the donor vary according to the composition of the family, with differences observed between heterosexual and same-sex parents, and between single or two-parent families (Beeson et al., 2011; Hertz et al., 2013) with, for example, a more pronounced interest in sperm donors in families without a father (Freeman et al., 2014).
With regards to wanting to establish a relationship with the donor, qualitative findings show that donor-conceived offspring most often want to establish a cordial or friendly relationship (Cushing, 2010; Zadeh, 2016), with only a small percentage (6.9%) reporting a desire to establish a father-child relationship (Hewitt, 2002; Scheib et al., 2005).
One of the few studies with a sample of donor-conceived offspring through oocyte donation and surrogacy concluded that adolescents search for their donors to learn about their motivation to donate, and about the donor’s family (Zadeh et al., 2018). A study in younger children (aged <6 years) with gay fathers reported that these children seem to have a clear understanding of the procedures taken to enable conception and showed more interest and gratefulness towards the surrogate than the oocyte donor, when informed of the latter (Carone et al., 2018). The researchers stated this can be justified by a more proximal relationship with the surrogate and might change in time as curiosity tends to increase with age.
Donor-conceived offspring rated all types of information about the donor, including photographs, education, hobbies or religion, as important (Rodino et al., 2011). When forced to make a choice, donor-conceived offspring ranked name first, followed by health and information about the donor’s family. In most countries where anonymity has been lifted, it is possible to find out the name and some health information to avoid medical risks and consanguineous relationships (Ravelingien et al., 2015a).
When asked about the three most important pieces of information to give to future donors, almost half of donor-conceived offspring recommended that donors should make themselves known, one-quarter reported that they should take responsibility for their donation, and one-fifth think it is important that donors know that future offspring might want to contact them (Hertz et al., 2013). Donor-conceived children with single mothers mentioned more often, compared to those with two-parent families, that donors should know that they have children (Beeson et al., 2011).
Recommendations.
Donor-conceived offspring should be informed about any donor registries that are relevant to their situation and jurisdiction.
As reasons for wanting to search for donors change over the life course and vary according to the type of family, the child’s age and the age at which s/he learned about being donor-conceived, counselling should be available at all ages.
Donor-conceived offspring should be informed that the information that will be available to them in the future may not match their expectations and that this information may have changed since being provided by the donor.
Establishing contact with the donor
Exact numbers on how many donor-conceived offspring have established contact with their donor are not available. In one study, around a third of donor-conceived adults requested the donor’s identity (allowed at 18 years of age), and 75% of these completed the process (Scheib et al., 2017). The majority of requests were made immediately after reaching the legal age. Three-quarters of the offspring who completed the request process expressed a wish to contact the donor and most received a positive reply. Of those who had approval from the donor to be contacted, expectations of establishing a relationship were low or non-existent. Another study followed a cohort of lesbian-parent offspring (n = 76) for more than 30 years (Koh et al., 2020). At 25 years of age, around 40% had unknown sperm donors (n = 30), with most reported feeling neutral or comfortable with this (n = 22). Of the remaining 46 offspring, 22 had always known the identity of their donor, and 24 had an open-identity regime: of the latter group, only eight had actually met their donors. Of the ones that did make contact with their donors (n = 30), half of them considered them as acquaintances, while nearly all others considered themselves close to the donor. A small faction expressed discomfort or conflicted feelings towards the sperm donor (Koh et al., 2020).
In effect, most studies focusing on the relationship between donor-conceived offspring and donors from the offspring’s perspective seem to describe positive perceptions of donors, both from adolescents (Jadva et al., 2010; Zadeh et al., 2018) or adults (e.g. Goldberg and Allen, 2013; Freeman, 2015; van den Akker et al., 2015).
Relationships are described as friendships (Beeson et al., 2011) or ‘extended family members’ (Goldberg and Allen, 2013), in line with the intentions of donor-conceived offspring (Cushing, 2010; Zadeh, 2016). Regarding family types of sperm donor offspring, there is both evidence pointing towards no differences (Beeson et al., 2011), and a perception of less positive relationships by those who do not have an obvious lack of genetic parenthood (heterosexual parent families) than by those who have (lesbian and single-parent families) (Jadva et al., 2010). Contact is mostly described as regular but not frequent (e.g. a few times a year) (van den Akker et al., 2015).
With regards to offspring-donor relationships, a considerable minority of linked donor-conceived adults reported some negative consequences with ambivalent emotions (van den Akker et al., 2015) or indicated that they still desired more information or contact even though they were satisfied with the current relationships (Goldberg and Allen, 2013). Lack of communication regarding the parties’ expectations and boundaries seems to be one of the main risk factors (Freeman, 2015).
There is a minority of cases in which donors say that they are not open to contact, hence creating feelings of disappointment and distress (Scheib et al., 2017). Disappointment can also arise for those who want limited information or occasional contact that does not correspond to what the donor wants (Freeman et al., 2014). In registries set up to facilitate contact, one study found that there was a significant number of donor-conceived offspring registrants that had not yet been linked. This can lead to anxiety and a greater propensity to report uncertainty about the impact of contact on themselves and significant others (van den Akker et al., 2015).
So far, almost all the available evidence refers to relationships with previously unknown sperm donors who provided no or little information to MAR centres/gamete banks at the time of donation. Studies on the long-term psychosocial effects of donor-offspring relationships with their donor do not, as yet, exist.
Recommendations.
MAR centres/gamete banks should be prepared to manage the requests of donor-conceived offspring to access information about their donors, according to national legislation.
Where donor-linking counselling services from national registries are available, these should be involved in facilitating the donor/offspring contact. When not available, the mediating MAR centres/gamete banks should have relevant support structures available.
Donor-conceived offspring should be informed that they can access available information and still choose not to contact the donor.
Recipients and donor-conceived offspring with anonymous donors should be encouraged to consider that the donor may not expect to be found and will not have had counselling to prepare them.
If attempting to contact the donor, donor-conceived offspring should be prepared for different outcomes, including that their donor may not be open to contact or may not have the same expectations in terms of establishing a relationship. If donor-conceived offspring are able to contact the donor (and start a relationship), counselling should be available so they can discuss their expectations and inclusion of the donor in their lives.
Donor-conceived offspring should be informed that some people report negative experiences when establishing contact with their donors.
Searching and contact with same-donor offspring and other relatives
The available evidence regarding frequency and nature of contact established between same-donor offspring with no previous knowledge of each other’s existence is similar to the evidence regarding contact with donors. Most donor-conceived offspring are curious to know other donor-conceived offspring that share genetic material (Zadeh et al., 2018), and most report positive relationships (Scheib and Ruby, 2008; Jadva et al., 2010; Blyth, 2012). Also like the evidence available on contact with donors, the ones who cannot find any same-donor offspring experience frustration and disillusionment (Cushing, 2010). Of those who report neutral or negative experiences, there is a greater proportion from heterosexual and single-parent families compared to lesbian parent families (Jadva et al., 2010). The possible differences between family types (parents, presence of social siblings) have not been addressed in comparative studies and warrants further investigation.
Blyth interviewed eight offspring from the same sperm donor with ages ranging from 44 to 65 years who had been in contact with each other for several years. Their narratives illustrated that there seems to be a journey of discovering the relationship between genetic and social ties (Blyth, 2012). All participants started by trying to look for similarities in physical as well as behavioural characteristics, with some sharing physical resemblance and others not identifying any shared characteristics. Participants recognized some shared behavioural characteristics but were unsure that these could be entirely attributed to genes.
Recommendations.
Donor-conceived offspring should be informed about the implications of using direct-to-consumer genetic testing.
Donor-conceived offspring should be counselled regarding their expectations and inclusion of same-donor offspring in their lives.
Donor-conceived offspring should be informed before revealing their identity that this is an irreversible decision.
Donor-conceived offspring should be informed of the possibility of finding many same-donor offspring or none.
Donor-conceived offspring should be informed of the possibility of identifying similarities between themselves and same-donor offspring regarding physical or behavioural characteristics, but should also be aware they may find no similarities.
Donor-conceived offspring should understand that, like any other personal relationship, when meeting same donor offspring there are no guarantees of forming a good relationship.
Contact and the impact on family relationships
Jadva et al. (2010) examined the impact of trying to contact donors on the parent–child relationship from the perspective of donor-conceived offspring. Most sperm donor-conceived adolescents and adults told their mothers (80%) that they were searching for their sperm donor or same-donor offspring. However, only around a quarter of those with heterosexual parents told their fathers, compared to almost 65% of those living with lesbian mothers. Regardless of family type, most adolescents/adults reported that the search for the donor and same-donor offspring had either a neutral impact or a mix of positive and negative consequences on the relationship with their mothers. While values were similar regarding the impact of the search for their donor on the relationship with mothers, the percentage of the ones who reported a positive impact in the relationship with their father or co-parent was higher in lesbian parents’ families than in heterosexual families. The percentage of ones who reported a positive impact of the search for same-donor offspring seems to be higher in lesbian parents’ families than in heterosexual and single-mother families, both for the mothers and the fathers or co-parents (excluding single-mother families in this last comparison). These findings were corroborated by other results describing concerns from donor-conceived offspring regarding how the parent with no genetic connection would be affected (Zadeh et al., 2018), but also perceptions that s/he would be less supportive of the process of searching (Beeson et al., 2011). Despite this, disclosure or contact with the donor by donor-conceived offspring does not seem to affect the existing family relationships (van den Akker et al., 2015).
According to donor-conceived offspring, counselling offered to prospective parents using third-party reproduction should offer reassurance that contact between the child and the donor generally does not disrupt the parent–child relationships (33%) and that offspring should be the ones deciding whether or not to initiate that contact (28%) (Hertz et al., 2013).
Recommendations.
Counsellors should discuss with donor-conceived offspring requesting support the implications and consequences of telling or not telling parents and other relatives (e.g. siblings) about their search for the donor or same-donor offspring, including the parent to which there is no genetic connection.
Parents and donor-conceived offspring should be able to access counselling before organizing contact with donor (or the donor’s family).
Donor-conceived offspring should be able to access counselling when considering trying to find donors and/or siblings through direct-to-consumer genetic testing.
Need for further research
Given the closed (secretive) way in which donor conception has been practised and developed historically, the slow development of an evidence base on which future practice and policy could be built was inevitable. Much of the available research evidence concerns individuals conceived through sperm donation conducted under a regime that promoted both anonymity and non-disclosure. Consequently, there is much less research that pertains to those conceived through other forms of collaborative reproduction, such as oocyte donation, embryo donation, or surrogacy, and on late disclosure. Furthermore, there is little adequate research on those conceived under regimes in which early parental disclosure is both advocated and practised, where the identity of the donor is known to the recipient from the outset (as in oocyte donation between friends or family members), and/or regarding donor-conceived people’s interests in learning about genetic relatives other than their donor. Furthermore, few studies have provided a longer-term perspective on individuals’ experiences and perceptions as they develop throughout their lives.
In addition, one should consider that the available data compiled in this paper are largely context-specific, often based on surveys or studies that had small numbers of participants (from Western populations). Specifically, with regards to disclosure, much of the evidence is specific to heterosexual couples and these findings cannot be generalizable to non-heterosexual couples. There is also a lack of longitudinal studies on a range of different populations.
Those seeking to shape policy in this area have noted their reliance on speculation in the absence of research on donor-conceived people’s views, and there have been calls for long-term follow-up studies since the 1970s. In light of the limitations of current ‘formal’ research and the evidence gaps highlighted above, future research could usefully include lessons from the grey literature, and an increased emphasis on theory and the values/principles underpinning policies and regulations. The development of a wider and more theoretically informed evidence base would be invaluable for both policymakers and practitioners.
Recommendation.
More research is needed into the long-term psychosocial aspects of forming families through donated gametes and embryos, examining a range of family types and different cultural and social contexts of reproductive donation.
Conclusion
The collected findings and recommendations in this document provide, for the first time, a roadmap for the global fertility sector in the minimum requirements for information that should be provided to all parties involved in reproductive donation.
We hope that the recommendations will enable donors, recipients, and donor-conceived offspring to be as well-informed as possible and experience donor-assisted conception positively. Direct-to-consumer genetic testing and social media platforms are evolving at an exponentially faster rate than either our psychosocial understandings of the implications for donor conception or national legal systems’ or regulatory bodies’ capacity to respond. Therefore, providing opportunities for all parties in donor conception to have access to counselling and the current best evidence and information is critical.
The recommendations outlined in this document should also be considered by regulatory bodies and policymakers at a national and international level and are crucial to guiding future regulatory and legislative efforts towards ensuring minimum standards for the ethical and safe practice of donor conception.
Data availability
All available data are included in the paper, its supplementary data and the review report available on www.eshre.eu/guidelines.
Supplementary Material
Acknowledgements
The authors would like to thank Paul Devroey for his input towards the project and the experts that participated in the stakeholder review for their constructive and helpful comments.
Authors’ roles
The working group was chaired by J.K.-B. and L.F. N.V. provided methodological support. All other authors contributed equally to writing the recommendations. All authors have revised and approved the final version.
Funding
The development of this paper was funded by ESHRE, covering expenses associated with the working group meetings, the literature searches and dissemination. The working group members did not receive payment.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Acharya S, Bryant L, Twiddy M.. Altruism or obligation? The motivations and experience of women who donate oocytes to known recipients in assisted conception treatment: an interpretative phenomenological analysis study. J Psychosom Obstet Gynaecol 2017;38:4–11. [DOI] [PubMed] [Google Scholar]
- Adams DH, Ullah S, de Lacey S.. Does the removal of anonymity reduce sperm donors in Australia? J Law Med 2016;23:628–636. [PubMed] [Google Scholar]
- Ahuja KK, Simons EG, Mostyn BJ, Bowen-Simpkins P.. An assessment of the motives and morals of egg share donors: policy of ‘payments’ to egg donors requires a fair review. Hum Reprod 1998;13:2671–2678. [DOI] [PubMed] [Google Scholar]
- Amor DJ, Kerr A, Somanathan N, McEwen A, Tome M, Hodgson J, Lewis S.. Attitudes of sperm, egg and embryo donors and recipients towards genetic information and screening of donors. Reprod Health 2018;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegarth LD, Kaufman NL, Josephs-Sohan M, Christos PJ, Rosenwaks Z.. Parental disclosure to offspring created with oocyte donation: intentions versus reality. Hum Reprod 2016;31:1809–1815. [DOI] [PubMed] [Google Scholar]
- Bass C, Gregorio J.. Conflicts of interest for physicians treating egg donors. Virtual Mentor 2014;16:822–826. [DOI] [PubMed] [Google Scholar]
- Bay B, Larsen PB, Kesmodel US, Ingerslev HJ.. Danish sperm donors across three decades: motivations and attitudes. Fertil Steril 2014;101:252–257.e1. [DOI] [PubMed] [Google Scholar]
- Baylis F, Widdows H.. Human embryos and eggs: from long-term storage to biobanking. Monash Bioeth Rev 2015;33:340–359. [DOI] [PubMed] [Google Scholar]
- Beeson DR, Jennings PK, Kramer W.. Offspring searching for their sperm donors: how family type shapes the process. Hum Reprod 2011;26:2415–2424. [DOI] [PubMed] [Google Scholar]
- Berntsen S, Söderström-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A.. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update 2019;25:137–158. [DOI] [PubMed] [Google Scholar]
- Black JJ. Egg donation: issues & concerns. MCN Am J Matern Child Nurs 2010;35:132–137; quiz 137–139. [DOI] [PubMed] [Google Scholar]
- Blake L, Carone N, Slutsky J, Raffanello E, Ehrhardt AA, Golombok S.. Gay father surrogacy families: relationships with surrogates and egg donors and parental disclosure of children's origins. Fertil Steril 2016;106:1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake L, Jadva V, Golombok S.. Parent psychological adjustment, donor conception and disclosure: a follow-up over 10 years. Hum Reprod 2014;29:2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth E. Genes r us? Making sense of genetic and non-genetic relationships following anonymous donor insemination. Reprod Biomed Online 2012;24:719–726. [DOI] [PubMed] [Google Scholar]
- Blyth E, Crawshaw M, Frith L, Jones C.. Donor-conceived people's views and experiences of their genetic origins: a critical analysis of the research evidence. J Law Med 2012;19:769–789. [PubMed] [Google Scholar]
- Blyth E, Crawshaw M, Frith L, van den Akker O.. Gamete donors' reasons for, and expectations and experiences of, registration with a voluntary donor linking register. Hum Fertil (Camb) 2017;20:268–278. [DOI] [PubMed] [Google Scholar]
- Blyth E, Farrand A.. Anonymity in donor-assisted conception and the UN Convention on the Rights of the Child. Int J Child Rights 2004;12:89–104. [Google Scholar]
- Blyth E, Frith L.. Access to genetic and biographical history in donor conception: an analysis of recent trends and future possibilities. In: K. Horsey (ed), Revisiting the Regulation of Human Fertilisation and Embryology Biomedical Law and Ethics Library. London, UK: Routledge, 2015, 136–152. [Google Scholar]
- Blyth E, Kramer W, Schneider J.. Perspectives, experiences, and choices of parents of children conceived following oocyte donation. Reprod Biomed Online 2013;26:179–188. [DOI] [PubMed] [Google Scholar]
- Bolt S, Postema D, van der Heij A, Bmm AJ.. Anonymous Dutch sperm donors releasing their identity. Hum Fertil (Camb) 2021;24:24–30. [DOI] [PubMed] [Google Scholar]
- Borgstrom MB, Nygaard SS, Danielsen AK, Kesmodel US.. Exploring motivations, attitudes and experiences of oocyte donors: a qualitative study. Acta Obstet Gynecol Scand 2019;98:1055–1062. [DOI] [PubMed] [Google Scholar]
- Bos HM, Gartrell NK.. Adolescents of the US National Longitudinal Lesbian Family Study: the impact of having a known or an unknown donor on the stability of psychological adjustment. Hum Reprod 2011;26:630–637. [DOI] [PubMed] [Google Scholar]
- Bracewell-Milnes T, Saso S, Abdalla H, Thum MY.. A systematic review investigating psychosocial aspects of egg sharing in the United Kingdom and their potential effects on egg donation numbers. Hum Fertil (Camb) 2018;21:163–173. [DOI] [PubMed] [Google Scholar]
- Bracewell-Milnes T, Saso S, Bora S, Ismail AM, Al-Memar M, Hamed AH, Abdalla H, Thum MY.. Investigating psychosocial attitudes, motivations and experiences of oocyte donors, recipients and egg sharers: a systematic review. Hum Reprod Update 2016;22:450–465. [DOI] [PubMed] [Google Scholar]
- Burrell R. The first years of the Finnish Act on Assisted Fertility Treatments—observations from the viewpoint of a supervisory authority. Med Law 2012;31:473–489. [PubMed] [Google Scholar]
- Calhaz-Jorge C, De Geyter CH, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V.. Survey on ART and IUI: legislation, regulation, funding and registries in European countries: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod Open 2020;2020:hoz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone N, Baiocco R, Manzi D, Antoniucci C, Caricato V, Pagliarulo E, Lingiardi V.. Surrogacy families headed by gay men: relationships with surrogates and egg donors, fathers' decisions over disclosure and children's views on their surrogacy origins. Hum Reprod 2018;33:248–257. [DOI] [PubMed] [Google Scholar]
- Cattapan AR. Good eggs? Evaluating consent forms for egg donation. J Med Ethics 2016;42:455–459. [DOI] [PubMed] [Google Scholar]
- Cohen CB. New Ways of Making Babies: The Case of Egg Donation. Bioethics1996;12:86–87. [Google Scholar]
- Cohen G, Coan T, Ottey M, Boyd C.. Sperm donor anonymity and compensation: an experiment with American sperm donors. J Law Biosci 2016;3:468–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier C, Ducrocq B, Fry J, Catteau-Jonard S.. Views of French oocyte donors at least 3 years after donation. Reprod Biomed Online 2020;40:819–826. [DOI] [PubMed] [Google Scholar]
- Crawshaw M. Direct-to-consumer DNA testing: the fallout for individuals and their families unexpectedly learning of their donor conception origins. Hum Fertil (Camb) 2018;21:225–228. [DOI] [PubMed] [Google Scholar]
- Crawshaw M, Montuschi O.. It ‘did what it said on the tin’—participant's views of the content and process of donor conception parenthood preparation workshops. Hum Fertil (Camb) 2014;17:11–20. [DOI] [PubMed] [Google Scholar]
- Crawshaw MA, Blyth ED, Daniels KD.. Past semen donors' views about the use of a voluntary contact register. Reprod Biomed Online 2007;14:411–417. [DOI] [PubMed] [Google Scholar]
- Cushing AL. I just want more information about who I am’: the search experience of sperm-donor offspring, searching for information about their donors and genetic heritage. Information Research 2010;15:15–12. [Google Scholar]
- Daniels KR, Kramer W, Perez-y-Perez MV.. Semen donors who are open to contact with their offspring: issues and implications for them and their families. Reprod Biomed Online 2012;25:670–677. [DOI] [PubMed] [Google Scholar]
- de Melo-Martin I. How best to protect the vital interests of donor-conceived individuals: prohibiting or mandating anonymity in gamete donations? Reprod Biomed Soc Online 2016;3:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deech R. Legal and ethical responsibilities of gamete banks. Hum Reprod 1998;13(Suppl 2):80–83; discussion 84–89. [DOI] [PubMed] [Google Scholar]
- Dillon KE, Fiester AM.. Sperm and oocyte cryopreservation: comprehensive consent and the protection of patient autonomy. Hum Reprod 2012;27:2894–2898. [DOI] [PubMed] [Google Scholar]
- Dondorp W, De Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, Barri P, Diedrich K, Eichenlaub-Ritter U, Tüttelmann F. et al. ESHRE Task Force on Ethics and Law 21: genetic screening of gamete donors: ethical issues. Hum Reprod 2014;29:1353–1359. [DOI] [PubMed] [Google Scholar]
- Donor Conceived Register. 2004. https://www.donorconceivedregister.co.uk/ (31 January 2022, date last accessed).
- Ethics Committee of the American Society for Reproductive Medicine. Financial incentives in recruitment of oocyte donors. Fertil Steril 2004;82:240–244. [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Interests, obligations, and rights of the donor in gamete donation. Fertil Steril 2009;91:22–27. [DOI] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Informing offspring of their conception by gamete or embryo donation: an Ethics Committee opinion. Fertil Steril 2018;109:601–605. [DOI] [PubMed] [Google Scholar]
- European Commission. COMMISSION DIRECTIVE 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Official Journal of the European Union2006;9.2.2006 L38:40–52.
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Donation of Oocytes: A Guide for Women to Support Informed Decisions. 2018. www.edqm.eu (31 January 2022, date last accessed).
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th edn. European Committee (Partial Agreement) on Organ Transplantation (CD-P-TO; ), 2019. www.edqm.eu (31 January 2022, date last accessed). [Google Scholar]
- European Parliament and Council of the European Union. DIRECTIVE 2004/23/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Official Journal of the European Union2004;7.4.2004 L102:48–58.
- Freeman T. Gamete donation, information sharing and the best interests of the child: an overview of the psychosocial evidence. Monash Bioeth Rev 2015;33:45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T, Bourne K, Jadva V, Smith V.. Making connections: contact between sperm donor relations. In: Ebtehaj F, Richards M, Graham S, Freeman T (eds). Relatedness in Assisted Reproduction: Families, Origins and Identities. Cambridge: Cambridge University Press, 2014, 270–295. [Google Scholar]
- Freeman T, Zadeh S, Smith V, Golombok S.. Disclosure of sperm donation: a comparison between solo mother and two-parent families with identifiable donors. Reprod Biomed Online 2016;33:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith L. The limits of evidence: evidence based policy and the removal of gamete donor anonymity in the UK. Monash Bioeth Rev 2015;33:29–44. [DOI] [PubMed] [Google Scholar]
- Frith L, Blyth E, Crawshaw M, van den Akker O.. Secrets and disclosure in donor conception. Sociol Health Illn 2018;40:188–203. [DOI] [PubMed] [Google Scholar]
- Frith L, Blyth E, Lui S.. Family building using embryo adoption: relationships and contact arrangements between provider and recipient families-a mixed-methods study. Hum Reprod 2017;32:1092–1099. [DOI] [PubMed] [Google Scholar]
- Frith L, Blyth E, Paul MS, Berger R.. Conditional embryo relinquishment: choosing to relinquish embryos for family-building through a Christian embryo ‘adoption’ programme. Hum Reprod 2011;26:3327–3338. [DOI] [PubMed] [Google Scholar]
- Gebhardt AJ, Sydsjo G, Skoog Svanberg A, Indekeu A, Lampic C.. Parenting stress and its association with perceived agreement about the disclosure decision in parents following donor conception. Acta Obstet Gynecol Scand 2017;96:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman L. Toxic money or paid altruism: the meaning of payments for identity-release gamete donors. Sociol Health Illn 2018;40:702–717. [DOI] [PubMed] [Google Scholar]
- Glennon T. 3 Legal regulation of family creation through gamete donation. In: S. Golombok, R. Scott, JB. Appleby, M. Richards, S. Wilkinson (eds), Regulating Reproductive Donation. Cambridge (UK): Cambridge University Press, 2016, 60. [Google Scholar]
- Goedeke S, Daniels K.. We wanted to choose us: how embryo donors choose recipients for their surplus embryos. J Reprod Infant Psychol 2018;36:132–143. [DOI] [PubMed] [Google Scholar]
- Goedeke S, Daniels K, Thorpe M, Du Preez E.. Building extended families through embryo donation: the experiences of donors and recipients. Hum Reprod 2015;30:2340–2350. [DOI] [PubMed] [Google Scholar]
- Goldberg AE, Allen KR.. Donor, dad, or…? Young adults with lesbian parents' experiences with known donors. Fam Process 2013;52:338–350. [DOI] [PubMed] [Google Scholar]
- Goldberg AE, Scheib JE.. Female-partnered and single women's contact motivations and experiences with donor-linked families. Hum Reprod 2015;30:1375–1385. [DOI] [PubMed] [Google Scholar]
- Golombok S. Disclosure and donor-conceived children. Hum Reprod 2017;32:1532–1536. [DOI] [PubMed] [Google Scholar]
- Golombok S, Ilioi E, Blake L, Roman G, Jadva V.. A longitudinal study of families formed through reproductive donation: parent-adolescent relationships and adolescent adjustment at age 14. Dev Psychol 2017;53:1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo J, Perul M, Corral M, Caballero M, Conti C, García D, Vassena R, Rodríguez A.. A follow-up study of the long-term satisfaction, reproductive experiences, and self-reported health status of oocyte donors in Spain. Eur J Contracept Reprod Health Care 2019;24:227–232. [DOI] [PubMed] [Google Scholar]
- Graham S, Jadva V, Freeman T, Ahuja K, Golombok S.. Being an identity-release donor: a qualitative study exploring the motivations, experiences and future expectations of current UK egg donors. Hum Fertil (Camb) 2016;19:230–241. [DOI] [PubMed] [Google Scholar]
- Greenfeld DA. Effects and outcomes of third-party reproduction: parents. Fertil Steril 2015;104:520–524. [DOI] [PubMed] [Google Scholar]
- Hadizadeh-Talasaz F, Roudsari RL, Simbar M.. Decision for disclosure: the experiences of Iranian infertile couples undergoing assisted reproductive donation procedures. Hum Fertil (Camb) 2015;18:265–275. [DOI] [PubMed] [Google Scholar]
- Hammond K. The role of normative ideologies of motherhood in intended mothers' experiences of egg donation in Canada. Anthropol Med 2018;25:265–279. [DOI] [PubMed] [Google Scholar]
- Hard DA. Artificial impregnation. Medical World 1909;27:163–164. [Google Scholar]
- Harper JC, Kennett D, Reisel D.. The end of donor anonymity: how genetic testing is likely to drive anonymous gamete donation out of business. Hum Reprod 2016;31:1135–1140. [DOI] [PubMed] [Google Scholar]
- Haskovic M, Poot WJ, van Golde RJT, Benneheij SH, Oussoren E, de Wert G, Krumeich A, Rubio-Gozalbo ME.. Intrafamilial oocyte donation in classic galactosemia: ethical and societal aspects. J Inherit Metab Dis 2018;41:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks S, Peeraer K, Bos H, Repping S, Dancet EAF.. The importance of genetic parenthood for infertile men and women. Hum Reprod 2017;32:2076–2087. [DOI] [PubMed] [Google Scholar]
- Hendriks S, van Wely M, D'Hooghe TM, Meissner A, Mol F, Peeraer K, Repping S, Dancet EAF.. The relative importance of genetic parenthood. Reprod Biomed Online 2019;39:103–110. [DOI] [PubMed] [Google Scholar]
- Hershberger PE, Driessnack M, Kavanaugh K, Klock SC.. Oocyte donation disclosure decisions: a longitudinal follow-up at middle childhood. Hum Fertil (Camb) 2021;24:31–45. [DOI] [PubMed] [Google Scholar]
- Hertz R, Nelson MK.. Acceptance and Disclosure: Comparing genetic symmetry and genetic asymmetry in heterosexual couples between egg recipients and embryo recipients. Facts Views Vis Obgyn 2016;8:11–22. [PMC free article] [PubMed] [Google Scholar]
- Hertz R, Nelson MK, Kramer W.. Donor conceived offspring conceive of the donor: the relevance of age, awareness, and family form. Soc Sci Med 2013;86:52–65. [DOI] [PubMed] [Google Scholar]
- Hertz R, Nelson MK, Sunol J.. Attitudes toward regulations of reproductive care in the European Union: a comparison between travellers for cross-border reproductive care and citizens of the local country. Facts Views Vis Obgyn 2016;8:147–160. [PMC free article] [PubMed] [Google Scholar]
- Hewitt G. Missing links: identity issues of donor conceived people. J Fertil Counsell 2002;9:14–19. [Google Scholar]
- Human Fertilisation and Embryology Act. Human Fertilisation and Embryology Act 1990. UK Legislation, Chapter 37 (Passed 1 November 1990). 1990. http://wwwlegislationgovuk/ukpga/1990/37/contents (26 April 2021, date last accessed).
- Ilioi E, Blake L, Jadva V, Roman G, Golombok S.. The role of age of disclosure of biological origins in the psychological wellbeing of adolescents conceived by reproductive donation: a longitudinal study from age 1 to age 14. J Child Psychol Psychiatry 2017;58:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilioi EC, Golombok S.. Psychological adjustment in adolescents conceived by assisted reproduction techniques: a systematic review. Hum Reprod Update 2015;21:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrie S, Golombok S.. Long-term outcomes of children conceived through egg donation and their parents: a review of the literature. Fertil Steril 2018;110:1187–1193. [DOI] [PubMed] [Google Scholar]
- Imrie S, Jadva V, Golombok S.. "Making the child mine": Mothers' thoughts and feelings about the mother-infant relationship in egg donation families. J Fam Psychol 2020;34:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indekeu A, D'Hooghe T, Daniels KR, Dierickx K, Rober P.. ‘Of course he’s our child': transitions in social parenthood in donor sperm recipient families. Reprod Biomed Online 2014;28:106–115. [DOI] [PubMed] [Google Scholar]
- Indekeu A, Lampic C.. The interaction between donor-conceived families and their environment: parents' perceptions of societal understanding and attitudes regarding their family-building. Hum Fertil (Camb) 2021;24:14–23. [DOI] [PubMed] [Google Scholar]
- Inhorn MC, Gurtin ZB.. Cross-border reproductive care: a future research agenda. Reprod Biomed Online 2011;23:665–676. [DOI] [PubMed] [Google Scholar]
- International Federation of Fertility Societies. International Federation of Fertility Societies’ Surveillance (IFFS) 2019: Global Trends in Reproductive Policy and Practice, 8th Edition. Glob Reprod Health 2019;4:e29. [Google Scholar]
- Isaksson S, Skoog-Svanberg A, Sydsjo G, Linell L, Lampic C.. It takes two to tango: information-sharing with offspring among heterosexual parents following identity-release sperm donation. Hum Reprod 2016;31:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson S, Sydsjo G, Skoog Svanberg A, Lampic C.. Preferences and needs regarding future contact with donation offspring among identity-release gamete donors: results from the Swedish Study on Gamete Donation. Fertil Steril 2014;102:1160–1166. [DOI] [PubMed] [Google Scholar]
- Jadva V, Blake L, Casey P, Golombok S.. Surrogacy families 10 years on: relationship with the surrogate, decisions over disclosure and children's understanding of their surrogacy origins. Hum Reprod 2012;27:3008–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadva V, Freeman T, Kramer W, Golombok S.. The experiences of adolescents and adults conceived by sperm donation: comparisons by age of disclosure and family type. Hum Reprod 2009;24:1909–1919. [DOI] [PubMed] [Google Scholar]
- Jadva V, Freeman T, Kramer W, Golombok S.. Experiences of offspring searching for and contacting their donor siblings and donor. Reprod Biomed Online 2010;20:523–532. [DOI] [PubMed] [Google Scholar]
- Jadva V, Freeman T, Tranfield E, Golombok S.. Why search for a sperm donor online? The experiences of women searching for and contacting sperm donors on the internet. Hum Fertil (Camb) 2018;21:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens PM, Thorn P, Castilla JA, Frith L, Crawshaw M, Mochtar M, Bjorndahl L, Kvist U, Kirkman-Brown JC.. Evolving minimum standards in responsible international sperm donor offspring quota. Reprod Biomed Online 2015;30:568–580. [DOI] [PubMed] [Google Scholar]
- Janzen T, Nolte AW, Traulsen A.. The breakdown of genomic ancestry blocks in hybrid lineages given a finite number of recombination sites. Evolution 2018;72:735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalampalikis N, Doumergue M, Zadeh S; French Federation of CECOS. Sperm donor regulation and disclosure intentions: Results from a nationwide multi-centre study in France. Reprod Biomed Soc Online 2018;5:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman M, Bourne K, Fisher J, Johnson L, Hammarberg K.. Gamete donors' expectations and experiences of contact with their donor offspring. Hum Reprod 2014;29:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein S, Chen A, Samplaski M.. The effect of loss of anonymity from direct-to-consumer DNA databases on sperm donation attitudes and practices of American sperm donors. J Urol 2020;204:1125–1126. [DOI] [PubMed] [Google Scholar]
- Klotz M. Wayward relations: novel searches of the donor-conceived for genetic kinship. Med Anthropol 2016;35:45–57. [DOI] [PubMed] [Google Scholar]
- Koh AS, van Beusekom G, Gartrell NK, Bos H.. Adult offspring of lesbian parents: how do they relate to their sperm donors? Fertil Steril 2020;114:879–887. [DOI] [PubMed] [Google Scholar]
- Kool EM, Bos AME, van der Graaf R, Fauser B, Bredenoord AL.. Ethics of oocyte banking for third-party assisted reproduction: a systematic review. Hum Reprod Update 2018;24:615–635. [DOI] [PubMed] [Google Scholar]
- Kovacs GT, Wise S, Finch S.. Keeping a child's donor sperm conception secret is not linked to family and child functioning during middle childhood: an Australian comparative study. Aust N Z J Obstet Gynaecol 2015;55:390–396. [DOI] [PubMed] [Google Scholar]
- Lampic C, Skoog Svanberg A, Sydsjo G.. Attitudes towards disclosure and relationship to donor offspring among a national cohort of identity-release oocyte and sperm donors. Hum Reprod 2014;29:1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle C, Place I, Demeestere I, Englert Y, Delbaere A.. Anonymity and secrecy options of recipient couples and donors, and ethnic origin influence in three types of oocyte donation. Hum Reprod 2011;26:382–390. [DOI] [PubMed] [Google Scholar]
- Lassalzede T, Paci M, Rouzier J, Carez S, Gnisci A, Saias-Magnan J, Deveze C, Perrin J, Metzler-Guillemain C.. Sperm donor conception and disclosure to children: a 10-year retrospective follow-up study of parental attitudes in one French center for the study and preservation of eggs and sperm (CECOS). Fertil Steril 2017;108:247–253. [DOI] [PubMed] [Google Scholar]
- Lingiardi V, Carone N, Morelli M, Baiocco R.. ‘It’s a bit too much fathering this seed': the meaning-making of the sperm donor in Italian lesbian mother families. Reprod Biomed Online 2016;33:412–424. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Wantman E, Stern JE, Baker VL, Widra E, Coddington CC 3rd, Gibbons WE, Ball GD.. A prediction model for live birth and multiple births within the first three cycles of assisted reproductive technology. Fertil Steril 2014;102:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahieu F, Decleer W, Osmanagaoglu K, Provoost V.. Anonymous sperm donors' attitude towards donation and the release of identifying information. J Assist Reprod Genet 2019;36:2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas M, Sunkara SK, Antonisamy B, Kamath MS.. Higher risk of preterm birth and low birth weight following oocyte donation: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2017;218:60–67. [DOI] [PubMed] [Google Scholar]
- McGovern PG, Schlaff WD.. Sperm donor anonymity: a concept rendered obsolete by modern technology. Fertil Steril 2018;109:230–231. [DOI] [PubMed] [Google Scholar]
- Mertes H, Lindheim SR, Pennings G.. Ethical quandaries around expanded carrier screening in third-party reproduction. Fertil Steril 2018;109:190–194. [DOI] [PubMed] [Google Scholar]
- Miettinen A, Rotkirch A, Suikkari A-M, Söderström-Anttila V.. Attitudes of anonymous and identity-release oocyte donors towards future contact with donor offspring. Hum Reprod 2019;34:672–678. [DOI] [PubMed] [Google Scholar]
- Millbank J. Numerical limits in donor conception regimes: genetic links and ‘extended family’ in the era of identity disclosure. Med Law Rev 2014;22:325–356. [DOI] [PubMed] [Google Scholar]
- Millbank J, Stuhmcke A, Karpin I.. Embryo donation and understanding of kinship: the impact of law and policy. Hum Reprod 2017;32:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moray N, Pink KE, Borry P, Larmuseau MH.. Paternity testing under the cloak of recreational genetics. Eur J Hum Genet 2017;25:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motluk A. Anonymous sperm donor traced on internet. New Scientist 2005, 2524. [Google Scholar]
- Nachtigall RD. Secrecy: an unresolved issue in the practice of donor insemination. Am J Obstet Gynecol 1993;168:1846–1849; discussion 1849–1851. [DOI] [PubMed] [Google Scholar]
- Nelson MK, Hertz R, Kramer W.. Gamete donor anonymity and limits on numbers of offspring: the views of three stakeholders. J Law Biosci 2016;3:39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaes SB. The medical management of donor insemination. In: KDEH (ed). Donor Insemination: International Social Science Perspectives. Cambridge: Cambridge University Press, 1998, 105–130. doi:110.1017/CBO9780511557804.9780511557006. [Google Scholar]
- Nuffield Council on Bioethics. Donor Conception: Ethical Aspects of Information Sharing. 2013. http://www.nuffieldbioethics.org (31 January 2022, date last accessed).
- Pennings G. Disclosure of donor conception, age of disclosure and the well-being of donor offspring. Hum Reprod 2017;32:969–973. [DOI] [PubMed] [Google Scholar]
- Pennings G. Genetic databases and the future of donor anonymity. Hum Reprod 2019;34:786–790. [DOI] [PubMed] [Google Scholar]
- Pennings G, de Mouzon J, Shenfield F, Ferraretti AP, Mardesic T, Ruiz A, Goossens V.. Socio-demographic and fertility-related characteristics and motivations of oocyte donors in eleven European countries. Hum Reprod 2014;29:1076–1089. [DOI] [PubMed] [Google Scholar]
- Pennings G, de Wert G, Shenfield F, Cohen J, Tarlatzis B, Devroey P.. ESHRE Task Force on Ethics and Law 12: oocyte donation for non-reproductive purposes. Hum Reprod 2007;22:1210–1213. [DOI] [PubMed] [Google Scholar]
- Pennings G, Gürtin Z.. The legal and ethical regulation of transnational donation. In: M. Richards, G. Pennings and JB Appleby (eds), Reproductive Donation: Policy, Practice, and Bioethics. Cambridge (UK): Cambridge University Press, 2012, 130–149. [Google Scholar]
- Platts S, Bracewell-Milnes T, Saso S, Jones B, Parikh R, Thum MY.. Investigating attitudes towards oocyte donation amongst potential donors and the general population: a systematic review. Hum Fertil (Camb) 2021;24:169–181. [DOI] [PubMed] [Google Scholar]
- Ravelingien A, Provoost V, Wyverkens E, Buysse A, De Sutter P, Pennings G.. Lesbian couples' views about and experiences of not being able to choose their sperm donor. Cult Health Sex 2015a;17:592–606. [DOI] [PubMed] [Google Scholar]
- Ravelingien A, Provoost V, Wyverkens E, Buysse A, De Sutter P, Pennings G.. Recipients' views on payment of sperm donors. Reprod Biomed Online 2015b;31:225–231. [DOI] [PubMed] [Google Scholar]
- Ravitsky V. The right to know one's genetic origins and cross-border medically assisted reproduction. Isr J Health Policy Res 2017;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. A British history of collaborative reproduction and the rise of the genetic connection. In: T. Freeman, S. Graham, F. Ebtehaj, M. Richards (eds), Relatedness in Assisted Reproduction: families, Origins and Identities. Cambridge (UK): Cambridge University Press, 2014, 21–43. [Google Scholar]
- Rodino IS, Burton PJ, Sanders KA.. Donor information considered important to donors, recipients and offspring: an Australian perspective. Reprod Biomed Online 2011;22:303–311. [DOI] [PubMed] [Google Scholar]
- Rubin LR, de Melo-Martin I, Rosenwaks Z, Cholst IN.. Once you're choosing, nobody's perfect: is more information necessarily better in oocyte donor selection? Reprod Biomed Online 2015;30:311–318. [DOI] [PubMed] [Google Scholar]
- Rumpikova T, Oborna I, Belaskova S, Konecna H, Rumpik D.. The attitudes of IVF patients treated in the Czech Republic towards informing children born after gamete donation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018;162:26–31. [DOI] [PubMed] [Google Scholar]
- Scheib JE, Riordan M, Rubin S.. Adolescents with open-identity sperm donors: reports from 12-17 year olds. Hum Reprod 2005;20:239–252. [DOI] [PubMed] [Google Scholar]
- Scheib JE, Ruby A.. Contact among families who share the same sperm donor. Fertil Steril 2008;90:33–43. [DOI] [PubMed] [Google Scholar]
- Scheib JE, Ruby A, Benward J.. Who requests their sperm donor's identity? The first ten years of information releases to adults with open-identity donors. Fertil Steril 2017;107:483–493. [DOI] [PubMed] [Google Scholar]
- Schrijvers A, Bos H, van Rooij F, Gerrits T, van der Veen F, Mochtar M, Visser M.. Being a donor-child: wishes for parental support, peer support and counseling. J Psychosom Obstet Gynaecol 2019;40:29–37. [DOI] [PubMed] [Google Scholar]
- Schrijvers AM, van Rooij FB, Overbeek G, de Reus E, Schoonenberg M, van der Veen F, Visser M, Bos HMW, Mochtar MH.. Psychosocial counselling for intended parents who opt for donor sperm treatment: which topics do they find relevant? J Reprod Infant Psychol 2020;38:474–484. [DOI] [PubMed] [Google Scholar]
- Shapiro DB. Payment to egg donors is the best way to ensure supply meets demand. Best Pract Res Clin Obstet Gynaecol 2018;53:73–84. [DOI] [PubMed] [Google Scholar]
- Shenfield F, de Mouzon J, Pennings G, Ferraretti AP, Andersen AN, de Wert G, Goossens V; ESHRE Taskforce on Cross Border Reproductive Care. Cross border reproductive care in six European countries. Hum Reprod 2010;25:1361–1368. [DOI] [PubMed] [Google Scholar]
- Slutsky J, Jadva V, Freeman T, Persaud S, Steele M, Steele H, Kramer W, Golombok S.. Integrating donor conception into identity development: adolescents in fatherless families. Fertil Steril 2016;106:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström-Anttila V, Foudila T, Hovatta O.. Oocyte donation in infertility treatment—a review. Acta Obstet Gynecol Scand 2001;80:191–199. [PubMed] [Google Scholar]
- Soderstrom-Anttila V, Miettinen A, Rotkirch A, Nuojua-Huttunen S, Poranen AK, Salevaara M, Suikkari AM.. Short- and long-term health consequences and current satisfaction levels for altruistic anonymous, identity-release and known oocyte donors. Hum Reprod 2016;31:597–606. [DOI] [PubMed] [Google Scholar]
- Somers S, Van Parys H, Provoost V, Buysse A, Pennings G, De Sutter P.. How to create a family? Decision making in lesbian couples using donor sperm. Sex Reprod Healthc 2017;11:13–18. [DOI] [PubMed] [Google Scholar]
- Storgaard M, Loft A, Bergh C, Wennerholm UB, Söderström-Anttila V, Romundstad LB, Aittomaki K, Oldereid N, Forman J, Pinborg A.. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: a systematic review and meta-analysis. BJOG 2017;124:561–572. [DOI] [PubMed] [Google Scholar]
- Stroud K, O'Doherty KC.. Ethically sustainable governance in the biobanking of eggs and embryos for research. Monash Bioeth Rev 2015;33:277–294. [DOI] [PubMed] [Google Scholar]
- Sydsjo G, Kvist U, Bladh M, Nordgaard A.. The optimal number of offspring per gamete donor. Acta Obstet Gynecol Scand 2015;94:1022–1026. [DOI] [PubMed] [Google Scholar]
- Tallandini MA, Zanchettin L, Gronchi G, Morsan V.. Parental disclosure of assisted reproductive technology (ART) conception to their children: a systematic and meta-analytic review. Hum Reprod 2016;31:1275–1287. [DOI] [PubMed] [Google Scholar]
- The European IVF-monitoring Consortium for ESHRE, Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A.. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen A, Provoost V, Vandormael E, Dhont N, Pennings G, Ombelet W.. Motivations and attitudes of candidate sperm donors in Belgium. Fertil Steril 2017;108:539–547. [DOI] [PubMed] [Google Scholar]
- Tulay P, Atilan O.. Oocyte donors' awareness on donation procedure and risks: a call for developing guidelines for health tourism in oocyte donation programmes. J Turk Ger Gynecol Assoc 2019;20:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Coyle A.. What does it mean to be a donor offspring? The identity experiences of adults conceived by donor insemination and the implications for counselling and therapy. Hum Reprod 2000;15:2041–2051. [DOI] [PubMed] [Google Scholar]
- van den Akker OB, Crawshaw MA, Blyth ED, Frith LJ.. Expectations and experiences of gamete donors and donor-conceived adults searching for genetic relatives using DNA linking through a voluntary register. Hum Reprod 2015;30:111–121. [DOI] [PubMed] [Google Scholar]
- Van den Broeck U, Vandermeeren M, Vanderschueren D, Enzlin P, Demyttenaere K, D'Hooghe T.. A systematic review of sperm donors: demographic characteristics, attitudes, motives and experiences of the process of sperm donation. Hum Reprod Update 2013;19:37–51. [DOI] [PubMed] [Google Scholar]
- Van Hoof W, Pennings G.. Extraterritoriality for cross-border reproductive care: should states act against citizens travelling abroad for illegal infertility treatment? Reprod Biomed Online 2011;23:546–554. [DOI] [PubMed] [Google Scholar]
- Van Parys H, Wyverkens E, Provoost V, De Sutter P, Pennings G, Buysse A.. Family communication about donor conception: a qualitative study with lesbian parents. Fam Process 2016;55:139–154. [DOI] [PubMed] [Google Scholar]
- Visser M, Gerrits T, Kop F, van der Veen F, Mochtar M.. Exploring parents' feelings about counseling in donor sperm treatment. J Psychosom Obstet Gynaecol 2016a;37:156–163. [DOI] [PubMed] [Google Scholar]
- Visser M, Mochtar MH, de Melker AA, van der Veen F, Repping S, Gerrits T.. Psychosocial counselling of identifiable sperm donors. Hum Reprod 2016b;31:1066–1074. [DOI] [PubMed] [Google Scholar]
- Wyverkens E, Provoost V, Ravelingien A, De Sutter P, Pennings G, Buysse A.. Beyond sperm cells: a qualitative study on constructed meanings of the sperm donor in lesbian families. Hum Reprod 2014;29:1248–1254. [DOI] [PubMed] [Google Scholar]
- Wyverkens E, Provoost V, Ravelingien A, Pennings G, De Sutter P, Buysse A.. The meaning of the sperm donor for heterosexual couples: confirming the position of the father. Fam Process 2017;56:203–216. [DOI] [PubMed] [Google Scholar]
- Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ.. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: an analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil Steril 2014;102:399–404. [DOI] [PubMed] [Google Scholar]
- Zadeh S. Disclosure of donor conception in the era of non-anonymity: safeguarding and promoting the interests of donor-conceived individuals? Hum Reprod 2016;31:2416–2420. [DOI] [PubMed] [Google Scholar]
- Zadeh S, Freeman T, Golombok S.. Absence or presence? Complexities in the donor narratives of single mothers using sperm donation. Hum Reprod 2016;31:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh S, Ilioi EC, Jadva V, Golombok S.. The perspectives of adolescents conceived using surrogacy, egg or sperm donation. Hum Reprod 2018;33:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh S, Jones CM, Basi T, Golombok S.. Children's thoughts and feelings about their donor and security of attachment to their solo mothers in middle childhood. Hum Reprod 2017;32:868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel JE. Donor conception from the viewpoint of the child: positives, negatives, and promoting the welfare of the child. Fertil Steril 2015;104:513–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data are included in the paper, its supplementary data and the review report available on www.eshre.eu/guidelines.