Abstract
Aims/Introduction
To examine the incidence rate of severe non‐proliferative and proliferative diabetic retinopathy (severe‐NPDR/PDR) and determine its potential risk factors.
Materials and Methods
The study consisted of 1,169 participants (675 women) with type 2 diabetes mellitus, aged ≥20 years. A trained interviewer collected information about the history of pan‐retinal photocoagulation as a result of diabetic retinopathy. Multivariable Cox proportional hazards regression models were applied.
Results
We found 187 cases (126 women) of severe‐NPDR/PDR during a median follow‐up period of 12.7 years; the corresponding incidence rate was 13.6 per 1,000 person‐years. Being overweight (hazard ratio [HR], 95% confidence interval [CI] 0.60, 0.39–0.92) and obese (HR 0.48, 95% CI 0.27–0.83) were associated with lower risk, whereas being smoker (HR 1.75, 95% CI 1.12–2.74), having fasting plasma glucose levels 7.22–10.0 mmol/L (HR 2.81, 95% CI 1.70–4.62), fasting plasma glucose ≥10 mmol/L (HR 5.87, 95% CI 3.67–9.41), taking glucose‐lowering medications (HR 2.58, 95% CI 1.87–3.56), prehypertension status (HR 1.65, 95% CI 1.05–2.58) and newly diagnosed hypertension (HR 1.96, 95% CI 1.06–3.65) increased the risk of severe‐NPDR/PDR. Among newly diagnosed diabetes patients, being male was associated with a 59% lower risk of severe‐NPDR/PDR (HR 0.41, 95% CI 0.21–0.79). Furthermore, patients who had an intermediate level of education (6–12 years) had a higher risk of developing PDR (HR 1.86, 95% CI 1.05–3.30) compared with those who had <6 years of education.
Conclusions
Among Iranians with type 2 diabetes mellitus, 1.36% developed severe‐NPDR/PDR annually. Normal bodyweight, being a smoker, out of target fasting plasma glucose level, prehypertension and newly diagnosed hypertension status were independent risk factors of severe‐NPDR/PDR. Regarding the sight‐threatening entity of advanced diabetic retinopathy, the multicomponent strategy to control diabetes, abstinence of smoking and tight control of blood pressure should be considered.
Keywords: Proliferative diabetic retinopathy, Microvascular complication, Visual impairment
The present study showed that each year, approximately 1% of Iranian patients with type 2 diabetes mellitus suffered from severe non‐proliferative and proliferative diabetic retinopathy. Poor control of diabetes, current smoking, prehypertension, newly diagnosed hypertension and normal bodyweight were associated with a higher risk of severe non‐proliferative and proliferative diabetic retinopathy.

INTRODUCTION
Over the past few decades, increasing prevalence and incidence rates of type 2 diabetes mellitus have made it the most critical key health priority. Currently, type 2 diabetes mellitus affects 425 million adults worldwide with a prevalence rate of 8.8%, and it is estimated to reach 500 million by the year 2030 1 , 2 . With increasing diabetes trends globally, devastating diabetes‐related complications and morbidities, including diabetic retinopathy (DR), are rising and have emerged as public health concerns 3 .
DR is the most common microvascular complication of diabetes, and is a leading cause of visual impairment and blindness in the working‐age population 4 , 5 . Additionally, DR, especially in the advanced stages, has a strong association with a two‐ to threefold excess risk of cardiovascular diseases and ischemic stroke 6 , 7 . Hence, timely diagnosis and management of DR seem vital. The American Diabetes Association and the American Academy of Ophthalmology recommend that after the diagnosis of type 2 diabetes mellitus, patients with type 2 diabetes mellitus should undergo a complete ophthalmic examination at least once a year 8 , 9 .
Several potential risk factors are introduced to initiate or progress DR among patients with type 2 diabetes mellitus, including the duration of diabetes, hypertension, advanced diabetic nephropathy, severe carotid artery occlusive disease and pregnancy 10 , 11 . Furthermore, Scanlon et al. showed that race plays an important role in the progression of DR among various populations 12 .
Between 2008 and 2011, the prevalence of type 2 diabetes mellitus among the Tehran population was reported to be >13%; >1% of the Iranian population developed diabetes each year 2 . A previous cross‐sectional study in Iran reported a high prevalence of DR among type 2 diabetes mellitus patients; the value reached 27.3% for non‐proliferative DR (NPDR) and 9.6% for proliferative DR (PDR) 13 .
In the present study, we aimed to determine the incidence and risk factors of severe‐NPDR/PDR over 10 years of follow up in the population‐based cohort of the Tehran Lipid and Glucose Study (TLGS).
MATERIALS AND METHODS
Study population
The TLGS is a prospective cohort study that was carried out with individuals who lived in district 13 of Tehran. It aimed at determining the prevalence, incidence and other epidemiological aspects of non‐communicable diseases. It also looked at counteracting the non‐communicable diseases burden by developing a healthier lifestyle. The TLGS enrollment was carried out in two phases, the first of which was from 31 January 1999 to 3 July 2001, the second phase was from 20 October 2001 until 22 September 2005. It is planned that data collection will continue for at least 20 years, with approximately 3‐year intervals. Further details of the TLGS have been reported elsewhere 14 .
Among a total of 1,375 participants with type 2 diabetes mellitus aged ≥20 years (1,164 individuals from phase I and 211 participants from phase II), we excluded individuals with 42% missing data regarding body mass index (BMI), waist circumference (WC), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking status, education level, serum creatinine and physical activity at baseline (n = 54, considering overlaps between numbers). After excluding individuals without any follow‐up measurements after baseline recruitment (n = 154), a total of 1,169 participants (675 women) were followed until 20 March 2016 for the current study analyses. No patients had a history of PRP at baseline.
The ethics committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences approved the study proposal (ethics number: IR.SBMU.ENDOCRINE.REC.1400.006 in May 2021) and written informed consent was obtained from all participants.
Clinical and laboratory measurements
Using a standard questionnaire, a trained interviewer collected information, which included demographic data, history of pan‐retinal photocoagulation as a result of DR, medication history, cardiovascular disease, smoking habits and education level. Body measurements (weight and height) were carried out while participants were wearing light clothing with shoes removed. Weight was recorded to the nearest 100 g. Height was recorded in a standing position, using a tape measure, while shoulders were in normal alignment. At the level of the umbilicus, WC was measured with light clothing 14 . Using a standard mercury sphygmomanometer (calibrated by the Iranian Institute of Standards and Industrial Research), after 15 min of rest, blood pressure (BP) was measured twice in a seated position. The mean of these two BP measurements was considered as the BP level. After 12–14 h of overnight fasting, blood samples were taken from all participants. Details for laboratory measurements, including FPG, TG, TC, HDL‐C and serum creatinine, are published elsewhere 14 . A standard oral glucose tolerance test was carried out in participants with untreated diabetes.
Definition of terms
General obesity was classified into three groups: (i) BMI <25 kg/m2 as normal; (ii) 25 ≤ BMI ˂ 30 kg/m2 as overweight; and (iii) ≥30 kg/m2 as obese. As recommended by ‘The Iranian National Committee of Obesity’, and based on multiple cross‐sectional and prospective studies, we defined central obesity as WC ≥95 cm for both sexes 15 , 16 . For age categorization, we classified our study population into three groups: (i) 20–40 years; (ii) 40–60 years; and (iii) ≥60 years. Education was sorted into three groups: (i) formal education lasting <6 years; (ii) 6–12 years; and (iii) >12 years. For smoking status categorization, three groups were defined: (i) current smokers (who smoke cigarettes or pipe water daily or occasionally); (ii) former smokers (those who used to smoke); and (iii) never smokers. For phase I‐enrolled participants, we used the Lipid Research Clinic questionnaire to evaluate weekly physical activity levels; low physical activity was defined as being physically active for <3 days per week. For phase II‐enrolled participants, we also used the Modifiable Activity Questionnaire and individuals with fewer than 600 MET (metabolic equivalent task)‐minutes per week were categorized as being in the low physical activity group 14 , 17 . Regarding hypertension status, we classified BP levels into five groups: normotensive individuals were individuals with SBP <120 mmHg and DBP <80 mmHg; prehypertensive individuals were individuals with 120 ≤ SBP < 140 or 80 ≤DBP < 90 not on any antihypertensive drug; newly diagnosed hypertensive patients were those who had SBP ≥140 mmHg or DBP ≥90 mmHg without taking any antihypertensive drug; controlled treated hypertensive individuals with SBP <140 mmHg and DBP <90 mmHg, and uncontrolled treated hypertensive individuals with SBP ≥140 mmHg or DBP ≥90 mmHg. Regarding blood glucose level, patients were categorized into three groups: (i) FPG categories were defined as FPG <7.22 mmol/L; (ii) FPG 7.22 to <10.0 mmol/L; and (iii) FPG ≥10 mmol/L, corresponding to hemoglobin A1c levels of <7, 7–8 and ≥8%, respectively 18 ; a similar approach was applied in our previous study 19 . High TC and high TG were defined as TC ≥5.18 mmol/L and TG ≥1.695 mmol/L, respectively. Low HDL‐C was defined as HDL‐C <1.036 mmol/L for men or <1.295 mmol/L for women.
Glomerular filtration rate (GFR) was estimated using the abbreviated prediction equation, provided by the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) study as follows 20 :
where κ = 0.7 for women and 0.9 for men, α = −0.329 for women and −0.411 for men.
In this equation, estimated GFR (eGFR) is expressed as mL/min per 1.73 m2 and serum creatinine as mg/dL. CKD was an eGFR of <60 mL/min per 1.73 m2 (CKD stage 3–5) occurring at any time during the follow‐up period.
Outcome: severe‐NPDR/PDR
According to the previously published article on outcomes in the TLGS cohort 21 , for each participant, any medical event leading to hospitalization was followed up by a telephone call annually. Individuals were asked about any medical conditions by a trained nurse, and later, a trained physician collected complementary data regarding that event during a home visit and by the acquisition of data from medical files. The collected data were then evaluated by an outcome committee consisting of an internist, endocrinologist, cardiologist, epidemiologist and other experts, if required, to assign a specific outcome for every event. Importantly, the outcome committee is blinded to the status of baseline risk factors. Following the TLGS protocol, severe‐NPDR/PDR was defined as the history of the first pan‐retinal photocoagulation (PRP), which was carried out in the follow‐up period. The recommendation of a trial of PRP for severe NPDR and PDR was addressed in several studies 22 , 23 . Furthermore, carrying out PRP at the severe NPDR stage is likely to be cost‐effective compared with delaying photocoagulation until PDR develops 24 .
Statistical analysis
Baseline characteristics of the study population are described as the mean (standard deviation; SD) values for continuous variables, and as frequencies (%) for categorical variables. Comparison of the baseline characteristics between male and female participants, and also respondents (entered in the study) and non‐respondent (including those with missing data at the baseline or with no follow‐up data) individuals was carried out using the Student’s t‐test for normally distributed continuous variables, the χ2‐test for categorical variables, and the Mann–Whitney U‐statistic for the skewed and ordered variables. The cumulative incidence rate of retinopathy was calculated by dividing the number of event cases by the total number of participants. The crude incidence rate (95% confidence interval [CI]) of retinopathy was calculated by dividing the number of new cases of retinopathy by person‐years at risk for each sex and the whole population.
Cox proportional hazards models were applied to evaluate the association of the potential risk factors with incident retinopathy. Univariable analysis was carried out for each potential retinopathy risk factor including sex (reference: women), age (reference: 20–39 years), BMI (reference: normal), smoking status (reference: never), education levels (reference: <6 years), low physical activity, BP level (reference: normal), low HDL‐C, high TC, high TG and FPG level categories (reference: <7.22 mmol/L), as well as for central obesity, prevalent cardiovascular disease, CKD and aspirin medication. Those covariates with a P‐value <0.2 were entered in the multivariable analysis.
The hazard ratios (HRs) and 95% CIs were reported for adjusted risk factors. The proportionality in the Cox model was evaluated with the Schoenfeld residual test and, generally, all proportionality assumptions were appropriate. The event date was defined as the date of the incident PDR. Those who met the following criteria were considered to be censored: leaving the residential area, loss to follow up or end of follow up. For individuals with incident PDR, survival time was considered as the time between the entered date and the severe‐NPDR/PDR date. Additionally, for the censored participants, the survival time was considered as the difference between the entered date and the last available follow‐up date.
As a sensitivity analysis, a multivariable analysis was carried out among those who had not been on diabetes medications (newly diagnosed patients with type 2 diabetes mellitus) at enrollment.
As another sensitivity analysis, to minimize selection bias, due to missing data at the baseline, the propensity score was calculated and adjusted in the analysis. The propensity score calculated the estimated probability of non‐responders based on individual characteristics at baseline. This measure was computed using maximum likelihood logistic regression analysis 25 .
All tests were carried out using Stata version 14 SE (StataCorp LP, College Station, TX, USA), which was considered to be significant with a two‐tailed P‐value of <0.05.
RESULTS
The study population consisted of 494 men and 675 women at baseline with a mean age of 56.93 (SD 12.37) and 54.32 (SD 10.66) years, respectively. We compared baseline characteristics of respondent individuals with non‐respondent individuals. As shown in Table S1, compared with non‐respondents, respondents were less likely to be obese, had higher TG and lower FPG levels. The baseline characteristics of men and women, and also according to BMI categories of the study population are shown in Table 1 and Table S2. There were significant differences between men and women; women who were younger had higher levels of BMI, WC, FPG, TC and HDL‐C, and higher frequencies of hypertension and CKD. They were less educated and less likely to be smokers, whereas men reported higher frequencies of aspirin medications and having a positive history of cardiovascular disease. Just 27.9% of the patients with type 2 diabetes mellitus reported use of glucose‐lowering medication at the baseline recruitment. The distribution of the medications is shown in Figure 1. Accordingly, sulfonylurea in combination with other glucose‐lowering medications was the most common category.
Table 1.
Baseline characteristics of the study participants in men, women and total population: Tehran Lipid and Glucose Study 1999–2016
| Total (n = 1,169) | Men (n = 494) | Women (n = 675) | P‐value | |
|---|---|---|---|---|
| Continuous variables | ||||
| Age (years) | 55.4 (11.5) | 56.9 (12.4) | 54.3 (10.7) | <0.01 |
| BMI (kg/m2) | 28.8 (4.6) | 27.6 (3.7) | 29.8 (4.8) | <0.01 |
| WC (cm) | 96.6 (10.8) | 95.9 (10.3) | 97.2 (11.1) | 0.06 |
| SBP (mmHg) | 134.4 (22.9) | 133.2 (23.2) | 135.3 (22.7) | 0.11 |
| DBP (mmHg) | 82.3 (11.6) | 81.6 (12.2) | 82.9 (11.1) | 0.07 |
| FPG (mmol/L) | 9.1 (3.4) | 8.7 (3.1) | 9.3 (3.6) | <0.01 |
| eGFR (mL/min/1.73 m2) | 66.4 (13.4) | 68.7 (14.1) | 64.8 (12.7) | <0.01 |
| TC (mmol/L) | 6.02 (1.3) | 5.6 (1.2) | 6.3 (1.4) | <0.01 |
| HDL‐C (mmol/L) | 1.1 (0.3) | 1.0 (0.3) | 1.1 (0.3) | <0.01 |
| TG (mmol/L) | 2.4 (1.6) | 2.3 (1.7) | 2.4 (1.5) | 0.18 |
| Categorical variables | ||||
| Age categories (years) | <0.01 | |||
| ‐20–39 | 117 (10.0) | 52 (10.5) | 65 (9.6) | |
| ‐40–59 | 586 (50.1) | 217 (43.9) | 369 (54.7) | |
| ‐≥60 | 466 (39.9) | 225 (45.5) | 241 (35.7) | |
| Central obesity | 676 (57.8) | 284 (57.5) | 392 (58.1) | 0.84 |
| BMI categories (kg/m2) | <0.01 | |||
| ‐<25 | 222 (19.0) | 121 (24.5) | 101 (15.0) | |
| ‐25–30 | 519 (44.4) | 247 (50.5) | 272 (40.3) | |
| ‐≥30 | 428 (36.6) | 126 (25.5) | 302 (44.7) | |
| Smoking status | <0.01 | |||
| ‐Never | 884 (75.6) | 271 (54.9) | 613 (90.8) | |
| ‐ Former | 135 (11.5) | 106 (21.5) | 29 (4.3) | |
| ‐Current | 150 (12.8) | 117 (23.7) | 33 (4.9) | |
| Education level (years) | <0.01 | |||
| ‐<6 | 728 (62.3) | 233 (47.2) | 495 (73.3) | |
| ‐6–12 | 366 (31.3) | 199 (40.3) | 167 (24.7) | |
| ‐>12 | 75 (6.4) | 62 (12.6) | 13 (1.9) | |
| Low physical activity (yes) | 830 (71.0) | 348 (70.4) | 482 (71.4) | 0.72 |
| Blood pressure categories | <0.01 | |||
| ‐Normal | 210 (18.0) | 99 (14.7) | 111 (22.5) | |
| ‐Prehypertension | 378 (32.3) | 212 (31.4) | 166 (33.6) | |
| ‐Newly diagnosed hypertensive | 101 (8.6) | 72 (10.7) | 29 (5.9) | |
| ‐Controlled treated hypertensive | 169 (14.5) | 123 (18.2) | 46 (9.3) | |
| ‐Uncontrolled treated hypertensive | 311 (26.6) | 169 (25.0) | 142 (28.7) | |
| FPG level categories (mmol/L) | 0.13 | |||
| ‐<7.22 | 447 (38.2) | 201 (40.7) | 246 (36.4) | |
| ‐7.22–10.0 | 357 (30.5) | 154 (31.2) | 203 (30.1) | |
| ‐≥10 | 365 (31.2) | 139 (28.1) | 226 (33.5) | |
| Low HDL‐C | 899 (76.9) | 346 (70.0) | 553 (81.9) | <0.01 |
| High TC | 315 (26.9) | 179 (36.2) | 136 (20.1) | <0.01 |
| High TG | 902 (77.2) | 366 (74.1) | 536 (79.4) | 0.03 |
| CKD (yes) | 377 (32.2) | 139 (28.1) | 238 (35.3) | 0.01 |
| Prevalence CVD (yes) | 160 (13.7) | 82 (16.6) | 78 (11.6) | 0.01 |
| Aspirin medication (yes) | 257 (22.0) | 124 (25.1) | 133 (19.7) | 0.03 |
| Glucose‐lowering medications (yes) | 450 (38.5) | 165 (33.4) | 285 (42.2) | <0.01 |
| Incident retinopathy (yes) | 187 (16.0) | 61 (12.3) | 126 (18.7) | <0.01 |
Values are shown as the mean (standard deviation) and number (%), for continuous and categorical variables, respectively. Triglycerides (TG) had skewed distribution, so it is shown as the median (interquartile range).
BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; WC, waist circumference.
Figure 1.
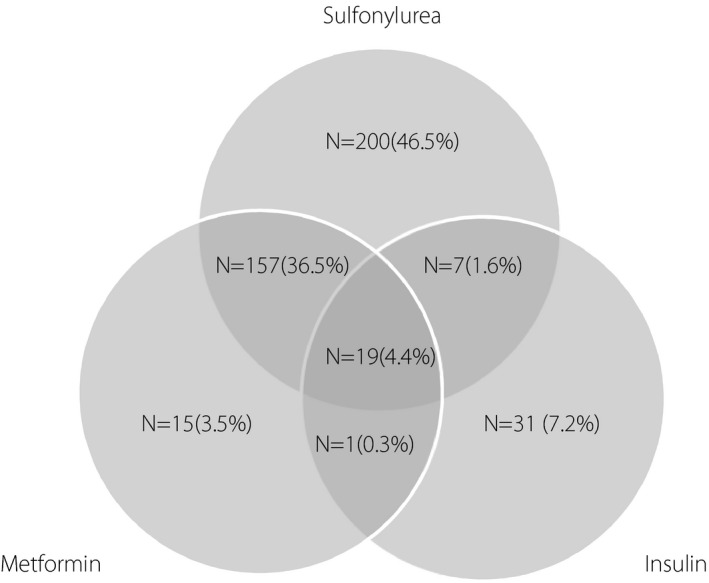
The number of patients with different types of glucose‐lowering medications at baseline or first follow up. The percentage of each group was calculated only among 430 patients with complete information, considering that for 20 patients, types of glucose‐lowering medications that they had used were missing.
During the median follow‐up period of 12.7 years (interquartile range 7.8–16.1), 187 PDR (126 women) were recorded. The crude and age‐standardized incidence rates of incident severe‐NPDR/PDR in the whole population were 13.6 (95% CI 11.7–15.6) and 11.0 (95% CI 8.7–13.7) per 1,000 person‐years. The sex‐specific crude incidence rates were 10.8 (95% CI 8.4–13.9) and 15.5 (95% CI 13.0–18.4) per 1,000 person‐years in men and women, respectively. The age‐standardized incidence of PDR among men and women was 9.5 (95% CI 6.2–13.8) and 12.0 (95% CI 9.0–15.7) per 1,000 person‐years, respectively.
Univariable HRs (95% CI) of potential categorical risk factors of developing severe‐NPDR/PDR are shown in Table 2. Table 3 shows multivariable‐adjusted hazard ratios and 95% CIs of potential severe‐NPDR/PDR risk factors. Being overweight and obese was associated with a 40% (HR 0.60, 95% CI 0.39–0.92, P = 0.02) and 52% (HR 0.48, 95% CI 0.27–0.83, P = 0.01) lower risk of PDR respectively. Furthermore, being a current smoker was associated with a 75% higher risk of PDR (HR 1.75, 95% CI 1.12–2.74, P = 0.02). Furthermore, there were significant positive associations between FPG categories ≥7.22 mmol/L, and diabetes medication with severe‐NPDR/PDR. The present results showed that prehypertension and newly diagnosed hypertension were also associated with a higher risk of PDR (HR 1.65, 95% CI 1.05–2.58, P = 0.03 and HR 1.96, 95% CI 1.06–3.65, P = 0.03, respectively). Furthermore, uncontrolled treated hypertensive patients showed a 42% non‐significant higher risk of severe‐NPDR/PDR (HR 1.42, 95% CI 0.87–2.31).
Table 2.
Hazard ratios and 95% confidence intervals from the univariable analysis of categorical potential risk factors for incident severe non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy: Tehran Lipid and Glucose Study 1999–2018
| Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|
| Sex (male) | 0.72 | 0.53–0.98 | 0.04 |
| Age categories, years | |||
| ‐20–39 | Reference | Reference | – |
| ‐40–59 | 2.21 | 1.22–4.01 | 0.01 |
| ‐≥60 | 1.74 | 0.93–3.25 | 0.08 |
| Central obesity (yes) | 0.80 | 0.60–1.07 | 0.15 |
| BMI categories (kg/m2) | |||
| ‐<25 | Reference | Reference | |
| ‐25–30 | 0.62 | 0.43–0.89 | 0.01 |
| ‐≥30 | 0.52 | 0.35–0.77 | <0.01 |
| Smoking status | |||
| ‐Never | Reference | Reference | |
| ‐Former | 0.86 | 0.52–1.43 | 0.57 |
| ‐Current | 1.39 | 0.92–2.09 | 0.11 |
| Education level (years) | |||
| ‐<6 | Reference | Reference | |
| ‐6–12 | 0.84 | 0.61–1.14 | 0.27 |
| ‐>12 | 0.49 | 0.23–1.06 | 0.07 |
| Low physical activity (yes) | 0.91 | 0.66–1.25 | 0.57 |
| Blood pressure categories (yes) | |||
| ‐Normal | Reference | Reference | |
| ‐Prehypertension | 1.44 | 0.93–2.22 | 0.11 |
| ‐Newly diagnosed hypertensive | 1.68 | 0.94–3.02 | 0.08 |
| ‐Controlled treated hypertensive | 1.15 | 0.66–2.00 | 0.61 |
| ‐Uncontrolled treated hypertensive | 1.21 | 0.75–1.93 | 0.44 |
| FPG level categories (mmol/L) | |||
| ‐<7.22 | Reference | Reference | |
| ‐7.22–10.0 | 3.06 | 1.87–5.00 | <0.01 |
| ‐≥10 | 8.67 | 5.53–13.60 | <0.01 |
| Low HDL‐C (yes) | 1.26 | 0.87–1.81 | 0.23 |
| High TC (yes) | 1.01 | 0.98–1.05 | 0.44 |
| High TG (yes) | 0.86 | 0.61–1.22 | 0.40 |
| CKD (yes) | 1.12 | 0.81–1.54 | 0.46 |
| Prevalence CVD (yes) | 1.01 | 0.62–1.64 | 0.98 |
| Glucose‐lowering medications (yes) | 4.06 | 3.01–5.47 | <0.01 |
| Aspirin medications (yes) | 1.22 | 0.86–1.73 | 0.26 |
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Table 3.
Hazard ratios and 95% confidence intervals from the multivariable analysis of categorical potential risk factors for incident severe non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy: Tehran Lipid and Glucose Study 1999–2018
| Event/n | HR (95% CI) | P‐value | |
|---|---|---|---|
| Sex (male) | 61/494 | 0.79 (0.54–1.16) | 0.23 |
| Age (years) | 187/1169 | 1.00 (0.98–1.02) | 0.93 |
| Central obesity (yes) | 99/676 | 1.04 (0.70–1.57) | 0.84 |
| BMI categories (kg/m2) | |||
| ‐<25 | 44/222 | Reference | |
| ‐25–30 | 81/519 | 0.60 (0.39–0.92) | 0.02 |
| ‐≥30 | 62/428 | 0.48 (0.27–0.83) | 0.01 |
| Smoking status | |||
| ‐Never | 142/884 | Reference | |
| ‐Former | 17/135 | 0.88 (0.51–1.51) | 0.64 |
| ‐Current | 28/150 | 1.75 (1.12–2.74) | 0.02 |
| Education level, years | |||
| ‐<6 | 122/728 | Reference | |
| ‐6–12 | 58/366 | 0.92 (0.63–1.34) | 0.66 |
| ‐>12 | 7/75 | 0.69 (0.31–1.57) | 0.38 |
| Blood pressure categories (yes) | |||
| ‐Normal | 28/210 | Reference | |
| ‐Prehypertension | 71/378 | 1.65 (1.05–2.58) | 0.03 |
| ‐Newly diagnosed hypertensive | 19/101 | 1.96 (1.06–3.65) | 0.03 |
| ‐Controlled treated hypertensive | 23/169 | 1.14 (0.63–2.08) | 0.66 |
| ‐Uncontrolled treated hypertensive | 46/311 | 1.42 (0.87–2.31) | 0.16 |
| FPG level categories (mmol/L) | |||
| ‐<7.22 | 23/447 | Reference | |
| ‐7.22–10.0 | 52/357 | 2.81(1.70–4.62) | <0.01 |
| ‐≥10 | 112/365 | 5.87 (3.67–9.41) | <0.01 |
| Glucose‐lowering medications (yes) | 116/450 | 2.58 (1.87–3.56) | <0.01 |
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; FPG, fasting plasma glucose; HR, hazard ratio.
As a sensitivity analysis, when we excluded known diabetes cases (on glucose‐lowering medications) from our data analysis, being male was associated with a 59% lower risk of severe‐NPDR/PDR (HR 0.41, 95% CI 0.21–0.79, P = 0.01). We observed that the risk of incident severe‐NPDR/PDR was significantly related to higher FPG levels. Patients who had an intermediate level of education (6–12 years) had a higher risk of developing PDR (HR 1.86, 95% CI 1.05–3.30, P = 0.03) (Table 4).
Table 4.
Hazard ratios and 95% confidence intervals from the multivariable analysis of categorical potential risk factors for incident severe non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy among those not on glucose‐lowering medications: Tehran Lipid and Glucose Study 1999–2018
| Event/n | HR (95% CI) | P‐value | |
|---|---|---|---|
| Sex (male) | 23/329 | 0.41 (0.21–0.79) | 0.01 |
| Age (years) | 71/719 | 1.01 (0.99–1.04) | 0.31 |
| Central obesity (yes) | 46/436 | 1.10 (0.55–2.17) | 0.79 |
| BMI categories (kg/m2) | |||
| ‐<25 | 12/117 | Reference | |
| ‐25–30 | 23/317 | 0.52 (0.24–1.15) | 0.11 |
| ‐≥30 | 36/285 | 0.79 (0.32–1.95) | 0.60 |
| Smoking status | |||
| ‐Never | 51/532 | Reference | |
| ‐Former | 8/88 | 1.58 (0.67–3.72) | 0.29 |
| ‐Current | 12/99 | 1.87 (0.91–3.86) | 0.09 |
| Education level, years | |||
| ‐<6 | 39/414 | Reference | |
| ‐6–12 | 31/251 | 1.86 (1.05–3.30) | 0.03 |
| ‐>12 | 1/51 | 0.36 (0.05–2.85) | 0.34 |
| Blood pressure categories (yes) | |||
| ‐Normal | 10/128 | Reference | |
| ‐Prehypertension | 26/245 | 1.44 (0.68–3.05) | 0.34 |
| ‐Newly diagnosed hypertensive | 5/46 | 1.68 (0.55–5.15) | 0.37 |
| ‐Controlled treated hypertensive | 6/85 | 0.89 (0.30–2.62) | 0.84 |
| ‐Uncontrolled treated hypertensive | 24/215 | 1.75 (0.81–3.76) | 0.15 |
| FPG level categories (mmol/L) | |||
| ‐<7.22 | 13/363 | Reference | |
| ‐7.22–10.0 | 26/223 | 3.51 (1.79–6.87) | <0.01 |
| ‐≥10 | 32/133 | 8.49 (4.36–16.50) | <0.01 |
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; FPG, fasting plasma glucose; HR, hazard ratio.
Furthermore, after further adjustment for inverse probability weighting (1 / propensity score), the results remained essentially unchanged (Tables S3 and S4).
DISCUSSION
Using data from a decade follow up of Iranian patients with type 2 diabetes mellitus, we assessed the incidence rate and potential risk factors of severe‐NPDR/PDR. Accordingly, approximately 1.36% of patients with type 2 diabetes mellitus developed severe‐NPDR/PDR each year. The present study showed strong associations of higher FPG levels, glucose‐lowering medications, smoking, prehypertension and newly diagnosed hypertension with an incidence of severe‐NPDR/PDR. Additionally, we found that having a BMI >25 kg/m2 was generally associated with a lower risk.
The high prevalence of diabetes leads to an increased rate of severe‐NPDR/PDR, which has been identified as one of the most common causes of visual impairment worldwide. In the present study, the incidence rate of severe‐NPDR/PDR in individuals with type 2 diabetes mellitus accounted for 13.6 per 1,000 person‐years. In a large USA managed‐care network, 19.6 cases per 1,000 person‐years with type 2 diabetes mellitus received the diagnosis of DR during 3 years’ follow up. Over 6 years of follow up, among Indians with type 2 diabetes mellitus who were treated, 42.7% developed an advanced form of DR (excluding PDR) in at least one eye 26 . Population‐based studies, such as the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) from the 1980s, reported a 4‐year incidence rate of PDR of 2–7% in patients with type 2 diabetes mellitus 27 . Additionally, in a systematic review carried out in 2018, the annual incidence rate of PDR ranged from 0.03% in the Singapore Indian Eye Study (SINDI) to 0.72% in the Nakuru Study 28 , 29 . Fang et al. 30 showed that despite the improvements in early diagnosis, control, and treatment of diabetes and its complications, the prevalence of DR during the first 2 years of diabetes diagnosis had remained high over the past three decades in the USA (1988–1994 to 1999–2008; 13.2–12.1% respectively, P for trend = 0.86) 30 . Furthermore, they also showed that during a decade of progress from 1999 to early the 2010s, glycemic and BO control declined in adult National Health and Nutrition Examination Survey participants with diabetes, whereas lipid control leveled off; it potentially contributes to the high burden of DR 31 . This discrepancy in the incidence of severe‐NPDR/PDR might be associated with a variety in participant age range, ethnicity, follow‐up duration, sample size, different methods in retinopathy assessment (i.e., dilated radiography, imaging and using patients’ claims) and facility to the acquisition of healthcare 32 .
Surprisingly, considering BMI, the present results showed that both overweight and obesity status were correlated with the reduced risk of severe‐NPDR/PDR; the value reached a significant level only for obese patients. Furthermore, after excluding the patients taking glucose‐lowering medications, being overweight and obese were still associated with a lower, but not significant, risk of severe‐NPDR/PDR; the issue might be suggestive of the increasing impact of glucose‐lowering medication on bodyweight, considering sulfonylurea was the most common medication among the study population. However, we did not find the association between central adiposity and severe‐NPDR/PDR, as addressed in some, but not all, studies 33 , 34 , 35 . There is controversy surrounding the effect of a high level of BMI on the incidence of DR. Similar to the present results, Rema et al. 36 found the inverse correlation between BMI and DR. They found that a greater level of BMI could decrease the 6‐year risk of DR in Indian populations 36 . This lower risk of higher BMI level on DR could be interpreted in several ways. First, patients with higher BMI levels have elevated C‐peptide levels, which could reduce the risk of DR 37 . Furthermore, the higher risk of DR among normal weight type 2 diabetes mellitus patients might be attributable to the long duration of disease in these patients, contributing to β‐cell failure, insulin deficiency and weight loss 36 . As shown in Table S2, we demonstrated that obese patients with type 2 diabetes mellitus had lower values of FPG and current smoking status compared with normal‐weight counterparts, despite having higher levels of hypertension and dyslipidemia, we did not have data regarding the duration. However, among our newly diagnosed cases of type 2 diabetes mellitus, the paradoxical association between BMI and severe‐NPDR/PDR was shown, although it did not reach a significant level. In contrast, several studies showed a high BMI level as a significant risk factor for DR 38 . A recent meta‐analysis showed that obesity status was not associated with PDR in a significant manner; however, an association was shown for NPDR, with high heterogeneity between included studies 39 . This inconsistent correlation between BMI level and severe‐NPDR/PDR could be explained by variation in ethnicity, socioeconomic status and also obesity paradox issues 33 .
The present data showed that regularly smoking could impose deterioration of DR on the proliferative state; this finding had been shown in several studies 40 , 41 , 42 . In comparison with non‐smokers, smokers are found to have high levels of carboxyhemoglobin, which induces a decrease in the oxygen‐carrying capacity of the blood, with retinal hypoxia leading to the progression of DR 42 , 43 . Furthermore, nicotine could lead to vasoconstriction, which might aggravate DR 44 . A recent meta‐analysis showed that in contrast to patients without type 2 diabetes mellitus, smoking could significantly decrease the risk of DR in patients with type 2 diabetes mellitus. However, as mentioned by the authors, there are several concerns regarding their findings, including that smokers with type 2 diabetes mellitus had lower survival compared with their non‐smoker counterparts and had not enough time to develop PDR. Hence, they suggested further population studies in this field 45 .
In line with previous studies, we found that use of glucose‐lowering medications could be defined as a major predictor for severe‐NPDR/PDR. Klein et al. 46 declared that the prevalence of DR is 70% in patients with type 2 diabetes mellitus using insulin, compared with just 39% in those not receiving insulin treatment. At the baseline in the present population, just 7% of the patients were treated with insulin. The use of glucose‐lowering medications would be an indicator of both the long duration of diabetes and the level of glycemic control that were associated with an increased risk of DR and PDR 47 .
One intriguing result reached through the present study was that prehypertension and newly diagnosed hypertensive status could increase the risk of severe‐NPDR/PDR by approximately 64 and 96%. Additionally, analysis from the UK Prospective Several studies Diabetes Study (UKPDS) showed that tight control of BP even in prehypertensive cases could lessen the risk of microvascular diabetes complication including progressive retinopathy up to 37% 48 . The present findings regarding the significant association between prehypertension status and PDR might be another reason that justifies the American College of Cardiology/American Heart Association recommendation threshold for initiation of antihypertension medications among patients with type 2 diabetes mellitus who had BP ≥130/80 mmHg 49 . Furthermore, the present result showed that uncontrolled treated hypertension could impose a 42% increase in the risk of development of DR, but in a non‐significant manner.
In line with the present results, Nwanyanwu et al. 50 found that there is no association between hypertension and progression of DR to PDR. These inconsistencies might reflect the fact that patients with type 2 diabetes mellitus who have BP ≥140/90 mmHg are educated to be more cautious about control of BP by using antihypertension drugs regularly. Increased BP could lead to endothelial dysfunction and inflammation, and vice versa, which have been hypothesized as key mechanisms that contribute to the development of diabetes and its vascular complications, such as DR 51 , 52 . Additionally, venular dilation and arterial narrowing after prehypertension could impose the progression of DR and the incidence of severe‐NPDR/PDR 53 , 54 , 55 .
To the best of our knowledge, this is the first long‐term study that has reported the various risk factors related to the progression of severe‐NPDR/PDR in the Middle East and North Africa region with a high burden of diabetes 56 . Furthermore, the present findings stem from a population‐based study rather than data derived from hospitalized surveys, which might confound the results.
The present results should be interpreted in light of some limitations. First, the evidence of pan‐retinal photocoagulation as a marker of progression of severe‐NPDR/PDR was based on patients' claims data, not an ophthalmic examination or imaging, so data might be slightly underestimated. Second, some physicians carried out PRP in eyes with severe‐NPDR in selected patients, and our population might include some of them, hence, we used the term “severe‐NPDR/PDR” to avoid results’ misinterpretation. Third, we did not have data on two important variables of the duration of diabetes and level of diabetes control as manifested by hemoglobin A1c levels (considering the cost and the absence of a precise method for its measurement); however, to partly address this concern, we re‐ran our data analysis only among newly diagnosed type 2 diabetes patients and we adjusted FPG level as a surrogate of hemoglobin A1c levels 18 . Fourth, the present study was carried out on a sample of the Iranian population, and our results might not be extrapolated to other populations with different ethnicities. Last, but not least, we did not have data regarding the retinal status of patients at the baseline recruitment, hence, some of the individuals might have had some degree of severe‐NPDR/PDR in the baseline.
In conclusion, the present study showed that each year approximately 1% of Iranian patients with type 2 diabetes mellitus suffered from severe‐NPDR/PDR. Poor control of diabetes, current smoking, prehypertension, newly diagnosed hypertension and normal bodyweight were associated with a higher risk of severe‐NPDR/PDR. Regarding the sight‐threatening entity of advanced DR, the multicomponent strategy to control diabetes, abstain from smoking and tight control of BP in the prehypertension status should be considered.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of the ethics committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences.
Informed Consent: All informed consent was obtained from the participants.
Approval date of Registry and the Registration No. of the study/trial: 8 May 2021. Approval ethics number: IR.SBMU.ENDOCRINE.REC.1400.006 ‐.
Animal Studies: N/A.
Supporting information
Table S1 | Baseline characteristics of the respondents (study participants) and non‐respondents (including those with missing data at the baseline or with no follow‐up data): Tehran Lipid and Glucose Study.
Table S2 | Baseline characteristics of the study participants in different groups of body mass index <25, body mass index 25–30, body mass index ≥30 and total population: Tehran Lipid and Glucose Study 1999–2016.
Table S3 | Hazard ratios and 95% confidence intervals from the multivariable analysis with propensity score of categorical potential risk factors for incident severe non‐proliferative and proliferative diabetic retinopathy: Tehran Lipid and Glucose Study (1999–2018).
Table S4 | Hazard ratios and 95% confidence intervals from the multivariable analysis with propensity score of categorical potential risk factors for incident severe non‐proliferative and proliferative diabetic retinopathy among those not on glucose‐lowering medications: Tehran Lipid and Glucose Study (1999–2018).
ACKNOWLEDGMENTS
We express our appreciation to the participants of district 13 of Tehran for their enthusiastic support of this study, and would acknowledge Dr Pasha Anvari, ophthalmologist, and Dr Seyyed Saeed Moazzeni for their valuable comments. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2022; 13: 317–327
REFERENCES
- 1. Neuenschwander M, Ballon A, Weber KS, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta‐analyses of prospective observational studies. BMJ 2019; 366: l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Derakhshan A, Sardarinia M, Khalili D, et al. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow‐up: Tehran Lipid and Glucose Study. PLoS One 2014; 9: e102563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 2019; 366: l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shotliff K, Davies N. Diabetes and the Eye. Diabetes Oxford, UK: Wiley‐Blackwell, 2012; 1–33. [Google Scholar]
- 5. Cheloni R, Gandolfi SA, Signorelli C, et al. Global prevalence of diabetic retinopathy: protocol for a systematic review and meta‐analysis. BMJ 2019; 9: e022188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in Type 2 diabetic patients. Diabet Med 2008; 25: 45–50. [DOI] [PubMed] [Google Scholar]
- 7. Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007; 38: 398–401. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association . Standards of medical care in diabetes–2012. Diabetes Care 2012; 35; S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh DV. Updates in diabetic retinopathy management in last decade. RSSDI Diabetes Update 2018; 2019: 28. [Google Scholar]
- 10. Liu L, Wu J, Yue S, et al. Incidence density and risk factors of diabetic retinopathy within type 2 diabetes: a five‐year cohort study in China (Report 1). Int J Environ Res Public Health 2015; 12: 7899–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pomytkina N, Sorokin E. Clinical features of diabetic retinopathy in pregnancy. Vestn Oftalmol 2019; 135: 55. [DOI] [PubMed] [Google Scholar]
- 12. Scanlon PH, Aldington SJ, Stratton IM. Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol 2013; 20: 293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Javadi MA, Katibeh M, Rafati N, et al. Prevalence of diabetic retinopathy in Tehran province: a population‐based study. BMC Ophthalmol 2009; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azizi F, Ghanbarian A, Momenan AA, et al. Prevention of non‐communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials 2009; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hadaegh F, Zabetian A, Sarbakhsh P, et al. Appropriate cutoff values of anthropometric variables to predict cardiovascular outcomes: 7.6 years follow‐up in an Iranian population. Int J Obes 2009; 33: 1437. [DOI] [PubMed] [Google Scholar]
- 16. Azizi F, Khalili D, Aghajani H, et al. Appropriate waist circumference cut‐off points among Iranian adults: the first report of the Iranian National Committee of Obesity. Arch Iran Med 2010; 13: 243–244. [PubMed] [Google Scholar]
- 17. Committee IR . Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)‐short and long forms. 2005. Available from: http://www.ipaq.ki.se/scoring.pdf Accessed: November 2, 2019.
- 18. Association AD . 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care 2020; 43: S66–S76. [DOI] [PubMed] [Google Scholar]
- 19. Moazzeni SS, Arani RH, Hasheminia M, et al. High incidence of chronic kidney disease among Iranian diabetic adults: using CKD‐EPI and MDRD equations for estimated glomerular filtration rate. Diabetes Metab J 2021. 10.4093/dmj.2020.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD‐EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013; 61: 57–66. [DOI] [PubMed] [Google Scholar]
- 21. Hadaegh F, Harati H, Ghanbarian A, et al. Association of total cholesterol versus other serum lipid parameters with the short‐term prediction of cardiovascular outcomes: Tehran Lipid and Glucose Study. Eur J Cardiovasc Prev Rehabil 2006; 13: 571–577. [DOI] [PubMed] [Google Scholar]
- 22. Moutray T, Evans JR, Lois N, et al. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2018; 3: CD012314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Royle P, Mistry H, Auguste P, et al. Pan‐retinal photocoagulation and other forms of laser treatment and drug therapies for non‐proliferative diabetic retinopathy: systematic review and economic evaluation. Health Technol Assess 2015; 19: v‐xxviii; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mistry H, Auguste P, Lois N, et al. Diabetic retinopathy and the use of laser photocoagulation: is it cost‐effective to treat early? BMJ Open Ophthalmol 2017; 2: e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016; 352: i189. [DOI] [PubMed] [Google Scholar]
- 26. Gupta P, Liang Gan AT, Kidd Man RE, et al. Impact of incidence and progression of diabetic retinopathy on vision‐specific functioning. Ophthalmology 2018; 125: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 27. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: X. Four‐year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989; 107: 244–249. [DOI] [PubMed] [Google Scholar]
- 28. Bastawrous A, Mathenge W, Wing K, et al. The incidence of diabetes mellitus and diabetic retinopathy in a population‐based cohort study of people age 50 years and over in Nakuru, Kenya. BBMC Endocr Disord 2017; 17: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabanayagam C, Yip W, Gupta P, et al. Singapore Indian Eye Study‐2: methodology and impact of migration on systemic and eye outcomes. Clin Exp Ophthalmol. 2017; 45: 779–789. [DOI] [PubMed] [Google Scholar]
- 30. Fang M, Selvin E. Thirty‐year trends in complications in US adults with newly diagnosed type 2 diabetes. Diabetes Care 2021; 44: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang M, Wang D, Coresh J, et al. Trends in diabetes treatment and control in US adults, 1999–2018. N Engl J Med 2021; 384: 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang SY, Andrews CA, Herman WH, et al. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology 2017; 124: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou J‐B, Yuan J, Tang X‐Y, et al. Is central obesity associated with diabetic retinopathy in Chinese individuals? An exploratory study. J Int Med Res 2019; 47: 5601–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Penman A, Hancock H, Papavasileiou E, et al. Risk factors for proliferative diabetic retinopathy in African Americans with type 2 diabetes. Ophthalmic Epidemiol 2016; 23: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang IC, Bae JH, Kim JM. Relationship between body fat and diabetic retinopathy in patients with type 2 diabetes: a nationwide survey in Korea. Eye 2019; 33: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raman R, Rani PK, Gnanamoorthy P, et al. Association of obesity with diabetic retinopathy: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study (SN‐DREAMS Report no. 8). Acta Diabetol 2010; 47: 209–215. [DOI] [PubMed] [Google Scholar]
- 37. Cai X, Han X, Zhang S, et al. Age at diagnosis and C‐peptide level are associated with diabetic retinopathy in Chinese. PLoS One 2014; 9: e91174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laiginhas R, Madeira C, Lopes M, et al. Risk factors for prevalent diabetic retinopathy and proliferative diabetic retinopathy in type 1 diabetes. Endocrine 2019; 66: 201–209. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Y, Zhang Y, Shi KE, et al. Body mass index and risk of diabetic retinopathy: a meta‐analysis and systematic review. Medicine 2017; 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song KH, Jeong JS, Kim MK, et al. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J Diabetes Investig 2019; 10: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Control D, Trial C, EoD I, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow‐up in the DCCT/EDIC. Diabetes 2015; 64: 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong Z‐L, Chen S. Plasma plasminogen activator inhibitor‐1 is associated with end‐stage proliferative diabetic retinopathy in the Northern Chinese Han population. Exp Diabetes Res 2012; 2012: 350852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leone A. Biochemical markers of cardiovascular damage from tobacco smoke. Curr Pharm Des 2005; 11: 2199–2208. [DOI] [PubMed] [Google Scholar]
- 44. Costa F, Soares R. Nicotine: a pro‐angiogenic factor. Life Sci 2009; 84: 785–790. [DOI] [PubMed] [Google Scholar]
- 45. Cai X, Chen Y, Yang W, et al. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: a meta‐analysis. Endocrine 2018; 62: 299–306. [DOI] [PubMed] [Google Scholar]
- 46. Klein R, Klein BE, Moss SE, et al. The wisconsin epidemiologic study of diabetic retinopathy: III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthal 1984; 102: 527–532. [DOI] [PubMed] [Google Scholar]
- 47. Association AD . 11. Microvascular complications and foot care: standards of medical care in diabetes—2021. Diabetes Care 2021; 44: S151–S167. [DOI] [PubMed] [Google Scholar]
- 48. Group UPDS . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713. [PMC free article] [PubMed] [Google Scholar]
- 49. Grossman E. Should we treat prehypertension in diabetes?: What are the cons? Diabetes Care 2009; 32: S280–S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harris Nwanyanwu K, Talwar N, Gardner TW, et al. Predicting development of proliferative diabetic retinopathy. Diabetes Care 2013; 36: 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang H‐N, Xu Q‐Q, Thakur A, et al. Endothelial dysfunction in diabetes and hypertension: role of microRNAs and long non‐coding RNAs. Life Sci 2018; 213: 258–268. [DOI] [PubMed] [Google Scholar]
- 52. Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre‐diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 2007; 30: 2708–2715. [DOI] [PubMed] [Google Scholar]
- 53. Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi‐Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008; 31: 544–549. [DOI] [PubMed] [Google Scholar]
- 54. Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progressionof diabetic retinopathy: Xix: the wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthal 2004; 122: 76–83. [DOI] [PubMed] [Google Scholar]
- 55. Murgan I, Beyer S, Kotliar KE, et al. Arterial and retinal vascular changes in hypertensive and prehypertensive adolescents. Am J Hypertens 2013; 26: 400–408. [DOI] [PubMed] [Google Scholar]
- 56. Azizi F, Hadaegh F, Hosseinpanah F, et al. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol 2019; 7: 866–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Baseline characteristics of the respondents (study participants) and non‐respondents (including those with missing data at the baseline or with no follow‐up data): Tehran Lipid and Glucose Study.
Table S2 | Baseline characteristics of the study participants in different groups of body mass index <25, body mass index 25–30, body mass index ≥30 and total population: Tehran Lipid and Glucose Study 1999–2016.
Table S3 | Hazard ratios and 95% confidence intervals from the multivariable analysis with propensity score of categorical potential risk factors for incident severe non‐proliferative and proliferative diabetic retinopathy: Tehran Lipid and Glucose Study (1999–2018).
Table S4 | Hazard ratios and 95% confidence intervals from the multivariable analysis with propensity score of categorical potential risk factors for incident severe non‐proliferative and proliferative diabetic retinopathy among those not on glucose‐lowering medications: Tehran Lipid and Glucose Study (1999–2018).


