Abstract
Purpose
Measure the effect of inhaled pulmonary vasodilators on gas exchange in mechanically ventilated patients with COVID-19.
Methods
A retrospective observational cohort study at three New York University Hospitals was performed including eighty-four mechanically ventilated SARS Cov-2 nasopharyngeal PCR positive patients, sixty nine treated with inhaled nitric oxide (iNO) and fifteen with inhaled epoprostenol (iEPO). The primary outcomes were change in PAO2:FIO2 ratio, oxygenation Index (OI), and ventilatory ratio (VR) after initiation of inhaled pulmonary vasodilators.
Results
There was no significant change in PAO2:FIO2ratio after initiation of iNO (mean − 4.1, 95% CI -17.3-9.0, P = 0.54) or iEPO (mean − 3.4, 95% CI -19.7-12.9, P = 0.66), in OI after initiation of iNO (mean 2.1, 95% CI-0.04-4.2, P = 0.054) or iEPO (mean − 3.4, 95% CI -19.7-12.9, P = 0.75), or in VR after initiation of iNO (mean 0.17, 95% CI -0.03-0.36, P = 0.25) or iEPO (mean 0.33, 95% CI -0.0847-0.74, P = 0.11). PAO2:FIO2, OI and VR did not significantly change over a five day period starting the day prior to drug initiation in patients who received either iNO or iEPO assessed with a fixed effects model.
Conclusion
Inhaled pulmonary vasodilators were not associated with significant improvement in gas exchange in mechanically ventilated patients with COVID-19.
Keywords: COVID-19, Inhaled nitric oxide, Epoprostenol, Ventilatory ratio, ARDS, Respiratory failure
Abbreviations: ANOVA, analysis of variance; ARDS, Acute Respiratory Distress Syndrome; c-AMP, cyclic adenosine monophosphate; c-GMP, cyclic guanosine monophosphate; CAD, Coronary Artery Disease; COVID-19, Coronavirus disease 2019; ECMO, Extracorporeal Membrane Oxygenation; FIO2, Fraction of inspired oxygen; ICU, intensive care unit; iEPO, inhaled epoprostenol; iNO, inhaled nitric oxide; IQR, interquartile range; NYU, New York University; OI, Oxygenation Index; PAO2, Partial pressure of arterial oxygen; PBW, predicted body weight; RT-PCR, Real Time Polymerase chain reaction; SARS-Cov-2, Severe acute respiratory syndrome coronavirus 2; SOFA Score, Sequential Organ Failure Assessment Score; VR, Ventilatory Ratio
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and can lead to hypoxemic respiratory failure requiring hospitalization, intensive care unit admission (ICU), and mechanical ventilation. Severe acute hypoxemic respiratory failure due to coronavirus disease 2019 (COVID-19) frequently meets criteria for the acute respiratory distress syndrome (ARDS) and has been correlated with diffuse alveolar damage on autopsy histopathology of the lung [[1], [2], [3]]. Management of COVID-19 with ARDS consists of lung protective ventilation with low tidal volumes and low driving pressure, prone body position when PaO2:FiO2 ratio is under 150, and in some cases extracorporeal membrane oxygenation (ECMO) [[4], [5], [6], [7]]. ARDS is characterized by a variable degree of microvascular occlusion, and early findings suggest that vascular and endothelial injury may be more prominent in ARDS due to COVID-19 [8,9]. Contrast enhanced chest CT scans of patients with COVID-19 have suggested abnormality of small blood vessels [10,11]. Post mortem examination of pulmonary endothelial cells via electron microscopy in patients who died of COVID-19 suggests pulmonary capillary endotheliopathy [12,13]. Immunothrombosis, neutrophil extracellular trap formation and innate immune activation are more prominent in the lung of patients COVID-19 as compared to influenza [14].
Inhaled pulmonary vasodilators including inhaled nitric oxide (iNO) and inhaled prostaglandins such as inhaled epoprostenol (iEPO) cause selective pulmonary vasodilation through cyclic guanosine monophosphate (c-GMP) and cyclic adenosine monophosphate (c-AMP) mediated smooth muscle relaxation, respectively, resulting in increased blood flow to ventilated lung units improving ventilation and perfusion matching [15]. Both agents may also possess anti-inflammatory and anti-platelet aggregation effects [16]. Both iNO and iEPO have been studied in ARDS and have been shown to improve the PAO2:FIO2 ratio, though they have been not found to reduce 30-day mortality or increase the number of ventilator-free days [[17], [18], [19], [20]]. iNO decreases the alveolar dead space in animal models of acute lung injury [21,22]. Because COVID-19 appears to have a specific effect on blood vessels including the pulmonary vasculature, the effect of selective pulmonary vasodilators in COVID-19 may differ from that observed in ARDS due to other causes. We hypothesized that inhaled pulmonary vasodilators would improve oxygenation and decrease impairment in CO2 elimination in mechanically ventilated patients with COVID-19.
2. Materials and methods
2.1. Study setting and patient population
We performed a retrospective, observational cohort study in three New York University (NYU) Langone Health hospitals in in close proximity to New York, NY. Adult patients (≥18 yr.) with positive Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) nasopharyngeal real time – polymerase chain reaction (RT-PCR) specimen admitted to NYU Langone Medical Center, NYU Langone Brooklyn Hospital, and NYU Long Island Hospital and receiving invasive mechanical ventilation between March 1, 2020 and June 30, 2020 who were treated with iNO or iEPO were included. Exclusion criteria were absence of ventilator or arterial blood gas data on the day of starting pulmonary vasodilators, ECMO prior to or at the time of pulmonary vasodilator initiation, and cardiac arrest immediately before or after initiation of pulmonary vasodilators. The study was approved by the NYU Institutional Review Board (i20–00770).
2.2. Pulmonary vasodilators
An institutional guideline suggested inhaled pulmonary vasodilators can be considered in ARDS with PaO2:FiO2 ratio < 200, after optimization of lung protective mechanical ventilation and consideration of prone body position. The ultimate decision to start inhaled pulmonary vasodilators rested with the treating intensivist.
iNO was provided with the INOMAX system (Mallinckrodt Pharmaceuticals, Bedminster NJ). The injector module for the nitric oxide system was placed on the dry side of the ventilator circuit heated humidifier (Fischer and Paykel, Aukland NZ). The starting dose of iNO was 10–40 ppm and determined by the treating clinician. iNO was the only inhaled pulmonary vasodilator provided at NYU Langone Medical Center and NYU Langone Hospital - Long Island during the study period. iEPO was administered using a Medfusion 3500 syringe pump (Cary, NC), and syringe connected to an Aerogen Solo vibratory mesh nebulizer system (Aerogen, Mountain View CA) placed on the dry side of the ventilator circuit heated humidifier. The initial dose of iEPO was 50 ng/kg/min based on ideal body weight and titrated by the treating intensivist as tolerated based on clinical response. iEPO was the only pulmonary vasodilator provided at NYU Langone Brooklyn Hospital during the study period.
2.3. Data collection
Patient data were obtained from the electronic Medical Record (EMR; EPIC, Verona WI). All adult patients with positive SARS-CoV-2 nasopharyngeal RT-PCR admitted during the study period with orders or flowsheet documentation of iNO were reviewed. All patients receiving iEPO were obtained from a query of pharmacy records. Patients meeting all inclusion and no exclusion criteria were included for analysis. Mechanical ventilator, arterial blood gas and clinical data were collected on the date of mechanical ventilation, the day prior to initiation of inhaled pulmonary vasodilators, the day of initiation of inhaled pulmonary vasodilators before and after initiation of inhaled pulmonary vasodilators, and on the three subsequent days. The ventilator data and arterial blood gas with highest PAO2:FIO2 in supine position were collected on each study day. On the day selective pulmonary vasodilators were started ventilator parameters and arterial blood gas values were collected within four hours before, and after initiation of the selective pulmonary vasodilator. The primary endpoints were change in gas exchange after initiation of inhaled pulmonary vasodilators. Oxygenation was assessed by PAO2:FIO2 ratio, and the oxygenation Index (OI) (FiO2*Mean Airway Pressure/PaO2) [23]. Impairment in CO2 elimination was estimated with ventilatory ratio (VR) (VEmeasured* PaCO2measured/ V̇E predicted* PaCO2predicted) [24,25].
2.4. Statistical analysis
Continuous variables are expressed as mean +/− standard deviation or medians and interquartile ranges (IQR). Categorical variables are expressed as frequencies and percentages. Comparisons between groups were performed with Kruskall Wallis test for continuous variables and Chi Square or Fischer's Exact tests for categorical variables. Change in PAO2:FIO2, OI, and VR were assessed using a paired Student's t-test, and serial measures were assessed with analysis of variance using a fixed effects model. A P value of <0.05 was considered statistically significant. Statistical analysis was performed in SAS 9.4 (Carey, NC).
3. Results
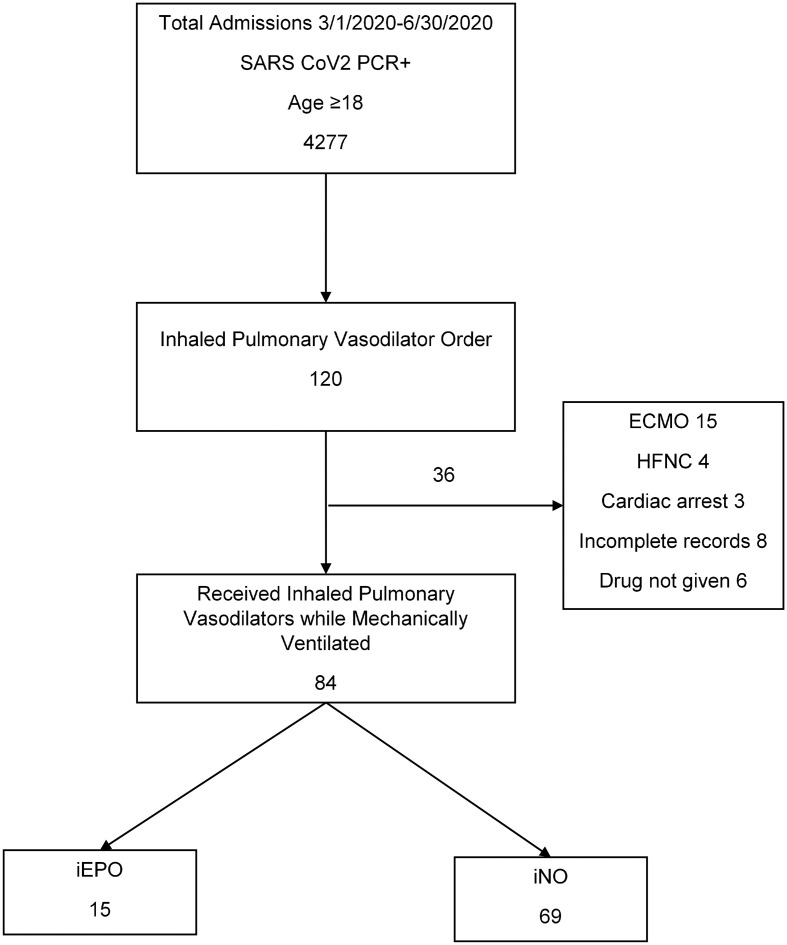
We screened 120 patients and excluded 36 for the following reasons: ECMO (n = 15), inadequate records (n = 14), spontaneously breathing at initiation of selective pulmonary vasodilators (n = 4), cardiac arrest at time of selective pulmonary vasodilator initiation (n = 3). The final study population included 84 patients, of which 69 received iNO and 15 received iEPO. (See Fig 1 .) Patients were predominantly male (73% male iNO vs 93% male iEPO p = 0.1). Demographic and medical history of the groups is summarized in Table 1 . Pharmacotherapy and immunomodulatory treatments reflected the current standards during the study period. Mortality by hospital day 30 was high in both groups but significantly worse in the iEPO group without correcting for severity of illness (52 vs 93%, P = 0.0031).
Fig. 1.
Patient flow figure.
Table 1.
Subject demographic characteristics and clinical outcomes.
| iNO |
iEpo |
P value | |||
|---|---|---|---|---|---|
| Number |
Percent | Number |
Percent | ||
| N = 69 | N = 15 | ||||
| Male | 49 | 71.0 | 14 | 93.3 | 0.1 |
| Female | 20 | 29.0 | 1 | 6.7 | 0.1 |
| White | 29 | 42.0 | 3 | 20.0 | 0.08 |
| African american | 8 | 11.6 | 0 | 0 | 0.08 |
| Asian | 4 | 5.8 | 1 | 6.7 | 0.08 |
| Other race | 19 | 27.5 | 8 | 53.3 | 0.08 |
| Unknown | 5 | 7.2 | 3 | 20.0 | 0.08 |
| Latino | 24 | 34.8 | 9 | 60.0 | 0.093 |
| DM | 24 | 34.8 | 3 | 20.0 | 0.22 |
| HTN | 34 | 49.3 | 5 | 33.3 | 0.40 |
| Congestive Heart Failure | 3 | 4.3 | 0 | 0 | 0.45 |
| Chronic Renal Disease | 8 | 11.6 | 1 | 6.7 | 0.28 |
| Liver disease | 6 | 8.7 | 0 | 0.0 | 0.24 |
| Asthma | 7 | 10.1 | 0 | 0.0 | 0.46 |
| HIV | 1 | 1.4 | 1 | 6.7 | 0.33 |
| Cancer | 4 | 5.8 | 0 | 0.0 | 0.36 |
| Organ transplant | 3 | 4.3 | 0 | 0 | 0.54 |
| Tracheostomy | 31 | 44.9 | 2 | 13.3 | 0.039 |
| Mortality day 30 | 36 | 52.2 | 14 | 93.3 | 0.0031 |
| Renal Replacement Therapy | 15 | 21.7 | 7 | 46.7 | 0.58 |
| Median | IQR | Median | IQR | ||
| Age | 62 | 10.0 | 54 | 22.0 | 0.0032 |
| BMI | 30.62 | 10.1 | 30.5 | 7.7 | 0.85 |
| Days Intubation to Inhaled Pulmonary vasodilator | 6 | 11.0 | 7 | 11.0 | 0.40 |
| Inhaled Pulmonary Vasodilator Hours | 106 | 164.0 | 53 | 58.0 | 0.0048 |
Clinical and laboratory parameters on the day of pulmonary vasodilator initiation are summarized in Table 2 . On the day of initiating inhaled pulmonary vasodilators patients in both groups had predominantly moderate or severe ARDS. Patients who received iEPO had more disturbed gas exchange and significantly lower PAO2:FIO2, and higher OI and VR than those who received iNO. Neuromuscular blockade was more common in patients receiving iEPO (55 vs 87%, P = 0.02). A majority of patients in both groups received vasopressors, and anticoagulation.
Table 2.
Mechanical ventilation variables, laboratory variables, and treatments.
| iNO |
iEPO |
P value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Tidal volume, (ml/kg of PBW) | 7.769 | 1.360 | 7.25 | 2.6 | 0.50 |
| V̇E, (liters/min) | 12.6 | 3.1 | 15.3 | 7.8 | 0.17 |
| PEEP, (cm H2O) | 12 | 5.0 | 14 | 4.0 | 0.096 |
| Mean airway pressure, (cm H2O) | 19 | 6.0 | 21 | 7.0 | 0.036 |
| FIO2 | 100 | 30.0 | 100 | 0.0 | 0.020 |
| PAO2:FIO2, (mmHg) | 93.7 | 59.7 | 68 | 65.0 | 0.025 |
| OI | 21.9 | 12.2 | 29.4 | 29.4 | 0.012 |
| VR | 2.63 | 1.2 | 4.08 | 1.4 | 0.008 |
| pH | 7.33 | 0.13 | 7.21 | 0.17 | 0.006 |
| paCO2, (mmHg) | 49 | 14.0 | 68 | 26.0 | 0.001 |
| paO2, (mmHg) | 82 | 51.0 | 79 | 84.0 | 0.65 |
| SOFA | 8 | 4.0 | 9.5 | 4.0 | 0.035 |
| CRP, mg/L | 140 | 169.0 | 231 | 171.0 | 0.063 |
| WBC x103 /μL | 12.75 | 6.1 | 22.8 | 13.2 | 0.002 |
| Hemoglobin, g/dl | 10.9 | 3.4 | 9.15 | 3.3 | 0.24 |
| Platelets x103 /μL | 239.5 | 118.0 | 227 | 99.0 | 0.97 |
| Total bilirubin, mg/dl | 0.65 | 0.7 | 0.6 | 0.7 | 0.99 |
| Albumin, g/dl | 2.5 | 0.4 | 1.6 | 0.7 | <0.0001 |
| D-dimer, ng/ml | 2126 | 3477.0 | 2096 | 6710.0 | 0.72 |
| Creatinine, ng/dl | 1.69 | 2.2 | 2.42 | 3.5 | 0.32 |
| n (percent) | n (percent) | ||||
| Vasopressors | 48 (73%) | 13 (87%) | 0.34 | ||
| Proning | 30 (46%) | 8 (53%) | 0.58 | ||
| Therapeutic Anticoagulation | 44 (67%) | 13 (87%) | 0.13 | ||
| Neuromuscular Blockade | 36 (55%) | 13 (87%) | 0.020 | ||
| Azithromycin | 61 (88%) | 12 (80%) | 0.41 | ||
| Hydroxychloroquine | 67 (97%) | 15 (100%) | 0.67 | ||
| Nitazoxanide | 4 (6%) | 8 (53%) | <0.0001 | ||
| Remdesivir | 11 (16%) | 0 (0%) | 0.20 | ||
| Corticosteroids | 52 (75%) | 13 (87%) | 0.50 | ||
| Convalescent Plasma | 14 (20%) | 0 (0%) | 0.064 | ||
| anti-IL-6 therapy | 42 (61%) | 3 (20%) | 0.0085 | ||
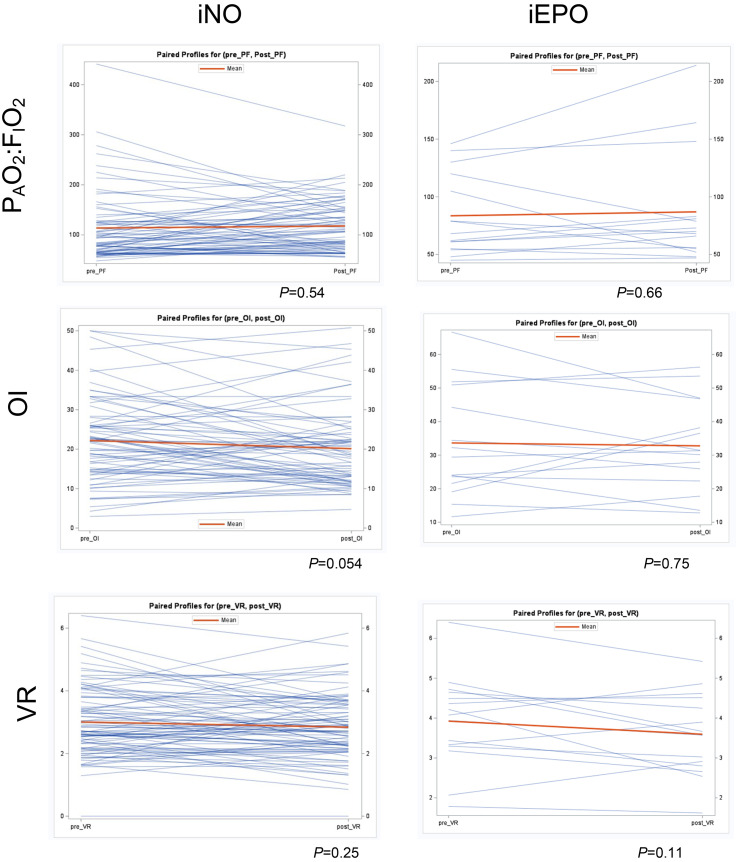
iNO was started a median of 6 days after the initiation of mechanical ventilation and continued for a median of 106 h. iEPO was started a median of 7 days after the initiation of mechanical ventilation and continued for a median of 53 h. We performed paired t-tests of PAO2:FIO2, OI and VR before and after iNO or iEPO, shown in Fig. 2 . There was no significant increase in PAO2:FIO2in patients who received iNO (mean − 4.1 mmHg, 95% CI -17.3- 9.0 mmHg, P = 0.54), or iEPO (mean − 3.4 mmHg, 95% CI -19.7- 12.9 mmHg, P = 0.66). Similarly, there was no significant improvement in OI in patients who received iNO (mean 2.1, 95% CI-0.04- 4.2, P = 0.054), or iEPO (mean − 3.4, 95% CI -19.7- 12.9, P = 0.75). Finally, there was no significant improvement in VR in patients who received iNO (mean 0.17, 95% CI -0.03- 0.36, P = 0.25), or iEPO (mean 0.33, 95% CI -0.0847- 0.74, P = 0.11). The proportion of patients with greater than 20% increase in PAO2:FIO2 was 32/84 (38%). There was no significant correlation between time from initiation of mechanical ventilation to start of selective pulmonary vasodilators and the responses to selective pulmonary vasodilators as measured by changes in PAO2:FIO2, OI or VR and (Supplementary Fig. 1). Similarly there was no significant correlation between PAO2:FIO2, OI or VR prior to the start of selective pulmonary vasodilators and the responses to selective pulmonary vasodilators as measured by changes in PAO2:FIO2, OI or VR (Supplementary Fig. 2).
Fig. 2.
Paired student's t-tests of PAO2:FIO2, OI, and VR before and after initiation of iNO or iEPO.
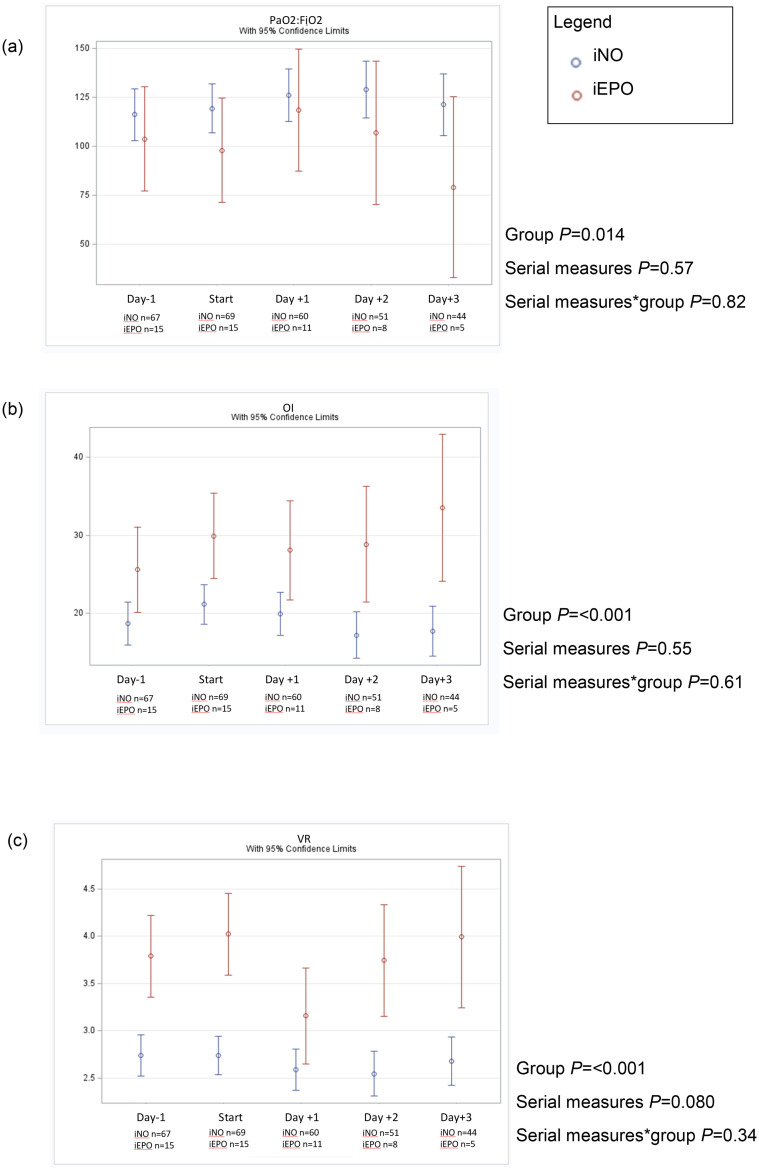
We assessed gas exchange over 5 days starting the day prior to initiation of inhaled pulmonary vasodilators and ending on day 3 after start of inhaled pulmonary vasodilators using a fixed effects model shown in Fig. 3 . There was substantial dropout in both groups due to death, and one patient in the iNO group was placed on ECMO. Gas exchange as assessed by PAO2:FIO2, OI and VR in patients who received iEPO was significantly worse than patients who were received iNO. We did not observe a significant change in PAO2:FIO2 ratio, OI, or VR over the five-day study period.
Fig. 3.
Fixed effects model serial measures ANOVA of PAO2:FIO2 (a), OI (b), VR (c) over five consecutive days beginning one day prior to initiation of iNO or iEPO.
Adverse consequences of selective pulmonary dilators were minimal and similar in both groups. The highest carboxyhemoglobin percentage observed after initiation of inhaled pulmonary vasodilators was 3% in the iEPO group and 2.9% in the iNO group. The highest methemoglobin percentage observed after initiation of inhaled pulmonary vasodilators was 2.1% in the iEPO group and 2.7% in the iNO group. The lowest observed platelet count in the iEPO group was 54,000 per mm3 and observed on the second day after initiation of iEPO. The lowest observed platelet count in the iNO group was 49,000 per mm3 and observed on the third day after initiation of iNO. Carboxyhemoglobin percentage, methemoglobin percentage and platelet count were not significantly different in either group at any study time point.
4. Discussion
Here we report our observations of a retrospective observational cohort study of the effects of selective inhaled pulmonary vasodilators on gas exchange in a relatively large group of mechanically ventilated patients with ARDS due to COVID-19. We analyzed both oxygenation (using PAO2:FIO2 ratio and OI) and carbon dioxide removal (VR), and our observations suggest that both iNO and iEPO do not significantly change gas exchange in this population.
A limited number of studies have evaluated the effects of inhaled pulmonary vasodilators in mechanically ventilated patients with COVID-19.DeGrado et al. did not find a significant increase in PAO2:FIO2 in a cohort of 38 patients with COVID-19 who were treated with iEPO and 11 who were treated with iNO [26]. Longobaro et al. did not find a significant increase in PAO2:FIO2 in 27 patients with COVID-19 who received iNO, as compared to a significant increase in a group of 11 patients with ARDS [27]. Lotz et al. reported a significant improvement in PaO2:FiO2 after initiation of iNO at 20 ppm in a group of 7 patients with severe COVID-19 ARDS [28]. A majority (65%) of 34 patients treated with iNO were defined as responders in a single center prospective cohort study by Abou-Arab [29]. In a cohort of 80 intubated patients with COVID-19 Sonti et al. found a median change in PAO2:FIO2 of 9 mmHg (IQR-9, +73) after initiation of iEPO with half of these patients showing a positive response. Responders were more likely to have lower initial PAO2:FIO2, and prone body position [30]. Almitrine bismesylate is a respiratory stimulant and pulmonary arterial vasoconstrictive agent which can improve ventilation-perfusion matching. Studies of the effect of almitrine bismesylate on oxygenation in COVID-19 are similarly mixed, with reports of significant improvement in PAO2:FIO2, and of no change in PAO2:FIO2 alone or in combination with iNO [31,32].
Both iNO and iEPO selectively dilate pulmonary vasculature of well-ventilated alveoli but with different pharmacological mechanisms and anticipated adverse effects [15]. iNO is a lipophilic gas that readily diffuses through alveolar membranes and into the surrounding capillaries where it stimulates cGMP-mediated processes including vascular smooth muscle relaxation leading to pulmonary vasodilation, platelet inhibition and regulation of cytokine production by leukocytes. Epoprostenol is a synthetic analog of endogenous prostacyclin, which is produced in the endothelium and is responsible for vascular smooth muscle relaxation and maintaining platelet stabilization. By binding to prostanoid IP receptors in the endothelium it causes a cascade of cAMP-mediated reactions resulting in vascular smooth muscle relaxation and pulmonary vasodilation. It also increases intracellular cAMP in platelets which enhances calcium release from thrombocytes rendering them inactive [33]. Inhaled selective pulmonary vasodilators improve gas exchange, but not risk of death or ventilator-free days, in patients with ARDS.
We observed a response rate of approximately 38% in the population we studied, which is lower than the 65% response rate reported in previous studies of inhaled selective pulmonary vasodilators [34]. The mechanisms responsible for the lack of response in patients with COVID-19 ARDS are unknown. We speculate that the lower response rate we observed among COVID-19-ARDS patients may relate to the pulmonary vascular effects of COVID-19. COVID-19 ARDS has been associated with thrombosis as well as abnormalities of pulmonary capillary endothelial cells [12]; these findings have also been reported in non-COVID-19-ARDS [9]. Pulmonary arterioles in patients with COVID-19 ARDS have been shown to have an abnormal appearance on contrast enhanced CT scan of the chest, described as a “vascular tree in bud pattern” [13]. Heterogeneity of lung perfusion has been described in contrast enhanced CT scans of patients with COVID-19 with and without pulmonary embolism [10,13]. Maldistribution of aerated lung tissue and lung perfusion correlated with impairment in PAO2:FIO2 in spontaneously breathing and mechanically ventilated patients with COVID-19 [35]. Pulmonary vascular involvement including neutrophil recruitment, neutrophil extracellular trap formation, and vascular occlusion has been described in COVID-19 ARDS and found to be more severe than in influenza [14]. Whether the prevalence of pulmonary vascular thrombosis is different in COVID-19-ARDS compared with ARDS from other causes is unknown. The physiologic effects of inhaled selective pulmonary vasodilators depends on a complex balance of vasodilation, elaboration of vasoconstrictors, and vascular smooth muscle response to these mediators [16]. Whether direct effects of viral infection or the inflammatory response to it explain the lower response rate that we observe remains a subject for future exploration. Because we use surrogate measures of the efficiency of oxygen and carbon dioxide transfer to estimate the effects of iNO and iEPO, we can only speculate about what mechanisms might underlie our observations. Mechanistic research would require more precise measures such as mixed inert gas elimination technique (MIGET), but this technique is cumbersome and currently available for research purposes at only a few centers worldwide. Whether exhaled gas might be safely collected and analyzed during the COVID-19 pandemic remains uncertain.
Our study has several strengths. We were able to include a relatively large cohort of patients who received iNO and a smaller group who received iEPO, included measurements of oxygenation as well as an estimate of ventilatory dead space, and an assessment of gas exchange over a 5-day period. Doses of iNO and iEPO are typical of those used in clinical practice, including the COVID-19 pandemic era..
The weaknesses of our study relate to a retrospective study design. We are not able to assess the effect of inhaled pulmonary vasodilators on clinical outcomes such as mortality or duration of mechanical ventilation. The higher 30 day mortality in patients who received iEPO likely relates to a higher baseline severity of illness in those patients. Patients treated during the study period received the contemporary standard of care pharmacotherapy for COVID-19, which has subsequently evolved. It is possible that the effect of selective pulmonary vasodilators on gas exchange may be different in patients with a different severity of illness, or at a different time point in the disease. We cannot exclude the possibility of a small response to these drugs, or that there are subgroups which may have a response to iEPO or iNO. Patients in our study started selective inhaled pulmonary vasodilators relatively late in the course of ARDS. However, we did not find a correlation between time from the initiation of mechanical ventilation and the start of selective inhaled pulmonary vasodilators and change in gas exchange. Nor did we observe an association between the severity of disordered gas exchange and a favorable response to selective inhaled pulmonary vasodilators.
COVID-19 causes severe acute respiratory failure, which is both similar to ARDS from other causes, and a source of unique challenges. We hypothesized that selective inhaled pulmonary vasodilators will improve oxygenation and decrease alveolar dead space. Neither oxygenation nor efficiency of pulmonary CO2 elimination were significantly improved after initiation of inhaled pulmonary vasodilators in a group of 84 mechanically ventilated adult patients with ARDS due to COVID-19. It is possible that the vascular manifestations of COVID-19 result in a specific subtype of lung injury in which the pulmonary vasculature is less responsive to selective inhaled pulmonary vasodilators.
Funding statement
This project did not receive any specific grant funding from agencies in public, commercial or not-for-profit sectors.
Prior presentation
This work has not been previously published in abstract or print form.
Conflicts of interest
ASL, AL, OE, BG, SB, JH, NA, MB, LB none.
AA Dr. Artigas's institution received funding from Lilly Foundation, and received research funding from Grifols, Fisher&Paykel and Aerogen.
DK Dr. Kaufman has received research funding from Fisher & Paykel, Cheetah Medical, and the NIH/NHLBI. He is a member of the medical advisory board of Pulsion Medical Systems.
Author statement
ASL was responsible for the design, data analysis, and main authorship of this manuscript, SBB, MTB, NA, DAK, AA, LB, OE, BG contributed to study design. OE, AL, JH, BG contributed to data collection. All authors contributed to final manuscript and revisions.
Acknowledgements
We thank the Respiratory Care Departments at NYU Langone Medical Center, NYU Langone Brooklyn Hospital, and NYU-Long Island Hospital for their service during the COVID-19 pandemic with special thanks to Nathanael Albright, Robert Sparaco, Lisa Hoffman, Vernan Druses, Judy Ackermann, and Joy Thomas.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2022.153990.
Appendix A. Supplementary data
Supplementary Figure 1. Linear regression analysis of response to inhaled pulmonary vasodilator and days from endotrachial intubation to the initation of inhaled pulmonary vasodilator. Supplementary Figure 2. Linear regression analysis of response to inhaled pulmonary vasodilator and initial gas exchange parameters.
References
- 1.Ranieri V.M., Rubenfeld G.D., Thompson B.T., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 doi: 10.1001/jama.2012.5669. Published online. [DOI] [PubMed] [Google Scholar]
- 2.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-Centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guérin C., Reignier J., Richard J.-C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 doi: 10.1056/nejmoa1214103. Published online. [DOI] [PubMed] [Google Scholar]
- 5.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018 doi: 10.1056/nejmoa1800385. Published online. [DOI] [PubMed] [Google Scholar]
- 6.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 doi: 10.1056/nejm200005043421801. Published online. [DOI] [PubMed] [Google Scholar]
- 7.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 doi: 10.1016/S0140-6736(09)61069-2. Published online. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski J.F., Davies P., Boggis C., Greene R., Zapol W.M., Reid L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112(1):112–126. [PMC free article] [PubMed] [Google Scholar]
- 9.Tomashefski J.F. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000 doi: 10.1016/S0272-5231(05)70158-1. Published online. [DOI] [PubMed] [Google Scholar]
- 10.Beenen L.F.M., Bos L.D., Scheerder M.J., et al. Extensive pulmonary perfusion defects compatible with microthrombosis and thromboembolic disease in severe Covid-19 pneumonia. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.08.026. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillet F., Busse-Coté A., Calame P., Behr J., Delabrousse E., Aubry S. COVID-19 pneumonia: microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant Imaging Med Surg. 2020 doi: 10.21037/QIMS-20-708. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020 doi: 10.1056/nejmoa2015432. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel B.V., Arachchillage D.J., Ridge C.A., et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1412OC. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolai L., Leunig A., Brambs S., et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.15179. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzierba A.L., Abel E.E., Buckley M.S., Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279–290. doi: 10.1002/phar.1365. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths M.J.D., Evans T.W. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005 doi: 10.1056/nejmra051884. Published online. [DOI] [PubMed] [Google Scholar]
- 17.Gebistorf F., Karam O., Wetterslev J., Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD002787.pub3. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor R.W., Zimmerman J.L., Dellinger R.P., et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004 doi: 10.1001/jama.291.13.1603. Published online. [DOI] [PubMed] [Google Scholar]
- 19.Torbic H., Szumita P.M., Anger K.E., Nuccio P., LaGambina S., Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care. 2013;28(5):844–848. doi: 10.1016/j.jcrc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Ammar M.A., Bauer S.R., Bass S.N., Sasidhar M., Mullin R., Lam S.W. Noninferiority of inhaled epoprostenol to inhaled nitric oxide for the treatment of ARDS. Ann Pharmacother. 2015;49(10):1105–1112. doi: 10.1177/1060028015595642. [DOI] [PubMed] [Google Scholar]
- 21.Skimming J.W., Banner M.J., Spalding H.K., Jaeger M.J., Burchfield D.J., Davenport P.W. Nitric oxide inhalation increases alveolar gas exchange by decreasing deadspace volume. Crit Care Med. 2001 doi: 10.1097/00003246-200106000-00022. Published online. [DOI] [PubMed] [Google Scholar]
- 22.Dahm P.L., Jonson B., De Robertis E., et al. The effects of nitric oxide inhalation on respiratory mechanics and gas exchange during endotoxaemia in the pig. Acta Anaesthesiol Scand. 1998 doi: 10.1111/j.1399-6576.1998.tb05163.x. Published online. [DOI] [PubMed] [Google Scholar]
- 23.Trachsel D., McCrindle B.W., Nakagawa S., Bonn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005 doi: 10.1164/rccm.200405-625OC. Published online. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Quinteros L., Schultz M.J., Bringué J., et al. Estimated dead space fraction and the ventilatory ratio are associated with mortality in early ARDS. Ann Intensive Care. 2019;9(1) doi: 10.1186/s13613-019-0601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha P., Calfee C.S., Beitler J.R., et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019 doi: 10.1164/rccm.201804-0692OC. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeGrado J.R., Szumita P.M., Schuler B.R., et al. Evaluation of the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide for refractory hypoxemia in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10) doi: 10.1097/cce.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longobardo A., Montanari C., Shulman R., Benhalim S., Singer M., Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021 doi: 10.1016/j.bja.2020.10.011. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotz C., Muellenbach R.M., Meybohm P., et al. Effects of inhaled nitric oxide in COVID-19–induced ARDS – is it worthwhile? Acta Anaesthesiol Scand. 2021 doi: 10.1111/aas.13757. Published online. [DOI] [PubMed] [Google Scholar]
- 29.Abou-Arab O., Huette P., Debouvries F., Dupont H., Jounieaux V., Mahjoub Y. Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study. Crit Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonti R., Pike C.W., Cobb N. Responsiveness of inhaled epoprostenol in respiratory failure due to COVID-19. J Intensive Care Med. 2020 doi: 10.1177/0885066620976525. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthélémy R., Blot P.L., Tiepolo A., et al. Efficacy of almitrine in the treatment of hypoxemia in Sars-Cov-2 acute respiratory distress syndrome. Chest. 2020 doi: 10.1016/j.chest.2020.05.573. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardinale M., Esnault P., Cotte J., Cungi P.J., Goutorbe P. Effect of almitrine bismesylate and inhaled nitric oxide on oxygenation in COVID-19 acute respiratory distress syndrome. Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.05.014. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuller B.M., Mohr N.M., Skrupky L., Fowler S., Kollef M.H., Carpenter C.R. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147(6):1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Arab O., Huette P., Debouvries F., Dupont H., Jounieaux V., Mahjoub Y. Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study. Crit Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball L., Robba C., et al. Lung distribution of gas and blood volume in critically ill COVID-19 patients: a quantitative dual-energy computed tomography study. Crit Care. 2021;25 doi: 10.1186/s13054-021-03610-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Linear regression analysis of response to inhaled pulmonary vasodilator and days from endotrachial intubation to the initation of inhaled pulmonary vasodilator. Supplementary Figure 2. Linear regression analysis of response to inhaled pulmonary vasodilator and initial gas exchange parameters.