Abstract
Introduction
Serological testing is an important tool to assist with assessing the immune response to SARS-CoV-2 infections, the causative agent of COVID-19. A quantitative assay was recently developed by Abbott Laboratories to measures antibodies against the receptor binding domain of the spike protein. In addition to assessing disease prevalence, this assay is useful towards determining the scale and duration of the humoral response to infection and vaccination. Here we evaluated the clinical and analytical performance of the quantitative Abbott AdviseDx SARS-CoV-2 IgG II assay and characterized the longitudinal dynamics of the IgG response against SARS-CoV-2 in 402 infected individuals up to 322 days post-symptom onset.
Methods
To assess test sensitivity, 1257 serum specimens derived from 402 patients positive for SARS-CoV-2 by RT-PCR were analyzed on the Abbott Alinity platform. To evaluate test specificity, 394 specimens were tested from patients who were symptomatic but PCR negative for SARS-CoV-2, as well as 305 archived pre-pandemic samples. To further characterize test performance metrics, we evaluated assay precision and linearity.
Results
The Abbott AdviseDx SARS-CoV-2 IgG II assay exhibited diagnostic specificity of 99.02% using 305 pre − COVID-19 serum specimens and 98.73% using 394 PCR negative specimens. Using 1257 sequential serum samples collected from PCR-confirmed individuals, clinical test sensitivity of the assay was 39.7% at 3–7 days, 75.9% at 8–14 days, 95.6% at 15–21 days, and 98.7% at 4–5 weeks post-symptom onset. The assay is linear across the analytical measurement range claimed by the manufacturer (22–25,000 AU/mL) and exhibited good analytical precision. The median concentration of IgG increased steadily from <22 AU/mL at 3–7 days post-symptom onset, to a peak of 14,421 AU/mL at 6–7 weeks. Although antibody concentration started to decline at 8–9 weeks following symptom onset, all patients remained seropositive during the observation period. When the positivity rate of this assay was compared with the Abbott anti-NP IgG and EUROIMMUN anti-S1 IgG tests, clinical sensitivity of the Abbott AdviseDx SARS-CoV-2 IgG II assay was the highest at all time points with the exception of 4–5 weeks after symptom onset.
Conclusion
The Abbott AdviseDx SARS-CoV-2 IgG II assay offers high test specificity and sensitivity across a broad reportable range. We anticipate this assay will be a useful towards quantitatively assessing the humoral immune response to COVID-19 infection and vaccination.
Keywords: Coronavirus, Anti-spike, Anti-RBD, SARS-CoV-2 IgG, Abbott
1. Introduction
Serological testing is an important tool towards assessing immune responsiveness to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. Almost all patients with COVID-19 develop detectable antibodies to the spike and nucleocapsid proteins (NP) within several weeks of symptom onset, making serologic assays a useful tool in assessing infection prevalence and aiding diagnosis at later time points following infection (Hanson et al., 2020; To et al., 2020). In addition to serving as a signature of immune responsiveness, the circulating antibodies likely play an important role in the clearance of the virus, most notably spike protein-specific antibodies with neutralizing capabilities (Hoffmann et al., 2020). A large collaborative effort between the pharmaceutical industry, healthcare and academic sectors, and multiple government agencies worldwide, has led to the development of several COVID-19 vaccines. The three vaccines that have been approved and administered in the United States all deliver genetic material that encodes for the spike protein of SARS-CoV-2. A longitudinal characterization of antibody concentration post-infection and vaccination is lacking. Assessing spike protein-specific antibody levels in a quantitative manner may be especially important in certain populations including immunocompromised and elderly individuals, since their immune response to infection and vaccination may be impaired or delayed (Zilla et al., 2021; Bajaj et al., 2020; Boyarsky et al., 2021). A quantitative serologic assay may also be helpful towards determining the scale and duration of the humoral response in both circumstances.
Here we evaluated the Abbott AdviseDx SARS-CoV-2 IgG II assay, a quantitative test that measures IgG antibody against the receptor binding domain (RBD) of the spike protein. Furthermore, we characterized the longitudinal dynamics of the IgG humoral response against SARS-CoV-2 in 402 infected individuals up to 322 days post symptom onset.
2. Methods
2.1. Instrumentation and analysis
The Abbott AdviseDx SARS-CoV-2 IgG II assay was evaluated for use on the Abbott Alinity immunoassay system (Abbott Park, IL, USA). The AdviseDx SARS-CoV-2 IgG II assay is a two-step chemiluminescent microparticle immunoassay (CMIA) for the semi-quantitative detection of IgG antibody against the spike protein receptor binding domain (RBD). During the first step of the assay, RBD-specific IgG antibodies present in the sample bind to antigen coated paramagnetic microparticles (Abbott AdviseDx SARS-CoV-2 IgG II Assay, 2021). Following a wash cycle, acridinium-labeled anti-human IgG is added to the reaction vessel. The paramagnetic particles are subsequently washed, and an alkaline hydrogen peroxide solution is added to react with the immobilized acridinium-conjugated anti-human IgG complex, resulting in the emission of light. The chemiluminescent reaction is measured as relative light units (RLU), and the RLU detected is directly proportional to the amount of RBD-specific IgG antibody present in the sample.
The measurements are reported in standard arbitrary units (AU/mL), and the manufacturer's recommended cutoff of 50 AU/mL was used. The correlation and relationship of the Abbott AdviseDx SARS-CoV-2 IgG II assay to the first WHO international standard for anti-SARS-CoV-2 immunoglobulin (NIBSC Code 20/136) (World Health Organization, 2020) was established based on an internal study from Abbott (provided in a letter to customers). A linear regression analysis was performed using seven samples with concentrations just below the AdviseDx SARS-CoV-2 IgG II limit of quantitation up to the concentration of the neat WHO international standard. The conversion factor between AU/mL and the WHO binding antibody units (BAU/mL) was established as 1 BAU/mL = 0.142 AU/mL. To determine the presence of SARS-CoV-2 infections, viral RNA was measured in nasopharyngeal swabs (main specimen type) and oropharyngeal swabs by RT-PCR as previously described (Maine et al., 2020).
This assay has an analytical measurement range (AMR) of 22–25,000 AU/mL (up to 50,000 AU/mL with on-board 1:2 dilution). Linearity across the AMR was assessed by two protocols. First, a high patient sample (24,736 AU/mL) was diluted with assay diluent provided by the manufacturer to create seven samples, including the non-diluted high sample (neat). Secondly, linearity was assessed by mixing high (20,288 AU/mL) and low (<22 AU/mL) patient sample pools to create nine samples, including the non-diluted high and low samples. Linearity was assessed by comparing the measured to expected values.
For precision studies, QC materials representing 3 concentrations were obtained from Abbott. Reproducibility was assessed by analyzing 10 replicates on one day. Total precision was assessed by measuring each QC sample in duplicate over 10 days.
2.2. Patient Cohorts
Specimens for clinical sensitivity and specificity validation were obtained from residual patient serum samples under a protocol approved by the Institutional Review Board at Beaumont Health (#2020–233). To assess test sensitivity, 1257 specimens derived from 402 patients who tested positive for SARS-CoV-2 by RT-PCR were used. Approximately 80% of these patients were admitted to Beaumont Hospital at Royal Oak between March and August 2020, which corresponds to the first COVID-19 hospitalization surge in Michigan. The remaining 20% of the cohort was from specimens collected from individuals in an outpatient setting. Duration from symptom onset was determined by review of the electronic medical record and inferred from physician encounter notes. To evaluate test specificity, 394 specimens from patients who were symptomatic but PCR negative for SARS-CoV-2 were utilized. Furthermore, a cohort of 305 archived samples collected pre-pandemic (between 2010 and 2015) was included to assess test specificity. Individuals with known conditions or treatments associated with immune impairment, such as cancer and chemotherapy, were excluded from this cohort. Archived samples were stored at −80 °C until analysis.
2.3. Statistical analysis
95% CI was calculated in accordance with CLSI EP12-A2 guideline. Cohen's Kappa was calculated with EP Evaluator (Data Innovations). All data visualization was performed with GraphPad Prism 8.
3. Results
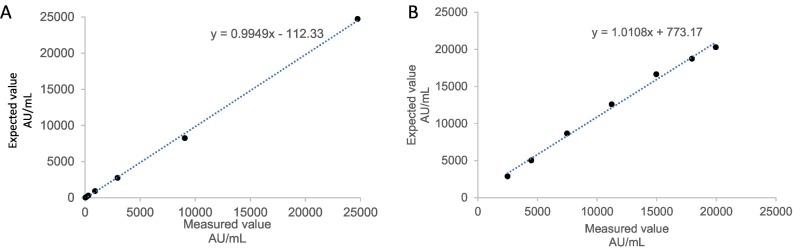
We first assessed linearity of the Abbott AdviseDx SARS-CoV-2 IgG II assay by diluting a high patient sample with assay diluent provided by the manufacturer. The results demonstrated a linear response across the analytical measurement range of 22–25,000 AU/mL, with an average percent difference of 2.2% from the expected values (Fig. 1A). Mixing high (22,451 AU/mL) and low (2 AU/mL) patient sample pools also exhibited a linear relationship, with average difference of 10.4% from the expected concentrations (Fig. 1B).
Fig. 1.
Linear dilution analysis. (A) Measured and expected values for dilution of a positive sample up to 1:729 with manufacturer provided diluent. (B) Measured and expected values after mixing high (22451 AU/mL) and low (2 AU/mL) patient serum sample pools to create 7 samples.
Assay imprecision was assessed using control materials. For QC level 2 (mean: 168.42), the coefficient of variation (%CV), which is a reflection of assay variability, was 2.5% within the same run (Supplemental Fig. 1). For QC level 3 (mean: 661.42), the %CV was 1.8%. Total imprecision was 3.2% and 2.7% across ten days for QC level 2 and level 3, respectively. The assay demonstrated acceptable precision for negative QC (level 1). Imprecision was unable to be calculated for this level because all results were below the limit of quantification (22 AU/mL).
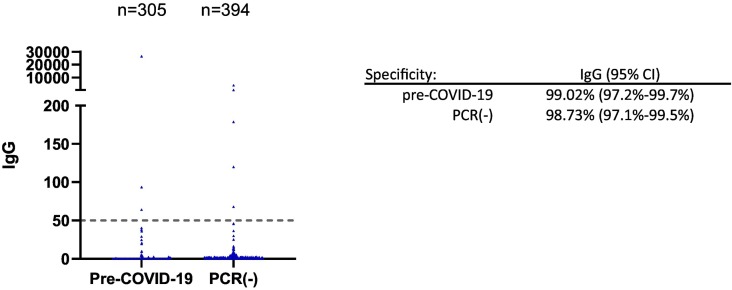
To assess clinical specificity of the assay, 305 archived specimens collected before the pandemic (between 2010 and 2015) from healthy individuals were assayed. Three specimens were above the cutoff of 50 AU/mL, resulting in an assay specificity of 99.02% (95%CI: 97.2%–99.7%) for the pre-COVID-19 cohort (Fig. 2 ). In addition, we tested 394 specimens from 205 patients who presented to Beaumont Hospital between March and April 2020 with symptoms consistent with a respiratory infection but tested negative by PCR for COVID-19 (Fig. 2). Five specimens in this cohort tested positive, resulting in an assay specificity of 98.73% (95% CI: 97.1%–99.5%). When these samples were analyzed using an orthogonal anti-NP IgG test from Abbott, three out of the five specimens tested positive. If these three specimens were excluded from analysis, the diagnostic specificity would be 99.49% for the Abbott AdviseDx SARS-CoV-2 IgG II assay in the PCR negative cohort.
Fig. 2.
Clinical specificity of the Abbott AdviseDx SARS-CoV-2 IgG II assays. 305 pre-COVID-19 serum specimens were collected from healthy individuals between 2010 and 2015. PCR(−) specimens were from patients presenting to the hospital with symptoms of respiratory infection and negative for COVID-19 by PCR.
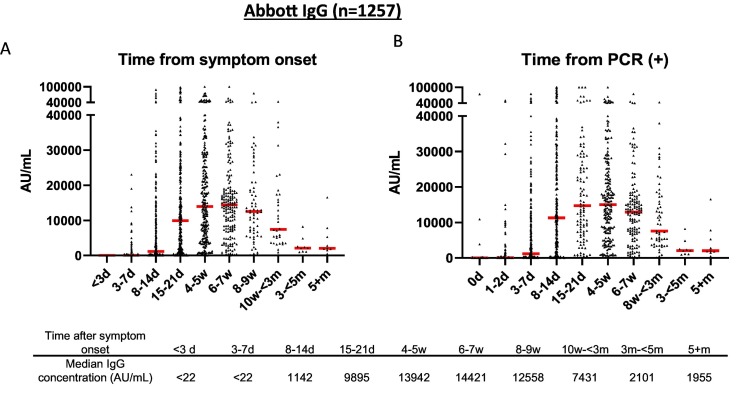
To determine assay sensitivity, we used 1257 specimens from 402 patients who tested positive for SARS-CoV-2 by PCR. Over 80% of these samples were collected from inpatients at Beaumont Hospital who were diagnosed with COVID-19 between March and May 2020. The sensitivity of assay relative to date of symptom onset was 39.7% at 3–7 days (95%CI: 31.2%–48.8%) with median IgG concentration of 17 AU/mL, 75.9% at 8–14 days (95%CI: 71.1%–80.1%) with median concentration of 1142 AU/mL, and 95.6% at 15–21 days (95%CI: 92.3%–97.5%) with median concentration of 9895 AU/mL (Fig. 3 ). The median IgG concentration reached a peak of 14,421 AU/mL at 6–7 weeks after symptom onset with a positivity rate of 99.4% (95% CI: 96.8%–99.9%) during this time frame (Fig. 3 and Table 1 ). There was only one patient who had not mounted an immune response for anti-RBD IgG by week 4. When the sample from this patient were analyzed using an orthogonal anti-S1 (Spike protein subunit 1) IgG test from EUROIMMUN they also tested negative (OD ratio: 0.08–0.25; cutoff: 1.1). The patient had no documented medications or disorders that may impair the immune system.
Fig. 3.
Seropositivity of Abbott semi-quantitative SARS-CoV-2 IgG immunoassay. A) Seropositivity in 1257 specimens from 402 patients with positive PCR results relative to days from symptom onset and B) relative to days from testing positive by PCR. Red line represents the median antibody concentration for each time period. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Positive rate of Abbott semi-quantitative anti-RBD, Abbott qualitative anti-NP, and EUROIMMUN (EI) anti-S1 assays. N: number of specimens for each time period.
| Time after symptom onset | Abbott anti-RBD | n | Abbott anti-NP | n | EI anti-S1 | n |
|---|---|---|---|---|---|---|
| <3 d | 0.0% | 14 | 0% | 14 | 0% | 14 |
| 3-7d | 39.7% | 116 | 29.10% | 110 | 27.60% | 123 |
| 8-14d | 75.9% | 340 | 69.50% | 367 | 61.10% | 357 |
| 15-21d | 95.6% | 250 | 93.60% | 297 | 89.60% | 297 |
| 4-5w | 98.7% | 239 | 99.60% | 248 | 93.60% | 235 |
| 6-7w | 99.4% | 175 | 99.40% | 179 | 98.30% | 176 |
| 8-9w | 100% | 66 | 97.20% | 71 | 98.50% | 68 |
| 10w- < 3 m | 100% | 34 | 97.10% | 34 | 100% | 35 |
| 3 m- < 5 m | 100% | 9 | 88.90% | 9 | 100% | 8 |
| 5 + m | 100% | 10 | 85.7% | 7 | 100% | 6 |
We observed a wide range of antibody concentration for positive samples, with 15.4% of samples having a concentration greater than the upper limit of the AMR of 25,000 AU/mL. With the 1:2 on-board dilution, the reportable range is extended to 50,000 AU/mL. Out of the positive patient cohort, 2.9% of samples yielded a concentration > 50,000 AU/mL. For those samples, we validated a manual 1:4 dilution with assay diluent provided by the manufacturer.
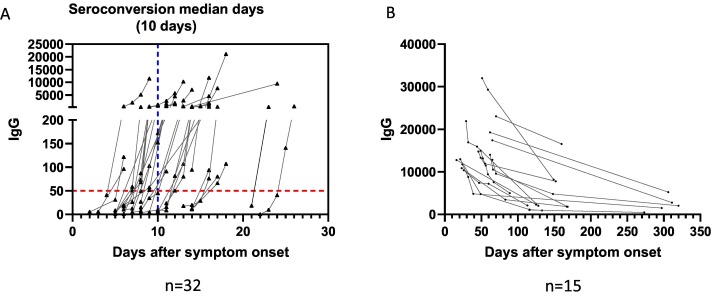
The antibody concentrations were examined in the positive patient cohort using sequential serum samples collected up to 322 days post onset of symptom. Among these patients, we observed seroconversion in 32 patients, with a median time to seroconversion of 10 days (range: 5–25 days) (Fig. 4 ). A decline was observed in the antibody concentration starting at 8–9 weeks following symptom onset (Fig. 3). However, the decline appeared to slow down, and antibody concentrations stabilized starting at approximately the third month post symptom onset (Fig. 4B). In addition, all of the patients in this cohort remained seropositive during the observation period (Fig. 3, Fig. 4 and Table 1).
Fig. 4.
Serological kinetics post-infection using Abbott semi-quantitative SARS-CoV-2 IgG assay (A) for patients who were initially seronegative and then underwent seroconversion during the observation period. Blue dashed lines represents the median time to seroconversion. (B) in 15 patients who were followed for >90 days. The first result of each patient was the peak level captured during the observation period. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We previously assessed the analytical and clinical performance of two qualitative anti-SARS-CoV-2 IgG assays, including the Abbott anti-NP (Nucleocapsid Protein) assay (Maine et al., 2020) and the EUROIMMUN (EI) anti-S1 assay, using the same patient cohorts. Here we evaluated concordance between Abbott quantitative anti-RBD and Abbott qualitative anti-NP assays, as well as between Abbott anti-RBD and EI anti-S1 assays. A total of 1931 specimens from both the positive and negative cohorts were used. Positive percent agreement and negative percent agreement for the comparison are shown in Table 2 . When the positivity rates were compared among the three assays, clinical sensitivity of the Abbott quantitative assay was the highest at all time points, with the exception of 4–5 weeks after symptom onset (Table 1).
Table 2.
Concordance between Abbott semi-quantitative anti-RBD and Abbott qualitative anti-NP, as well as EUROIMMUN (EI) anti-S1 antibody assays.
| Abbott anti-NP | EI anti-S1 | |
|---|---|---|
| (+) % Agreement | 94.6% (1021/1079) | 90.1% (972/1079) |
| (−) % Agreement | 97.0% (825/851) | 97.2% (827/851) |
| Cohen's Kappa | 91.2% (89.4%–93.0%) | 86.4% (84.1%–88.6%) |
4. Discussion
Here we report the performance characteristics of the Abbott AdviseDx SARS-CoV-2 IgG II (anti-RBD) assay that was issued Emergency Use Authorization for clinal use by the Food and Drug Administration in March of 2021. We demonstrated that the quantitative assay is linear across the AMR claimed by the manufacturer (22–25,000 AU/mL). The assay also exhibited good analytical precision. We report a diagnostic specificity of 99.02% using 305 pre − COVID-19 serum specimens and 98.73% using 394 PCR negative specimens. The median concentration of IgG increased steadily to reach a peak of 14,421 AU/mL at 6–7 weeks post-symptom onset. Although antibody concentrations started to decline at 8–9 weeks, all patients remained seropositive during the observation period. When the positivity rate of this assay was compared with the Abbott anti-NP IgG and EUROIMMUN anti-S1 IgG tests, clinical sensitivity of the Abbott AdviseDx SARS-CoV-2 IgG II assay was the highest at all time points with the exception of 4–5 weeks after symptom onset.
One significant finding of our study is that all of the infected patients remained seropositive for anti-RBD IgG up to 322 days post onset of symptoms. In 10 patients followed for more than 5 months, antibody concentrations ranged from 468 AU/mL to 16,534 AU/mL, all well above the cutoff value of 50 AU/mL. This is consistent with previous studies showing that although there is modest decline, neutralizing antibodies against the SARS-CoV-2 spike protein persisted for at least 4–5 months after infection (Wajnberg et al., 2020; Crawford et al., 2021). However, early studies in small cohorts of asymptomatic individuals or patients with mild COVID-19 showed antibody levels decayed rapidly in the early convalescent phase (Long et al., 2020a; Ibarrondo et al., 2020). Part of the reason for the discrepant findings is that individuals with mild disease typically may have lower levels of antibodies than hospitalized patients with severe illness (Klein et al., 2020; Long et al., 2020b). In addition, our study suggests that anti-RBD and anti-S IgG are longer lasting than the anti-NP antibody, which has been similarly shown in other reports (Muecksch et al., 2021).
We found that the Abbott AdviseDx SARS-CoV-2 IgG II assay offered good specificity, which is essential for achieving a high positive predictive value (Hanson et al., 2020; Centers for Disease Control and Prevention, 2021a). The clinical specificity in the PCR negative cohort is slightly below 99%, which is lower than that observed using a pre-pandemic sample cohort. Similar observations have been claimed by the manufacturer. One explanation for this is that the PCR results could be falsely negative. Since SARS-CoV-2 RNA starts to decline 7–10 days after symptom onset and becomes undetectable in most patients around 20 days (To et al., 2020), it is likely that we missed the window in which patients had detectable level of RNA. Furthermore, when the five specimens that showed discrepant results from PCR testing were analyzed using an orthogonal anti-NP IgG test from Abbott, three samples tested positive. Taken together, our observations confirm the recommendation from the Infectious Diseases Society of America (IDSA) that a second serological assay could serve as an adjunctive diagnostic tool for symptomatic patients with negative PCR results.
One of the concerns for COVID-19 serological testing is whether commercial assays can appropriately detect the antibody response in individuals infected with different SARS-CoV-2 strains, including the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B. 1.1.529) variants. Each of these variants harbor multiple mutations. Several of the mutations reside in the region encoding the spike protein (Centers for Disease Control and Prevention, 2021a). It is therefore possible that current serologic assays targeting the spike protein or RBD could have decreased sensitivity in detecting antibodies generated against the variants. On the other hand, since both natural infection and vaccination produce a polyclonal antibody response that targets several regions of the spike protein (Centers for Disease Control and Prevention, 2021b), it is unlikely that the current serologic testing will substantially impact test sensitivity. More studies characterizing the humoral response in individuals infected with the different variants of SARS-CoV-2 are needed to further address this issue.
Our study was limited by using specimens from patients who mostly required hospitalization with multiple comorbidities. Furthermore, a large proportion (65.3%) of patients were > 60 years old and may have decreased immune responsiveness (Montecino-Rodriguez et al., 2013). These reasons may account for why we observed a lower sensitivity than that claimed in the manufacturer's package insert.
In conclusion, the Abbott AdviseDx SARS-CoV-2 IgG II assay provides high test specificity and sensitivity and demonstrates linearity across a broad reportable range. We anticipate this assay will aid in determining immune responsiveness to COVID-19 infection and vaccination.
Funding
None.
Declaration of Competing Interest
The authors do not have conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2022.113243.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Abbott AdviseDx SARS-CoV-2 IgG II Assay . Abbott Laboratories; Abbott Park, IL: 2021. package insert. [Google Scholar]
- Bajaj V., et al. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky B.J., et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K.H.D., et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021;223(2):197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.E., et al. Infectious diseases society of America guidelines on the diagnosis of COVID-19:serologic testing. Clin. Infect. Dis. 2020;ciaa1343 doi: 10.1093/cid/ciaa1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo F.J., et al. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Long Q.X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Maine G.N., et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes, consequences, and reversal of immune system aging. J. Clin. Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., et al. Longitudinal serological analysis and neutralizing antibody levels in Coronavirus Disease 2019 convalescent patients. J. Infect. Dis. 2021;223(3):389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2021. Interim Guidelines for COVID-19 Antibody Testing. [Google Scholar]
- Centers for Disease Control and Prevention . 2021. SARS-CoV-2 Variant Classifications and Definitions. [Google Scholar]
- To K.K., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. 2020. https://www.who.int/publications/m/item/WHO-BS-2020.2403 Available at.
- Zilla M.L., et al. SARS-CoV-2 serologic immune response in exogenously immunosuppressed patients. J Appl Lab Med. 2021;6(2):486–490. doi: 10.1093/jalm/jfaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.