Abstract
The COVID-19 pandemic has led to significant mortality in the United States with more than 800,000 deaths in 2020 and 2021. The proportion of patients with COVID-19 who develop severe disease varies but is decreasing over time with growing population immunity and improved therapeutic options. Patients who are 65 years and older represent the largest proportion of deaths from COVID-19. Additional risk factors include immunosuppression and chronic medical conditions. Vaccination dramatically reduces the risk of severe COVID-19. Although critical illness from COVID-19 is mostly driven by respiratory disease, critical illness can manifest in several ways and affect several organ systems.
Keywords: COVID-19, COVID, SARS-CoV-2, Critically ill, Critical care, ARDS, Acute respiratory distress syndrome, Coronavirus
Key points
-
•
Severe COVID-19 is characterized by dyspnea, tachypnea, hypoxemia, and bilateral pulmonary infiltrates. The proportion of patients with COVID who develop severe disease has been decreasing over time.
-
•
Age greater than or equal to 65 years is the strongest risk factor for developing severe COVID-19. Additional risk factors include obesity, anxiety disorders and depression, chronic diseases, and complicated diabetes.
-
•
Although the primary driver of critical illness from COVID-19 is the acute respiratory disease syndrome, many other organ systems may be involved due to direct viral injury, hyperinflammatory responses, or complications of critical illness.
-
•
Therapeutic agents generally attempt to target the earlier stage of illness mediated by viral replication or the later inflammatory stages with immunomodulatory agents.
Introduction
COVID-19 has become the fourth leading cause of mortality worldwide since the beginning of 2020, accounting for just less than 1 in every 20 deaths in the United States. However, when accounting for excess deaths, the estimates could be as high as 1 in every 10 deaths.1 In the United States, the pandemic caused approximately 375,000 deaths in 2020, which was surpassed in 2021 with more than 450,000 deaths.2 However, with vaccination, the rate of critical illness and death is dramatically reduced.3 The epidemiology, pathogenesis, and treatment of severe and critical COVID-19 are discussed in this chapter.
Epidemiology
For epidemiologic purposes, severe COVID-19 is defined as subjective dyspnea, a respiratory rate of 30 or more breaths per minute, a blood oxygen saturation of 93% or less, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao 2:Fio 2 or P/F ratio) of less than 300 mm Hg, or infiltrates in more than 50% of the lung field.4 Studies demonstrate that the proportion of patients infected with SARS-CoV-2 who develop severe COVID-19 requiring hospitalization varies widely and depend on several factors—including geographic differences in admission patterns and population characteristics. Early reports from Wuhan, China suggested admission rates as high as 20% with around 25% of admitted patients needing intensive care (around 5% of all cases).5 , 6 Reports from the Lombard region of Italy showed the proportion of intensive care unit (ICU) admissions ranged from to 5% to 16%.7 , 8
The proportion of cases admitted to the ICU seems to be decreasing over time. In Germany the proportion of hospitalized patients requiring ICU care dropped from 30% at the beginning of the year to 14% in December 2020, corresponding to a relative drop of more than 50%.9 There are several underlying reasons for this trend—including increasing experience managing patient with high oxygen needs outside of the ICU, improvements in therapeutic options, and growing population immunity.
Risk Factors for Severe Disease
Several risk factors have been linked to worse outcomes from COVID-19 (Table 1 ). Age is the strongest risk factor, with those aged 65 years and older representing 81% of US COVID-19–related deaths despite making up around 17% of the population.2 In an analysis of 540,667 adults hospitalized for COVID-19, 94.9% of admissions were in patients with at least one underlying medical condition. Essential hypertension (50.4%), disorders of lipid metabolism (49.4%), and obesity (33.0%) were the most common conditions.10 (p667) The strongest risk factors for death were obesity (absolute risk reduction [aRR] = 1.30), anxiety disorders (aRR = 1.28), and diabetes with complication (aRR = 1.26). In addition, the total number of conditions was linked with increased risk for mortality, with aRRs of death ranging from 1.53 for patients with 1 condition to 3.82 for patients with more than 10 conditions (compared with patients with no underlying conditions).
Table 1.
Risk factors for severe COVID-19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviation: BMI, body mass index.
Adapted from CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. Published 2020 Mar 27. https://doi.org/10.15585/mmwr.mm6912e2.
Racial and Ethnic Disparities
When compared with White populations, higher rates of infection, hospitalization, and death have been observed in Black, Hispanic, and Asian Americans and Pacific Islander (AAPI) populations.11 According to a Kaiser Family Foundation and the Epic Health Research Network analysis, hospitalization and deaths rates were between 2 and 3 times higher for Black, Hispanic, and AAPI patients.12 The disparity has been linked with higher rates of chronic conditions and poorer access to health care in marginalized communities.13 In a study that compared mortality rates due to SARS-CoV-2 infection by race among 11,210 hospitalized adults, there was no difference in all-cause, in-hospital mortality between White and Black patients after adjusting for age, sex, insurance status, comorbidity, neighborhood deprivation, and site of care14; this argues against an independent predisposition to severe disease driven by genetics, but rather the impacts of structural racism on health and access to health care in communities of color (see Chapter 4 for further discussion of racial and ethnic disparities in COVID-19).
Protection from Vaccination
The risk of severe COVID is substantially reduced with vaccination. In an observational study of more than 6.5 million individuals in Israel, the BNT162b2 (Pfizer-BioNTech) vaccine demonstrated 97% efficacy for both COVID-19–related hospitalization and COVID-19–related death before the spread of the Delta variant.15 (p2) Among adults aged 65 years and older, an evaluation of 417 COVID-19–related hospitalizations demonstrated an adjusted vaccine effectiveness of 94% against COVID-19–associated hospitalization for full vaccination and 64% for partial vaccination with either Pfizer-BioNTech or the mRNA-1273 (Moderna) vaccines.16 In comparison to the mRNA vaccines, a single dose of the Ad26.COV2.S (Johnson and Johnson) vaccine has shown lower efficacy in preventing moderate-to-severe COVID-19. In a phase III efficacy trial, a single dose had 66.9% efficacy in preventing moderate-to-severe/critical COVID-19 beginning at or after 14 days postvaccination.17 Vaccination against COVID-19 is covered in detail in Chapter 13.
Although vaccination has consistently shown protection from severe outcomes from COVID-19, protection from infection has been shown to wane with the emergence of the Delta and Omicron variants. In a cohort of health care workers, vaccine effectiveness during the Delta predominant period was 66% compared with 91% during the months preceding Delta predominance.18 Further erosion in vaccine effectiveness have been observed with the Omicron variant,19 although protection from severe illness remains robust for most vaccine recipients. In an analysis of more than 1 million people who had completed primary vaccination, the risk for severe outcomes was higher among persons who were aged 65 years and older, were immunosuppressed, or had at least 1 of 6 underlying chronic conditions—including pulmonary, liver, kidney, cardiac, neurologic disease, and diabetes mellitus.20 Further, 78% of those who died had 4 or more risk factors.
Pathogenesis and clinical features
Although the primary driver of critical illness from COVID-19 is respiratory disease, critical illness can manifest in several ways and affect several organ systems. For critically ill patients there may be multiple mechanisms involved, although a common link to complications stem from hyperinflammatory responses.
Viral-Mediated Phase Versus the Immune-Mediated Phase
COVID-19 is characterized by 3 stages. In the first stage mild symptoms typical of other viral respiratory tract infections predominate. As viral replication continues and the immune response develops, ongoing infection can lead to complications from stage 2 disease, characterized by moderate respiratory symptoms. In the case of mild disease, viral replication decreases during this stage as well as the immune response during convalescence. For patients who subsequently develop worsening disease, a hyperinflammatory state leads to stage 3 disease with acute respiratory distress syndrome (ARDS), which may be complicated by circulatory failure/cardiac failure and multiorgan dysfunction syndrome.21 Therapeutic agents for COVID generally target the earlier stage of viral replication with antiviral agents or the later stage with immunomodulatory agents.22
Pulmonary Manifestations
Viral pneumonia is the most frequent serious manifestation of infection, characterized by fever, cough, dyspnea, and bilateral infiltrates on chest imaging characteristic of ARDS.5 , 6 Viral replication in the upper and lower respiratory tract induces cellular death and injury in airway epithelial cells, causing the release of various damage-associated molecular patterns and pathogen-associated molecular patterns. These mediators lead to the induction of proinflammatory cytokines and subsequent filling of the alveolar space, impairing gas exchange and respiratory mechanics.23 For patients who require mechanical ventilation complications from alveolar stretch injury can occur—such as barotrauma and ventilator induced lung injury, pneumothorax, and pneumomediastinum.24 Hypercapnia is rare unless there is concomitant chronic obstructive pulmonary disease or a history of narcotic overdose.
Cardiovascular Manifestations
Cardiac injury is a common complication in critically ill patients, especially in those with concomitant severe pulmonary disease. Several mechanisms may be involved, including myocarditis, hypoxia-induced injury, physiologic stress (Takotsubo) cardiomyopathy, vasculopathy, pulmonary embolism, ARDS, shock, and cytokine storm. In one New York City cohort of patients on mechanical ventilation, multiple cardiac complications were described—including arrhythmias (18%), myocardial infarction (8%), and heart failure (2%).25
Neurologic Manifestations
Delirium, encephalopathy, and other neurologic manifestations are common findings in critically ill patients with COVID-19. In one review of 214 hospitalized patients in Wuhan, 36.4% had neurologic manifestations. In comparison to patients with nonsevere disease, those with severe infection had a higher chance of neurologic manifestations, such as acute cerebrovascular diseases (5.7% vs 1%), impaired consciousness (14.8% vs 2.4%), and skeletal muscle injury (19.3% vs 4.8%).26 In a review of critically ill patients in France 13 patients underwent MRI for unexplained encephalopathy without a localizing neurologic examination. Leptomeningeal enhancement was noted in 8 patients (61.5%) and bilateral frontotemporal hypoperfusion was noted in all 11 patients who underwent perfusion imaging. In addition, 2 patients (15.4%) were noted to have acute or subacute ischemic strokes.27
Renal Manifestations
Acute kidney injury (AKI) commonly occurrence in patients with critical illness from COVID-19. In a meta-analysis of around 13,000 hospitalized patients the overall prevalence of AKI was 17%, with 5% of overall patients requiring renal replacement therapy. In addition, AKI was associated with an increased odds of death (pooled odds ratio 15.27; 95% confidence interval [CI] 4.82–48.36).28 The cause of AKI is likely multifactorial and includes virus-mediated injury, cytokine storm, angiotensin II pathway activation, complement activation, hypercoagulation, and microangiopathy interacting with known risk factors for AKI in critically ill patients (Fig. 1 ).29 In an autopsy series of 42 patients who died from COVID-19, the most common finding on kidney histopathology was acute tubular necrosis.30
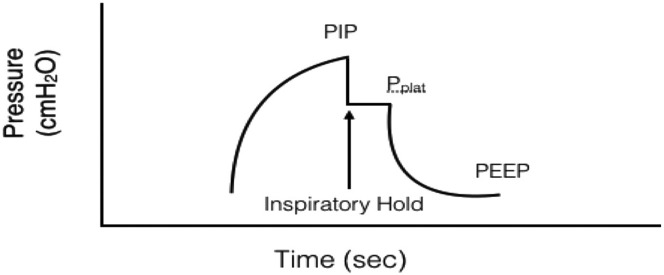
Fig. 1.
Measuring plateau pressure for mechanical ventilation.
(From Pacheco GS, Mendelson J, Gaspers M. Pediatric Ventilator Management in the Emergency Department. Emerg Med Clin North Am. 2018;36(2):401-413. https://doi.org/10.1016/j.emc.2017.12.008; with permission.)
Gastrointestinal Manifestations
Patients with severe COVID-19 are at high risk for developing a range of gastrointestinal complications, including bowel ischemia, transaminase elevation, acalculous cholecystitis, gastrointestinal bleeding, pancreatitis, Ogilvie syndrome, and severe ileus.31 In one evaluation of propensity-matched patients of critically ill patients with or without COVID-19, those with COVID-19 were more likely to develop gastrointestinal complications (74% vs 37%). Specifically, patients with COVID-19 developed more transaminase elevation (55% vs 27%), severe ileus (48% vs 22%), and bowel ischemia (4% vs 0%).32
Thrombosis and Vasculopathy
Estimates of venous thromboembolism (VTE) in critically ill patients with COVID-19 have varied widely but seem to have declined from 20% to 25% in the earlier phase of the pandemic to around 7% to 15%.33, 34, 35, 36 Rates of deep venous thrombosis have generally been higher in studies that routinely perform surveillance. The mechanism of hypercoagulability is likely related to endothelial injury, microvascular inflammation, immobilization in critically ill patients, and microangiopathy.37 In many instances, VTE has been noted despite the routine use of prophylactic anticoagulation. The American Society of Hematology recommends routine prophylactic intensity pharmacologic prophylaxis over intermediate or therapeutic anticoagulation, as trials demonstrated an increased risk of bleeding without mortality benefit among critically ill patients.38 Select patients may benefit from therapeutic dose anticoagulation.39
Secondary Infection
Prolonged ICU admissions place critically ill COVID-19 patients at risk for hospital-acquired infections. Common complications include secondary pneumonia, central line–associated bloodstream infection, and urinary tract infections. In endemic areas, strongyloidiasis reactivation is also of concern, particularly in those who receive immunosuppressive agents, such as dexamethasone. Several reports have described invasive aspergillus in immunocompetent hosts, although the true prevalence is uncertain.40 , 41
Secondary bacterial infections have been noted in COVID-19 patients, and patients are frequently treated with empirical antimicrobial agents. However, secondary bacterial infections do not seem to be common. A meta-analysis found that 71.9% of patients hospitalized with COVID-19 before mid-April 2020 received antibiotics despite the fact that only 6.9% of these admissions were associated with bacterial infections.42 The Infectious Disease Society of America and National Institutes of Health COVID-19 treatment guidelines do not recommend either for or against empirical broad spectrum antimicrobial therapy.39 , 43 However, if broad spectrum antimicrobials are used, effort should be made to obtain culture data and there should be regular reassessment of the appropriateness of ongoing use.
Diagnosis
Laboratory Findings
Laboratory findings in critically ill patients with COVID-19 are varied and include leukopenia or leukocytosis, lymphopenia, thrombocytopenia, and elevated D-dimer, aminotransferases, lactate dehydrogenase, and ferritin levels.44 Many patients with severe COVID-19 also display evidence of a pronounced inflammatory response that is similar to the cytokine release syndrome with elevated serum levels of C-reactive protein, ferritin, and interleukin-6. Patients with evidence of a hyperinflammatory response have a higher risk of progression to mechanical ventilation and death.45
Imaging
Chest imaging findings can be nonspecific but frequent findings in critically ill patients include multifocal consolidation and ground-glass opacities.46 Peak severity in lung findings usually occurs after the first week of symptom onset. In one systemic review of computed tomography findings in more than 2700 patients with COVID-19, the following pulmonary manifestations were noted (Table 2 ):47
Table 2.
Computed tomographic manifestations of COVID-19
| CT Finding | Proportion |
|---|---|
| Ground glass opacities(GGO) | 83.3% |
| GGO mixed with consolidation | 58.4% |
| Adjacent pleural thickening | 52.5% |
| Consolidation | 44% |
| Interlobular septal thickening | 48.5% |
| Air bronchogram | 46.5% |
Modified from Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2020;17(6):701-709. https://doi.org/10.1016/j.jacr.2020.03.006; with permission.
Treatment
The key principles in treatment of patients with severe COVID-19 include high-quality supportive care, antiviral therapies, immunomodulatory therapies, prevention of hospital-acquired conditions, and rigorous infection control procedures to limit nosocomial spread. In addition, managing surges of critically ill patients requires proactive management of hospital staff, bed allocation, and equipment resources to adequately meet the needs of the pool of critically ill patients. In extreme circumstances, crisis of care standard may need to be implemented.48
Basic Management
Patients admitted with severe COVID-19 should be monitored closely for disease progression with close observation and pulse oximetry. Oxygen support should be titrated to achieve a saturation between 90% and 96%. For patients with hypotension or shock, fluid resuscitation should be conservative and norepinephrine should be used as the first-line vasoactive agent to achieve a mean arterial pressure of 60 to 65 mm Hg.49
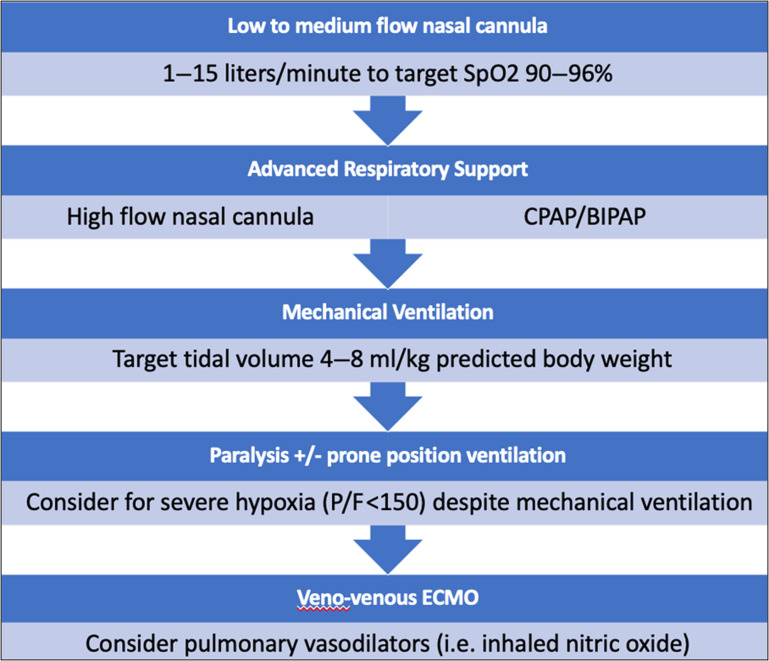
Advanced Noninvasive Respiratory Support
In patients unable to achieve adequate oxygen saturation with supplemental nasal cannula, either high-flow nasal cannula or noninvasive ventilation (continuous positive airway pressure/bilevel positive airway pressure) should be used. Deciding if and when to intubate is a critical decision that should be considered for patients on advanced respiratory support. Clinicians should consider the anticipated clinical course and weigh both the risk of premature intubation against the risk of respiratory arrest and emergency intubation.4 Signs of labored breathing, refractory hypoxemia, and encephalopathy indicate impending respiratory arrest and should prompt urgent endotracheal intubation and mechanical ventilation.
Mechanical Ventilation
The primary lung pathology from severe COVID-19 is ARDS. Therefore, patients on mechanical ventilation should be managed according to established principles of managing ARDS to avoid alveolar collapse, overdistension, and hyperoxia-induced injury.
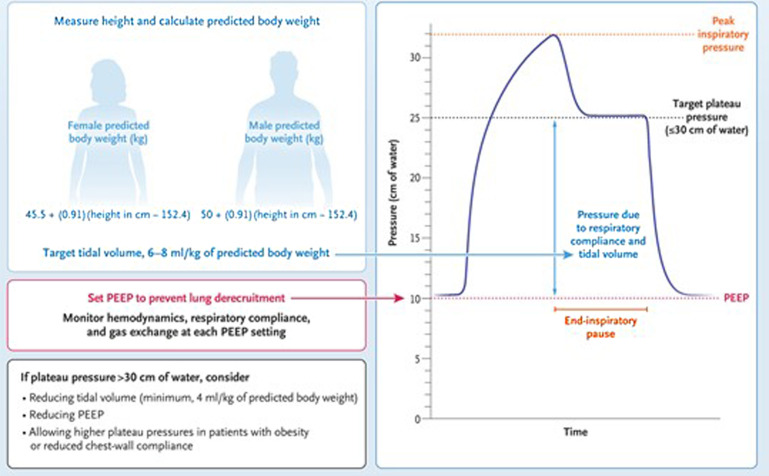
Sufficient positive end-expiratory pressure (PEEP) should be used to prevent end-expiratory alveolar collapse, and tidal volumes should be limited to between 4 and 8 mL/kg of predicted body weight to achieve an end-inspiratory pressure (plateau pressure [Pplat]) of less than 30 cm H2O (Fig. 2 ).50 To achieve low tidal volume lung protective ventilation, permissive hypercapnia should be tolerated to maintain arterial pH less than 7.20. Clinicians should be mindful that excessive PEEP may cause alveolar overdistension and hemodynamic instability from decreased venous filling to the heart. Achieving the lowest driving pressure possible (Pplat–PEEP) is associated with improved mortality and indicates the ideal balance between alveolar collapse and overdistension.51 Sedation and pain control agents should be used to achieve comfort and synchrony with mechanical ventilation.
Fig. 2.
Goals of mechanical ventilation.
(Adapted from Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451-2460. https://doi.org/10.1056/NEJMcp2009575; with permission.)
In the case of severe hypoxia (P/F < 150 mm Hg) despite conventional lung protective ventilation, paralysis and prone ventilation should be considered (Fig. 3 ). In a pre-COVID randomized trial of intubated patients with ARDS, prone ventilation for 16 hours per day improved oxygenation and mortality.52 If severe hypoxia persists despite these efforts, rescue therapies, such as inhaled pulmonary vasodilators and extracorporeal membrane oxygenation (ECMO), should be considered.4
Fig. 3.
Respiratory support in patients with COVID-19.
(Modified from Farkas J. Management of COVID-19 patients admitted to stepdown or ICU. EMCrit Project. Available at https://emcrit.org/ibcc/covid19/. Accessed; with permission.)
Antiviral Therapies
Remdesivir is a nucleoside analogue the inhibits the RNA-dependent RNA polymerase of coronaviruses and is recommended for use in combination with dexamethasone for patients admitted and requiring supplemental oxygen.39 A randomized trial demonstrated an improved time to recovery for hospitalized patients, although benefit was greatest in patients earlier in disease course who were receiving supplemental oxygen but not intubated.53 A large open-label trial did not demonstrate an in-hospital mortality benefit.54
Monoclonal antibodies are generally recommended for outpatients with COVID-19 who are at high risk of progression to severe disease. However, they may be considered for immunocompromised patients on supplemental oxygen therapy through expanded access programs but is otherwise generally not authorized nor recommended for patients with severe or critical COVID-19, based on negative results from several randomized controlled studies.55 , 56 Convalescent plasma has not been demonstrated to improve outcomes in hospitalized patients and is not recommended.
Immunomodulatory Therapies
Dexamethasone is considered standard of care for patients with severe COVID-19. A randomized trial of more than 6400 hospitalized patients with COVID-19 showed a reduction in 30-day mortality (22.9% vs 25.7%) with the benefit most pronounced in patients requiring mechanical ventilation (29.3% vs 41.4%).57
A second immunomodulatory agent—such as tocilizumab, sarilumab, or baricitinib—should be considered for patients with rapidly escalating oxygen needs or those requiring advanced respiratory support, mechanical ventilation, or ECMO. Tocilizumab and sarilumab are monoclonal antibodies that competitively inhibit interleukin-6 (IL-6) receptor binding. In a meta-analysis of more than 10,000 hospitalized patients receiving anti-IL-6 inhibitors the odd ratios for the association with mortality compared with usual care or placebo were 0.77 (95% CI, 0.68–0.87) for tocilizumab and 0.92 (95% CI, 0.61–1.38) for sarilumab.58 Anti-IL-6 inhibitors should not be used in patients with a concurrent active infection other than the SARS-CoV-2 infection (including localized infection) or with elevated alanine aminotransferase or aspartate transaminase (AST) greater than 10 times the upper limit of the reference range.
Baricitinib is a selective Janus kinase 1 and 2 inhibitor and can be used as an alternative to the anti-IL-6 inhibitors. In a randomized trial of 1525 hospitalized adults with COVID-19 and elevated inflammatory markers but not receiving invasive mechanical ventilation, adding baricitinib to standard of care reduced 28-day mortality compared with standard of care (8.1% vs 13.1%).59 It should not be administered if estimated glomerular filtration rate is less than 15 mL/min, the absolute lymphocyte count is less than 200 cells/uL, or if the absolute neutrophil count is less than 500 cells/uL.
Clinics care points
-
•
Patients with severe COVID-19 should be hospitalized and monitored closely for signs of deterioration.
-
•
When to intubate is a critical decision that should depend on the anticipated clinical course and weigh both the risk of premature intubation against the risk of respiratory arrest and emergency intubation.
-
•
Remdesivir and dexamethasone are standard of care for most patients with severe COVID-19. Additional immunomodulatory agents (baricitinib, anti-IL-6 inhibitors) should be considered for patients on high-flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
-
•
The evidenced-based principles for ARDS should be used in patients on mechanical ventilation: low tidal volume ventilation, permissive hypercapnia, and adequate PEEP.
Acknowledgments
Disclosure
The author has nothing to disclose.
References
- 1.Causes of death (COD) visualization. institute for health metrics and evaluation. 2014. https://www.healthdata.org/data-visualization/causes-death-cod-visualization Available at: Accessed December 26, 2021.
- 2.Ahmad F.B. Provisional mortality data — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021:70. doi: 10.15585/mmwr.mm7014e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S. COVID-19 vaccination and non–COVID-19 mortality risk — seven integrated health care organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7043e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in lombardy, italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 8.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 9.Karagiannidis C., Windisch W., McAuley D.F., et al. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med. 2021;9(5):e47–e48. doi: 10.1016/S2213-2600(21)00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kompaniyets L. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev Chronic Dis. 2021:18. doi: 10.5888/pcd18.210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez L., III, Hart L.H., III, Katz M.H. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719–720. doi: 10.1001/jama.2020.26443. [DOI] [PubMed] [Google Scholar]
- 12.Rubin-Miller L., Alban C., Sep 16 SSP COVID-19 racial disparities in testing, infection, hospitalization, and death: analysis of epic patient data. KFF. 2020. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-racial-disparities-testing-infection-hospitalization-death-analysis-epic-patient-data/ Published September 16, 2020. Accessed January 24, 2022. Available at.
- 13.Price J.H., Khubchandani J., McKinney M., et al. Racial/Ethnic Disparities in Chronic Diseases of Youths and Access to Health Care in the United States. Biomed Res Int. 2013;2013:787616. doi: 10.1155/2013/787616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehia B.R., Winegar A., Fogel R., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8):e2018039. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas E.J., Angulo F.J., McLaughlin J.M., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. Lond Engl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenforde M.W., Olson S.M., Self W.H., et al. Effectiveness of Pfizer-biontech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowlkes A., Gaglani M., Groover K., et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance — eight U.S. locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enhancing response to Omicron SARS-CoV-2 variant. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states Available at: Accessed January 24, 2022.
- 20.Yek C. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series — 465 health care facilities, United States, December 2020–October 2021. MMWR Morb Mortal Wkly Rep. 2022:71. doi: 10.15585/mmwr.mm7101a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transpl. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 23.Bohn M.K., Hall A., Sepiashvili L., et al. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim G.S., Alkandari B.M., Shady I.A.A., et al. Invasive mechanical ventilation complications in COVID-19 patients. Egypt J Radiol Nucl Med. 2021;52(1):226. [Google Scholar]
- 25.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins-Juarez S.Y., Qian L., King K.L., et al. Outcomes for patients with COVID-19 and Acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batlle D., Soler M.J., Sparks M.A., et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoriello D., Khairallah P., Bomback A.S., et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272(2):e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Moheb M., Naar L., Christensen M.A., et al. Gastrointestinal complications in critically ill patients with and without COVID-19. JAMA. 2020;324(18):1899–1901. doi: 10.1001/jama.2020.19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui S., Chen S., Li X., et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill J.B., Garcia D., Crowther M., et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4(21):5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuker A., Tseng E.K., Nieuwlaat R., et al. American society of hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NIH COVID-19 Treatment Guidelines COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ Available at: Accessed January 26, 2022.
- 40.Bartoletti M., Pascale R., Cricca M., et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect. 2021;73(11):e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koehler P., Cornely O.A., Böttiger B.W., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langford B.J., So M., Raybardhan S., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19. Clin Infect. 2020;27:ciaa478. doi: 10.1093/cid/ciaa478. Published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb B.J., Peltan I.D., Jensen P., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong H.Y.F., Lam H.Y.S., Fong A.H.T., et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2):E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao C., Liu X., Zhang H., et al. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read “Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic (March 28, 2020)” at NAP.Edu. doi:10.17226/25765.
- 49.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 51.Guérin C., Papazian L., Reignier J., et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care Lond Engl. 2016;20(1):384. doi: 10.1186/s13054-016-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 53.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 54.Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results | medRxiv. https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1 Available at: Accessed January 26, 2022. [DOI] [PMC free article] [PubMed]
- 55.Self W.H., Sandkovsky U., Reilly C.S., et al. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021;0(0) doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ACTIV-3/TICO LY-CoV555 Study Group, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.RECOVERY Collaborative Group, Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shankar-Hari M., Vale C.L., Godolphin P.J., et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marconi V.C., Ramanan A.V., de Bono S., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]