Abstract
The COVID-19 pandemic has raised international awareness of the importance of rigorous scientific evidence and the havoc caused by uncontrolled excessive inflammation. Here we consider the evidence on whether the specialized pro-resolving mediators (SPMs) are ready to meet this challenge as well as targeted metabololipidomics of the resolution-inflammation metabolomes. Specific stereochemical mechanisms in the biosynthesis of SPMs from omega-3 essential fatty acids give rise to unique local-acting lipid mediators. SPMs possess stereochemically defined potent bioactive structures that are high-affinity ligands for cognate G protein-coupled surface receptors that evoke the cellular responses required for efficient resolution of acute inflammation. The SPMs biosynthesized from the major omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are coined Resolvins (resolution phase interaction products; E series and D-series), Protectins and Maresins (macrophage mediators in resolving inflammation). Their biosynthesis and stereochemical assignments are established and confirmed (>1,441 resolvin publications in PubMed.gov) as well as their functional roles on innate immune cells and adaptive immune cells (both lymphocyte T-cell subsets and B-cells). The resolution of a protective acute inflammatory response is governed mainly by phagocytes that actively clear apoptotic cells, debris, blood clots and pathogens. These resolution phase functions of the acute inflammatory response are enhanced by SPMs, which together prepare the inflammatory loci for homeostasis and stimulate tissue regeneration via activating stem cells and the biosynthesis of novel cys-SPMs (e.g. MCTRs, PCTRs and RCTRs). These cys-SPMs also activate regeneration, are organ protective and stimulate resolution of local inflammation. Herein, we review the biosynthesis and functions of the E-series resolvins, namely resolvin E1 (the first n-3 resolvin identified), resolvin E2, resolvin E3 and resolvin E4 biosynthesized from their precursor eicosapentaenoic acid (EPA), and the critical role of total organic synthesis in confirming SPM complete stereochemistry, establishing their potent functions in resolution of inflammation, and novel structures. The physical properties of each biologically derived SPM, i.e., ultra-violet (UV) absorbance, chromatographic behavior, and tandem mass spectrometry (MS2) fragmentation, were matched to SPMs biosynthesized and prepared by stereospecific total organic synthesis. We briefly review this approach, also used with the endogenous D-series resolvins, protectins and maresins confirming their potent functions in resolution of inflammation, that paves the way for their rigorous evaluation in human tissues and clinical trials. The assignment of complete stereochemistry for each of the E and D series Resolvins, Protectins and Maresins was a critical and required step that enabled human clinical studies as in SPM profiling in COVID-19 infections and experimental animal disease models that also opened the promise of resolution physiology, resolution pharmacology and targeted precision nutrition as new areas for monitoring health and disease mechanisms.
Keywords: Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Omega-3 polyunsaturated fatty acids, Human phagocytes, M2 macrophages, Neutrophils
1. Introduction
The current pandemic and COVID-19 infections have painfully underscored the important contribution of excessive, uncontrolled inflammation and collateral tissue damage to disease pathology that can amplify inflammation and lead to untimely death of patients [1,2]. The acute inflammatory response is an essential and temporal process evoked in the host defense of infection as well as by tissue injury that normally is self-limited and resolves to return to tissue homeostasis on its own [3,4]. In the acute inflammatory response, human phagocytes, both neutrophils and macrophages, play central roles in host defense and produce endogenous lipid mediators (LMs), e.g. eicosanoids such as prostaglandins and leukotrienes, that are potent pro-inflammatory mediators critical to host defense [5,6].The acute inflammatory response is functionally characterized in two separate and discrete phases, the initiation phase (Fig. 1 A) and resolution phase [[7], [8], [9]]. Mediators such as complement components, chemokines and cytokines have well appreciated roles in the initiation phase of the acute inflammatory response summoning neutrophils to the local site(s) of invasion or tissue injury [10] as do lipid mediators derived from the precursor arachidonic acid [6,7]. In the initiation phase, prostaglandins such as PGE2 regulate the cardinal signs of inflammation: local capillary blood flow, heat, swelling and pain [7]. At sites of inflammation-resolution, physiologic hypoxia is transient in the local milieu that was modeled and played a triggering role in our original studies [7,8] on the biosynthesis and functions of the resolvins (vide infra). In our studies on the resolution phase of acute inflammation in self-limited or self-resolving acute inflammation [7], infection [11], and collateral organ damage [4], a novel superfamily of potent endogenous LMs emerged, which one of the authors coined specialized pro-resolving mediators (SPMs: resolvins, maresins, protectins and lipoxins, derived from the major essential omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) that were first uncovered in mouse resolving inflammatory exudates), given the potent and special functions in vivo of this superfamily of mediators [7]. These inflammatory exudates (i.e., pus) were temporally interrogated along the time course and were comprised of a mixture of leukocytes recruited in vivo to form the exudates [8,12,13]. Each family of mediators was named given their potent bioactivity and unique structures [7,8,14,15]. The biosynthesis of the SPMs was investigated in detail and confirmed with isolated human leukocytes. SPMs play a fundamental role in the resolution of acute inflammation by governing the temporal and spatial regulation of neutrophil traffic and down regulation of the pro-inflammatory mediators, e.g., eicosanoids, cytokines, and chemokines [7]. By their defined criteria as a pro-resolving molecule as originally proposed [7,8], each SPM specifically limits further neutrophil infiltration to the site and enhances the clearance of apoptotic cells by macrophages, reduces both cytokine and eicosanoid storms, reduces pain (Fig. 1 A) and shortens the resolution interval in vivo [4,7,12,16]. Macrophages play a central function in the resolution phase of inflammation by actively clearing dead cell debris, apoptotic neutrophil cells [16] and biosynthesizing distinct families of LMs that are dependent on the macrophage phenotype [17,18]; see Fig. 1. The mediators biosynthesized by macrophages are either pro-inflammatory or anti-inflammatory-pro-resolving depending on the specific macrophage agonists and the availability of the required precursor [18,19]. In this context, human macrophages incubated with the high-mobility group box 1 protein (HMGB1) stimulate the production of pro-inflammatory cytokines and leukotrienes, whereas the high-mobility group box 1 protein (HMGBI) exposed together with the complement component 1q (C1q) switches these macrophages to produce SPMs [20]. Apoptotic PMN, M2 macrophages and microglial cells are major human cell types that produce and release SPMs [17,18] (Fig. 1 B). Human PMN also biosynthesize resolvins via transcellular biosynthesis routes when interacting with vascular endothelial cells or mucosal epithelial cells (e.g., lung airway, gastrointestinal tract) [8,13,21,22].
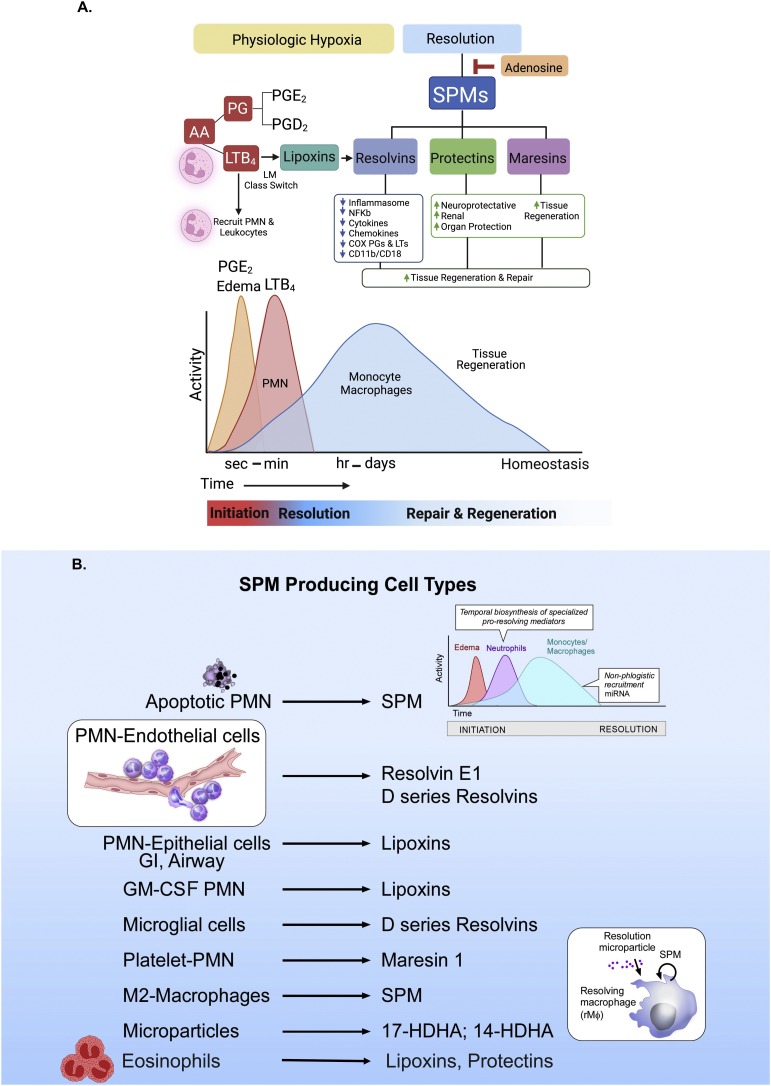
Fig. 1.
Schematic illustration of the acute inflammatory response with the strategic positions of lipid mediators in this local protective response.
Panel A: Initiation of the acute inflammatory response ideally proceeds to complete resolution and homeostasis. Edema, regulated by PGE2 in seconds to minutes, enables neutrophil tissue/exudate influx by chemotaxis to the arachidonic-acid-derived leukotriene B4 (LTB4). The prostaglandins PGE2 and PGD2 activate the lipid mediator class switch [13], triggering the increase in 15-lipoxygenase [211] that leads to production of lipoxins starting the decrease in neutrophil numbers in the inflammatory exudate and beginning the resolution phase and biosynthesis of resolvins [8,21], protectins, and maresins (the SPM), which carry functions critical to timely resolution and homeostasis [4]. These include limiting further PMN recruitment to the site and the uptake and removal of apoptotic PMN and cell debris by macrophages. The resolvins down regulate and counteract the cytokines, chemokines, and eicosanoids, e.g., prostaglandins and leukotrienes, the pro-inflammatory signals produced via inflammasomes, and NF-κB are reduced and cellular adhesion molecules C11b/CD18 are also downregulated. The production of protectins and maresins from DHA is organ protective and activates tissue regeneration as needed to repair the injured site for homeostasis (see text for further details). Adenosine blocks the biosynthesis of SPMs from omega-3 fatty acids [194], illustrating that there are many checkpoint regulators involved in the resolution of the acute inflammatory response and the resolution of this complex leukocyte trafficking event that is critical to host defense, elimination of invading bacteria, and survival. Each SPM reduces proinflammatory mediator production and expression as well as enhances PMN and macrophage-mediated bacterial phagocytosis and killing.
Panel B: SPM-producing cell types. Apoptotic PMN, brain microglial cells and M2 macrophages biosynthesize SPM from the single cell type, while intact neutrophil and vascular endothelial cells biosynthesize SPM via transcellular biosynthesis (see text for details and original references; for example, see Fig. 3). Eosinophils are a source of SPM, lipoxins, and protectins and are rich in 15-lipoxygenase [209,212], giving a new role for these cells in resolution and wound healing via their production of SPM.
2. SPMs in COVID-19 infections and other human studies
The stereochemical assignments of each of the SPMs originally reported by the Serhan Lab (reviewed herein) permitted SPM identification by other research teams in COVID-19 patients (Table 1 ) along with eicosanoids, i.e., prostaglandins and leukotriene [[23], [24], [25], [26]]. New rigorous LC-MS-MS-based profiling methods introduced using synthetic SPM [27], internal standards (for some of the original publications, see Refs. [[28], [29], [30]]) helped to establish criteria for endogenous production and identification of resolvins in tissues enabling the recent publications from independent laboratories with human subjects providing results (Table 1) suggesting that, in COVID-19 infections, endogenous SPM production is dysregulated in vivo during these viral infections [23,31]. Each of these studies used LC-MS-MS-based identification enabled by the availability of synthetic SPMs and deuterium-labeled internal standards that were first introduced by the Serhan laboratory (reviewed in Ref. [27]). SPMs are now commercially available from several sources based on our original studies determining the stereochemical assignments of each SPM with human leukocyte, mouse exudates and tissues reviewed here and accomplished with support from NIH Program Project grant P01GM095467 to the CN Serhan research team.
Table 1.
Resolvins*, SPMs and Eicosanoids in Human Subjects with COVID-19 Infections.
| Reference | Findings | Source |
|---|---|---|
| Schwarz et al. [31] | Dysregulation of eicosanoids increases 5-lipoxygenase, leukotriene, prostaglandins, EETs, specific resolvins in severe illness, decreases 12-LOX (ALOX12) and COX-2; immune lipid mediator metabolome imbalance in severe COVID-19 | Serum |
| Archambault et al. [23] | Predominance of prostaglandins, thromboxane, LTB4 and leukotrienes; increased lipoxin A4 and D-series resolvins | Bronchoalveolar lavages (BAL) |
| Proinflammatory eicosanoids and SPMs were elevated in COVID-19 BAL | ||
| Koenis et al. [25] | Disrupted resolution and altered phagocyte responses | Plasma |
| SPM and eicosanoids identified | ||
| Turnbull et al. [26] | SPM and eicosanoid metabolomes identified in critically ill COVID-19 showing dysregulation during infection | Serum |
| Arnardottir et al. [195] | Omega-3 in resolution of COVID-19; randomized clinical trial | COVID-Omega-F |
In most COVID-19 infection studies, there appears to be an excess of proinflammatory lipid mediators PGs and LTB4 with diminished amounts of SPMs compared to peripheral blood of control subjects (Table 1). The increase in pro-inflammatory eicosanoids may drive as well as amplify the uncontrolled cytokine storms and excessive lung inflammation that possibly reflect systemic inflammation characteristic of COVID-19 infections [32,33]. In one published study analyzing bronchial alveolar lavage fluids from COVID-19 patients, in addition to eicosanoids such as leukotrienes and prostaglandins, the COVID-19 lung lavages (Table 1) contained high amounts of resolvins and lipoxins [23]. Given the potent pro-resolving functions of these SPMs, it appears that in this study endogenous SPM produced in vivo that appear in BAL were functionally unable to counteract the excessive pro-inflammatory phenotype in COVID lung infections and/or were unable to engage leukocytes to activate the endogenous resolution response needed in these patients. Much still needs to be learned about the potential role of specific SPMs in COVID-19 infections during the time course of human infection and disease. Recent in vitro studies demonstrated that both Resolvin D1 and Resolvin D2 each are potent in reducing human macrophage production of pro-inflammatory cytokines and chemokines in response to the virus spike protein [34]. It is appropriate to emphasize that these early fundamental studies are vital to understanding the pathogenesis of COVID-19 infections and the potential role of lipid mediators that could give useful new directions for treatments of COVID-19, long COVID and in lasting immunity. These new findings (Table 1) and many other human studies for SPMs from international experts [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]] would not have been possible without the i) complete stereochemical assignments of each of the potent bioactive SPMs produced by human and mouse leukocytes, ii) their biosynthesis from n-3 essential fatty acids (EPA and DHA) and iii) total organic synthesis with rigorous matching studies with endogenous SPMs along with our dedicated and talented organic chemistry collaborators summarized herein. In this relatively new SPM field, the Serhan lab has collaborated for many years with Professor Nicos Petasis and colleagues as part of the total synthesis core of our NIH-supported Program Project grant P01GM095467, Professor Trond Hansen and team in Oslo, Norway [56], Professor Bernd Spur and Anna Rodriguez [[57], [58], [59]], and the organic synthesis group at Cayman Chemical; for examples, see the custom synthesis of benzo-RvD1 analog mimetic [60] and the new RvE4 [61].
Importantly, the many other elegant human studies on SPMs would not have been possible without reliance on the complete stereochemically defined synthetic SPMs and their internal standards for LC-MS-MS-based analyses with adult tissues [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]] and adolescent human peripheral blood samples [38] as well as their scale-up for commercial availability [62]. A recent clinical trial reported that local topical treatment of gingival inflammation with a proresolving lipoxin/resolvin stable analog is safe and effective as well as activated endogenous production of SPMs present in peripheral blood [63]. A new generation of lipoxin stable analog mimetics introduced by investigators in Dublin hold exceptional promise for novel pro-resolving treatments in inflammation-resolution for kidney diseases and the many diabetic complications [[64], [65], [66]]. They demonstrated with analyses of nasal lavages that SPMs, e.g., RvD2, are dysregulated in human rhinosinusitis. This may be an indication where treatment with a pro-resolving molecule can be a useful new treatment approach [67].
3. E series Resolvins (resolution phase interaction products) and new bioactive members: stereochemistry
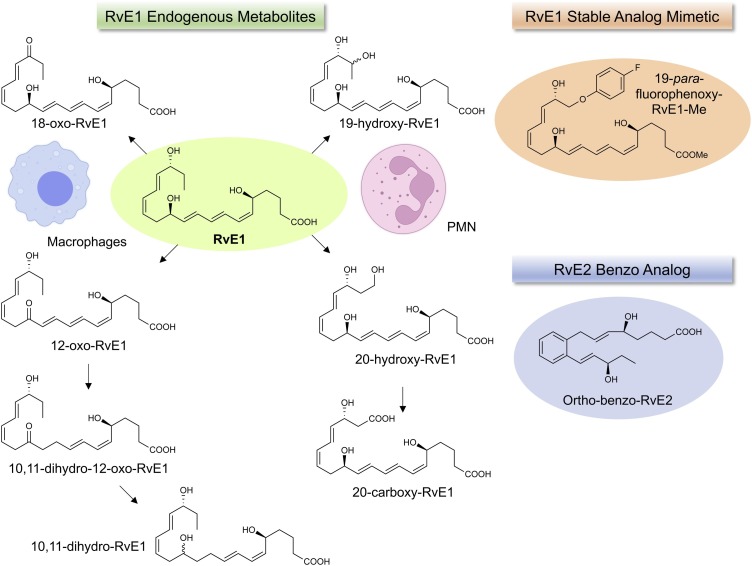
In the original studies from the Serhan lab, eicosapentaenoic acid (EPA) was found to be the obligatory precursor to the first resolvins, namely resolvin E1 [21] and the E-series resolvins biosynthesis [8]. Resolvin E1 is produced via transcellular biosynthesis with interactions between human neutrophil (PMN) and hypoxic vascular endothelial cells that release 18-HEPE, which is converted to the bioactive resolvin E1 [21] (Fig. 2 ). The therapeutic uses of EPA in cardiovascular medicine and health are still heatedly debated [68] even today, 50 years after the landmark Greenland study and publication clearly pointing to reduced cardiovascular disease in the indigenous population study participants and their marine-based nutrition [69]. Prescription omega-3 fatty acids are used clinically to reduce triglycerides in patients. Employing Icosapent ethyl ester of EPA, a human clinical trial with hundreds of subjects enrolled showed a statistically significant reduction in risk of cardiovascular disease (CVD) [70]. The omega-3 essential fatty acids are also being considered in COVID-19 patients with cardiovascular complications as additional therapies in these patients [71]. These are significant advancements for the field of omega-3-based therapeutics given the worldwide unmet medical needs of our times. The mechanism(s) of EPA efficacy in reducing cardiovascular disease remains to be determined and is an ongoing interest of many investigators along with the potential role of the EPA resolution metabolome in the resolution of inflammation in human disease including COVID-19 infections (Table 1).
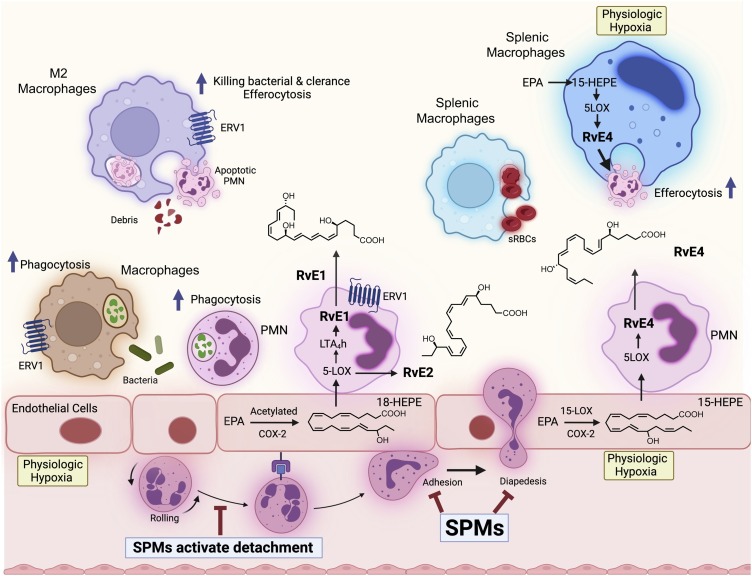
Fig. 2.
Schematic illustration of the transcellular and single cell biosynthesis and functions of the E-series Resolvins in efferocytosis, phagocytosis and limiting further PMN infiltration. The transcellular biosynthesis of E-series resolvins involves hypoxic vascular endothelial cells and neutrophils. Physiologic hypoxia activates the conversion of EPA by COX-2 and the upregulation of this enzyme [8,21]. Each SPM limits further PMN recruitment by blocking diapedesis. RvE1 and RvE2 activate specific GPCRs such as ERV1, ChemR23 and BLT1 [72,213].
In our original experiments, we found that hypoxic human vascular endothelial cells converted EPA to a novel 18-hydroxyeicosapentaenoic acid (18-HEPE) that is released from the cells and is precursor for transcellular biosynthesis of resolvin E1 (RvE1) [8,21]. The complete stereochemistry of resolvin E1 was determined, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid, and its proposed structure and potent bioactions [21] were confirmed with materials prepared by total organic synthesis [21,72]. These matched the products from human neutrophils produced during coincubations with hypoxic endothelial cells and in mouse resolving exudates, given the vast surface area of the vascular endothelium and vessels in vivo and high number of circulating PMN that roll on the endothelial surface in vivo from head to toe [73]. This is a considerably massive cellular architecture in vivo for cell-cell interactions and transcellular biosynthesis of several SPMs [73,74], interactions that can also stimulate resolvin biosynthesis from EPA on command to function on surrounding blood cells [75]. RvE1 is produced via transcellular biosynthesis with human neutrophils via acetylated cyclooxygenase-2 (COX-2) [21] or the microbial cytochrome P450 [76]. The RvE1 produced via co-incubations of human cells matched the properties of the product found in mouse resolving inflammatory exudates and its bioactivity in blocking transmigration and the binding of LTB4 to its receptor BLT1 [21].
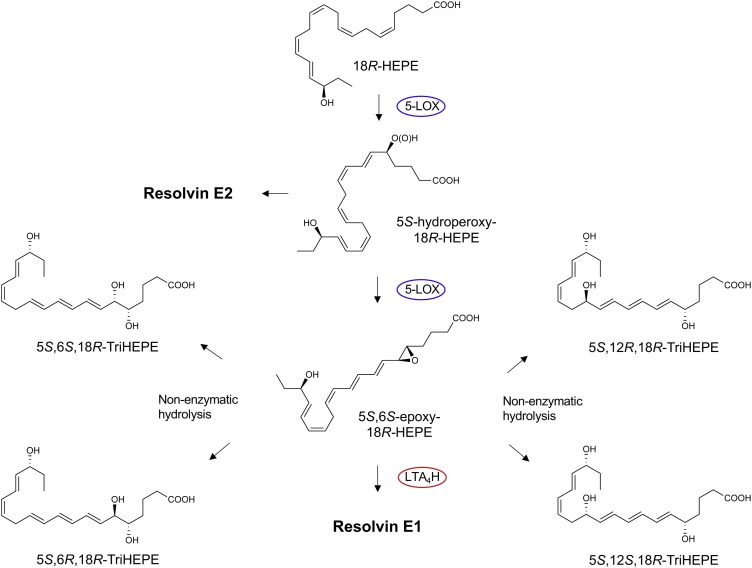
The 18-HEPE released from endothelial cells is converted by the human neutrophil 5-LOX to a 5(6)-epoxide-containing intermediate that was identified by trapping experiments and analyses of the products using both GC–MS and LC-MS-MS as well as derivatives. This 5(6)-epoxide intermediate is converted to RvE1 via the LTA4 hydrolase (LTA4H) in human neutrophils (see Fig. 3, Fig. 4 ). This step of the RvE1 biosynthesis was confirmed with human recombinant LTA4 hydrolase [77,78]. Chiral LC-MS-MS analyses were also carried out with the RvE1 biosynthesis pathway with human leukocytes as well as functional studies with 18S-RvE1 compared directly with RvE1, where both isomers proved to be equally potent with human cells and in vivo in mice promoting resolution [77]. Of interest, the 18S-RvE1 isomer is more rapidly inactivated via metabolic conversion than RvE1 given the stereoselectivity of the dehydrogenase [77], which may underlie the finding that RvE1 (which carries its carbon 18 position alcohol in the 18R configuration) is identified in vivo using MS3 with human plasma [72] and in other human studies (vide infra).
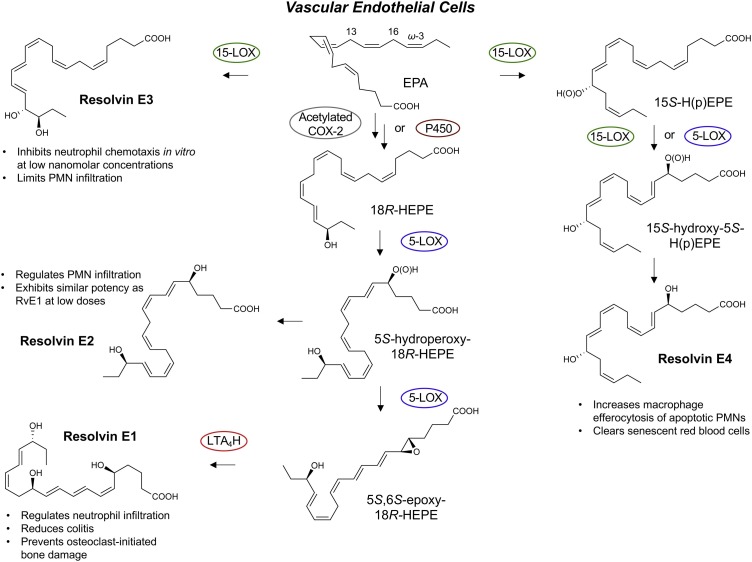
Fig. 3.
Proposed Biosynthesis of the E-series Resolvins.
18R-HEPE produced from unesterified EPA via acetylated or modified COX-2 as well as p450 [21] is next converted by leukocyte 5-lipoxygenase to the intermediate 5S-hydroperoxy-18R-HEPE, which is either reduced to RvE2 or converted to the epoxide intermediate 5S,6S-epoxy-18R-HEPE to produce RvE1 [77] to carry out their specific functions listed above; see text for details. RvE1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) [8,21,72]. EPA is also a substrate for lipoxygenation by 15-LOX to produce RvE3 (17R,18R-dihydroxy-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid) [83]. Stereochemistry and proposed biosynthetic route of RvE4 are confirmed as well as RvE4’s potent actions and functions in vivo [61,87].
Fig. 4.
Biosynthetic Mechanism and Intermediates in Resolvin E1 Production from EPA. The enzymes and structures of 5-LOX and LTA4H were determined with recombinant enzymes and the 5,6-epoxide intermediate identified by methanol trapping [78]. The biosynthesis of the 18S E-series resolvins and their potent functions were described in [77,78].
The biosynthesis of RvE1 [21] from EPA (Fig. 2, Fig. 3, Fig. 4) also yields in this pathway the bioactive resolvin E2 structure 5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid [77,78]; each possesses potent anti-inflammatory-pro-resolving actions. RvE2 is also produced with hypoxic endothelial cells on incubation with human neutrophils [78], and both RvE1 and RvE2 each stereoselectively activate the receptor ChemR23, a G-protein coupled surface receptor [72,[77], [78], [79]]. Anti-ChemR23 (i.e., resolvin E1 receptor) antibodies that activate this RvE1 receptor as an agonist stimulate resolution of inflammation and reduce the cancer burden in mice [79], as does Resolvin E1 and protectin D1 in the activation of endogenous resolution programs in vivo [80] in mice and resolvin E1 in rabbits [81], which demonstrates the potential for resolution pharmacology with agonists to control excess inflammation. This principle was also demonstrated (Fig. 5 ) with metabolically stable analogs prepared for RvE1, 19-phenoxy-parafluoro-RvE1 [82]. The discovery of agonist antibodies for the proresolving receptors that activate endogenous resolution programs [79] is a very exciting new addition to the potential novel therapeutic approaches to control excessive inflammation and cancer.
Fig. 5.
Further Local Metabolism of Resolvin E1 and Structures: metabolic inactivation. RvE1 is converted to less active metabolites by neutrophils and macrophages [214]. The Resolvin E1 further metabolome is cell-type specific, macrophages carry out dehydrogenation, and neutrophil p450 converts RvE1 to 20-carboxy-metabolites [214]. We prepared and designed stable analogs of Rv [60,82] that delay their rapid local inactivation. For examples, benzo RvD1 analog has been prepared [60] that retains potent bioactions of RvD1, and the 19-p-fluorophenoxy-RvE1 analog delays rapid leukocyte inactivation and is a potent anti-inflammatory and proresolving molecule [82]. Also, the benzo-Resolvin E2 has femtomolar potencies in mouse peritonitis [124].
Resolvin E3 was the next bioactive mediator to join the E-series of resolvins, discovered by Professor Makoto Arita and his colleagues in Japan. Resolvin E3 proved to have the complete stereochemistry 17R,18R-dihydroxyicosa-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid, containing a vicinal diol that potently blocks neutrophil migration and is biosynthesized via the actions of 15-lipoxygenase and confirmed by total organic synthesis [83]. RvE3 is identified in human tissues in vivo, for example in arthritic exudates [46]. The resolvin E1 and RvE2 precursor, 18-HEPE, is a major product of EPA identified in humans in vivo (Table 1) that also carries its own bioactivity with potent actions on cardiovascular tissues [84].
Recently, we encountered [85] and elucidated the complete structure [61] of a new bioactive member of the EPA–derived E-series resolvins termed Resolvin E4 (RvE4) given its potent actions and in vivo production. The stereochemistry of RvE4 was deduced and the double-bond geometry proposed based on the activity of 15-lipoxygenase (15-LOX) with EPA via double lipoxygenation to produce the potent bioactive molecule 5S,15S-dihydroxy-6E,8Z,11Z,13E,17Z-eicosapentaenoic acid. RvE4 is produced in physiologic hypoxia by human neutrophils and macrophages. Given that RvE4 proved to be a potent agonist for efferocytosis of both senescent erythrocytes (sRBCs) and apoptotic neutrophils, it is classified as having resolving functions, and with EPA as the precursor and backbone we grouped it with the E-series resolvin members. This novel bioactive structure was accordingly named RvE4 because it increases the resolution of hemorrhagic exudate in vivo in mice [85].
The proposed biosynthesis of RvE4 was recently independently confirmed with purified recombinant human enzymes using 5-lipoxygenase (5-LOX) and 15-LOX with substrate EPA in vitro [86]. We establish the complete stereochemistry of RvE4 and its potent functions with isolated human macrophage-mediated efferocytosis employing results from detailed matching experiments [61]. To assign the complete stereochemistry of RvE4 as well as determine whether synthetic RvE4 shares reported physical and biological functions [85], it was deemed essential to establish the physical properties of the newly synthesized RvE4 [87].
The structure and stereochemistry of synthetic RvE4 were determined using total organic synthesis from chiral starting materials of known stereochemistry as well as stereocontrolled chemical reactions. This approach was successfully used with each of the potent bioactive SPMs. Synthetic RvE4 was directly compared to the target RvE4 structure that was deduced earlier using the isolated and well-studied 15-lipoxygenase (LOX) enzyme that inserts molecular oxygen into 1,4-cis-pentadienes predominantly in the S configuration [85]. Co-injections of biogenic RvE4 with synthetic RvE4 confirmed their identical chromatographic behavior. MS/MS analysis indicated that co-injection gave identical fragmentation with parent ion m/z 333 = M-H and daughter ions at m/z 315 = M-H-H2O, m/z 271 = M-H-H2O-CO2, m/z 253 = M-H-2H2O-CO2, m/z 217 = 235-H2O, m/z 199 = 217-H2O, and m/z 173 = 235-H2O-CO2 essentially identical to that obtained in [61,85,87].
Neutrophils are often the first leukocytes to infiltrate an inflammatory site, recruited from post-capillary venules in large numbers, and therefore their effective removal from the inflammatory site following neuralization of invading microbes is a prerequisite for the resolution of inflammation and return to tissue homeostasis [7,16,88,89]. SPMs, including RvE4, enhance macrophage efferocytosis of apoptotic neutrophils in vitro and in vivo with both human cells and in murine models of inflammation [7,85]. RvE4 enhanced efferocytosis of sRBCs by M2 macrophages confirming the function of endogenous RvE4 [61]. From results of matching studies using LC-MS-MS, co-elution in LC and fragmentation, UV spectrum absorbance and biological actions, we assigned the complete double-bond geometry of RvE4, namely 5S,15S-dihydroxy-6E,8Z,11Z,13E,17Z-eicosapentaenoic acid [61,85,87]. Hence, the synthetic RvE4 now commercially available is suitable for use as both a standard for LC-MS-MS-based mediator lipidomics and for further functional investigations. In line with this and of interest, endogenous human RvE4 has already been identified in human cerebrospinal fluid indicating its production in vivo in human tissue [90] and is present in plasma obtained from COVID-19 patients [26].
RvE4 was first uncovered from physiological hypoxic triggered biosynthesis of EPA, via lipoxygenation, by human macrophages and neutrophils exposed to stimuli [85]. M2 macrophages play critical roles in the resolution of inflammation by virtue of their capacity to carry out efferocytosis [7,16], wound repair [7,91], and production of SPMs [18]. RvE4 proved to be a potent agonist of M2 macrophage efferocytosis of neutrophils: EC50 ∼0.23 nM and an EC50 ∼0.29 nM for efferocytosis of sRBCs [61]. In human macrophages and neutrophils, RvE4 biosynthesis is solely dependent on the substrate availability, where we found that EPA is released from both phospholipids and triglycerides [85]. EPA is converted by 15-LOX to 15S-HpEPE, which becomes a substrate for further lipoxygenation by either 5-LOX or a second enzymatic turn of 15-LOX to produce 15S-hydroxy-5S-HpEPE; this is further reduced to RvE4 (Fig. 3, Fig. 4). This route of RvE4 biosynthesis by human phagocytes was subsequently also confirmed by Kutzner et al. using recombinant human 5- and 15-LOX co-incubations in vitro [86]. EPA is converted by the wild type and recombinant engineered lipoxygenases to double dioxygenation products including RvE4 on a larger synthesis scale [92]. It is likely that the lipoxygenation of EPA is initiated via hydrogen abstraction at carbon position C7 by 5-LOX to biosynthesize RvE4 in human neutrophils and/or via transcellular biosynthesis [21,93] as depicted in the cellular interactions in Fig. 2 illustration.
Earlier results demonstrated conversion of EPA to 15R-HEPE via acetylated COX-2 [21,76]; hence it is likely that this product can be a substrate for lipoxygenation by 5-LOX that can lead to further conversion to the corresponding alcohol giving rise to a novel 15R epimer of the RvE4. This 15 epi-RvE4 could also be biosynthesized following acetylation of COX-2 by acetyl-CoA and sphingosine via sphingosine kinase 1 (SphK1) [94] and/or modified possibly by S-nitrosylation of COX-2 [95]. Since 5-LOX has been shown to be phosphorylated to produce 15R-HETE and further converted to 15R-lipoxin A4 (LXA4) [[96], [97], [98]], it is likely that, with EPA as a carbon 20:5 substrate like C20:4 arachidonate, phosphorylated 5-LOX can produce 15R-HEPE that can be subsequently converted to the novel 15R-RvE4 structure. Along these lines, RvE4 can be biosynthesized from EPA via several separate biosynthetic routes and can now be included in the E-series of pro-resolving mediators; see Table 2 . At this juncture, the actions of RvE4 include stimulating human macrophage efferocytosis of apoptotic neutrophils and senescent red blood cells, as well as reducing mouse hemorrhagic exudates by increasing efferocytosis and decreasing neutrophil infiltration in vivo [85]. The abilities of RvE4 to limit neutrophil tissue infiltration and to enhance efferocytosis are also functions shared by other SPMs and members of the E-series resolvins (Table 2).
Table 2.
Inflammation-Resolution EPA Bioactive Functional Metabolome and Related Analogs and Structures.
| Name and Abbreviation | Stereochemical name | Structure | Pro-resolving cellular functions and in vivo actions | Organ protection | Locations/origins in humans and animal models |
|---|---|---|---|---|---|
| Resolvin E1 (RvE1) | 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid | 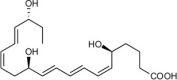 |
Limits PMN [21,77,78,80,99] and reduces dendritic cell further tissue infiltration [72,100]. | Peritonitis [21,72,77,78,80], air pouch [21], CLP/sepsis [106], bacterial [101], I/R injury [107], diabetes [108], obesity [112], colitis [99,109,110]; lung inflammation [111], kidney injury [199], depression, Alzheimer’s [200], atherosclerosis [113,201], bone [81], reduces tumor growth [114], Dermatitis [115], Candida albicans [116], pain [118], and reduces herpes [117]. | Plasma [119] |
| Nano-delivery of RvE1 repairs gastrointestinal injury and activates wound healing [110,202] | Plasma from arthritis patients [46] | ||||
| Reduces pro-inflammatory cytokines [101]. | Arthritic synovial fluid exudates [46] | ||||
| Enhances MΦ phagocytosis & efferocytosis [77,80]. | Plasma of Type 2 diabetes mellitus [54] | ||||
| Enhances bacterial clearance [101]. | Plasma of peripheral artery disease (OMEGA-PAD II trial) | ||||
| Inhibit pain TRP channels [102]. | 0.32−0.62 pg/mL [120] | ||||
| Regeneration of stem cells isolated from periodontal ligaments [103]. | Cord blood [55] | ||||
| Blocks ADP dependent platelet aggregation [198]. | Human breast milk [121] | ||||
| Blister [122] | |||||
| Metabolic syndrome | |||||
| (weight loss) PMN [45] | |||||
| 18S-Resolvin E1 (18S-RvE1) | 5S,12R,18S-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid | 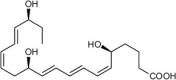 |
Stops PMN migration, reduces pro-inflammatory cytokines, and enhances MΦ phagocytosis & efferocytosis [77]. | Mouse Peritonitis [77]. | |
| Resolvin E4 (RvE4) | 5S,15S-dihydroxy-6E,8Z,11Z,13E,17Z-eicosapentaenoic acid | 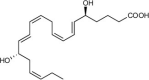 |
RvE4 stimulates macrophage efferocytosis and phagocytosis [61,85]. | Mouse Hemorrhagic Exudate [85] | |
| Resolvin E2 (RvE2) | 5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid | 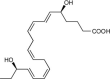 |
Stops PMN migration [77,78]. | Resolves Peritonitis [77,78], and reduces depression [123]. | Plasma 2.3−2.7 pg/mL [119] |
| Down-regulates leukocyte integrins [110]. | Plasma of arthritis 68.8 pg/mL [46] | ||||
| Arthritis synovial fluid | |||||
| 774.2 pg/mL [46] | |||||
| Plasma of Type 2 diabetes mellitus [54] | |||||
| Cord blood [55] | |||||
| Plasma of peripheral artery disease (OMEGA-PAD II trial) [120] | |||||
| Human breast milk [121] | |||||
| Human skin blisters [122] | |||||
| Ortho-Benzo-Resolvin E2 (o-BZ-RvE2) | (S,E)-5-hydroxy-8-(2-((R,E)-3-hydroxypent-1-en-1-yl)phenyl)oct-6-enoic acid | 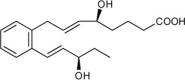 |
Reduces PMN infiltration in vivo [124]. | Resolves mouse Peritonitis [124]. | |
| 18R-Resolvin E3 (18R-RvE3) | 17R,18R-dihydroxy-5Z,8Z,11Z,13E,15E- eicosapentaenoic acid | 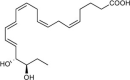 |
Inhibits leukocyte migration [83]. | Peritonitis [83], Mouse lung inflammation [125], reduces murine depression behavior [126], premature birth [127]. | Plasma of arthritis [46] |
| Reduces IL-23 and IL-17 [125]. | Arthritis synovial fluid [46] | ||||
| BLT1R antagonist [125]. | Plasma of Type 2 diabetes mellitus [54] | ||||
| Plasma of peripheral artery disease (OMEGA-PAD II trial) [120] | |||||
| Cord blood [55] | |||||
| Human breast milk [121] | |||||
| Human skin blisters [122] | |||||
| Metabolic syndrome (weight loss) PMN [45] | |||||
| 18S-Resolvin E3 (18S-RvE3) | 17R,18S-dihydroxy-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid | 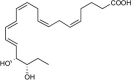 |
Inhibits PMN migration [83,203]. | Reduces mouse peritonitis [83,203]. | |
| 18-hydroxy-eicosapenta-enoic acid (18-HEPE) | 18S/R-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid | 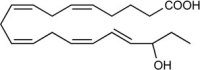 |
Reduces pro-inflammatory cytokines [84]. | Prevents overload-induced maladaptive cardiac remodeling [84] and metastasis [204]. |
RvE1’s pro-resolving actions include stopping neutrophil [21,77,78,80,99] and dendritic cell migration [72,100], reducing pro-inflammatory cytokines [101], enhancing macrophage phagocytosis and efferocytosis [77,80], enhancing bacterial clearance [101], modulating T cell responses [102] and regenerating periodontal ligament stem cells [103]. RvE1 has unique actions on platelets by inhibiting adenosine diphosphate (ADP)-activated mobilization of P-selectin to minimize platelet aggregation, which is very useful for prolonged storage of blood that can be useful for needed battlefield transfusions [104]. In addition, RvE1 and the other SPMs have potent actions in the nanogram range in vivo that include organ protection, clearing infections (by increasing killing [105]), and stimulating resolution as receptor agonists in, e.g., peritonitis [21,72,77,78,80], sepsis [106], ischemia-reperfusion injury [107], diabetes [108], colitis [99,109,110], lung inflammation [111], obesity [112], atherosclerosis [113], tumor burden [114], dermatitis [115], Candida albicans [116], and herpes simplex virus infections [117] as well as in pain [118]. In humans, RvE1 has been identified in the plasma of healthy individuals [119], plasma and synovial fluid from arthritic patients [46], plasma of type II diabetes mellitus patients [54], plasma of peripheral artery disease patients [120], cord blood [55], breast milk [121], and blisters induced by UV-killed E. coli [122]. Recently, RvE1 was found by Barden and colleagues to be increased in human neutrophils from subjects with metabolic syndrome following weight loss upon stimulation ex vivo [45]. The RvE1 analog mimetic (Fig. 5) evokes anti-inflammatory actions by reducing both neutrophil infiltration and pro-inflammatory cytokine/chemokine production in vivo [82]. These findings demonstrate tissue/organ- and cell type-specific actions of RvE1 in the control of a wide range of diseases where excessive inflammation is an underlying pathobiology driven by excessive PMN infiltration and resulting collateral tissue damage.
In the E-series bioactive metabolome, RvE2 stops chemoattractant-stimulated PMN recruitment in vivo in murine peritonitis [78] and decreases depression-like behavior in mice [123]. RvE2 has also been identified in the plasma of healthy individuals [119], plasma and synovial fluid of arthritis patients correlating with pain reduction in these patients [46], plasma of type II diabetes mellitus patients [54], plasma of peripheral artery disease patients, breast milk [121], and in human blisters induced by UV-killed E. coli [122]. Of interest, a novel RvE2 analog, benzo-resolvin E2, was found to display exceptional potency (in the femtomole range) at reducing inflammation in vivo [124] (Table 2).
RvE3 decreases allergic airway inflammation via the IL-23/IL-17A pathway [125], reduces depression-like behavior in mice [126], and lowers the incidence of preterm birth in lipopolysaccharide-exposed pregnant mice [127]. Also, RvE3 was identified in both plasma and synovial fluid of arthritic patients [46], plasma of type II diabetes mellitus patients [54], cord blood [55], breast milk [121], and E. coli-stimulated human skin blisters [122] (Table 2). Considering the E series metabolome, the precursor of several E-series resolvins, namely 18-HEPE (Fig. 2, Fig. 3, Fig. 4), is also bioactive, inhibiting macrophage-mediated pro-inflammatory activation of cardiac fibroblasts as well as preventing overload-induced maladaptive cardiac remodeling in vivo, demonstrating potent actions in cardiac tissue [84]. Considering the structure-activity relationships for the E series resolvins, the 18-deoxy-resolvin E3 (18-deoxy-RvE3) shows potent bioactivity in stopping neutrophil infiltration in mice as well as resolves peritonitis [128]. Recent results indicate that RvE3 is reduced in COVID-19 patients with severe disease compared to moderate disease [24] (Table 1), which highlight the potential significance of SPMs in human disease. E-series resolvins are also found to be endogenous anti-depressants in mouse models [129]. Together these new findings from many independent investigators emphasize the potent structure-based function and bioactions of the E-series resolvins (Table 2).
The EPA resolution bioactive metabolome to the E-series bioactive resolvins produces several potent mediators in this biosynthesis pathway (Table 2 and Fig. 3) that target diverse cell types relevant to inflammation, resolution, depression, and resolution of vascular inflammation. This EPA precursor metabolome may contribute to the clinical impact of omega-3 supplementation as in recent results from cardiovascular disease patients [130] and in human randomized trials, where omega-3 lowered systemic levels of potent pro-inflammatory cytokines associated with inflammaging [131]. It is now clearly demonstrated in humans that omega-3 supplementation is required to increase RvE1, as reported in both serum and plasma of healthy individuals, RvE3 in plasma of patients with peripheral artery disease [120], and circulating RvE1, RvE2, and RvE3 [132]. Welty et al. [133] found that the ratio of the sum of 18-HEPE plus RvE1 to the amounts of leukotriene B4 is useful in assessing the regression of coronary artery plaques in humans. Keeley et al. [134] recently reported identification of SPMs in women with coronary microvascular dysfunction.
4. D-series resolvins: biosynthesis and stereochemistry
The DHA-derived resolvins and protectins were first uncovered in resolving inflammatory exudates [8,14] and named given their potent actions. The D-series resolvins are biosynthesized via two separate pathways to yield the 17R series [8] and 17S-series resolvins that can involve transcellular biosynthesis with hypoxic vascular endothelial cells and human PMN. There are six potent bioactive members in each, named RvD1, RvD2, RvD3, RvD4, RvD5 and RvD6 [8]. The 17R resolvins are produced from DHA conversion to 17R-hydroperoxy intermediate via acetylated COX-2 and 17S-series resolvins from DHA conversion via 15-lipoxygenase reaction [8,14]. Each of the D series Resolvins and other SPMs are produced by specific agonist and cell types (Table 3 ) in well-defined in vitro conditions. Resolvin D1 and Resolvin D2 are produced from a transient 7(8)-epoxide intermediate produced via the 5-LOX. Their structures were determined using both GC–MS and LC-MS-MS together with bioassays. The complete stereochemistry of RvD1 and 17R-RvD1 was systematically determined with total organic synthesis and results from rigorous matching studies [135]. The complete structure of Resolvin D2 was also established using this approach and proved to be a potent proresolving molecule [136].
Table 3.
Agonist and Cell Types Producing SPM in vitro.
| Agonist | Cell Type | Incubation | SPM | Reference |
|---|---|---|---|---|
| ASA (500 μg/air pouch) | TNF-α induced exudate (6 h) | 2.3 × 106 leukocytes/ pouch FVB mice | 18R HEPE | [21] |
| EPA (500 μg/ air pouch) | Air pouch leukocyte | Resolvin E1 | ||
| IL-1β-treated | HUVEC | IL-1β (24 h) and ASA | 18R HEPE | [21] |
| Human HUVEC | ||||
| Serum-treated zymosan 100 ng/ mL | Human PMN | PMN 30 × 106, 30 min | Resolvin E1 | [21] |
| ASA recombinant COX-2 EPA products | STZ (100 ng/mL) | Lipoxin A5 | ||
| FMLP 10−7 M | GM-CSF Human PMN | PMN 30 × 106 | Lipoxin A4 | [205] |
| 200 pM rhGMCSF | Lipoxin B4 | |||
| 90 minutes | ||||
| A23187 (20 min) | Trout Macrophages | 10 × 106 cells, A23187 | Lipoxin A4 | [206] |
| 20 min, 18 °C | Lipoxin A5 | |||
| TNF-α (50 ng/mL) 24 h | Human Microglia | ASA, 30 min | 17R-HDHA | [8] |
| Hypoxia | HUVEC | ASA, TNF-α, IL-1β + Hypoxia | 17R-HDHA | [8] |
| DHA (20 μg/ 1 × 106) | ||||
| ASA | Inflammatory exudates | 6 h, FVB mice | 17R-Resolvin D5 | [8] |
| Mouse ASA | 17R-Resolvin D3 | |||
| Zymosan | Human PMN | PMN (50 × 106 cells/ ml) | D series Resolvins | [8] |
| Zymosan 100 ng/mL | ||||
| 17R HDHA (5μg/mL) | ||||
| IL-1β or | Retinal ARPE-19 cells | IL-1β (6 h) | NPD1 | [166] |
| A23187 | ||||
| TLR-7 Agonist (R-848) | Human monocytes | 10 × 106 cells + | Resolvin D5 | [207] |
| R-848 (100 μM) | Protectin D1 | |||
| 1 h, 37 °C | ||||
| Apoptotic PMN | 10 × 106 cells | E series Resolvins | [17] | |
| D series Resolvins | ||||
| Mouse apoptotic PMN | M0 Macrophages | Time course 120 min efferocytosis | Lipoxin A4, Resolvin E1 | [80] |
| Protectin D1 | ||||
| ASA + EPA | Human M0 Macrophages | EPA (20μM) for 45 min | Resolvin E1 | [80] |
| E. coli | M2 human Macrophages | 5 × 106 M2 Macrophages | Resolvin D5 | [18] |
| 90 min, 37 °C | ||||
| sPLA2 Type V | Microparticle | Zymosan peritonitis (1 mg) exudates 48 h | 17-HDHA | [191] |
| 14-HDHA | ||||
| E. coli (105 CFU) | Infectious Leukocytes | In vivo collected exudate 24 h | Resolvin D3 | [138] |
| Exudate | ||||
| 18-HEPE (5μg) | Human PMN | Hypoxia PMN plus | Resolvin E1 | [78] |
| Zymosan (100 μg/mL) | Resolvin E2 | |||
| LPS/ FMLP | GM-CSF Human Macrophages | LPS (1μg/mL) 20 min | Lipoxin A4 | [208] |
| + FMLP (1μM) 10 min | 17-HDHA | |||
| 37 °C | ||||
| Zymosan peritonitis (1 mg) | Eosinophils | In vivo collected exudate 24 h | Lipoxin A4 | [209] |
| Protectin D1 | ||||
| HMGB1+ C1q | Human Monocytes | HMGB1 (1μg/mL) | Lipoxin A4 | [20] |
| C1q (25 μg/mL) | Resolvin D1 | |||
| 6 h | Resolvin D2 | |||
| HMGB1 alone | Human Monocytes | HMGB1 (1μg/mL) | Leukotriene B4 | [20] |
| Carbon monoxide (CO) + zymosan in vivo in mice | Mouse Peritoneal Exudates | 250 ppm CO | Resolvin E2 | [210] |
| 0.1 mg/ zymosan | Resolvin D1 | |||
| Resolvin D2 | ||||
| Maresin 1 | ||||
| Zymosan peritonitis (1 mg) | Human MCSF Macrophages | Endogenous Maresin 1 | Maresin 1 | [15,175] |
| Stereochemistry | ||||
| Physiologic Hypoxia (1 % oxygen) | Human M2 Macrophages | 24 h hypoxia chamber | RvE4 | [85] |
| D-Series Resolvins | ||||
| EPA (1 g) | Human plasma collected (3 h) | 18-HEPE | [77] |
Resolvin D3 and Resolvin D4 are produced [8,14] later in the time course of the acute inflammatory response, appearing in inflammatory exudates after both resolvin D1 and D2 [137]. The complete stereochemical assignment of RvD3’s structure was established and its potent bioactions confirmed by total organic synthesis confirmed in two separate series of experiments as 4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid [[137], [138], [139]]. The second synthesis undertaken afforded larger-scale production of RvD3 [138]. The complete stereochemistry of Resolvin D4 was also established using matching studies with materials prepared from total organic synthesis [140,141].
RvD4’s complete structure is 4S,5R,17S-trihydroxydocosa-6E,8E,10Z,13Z,15E,19Z-hexaenoic acid. Both RvD3 and RvD4 proved to be potent immunoresolvents stopping further neutrophil infiltration and transmigration, enhancing macrophage phagocytosis and efferocytosis, and reducing cytokines and chemokines, promoting bacterial clearance in vivo in mice. Resolvin D3 and Resolvin D4 are produced in resolving inflammatory exudate from the same 4(5)-epoxy-resolvin intermediate as proposed in Ref. [8]. The 4S,5S-epoxy-resolvin intermediate was recently prepared by total organic synthesis and is selectively converted to Resolvin D3 by human neutrophils and to Resolvin D4 by human neutrophils and M2 macrophages [142] by specific enzymes in these phagocytes. RvD4 reduced thrombus in pathologic thrombosis [[143], [144], [145]]. Morita et al. [146] also reported a stereocontrolled synthesis of resolvin D4.
Morbidly obese human subjects have higher amounts of RvD4 and other SPMs that are reduced post-surgery in diabetes, suggesting ongoing inflammation in these patients [42]. Resolvin D5 plays a critical role in host defense, enhancing bacterial killing and clearance [11,147]. The structure of resolvin D5 was established and confirmed with results from total organic synthesis [148,149], leukocyte biosynthesis, and from enzymatic studies [150] with 15-lipoxygenase-2. RvD5 is increased in human blood with n-3 supplementation [38] and is present in human skin in lesions of psoriasis [151]. With skin cells, RvD5 reduces expression of IL-24 and S100A12 with human keratinocytes [151] and remains elevated one year after discharge in ICU patients with abdominal septic shock [152], suggesting the need for long-term follow-up of ICU patients. Resolvin D5 and other D-series resolvins reduce both inflammatory pain and neuropathic pain, showing sex-dependent dimorphism in mice [153]. In addition to the innate system, SPMs are also biosynthesized [154] and function in the adaptive immune system regulating T-cell responses [155] and B cell antibody production [156] that is relevant in infections such as COVID-19. Recently, RvD5 was found to also act on T cells to reduce experimental arthritis [157].
Resolvin D6 was first uncovered in resolving inflammatory exudates along with the other D-series resolvins and protectins biosynthesized from DHA [8]. These novel and unique structures were determined using both LC-MS-MS and GC–MS fragmentation and bioassays. Resolvin D6 has a role in reducing muscle inflammation associated with injury and aging [48] and in tendon overuse [49]. Recently, a Resolvin D6 isomer and Elovanoid-N32 were discovered to reduce ACE2 and binding of the virus spike protein to human corneal epithelial cells of the eye following injury or exposure to IFNγ [158,159] and to stimulate nerve regeneration, wound healing and pain [160]. Human periodontal stem cells also produce Resolvin D6 and other SPM [161]. The complete structure of the Resolvin D6 novel isomer responsible for reducing neuropathic pain and nerve regeneration was recently elucidated and found to be RR-RvD6 prepared by total organic synthesis [162].
DHA is enriched in neural tissues, nerves, and brain. SPMs are bioactive products via enzymatic pathways that are highly conserved in evolution from fish to humans [163]. Trout brain produces Resolvin D5, RvD1 and RvD2 as well as neuroprotectin D1, indicating that these chemical signals are conserved structures from fish to humans [164]. Atlantic salmon also produce resolvins de novo, which are reduced in amounts with baking [165]. DPA of the n-3 pathways is also a precursor to SPMs [56]. The consequence of consuming fish that contain resolvins and/or other SPM as well as prostaglandins remains to be determined.
5. The Protectin and Maresin families of potent bioactive mediators: biosynthesis and stereochemistry
Within the inflammatory exudates (Fig. 1) as neutrophils enter the exudates [8], DHA is converted to conjugated triene structures, e.g. 10,17-diHDHA, that include neuroprotectin D1, which protects retinal pigmented epithelial cells of the eye [166] and stops PMN infiltration to the brain, protecting neural tissues from leukocyte-mediated collateral tissue damage and inflammation [167], and stimulates wound healing of corneal epithelial cells [168]. Both 17S-resolvins and protectins are produced from the 17S-HpDHA intermediate that is the biosynthetic product of human 15-LOX (12/15-lipoxygenase of the mouse) with DHA [14] and subsequent enzymatic steps to produce each of the potent bioactive molecules. The name protectins was coined from the potent organ-protective actions and anti-inflammatory—pro-resolving actions of this family of mediators. The prefix neuro in neuroprotectin D1 (NPD1) reports the tissue of origin of these bioactive molecules [166], which are also produced by exudate leukocytes of the innate immune system [8,14]. NPD1 reduced stroke damage [167], protects lung tissue [169] and kidneys from injury [170], and increases neural cell survival [171]. Macrophages, T cells and neutrophils produce protectins from DHA. The complete stereochemistries of the bioactive neuroprotectin D1/protectin D1 and its natural biosynthesis isomers produced on enzymatic processing of DHA were established using materials prepared by total organic synthesis and qualified with NMR [172]. NPD1/PD1 activates endogenous resolution programs, shortening the time to resolve [80].
The 17-epi-protectins such as 17R-PD1/NPD1 are biosynthesized via aspirin-acetylated COX-2 enzyme, as for example in hypoxic vascular endothelial cells [173], which proved to be potent protective and pro-resolving molecules [reviewed in Ref. 174]. The complete stereochemistry of the 17-epi-NPD1/PD1, also known as the aspirin-triggered (AT)-NPD1/PD1, was assigned using materials prepared by total organic synthesis. The endogenous AT- or R epimer of NPD1/PD1 proved to be 10R,17R-dihydroxy-docosa-4Z,7Z,10E,12E,14Z,19Z-hexaenoic acid with potent proresolving actions [reviewed in Ref. 174, cf. and see references within]. Other stereo and geometric isomers of NPD1/PD1 do not possess these potent anti-inflammatory—pro-resolving actions. These properties of NPD1/PD1 may be useful in the design of novel therapeutic agents for neurodegenerative diseases where neural inflammation plays a central role in disease pathology.
Later in the progression of the acute inflammatory response (Fig. 1), when macrophages appear and enter the exudates with directives for wound healing and homeostasis, these cells convert DHA to the maresins (macrophage mediators in resolving inflammation), which are proresolving and stimulate tissue regeneration [15,175]. The complete stereochemistry of Maresin 1 (MaR1) is 7R,14S,dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid and was determined by matching the endogenous MaR1 produced by macrophages to compounds prepared by total organic synthesis. MaR1’s proresolving actions are complemented by this molecule’s ability to stimulate tissue regeneration and reduce pain. MaR1 inhibits capsaicin-induced TRPV1 currents in neurons [175]. MaR1 is biosynthesized via an epoxide intermediate enzymatically produced from the DHA-derived lipoxygenase product 14-HpDHA [15] as determined by acid methanol trapping studies and analyses of the resulting products. The role of this unique 13S,14S-epoxy-maresin was confirmed by total organic synthesis and its conversion to MaR1 [176,177] by M2 macrophages, where this epoxide also proved to be bioactive, stimulating the phenotype switch from M1 to M2 macrophages.
6. Cys-SPM
The enzymatic conversion of lipoxygenase produces hydro(peroxy)-containing intermediates that are transient and rapidly transformed to epoxide-containing intermediates that play critical roles in the stereochemical specific biosynthesis of Resolvins, Protectins and Maresins in each pathway [for review, see Ref. 174]. Evidence for the cellular production of unstable epoxide intermediates within each SPM biosynthesis pathway was obtained using acid-methanol trapping [8,14,15,135,154]. Confirmation of the role of these transient epoxide-containing intermediates was obtained from studies with leukocytes, isolated enzymes, and their stereoselective total organic synthesis. The 13S,14S-epoxy-maresin was prepared with Professor Nicos Petasis and his team [176], and the 16,17-epoxy-protectin was prepared with Professor Trond Hansen, Jørn Tungen and Marius Aursnes [178]. Recently, the 4S,5S-epoxy-resolvin was synthesized by total organic synthesis, and its enzymatic conversion to Resolvin D3 and Resolvin D4 proved to be cell type specific with human phagocytes [142]. Each epoxide intermediate is also pivotal in the enzymatic biosynthesis of the cys-SPM, e.g. MCTRs [179], which stimulate tissue regeneration and are proresolving, PCTRs [178] and RCTRs, each with 3 members in their biosynthetic pathways; their complete stereochemistry has been determined and confirmed their potent bioactivities [180] with both Dr. Bernd Spur and Dr. Nicos Petasis [181,182]. With these epoxides in hand, we’ve focused on the enzymes responsible for their conversion to potent cys-SPMs [142,183], as well as specific SPMs.
Essential fatty acids, i.e., EPA and DHA, can undergo autooxidation reactions that are widely known to lead to products that are pro-inflammatory; however, these non-enzymatic reaction products are not stereoselective for the most part in their production and give rise to racemic mixtures of products that do not share the properties or potent biologic functions of the SPMs reviewed herein. The stereoselective biosynthesis of the resolvins within human leukocytes leads to their stereochemically defined structures for the SPMs that activate specific cell surface GPC receptors with nanomolar binding constants [reviewed recently in Ref. 184]. The biosynthesis of SPM in humans requires substrate EPA and DHA in adequate amounts and locations in vivo to enable SPM biosynthesis in vivo in humans; for examples, see [[38], [39], [40],50,[185], [186], [187]], to yield precise chemical structures of the SPMs with protective function for the host [188]. These early results of our studies underscore the importance of precision nutrition in the optimal progression of host defense and resolution of the acute inflammatory response in humans and model organisms [189].
7. In summation
The results reviewed here provide evidence that synthetic stereochemically defined potent bioactive SPMs matching the endogenous molecules produced by human cell types, e.g., neutrophils, lymphocytes, and macrophages (Fig. 1 B), are now in wide use worldwide in experimental disease studies and as standards for targeted LC-MS/MS-based profiling and lipidomics/metabolomic human studies from many independent investigators worldwide confirming that SPMs are produced in vivo in humans. Omega-3 supplementation is required and increases SPM production in vivo [50]. Each member of the E-series resolvins, i.e., RvE1, RvE2, RvE3 and RvE4, are now documented in human tissues in several independent investigations that confirm their original structural elucidation. The physiologic function(s) of the E series resolvin metabolomes in humans are still evolving. The current results available from human studies indicate that the SPMs are indeed produced in humans when suitable substrates are available in vivo.
The potent functions of each of the stereochemically defined SPMs in resolving and reducing the magnitude of inflammation in vivo are now demonstrated by many independent investigations around the world with diverse experimental animal models of disease: >1420 publications were reported for resolvins in PubMed.gov as of Dec. 2021 (with resolvins as search term) that confirm the potent pro-resolving properties of each of the SPMs and their novel structures as originally described, triggered via local hypoxia in the resolving inflammatory milieu [8,14,15]. Given the current pandemic and the identification of resolvins as well as the other SPMs in human COVID-19 (Table 1), the availability of SPM and several of their deuterium-containing labels for use as standards and rigorous determinations and authentication methods enable targeted LC-MS-MS-based profiling that the Serhan lab initially introduced [[27], [28], [29]]. These can be tested in long-COVID patients to assess SPM, their de novo production and potential local cellular and organ functions with precision nutrition interventions that can now be rigorously tested as, e.g. to consider whether they can reduce the symptoms of long COVID and acute COVID-19 infections. The SPMs have proven to be potent proresolving molecules in resolving inflammation and pain in experimental animals and with isolated single cell analysts with human leukocytes. Given that SPMs are conserved structures in evolution as discussed herein, it is very possible that increasing SPM production in vivo can permit them to function in humans to reduce pain, resolve tissue inflammation and clear microbes as well as activate regeneration of damaged tissues.
Clinical studies along these lines with interventions for precision nutrition [190] are now made possible with the stereochemically defined SPMs and targeted LC-MS-MS-based metabololipidomic profiling [27,28] as required tools for this endeavor. In a clinical trial where a proresolving mimetic LX/Rv stable analog was employed topically on inflamed gingival tissue, subjects had reduced gingival inflammation as well as increased production of SPMs in their peripheral blood samples [63]. These human results suggest that SPM therapies can increase endogenous SPM and resolution in a positive feed-forward mechanism as uncovered earlier in experimental animals, where we found that SPMs each activate endogenous resolution programs to shorten recovery times from inflammatory challenge [80]. Local delivery of pro-resolving molecules with nano-pro-resolving medicines such as the resolvins and lipoxins delivered in humanized nano particles has advantages as well in resolving inflammation and activating wound healing and repair [191]. This concept for the local delivery of resolvins has now been studied in a wide range of engineered materials to successfully activate endogenous resolution programs in vivo [192,193]. Hence, rigorous determinations of whether the state of resolution of inflammation can be achieved in humans with precision nutrition is of vital importance given the impact of the COVID-19 variants and long COVID on human health, the ongoing pandemic, and the unmet need in public health. Have the stereochemistry studies of the SPM and their human biosynthesis pathway(s)-metabolomes each from omega-3 precursors prepared us for this paramount challenge? Are we ready?
Can precision nutrition targeted to increase SPMs in vivo enable human subjects to return to homeostasis more rapidly (recover more swiftly) following the initial insult of the COVID viral infection? It can also be considered whether there are functional roles for SPMs in peripheral blood circulation. Are they functional in the local milieu, where they can directly regulate leukocyte response in whole human blood [75,194], or in transit and/or leaking into peripheral blood from other sites/organs within the body, possibly from sites of infectious inflammation in multiple organs? Our field is prepared to address these challenges and is armed with the required tools and ample experimental evidence now available from investigators around the world’s scientific community interested in resolving inflammation and infection (cited herein). Only time, resources and rigorous testing-documentation will give us the much-needed answers on precision nutrition and its potential in human public health and the potential for SPMs in resolution physiology-pharmacology.
Author contributions
C.N.S., S.L. and R.N. composed and contributed to the preparation of this manuscript, tables, and figures.
Funding
C.N.S is supported by the National Institutes of Health (grant no. R35GM095467) and S.L. is supported by NIH grant no. K99HL153673.
Acknowledgments
We thank M. H. Small for expert assistance in manuscript preparation. We thank the many investigators that have published exciting results on the potent functions and pharmacology of the SPMs and their total organic syntheses reviewed herein.
References
- 1.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stasi C., Fallani S., Voller F., Silvestri C. Treatment for COVID-19: an overview. Eur. J. Pharmacol. 2020;889 doi: 10.1016/j.ejphar.2020.173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., Golenbock D., Gresnigt M.S., Heneka M.T., Hoffman H.M., Hotchkiss R., Joosten L.A.B., Kastner D.L., Korte M., Latz E., Libby P., Mandrup-Poulsen T., Mantovani A., Mills K.H.G., Nowak K.L., O’Neill L.A., Pickkers P., van der Poll T., Ridker P.M., Schalkwijk J., Schwartz D.A., Siegmund B., Steer C.J., Tilg H., van der Meer J.W.M., van de Veerdonk F.L., Dinarello C.A. A guiding map for inflammation. Nat. Immunol. 2017;18(8):826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C.N., Ward P.A., Gilroy D.W. Cambridge University Press; New York: 2010. Fundamentals of Inflammation. [Google Scholar]
- 5.Haeggstrom J.Z. Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Invest. 2018;128(7):2680–2690. doi: 10.1172/JCI97945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 7.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindbom L. Regulation of vascular permeability by neutrophils in acute inflammation. Chem. Immunol. Allergy. 2003;83:146–166. doi: 10.1159/000071559. [DOI] [PubMed] [Google Scholar]
- 11.Chiang N., Fredman G., Bäckhed F., Oh S.F., Vickery T.W., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannenberg G.L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K.H., Hong S., Serhan C.N. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 13.Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 14.Hong S., Gronert K., Devchand P., Moussignac R.-L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 15.Serhan C.N., Yang R., Martinod K., Kasuga K., Pillai P.S., Porter T.F., Oh S.F., Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arienti S., Barth N.D., Dorward D.A., Rossi A.G., Dransfield I. Regulation of apoptotic cell clearance during resolution of inflammation. Front. Pharmacol. 2019;10:891. doi: 10.3389/fphar.2019.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalli J., Serhan C.N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werz O., Gerstmeier J., Libreros S., De la Rosa X., Werner M., Norris P.C., Chiang N., Serhan C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018;9(1):59. doi: 10.1038/s41467-017-02538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan P.M., Gerstmeier J., Pace S., Bilancia R., Rao Z., Borner F., Miek L., Gutierrez-Gutierrez O., Arakandy V., Rossi A., Ialenti A., Gonzalez-Estevez C., Loffler B., Tuchscherr L., Serhan C.N., Werz O. Staphylococcus aureus-derived alpha-hemolysin evokes generation of specialized pro-resolving mediators promoting inflammation resolution. Cell Rep. 2020;33(2) doi: 10.1016/j.celrep.2020.108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T., Xiang A., Peng T., Doran A.C., Tracey K.J., Barnes B.J., Tabas I., Son M., Diamond B. HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 2019;116(46):23254–23263. doi: 10.1073/pnas.1907490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang N., Serhan C.N. Cell-cell interaction in the transcellular biosynthesis of novel omega-3-derived lipid mediators. Methods Mol. Biol. 2006;341:227–250. doi: 10.1385/1-59745-113-4:227. [DOI] [PubMed] [Google Scholar]
- 23.Archambault A.S., Zaid Y., Rakotoarivelo V., Turcotte C., Doré É., Dubuc I., Martin C., Flamand O., Amar Y., Cheikh A., Fares H., El Hassani A., Tijani Y., Côté A., Laviolette M., Boilard É., Flamand L., Flamand N. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB J. 2021;35(6) doi: 10.1096/fj.202100540R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz B., Sharma L., Roberts L., Peng X., Bermejo S., Leighton I., Massana A.C., Farhadian S., Ko A., DelaCruz C., Bosio C.M. Severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome resulting in dysregulation of eicosanoid immune mediators. medRxiv. 2020 doi: 10.1101/2020.07.09.20149849. Preprint, July 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenis D., Beegun I., Jouvene C., Aguirre G.A., Souza P.R., Gonzalez-Nunez M., Ly L., Pistorius K., Kocher H.M., Ricketts W., Thomas G., Perretti M., Alusi G., Pfeffer P., Dalli J. Disrupted resolution mechanisms favor altered phagocyte responses in Covid-19. Circ. Res. 2021;129:e54–e71. doi: 10.1161/CIRCRESAHA.121.319142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbull J., Jha R., Ortori C.A., Lunt E., Tighe P.J., Irving W.L., Gohir S.A., Kim D.H., Valdes A.M., Tarr A.W., Barrett D.A., Chapman V. Serum levels of pro-inflammatory lipid mediators and specialised pro-resolving molecules are increased in SARS-CoV-2 patients and correlate with markers of the adaptive immune response. J. Infect. Dis. 2022 doi: 10.1093/infdis/jiab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang R., Chiang N., Oh S.F., Serhan C.N. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr. Protoc. Immunol. Suppl. 2011;95:14.26.1–14.26.26. doi: 10.1002/0471142735.im1426s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan C.N., Lu Y., Hong S., Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins and protectins. Meth. Enzymol. 2007;432:275–317. doi: 10.1016/S0076-6879(07)32012-0. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y., Hong S., Yang R., Uddin J., Gotlinger K.H., Petasis N.A., Serhan C.N. Identification of endogenous resolvin E1 and other lipid mediators derived from eicosapentaenoic acid via electrospray low energy tandem mass spectrometry: spectra and fragmentation mechanisms. Rapid Commun. Mass Spectrom. 2007;21:7–22. doi: 10.1002/rcm.2798. [DOI] [PubMed] [Google Scholar]
- 30.Hong S., Lu Y., Yang R., Gotlinger K.H., Petasis N.A., Serhan C.N. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz B., Sharma L., Roberts L., Peng X., Bermejo S., Leighton I., Casanovas-Massana A., Minasyan M., Farhadian S., Ko A.I., Dela Cruz C.S., Bosio C.M. Cutting edge: severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J. Immunol. 2021;206(2):329–334. doi: 10.4049/jimmunol.2001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreakos E., Papadaki M., Serhan C.N. Dexamethasone, pro-resolving lipid mediators and resolution of inflammation in COVID-19. Allergy. 2021;76(3):626–628. doi: 10.1111/all.14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J., Phipps R.P., Serhan C.N., Hammock B.D. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39:337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recchiuti A., Patruno S., Mattoscio D., Isopi E., Pomilio A., Lamolinara A., Iezzi M., Pecce R., Romano M. Resolvin D1 and D2 reduce SARS-CoV-2-induced inflammatory responses in cystic fibrosis macrophages. FASEB J. 2021;35(4) doi: 10.1096/fj.202001952R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barden A.E., Mas E., Croft K.D., Phillips M., Mori T.A. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am. J. Clin. Nutr. 2015;102(6):1357–1364. doi: 10.3945/ajcn.115.116384. [DOI] [PubMed] [Google Scholar]
- 36.Bazan H.A., Lu Y., Jun B., Fang Z., Woods T.C., Hong S. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fatty Acids. 2017;125:43–47. doi: 10.1016/j.plefa.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barden A.E., Shinde S., Burke V., Puddey I.B., Beilin L.J., Irish A.B., Watts G.F., Mori T.A. The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease. Prostaglandins Other Lipid Mediat. 2018;136:1–8. doi: 10.1016/j.prostaglandins.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Hartling I., Cremonesi A., Osuna E., Lou P.H., Lucchinetti E., Zaugg M., Hersberger M. Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS. Clin. Chem. Lab. Med. 2021;59:1811–1823. doi: 10.1515/cclm-2021-0644. [DOI] [PubMed] [Google Scholar]
- 39.Mas E., Barden A., Burke V., Beilin L.J., Watts G.F., Huang R.C., Puddey I.B., Irish A.B., Mori T.A. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr. (Edinburgh, Scotland) 2016;35(2):331–336. doi: 10.1016/j.clnu.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Mas E., Croft K.D., Zahra P., Barden A., Mori T.A. Resolvins D.1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 41.Polinski K.J., Armstrong M., Manke J., Seifert J., Crume T., Yang F., Clare-Salzler M., Holers V.M., Reisdorph N., Norris J.M. Collection and storage of human plasma for measurement of oxylipins. Metabolites. 2021;11(3):137. doi: 10.3390/metabo11030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulte F., Asbeutah A.A., Benotti P.N., Wood G.C., Still C., Bistrian B.R., Hardt M., Welty F.K. The relationship between specialized pro-resolving lipid mediators, morbid obesity and weight loss after bariatric surgery. Sci. Rep. 2020;10(1):20128. doi: 10.1038/s41598-020-75353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barden A., Mas E., Croft K.D., Phillips M., Mori T.A. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014;55:2401–2407. doi: 10.1194/jlr.M045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss G.A., Troxler H., Klinke G., Rogler D., Braegger C., Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barden A., Shinde S., Tsai I.J., Croft K.D., Beilin L.J., Puddey I.B., Mori T.A. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot. Essent. Fatty Acids. 2019;148:25–29. doi: 10.1016/j.plefa.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Barden A.E., Moghaddami M., Mas E., Phillips M., Cleland L.G., Mori T.A. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids. 2016;107:24–29. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Keelan J.A., Mas E., D’Vaz N., Dunstan J.A., Li S., Barden A.E., Mark P.J., Waddell B.J., Prescott S.L., Mori T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction. 2015;149(2):171–178. doi: 10.1530/REP-14-0549. [DOI] [PubMed] [Google Scholar]
- 48.Markworth J.F., Brown L.A., Lim E., Castor-Macias J.A., Larouche J., Macpherson P.C.D., Davis C., Aguilar C.A., Maddipati K.R., Brooks S.V. Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell. 2021;20(6) doi: 10.1111/acel.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markworth J.F., Sugg K.B., Sarver D.C., Maddipati K.R., Brooks S.V. Local shifts in inflammatory and resolving lipid mediators in response to tendon overuse. FASEB J. 2021;35(6) doi: 10.1096/fj.202100078R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamon-Fava S., So J., Mischoulon D., Ziegler T.R., Dunlop B.W., Kinkead B., Schettler P.J., Nierenberg A.A., Felger J.C., Maddipati K.R., Fava M., Rapaport M.H. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2021;164 doi: 10.1016/j.plefa.2020.102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markworth J.F., Vella L.D., Lingard B.S., Tull D.L., Rupasinghe T.W., Sinclair A.J., Maddipati K.R., Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Compar. Physiol. 2013;305:R1281–R1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terrando N., Park J.J., Devinney M., Chan C., Cooter M., Avasarala P., Mathew J.P., Quinones Q.J., Maddipati K.R., Berger M. Immunomodulatory lipid mediator profiling of cerebrospinal fluid following surgery in older adults. Sci. Rep. 2021;11(1):3047. doi: 10.1038/s41598-021-82606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eickmeier O., Fussbroich D., Mueller K., Serve F., Smaczny C., Zielen S., Schubert R. Pro-resolving lipid mediator Resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barden A., Shinde S., Phillips M., Beilin L., Mas E., Hodgson J.M., Puddey I., Mori T.A. The effects of alcohol on plasma lipid mediators of inflammation resolution in patients with Type 2 diabetes mellitus. Prostaglandins Leukot. Essent. Fatty Acids. 2018;133:29–34. doi: 10.1016/j.plefa.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 55.See V.H.L., Mas E., Prescott S.L., Beilin L.J., Burrows S., Barden A.E., Huang R.C., Mori T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: a double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017;118:971–980. doi: 10.1017/S0007114517002914. [DOI] [PubMed] [Google Scholar]
- 56.Hansen T.V., Vik A., Serhan C.N. The protectin family of specialized pro-resolving mediators: potent immunoresolvents enabling innovative approaches to target obesity and diabetes. Front. Pharmacol. 2019;9:1582. doi: 10.3389/fphar.2018.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez A.R., Spur B.W. First total syntheses of the pro-resolving lipid mediators 7(S),13(R),20(S)-Resolvin T1 and 7(S),13(R)-Resolvin T4. Tetrahedron Lett. 2020;61 doi: 10.1016/j.tetlet.2019.151473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez A.R., Spur B.W. First total synthesis of the pro-resolving lipid mediator 7(S),12(R),13(S)-Resolvin T2 and its 13(R)-epimer. Tetrahedron Lett. 2020;61(20) doi: 10.1016/j.tetlet.2020.151857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang N., Sakuma M., Rodriguez A.R., Spur B.W., Irimia D., Serhan C.N. Resolvin T-series reduce neutrophil extracellular traps. Blood. 2021 doi: 10.1182/blood.2021013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orr S.K., Colas R.A., Dalli J., Chiang N., Serhan C.N. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(9):L904–11. doi: 10.1152/ajplung.00370.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Libreros S., Shay A.E., Nshimiyimana R., Fichtner D., Martin M.J., Wourms N., Serhan C.N. A new E-series resolvin: RvE4 stereochemistry and function in efferocytosis of inflammation-resolution. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.631319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edin M.L., Zeldin D.C. Commercial scale production of RvD4 opens the resolving door to new research. J. Leukoc. Biol. 2018 doi: 10.1002/jlb.3ce0118-032r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasturk H., Schulte F., Martins M., Sherzai H., Floros C., Cugini M., Chiu C.J., Hardt M., Van Dyke T. Safety and preliminary efficacy of a novel host-modulatory therapy for reducing gingival inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brennan E., Kantharidis P., Cooper M.E., Godson C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021;17(11):725–739. doi: 10.1038/s41581-021-00454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vartak T., Godson C., Brennan E. Therapeutic potential of pro-resolving mediators in diabetic kidney disease. Adv. Drug Deliv. Rev. 2021;178 doi: 10.1016/j.addr.2021.113965. [DOI] [PubMed] [Google Scholar]
- 66.Vickery T.W., Armstrong M., Kofonow J.M., Robertson C.E., Kroehl M.E., Reisdorph N.A., Ramakrishnan V.R., Frank D.N. Altered tissue specialized pro-resolving mediators in chronic rhinosinusitis. Prostaglandins Leukot. Essent. Fatty Acids. 2021;164 doi: 10.1016/j.plefa.2020.102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pérez-Novo C.A., Watelet J.B., Claeys C., Van Cauwenberge P., Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J. Allergy Clin. Immunol. 2005;115(6):1189–1196. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 68.De Caterina R. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011;364(25):2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 69.Harris W.S., Calder P.C., Mozaffarian D., Serhan C.N. Bang and Dyerberg’s omega-3 discovery turns fifty. Nat. Food. 2021;2:303–305. doi: 10.1038/s43016-021-00289-7. [DOI] [PubMed] [Google Scholar]
- 70.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M., Investigators R.-I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 71.Darwesh A.M., Bassiouni W., Sosnowski D.K., Seubert J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021;219 doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cotran R.S., Kumar V., Collins T. W.B. Saunders Co.; Philadelphia: 1999. Robbins Pathologic Basis of Disease. [Google Scholar]
- 74.Colgan S.P. In: Methods in Molecular Biology. Walker J.M., editor. Humana Press; Totowa, NJ: 2006. Cell-cell interactions: methods and protocols. [Google Scholar]
- 75.Dona M., Fredman G., Schwab J.M., Chiang N., Arita M., Goodarzi A., Cheng G., von Andrian U.H., Serhan C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arita M., Clish C.B., Serhan C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005;338(1):149–157. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- 77.Oh S.F., Pillai P.S., Recchiuti A., Yang R., Serhan C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tjonahen E., Oh S.F., Siegelman J., Elangovan S., Percarpio K.B., Hong S., Arita M., Serhan C.N. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Trilleaud C., Gauttier V., Biteau K., Girault I., Belarif L., Mary C., Pengam S., Teppaz G., Thepenier V., Danger R., Robert-Siegwald G., Néel M., Bruneau S., Glémain A., Néel A., Poupon A., Mosnier J.F., Chêne G., Dubourdeau M., Blancho G., Vanhove B., Poirier N. Agonist anti-ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci. Adv. 2021;7(14) doi: 10.1126/sciadv.abd1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N.A., Levy B.D., Serhan C.N., Van Dyke T.E. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 82.Arita M., Oh S., Chonan T., Hong S., Elangovan S., Sun Y.-P., Uddin J., Petasis N.A., Serhan C.N. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 83.Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., Taguchi R., Masuda K., Sasaki K., Urabe D., Inoue M., Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012;287(13):10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Endo J., Sano M., Isobe Y., Fukuda K., Kang J.X., Arai H., Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J. Exp. Med. 2014;211(8):1673–1687. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norris P.C., Libreros S., Serhan C.N. Resolution metabolomes activated by hypoxic environment. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kutzner L., Goloshchapova K., Rund K.M., Jubermann M., Blum M., Rothe M., Kirsch S.F., Schunck W.H., Kuhn H., Schebb N.H. Human lipoxygenase isoforms form complex patterns of double and triple oxygenated compounds from eicosapentaenoic acid. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865(12) doi: 10.1016/j.bbalip.2020.158806. [DOI] [PubMed] [Google Scholar]
- 87.Reinertsen A.F., Primdahl K.G., Shay A.E., Serhan C.N., Hansen T.V., Aursnes M. Stereoselective synthesis and structural confirmation of the specialized pro-resolving mediator resolvin E4. J. Org. Chem. 2021;86(4):3535–3545. doi: 10.1021/acs.joc.0c02913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cassatella M.A. 2003. The Neutrophil an Emergenging Regulator of Inflammatory and Immune Response. [Google Scholar]
- 89.Nauseef W.M., Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15(7):602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 90.Do K.V., Hjorth E., Wang Y., Jun B., Kautzmann M.A., Eriksdotter M., Schultzberg M., Bazan N.G. Cerebrospinal fluid profile of lipid mediators in Alzheimer’s disease. Res. Sq. 2021 doi: 10.21203/rs.3.rs-1029034/v1. [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okabe Y., Medzhitov R. Tissue biology perspective on macrophages. Nat. Immunol. 2016;17(1):9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 92.Lee J., An J.U., Kim T.H., Ko Y.J., Park J.B., Oh D.K. Discovery and engineering of a microbial double-oxygenating lipoxygenase for synthesis of dihydroxy fatty acids as specialized proresolving mediators. ACS Sustain. Chem. Eng. 2020;8:16172–16183. [Google Scholar]
- 93.Clària J., Serhan C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee J.Y., Han S.H., Park M.H., Song I.S., Choi M.K., Yu E., Park C.M., Kim H.J., Kim S.H., Schuchman E.H., Jin H.K., Bae J.S. N-AS-triggered SPMs are direct regulators of microglia in a model of Alzheimer’s disease. Nat. Commun. 2020;11(1):2358. doi: 10.1038/s41467-020-16080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dalli J., Chiang N., Serhan C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 2015;21(9):1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Radmark O., Werz O., Steinhilber D., Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta. 2015;1851(4):331–339. doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 97.He Z., Tao D., Xiong J., Lou F., Zhang J., Chen J., Dai W., Sun J., Wang Y. Phosphorylation of 5-LOX: the potential set-point of inflammation. Neurochem. Res. 2020;45(10):2245–2257. doi: 10.1007/s11064-020-03090-3. [DOI] [PubMed] [Google Scholar]
- 98.Ye Y., Lin Y., Perez-Polo J.R., Uretsky B.F., Ye Z., Tieu B.C., Birnbaum Y. Phosphorylation of 5-lipoxygenase at ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin a4 production. J. Immunol. 2008;181(5):3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 99.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J.N., Petasis N.A., Blumberg R.S., Serhan C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U. S. A. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawada Y., Honda T., Hanakawa S., Nakamizo S., Murata T., Ueharaguchi-Tanada Y., Ono S., Amano W., Nakajima S., Egawa G., Tanizaki H., Otsuka A., Kitoh A., Dainichi T., Ogawa N., Kobayashi Y., Yokomizo T., Arita M., Nakamura M., Miyachi Y., Kabashima K. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015;212(11):1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C.T., Teles R., Kantarci A., Chen T., McCafferty J., Starr J.R., Brito L.C., Paster B.J., Van Dyke T.E. Resolvin E1 reverses experimental periodontitis and dysbiosis. J. Immunol. 2016;197(7):2796–2806. doi: 10.4049/jimmunol.1600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park C.K., Xu Z.Z., Liu T., Lu N., Serhan C.N., Ji R.R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J. Neurosci. 2011;31(50):18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albuquerque-Souza E., Schulte F., Chen T., Hardt M., Hasturk H., Van Dyke T.E., Holzhausen M., Kantarci A. Maresin-1 and resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.585530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reddoch-Cardenas K.M., Sharma U., Salgado C.L., Cantu C., Darlington D.N., Pidcoke H.F., Bynum J.A., Cap A.P. Use of specialized pro-resolving mediators to alleviate cold platelet storage lesion. Transfusion. 2020;60(Suppl. 3):S112–s118. doi: 10.1111/trf.15750. [DOI] [PubMed] [Google Scholar]
- 105.Thornton J.M., Yin K. Role of specialized pro-resolving mediators in modifying host defense and decreasing bacterial virulence. Molecules. 2021;26(22):6970. doi: 10.3390/molecules26226970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen J., Purvis G.S.D., Collotta D., Al Zoubi S., Sugimoto M.A., Cacace A., Martin L., Colas R.A., Collino M., Dalli J., Thiemermann C. RvE1 attenuates polymicrobial sepsis-induced cardiac dysfunction and enhances bacterial clearance. Front. Immunol. 2020;11:2080. doi: 10.3389/fimmu.2020.02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keyes K.T., Ye Y., Lin Y., Zhang C., Perez-Polo J.R., Gjorstrup P., Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;299(1):H153–64. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 108.Freire M.O., Dalli J., Serhan C.N., Van Dyke T.E. Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J. Immunol. 2017;198(2):718–728. doi: 10.4049/jimmunol.1601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campbell E.L., MacManus C.F., Kominsky D.J., Keely S., Glover L.E., Bowers B.E., Scully M., Bruyninckx W.J., Colgan S.P. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quiros M., Feier D., Birkl D., Agarwal R., Zhou D.W., Garcia A.J., Parkos C.A., Nusrat A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9477–9482. doi: 10.1073/pnas.1921335117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haworth O., Cernadas M., Levy B.D. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J. Immunol. 2011;186(11):6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Periz A., Horrillo R., Ferre N., Gronert K., Dong B., Moran-Salvador E., Titos E., Martinez-Clemente M., Lopez-Parra M., Arroyo V., Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23(6):1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ho K.J., Spite M., Owens C.D., Lancero H., Kroemer A.H., Pande R., Creager M.A., Serhan C.N., Conte M.S. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 2010;177(4):2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sulciner M.L., Serhan C.N., Gilligan M.M., Mudge D.K., Chang J., Gartung A., Lehner K.A., Bielenberg D.R., Schmidt B., Dalli J., Greene E.R., Gus-Brautbar Y., Piwowarski J., Mammoto T., Zurakowski D., Perretti M., Sukhatme V.P., Kaipainen A., Kieran M.W., Huang S., Panigrahy D. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018;215(1):115–140. doi: 10.1084/jem.20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sawada Y., Honda T., Nakamizo S., Otsuka A., Ogawa N., Kobayashi Y., Nakamura M., Kabashima K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018;8(1):11873. doi: 10.1038/s41598-018-30373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haas-Stapleton E.J., Lu Y., Hong S., Arita M., Favoreto S., Nigam S., Serhan C.N., Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2(12):e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rajasagi N.K., Reddy P.B., Suryawanshi A., Mulik S., Gjorstrup P., Rouse B.T. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol. 2011;186(3):1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16(5):592–597. doi: 10.1038/nm.2123. 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colas R.A., Souza P.R., Walker M.E., Burton M., Zaslona Z., Curtis A.M., Marques R.M., Dalli J. Impaired production and diurnal regulation of vascular RvDn-3 DPA increase systemic inflammation and cardiovascular disease. Circ. Res. 2018;122(6):855–863. doi: 10.1161/CIRCRESAHA.117.312472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramirez J.L., Gasper W.J., Khetani S.A., Zahner G.J., Hills N.K., Mitchell P.T., Sansbury B.E., Conte M.S., Spite M., Grenon S.M. Fish oil increases specialized pro-resolving lipid mediators in PAD (The OMEGA-PAD II trial) J. Surg. Res. 2019;238:164–174. doi: 10.1016/j.jss.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnardottir H., Orr S.K., Dalli J., Serhan C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016;9(3):757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Motwani M.P., Colas R.A., George M.J., Flint J.D., Dalli J., Richard-Loendt A., Dee Maeyer R.P., Serhan C.N., Gilroy D.W. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.94463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deyama S., Shimoda K., Suzuki H., Ishikawa Y., Ishimura K., Fukuda H., Hitora-Imamura N., Ide S., Satoh M., Kaneda K., Shuto S., Minami M. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology (Berl.) 2018;235(1):329–336. doi: 10.1007/s00213-017-4774-7. [DOI] [PubMed] [Google Scholar]
- 124.Murakami Y., Fukuda H., Muromoto R., Hirashima K., Ishimura K., Fujiwara K., Ishihara J., Matsuda T., Watanabe M., Shuto S. Design and synthesis of benzene congeners of resolvin E2, a proresolving lipid mediator, as its stable equivalents. ACS Med. Chem. Lett. 2020;11(4):479–484. doi: 10.1021/acsmedchemlett.9b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sato M., Aoki-Saito H., Fukuda H., Ikeda H., Koga Y., Yatomi M., Tsurumaki H., Maeno T., Saito T., Nakakura T., Mori T., Yanagawa M., Abe M., Sako Y., Dobashi K., Ishizuka T., Yamada M., Shuto S., Hisada T. Resolvin E3 attenuates allergic airway inflammation via the interleukin-23-interleukin-17A pathway. FASEB J. 2019;33(11):12750–12759. doi: 10.1096/fj.201900283R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deyama S., Shimoda K., Ikeda H., Fukuda H., Shuto S., Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J. Pharmacol. Sci. 2018;138(1):86–88. doi: 10.1016/j.jphs.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 127.Yamashita A., Kawana K., Tomio K., Taguchi A., Isobe Y., Iwamoto R., Masuda K., Furuya H., Nagamatsu T., Nagasaka K., Arimoto T., Oda K., Wada-Hiraike O., Yamashita T., Taketani Y., Kang J.X., Kozuma S., Arai H., Arita M., Osuga Y., Fujii T. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci. Rep. 2013;3:3113. doi: 10.1038/srep03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fukuda H., Ikeda H., Muromoto R., Hirashima K., Ishimura K., Fujiwara K., Aoki-Saito H., Hisada T., Watanabe M., Ishihara J., Matsuda T., Shuto S. Synthesis of resolvin E3, a proresolving lipid mediator, and its deoxy derivatives: identification of 18-deoxy-resolvin E3 as a potent anti-inflammatory agent. J. Org. Chem. 2020;85(21):14190–14200. doi: 10.1021/acs.joc.0c01701. [DOI] [PubMed] [Google Scholar]
- 129.Deyama S. Resolvins as novel targets for rapid-acting antidepressants. Folia Pharmacol. Jpn. 2020;155(6):381–385. doi: 10.1254/fpj.20044. [DOI] [PubMed] [Google Scholar]
- 130.Bhatt D.L., Steg P.G., Miller M. Cardiovascular risk reduction with icosapent ethyl. reply. N. Engl. J. Med. 2019;380(17):1678. doi: 10.1056/NEJMc1902165. [DOI] [PubMed] [Google Scholar]
- 131.Tan A., Sullenbarger B., Prakash R., McDaniel J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Souza P.R., Marques R.M., Gomez E.A., Colas R.A., De Matteis R., Zak A., Patel M., Collier D.J., Dalli J. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ. Res. 2020;126(1):75–90. doi: 10.1161/CIRCRESAHA.119.315506. [DOI] [PubMed] [Google Scholar]
- 133.Welty F.K., Schulte F., Alfaddagh A., Elajami T.K., Bistrian B.R., Hardt M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B(4) FASEB J. 2021;35(4) doi: 10.1096/fj.202002471R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keeley E.C., Li H.J., Cogle C.R., Handberg E.M., Merz C.N.B., Pepine C.J. Specialized proresolving mediators in symptomatic women with coronary microvascular dysfunction (from the women’s ischemia trial to reduce events in nonobstructive CAD [WARRIOR] trial) Am. J. Cardiol. 2022;162:1–5. doi: 10.1016/j.amjcard.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun Y.-P., Oh S.F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S.P., Petasis N.A., Serhan C.N. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 136.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dalli J., Winkler J.W., Colas R.A., Arnardottir H., Cheng C.Y.C., Chiang N., Petasis N.A., Serhan C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Norris P.C., Arnardottir H., Sanger J.M., Fichtner D., Keyes G.S., Serhan C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fatty Acids. 2018;138:81–89. doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Winkler J.W., Uddin J., Serhan C.N., Petasis N.A. Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17R-epimer. Org. Lett. 2013;15(7):1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Winkler J.W., Libreros S., De La Rosa X., Sansbury B.E., Norris P.C., Chiang N., Fichtner D., Keyes G.S., Wourms N., Spite M., Serhan C.N. Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukoc. Biol. 2018;103:995–1010. doi: 10.1002/JLB.3MI0617-254R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winkler J.W., Orr S.K., Dalli J., Cheng C.Y., Sanger J.M., Chiang N., Petasis N.A., Serhan C.N. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shay A.E., Nshimiyimana R., Samuelsson B., Petasis N.A., Haeggstrom J.Z., Serhan C.N. Human leukocytes selectively convert 4S,5S-epoxy-resolvin to resolvin D3, resolvin D4, and a cys-resolvin isomer. Proc. Natl. Acad. Sci. U. S. A. 2021;118(51) doi: 10.1073/pnas.2116559118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cherpokova D., Jouvene C.C., Libreros S., DeRoo E., Chu L., de la Rosa X., Norris P., Wagner D.D., Serhan C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134:1458–1468. doi: 10.1182/blood.2018886317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holinstat M. Resolvin the clot: DVT resolution through RvD4. Blood. 2019;134:1370–1371. doi: 10.1182/blood.2019002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huynh K. Resolution of thrombus burden by targeting inflammatory mediators to treat DVT. Nat. Rev. Cardiol. 2019;16(10):577. doi: 10.1038/s41569-019-0259-1. [DOI] [PubMed] [Google Scholar]
- 146.Morita M., Kobayashi Y. Stereocontrolled synthesis of resolvin D4. J. Org. Chem. 2018;83(7):3906–3914. doi: 10.1021/acs.joc.8b00256. [DOI] [PubMed] [Google Scholar]
- 147.Diaz L.A., Altman N.H., Khan W.N., Serhan C.N., Adkins B. Specialized proresolving mediators rescue infant mice from lethal Citrobacter rodentium infection and promote immunity against reinfection. Infect. Immun. 2017;85(10):e00464–17. doi: 10.1128/IAI.00464-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodriguez A.R., Spur B.W. First total synthesis of 7(S),17(S)-resolvin D5, a potent anti-inflammatory docosanoid. Tetrahedron Lett. 2005;46:3623–3627. [Google Scholar]
- 149.Ogawa N., Sugiyama T., Morita M., Suganuma Y., Kobayashi Y. Total synthesis of resolvin D5. J. Org. Chem. 2017;82(4):2032–2039. doi: 10.1021/acs.joc.6b02870. [DOI] [PubMed] [Google Scholar]
- 150.Perry S.C., Kalyanaraman C., Tourdot B.E., Conrad W.S., Akinkugbe O., Freedman J.C., Holinstat M., Jacobson M.P., Holman T.R. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5. J. Lipid Res. 2020;61(7):1087–1103. doi: 10.1194/jlr.RA120000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sorokin A.V., Norris P., English J., Dey A.K., Chaturvedi A., Baumer Y., Silverman J., Playford M.P., Serhan C.N., Mehta N.N. Identification of pro-resolving and inflammatory lipid mediators in human psoriasis. J. Clin. Lipidol. 2018;12(4):1047–1060. doi: 10.1016/j.jacl.2018.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riché F., Chousterman B.G., Valleur P., Mebazaa A., Launay J.M., Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit. Care. 2018;22:42. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo X., Gu Y., Tao X., Serhan C.N., Ji R.R. Resolvin D5 inhibits neuropathic and inflammatory pain in male but not female mice: distinct actions of D-series resolvins in chemotherapy-induced peripheral neuropathy. Front. Pharmacol. 2019;10:745. doi: 10.3389/fphar.2019.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ariel A., Li P.-L., Wang W., Tang W.-X., Fredman G., Hong S., Gotlinger K.H., Serhan C.N. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J. Biol. Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 155.Chiurchiu V., Leuti A., Dalli J., Jacobsson A., Battistini L., Maccarrone M., Serhan C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016;8(353) doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ramon S., Bancos S., Thatcher T.H., Murant T.I., Moshkani S., Sahler J.M., Bottaro A., Sime P.J., Phipps R.P. Peroxisome proliferator-activated receptor gamma B cell-specific-deficient mice have an impaired antibody response. J. Immunol. 2012;189(10):4740–4747. doi: 10.4049/jimmunol.1200956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamada H., Saegusa J., Sendo S., Ueda Y., Okano T., Shinohara M., Morinobu A. Effect of resolvin D5 on T cell differentiation and osteoclastogenesis analyzed by lipid mediator profiling in the experimental arthritis. Sci. Rep. 2021;11(1):17312. doi: 10.1038/s41598-021-96530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pham T.L., He J., Kakazu A.H., Calandria J., Do K.V., Nshimiyimana R., Petasis N.A., Bazan H.E.P., Bazan N.G. Elovanoid-N32 or RvD6-isomer decrease ACE2 and binding of S protein RBD after injury or INFγ in the eye. Res. Sq. 2020 doi: 10.21203/rs.3.rs-55764/v1. [Preprint] [DOI] [Google Scholar]
- 159.Pham T.L., He J., Kakazu A.H., Calandria J., Do K.V., Nshimiyimana R., Lam T.F., Petasis N.A., Bazan H.E.P., Bazan N.G. ELV-N32 and RvD6 isomer decrease pro-inflammatory cytokines, senescence programming, ACE2 and SARS-CoV-2-spike protein RBD binding in injured cornea. Sci. Rep. 2021;11(1):12787. doi: 10.1038/s41598-021-92293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pham T.L., Bazan H.E.P. Docosanoid signaling modulates corneal nerve regeneration: effect on tear secretion, wound healing, and neuropathic pain. J. Lipid Res. 2021;62 doi: 10.1194/jlr.TR120000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cianci E., Recchiuti A., Trubiani O., Diomede F., Marchisio M., Miscia S., Colas R.A., Dalli J., Serhan C.N., Romano M. Human periodontal stem cells release specialized proresolving mediators and carry immunomodulatory and prohealing properties regulated by lipoxins. Stem Cells Transl. Med. 2016;5(1):20–32. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pham T.L., Kakazu A.H., He J., Nshimiyimana R., Petasis N.A., Jun B., Bazan N.G., Bazan H.E.P. Elucidating the structure and functions of Resolvin D6 isomers on nerve regeneration with a distinctive trigeminal transcriptome. FASEB J. 2021;35(8) doi: 10.1096/fj.202100686R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hong S., Tjonahen E., Morgan E.L., Yu L., Serhan C.N., Rowley A.F. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins – mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 164.Calder P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie. 2020;178:105–123. doi: 10.1016/j.biochi.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 165.Raatz S.K., Golovko M.Y., Brose S.A., Rosenberger T.A., Burr G.S., Wolters W.R., Picklo M.J., Sr. Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar) J. Agric. Food Chem. 2011;59:11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mukherjee P.K., Marcheselli V.L., Serhan C.N., Bazan N.G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marcheselli V.L., Hong S., Lukiw W.J., Hua Tian X., Gronert K., Musto A., Hardy M., Gimenez J.M., Chiang N., Serhan C.N., Bazan N.G. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 168.Gronert K., Maheshwari N., Khan N., Hassan I.R., Dunn M., Schwartzman M.L. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 169.Levy B.D., Kohli P., Gotlinger K., Haworth O., Hong S., Kazani S., Israel E., Haley K.J., Serhan C.N. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J. Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duffield J.S., Hong S., Vaidya V., Lu Y., Fredman G., Serhan C.N., Bonventre J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 171.Lukiw W.J., Cui J.G., Marcheselli V.L., Bodker M., Botkjaer A., Gotlinger K., Serhan C.N., Bazan N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Serhan C.N., Gotlinger K., Hong S., Lu Y., Siegelman J., Baer T., Yang R., Colgan S.P., Petasis N.A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 173.Serhan C.N., Fredman G., Yang R., Karamnov S., Belayev L.S., Bazan N.G., Zhu M., Winkler J.W., Petasis N.A. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Serhan C.N., Dalli J., Colas R.A., Winkler J.W., Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Serhan C.N., Dalli J., Karamnov S., Choi A., Park C.K., Xu Z.Z., Ji R.R., Zhu M., Petasis N.A. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dalli J., Zhu M., Vlasenko N.A., Deng B., Haeggstrom J.Z., Petasis N.A., Serhan C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Deng B., Wang C.W., Arnardottir H.H., Li Y., Cheng C.Y., Dalli J., Serhan C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramon S., Dalli J., Sanger J.M., Winkler J.W., Aursnes M., Tungen J.E., Hansen T.V., Serhan C.N. The Protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am. J. Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dalli J., Chiang N., Serhan C.N. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4753–4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de la Rosa X., Norris P.C., Chiang N., Rodriguez A.R., Spur B.W., Serhan C.N. Identification and complete stereochemical assignments of the new Resolvin Conjugates in Tissue Regeneration (RCTR) in human tissues that stimulate proresolving phagocyte functions and tissue regeneration. Am. J. Pathol. 2018;188:950–966. doi: 10.1016/j.ajpath.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dalli J., Ramon S., Norris P.C., Colas R.A., Serhan C.N. Novel proresolving and tissue regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 2015;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dalli J., Vlasakov I., Riley I.R., Rodriguez A.R., Spur B., Petasis N.A., Chiang N., Serhan C.N. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. U. S. A. 2016;113:12232–12237. doi: 10.1073/pnas.1607003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jouvene C.C., Shay A.E., Soens M.A., Norris P.C., Haeggstrom J.Z., Serhan C.N. Biosynthetic metabolomes of cysteinyl-containing immunoresolvents. FASEB J. 2019;33:13794–13807. doi: 10.1096/fj.201902003R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chiang N., Serhan C.N. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64:443–462. doi: 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maddipati K.R. Non-inflammatory physiology of “Inflammatory” mediators - unalamation, a new paradigm. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.So J., Wu D., Lichtenstein A.H., Tai A.K., Matthan N.R., Maddipati K.R., Lamon-Fava S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: a randomized, double-blind, crossover study. Atherosclerosis. 2021;316:90–98. doi: 10.1016/j.atherosclerosis.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 187.Tsai W.C., Kalyanaraman C., Yamaguchi A., Holinstat M., Jacobson M.P., Holman T.R. In vitro biosynthetic pathway investigations of neuroprotectin D1 (NPD1) and protectin DX (PDX) by human 12-lipoxygenase, 15-lipoxygenase-1, and 15-lipoxygenase-2. Biochemistry. 2021;60(22):1741–1754. doi: 10.1021/acs.biochem.0c00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee C.R., Zeldin D.C. Resolvin infectious inflammation by targeting the host response. N. Engl. J. Med. 2015;373(22):2183–2185. doi: 10.1056/NEJMcibr1511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Serhan C.N., Chiang N., Dalli J., Levy B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014;7(2) doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Simopoulos A.P., Serhan C.N., Bazinet R.P. The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol. Aspects Med. 2021;77 doi: 10.1016/j.mam.2021.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Norling L.V., Spite M., Yang R., Flower R.J., Perretti M., Serhan C.N. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sok M.C.P., Tria M.C., Olingy C.E., San Emeterio C.L., Botchwey E.A. Aspirin-Triggered Resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling. Acta Biomater. 2017;53:109–122. doi: 10.1016/j.actbio.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Menon R., Krzyszczyk P., Berthiaume F. Pro-resolution potency of resolvins D1, D2 and E1 on neutrophil migration and in dermal wound healing. Nano Life. 2017;7(1) doi: 10.1142/S1793984417500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Norris P.C., Libreros S., Chiang N., Serhan C.N. A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aan1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arnardottir H., Pawelzik S.C., Öhlund Wistbacka U., Artiach G., Hofmann R., Reinholdsson I., Braunschweig F., Tornvall P., Religa D., Bäck M. Stimulating the resolution of inflammation through Omega-3 polyunsaturated fatty acids in COVID-19: rationale for the COVID-Omega-F trial. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.624657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 197.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 198.Fredman G., Van Dyke T.E., Serhan C.N. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol. 2010;30(10):2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Levy B.D., Zhang Q.Y., Bonnans C., Primo V., Reilly J.J., Perkins D.L., Liang Y., Amin Arnaout M., Nikolic B., Serhan C.N. The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins Leukot. Essent. Fatty Acids. 2011;84(1–2):43–50. doi: 10.1016/j.plefa.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kantarci A., Aytan N., Palaska I., Stephens D., Crabtree L., Benincasa C., Jenkins B.G., Carreras I., Dedeoglu A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018;300:111–120. doi: 10.1016/j.expneurol.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 201.Laguna-Fernandez A., Checa A., Carracedo M., Artiach G., Petri M.H., Baumgartner R., Forteza M.J., Jiang X., Andonova T., Walker M.E., Dalli J., Arnardottir H., Gistera A., Thul S., Wheelock C.E., Paulsson-Berne G., Ketelhuth D.F.J., Hansson G.K., Back M. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation. 2018;138(16):1693–1705. doi: 10.1161/CIRCULATIONAHA.117.032801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Colgan S.P. Resolvins resolve to heal mucosal wounds. Proc. Natl. Acad. Sci. U. S. A. 2020;117(20):10621–10622. doi: 10.1073/pnas.2005652117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Isobe Y., Arita M., Iwamoto R., Urabe D., Todoroki H., Masuda K., Inoue M., Arai H. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J. Biochem. 2013;153(4):355–360. doi: 10.1093/jb/mvs151. [DOI] [PubMed] [Google Scholar]
- 204.Li J., Chen C.Y., Arita M., Kim K., Li X., Zhang H., Kang J.X. An omega-3 polyunsaturated fatty acid derivative, 18-HEPE, protects against CXCR4-associated melanoma metastasis. Carcinogenesis. 2018;39(11):1380–1388. doi: 10.1093/carcin/bgy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fiore S., Serhan C.N. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J. Exp. Med. 1990;172(5):1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rowley A.F., Lloyd-Evans P., Barrow S.E., Serhan C.N. Lipoxin biosynthesis by trout macrophages involves the formation of epoxide intermediates. Biochemistry. 1994;33(4):856–863. doi: 10.1021/bi00170a002. [DOI] [PubMed] [Google Scholar]
- 207.Koltsida O., Karamnov S., Pyrillou K., Vickery T., Chairakaki A.D., Tamvakopoulos C., Sideras P., Serhan C.N., Andreakos E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol. Med. 2013;5(5):762–775. doi: 10.1002/emmm.201201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lukic A., Larssen P., Fauland A., Samuelsson B., Wheelock C.E., Gabrielsson S., Radmark O. GM-CSF- and M-CSF-primed macrophages present similar resolving but distinct inflammatory lipid mediator signatures. FASEB J. 2017;31(10):4370–4381. doi: 10.1096/fj.201700319R. [DOI] [PubMed] [Google Scholar]
- 209.Yamada T., Tani Y., Nakanishi H., Taguchi R., Arita M., Arai H. Eosinophils promote resolution of acute periotonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561–568. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- 210.Chiang N., Shinohara M., Dalli J., Mirakaj V., Kibi M., Choi A.M., Serhan C.N. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 2013;190(12):6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dahlén S.-E., Serhan C.N. In: Lipoxygenases and Their Products. Wong A., Crooke S.T., editors. Academic Press; San Diego: 1991. Lipoxins: bioactive lipoxygenase interaction products; pp. 235–276. [Google Scholar]
- 212.Ogawa M., Ishihara T., Isobe Y., Kato T., Kuba K., Imai Y., Uchino Y., Tsubota K., Arita M. Eosinophils promote corneal wound healing via the 12/15-lipoxygenase pathway. FASEB J. 2020;34(9):12492–12501. doi: 10.1096/fj.202000483R. [DOI] [PubMed] [Google Scholar]
- 213.Arita M., Ohira T., Sun Y.P., Elangovan S., Chiang N., Serhan C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 214.Hong S., Porter T.F., Lu Y., Oh S.F., Pillai P.S., Serhan C.N. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]