Abstract
Introduction
A mass vaccination campaign against SARS-CoV-2 was initiated in European countries on December 27, 2020. This study compared the antibody response in a sample of healthcare workers (HCWs) who, after the first dose of the BNT162b2 mRNA vaccine, were infected with SARS-CoV-2 (infection group) with the response in a control group of HCWs immunized with two doses (vaccine group).
Methods
This two-arm observational cohort study was carried out using routine health surveillance data obtained from HCWs at Bari Policlinico General Hospital (Italy). The antibody response was determined infection group and vaccine group.
Results
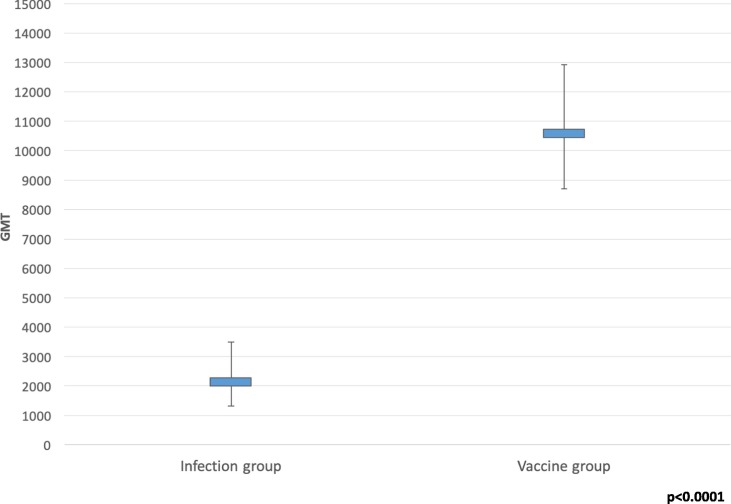
Among the 100 HCWs, 25 (25.0%) were in the infection group and 75 (75.0%) in the full-vaccine group. At the serological evaluation, all of the HCWs tested positive, with a geometric mean titer (GMT) of 7106.8 (95 %CI = 5628.5–8973.4) and a statistically significant difference (p < 0.0001) between the infection group (GMT = 2139.7; 95 %CI = 1310.4–3493.6) and the vaccine group (GMT = 10603.6; 95 %CI = 8698.0–12926.8).
Discussion
Our results shed light on the vaccine response of individuals in different risk categories. It also emphasizes the need for the continued use by HCWs of PPE and good practices during the window between the first and second anti-SARS-CoV-2 vaccinations.
Abbreviations: WHO, World Health Organization; HCWs, Healthcare workers; AIFA, Italian Drugs Agency
Keywords: Immunization, Biological risk, Healthcare workers, COVID-19, Vaccine
1. Introduction
COVID-19 is the infectious disease caused by the novel coronavirus SARS-CoV-2. Neither the virus nor the potentially fatal disease it causes were known before the occurrence of the first cases, in Wuhan, China, in December 2019. COVID-19 is now a pandemic, having reached global proportions [1], with > 140,000,000 confirmed cases and > 3,000,000 deaths worldwide [2]. Within the EU/EEA, there have been > 28,000,000 cases and > 645,000 deaths [3]. Among European countries, Italy ranks first in the number of COVID-19-related deaths (114,470; lethality: 3%) and second in absolute number of cases (n = 3,825,445), including 131,312 cases in healthcare workers (HCWs) [4].
Beginning in December 2020, four vaccines aimed at the prevention of SARS-CoV-2 infection and thus COVID-19 became available in Europe. A mass vaccination campaign was initiated in Italy and other European countries on December 27, 2020, but its effects will not be appreciable until late 2021. In Italy, the government opted to prioritize the vaccination of HCWs, a decision in line with the recommendations of the Center for Disease Control and Prevention [5]. By providing critical care to those who are or might be infected with SARS-CoV-2, HCWs are at high risk of exposure to the virus and thus to the development of COVID-19. Vaccinating HCWs safeguards healthcare capacity and can prevent patients hospitalized for reasons other than COVID-19 from becoming infected. The vaccine used to immunize HCWs in Italy was the BNT162b2 mRNA COVID-19 vaccine (Comirnaty), the first vaccine to be approved by the European Medicines Agency. The vaccine is administered in two-doses delivered at least 21 days apart and at the time of its release was indicated for individuals 16 years of age and older [6], [7].
The pre-licensure trial showed that the vaccine had 95% efficacy in preventing COVID-19, including severe disease. Aside from transient local and systemic reactions, no safety concerns were identified [8]. A subsequent study showed high titers of IgM and IgG against the SARS-CoV-2 spike protein, including the receptor-binding-domain, in vaccinated individuals 8 weeks after the second dose [9].
Because protection against COVID-19 is first gained ∼ 7 days after the second vaccine dose, SARS-CoV-2 infections may occur during the 21-day interval between the two doses. According to the Italian Drugs Agency (AIFA), in people who develop confirmed SARS-CoV-2 infection/disease after the first vaccine dose, the infection itself provides a powerful boost to the immune system that adds to the immune response induced by the first vaccine dose. Consequently, in Italy a second vaccine dose is considered unnecessary in this group [10]. However, AIFA’s decision was adopted without evidence of the immune response generated by a one dose schedule and this strategy is not described in the official Summary of Products Characteristics for Comirnaty [11].
Thus, the aim of this study was to compare the antibody response in HCWs who, after the first dose of the BNT162b2 mRNA vaccine, were diagnosed with SARS-CoV-2 infection with the response in HCWs immunized with two doses. Our study was carried out in Apulia (Southern Italy, ∼4,000,000 inhabitants), where, since February 2020, 166,237 confirmed cases of COVID-19 and 4,303 related deaths have been reported [12].
2. Methods
This observational cohort study was conducted at Bari Policlinico General University-Hospital (1000 beds, 6000 HCWs), where 180 hospital beds are reserved for COVID patients and the Emergency Room is charged with the triage and care of COVID-19 patients. December 27, 2020 marked the start of the vaccination campaign for HCWs, with scheduling and follow-up activities coordinated by the Hygiene and Occupational Medicine departments of Bari Policlinico.
Vaccination at the hospital is organized as follows: after requesting vaccination by completing an intranet on-line form, the HCW is contacted by the Hygiene Department to schedule an appointment for immunization, with the date confirmed by mail or phone. An appointment is also scheduled for the second dose, delivered 21–28 day after the first.
All vaccinations are administered by Public Health physicians with expertise in vaccinology. The two doses of BNT162b2 mRNA vaccine are delivered intramuscularly in the deltoid muscle, with a minimum interval of 21 days. The HCW can refuse vaccination, as vaccination prophylaxis is not mandatory. Informed consent is collected at the time of vaccination. All vaccinated HCWs are followed-up for 1 month to assess the development of adverse effects.
Policlinico Bari General Hospital also adopted a specific procedure for the control and prevention of SARS-CoV-2 infection among its HCWs. All asymptomatic HCWs are screened every 14 days for SARS-CoV-2 infection using molecular tests of naso-pharyngeal swabs obtained as recommended by the WHO [13]. Fast-track access to molecular testing is ensured for HCWs with signs and symptoms of COVID-19 (fever, cough, ageusia, etc.). Infected HCWs are included in an active surveillance program conducted by Public Health physicians and followed-up until they test negative for the virus. Data on infection control and prevention are entered into the computerized COVID-19 platform GIAVA, a regional immunization database developed in accordance with the WHO Go. Data outbreak investigation tool and set up to manage the pandemic emergency in Apulia.
Finally, according to hospital procedures, HCWs (the immunized, those with a history of SARS-COV-2 infection, and the “naïve”) undergo monthly serological testing to evaluate the general burden of SARS-CoV-2 circulation in the hospital.
The study population consisted of:
-
1.
Bari Policlinico HCWs who tested positive for SARS-CoV-2 after the first dose of vaccine, administered between December 27, 2020 and January 31, 2021 (infection group)
-
2.
A control group of HCWs who participated in the same vaccination program but who completed the two doses (vaccine group)
All HCWs included in the study completed and signed an informed consent form allowing the use of their personal and health data for scientific purposes. Ethical approval was not requested for this study because only data collected for routine health surveillance activity were used. The study was carried out in accordance with the Helsinki Declaration.
An allocation ratio of 1:3 was applied to improve the statistical analysis. The two groups were matched for age and sex using STATA MP16 software, with a final sample of 100 individuals: 25 in the infection group and 75 in the vaccine group.
To stratify HCWs in the infection group according to disease severity, a synthetic score was adopted (“S + D score”) that took into account the HCW’s presenting symptoms and the drugs used to treat the disease. However, less weight was assigned to the latter given that the type and amount of drugs used in the management of COVID-19 reflect the presence and severity of symptoms. Based on the score attributed to the individual items (Table 1 ), the final score ranged between 0 and 18.5.
Table 1.
S + D Score.
| Symptoms | Score |
|---|---|
| Fever | 2 |
| Cough | 2 |
| Asthenia | 0.2 |
| Rhinorrhea | 0.2 |
| Anosmia | 0.2 |
| Ageusia | 0.2 |
| Diarrhea | 1 |
| Conjunctivitis | 0.2 |
| Headache | 1 |
| Myalgia | 1 |
| Dyspnea | 2 |
| Drugs | |
| Paracetamol | 0.5 |
| Azithromycin | 1 |
| Cortisone | 1 |
| Cefixoral | 1 |
| Hospitalization | 5 |
| Total | 18.5 |
HCWs with a documented history of SARS-CoV-2 infection before enrollment were excluded from study participation. The overall vaccination status of HCWs and data on documented cases of SARS-CoV-2 infection were extracted from GIAVA, which stores data on COVID-19 patients and their contacts, patient demographics, laboratory and clinical values, the results of SARS-CoV-2 PCR testing, and the follow-up of COVID-19 patients over the course of the disease, with updates of their health status (clinical symptoms, hospitalization, death, recovery) [14].
For each of the HCWs in the infection group, the results of a quantitative serological test for IgG performed 30 days after the diagnosis of positivity for SARS-CoV-2 were recorded, and for HCWs in the vaccine group, the results of a quantitative serological test for IgG performed 30 days after the administration of the second vaccine.
Serum samples were tested for SARS-CoV-2-IgG using the Abbott Architect instrument. Testing consisted of a quantitative evaluation of serum lgG against the SARS-CoV-2 spike protein. The result are reported as previously described [15] and expressed as AU/mL.
The final dataset was created as an Excel spreadsheet that included information on sex, age at first vaccine dose, group (infection vs. vaccinated), documented infection (YES/NO), symptoms, hospitalization (YES/NO), and the results of the serological tests. An anonymized data analysis was performed using the STATA MP16 software.
Continuous variables are reported as the mean ± standard deviation and range, and categorical variables as proportions. Serological results are expressed as the geometric mean titer (GMT). Skewness and kurtosis tests were used to evaluate the normality of the continuous variables; when possible, non-normally distributed variables were normalized in a normalization test. Student’s t-test for independent data or Wilcoxon’s rank sum test was used to compare continuous variables between groups, and a chi-squared test to compare group proportions.
GMT determinants were assessed in a multivariate linear regression model, in which GMT was the outcome and group (infection vs. vaccine), sex (male vs. female), and age at first vaccine dose (years) were the determinants. Correlation coefficients were calculated together with their 95% confidence intervals (95 %CIs).
The relationship between the time elapsed since the vaccine dose, the COVID-19 diagnosis, the IgG value, and the S + D score was assessed in two univariate linear regressions. Correlation coefficients were calculated together with their 95 %CIs and R2 values.
For all tests, a two-sided p-value < 0.05 was considered to indicate statistical significance.
3. Results
The study population included 100 HCWs, of whom 25 (25.0%) were in the infection group and 75 (75.0%) in the vaccine group. Overall, 63 (63.0%) were females, without a significant difference between the female:male ratio in the infection group (n = 17/25; 68.0%) and the vaccine group (46/75; 61.3%; p = 0.550). The average age at first vaccine dose was 44.5 ± 13.7 (range: 20–69) years and did not differ significantly between groups (infection group: 46.0 ± 13.9 [range: 20–64] years; vaccine group: 44.0 ± 13.7 [range = 23–69] years; p = 0.575).
On average, COVID-19 was diagnosed 9.2 ± 5.1 (range: 0–18) days after the first vaccine dose; 4/25 (16.0%) HCWs were asymptomatic and 21/25 (84.0%) had at least one symptom, including fever (11/25, 44.0%), cough (10/25, 40.0%), asthenia (14/25, 56.0%), rhinorrhea (8/25, 32.0%), anosmia (14/25, 56.0%), ageusia (13/25, 52.0%), diarrhea (5/25, 20.0%), conjunctivitis (1/25, 4.0%), headache (11/25, 44.0%), myalgia (12/25, 48.0%), and dyspnea (7/25, 28.0%). Symptoms lasted for an average of 12.1 ± 12.6 (range: 2–60) days. One HCW required 22 days of hospitalization. The mean S + D score was 4.7 ± 3.7.
At serological evaluation, all HCWs tested positive; the mean GMT was 7,106.8 (95 %CI: 5,628.5–8,973.4), with a statistically significant difference (p < 0.0001) between the infection group (GMT = 2,139.7; 95 %CI = 1,310.4–3,493.6) and the vaccine group (GMT = 10,603.6; 95 %CI = 8,698.0–12,926.8; Fig. 1 ).
Fig. 1.
GMT values in the infection and vaccine groups.
In the linear multivariate analysis, the relationship between GMT and group was significant (coefficient = −10,037.6; 95 %CI = −14,816.6–5,258.6), whereas associations with sex and age were not (p > 0.05; Table 2 ).
Table 2.
Relationship between GMT and group, sex, and age in a multivariate linear regression model.
| Determinant | Coefficient | 95% confidence interval | p-value |
|---|---|---|---|
| Group (infection vs. vaccine) | −10037.6 | −14816.6 to −5258.6 | <0.0001 |
| Sex (male vs. female) | −3954.4 | −8244.9 to 366.1 | 0.070 |
| Age (years) | −41.8 | −193.6 to 109.9 | 0.585 |
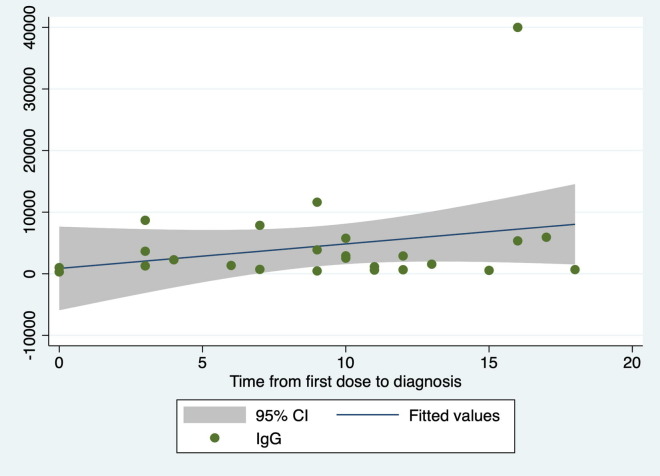
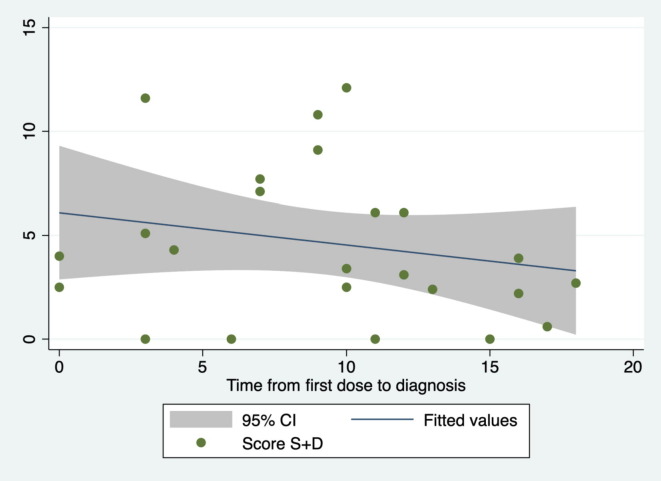
Among the infected HCWs, the time from vaccines dose to diagnosis was not significantly related to the IgG level (coefficient = 398.1; 95 %CI = 247.0 –1,043.2; p = 0.215; R2 = 0.07; Fig. 2 ) or the S + D score (coefficient = − 0.16; 95 %CI = −0.46 – 0.15; p = 0.304; R2 = 0.05; Fig. 3 ).
Fig. 2.
Relationship between time from the first vaccines dose to COVID-19 diagnosis and the IgG level.
Fig. 3.
Relationship between the time from the first vaccines dose to diagnosis and the S + D score.
4. Conclusions
All of the HCWs enrolled in our study tested positive at serological evaluation, but HCWs vaccinated with two doses had a significantly greater antibody response than those who were vaccinated with one dose and then became infected. This was evidenced by the GMT in the two groups (10,603.6 vs. 2139.7; p > 0.0001) and by the results of the multivariate regression model (coefficient = −10037.6; 95 %CI = −14816.6 to −5258.6).
Our results can be compared with those of a 2021 study [16] that analyzed 67 fully vaccinated HCWs without documented pre-existing SARS-CoV-2 immunity and 43 HCWs with documented pre-existing SARS-CoV-2 immunity who had received only one dose of the vaccine. The authors found that a single dose of mRNA vaccine elicited a rapid immune responses in seropositive participants, with postvaccination antibody titers that were similar to or higher than the titers measured in seronegative participants who received two vaccine doses. That study and our own together suggest that individuals vaccinated with one dose who then become infected develop a poorer response than those who are fully vaccinated, while individuals who are infected and then vaccinated with one dose develop a response similar to that of the fully vaccinated. Further studies are needed to examine the differences in the immune response of HCWs differing in their SARS-CoV-2 exposure.
Univariate linear regressions of HCWs infected after the first vaccine dose suggested that a longer time between the first vaccine dose and the diagnosis of infection is associated with a higher level of IgG and milder symptoms; however, this finding could not be supported statistically, presumably due to the small sample size.
To our knowledge this is the only study to have addressed SARS-CoV-2 infection in HCWs who received a single dose of a two-dose vaccine. It should be pointed out that the anti-spike IgG (and possibly IgG directed against other viral domains) used in the quantitative serological test is only able to evaluate immunity achieved after SARS-CoV-2 infection; thus, neither the extent of the total antibody response nor the differences between the infection and vaccine groups could be accurately determined. Although HCWs who received two doses of the vaccine had a higher antibody titer against the spike protein than those who received only one dose, whether the immunity conferred by actual infection has a greater protective effect against re-infection is unknown. The small sample size reflected the high rate of anti-SARS-CoV-2 vaccination within Bari Policlinico, with 6643 of its 10,702 HCWs and medical school students having been fully vaccinated thus far. This high rate of vaccination has reduced circulation of the virus within the hospital and therefore the risk of an outbreak of infection between the first and second doses. For this reason, our study is difficult to repeat or expand.
In conclusion, our study sheds light on the vaccine response in different risk categories. Its results highlight the importance of the continued use of both PPE and good practices in the hospital setting during the window between the first and second vaccinations. The false security that is common among health professionals soon after the first dose of vaccine can give rise to inappropriate and risky behavior. Our results also call into question mono-administration of the anti-SARS-CoV-2 vaccine in individuals with a confirmed positive SARS-CoV-2 infection in the previous 6 months, although this choice is clearly also dictated by the limited availability of vaccine. Thus, individuals with a history of SARS-CoV-2 infection scheduled for only one vaccine dose should be offered a close serological follow-up. Finally, we recommend the continued serological monitoring of individuals vaccinated with two vaccine doses, as a benchmark for evaluating the trend in the antibody response and possible susceptibility to SARS-CoV-2 infection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectorsThe authors have no competing interests to declare.The manuscript has not been presented at a meeting.
References
- 1.WHO. Q&A on coronaviruses (COVID-19). Updated 10 November 2020. Available on: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses. Last accessed on April 17, 2021.
- 2.WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int/. Last accessed on April 19, 2021.
- 3.ECDC. COVID-19 situation update for the EU/EEA, as of week 2 2021. Available on: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea. Last accessed on April 19, 2021.
- 4.Istituto Superiore di Sanità. Epidemiology for public health. COVID-19 integrated surveillance data in Italy. Available on: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard. Last accessed on April 19, 2021.
- 5.CDC. The Importance of COVID-19 Vaccination for Healthcare Personnel. Updated December 28, 2020. Available on: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/hcp.html. Last accessed on April 1, 2021.
- 6.CDC. Pfizer-BioNTech COVID-19 Vaccine. Available on: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html. Last accessed on April 12, 2021.
- 7.Italian Ministry of Health. Piano vaccini anti Covid-19. Vaccino Comirnaty di Pfizer/BioNTech. Available on: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5452&area=nuovoCoronavirus&menu=vuoto&tab=1. Last accessed on April 13, 2021.
- 8.Polack Fernando P., Thomas Stephen J., Kitchin Nicholas, Absalon Judith, Gurtman Alejandra, Lockhart Stephen, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, Oliveira TY, Yang Z, Abernathy ME, Huey-Tubman KE, Hurley A, Turroja M, West KA, Gordon K, Millard KG, Ramos V, Da Silva J, Xu J, Colbert RA, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Gazumyan A, Caskey M, Bjorkman PJ, Casellas R, Hatziioannou T, Bieniasz PD, Nussenzweig MC. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv [Preprint]. 2021 Jan 19:2021.01.15.426911.
- 10.AIFA. COVID-19 Vaccines. Available on: https://www.aifa.gov.it/domande-e-risposte-su-vaccini-covid-19. Last accessed on April 11, 2021.
- 11.European Medicine Agency. Vaxzevria. Available on: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca. Last accessed on March 16, 2021.
- 12.Apulia Region. COVID-19 Epidemiological Update. Available on: https://www.regione.puglia.it/documents/65725/216593/Bollettino+Covid_15032021.pdf/64801426-f249-72ea-01c1-6fc9fab8552b?t=1615816279358. Last accessed on March 15, 2021.
- 13.WHO. Use of laboratory methods for SARS diagnosis. Available on: https://www.who.int/health-topics/severe-acute-respiratory-syndrome/technical-guidance/laboratory/use-of-laboratory-methods-for-sars-diagnosis. Last accessed on April 9, 2021.
- 14.Pedote P.D., Termite S., Gigliobianco A., Lopalco P.L., Bianchi F.P. Influenza Vaccination and Health Outcomes in COVID-19 Patients: A Retrospective Cohort Study. Vaccines. 2021;9(4):358. doi: 10.3390/vaccines9040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryan Andrew, Pepper Gregory, Wener Mark H., Fink Susan L., Morishima Chihiro, Chaudhary Anu, et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer Florian, Srivastava Komal, Alshammary Hala, Amoako Angela A., Awawda Mahmoud H., Beach Katherine F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]