Abstract
Background
Contact tracing is used for multiple infectious diseases, most recently for COVID-19, but data regarding its effectiveness in disease control are scarce. To address this knowledge gap and inform public health decision making for COVID-19, we systematically reviewed the existing literature to determine the effectiveness of contact tracing in the control of communicable illness.
Methods
We searched PubMed, Embase, and the Cochrane Library from database inception up to Nov 22, 2021, for published studies evaluating associations between provider-initiated contact tracing for transmissible infectious diseases and one of three outcomes of interest: case detection rates among contacts or at the community level, overall forward transmission, or overall disease incidence. Clinical trials and observational studies were eligible, with no language or date restrictions. Reference lists of reviews were searched for additional studies. We excluded studies without a control group, using only mathematical modelling, not reporting a primary outcome of interest, or solely examining patient-initiated contact tracing. One reviewer applied eligibility criteria to each screened abstract and full-text article, and two reviewers independently extracted summary effect estimates and additional data from eligible studies. Only data reported in published manuscripts or supplemental material was extracted. Risk of bias for each included study was assessed with the Cochrane Risk of Bias 2 tool (randomised studies) or the Newcastle–Ottawa Scale (non-randomised studies).
Findings
We identified 9050 unique citations, of which 47 studies met the inclusion criteria: six were focused on COVID-19, 20 on tuberculosis, eight on HIV, 12 on curable sexually transmitted infections (STIs), and one on measles. More than 2 million index patients were included across a variety of settings (both urban and rural areas and low-resource and high-resource settings). Of the 47 studies, 29 (61·7%) used observational designs, including all studies on COVID-19, and 18 (38·3%) were randomised controlled trials. 40 studies compared provider-initiated contact tracing with other interventions or evaluated expansions of provider-initiated contact tracing, and seven compared programmatic adaptations within provider-initiated contact tracing. 29 (72·5%) of the 40 studies evaluating the effect of provider-initiated contact tracing, including four (66·7%) of six COVID-19 studies, found contact tracing interventions were associated with improvements in at least one outcome of interest. 23 (48·9%) studies had low risk of bias, 22 (46·8%) studies had some risk of bias, and two (4·3%) studies (both randomised controlled trials on curable STIs) had high risk of bias.
Interpretation
Provider-initiated contact tracing can be an effective public health tool. However, the ability of authorities to make informed choices about its deployment might be limited by heterogenous approaches to contact tracing in studies, a scarcity of quantitative evidence on its effectiveness, and absence of specificity of tracing parameters most important for disease control.
Funding
The Sullivan Family Foundation, Massachusetts General Hospital Executive Committee on Research, and US National Institutes of Health.
Introduction
Contact tracing is defined by WHO as “the process of identifying, assessing, and managing people who have been exposed to a disease to prevent onward transmission”, which informs important follow-up actions such as diagnostic tests, post-exposure prophylaxis, and quarantine. Contact tracing has been used in the control of infections including smallpox,1 tuberculosis,2 HIV,3 other sexually transmitted infections (STIs),4 Ebola virus,5 and, now, COVID-19. The objectives of contact tracing include identifying potential new cases before they might infect others (with referrals to care and social supports when indicated), detecting clusters of cases before they expand, and improving overall understanding of disease dynamics (appendix p 2).
Although observers have highlighted apparent successes of contact tracing for COVID-19 in places including Taiwan,6 Vietnam,7 and US states such as Massachusetts, quantitative data are scarce on the strategy's effectiveness for disease control in practice. Furthermore, contact tracing has implementation challenges in some settings; in a study of 74 185 patients with COVID-19 in the USA, 49 480 (66·7%) patients were not reached to elicit contacts or reported zero contacts.8 Previous systematic reviews focused on tracing for other infections, including tuberculosis9, 10 and HIV,11 confirmed a high prevalence of these diseases among patients' close contacts, yet remained unable to conclude the exact “contribution of contact tracing to reducing the burden of disease in the population”.9 Improving our understanding of contact tracing's effectiveness in disease control has substantial public health importance, particularly given ongoing SARS-CoV-2 transmission and the potential for future pandemics. Although the yield of contact tracing is likely to be influenced by the specific pathogen, population, and strategy involved, lessons from tracing programmes within specific contexts might help to inform best practices for disease control within and across disease categories (including for novel diseases such as COVID-19).
Research in context.
Evidence before this study
Relatively few studies have attempted to quantify the effectiveness of contact tracing in the control of COVID-19 and other infectious diseases on the basis of experimental approaches or collection of observational data. Although many studies suggest contact tracing can interrupt COVID-19 spread, most studies base this conclusion either on mathematical modelling or narrative descriptions of programmes. We performed a preliminary search of PubMed, Embase, and the Cochrane Library for existing reviews evaluating contact tracing in the control of infectious diseases, using the search terms “contact tracing”, “contact investigation”, “contact examination”, “contact screen”, or “partner notification” or their permutations, and limiting the results to reviews and systematic reviews only that were published from database inception up to March 31, 2020, without language restrictions. This preliminary investigation revealed only a small number of existing systematic reviews assessing contact tracing for tuberculosis (six reviews) and HIV or other sexually transmitted infections (six reviews), which were unable to draw firm conclusions about the strategy's overall effectiveness in population-level disease control. Additionally, these previous reviews each focused on only one type of infectious disease, revealing a missed opportunity to evaluate whether lessons from tracing programmes within specific contexts might help to inform best practices for the control of other illnesses, including novel diseases such as COVID-19. To address these gaps, we searched PubMed, Embase, and the Cochrane Library without date or language restrictions for published studies evaluating the effect of provider-initiated contact tracing in stopping infectious disease spread. Our search used the terms “contact tracing”, “contact investigation”, “contact examination”, “contact screen”, or “partner notification” or their permutations and included studies published from database inception up to Nov 22, 2021.
Added value of this study
This systematic review of contact tracing considers evidence of disease control effectiveness across multiple infectious diseases (COVID-19, tuberculosis, HIV, curable sexually transmitted infections, and measles). To our knowledge, this review is the first to synthesise empirical data for all forms of COVID-19 contact tracing and how they relate to disease control. We assessed data from more than 2 million index patients across eight infectious diseases and a variety of settings, including both urban and rural areas, and both low-resource and high-resource settings. 29 (72·5%) of 40 studies across all diseases, including four (66·7%) of six studies on COVID-19, found that provider-initiated contact tracing was associated with improvements in case detection, forward transmission, or disease incidence. However, the heterogeneity of approaches and the fact that most studies used observational designs limit our ability to understand how, where, and when to deploy this public health tool most effectively when disease control is the objective. Furthermore, the existing evidence base does not fully answer how parameters such as speed or completeness of contact tracing affect success, and how effectiveness might change in the event of case numbers exceeding health system capacity.
Implications of all the available evidence
The evidence suggests contact tracing to be an effective public health tool; however, more evidence is needed to understand how to optimise the effectiveness of contact tracing for infectious diseases control across a range of settings and contexts. Large-scale comparative studies of contact tracing that clearly specify approach and measure factors such as contact tracing speed and completeness would aid in decision making over resource allocation. In the interim, lessons from existing studies, such as the importance of ensuring privacy for contacts during tracing, and leveraging technology to increase tracing rates, can help to inform existing programmes for COVID-19 and other diseases.
To address this knowledge gap and to inform public health decision making, we conducted a systematic review of published studies that used empirical data to evaluate the effectiveness of contact tracing to control the spread of infectious diseases.
Methods
Search strategy and selection criteria
We conducted a systematic review in accordance with the PRISMA guidelines.12 We searched PubMed, Embase, and the Cochrane Library for studies in the published literature starting from database inception up to Nov 22, 2021, with no date or language restrictions. Our search used the terms “contact tracing”, “contact investigation”, “contact examination”, “contact screen”, or “partner notification” or their permutations. The full protocol and search strategy are provided in the appendix (pp 4, 21–23). We manually reviewed reference lists of reviews identified during the search for additional study articles to include.
We included clinical trials and observational studies evaluating the effect of contact tracing interventions specifically implemented by public health or health-care workers (ie, provider-initiated contact tracing, as previously defined;13, 14, 15, 16 appendix p 5) on the control of transmissible infectious diseases. Transmissible infectious diseases were classed as all those transmitted through human-to-human contact. We included studies that had evaluated one of three outcomes of interest: case detection rates among contacts or at the community level (hypothesised to increase with contact tracing); overall forward transmission of disease as measured by the reproduction number (R; hypothesised to decrease), secondary attack rates (hypothesised to decrease), or similar measures (including cases and deaths prevented, reinfection rates among contacts, and treatment rates among contacts, where increased treatment rates were interpreted as a proxy for decreased forward transmission); and overall disease incidence (hypothesised to decrease). Other downstream disease outcomes, such as mortality, were considered but beyond the scope of this review. We included studies evaluating the effects of provider-initiated contact tracing compared with the absence of contact tracing or with patient-initiated contact tracing (appendix p 5). Studies evaluating expansions or programmatic adaptations of pre-existing provider-initiated tracing services and studies comparing provider-initiated tracing to tracing programmes without follow-up actions (such as instructions for contacts to present for testing or to avoid others for a specific period of time) were also included. We excluded studies that used mathematical modelling only; did not have a control group; did not report one of our outcomes of interest; or solely examined patient-initiated contact tracing.
We uploaded search results to Covidence software for deduplication, screening, and data extraction. After elimination of duplicate records, one reviewer (among ADH, JJ, AE, CXH, and AR) independently screened each abstract for full-text review and applied eligibility criteria to each full-text article; in cases of uncertainty, abstracts or full texts in question were reviewed by three reviewers and included or excluded by consensus.
Data analysis
Using a standardised form created for the review, two reviewers (among ADH, JJ, AE, CXH, and AR) independently extracted the following data from eligible studies: authors, infection studied, years of study, location and setting, study design, study population, sample size (numbers of index patients and contacts), details of contact tracing intervention, effect measured, and effect size. For the purposes of this study we considered summary effect estimates, with confidence intervals and p values when provided. Two reviewers (among the same group) then independently assessed risk of bias for each included study, using the Cochrane Risk of Bias 2 tool17 for randomised studies and the Newcastle–Ottawa Scale18 for non-randomised studies. Conflicts in data extraction or bias assessments were adjudicated by discussion between the two reviewers regarding each domain within the extraction form or bias tool to reach consensus, or by independent completion of the bias assessment by a third reviewer.
When possible, tracing and control approaches were categorised with consistent definitions (appendix p 5). Provider-initiated contact tracing included both provider referral (in which a trained health worker notifies contacts of their infection exposure and the need for evaluation or other actions) and contract referral (in which a trained health worker and index patient make an agreement that the health worker will not notify contacts until a prespecified time has passed during which time the index patient agrees to notify contacts and refer them for services themselves). Because tuberculosis is the most studied disease among all the diseases included, we additionally stratified tuberculosis studies by World Bank country classification (low income, lower-middle income, upper-middle income, or high income) and by burden of tuberculosis (high-burden or lower-burden settings, as defined in the appendix [p 6]).19
We report on studies categorised by disease and type of contact tracing intervention (studies on provider-initiated contract tracing and those on programmatic adaptations within provider-initiated contact tracing), summarising the findings of each study and the risk of bias for each study. We expected that interventions and outcomes would be too heterogeneous for meta-analysis and thus did not plan to meta-analyse the data.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
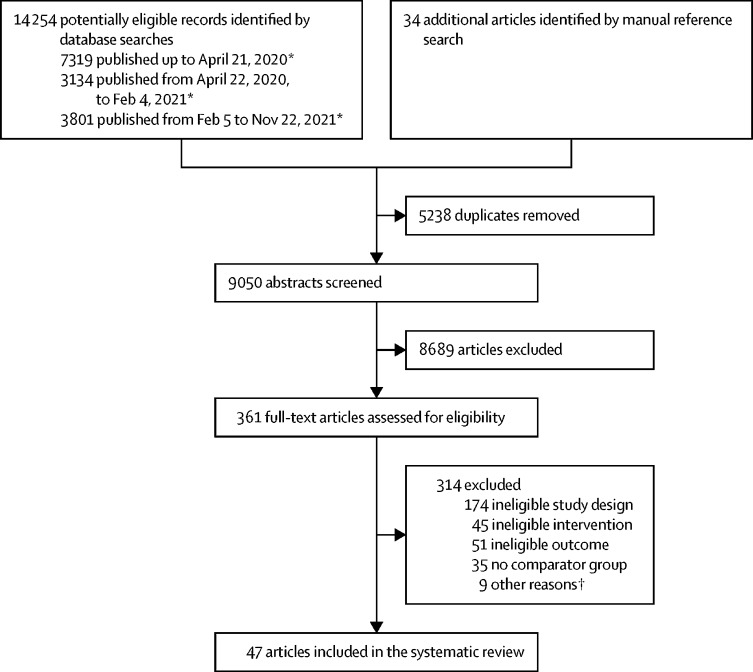
We identified 14 254 records, including 5238 duplicates, from the database search (figure ). 34 additional articles were identified by manually searching reference lists. We screened 9050 unique abstracts, yielding 361 full-text articles to be assessed for eligibility, of which 314 were excluded. Of the 47 included articles evaluating contact tracing interventions, six were focused on COVID-1920, 21, 22, 23, 24, 25 (table 1 ), 20 on tuberculosis26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 (table 2 ), eight on HIV46, 47, 48, 49, 50, 51, 52, 53 (appendix pp 11–14), 12 on curable STIs (syphilis,54, 55, 56 gonorrhea,57, 58, 59, 60 trichomoniasis,61 chlamydia,62, 63 and STI syndromes;64, 65 appendix pp 15–19), and one on measles66 (appendix p 20). Included studies were conducted across five continents (appendix p 3), with study years ranging from 1975 to 2020. The 47 studies comprised 18 (38·3%) randomised controlled trials (RCTs)26, 28, 29, 36, 38, 41, 43, 46, 47, 48, 50, 51, 56, 58, 61, 63, 64, 65 and 29 (61·7%) observational studies.20, 21, 22, 23, 24, 25, 27, 30, 31, 32, 33, 34, 35, 37, 39, 40, 42, 44, 45, 49, 52, 53, 54, 55, 57, 59, 60, 62, 66 40 studies compared provider-initiated contact tracing with other interventions or evaluated expansions of provider-initiated contact tracing,20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 55, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 and seven studies compared programmatic adaptations within provider-initiated contact tracing.29, 31, 33, 52, 53, 54, 56 23 (48·9%) studies had low risk of bias,20, 21, 22, 25, 26, 28, 29, 34, 36, 39, 40, 41, 46, 47, 48, 50, 57, 59, 60, 61, 64, 65, 66 22 (46·8%) studies had some risk of bias,23, 24, 27, 30, 31, 32, 33, 35, 37, 38, 42, 43, 44, 45, 49, 51, 52, 53, 54, 55, 58, 62 and two (4·3%) studies (both RCTs on curable STIs56, 63) had high risk of bias (appendix pp 7–8). 29 (72·5%) of the 40 studies evaluating the effect of provider-initiated contact tracing, including four (66·7%) of six COVID-19 studies and 11 (68·8%) of 16 RCTs and quasi-RCTs, found contact tracing interventions were associated with improvements in at least one outcome of interest.
Figure.
PRISMA diagram of studies evaluating the effect of contact tracing interventions on the control of infectious diseases transmitted by human-to-human contact
*The review protocol was exactly repeated twice after the original search of articles published up to April 21, 2020, with additional searches to capture studies published between April 22, 2020 and Feb 4, 2021, and between Feb 5, 2021 and Nov 22, 2021 (appendix pp 22–23). †Other reasons for exclusion were wrong article type (five articles: two conference abstracts, one opinion piece, one clinical trial registration, and one general summary of contact tracing), no separation of outcomes of contact tracing from a broader intervention (one article), only summarising the findings of another study already included in the review (one article), near-identical overlap in study dates, study population, and study results with another study already included in the review (one study), and not having an available manuscript (one study).
Table 1.
Summary of included studies of contact tracing* for COVID-19
| Country | Study year | Design | Setting | Sample | Intervention | Control | Outcome measured | Results† | Risk of bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fetzer and Graeber (2021)20 | UK | 2020 | Quasi-experimental design, with difference-in-differences regression | Lower tier local authorities (districts, boroughs, and city councils) around the country (urban and rural) | 15 841 COVID-19 cases from Sept 25 to Oct 2, 2020, with contact tracing accidentally not performed on an estimated 48 000 contacts due to a computer error; areas were compared based on the degree they were affected by the error, which was seemingly random | Timely contact tracing for close recent contacts (not fully described) including instructions to self-quarantine; other social distancing policies in place not fully described | Delayed contact tracing for close recent contacts (not fully described), including delayed instructions to self-quarantine (computer error); other social distancing policies in place not fully described | Forward transmission: cases not prevented, deaths not prevented | Accidental failure to conduct timely contact tracing for 15 841 index patients (around 20% of index patients during the one-week period) was estimated to have led to an additional 126 836–185 188 additional COVID-19 cases during the 6 weeks after the error was discovered (22·5–32·8% of all cases during the period), as well as an additional 1521–2049 deaths over the 6 weeks (30·6–41·2% of all deaths during the period); ie, there were an estimated 18·6 additional COVID-19 cases per late referral and 0·24 additional deaths per late referral | Low |
| Kendall et al (2020)21 | UK | 2020 | Retrospective cohort study | Isle of Wight compared with rest of the UK (urban and rural) | All COVID-19 cases in the UK from March 28 to June 29, 2020 (exact number not specified) | Isle of Wight: traditional contact tracing (not fully described; starting May 5, 2020) and digital contact tracing with a Bluetooth-powered mobile application (starting May 7, 2020), with related advertising and community discussions (not fully described); pre-existing social distancing policies simultaneously in place (not fully described) | Rest of UK: initially no contact tracing, followed by traditional contact tracing only (not fully described; starting May 28, 2020); pre-existing social distancing policies simultaneously in place (not fully described) | Forward transmission: R0 | After intervention initiation, the Isle of Wight had a decrease in R0 (1·3 to 0·5) between May 5 and June 29, 2020, and also had a lower R0 than the UK as a whole at the same timepoint (p<0·0001) | Low |
| Liu et al (2021)22 | 130 nations | 2020 | Country-level cohort study | 130 nations (urban and rural) | All COVID-19 cases in 130 nations from Jan 1 to June 22, 2020 | Contact tracing with intensities rated as comprehensive, limited, or none (not fully described; different countries likely to have employed different approaches); 12 other NPIs were also assessed and controlled for | No contact tracing or decreased intensity of contact tracing (not fully described) | Forward transmission: Rt | Contact tracing was associated with an increase in Rt (exact effect size not specified) at 10 days; this increase might have reflected an increase in case detection, or other temporally associated NPIs in the model (eg, testing policies) | Low |
| Malheiro et al (2020)23 | Portugal | 2020 | Retrospective cohort study | Eastern Porto (urban) | All COVID-19 cases in eastern Porto from March 1 to April 30, 2020: 551 index patients, 1627 close contacts | Identification as a potential COVID-19 case through contact tracing (interview with patient or caregiver) or travel history, with mandatory quarantine prior to testing positive; social distancing policies (limiting movements and business activities) simultaneously in place starting March 22, 2020 | Identification as a COVID-19 case through testing, with no mandatory quarantine prior to testing positive; social distancing policies (limiting movements and business activities) simultaneously in place starting March 22, 2020 | Forward transmission: secondary attack rate | There was no significant difference in the secondary attack rate for index cases between the intervention group (16 of 132, 12·1% [95% CI 7·1–18·9]) and the control group (138 of 1495, 9·2% [7·8–10·8], p=0·13), including when stratifying by the presence or absence of social distancing policies (p=0·72) | Some |
| Park et al (2020)24 | South Korea | 2020 | Pre–post design | Seoul (urban) | All COVID-19 cases in Seoul from Jan 24 to May 2, 2020: 637 index patients, 16 176 contacts | Post-period (March 9 to May 2, 2020): widespread testing of contacts associated with clusters of COVID-19 cases (identified via interviews, global positioning system data, credit card histories, drug utilisation review [not fully described], closed-circuit television records, and possibly other methods) and individuals with COVID-19 symptoms; all positive-testing individuals moved to health-care settings for quarantine; other social distancing policies in place not fully described | Pre-period (Jan 24 to March 8, 2020): widespread testing of individuals with COVID-19 symptoms only; all positive-testing individuals moved to health-care settings for quarantine; other social distancing policies in place not fully described | Forward transmission: Rt | The intervention was associated with a decrease in Rt across Seoul (1·3 during the pre-period, 0·6 during the post-period) | Some |
| Wymant et al (2021)25 | UK | 2020 | Retrospective cohort study | All of England and Wales (both urban and rural) | All COVID-19 cases in England and Wales from Oct 8 to Dec 31, 2020: 1 892 000 index patients (32 500 deaths) | Digital contact tracing with a Bluetooth-powered mobile application, with simultaneous manual contact tracing and additional restrictive and lockdown measures (not fully described) in place | No digital contact tracing, but with simultaneous manual contact tracing and additional restrictive and lockdown measures (not fully described) in place | Forward transmission: cases prevented, deaths prevented | With matched-neighbours regression, use of the contact tracing application by 16·5 million (27·8% of 59·4 million people in England and Wales) was estimated to prevent 594 000 COVID-19 cases (95% CI 317 000–914 000) and 8700 deaths (95% CI 4700–13 500) between Oct 8 and Dec 31, 2020; each percentage point increase in application usage was associated with a 2·26% (95% CI 1·50–3·00) reduction in cases for the total period (1·09% [0·04–2·14] reduction from Oct 8 to Nov 6 and 2·66% [1·75–3·56] reduction fromNov 7 to Dec 31 following application improvements released on Oct 29 that were not fully described) | Low |
R0=reproduction number. Rt=time-varying reproduction number. NPI=non-pharmaceutical intervention.
Provider-initiated contact tracing for all studies listed.
Significance or non-significance of result is not stated when it was not specified in the study.
Table 2.
Summary of included studies of contact tracing for tuberculosis
| Country | Study years | Design | Setting | Sample | Intervention | Control | Outcome measured | Results* | Risk of bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies evaluating the effect of provider-initiated contact tracing | ||||||||||
| Ayles et al (2013)26 | Zambia, South Africa | 2010 | Cluster RCT (two-by-two factorial design) | 16 communities in Zambia and eight communities in South Africa (urban and rural) | 64 463 individuals screened for tuberculosis | Three intervention groups: (1) complex household intervention including household contact tracing for tuberculosis, as well as HIV testing for household contacts and appropriate linkages to care; (2) complex community intervention including community mobilisation (eg, pamphlets, megaphone announcements) and establishment of additional and expedited sputum collection sites; (3) both the complex household intervention and complex community intervention | Passive case finding; all groups (intervention and control) also received improved clinic-based tuberculosis and HIV services (eg, diagnostics) | Overall active tuberculosis prevalence (among adults), and overall latent tuberculosis incidence (among children) | When comparing communities in the study groups with and without the complex household intervention (which included household contact tracing), there was no significant difference in the prevalence of active tuberculosis among adults 4 years after the start of the intervention (adjusted prevalence ratio 0·82 [95% CI 0·64–1·04], p=0·095, permutation test p=0·063), or in the incidence of latent tuberculosis among children (adjusted IRR 0·45 [95% CI 0·20–1·05], p=0·063, permutation test p=0·10) | Low |
| Becerra et al (2005)27 | Peru | 1996–97 | Prospective cohort study | Low-income neighbourhood in Lima (urban) | 208 index patients, 1094 household contacts, 2253 neighbours | Household contact tracing and screening of neighbours | Passive case finding: index patients told to instruct household contacts to present to clinic if the contacts developed tuberculosis-like symptoms | Case detection among contacts | A higher tuberculosis prevalence among household contacts was detected by household contact tracing (eight [0·7%] of 1094) than by passive case finding (two [0·1%] of 1094); the tuberculosis prevalence among neighbours of index cases was 0·1% for both groups (three of 2253 and two of 2253, respectively) | Some |
| Cavalcante et al (2010)28 | Brazil | 2000–04 | Cluster RCT | Eight neighbourhoods in Rio de Janeiro, pair-matched according to tuberculosis incidence (urban) | 650 index patients, 2147 household contacts | Enhanced DOTS with household contact tracing (DOTS-A), including treatment for contacts found to have active tuberculosis and prophylaxis for those found to have latent tuberculosis infection | Conventional DOTS with patient-initiated contact tracing | Overall disease incidence | During the 5-year study period, the incidence of tuberculosis in neighbourhoods receiving DOTS with household contact tracing (DOTS-A) decreased by 10·0% (from 339 to 305 cases per 100 000 residents), while the incidence in neighbourhoods receiving conventional DOTS increased by 5·3% (from 340 to 358 cases per 100 000 residents), resulting in a 14·8% lower incidence in DOTS-A neighbourhoods compared with conventional DOTS neighbourhoods (p=0·04) | Low |
| Dongo et al (2021)30 | Uganda | 2014–16 | Pre–post design | Two districts: Kabarole (rural) and Wakiso (peri-urban) | All detected tuberculosis cases in Kabarole and Wakiso (exact number not specified) | Post-period (July, 2015 to Dec, 2016): decentralisation of child tuberculosis services, which included household contact tracing after training of 178 CHWs, and health system improvements including repairs to laboratory equipment and training for laboratory staff | Pre-period (Jan, 2014, to June, 2015): child tuberculosis services prior to decentralisation, including before household contact tracing and before other health system improvements | Case detection at community level | There was a 138·7% increase in tuberculosis cases in children (age 0–14 years) detected during the post-period (647 cases) compared with the pre-period (271 cases); there was also a 31·7% increase in tuberculosis cases detected among adolescents and adults aged ≥15 years during the post-period (3693 cases) compared with the pre-period (2805 cases) | Some |
| Eyo et al (2021)32 | Nigeria | 2017–19 (historical comparison 2015–18) | Prospective cohort with historical comparison | Three states in southern Nigeria: Akwa Ibom State (intervention state), Cross River State (intervention state), and Rivers State (control state; none specified as urban or rural) | 509 768 individuals screened for tuberculosis (both household contacts of index patients and individuals screened in other places) | Several simultaneous interventions including: household contact tracing; screening of individuals in other homes and tents (H2H/T2T); screening of individuals in public areas (community screening); and promotion of the interventions via print media and public talks | Baseline tuberculosis services (not fully described) in the control state during the study period, and in the intervention states prior to the study period | Case detection at community level | Intervention states saw a 112·9% increase in all forms of tuberculosis detected versus baseline (1258 cases in 2018–19, versus 591 cases in 2017–18), or a 138·3% increase compared with the expected trend; this increase in all forms of tuberculosis detected was greater than the increase observed in the control state (410 cases in 2018–19, versus 204 cases in 2017–2018, or a 101·0% increase), or a 49·1% increase compared with the expected trend; household contact tracing resulted in a greater proportion of screened individuals diagnosed with presumptive tuberculosis (1350 [11·5%] of 11 700) compared with H2H/T2T (5786 [2·2%] of 269 069) and community outreach (4536 [2·2%] of 209 177) | Some |
| Gashu et al (2016)34 | Ethiopia | 2011–14 | Cross-sectional study | Six zones in Oromia and Amhara regions (rural) | 47 021 index patients, 272 441 close contacts | Retrospective tracing of close contacts for all tuberculosis cases in the previous 3 years, which included household contact tracing | Routine case detection strategy (not fully described) | Case detection among contacts | A higher tuberculosis prevalence among close contacts was detected by retrospective contact tracing (768 per 100 000) compared with the control strategy (baseline case notification rate reported to be more than 130 per 100 000 for these zones) | Low |
| Gurung et al (2021)35 | Nepal | 2017–18 (historical comparison 2014–17) | Prospective cohort study | Eight districts (not specified if urban or rural) | 54 239 individuals screened for tuberculosis | Three-part active case finding intervention: (1) contact tracing of close contacts (not fully described); (2) facility-based screening; (3) tuberculosis bases in various under-served communities where attendees were screened for symptoms | Passive case finding and limited household contact tracing (not fully described) | Case detection at community level | The intervention was associated with a 22·3% increase in detected tuberculosis cases in intervention districts compared with control districts, and was also associated with a 29·0% increase in detected tuberculosis cases within intervention districts compared with the previously expected trend in intervention districts | Some |
| Hanrahan et al (2019)36 | South Africa | 2017–19 | Cluster RCT | Catchment area of 56 primary care clinics in Limpopo province (rural) | 3655 index patients, 1677 contacts | Household contact tracing, or contacts invited for screenings at clinics with monetary incentive | Facility-based screening of all patients | Forward transmission: treatment initiation rates | There was no significant difference in tuberculosis treatment initiation rates between the facility-based screening group and household contact tracing group (treatment initiation rate ratio 1·04 [95% CI 0·83–1·30], p=0·73) | Low |
| Hernández-Garduño et al (2015)37 | Mexico | 1990–2010† | Longitudinal population-based study | Whole country (urban and rural) | 72 398 index patients with all forms of tuberculosis in Mexico (2007–10) | Contact investigation (not fully described) | Modelled the control group based on average number of contacts examined per reported tuberculosis case | Overall disease incidence | There was a negative correlation between the mean number of contacts examined per case and the incidence of tuberculosis (all forms; r2=−0·47, p=0·010), with a 33·0% reduction in (all forms of tuberculosis) incidence for each unit increase in the mean number of contacts examined | Some |
| Khatana et al (2019)38 | India | 2014–15 | Quasi-RCT‡ | Two communities in Kashmir (rural) | 282 index patients, 1191 household contacts | Household contact tracing | Passive case finding | Case detection among contacts | A higher tuberculosis prevalence among household contacts was detected by household contact tracing (27 [4·5%] of 598) compared with passive case finding (seven [1·2%] of 593; OR 3·97 [95% CI 1·73–9·11], p=0·001) | Some |
| Kliner et al (2013)39 | Eswatini | 2011–12 | Prospective cohort study with sequential interventions | Catchment area of a regional hospital (rural) | 122 index patients, 658 household contacts | Three sequential interventions for household contacts: (1) phone call reminders for contacts asking them to present for screening, followed by household contact tracing if contact does not present (November, 2011–February, 2012); (2) screening of contacts by phone, followed by household contact tracing if contact is not reachable by phone (March–July, 2012); (3) household contact tracing by contract referral (7 days) for high-risk contacts (≤5-years old, index patient with multidrug-resistant tuberculosis, or index patient with a high-grade [3+ grade] sputum smear), or a second invitation letter given to contacts not deemed high-risk (August–October, 2012) | Patient-initiated contact tracing, and screening of any contacts who accompanied index patients to clinic | Case detection among contacts | A greater number of tuberculosis cases was detected by patient-initiated contact tracing and screening of contacts who accompanied index patients to clinic (four [2·5%] of 157 screened contacts), compared with any of the intervention methods (0 of 137 contacts in the phone call intervention, 0 of 226 contacts in the phone screening intervention, 0 of 107 high-risk contacts in the household contact tracing by contract referral intervention, and 0 of 31 contacts who received a second invitation letter) | Low |
| Mandalakas et al (2017)40 | Eswatini | 2013–15 | Prospective cohort study | Seven basic management units (urban and rural) | 3258 index patients, 12 175 household contacts | Household contact tracing | No household contact tracing before the intervention (not fully specified) | Case detection at community level | The first year of the household contact tracing intervention was associated with a 32% increase in the number of children diagnosed with bacteriologically confirmed tuberculosis despite a concurrent decrease in the national tuberculosis case notification rate | Low |
| Morishita et al (2016)41 | Cambodia | 2012–14 | Cluster RCT (quasi-experimental) | 30 districts with high rates of poverty (not specified if urban or rural) | 25 668 index patients | Household contact tracing and screening of neighbours | Passive case finding | Case detection at community level | Household contact tracing was associated with an increase in all forms of tuberculosis detected compared with baseline (9·8% increase during year 1, when 15 of 30 districts were conducting the intervention; 23·4% increase during year 2, when 30 of 30 districts were conducting the intervention); 6 quarters after year 1, there was a decrease in all forms of tuberculosis detected (−218%) compared with the previously expected trend | Low |
| Sanaie et al (2016)42 | Afghanistan | 2011–12 | Prospective cohort study | Six provinces (not specified if urban or rural) | 2 022 127 health facility attendees, household contacts of patients with tuberculosis and internally displaced people | Household contact tracing | Facility-based screening of all patients, and screening all people in internally displaced person camps | Case detection among contacts | A higher prevalence of sputum smear-positive tuberculosis was detected by household contact tracing (268 [1·6%] of 16 645 household contacts) than by other strategies (4125 [0·2%] of 1 699 277 people presenting to health facilities with any concern; 653 [0·2%] of 306 205 people in internally displaced person camps) | Some |
| Shah et al (2020)43 | Peru | 2012–14 | Cluster RCT (stepped-wedge design) | Densely populated district in Lima (urban) | 3222 index patients, 12 566 household contacts | Household contact tracing | Passive case finding: index patients told to instruct household contacts to present to clinic if these contacts developed tuberculosis-like symptoms | Case detection among contacts | A higher incidence of tuberculosis among household contacts was detected by household contact tracing than by passive case finding (IRR 1·51 [95% CI 1·21–1·88], p<0·0001 for pulmonary tuberculosis; IRR 1·48 [1·19–1·84], p<0·0001 for all forms of tuberculosis) | Some |
| Young et al (2016)44 | USA | 2012 | Observational, with hypothetical control | All 50 states and Puerto Rico (urban and rural) | 9945 index patients, 105 100 contacts (not fully described) | Contact investigation (not fully described) with treatment of detected latent tuberculosis infection | No contact investigation or treatment of detected latent tuberculosis infection (theoretical control)§ | Forward transmission: cases prevented | Contact investigation and treatment of latent tuberculosis infection was estimated to prevent 128 cases (95% CI 64–252) of tuberculosis over 5 years | Some |
| Zachariah et al (2003)45 | Malawi | 2001–02 | Pre–post design | One district (rural) | 189 index patients, 985 household contacts | Post-period (2002): household contact tracing | Pre-period (2001): passive case finding (household visit performed only to record information on household contacts) | Case detection among contacts | A higher prevalence of tuberculosis among household contacts was observed during the post-period (eight [1·74%] of 461) compared with the pre-period (one [0·19%] of 524, p=0·01). | Some |
| Studies comparing programmatic adaptations within provider-initiated contact tracing | ||||||||||
| Davis et al (2019)29 | Uganda | 2016–17 | Cluster RCT | Catchment area of seven primary care clinics in Kampala (urban) | 372 index patients, 919 household contacts | Household contact tracing: in homes, CHWs collected sputum samples from household contacts with tuberculosis symptoms or those living with HIV, with automated results provided by mobile phone SMS | Household contact tracing: CHWs refer household contacts with tuberculosis symptoms and those living with HIV for clinic evaluations | Case detection among contacts | There was no significant difference in the odds of tuberculosis diagnosis among household contacts between the intervention group and control group (OR 1·34 [95% CI 0·42–4·24], p=0·62). | Low |
| Duarte et al (2012)31 | Portugal | 2001–06 | Pre–post design | City of Vila Nova de Gaia (urban) | 877 index patients, 3946 household and workplace contacts | Post-period (2004–06): household and workplace contact tracing | Pre-period (2001–03): investigation of contacts named by index patients | Forward transmission: cases prevented | A greater number of estimated active tuberculosis cases were prevented during the post-period (ten cases) compared with the pre-period (five cases) | Some |
| Fatima et al (2016)33 | Pakistan | 2011–15 | Pre–post design | Four districts with high concentrations of low-income neighbourhoods (urban) | 89 222 household contacts, 693 821 community contacts | Post-period (2013–15): tuberculosis screening among community contacts living within 50 m of index patients, and household contact tracing | Pre-period (2011–13): household contact tracing only | Case detection among contacts | The intervention was associated with an increase in tuberculosis case detection (108 341 cases in the post-period compared with 100 384 cases in the pre-period, or a 7·9% increase) | Some |
RCT=randomised controlled trial. IRR=incidence rate ratio. DOTS=directly observed therapy, short-course. DOTS-A=enhanced directly observed therapy, short-course. CHWs=community health workers. OR=odds ratio.
Significance or non-significance of result is not stated when it was not specified in the study.
Although Hernández-Garduño and colleagues37 collected data from 1990–2010, the outcome of interest for our review was measured from 2007–10.
Although the first index patient from each community in Khatana et al38 was randomly assigned to either household contact tracing (intervention group) or passive case finding (control group), all subsequent index patients were assigned to these groups via alternate assignment.
To calculate their theoretical control results, Young and colleagues44 assumed a 2·4% cumulative 5-year incidence of active tuberculosis among contacts with latent tuberculosis infection not receiving treatment.
All six studies examining contact tracing for COVID-19 were observational (table 1). The studies were primarily based in high-income countries, where additional social distancing measures were simultaneously in place. Four studies included a total of 1·9 million index patients,20, 23, 24, 25 and the other two studies were population based.21, 22 Fetzer and Graeber20 analysed a natural experiment enabled by a computer error in the UK in September, 2020, that delayed contact tracing for 15 841 index patients. By comparing areas more affected versus less affected by the computer error, they found that each late referral was associated with 18·6 additional COVID-19 cases and 0·24 additional deaths over 6 weeks. Two other UK-based studies also reported positive effects of contact tracing. On the Isle of Wight, initiation of traditional contact tracing and concurrent digital contact tracing (exposure notification via a smartphone application downloaded by more than 54 000 [>38%] of 141 536 in the population) was associated with a reduction in the basic reproduction number (R0) from 1·3 on May 5, 2020, to 0·5 on June 29, 2020.21 A separate matched-neighbours regression estimated that every percentage point increase in uptake of the same application across England and Wales between Oct 8 and Dec 31, 2020, led to a 2·26% (95% CI 1·50–3·00) reduction in COVID-19 cases.25 Another study from South Korea found widespread identification and testing of individuals linked with clusters of COVID-19 cases was associated with a reduction in the time-varying reproduction number (Rt) from 1·3 (Jan 24 to March 8, 2020) to 0·6 (March 9 to May 2, 2020).24 The other two studies did not find evidence in support of COVID-19 contact tracing. An analysis of all COVID-19 cases from 130 countries (Jan to June, 2020) found only a weak relationship between contact tracing and Rt, specifically an association with higher Rt values, which could not be well distinguished from other clustered interventions and was hypothesised as potentially reflecting increased case detection from tracing.22 A study from Portugal found no difference in COVID-19 secondary attack rates when tracing and quarantine measures were initiated either before laboratory confirmation of disease (12·1% [95% CI 7·1–18·9]) or after (9·2% [7·8–10·8], p=0·13).23 Of note, secondary attack rates might appear to be increased by contact tracing as a consequence of increased detection of cases among contacts.
20 studies examined contact tracing for tuberculosis (table 2).26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 These studies included more than 168 000 index patients across a diversity of settings, including urban and rural areas, high-burden and lower-burden areas (in terms of tuberculosis prevalence, incidence, or case notification rate), and all World Bank income levels (appendix pp 9–10). A variety of tracing approaches were described between and within studies, including household contact tracing (17 of 20 studies)26, 27, 28, 29, 30, 31, 32, 33, 34, 36, 38, 39, 40, 41, 42, 43, 45 and screenings of neighbours (three studies)27, 33, 41 and co-workers (one study;31 table 2).
17 studies evaluated the effect of provider-initiated contact tracing for tuberculosis compared with a control intervention. Four of six RCTs and quasi-RCTs comparing contact tracing for tuberculosis to passive case finding or facility-based screening found an association between contact tracing and an increase in case detection38, 41, 43 or decrease in incidence (when tracing was coupled with treatment for contacts with active disease and prophylaxis for contacts with latent infection);28 whereas, the other two RCTs did not observe a positive effect on outcomes of interest26, 36 (table 2). Among 11 observational studies comparing tracing programmes with control interventions, eight (72·7%) found an increase in tuberculosis case detection,27, 30, 32, 34, 35, 40, 42, 45 one (9·1%) identified an increase in cases averted,44 one (9·1%) found a decrease in incidence,37 and one (9·1%) found fewer cases detected with provider-initiated tracing than with patient-initiated tracing39 (table 2). Among these 17 studies overall, tracing for tuberculosis led to improved outcomes in lower-burden settings (three of three)37, 38, 44 and high-burden settings (11 [78·6%] of 14 studies);26, 27, 28, 30, 32, 34, 35, 36, 39, 40, 41, 42, 43, 45 and in low-income countries (six of six)30, 34, 35, 41, 42, 45 and countries in other income categories (eight [72·7%] of 11 studies;26, 27, 28, 32, 36, 37, 38, 39, 40, 43, 44 table 2, appendix pp 9–10). The largest study in scale examined annual data from 2007 to 2010 for reported tuberculosis cases across Mexico, finding that each unit increase in the mean number of contacts traced per patient was associated with a 33·0% decrease in national incidence (r 2=–0·47, p=0·010).37
Three studies compared programmatic adaptations within provider-initiated contact tracing for tuberculosis (table 2). A cluster RCT in Uganda found no difference in the odds of tuberculosis diagnosis among household contacts when sputum was collected at home (intervention group) versus when contacts were referred to clinics for sputum collection (odds ratio 1·34 [95% CI 0·42–4·24], p=0·62), with the authors noting that only 35 (38·5%) of 91 eligible contacts in the intervention group could produce sputum samples at home.29 Two observational studies evaluated expansions to existing tuberculosis tracing: one study in Portugal found that tracing household and workplace contacts prevented more cases than tracing only named contacts,31 and the other study in Pakistan found that screening neighbours increased case detection by 7·9% compared with household contact tracing alone.33
Eight studies examined the effect of contact tracing programmes for HIV, enrolling a total of 15 439 index patients (appendix pp 11–14).46, 47, 48, 49, 50, 51, 52, 53 Five RCTs, two from Malawi46, 47 and one each from China,50 Kenya,48 and the USA,51 found that provider-initiated contact tracing was associated with higher HIV case detection rates compared with patient-initiated contact tracing. However, the study in China could not separate the effect of provider-initiated tracing from patient-initiated tracing with self-testing kits.50 Among the observational studies, a pre–post study from Botswana found that offering provider-initiated contact tracing and other services to index patients did not increase the number of partners diagnosed with HIV per index patient compared with patient-initiated tracing (0·14 vs 0·13, p=0·50).49 Two cohort studies from the USA evaluated adaptations to HIV contact tracing programmes. Malave and colleagues showed that contact tracing by disease intervention specialists (DIS) versus community clinicians led to similar rates of new HIV diagnoses among tested partners (20 [27·0%] of 74 partners tested and 10 [22·2%] of 45, p=0·56), although DIS intervention elicited significantly more partners per index patient (0·87 vs 0·22, p<0·01).52 Udeagu and colleagues53 reported a reduced HIV case detection rate with provider-initiated tracing by mobile phone text message (five [1·5%] cases among 325 contacts) or email (three [1·1%] among 267), compared with traditional methods (telephone or in-person; 106 [5·3%] among 2009).
12 studies examined provider-initiated contact tracing for curable STIs, which included 2383 index patients for syphilis,54, 55, 56 45 228 for gonorrhea,57, 58, 59, 60 484 for trichomoniasis,61 862 for chlamydia,62, 63 and 1446 for non-specified STIs64, 65 (appendix pp 15–19). Five RCTs or quasi-RCTs compared provider-initiated contact tracing with control interventions. Two RCTs, one on chlamydia in the USA63 and one on syndromic STIs in Zambia,64 found that provider-initiated tracing for male patients led to an increase in the number of treated partners (as a proxy for forward transmission); however, the Zambian study did not specify how many participants chose provider-initiated contact tracing versus an alternative referral slip (patient-initiated) intervention within the overall intervention group,64 and the US study did not assess how many partners in the control group received care elsewhere (and was therefore rated at high risk of bias).63 Two other RCTs and one quasi-RCT did not find evidence in favour of provider-initiated contact tracing for STIs,58, 61, 65 although one study noted a potentially underpowered analysis due to low trichomoniasis reinfection rates.61 Among the five observational studies comparing provider-initiated contact tracing with other interventions, four on gonorrhoea or syphilis found that provider-initiated tracing was associated with improvements in one or more of our outcomes of interest.55, 57, 59, 60 The remaining observational study on chlamydia found significantly fewer partners completed treatment after provider-initiated tracing by DIS compared with a patient-initiated approach.62 In addition, two studies examined the effect of programmatic adaptations within provider-initiated contact tracing interventions: an RCT detected no difference in syphilis case detection between immediate provider referral compared with contract referral (but was assessed to have high risk of bias due to absence of randomisation concealment and deviations in administered interventions; appendix p 8),56 and an observational study found the proportion of syphilis patients with at least one partner treated increased from 26% to 28% (8% relative increase) when tracing by email was added to traditional in-person or telephone contact.54
The single observational study for measles took place in the USA during a measles outbreak in 2017. It assessed contact tracing plus recommendation to isolate if symptoms develop, compared with contact tracing plus exclusion, in which measles-exposed individuals were recommended to be temporarily excluded from high-risk settings and to avoid public places, public transportation, and home visitors (appendix p 11). The Rt for non-excluded individuals was 1·61 (95% CI 1·00–2·69) compared with 0·38 (0·20–0·73) for excluded individuals, for a relative transmissibility (unadjusted ratio of Rt values) of 4·2 (95% CI 1·9–9·6).66
Discussion
We identified 47 empirical studies that either evaluated the effectiveness of provider-initiated contact tracing or compared programmatic adaptations within contact tracing interventions,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 including six studies focused on COVID-19. Among the studies were a wide range of tracing strategies (including phone calls,39, 53, 54 text messaging,29, 50, 53 and household contact tracing26, 27, 28, 29, 30, 31, 32, 33, 34, 36, 38, 39, 40, 41, 42, 43, 45) and personnel involved with the contact tracing (ranging from public health specialists52, 62, 63 to volunteers41), highlighting the diversity of programmatic approaches often taken.
Overall, provider-initiated contact tracing was generally associated with improved control of communicable illnesses: 29 (72·5%) of 40 studies evaluating the effect of provider-initiated contact tracing, including four (66·7%) of six observational studies on COVID-19 and 11 (68·8%) of 16 RCTs and quasi-RCTs on other diseases, reported increased case detection, decreased forward transmission, or decreased disease incidence compared with control interventions. Tracing was associated with improved outcomes across pathogens with diverse transmission dynamics and across a variety of settings. However, our ability to use these observations to form precise estimates on the effectiveness of contact tracing in disease control is limited largely by the heterogeneity of approaches and also by a relative scarcity of RCT data. Most studies (29 [61·7%] of 47) used observational designs with inherent vulnerability to confounding and other biases.
Among studies of contact tracing for COVID-19, four of six studies reported notable reductions in cases,20, 25 R0,21 or Rt24 after provider-initiated contact tracing efforts, suggesting that such programmes can have effectiveness in mitigating disease spread. However, these studies were almost exclusively in high-resource settings20, 21, 23, 24, 25 and used observational designs with differing programme approaches,20, 21, 22, 23, 24, 25 limiting generalisability. Most studies took place during a dynamic public health situation; as such, fluctuations in COVID-19 transmission and other externalities might have reduced accuracy in estimations of tracing effectiveness.67 These six studies do not capture how tracing speed or completeness influence success, and such parameters are likely to be important for disease control when pre-symptomatic onward transmission occurs.68 The studies also do not quantify how tracing feasibility and effectiveness might change as cases exceed a contact tracing workforce or laboratory testing capacity.
Most studies of provider-initiated contact tracing for diseases other than COVID-19 similarly reported improvements in our outcomes of interest, suggesting some benefit in disease control. The repeatedly reported effectiveness of tracing interventions for tuberculosis, including in low-resource, high-burden settings, was notable. However, these studies similarly examined many distinct tracing interventions and largely relied on observational methods, once again preventing a summary effectiveness measure.
Of note, two studies found that provider-initiated contact tracing resulted in lower case detection39 or fewer partners completing treatment62 than patient-initiated tracing. Although inconsistent with most other identified studies, these findings suggest that in particular contexts, well designed, patient-initiated methods might be preferable to provider-initiated approaches. These results might alternatively reflect difficulties in connecting provider-traced contacts to follow-up services in these studies.
As a result, although contact tracing is shown to be an effective public health tool, additional evidence is needed to inform how, where, and when to deploy this tool most effectively for maximum effect in disease control, including against COVID-19. When feasible, further comparative evaluations, such as randomised, pragmatic trials within existing tracing systems, should be undertaken to evaluate different tracing interventions in real life and identify potential adaptations for unique infections and contexts.
In the interim, specific lessons learned from these studies, including those in which provider-initiated contact tracing was not shown to reduce disease spread or appeared to worsen outcomes, can inform elements of tracing programmes for COVID-19 and other diseases. Davis and colleagues29 concluded that low rates of home-based contact tracing for tuberculosis might have related to limited experience among community health workers in collecting sputum and a hesitation among contacts to expectorate in public, highlighting the importance of sufficient training for tracers and privacy for contacts. Two studies (Udeagu et al53 and Ehlman et al54) showed that contact tracing via email or text message had less success than traditional contract tracing, but nevertheless increased case detection53 or the number of index patients with at least one partner receiving treatment54 when added to traditional contact tracing, highlighting how technology might increase tracing impact. Notably, two UK studies found widespread use of a smartphone-based proximity detection and exposure notification application was associated with substantial reductions in COVID-19 cases25 and R0,21 although these studies were unable to fully isolate the effects of digital contact tracing from simultaneous traditional contact tracing and other interventions.
Strengths of this review include its consideration of multiple geographic regions and socioeconomic contexts across the included studies. Limitations include the predominance of observational studies, incomplete reporting in some studies, and the possibility of publication bias. We aimed to better understand how contact tracing would apply to the COVID-19 pandemic, but the unique characteristics of COVID-19, including frequent presymptomatic transmission69 and a relatively short serial interval,70 limit the generalisability of evidence from other diseases. Our study focused on disease control and did not evaluate other important outcomes of contact tracing such as how it helps understanding of disease dynamics or its ability to serve as an entry point to the health system for those at risk of disease. Further research should evaluate which patient groups (such as individuals experiencing homelessness or marginalised or minoritised communities) might differentially be excluded from or could be engaged by tracing programmes; how tracing might be affected by local values or customs, or by the accuracy and privacy of digital tools;71, 72 and how the cost and cost-effectiveness of tracing programmes compare with alternative interventions.
Our review confirms a premise for contact tracing as an effective public health tool for infectious disease control, however we found little empirical data to inform best practices for contact tracing, revealing an opportunity to further evaluate this common practice relative to other public health strategies. As health agencies continue their responses against COVID-19 while responding to other ongoing health threats and planning for future pandemics, addressing these knowledge gaps through additional studies or analyses of current tracing programmes is crucial to inform on the role of contact tracing in protecting the health of communities.
Data sharing
All data are available in this Article and in the appendix.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was funded by the US National Institute of Allergy and Infectious Diseases (grant number T32 AI007433 to JJ), the Massachusetts General Hospital Executive Committee on Research (Fund for Medical Discovery Fellowship; to JJ), and the Sullivan Family Foundation (to LCI).
Acknowledgments
Contributors
LCI conceived of the study. AR and LCI designed the study. ADH, JJ, AE, CXH, and AR were responsible for the search protocol, data extraction, and risk of bias ratings. ADH and JJ wrote the first draft of the manuscript. AE, CXH, AR, and LCI edited the manuscript. All authors had full access to all study data, approved the final version of the manuscript, and agreed to submit for publication. ADH and JJ accessed and verified all data.
Supplementary Material
References
- 1.Mooney G. “A menace to the public health”—contact tracing and the limits of persuasion. N Engl J Med. 2020;383:1806–1808. doi: 10.1056/NEJMp2021887. [DOI] [PubMed] [Google Scholar]
- 2.Baxter S, Goyder E, Chambers D, Johnson M, Preston L, Booth A. Interventions to improve contact tracing for tuberculosis in specific groups and in wider populations: an evidence synthesis. Health Serv Deliv Res. 2017;5:1–102. [PubMed] [Google Scholar]
- 3.Rutherford GW, Woo JM. Contact tracing and the control of human immunodeficiency virus infection. JAMA. 1988;259:3609–3610. [PubMed] [Google Scholar]
- 4.Henderson RH. Control of sexually transmitted diseases in the United States–a federal perspective. Br J Vener Dis. 1977;53:211–215. doi: 10.1136/sti.53.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson KC, Altare C, Wesseh CS, et al. Contact tracing performance during the Ebola epidemic in Liberia, 2014–2015. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Summers J, Cheng H-Y, Lin H-H, et al. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. Lancet Reg Health West Pac. 2020;4 doi: 10.1016/j.lanwpc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TV, Tran QD, Phan LT, et al. In the interest of public safety: rapid response to the COVID-19 epidemic in Vietnam. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lash RR, Moonan PK, Byers BL, et al. COVID-19 case investigation and contact tracing in the US, 2020. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 11.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. Am J Prev Med. 2007;33(suppl):S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Morb Mortal Wkly Rep. 2008;57:1–63. [PubMed] [Google Scholar]
- 14.WHO Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. December, 2016. https://apps.who.int/iris/bitstream/handle/10665/251655/9789241549868-eng.pdf?sequence=1 [PubMed]
- 15.WHO Systematic screening for active tuberculosis: principles and recommendations. 2013. https://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf [PubMed]
- 16.WHO Digital tools for COVID-19 contact tracing. June 2, 2020. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Contact_Tracing-Tools_Annex-2020.1
- 17.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O'Connel D, et al. Ottawa Hospital Research Institute; Ottawa, ON: 2000. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 19.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488–494. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fetzer T, Graeber T. Measuring the scientific effectiveness of contact tracing: Evidence from a natural experiment. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2100814118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall M, Milsom L, Abeler-Dörner L, et al. Epidemiological changes on the Isle of Wight after the launch of the NHS Test and Trace programme: a preliminary analysis. Lancet Digit Health. 2020;2:e658–e666. doi: 10.1016/S2589-7500(20)30241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Morgenstern C, Kelly J, Lowe R, Jit M. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021;19:40. doi: 10.1186/s12916-020-01872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malheiro R, Figueiredo AL, Magalhães JP, et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: a retrospective cohort study. Public Health. 2020;189:54–59. doi: 10.1016/j.puhe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park Y, Huh IS, Lee J, et al. Application of testing-tracing-treatment strategy in response to the COVID-19 outbreak in Seoul, Korea. J Korean Med Sci. 2020;35:e396. doi: 10.3346/jkms.2020.35.e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wymant C, Ferretti L, Tsallis D, et al. The epidemiological impact of the NHS COVID-19 app. Nature. 2021;594:408–412. doi: 10.1038/s41586-021-03606-z. [DOI] [PubMed] [Google Scholar]
- 26.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–1194. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 27.Becerra MC, Pachao-Torreblanca IF, Bayona J, et al. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005;120:271–277. doi: 10.1177/003335490512000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalcante SC, Durovni B, Barnes GL, et al. Community-randomized trial of enhanced DOTS for tuberculosis control in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2010;14:203–209. [PMC free article] [PubMed] [Google Scholar]
- 29.Davis JL, Turimumahoro P, Meyer AJ, et al. Home-based tuberculosis contact investigation in Uganda: a household randomised trial. ERJ Open Res. 2019;5:00112–02019. doi: 10.1183/23120541.00112-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dongo JP, Graham SM, Nsonga J, et al. Implementation of an effective decentralised programme for detection, treatment and prevention of tuberculosis in children. Trop Med Infect Dis. 2021;6:131. doi: 10.3390/tropicalmed6030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte R, Neto M, Carvalho A, Barros H. Improving tuberculosis contact tracing: the role of evaluations in the home and workplace. Int J Tuberc Lung Dis. 2012;16:55–59. doi: 10.5588/ijtld.10.0511. [DOI] [PubMed] [Google Scholar]
- 32.Eyo AS, Obot VO, Onyedinachi O, et al. A multi-faceted approach to tuberculosis active case finding among remote riverine communities in southern Nigeria. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18189424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatima R, Qadeer E, Yaqoob A, et al. Extending ‘contact tracing’ into the community within a 50-metre radius of an index tuberculosis patient using Xpert MTB/RIF in urban, Pakistan: did it increase case detection? PLoS One. 2016;11 doi: 10.1371/journal.pone.0165813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gashu Z, Jerene D, Ensermu M, et al. The yield of community-based “retrospective” tuberculosis contact investigation in a high burden setting in Ethiopia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurung SC, Dixit K, Rai B, et al. Comparative yield of tuberculosis during active case finding using GeneXpert or smear microscopy for diagnostic testing in Nepal: a cross-sectional study. Trop Med Infect Dis. 2021;6:50. doi: 10.3390/tropicalmed6020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanrahan CF, Nonyane BAS, Mmolawa L, et al. Contact tracing versus facility-based screening for active TB case finding in rural South Africa: a pragmatic cluster-randomized trial (Kharitode TB) PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Garduño E, Mendoza-Damián F, Garduño-Alanís A, Ayón-Garibaldo S. Tuberculosis in Mexico and the USA, comparison of trends over time 1990–2010. Tuberc Respir Dis (Seoul) 2015;78:246–252. doi: 10.4046/trd.2015.78.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatana GH, Haq I, Khan SMS. Effectiveness, acceptance and feasibility of home-based intervention model for tuberculosis contact tracing in Kashmir. J Clin Tuberc Other Mycobact Dis. 2019;14:19–25. doi: 10.1016/j.jctube.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kliner M, Knight A, Elston J, et al. Development and testing of models of tuberculosis contact tracing in rural southern Africa. Public Health Action. 2013;3:299–303. doi: 10.5588/pha.13.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandalakas AM, Ngo K, Alonso Ustero P, et al. BUTIMBA: intensifying the hunt for child TB in Swaziland through household contact tracing. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morishita F, Eang MT, Nishikiori N, Yadav R-P. Increased case notification through active case finding of tuberculosis among household and neighbourhood contacts in Cambodia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanaie A, Mergenthaler C, Nasrat A, et al. An evaluation of passive and active approaches to improve tuberculosis notifications in Afghanistan. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah L, Rojas Peña M, Mori O, et al. A pragmatic stepped-wedge cluster randomized trial to evaluate the effectiveness and cost-effectiveness of active case finding for household contacts within a routine tuberculosis program, San Juan de Lurigancho, Lima, Peru. Int J Infect Dis. 2020;100:95–103. doi: 10.1016/j.ijid.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Young KH, Ehman M, Reves R, et al. Tuberculosis contact investigations—United States, 2003–2012. MMWR Morb Mortal Wkly Rep. 2016;64:1369–1374. doi: 10.15585/mmwr.mm6450a1. [DOI] [PubMed] [Google Scholar]
- 45.Zachariah R, Spielmann MP, Harries AD, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–1039. [PubMed] [Google Scholar]
- 46.Brown LB, Miller WC, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JS, Matoga M, Pence BW, et al. A randomized controlled trial evaluating combination detection of HIV in Malawian sexually transmitted infections clinics. J Int AIDS Soc. 2021;24 doi: 10.1002/jia2.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherutich P, Golden MR, Wamuti B, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV. 2017;4:e74–e82. doi: 10.1016/S2352-3018(16)30214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grande M, Mawandia S, Bakae O, et al. Intensified assisted partner notification implementation in Botswana increased partner identification but not HIV case-finding: findings highlight the need for improved data monitoring. J Acquir Immune Defic Syndr. 2021;87:951–958. doi: 10.1097/QAI.0000000000002673. [DOI] [PubMed] [Google Scholar]
- 50.Hu Q-H, Qian H-Z, Li J-M, et al. Assisted partner notification and uptake of HIV testing among men who have sex with men: a randomized controlled trial in China. Lancet Reg Health West Pac. 2021;12 doi: 10.1016/j.lanwpc.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landis SE, Schoenbach VJ, Weber DJ, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med. 1992;326:101–106. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 52.Malave MC, Shah D, Sackoff JE, Rubin S, Begier EM. Human immunodeficiency virus partner elicitation and notification in New York City: public health does it better. Sex Transm Dis. 2008;35:869–876. doi: 10.1097/OLQ.0b013e31817d2f82. [DOI] [PubMed] [Google Scholar]
- 53.Udeagu C-CN, Bocour A, Shah S, Ramos Y, Gutierrez R, Shepard CW. Bringing HIV partner services into the age of social media and mobile connectivity. Sex Transm Dis. 2014;41:631–636. doi: 10.1097/OLQ.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 54.Ehlman DC, Jackson M, Saenz G, et al. Evaluation of an innovative internet-based partner notification program for early syphilis case management, Washington, DC, January 2007–June 2008. Sex Transm Dis. 2010;37:478–485. doi: 10.1097/OLQ.0b013e3181e212cb. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control Epidemic early syphilis—Montgomery County, Alabama, 1990–1991. MMWR Morb Mortal Wkly Rep. 1992;41:790–794. [PubMed] [Google Scholar]
- 56.Peterman TA, Toomey KE, Dicker LW, Zaidi AA, Wroten JE, Carolina J. Partner notification for syphilis: a randomized, controlled trial of three approaches. Sex Transm Dis. 1997;24:511–518. doi: 10.1097/00007435-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Du P, Coles FB, Gerber T, McNutt L-A. Effects of partner notification on reducing gonorrhea incidence rate. Sex Transm Dis. 2007;34:189–194. doi: 10.1097/01.olq.0000237861.47751.16. [DOI] [PubMed] [Google Scholar]
- 58.Potterat JJ, Rothenberg R. The case-finding effectiveness of self-referral system for gonorrhea: a preliminary report. Am J Public Health. 1977;67:174–176. doi: 10.2105/ajph.67.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schleihauf E, Leonard E, Phillips C, et al. Increase in gonorrhea incidence associated with enhanced partner notification strategy. Sex Transm Dis. 2019;46:706–712. doi: 10.1097/OLQ.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 60.Woodhouse DE, Potterat JJ, Muth JB, Pratts CI, Rothenberg RB, Fogle JS., 2nd A civilian-military partnership to reduce the incidence of gonorrhea. Public Health Rep. 1985;100:61–65. [PMC free article] [PubMed] [Google Scholar]
- 61.Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sex Transm Dis. 2010;37:392–396. doi: 10.1097/OLQ.0b013e3181dd1691. [DOI] [PubMed] [Google Scholar]
- 62.Jones AT, Craig-Kuhn MC, Schmidt N, et al. Adapting index/partner services for the treatment of chlamydia among young African American men in a community screening program. Sex Transm Dis. 2021;48:323–328. doi: 10.1097/OLQ.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katz BP, Danos CS, Quinn TS, Caine V, Jones RB. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis. 1988;15:11–16. doi: 10.1097/00007435-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Faxelid E, Tembo G, Ndulo J, Krantz I. Individual counseling of patients with sexually transmitted diseases. A way to improve partner notification in a Zambian setting? Sex Transm Dis. 1996;23:289–292. [PubMed] [Google Scholar]
- 65.Mathews C, Lombard C, Kalichman M, et al. Effects of enhanced STI partner notification counselling and provider-assisted partner services on partner referral and the incidence of STI diagnosis in Cape Town, South Africa: randomised controlled trial. Sex Transm Infect. 2021;97:38–44. doi: 10.1136/sextrans-2020-054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee E, Paul P, Griffith J, Laine E, Como-Sabetti K, Gastañaduy PA. Impact of isolation and exclusion as a public health strategy to contain measles virus transmission during a measles outbreak. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab939. published online Nov 10. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Huang J, Li C, et al. The role of seasonality in the spread of COVID-19 pandemic. Environ Res. 2021;195 doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hjorleifsson KE, Rognvaldsson S, Jonsson H, et al. Reconstruction of a large-scale outbreak of SARS-CoV-2 infection in Iceland informs vaccination strategies. medRxiv. 2021 doi: 10.1101/2021.06.11.21258741. published online June 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rai B, Shukla A, Dwivedi LK. Estimates of serial interval for COVID-19: a systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;9:157–161. doi: 10.1016/j.cegh.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivers LC, Weitzner DJ. Can digital contact tracing make up for lost time? Lancet Public Health. 2020;5:e417–e418. doi: 10.1016/S2468-2667(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toh A, Brown D. Human Rights Watch; June 4, 2020. How digital contact tracing for COVID-19 could worsen inequality.https://www.hrw.org/news/2020/06/04/how-digital-contact-tracing-covid-19-could-worsen-inequality [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in this Article and in the appendix.