Abstract
Aims/Introduction
Type 2 diabetes mellitus is associated with an increased incidence of osteoporosis and sarcopenia. However, the relationship between osteoporosis and sarcopenia in patients with type 2 diabetes mellitus remains to be unclear. Appendicular skeletal muscle was adjusted by height (appendicular skeletal muscle mass [ASM]/height2) as a marker of sarcopenia. This study aimed to explore the relationship between ASM/height2, osteoporosis and bone mineral density (BMD) in this population.
Materials and Methods
A total of 192 women and 225 men with type 2 diabetes mellitus were recruited. General information, laboratory and BMD data were collected. Spearman’s correlation, multiple regression analyses and receiver operating characteristic curve analysis were used to explore the correlation between ASM/height2, BMD and bone metabolism markers.
Results
Spearman’s correlation analysis showed that ASM/height2 had a positive correlation with serum calcium and BMD (r = 0.209–0.404, P < 0.01). In multivariate regression analysis, we found significant correlations between ASM/height2 and total lumbar spine, hip and femur neck BMD. According to the receiver operating characteristic curve, ASM/height2 was the best marker of osteoporosis, with a cut‐off value of 7.87 kg/m2 for men and 5.94 kg/m2 for women. When these cut‐off values were used to identify sarcopenia, the risk of osteoporosis increased 6.036‐fold in men and 4.079‐fold in women, respectively.
Conclusions
In patients with type 2 diabetes mellitus, ASM/height2 was positively correlated with BMD, and negatively correlated with osteoporosis.
Keywords: Bone mineral density, Muscle mass, Osteoporosis
In type 2 diabetes mellitus patients, appendicular skeletal muscle mass/height2 is positively correlated with bone mineral density and negatively correlated with osteoporosis. When the diagnostic cut‐off value of sarcopenia was 7.87 kg/m2 in men and 5.94 kg/m2 in women, the risk of osteoporosis increased.

INTRODUCTION
Osteoporosis and low‐energy fractures are on the rise in the elderly, which can lead to poor quality of life, disability and even death 1 . Patients with type 2 diabetes mellitus are generally more prone to fracture than the general population 2 , 3 . The risk of fracture remains different even after adjusting diabetes duration, age and body mass index (BMI) 4 , 5 . Previous studies have reported and analyzed the risk factors related to osteoporosis in the general population 6 . However, it is important to discuss the risk factors of osteoporosis that might differ in people with type 2 diabetes mellitus due to metabolic disorders.
Aging can lead to osteoporosis, but it can also result in a loss of muscle mass. This low muscle mass is named sarcopenia. The term sarcopenia is more than 20 years old. 7 Kwon et al. 8 reported that sarcopenia was 19.5% in women aged in their 50s, but increased to 22.1% in older women. Furthermore, Lima et al. 9 found the prevalence of osteoporosis to be 19.2% in pre‐sarcopenia, and 35.3% in sarcopenia. Therefore, the mass of muscle loss showing a relationship to aging increases the risks regarding sarcopenia and osteoporosis 10 .
The prevalence of sarcopenia in patients with type 2 diabetes mellitus is increased as a result of impaired insulin sensitivity 11 , ranging from 7% to 29.3%. The relationship between osteoporosis and sarcopenia in type 2 diabetes mellitus patients remains to be unclear. The present study investigated the relationship between lean mass (as a surrogate measure of mass of muscle), BMD and osteoporosis in patients with type 2 diabetes mellitus.
MATERIALS AND METHODS
Study population
The present study included 447 Chinese patients with type 2 diabetes mellitus (age >50 years). Participants underwent type 2 diabetes mellitus evaluation or treatment at the Second Affiliated Hospital of Wenzhou Medical University and Yuying Children's Hospital (Wenzhou, China) from January 2017 to December 2017. Exclusion criteria included: (i) malignant tumor and severe liver, kidney or heart disease (n = 35); (ii) diagnosis of parathyroid, pituitary, thyroid, gonadal and adrenal disease (n = 10); (iii) long use of vitamin D, calcium or other drugs that affect bone metabolism (n = 27); (iv) long‐term bedridden patients (n = 14); and (v) patients with a lack of available information (n = 55). The present study was approved by the ethics committee of the Second Affiliated Hospital of Wenzhou Medical University (No. LCKY2017‐21, date: July 2017), and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Clinical assessment and health history
Their weight and height were measured with light clothing and no shoes. We calculated BMI by dividing weight (kg) by the square of height (m2). Blood pressure (mmHg) was measured with a mercury sphygmomanometer after supine resting for 5 min. The duration of diabetes was calculated in years from the time of diagnosis of type 2 diabetes mellitus in the patient's medical records to our blood tests and BMD measurements. A history of smoking and alcohol consumption was defined as never or ever. The use history of oral hypoglycemic drugs, insulin, calcium channel blockers and statins were record.
Biochemical parameters
Serum samples were collected at 06.00 hours after overnight fasting (at least 8 h). Standard laboratory methods were used to measure markers of glucose metabolism, including fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c); serum lipid metabolism indices, including triglyceride (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C) levels; bone metabolism markers, including PINP, β‐CTX, parathyroid hormone and 25‐hydroxy‐vitamin. In addition, other laboratory markers, such as serum creatinine, calcium, albumin and uric acid, were recorded.
BMD measurement
The BMD of each patient was measured using a dual‐energy X‐ray absorptiometry at three sites (Hologic‐Discovery, Boston, MA, USA): total lumbar spine, total hip and femur neck. The World Health Organization standard for osteoporosis is a T score of <2.5 standard deviations of BMD 12 .
Body composition measurements
The lean body mass of the arms and legs, and the ASM was carried out by dual bioelectrical impedance analyzer (InBody 720; Biospace, Seoul, Korea). Bioelectrical impedance analyzer is now considered an accurate method for assessing body composition 11 . As total skeletal muscle mass has an effect on body size, the measured skeletal muscle mass needs to be corrected for the individual’s body size. The correction methods included dividing the skeletal muscle mass by the bodyweight (ASM / weight), body mass index (ASM / BMI) or height square (ASM / height2). Most of the current studies, including those published by the Asian Working Group of Sarcopenia, International Working Group on Sarcopenia and European Working Group on Sarcopenia in Older People, use ASM/height2 evaluated by DXA 13 .
Statistical analysis
Men and women were analyzed separately. We used mean ± standard deviation to describe continuous variables, and used proportion to describe categorical variables. One‐way anova analysis and Pearson’s χ2‐test were used to compare the mean and proportion. The ASM/height2, BMD and bone metabolism markers were analyzed by Spearman’s partial coefficient analysis adjusting for age. Multivariate linear regression analysis was used to analyze the correlation between the BMD of hip, lumbar spine and femoral neck, and other variables. Logistic analysis was used to calculate the odds ratios (ORs) and 95% confidence intervals. Based on the cut‐off value of osteoporosis, the effect of low mass of muscle was determined. To evaluate the clinical value of ASM/height2 in predicting osteoporosis, receiver operating characteristic (ROC) curves were plotted and the area under the curve was calculated. We carried out a post‐hoc analysis of the incidence of osteoporosis in the sarcopenia group and non‐sarcopenia group, and the post‐hoc analysis was 98.9%.
RESULTS
Basic characteristics
Patient characteristics are shown in Table 1. The present retrospective study included 447 patients with type 2 diabetes mellitus, average age of 66.1 ± 9.5 years and BMI of 24.4 ± 3.7 kg/m2. The BMD of the total lumbar spine, femur neck and total hip were higher in men (1.073 vs 0.925, 0.827 vs 0.738, 0.896 vs 0.809, all P < 0.01) compared with women. The levels of FBG, TC, TG, HDL‐C, PINP and the incidence of osteoporosis were significantly lower in men compared with women. The levels of ASM, ASM/height2 and proportion of smoking or alcohol consumption were significantly higher in men than those in women.
Table 1.
Patient characteristics, stratified by sex
|
Total patients (n = 447) |
Male patients (n = 255) |
Female patients (n = 192) |
P | |
|---|---|---|---|---|
| Age (years) | 66.1 ± 9.5 | 65.0 ± 9.8 | 67.6 ± 8.8 | 0.003 |
| Diabetes duration (years) | 8.0 ± 6.8 | 7.6 ± 6.9 | 8.5 ± 6.7 | 0.177 |
| Systolic blood pressure (mmHg) | 139.4 ± 22.3 | 136.9 ± 21.5 | 142.8 ± 22.8 | 0.006 |
| Diastolic blood pressure (mmHg) | 77.4 ± 12.6 | 78.3 ± 12.6 | 76.3 ± 12.5 | 0.103 |
| BMI (kg/m2) | 24.4 ± 3.7 | 24.0 ± 3.3 | 25.0 ± 4.1 | 0.003 |
| Medicine used (%) | ||||
| Insulin | 56.4% | 55.3% | 57.8% | 0.630 |
| Metformin | 46.5% | 48.6% | 43.8% | 0.338 |
| Statins | 32.9% | 31.8% | 34.4% | 0.611 |
| Calcium channel blockers | 42.3% | 40.0% | 45.3% | 0.288 |
| Smoking (current or ever) | 28.6% | 49.4% | 1.0% | <0.001 |
| Alcohol consumption (current or ever) | 21.7% | 36.9% | 1.6% | <0.001 |
| Laboratory findings | ||||
| FBG (mmol/L) | 8.9 ± 3.2 | 8.5 ± 2.7 | 9.3 ± 3.7 | 0.030 |
| HbA1c (mmol/L) | 9.9 ± 2.4 | 10.1 ± 2.6 | 9.5 ± 2.0 | 0.003 |
| TC (mmol/L) | 4.47 ± 1.22 | 4.29 ± 1.09 | 4.69 ± 1.35 | 0.001 |
| TG (mmol/L) | 1.82 ± 1.57 | 1.65 ± 1.10 | 2.05 ± 2.01 | 0.009 |
| HDL‐C (mmol/L) | 1.00 ± 0.26 | 0.96 ± 0.25 | 1.06 ± 0.26 | <0.001 |
| LDL‐C (mmol/L) | 2.58 ± 0.93 | 2.51 ± 0.88 | 2.67 ± 0.99 | 0.087 |
| Albumin (g/L) | 37.7 ± 4.7 | 38.3 ± 4.2 | 36.8 ± 5.0 | 0.001 |
| Creatinine (µmol/L) | 72.0 ± 31.8 | 78.6 ± 30.0 | 63.2 ± 31.9 | <0.001 |
| Uric acid (µmmol/L) | 314.2 ± 98.0 | 327.7 ± 101.1 | 296.2 ± 91.0 | 0.001 |
| PTH (pg/mL) | 43.60 ± 22.18 | 43.08 ± 21.04 | 44.43 ± 24.10 | 0.739 |
| PINP (ng/mL) | 14.95 ± 22.08 | 13.14 ± 19.76 | 17.36 ± 24.67 | 0.045 |
| β‐CTX (ng/mL) | 0.38 ± 0.24 | 0.35 ± 0.25 | 0.41 ± 0.23 | 0.072 |
| 25(OH)D (ng/mL) | 58.42 ± 22.71 | 60.54 ± 20.93 | 55.71 ± 24.66 | 0.155 |
| Calcium (mmol/L) | 2.19 ± 0.11 | 2.19 ± 0.11 | 2.19 ± 0.11 | 0.812 |
| ASM | 19.07 ± 4.04 | 21.72 ± 2.67 | 15.52 ± 2.55 | <0.001 |
| ASM/height2 | 7.23 ± 1.77 | 7.71 ± 0.72 | 6.44 ± 0.93 | <0.001 |
| BMD | ||||
| Total lumbar (g/cm2) | 1.009 ± 0.312 | 1.073 ± 0.363 | 0.925 ± 0.201 | <0.001 |
| Femur neck (g/cm2) | 0.788 ± 0.148 | 0.827 ± 0.135 | 0.738 ± 0.149 | <0.001 |
| Total hip (g/cm2) | 0.858 ± 0.162 | 0.896 ± 0.148 | 0.809 ± 0.166 | <0.001 |
| Osteoporosis | 15.1% | 9.8% | 21.9% | 0.001 |
Values are the mean ± standard deviation or number (%). P < 0.05 was deemed significant (comparison between men and women group). 25(OH)D, 25‐hydroxy‐vitamin; ASM, appendicular skeletal muscle mass; BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PTH, parathyroid hormone; TC, total cholesterol; TG, triglyceride.
Spearman’s partial correlations between ASM/height2, bone metabolism markers and BMD
Figure 1 shows that ASM/height2 was positively correlated with the lumbar spine BMD (r = 0.245 in men and 0.285 in women, P < 0.001), femoral neck BMD (r = 0.376 in men and 0.292 in women, P < 0.001) and total hip BMD (r = 0.394 in men and 0.332 in women, P < 0.001). After age adjustment, Spearman’s partial correlation analysis showed that ASM/height2 was still associated with BMD, procollagen of type 1 N‐propeptide, parathyroid hormone and albumin‐corrected calcium (Table 2). The Spearman’s partial correlation coefficients (r) of total lumbar, total hip, femur neck and serum calcium were 0.250, 0.404, 0.378 and 0.259 in male groups, and 0.209, 0.212, 0.228 and 0.314 in female groups, respectively. The ASM/height2 was negatively correlated with parathyroid hormone (r = −0.324 in men and −0.302 in women, P < 0.05).
Figure 1.
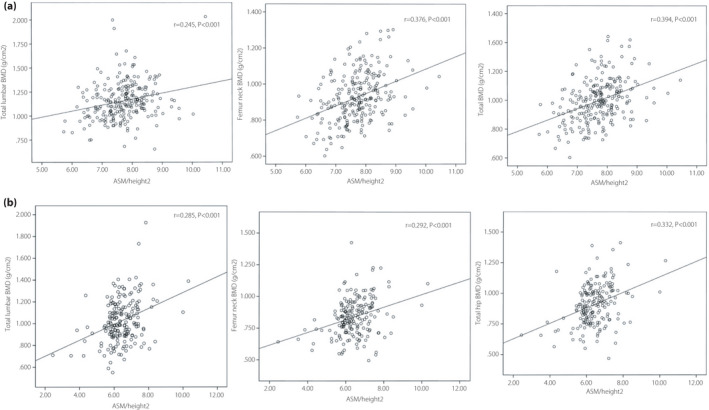
Scatter diagrams showing the correlation between the appendicular skeletal muscle mass (ASM)/height2 and bone mineral density (BMD).
Table 2.
Correlation analysis between appendicular skeletal muscle mass/height2, bone mineral density and bone metabolism markers adjusted for age
| Variables | Male | Female | ||
|---|---|---|---|---|
| r | P | r | P | |
| Total lumbar BMD | 0.250 | 0.000 | 0.209 | 0.001 |
| Total hip BMD | 0.404 | 0.000 | 0.212 | 0.002 |
| Femur neck BMD | 0.378 | 0.000 | 0.228 | 0.001 |
| PTH | −0.324 | 0.017 | −0.302 | 0.042 |
| PINP | 0.432 | 0.001 | 0.237 | 0.048 |
| β‐CTX | −0.046 | 0.740 | −0.019 | 0.909 |
| 25(OH)D | 0.013 | 0.925 | −0.190 | 0.246 |
| Albumin‐corrected Calcium | 0.259 | 0.050 | 0.314 | 0.040 |
25(OH)D, 25‐hydroxy‐vitamin; BMD, bone mineral density; PTH, parathyroid hormone.
Linear regression analysis for BMD
Tables 3 and 4 show multivariate linear regression analysis of BMD between age and BMI. Age, sex and BMI have a significant impact on the prevalence of osteoporosis. Therefore, we carried out subgroup analyses by sex, BMI and age. After adjusting age, duration of diabetic, systolic blood pressure, diastolic blood pressure, smoking, alcohol consumption, TG, TC, HDL‐C, LDL‐C, creatinine, HbA1c, uric and FBG, ASM/height2 showed an independent relationship to BMD in the total lumbar spine, hip and femoral neck in the group with BMI <24 kg/m2 (β values were 0.236, 0.323 and 0.260 in the male group, and 0.413, 0.504 and 0.449 in the female group, respectively). In women with a BMI >24 kg/m2, ASM/height2 was independently associated with BMD in the total lumbar spine, hip and femoral neck (β values were 0.374, 0.267 and 0.270, respectively), but not in the male group. ASM/height2 was independently associated with BMD in the total hip and femoral neck (β values were 0.347 and 0.317, respectively) in men aged <65 years. In women aged <65 years, ASM/height2 was independently associated with BMD at total lumbar, hip and femoral neck (β values were 0.349, 0.379 and 0.397, respectively). In men aged >65 years, ASM/height2 was independently associated with BMD at the total hip, lumbar and femoral neck (β values were 0.398, 0.284 and 0.399, respectively).
Table 3.
Appendicular skeletal muscle mass/height2 was independent association with bone mineral density based on the cross‐categorization of body mass index and sex
| 18.5 ≤ BMI < 24 | BMI ≥24 | |||
|---|---|---|---|---|
| β | P | β | P | |
| Male | ||||
| Total lumbar | 0.236 | 0.029 | 0.147 | 0.272 |
| Total hip | 0.323 | 0.008 | 0.096 | 0.478 |
| Femur neck | 0.260 | 0.033 | 0.145 | 0.279 |
| Female | ||||
| Total lumbar | 0.413 | <0.001 | 0.374 | 0.039 |
| Total hip | 0.504 | <0.001 | 0.267 | 0.048 |
| Femur neck | 0.449 | 0.001 | 0.270 | 0.042 |
BMI, body mass index.
Table 4.
Appendicular skeletal muscle mass/height2 was independent association with bone mineral density based on the cross‐categorization of age and sex
| Age <65 years | Age ≥65 years | |||
|---|---|---|---|---|
| β | P | β | P | |
| Male | ||||
| Total lumbar | 0.059 | 0.548 | 0.284 | 0.042 |
| Total hip | 0.347 | <0.001 | 0.398 | 0.033 |
| Femur neck | 0.317 | 0.001 | 0.399 | 0.030 |
| Female | ||||
| Total lumbar | 0.349 | 0.001 | 0.053 | 0.731 |
| Total hip | 0.379 | 0.001 | 0.185 | 0.210 |
| Femur neck | 0.397 | <0.001 | 0.053 | 0.731 |
Logistic regression analyses for osteoporosis
Table 5 shows a logistic regression analysis used to examine the relationship between ASM/height2 and osteoporosis. Although the OR decreased after adjusting age, diabetes duration, systolic blood pressure, diastolic blood pressure, smoking, alcohol consumption, TG, TC, HDL‐C, LDL‐C, albumin, creatinine, uric, HbA1c, FBG, the association between ASM/height2 and osteoporosis remained significant (OR 6.036 in men and OR 4.079 in women, P < 0.05).
Table 5.
Logistic regression analysis for osteoporosis
| Variables | SE | Male | SE | Female | ||
|---|---|---|---|---|---|---|
| odds ratio | P | odds ratio | P | |||
| Age | 0.037 | 0.961 (0.894, 1.033) | 0.961 | 0.027 | 1.055 (1.000, 1.112) | 0.050 |
| Diabetes duration | 0.050 | 1.010 (0.915, 1.114) | 0.846 | 0.036 | 1.013 (0.944, 1.087) | 0.725 |
| TC | 2.217 | 1.217 (0.068, 2.121) | 0.456 | 0.965 | 0.812 (0.122, 5.380) | 0.829 |
| TG | 1.442 | 0.133 (0.008, 2.243) | 0.162 | 0.389 | 1.017 (0.474, 2.181) | 0.966 |
| HDL‐C | 3.501 | 0.068 (0.000, 26.638) | 0.309 | 1.608 | 4.386 (0.187, 10.268) | 0.358 |
| LDL‐C | 2.343 | 0.206 (0.002, 20.326) | 0.500 | 1.080 | 0.999 (0.120, 8.297) | 0.999 |
| Albumin | 0.087 | 0.767 (0.647, 0.910) | 0.002 | 0.065 | 0.855 (0.753, 0.971) | 0.016 |
| Creatinine | 0.015 | 0.998 (0.969, 1.029) | 0.917 | 0.009 | 0.997 (0.981, 1.015) | 0.766 |
| Uric acid | 0.005 | 1.002 (0.993, 1.011) | 0.714 | 0.003 | 1.004 (0.998, 1.015) | 0.224 |
| HbA1c | 0.136 | 1.100 (0.843, 1.435) | 0.482 | 0.117 | 0.958 (0.761, 1.205) | 0.713 |
| FPG | 0.143 | 0.901 (0.681, 1.193) | 0.467 | 0.054 | 1.035 (0.930, 1.151) | 0.529 |
| ASM/height <7.87 kg/m2 in men or <5.94 kg/m2 in women | 0.528 | 6.036 (2.389, 15.325) | 0.001 | 0.531 | 4.079 (1.440, 11.559) | 0.008 |
ASM, appendicular skeletal muscle mass, FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SE, standard error; TC, total cholesterol; TG, triglyceride.
Prognostic value of ASM/height2
The ROC curve was used to analyze the influence of ASM/height2 on the diagnosis of osteoporosis (Figure 2). The area under the ROC curve reached 0.722 for men and 0.686 for women. In the male groups. the optimal cut‐off value for ASM/height2 in predicting of osteoporosis was 7.87kg/m2, with sensitivity of 43% and specificity 96%. The optimal cut‐off value for ASM/height2 in predicting osteoporosis was 5.94kg/m2, with sensitivity of 80% and specificity 57% in female groups.
Figure 2.
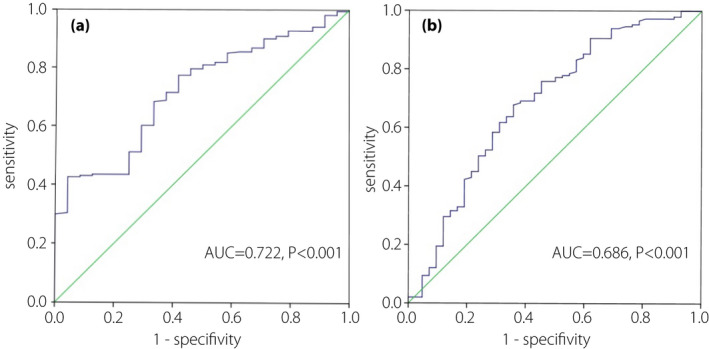
(a) Receiver operating characteristic analysis of the appendicular skeletal muscle mass (ASM)/height2 for osteoporosis among men. (b) Receiver operating characteristic analysis of the ASM/height2 for osteoporosis among women.
DISCUSSION
To the best of our knowledge, the present study is the first to explore the relationship between ASM/height2, BMD and osteoporosis in patients with type 2 diabetes mellitus. The main finding of our study was that in patients with type 2 diabetes mellitus, ASM/height2 was positively correlated with BMD, at femur neck, total lumbar and total hip, and negatively correlated with osteoporosis.
It is well known that type 2 diabetes mellitus patients are at an increased risk of osteoporosis and have accelerated skeletal muscle mass loss 3 , 14 . It is also known that patients with type 2 diabetes mellitus have a higher incidence of sarcopenia than non‐type 2 diabetes mellitus patients. However, the relationship between osteoporosis and sarcopenia in type 2 diabetes mellitus patients has been unknown so far. Considering the present findings and the results of previous studies, patients with sarcopenia, especially those with type 2 diabetes mellitus, might have a high risk for osteoporosis.
Increasing evidence shows that there are many common pathways between sarcopenia and osteoporosis 15 , and these conditions are present in a considerable number of individuals 10 , 16 . The previous cross‐sectional studies have reported an increase proportion of patients with sarcopenia with BMD values corresponding to osteoporosis. On the contrary, Locquet et al. 10 , 14 found that an increased incidence of sarcopenia, decreased strength of muscle, low muscle mass and impaired physical performance were increased in the elderly participants with low BMD levels. The association between sarcopenia and osteoporosis varied by publication, with reported risks increasing from twofold to 12‐fold. Some of these differences can be explained by cohort particularities (e.g., age, sex, comorbidities). Therefore, the causal relationship between the two diseases remains controversial.
Decreased muscle mass will lead to the deterioration of insulin sensitivity, which in turn leads to diabetes 17 . Diabetes mellitus and other systemic diseases caused by sarcopenia can cause both muscle loss and abnormal bone metabolism 18 . The present results show that low ASM/height2 is a significant risk factor for lower BMD and osteoporosis in patients with type 2 diabetes mellitus. To our knowledge, this is the first report showing a direct association between ASM/height2, BMD and osteoporosis in type 2 diabetes mellitus. ASM/height2 might be a simple predictor of BMD in patients with type 2 diabetes mellitus.
An association between sarcopenia and osteoporosis has been established in individuals with chronic liver disease 19 , inflammatory bowel disease, chronic obstructive pulmonary disease and healthy individuals 20 , 21 , 22 . The prevalence of osteoporosis and sarcopenia was increased in elderly women with low grip strength and muscle mass 23 . Therefore, ASM/height2 might be a simple predictor of osteoporosis in patients with these diseases.
According to the 2019 Asian Working Group of Sarcopenia, the height‐adjusted mass of muscle cut‐off points for men and women are 7.0kg/m2 and 5.7kg/m2 7 , respectively. An important aspect of the present study was the use of ROC curves to calculate cut‐off values. The ASM/height2 cut‐off value was 7.87kg/m2 for men and 5.94 kg/m2 for women. These values were slightly higher than the Asian Working Group of Sarcopenia guideline, and can be used as a value required for bone health.
In the present study, the effect was greater in men (OR 6.036; P = 0.001) compared with women (OR 4.079, P = 0.008) with sarcopenia, even after adjusting age, duration of diabetes, TG, TC, HDL‐C, LDL‐C albumin, creatinine, uric, HbA1c and FPG. This has been confirmed in previous studies, showing that a stronger relationship between muscle mass and bone mass is closer in men than in women 24 , 25 , 26 . The sex distinction in the relationship between muscle and bone can be explained by sex‐specific effects of sex hormones. In men, changes in bone and muscle are controlled by elevated testosterone and insulin‐like growth factor‐1 level, which lead to increased muscle strength and mass, whereas in women, higher levels of estrogen lead to bone mass tending to growth more rapidly in relation to muscle 24 , 25 . Aging causes muscle and bone loss in both men and women; and the relationship between muscle and bone mass is influenced by sex differences in the rate of muscle and bone loss 26 . In particular, age‐related declines in insulin‐like growth factor‐1 and testosterone levels might result in bone and muscle loss in men, whereas the absolute level and degree of decline in testosterone in women are much lower, and the muscle mass can be relatively preserved. Furthermore, mechanical stress and estrogen have a common pathway involving estrogen receptor α, and a decrease in estrogen receptor α reduces the ability of mechanical stress to induce an osteogenic response 27 , 28 , 29 . This resetting of mechanical parameters caused by estrogen deficiency can explain the decoupling between mass of muscle and BMD in women, whereas the relationship between muscle mass and actual BMD is stronger in men.
However, the current work had some limitations. First, the causal relationship between ASM/height2 and BMD was difficult to assess in this cross‐sectional study. As this study was based on the previous data, several vital parameters received limited analysis, which affects control selection. Second, we did not assess the strength of muscle and the degree of muscle function. Muscle strength and physical performance are considered to be components of sarcopenia according to the modern definition of the European Working Group on Sarcopenia in the Elderly 7 . Assessment of physical performance and muscle strength is also very important in the assessment of activities of daily living. In further research, we will use physical performance and muscle strength as the independent variables to determine the relationship between sarcopenia and osteoporosis. Third, in the present study population, blood glycemic control was poor, so there was no subgroup analysis of blood glucose levels. Further research is required to examine the relationship between osteoporosis and sarcopenia considering the influence of blood glucose levels. Fourth, some parameters that might affect the results have been ignored in the present study (e.g., physical activity, dietary habits, estrogen level, menopausal status).
To conclude, we report the first study that shows lower ASM/height2 is strongly associated with osteoporosis and low BMD in patients with type 2 diabetes mellitus. The risk of osteoporosis was increased when the diagnostic cut‐off value of sarcopenia was 7.87 kg/m2 in men and 5.94 kg/m2 in women, respectively.
Disclosure
The authors declare no conflict of interest.
Approval of the research protocol: This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University.
Informed Consent: The written informed consent of all subjects was obtained following the Declaration of Helsinki.
Approval date of Registry and the Registration No. of the study/trial: No. LCKY2017‐21, date: July 2017.
Animal Studies: N/A
Acknowledgments
The authors thank the staff of the Department of Endocrinology of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, as well as all the patients who participated in the study.
J Diabetes Investig 2022; 13: 351–358
REFERENCES
- 1. Lin X, Xiong D, Peng YQ, et al. Epidemiology and management of osteoporosis in the People's Republic of China: current perspectives. Clin Interv Aging 2015; 10: 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janghorbani M, Van Dam RM, Willett WC, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes a meta‐analysis. Osteoporos 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 4. Schacter GI, Leslie WD. DXA‐based measurements in diabetes: can they predict fracture risk? Calcif Tissue Int 2017; 100: 150–164. [DOI] [PubMed] [Google Scholar]
- 5. Moayeri A, Mohamadpour M, Mousavi SF, et al. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta‐analysis. Ther Clin Risk Manag 2017; 13: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holm JP, Hyldstrup L, Jensen JB. Time trends in osteoporosis risk factor profiles: a comparative analysis of risk factors, comorbidities, and medications over twelve years. Endocrine 2016; 54: 241–255. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med 2011; 27: 337–339. [DOI] [PubMed] [Google Scholar]
- 8. Kwon HJ, Ha YC, Park HM. Prevalence of sarcopenia in the korean woman based on the korean national health and nutritional examination surveys. J Bone Metab 2016; 23: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos 2019; 14: 38. [DOI] [PubMed] [Google Scholar]
- 10. Reginster JY, Beaudart C, Buckinx F, et al. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutrit Metab Care 2016; 19: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izzo A, Massimino E, Riccardi G, et al. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients 2021; 13: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Osteoporos Int 1994; 4: 368–381. [DOI] [PubMed] [Google Scholar]
- 13. Gojanovic M, Holloway‐Kew KL, Hyde NK, et al. The dietary inflammatory index is associated with low muscle mass and low muscle function in older australians. Nutrients 2021; 13: 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osuna JA, Gomez‐Perez R, Arata‐Bellabarba G, et al. Relationship between BMI, total testosterone, sex hormone‐binding‐globulin, leptin, insulin and insulin resistance in obese men. Arch Androl 2006; 52: 355–361. [DOI] [PubMed] [Google Scholar]
- 15. Tagliaferri C, Wittrant Y, Davicco MJ, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015; 21: 55–70. [DOI] [PubMed] [Google Scholar]
- 16. Di Monaco M, Castiglioni C, De Toma E, et al. Presarcopenia and sarcopenia in hip‐fracture women: prevalence and association with ability to function in activities of daily living. Aging Clin Exp Res 2015; 27: 465–472. [DOI] [PubMed] [Google Scholar]
- 17. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity‐associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 2010; 5: e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landi F, Cruz‐Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013; 42: 203–209. [DOI] [PubMed] [Google Scholar]
- 19. Hayashi M, Abe K, Fujita M, et al. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol Res 2018; 48: 893–904. [DOI] [PubMed] [Google Scholar]
- 20. Bryant RV, Ooi S, Schultz CG, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41: 895–906. [DOI] [PubMed] [Google Scholar]
- 21. Hwang JA, Kim YS, Leem AY, et al. Clinical implications of sarcopenia on decreased bone density in men with COPD. Chest 2017; 151: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 22. He H, Liu Y, Tian Q, et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int 2016; 27: 473–482. [DOI] [PubMed] [Google Scholar]
- 23. Lee K, Lee JY, Kim YH. Low grip strength and muscle mass increase the prevalence of osteopenia and osteoporosis in elderly women. Healthcare 2021; 9: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zofkova I. Hormonal aspects of the muscle‐bone unit. Physiol Res 2008; 57(Suppl 1): S159–169. [DOI] [PubMed] [Google Scholar]
- 25. Macdonald H, Kontulainen S, Petit M, et al. Bone strength and its determinants in pre‐ and early pubertal boys and girls. Bone 2006; 39: 598–608. [DOI] [PubMed] [Google Scholar]
- 26. Lang TF. The bone‐muscle relationship in men and women. J Osteoporos 2011; 2011: 702735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor alpha. Exe Sport Sci Rev 2004; 32: 64–68. [DOI] [PubMed] [Google Scholar]
- 28. Fricke O, Schoenau E. The 'Functional Muscle‐Bone Unit': probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm IGF Res 2007; 17: 1–9. [DOI] [PubMed] [Google Scholar]
- 29. Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Mineral Res 2001; 16: 1343–1352. [DOI] [PubMed] [Google Scholar]


