Abstract
Aims/Introduction
To investigate the national trend in the prescription of first‐line non‐insulin antidiabetic agents and total medical costs (TMCs) after prescribing the drug in Japanese patients with type 2 diabetes.
Materials and Methods
Using the National Database of Health Insurance Claims and Specific Health Check‐ups of Japan covering almost the entire Japanese population, we calculated the proportion of each antidiabetic drug from 2014 to 2017, and determined the factors associated with drug selection. The TMCs in the first year after starting the drugs were calculated, and factors associated with the costs were also determined.
Results
Among 1,136,723 new users of antidiabetic agents, dipeptidyl peptidase‐4 inhibitors were the most prescribed (65.1%), followed by biguanides (15.9%) and sodium–glucose cotransporter 2 inhibitors (7.6%). Sodium–glucose cotransporter 2 inhibitor and biguanide use increased during 2014–2017 (2.2%–11.4% and 13.7%–17.2%, respectively), whereas the others decreased. Biguanides were not prescribed at all in 38.2% of non‐Japan Diabetes Society‐certified facilities. The TMCs were the lowest among those who started with biguanides. Fiscal year, age, sex, facility, number of beds and comorbidities were associated with drug choice and TMCs. There were wide regional variations in the drug choice, but not in the TMCs.
Conclusions
Unlike in the USA and Europe, dipeptidyl peptidase‐4 inhibitor is the most prescribed first‐line medication for type 2 diabetes patients in Japan, while there is a wide variation in the drug choice by facility‐type and prefecture.
Keywords: Antidiabetic agents, First‐line therapy, Type 2 diabetes
In this large‐scale, nationwide study of Japanese patients with type 2 diabetes, dipeptidyl peptidase‐4 inhibitors were the most prescribed followed by biguanides, with a wide variation in the drug choice by facility and prefecture. The total medical costs were the lowest among patients who started with biguanides.

INTRODUCTION
The International Diabetes Federation recently reported that the global diabetes prevalence rate in 2019 was approximately 9.3% (463 million people), and this is projected to increase to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 1 . Type 2 diabetes is associated with microvascular complications (e.g., retinopathy and nephropathy) and significantly increases the risk of life‐threatening complications (e.g., cardiovascular disease [CVD]) 2 . These complications also worsen the quality of life 3 and impose a heavy burden on the health and social care systems 4 . Therefore, achieving and sustaining glycemic targets is essential for diabetes care, and many antidiabetic agents have been developed over the past 20 years. In Japan, dipeptidyl peptidase‐4 (DPP4) inhibitors, glucagon‐like peptide‐1 (GLP‐1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors have been available since 2009, 2010 and 2014, respectively.
Regarding the initial choice of antidiabetic agents, the American Diabetes Association and the European Association for the Study of Diabetes 5 , 6 recommend metformin as the first‐line drug for type 2 diabetes treatment since 2006 due to its low cost, high efficacy, and the potential for reducing cardiovascular events and death 7 . In the past decade, cardiovascular outcome trials have accumulated evidence to support the use of GLP‐1 receptor agonists and SGLT2 inhibitors in patients with type 2 diabetes who had or were at high risk for CVD 8 , 9 , 10 , 11 , 12 . Thereafter, the American Diabetes Association and European Association for the Study of Diabetes clarified the usefulness of these drugs for reducing the risk of CVD and chronic kidney disease (CKD) 13 , 14 . In contrast, Japanese patients with type 2 diabetes are more vulnerable to impaired insulin secretion; are less obese; have a lower prevalence of cardiovascular complications than type 2 diabetes patients in Europe and North America 15 , 16 , and the genetic background for the development of diabetes is different between Japanese and European individuals 17 . Therefore, the Japan Diabetes Society (JDS) does not position metformin or any other drugs as the first‐line treatment, and places emphasis on tailored therapy 18 . Given these current trends in diabetes management, it is assumed that there are differences in the prescription of antidiabetic agents between the USA/Europe and Japan. Although several studies have reported trends in antidiabetic medications, and compared the effectiveness and drug cost in Japan 19 , 20 , these were not population‐based studies, and might not represent general Japanese patients with type 2 diabetes 19 , 20 , 21 . Furthermore, they were limited by relatively small sample sizes 19 , 20 , 21 and lacked data on older age groups 19 , 21 . Therefore, the prescription patterns of antidiabetic agents in Japan are not well‐understood, and there is a need to provide firm evidence from a nationwide, population‐based study.
Within this context, the present study aimed to investigate the following: (i) national trends in the initial antidiabetic agents, except insulin, for the treatment of type 2 diabetes from fiscal year (FY) 2014 to FY 2017; (ii) total medical costs for 1 year after the administration of the initial antidiabetic agents; and (iii) factors associated with these phenomena, using the National Database of Health Insurance Claims and Specific Health Check‐ups of Japan (NDB).
MATERIALS AND METHODS
Study design and data source
This was a population‐based retrospective cohort study using the NDB dataset. NDB, one of the largest health‐related databases worldwide (>100 million IDs, comparable to the total population in Japan), deposits all electronic claims data reimbursed by Japan’s public medical insurance system, excluding public assistance. As of 2017, >98% of claims were issued electronically. Details are provided in the Supporting Information.
The institutional review board approved this study of the National Center for Global Health and Medicine (NCGM‐G‐002492‐02). The need for informed consent was waived, because the database was anonymized before being provided by the Ministry of Health, Labor and Welfare.
Definitions of diseases
The patients were identified from the database using the International Classification of Disease codes, 10th edition in the claims of the antidiabetic agents‐initiating month. Detailed definitions of diseases can be found in the Supporting Information.
For the antidiabetic agents, eight classes of antidiabetic agents, namely, biguanides, sulfonylureas, alpha‐glucosidase inhibitors, thiazolidinediones, DPP4 inhibitors, SGLT2 inhibitors, glinides and GLP‐1 receptor agonists, were available for the treatment of type 2 diabetes in Japan during the study period.
Participant selection
Figure 1 shows the participant selection process. Among 11,379,602 patients who had received any antidiabetic agent during the study period, those who: (i) had received antidiabetic agents within 6 months before the first visit; (ii) had been hospitalized and received any antidiabetic agent at the same month or earlier of the first prescription of antidiabetic agents; (iii) were aged <20 years; (iv) received insulin, had diabetes types other than type 2 diabetes and diabetes in pregnancy; (v) had no disease name “diabetes” on the claim; (vi) had unmatched information on the medical institution or those who had visited more than two hospitals/clinics in the same month when the antidiabetic agent was administered; (vii) had been treated by comprehensive medical care (and therefore their medication might not have been fully listed on the claims); and (viii) had been treated with two or more antidiabetic agents or combination drugs were excluded. Finally, 1,136,723 patients with type 2 diabetes (i.e., E11, E14, refer to the Supporting Information) who started antidiabetic medications between October 2014 and March 2018 were enrolled. To analyze the total medical cost after administering the initial antidiabetic agent, we further restricted the participants to the patients whose medical care was observed after 12 months to exclude any dropout of observation during the 12 months. Therefore, patients were limited to those who started antidiabetic medication between October 2014 and January 2017 to follow up of 12 months for medical costs, and an extra 3 months to detect any medical care after 12 months. Overall, 645,493 patients were included.
Figure 1.
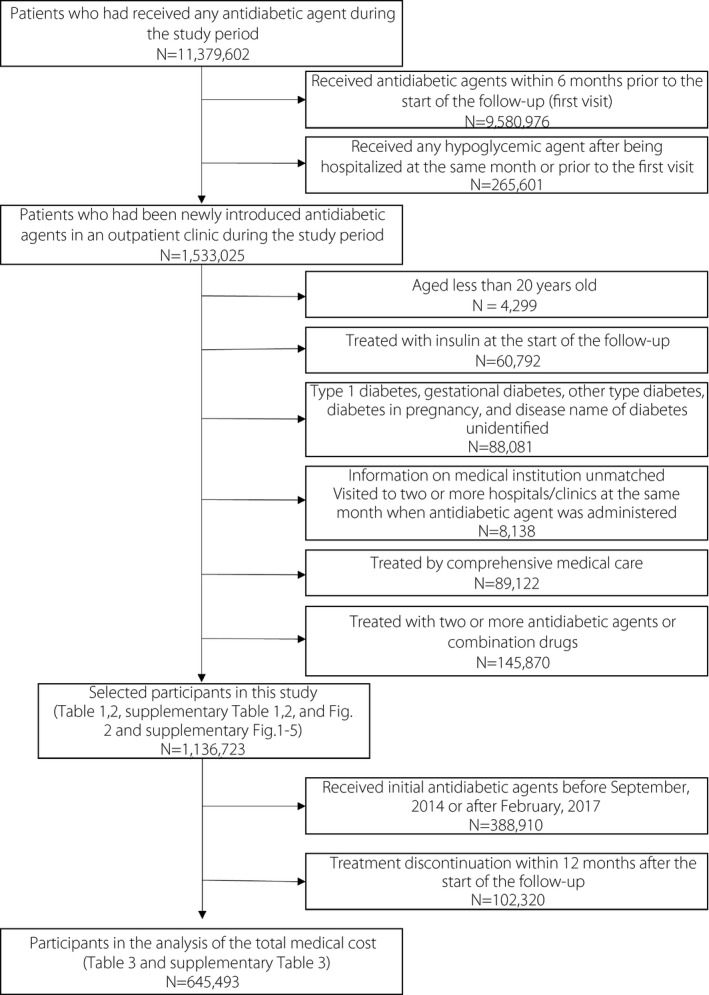
Flowchart of the study patients.
Outcomes
The prescription rate of each antidiabetic agent during the study period was determined stratified by age categories, sex, type of facilities, type of insurance, use of medications for non‐communicable diseases, such as hypertension, and comorbidities (e.g., ischemic heart disease [IHD] and CKD). We further evaluated the total medical costs in the first year after starting the initial antidiabetic agents. Detailed information on calculation of the costs can be found in the Supporting Information. All information about other variables, including sex, age at the time of prescription, insurance type and facility characteristics (prefecture, the first level of administrative division in Japan, whether the facility was certified by the JDS and the number of beds) were determined from claims data. Detailed information on insurance type can be found in the Supporting Information. Prescriptions of antihypertensives and lipid‐lowering agents were defined as the patients receiving these drugs by the antidiabetic agents‐initiating month. Disease names of IHD, CKD and renal transplantation or dialysis were identified similarly from the disease names by the month.
Statistical analysis
First, we described the characteristics of patients and the prescription rate of each antidiabetic agent based on the characteristics. We also investigated the trends in prescription patterns. As an additional analysis, we stratified the patients according to the presence or absence of IHD and the temporal trends of SGLT2 inhibitor and GLP‐1 receptor agonist prescriptions. The analysis was carried out to investigate the effect of evidence recently published on the cardioprotective action of SGLT2 inhibitors and GLP‐1 receptor agonists 8 , 9 , 10 , 11 , 12 on changes in initial prescription patterns, especially among those who already had IHD. We hereafter focused on biguanides, DPP4 inhibitors, SGLT2 inhibitors and sulfonylureas by considering their clinical importance. We carried out multiple logistic analyses for each prescription as dependent variables, and individual or facility characteristics as independent variables to address confounding covariates. Considering the possibility that hospitals with many beds might have many board‐certified diabetologists and the fact that the JDS‐certified facilities are required to have board‐certified diabetologists/trainers, we considered that number of beds and facility‐type were to be included as covariates. To consider the age‐specific aggregation of insurance‐type (i.e., patients aged ≥75 years are uniformly covered by the medical insurance for the elderly in the later stages of life), we included production terms of age category and insurance types. We then calculated the predicted proportion of each prescription after controlling for the covariates, using multiple logistic regression models.
Second, we further aggregated the prescription by facilities and determined the distributions of the prescription rates by facility‐type (JDS‐certified or non‐JDS‐certified). We primarily focused on facilities with ≥10 patients who initiated antidiabetic agents during the period. We then repeated the analyses restricted to facilities with more patients (≥20, ≥50 and ≥100). We also carried out the same analyses, including patients aged ≤65 years who neither had CKD nor underwent dialysis.
Furthermore, we described the 12‐month total medical costs of the eight antidiabetic medication classes. We investigated factors associated with higher medical costs using a generalized linear model. We adopted the generalized linear model with the log link and inverse Gaussian family to account for the strongly rightly skewed distribution. Detailed information on calculation of the 12‐month total medical costs can be found in the Supporting Information.
All statistical analyses were carried out using Stata 15.2 (StataCorp, College Station, TX, USA). Only complete data were included in the analyses.
RESULTS
Initial choice of antidiabetic agents during the study period
Among the participants, 57.5% were men, and 67.2% were aged >60 years. Approximately 10.7% visited JDS‐certified facilities, and patients in facilities with <20 hospital beds accounted for 66.8%. In total, 16.7% had a history of IHD. Table 1 shows the prescription rate of each antidiabetic agent according to age, sex, type of insurance, characteristics of facilities and comorbidities. DPP4 inhibitors were the most prescribed throughout the entire study period, followed by biguanides and SGLT2 inhibitors, whereas GLP‐1 receptor agonists were the least prescribed. As shown in Figure 2a, DPP4 inhibitor prescriptions accounted for >60% of prescriptions throughout the study period. However, the percentage gradually decreased, whereas a rapid increase in SGLT2 inhibitor prescription was observed. The prescription of biguanide also increased during the study period, but remained <20%. The prescription rates of sulfonylureas, α‐glucosidase inhibitors, glinides and thiazolidinediones gradually decreased.
Table 1.
Prescription of initial hypoglycemic agents by patient characteristics
| n | BG | SU | α‐Glucosidase inhibitor | TZD | DPP4 inhibitor | SGLT2 inhibitor | Glinide | GLP‐1 receptor agonist | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall, n (%) | 1,136,723 | 180,194 (15.9) | 46,373 (4.1) | 56,262 (4.9) | 17,758 (1.6) | 739,717 (65.1) | 86,890 (7.6) | 7,505 (0.7) | 2,024 (0.2) | 1,136,723 (100.0) |
| Sex | ||||||||||
| Male | 653,049 | 15.8 | 4.3 | 4.8 | 1.5 | 65.0 | 7.7 | 0.7 | 0.2 | 100.0 |
| Female | 483,674 | 15.9 | 3.8 | 5.1 | 1.6 | 65.1 | 7.6 | 0.6 | 0.2 | 100.0 |
| Age (years) | ||||||||||
| 20–29 | 9,831 | 45.1 | 2.4 | 5.0 | 3.3 | 27.5 | 15.5 | 0.4 | 0.7 | 100.0 |
| 30–39 | 39,431 | 36.8 | 3.3 | 4.3 | 2.3 | 36.8 | 15.6 | 0.4 | 0.5 | 100.0 |
| 40–49 | 123,582 | 25.7 | 3.7 | 4.2 | 1.7 | 48.8 | 15.1 | 0.5 | 0.4 | 100.0 |
| 50–59 | 200,077 | 20.8 | 3.8 | 4.6 | 1.6 | 56.8 | 11.6 | 0.6 | 0.2 | 100.0 |
| 60–69 | 325,490 | 16.3 | 4.1 | 5.1 | 1.6 | 65.4 | 6.7 | 0.7 | 0.1 | 100.0 |
| 70–79 | 290,673 | 9.7 | 4.2 | 5.4 | 1.4 | 74.4 | 4.1 | 0.7 | 0.1 | 100.0 |
| 80–89 | 131,471 | 4.9 | 4.7 | 5.1 | 1.3 | 80.7 | 2.4 | 0.7 | 0.1 | 100.0 |
| ≥90 | 16,168 | 2.9 | 6.4 | 4.5 | 0.8 | 82.6 | 2.0 | 0.6 | 0.2 | 100.0 |
| Insurance | ||||||||||
| NHI | 450,446 | 15.5 | 4.4 | 5.1 | 1.6 | 65.8 | 6.8 | 0.7 | 0.2 | 100.0 |
| HIAJ | 235,128 | 21.3 | 3.6 | 4.5 | 1.7 | 57.4 | 10.8 | 0.6 | 0.2 | 100.0 |
| SHIU | 145,890 | 23.8 | 3.2 | 4.7 | 1.7 | 53.5 | 12.3 | 0.6 | 0.3 | 100.0 |
| MMA | 41,977 | 24.4 | 3.2 | 5.2 | 2.0 | 51.9 | 12.4 | 0.6 | 0.3 | 100.0 |
| MIELSL | 263,282 | 6.0 | 4.6 | 5.2 | 1.3 | 79.2 | 2.9 | 0.7 | 0.1 | 100.0 |
| Facility | ||||||||||
| Non‐JDS certified | 1,015,068 | 15.3 | 4.2 | 4.9 | 1.6 | 65.5 | 7.7 | 0.6 | 0.2 | 100.0 |
| JDS certified | 121,655 | 20.8 | 2.9 | 5.0 | 1.1 | 61.8 | 6.9 | 1.1 | 0.4 | 100.0 |
| No. beds | ||||||||||
| ≤19 | 759,270 | 15.1 | 4.3 | 4.8 | 1.7 | 65.1 | 8.3 | 0.6 | 0.1 | 100.0 |
| 20–99 | 72,422 | 15.2 | 3.8 | 5.7 | 1.3 | 66.8 | 6.6 | 0.5 | 0.2 | 100.0 |
| 100–199 | 100,378 | 16.5 | 3.9 | 5.3 | 1.1 | 66.2 | 6.3 | 0.5 | 0.2 | 100.0 |
| ≥200 | 204,653 | 18.8 | 3.3 | 5.1 | 1.3 | 64.0 | 6.3 | 0.9 | 0.3 | 100.0 |
| Antihypertensives | ||||||||||
| No | 427,380 | 20.0 | 4.1 | 5.3 | 1.5 | 60.3 | 7.7 | 0.8 | 0.2 | 100.0 |
| Yes | 709,343 | 13.4 | 4.1 | 4.7 | 1.6 | 68.0 | 7.6 | 0.6 | 0.1 | 100.0 |
| Lipid‐lowering agents | ||||||||||
| No | 599,511 | 17.4 | 4.6 | 5.1 | 1.3 | 63.6 | 7.0 | 0.8 | 0.2 | 100.0 |
| Yes | 537,212 | 14.1 | 3.5 | 4.8 | 1.9 | 66.7 | 8.4 | 0.6 | 0.1 | 100.0 |
| Ischemic heart disease | ||||||||||
| No | 947,137 | 16.9 | 4.2 | 4.9 | 1.6 | 63.8 | 7.7 | 0.7 | 0.2 | 100.0 |
| Yes | 189,586 | 10.5 | 3.6 | 5.0 | 1.4 | 71.3 | 7.4 | 0.6 | 0.2 | 100.0 |
| Chronic kidney disease | ||||||||||
| No | 1,050,542 | 16.0 | 4.2 | 4.9 | 1.6 | 64.8 | 7.7 | 0.6 | 0.2 | 100.0 |
| Yes | 86,181 | 13.9 | 3.0 | 4.9 | 1.0 | 68.7 | 7.1 | 0.9 | 0.4 | 100.0 |
| Renal transplantation or dialysis | ||||||||||
| No | 1,131,293 | 15.9 | 4.1 | 4.9 | 1.6 | 65.0 | 7.7 | 0.7 | 0.2 | 100.0 |
| Yes | 5,430 | 0.3 | 0.8 | 7.9 | X | 87.8 | Y | 1.1 | 1.8 | 100.0 |
X and Y mean percentages cannot be shown because the numbers were <10. BG, biguanide; DPP4; dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; HIAJ, Health Insurance Association of Japan JDS, Japan Diabetes Society; MAA, Mutual Aid Association; MIELSL, Medical Insurance for the Elderly in the Later Stages of Life; NA, not available; NHI, National Health Insurance; SGLT2, sodium–glucose cotransporter 2; SHIU, Social Health Insurance Unions; TZD, thiazolidinedione.
Figure 2.
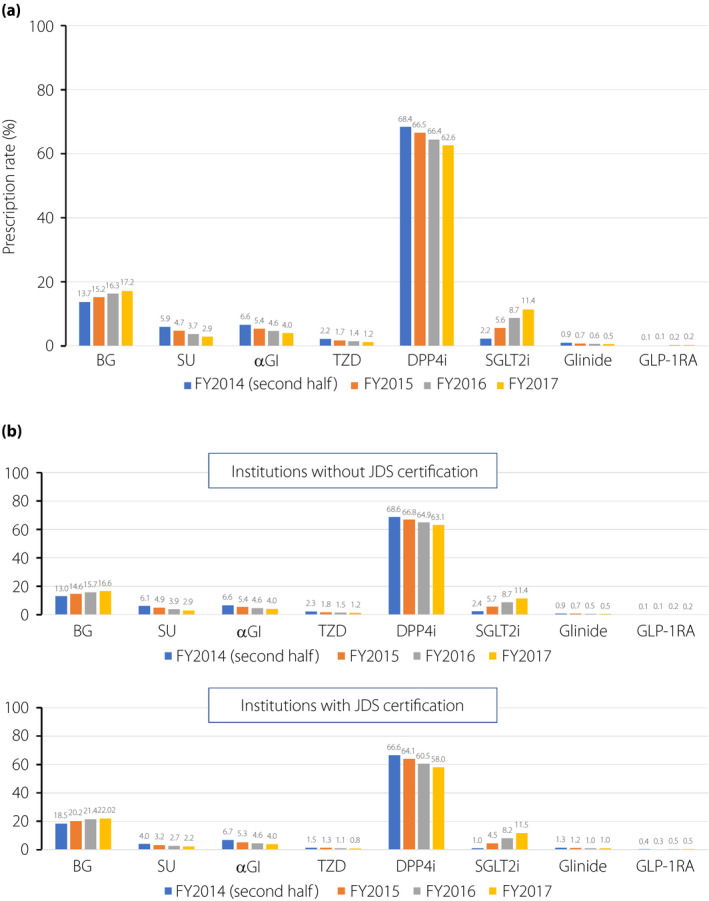
Prescription rate of antidiabetic agents during the study period in (a) the whole population, and (b) among patients cared for at facilities without JDS certification and among those cared for at facilities with JDS certification. αGI, α‐glucosidase inhibitor; BG, biguanide; DPP4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; SGLT2, sodium–glucose cotransporter 2; SU, sulfonylurea; TZD, thiazolidinedione
Initial choice of antidiabetic agents according to the patient characteristics
As shown in Table 1, there were no sex differences in the prescription of antidiabetic agents, whereas age strongly influenced the initial choice of antidiabetic agents. DPP4 inhibitor prescriptions markedly increased with age; conversely, both biguanide and SGLT2 inhibitor prescriptions markedly decreased with age. The prescriptions of sulfonylureas and glinides unexpectedly increased with age. Regarding the initial choice according to the type of insurance, patients who were covered by the Health Insurance Association of Japan, Social Health Insurance Unions or Mutual Aid Association were more likely to receive biguanides than those belonging to the National Health Insurance (NHI). In contrast, the prescription rate of DPP4 inhibitors was lower in those covered by Health Insurance Association of Japan, Social Health Insurance Unions or Mutual Aid Association than in those covered by NHI. Biguanides were more prescribed in the JDS‐certified facilities than in other facilities, and there was a positive association between medical beds and biguanide prescription. In contrast to biguanides, the prescriptions of DPP4 inhibitors, SGLT2 inhibitors and sulfonylureas were lower in the JDS‐certified facilities than in other facilities. There were no differences in prescription trends during the study period by facility characteristics (Figure 2b). For the regional factor, the prescriptions of biguanides and DPP4 inhibitors differed markedly by prefecture, with a maximum of 33.3% (Okinawa) and a minimum of 8.7% (Kagawa) in biguanides, and a maximum of 71.9% (Fukui) and a minimum of 47.2% (Okinawa) in DPP4 inhibitors (Table S1).
We carried out a sensitivity analysis according to the FY and the presence or absence of IHD (Table S2). The prescription rate of SGLT2 inhibitors in patients with IHD rapidly increased by more than sevenfold from FY 2014 to 2017. Furthermore, that in patients without IHD also increased by approximately fivefold. In contrast, the prescription rate of GLP‐1 receptor agonists remained very low throughout the study period regardless of the presence or absence of IHD.
Therefore, we further examined whether the proportion of the prescriptions of biguanides, DPP4 inhibitors, SGLT2 inhibitors and sulfonylureas in each facility differed by facility type. Regarding DPP4 inhibitors in facilities with ≥10 patients (Figure S1a), its proportion as an initial choice was concentrated on 40–70% (the peak was at 60–65%) among the JDS‐certified facilities, whereas other facilities showed a broad distribution (0–100%) with the peak at 95–100%. Focusing on facilities with more patients (Figure S1b–d), the DPP4 inhibitor prescription rate distribution in the non‐JDS‐certified facilities became more similar to that in the JDS‐certified facilities. As for biguanides (Figure S2a), the shape of the distribution was almost similar to that of DPP4 inhibitors in the JDS‐certified facilities, with the peak at 15–20%. In contrast, the distribution of the proportion of biguanides was strongly inclined toward 0% (biguanide was not prescribed at all in 38.2% of the facilities) in other facilities with ≥10 patients (Figure S2a) as the threshold number of patients for including the facilities increased (Figure S2b–2d), the proportion of facilities with no biguanide prescriptions decreased. As shown in Figures S3 and S4, the initial prescription rates of SGLT2 inhibitors and sulfonylureas were almost similar between the JDS‐certified facilities and others, regardless of the number of patients. A similar result was obtained in the sensitivity analysis, excluding patients aged ≥ 65 years and those with advanced kidney disease (Figure S5).
Factors associated with prescriptions of biguanides, DPP4 inhibitors, SGLT2 inhibitors and sulfonylureas
Table 2 shows the factors associated with biguanide, DPP4 inhibitor, SGLT2 inhibitor and sulfonylurea prescriptions in the multivariable logistic regression analysis. FY was positively associated with prescriptions of biguanides and SGLT2 inhibitors, whereas it was inversely associated with those of DPP4 inhibitors and sulfonylureas. Women were more likely to be initially treated with biguanides or SGLT2 inhibitors and were less likely to be treated with DPP4 inhibitors or sulfonylureas than men. The presence of cardiometabolic risk factors (hypertension and dyslipidemia) increased SGLT2 inhibitor prescriptions by 30%. The presence of IHD and CKD reduced sulfonylurea prescriptions by 14% and 29%, respectively. Biguanides and SGLT2 inhibitors were 1.3‐ and 1.1‐fold more likely to be prescribed in the JDS‐certified facilities than in the other facilities.
Table 2.
Factors associated with the initial choice of biguanide, dipeptidyl peptidase‐4 inhibitor, sodium–glucose cotransporter 2 inhibitor and sulfonylurea in the multivariable logistic regression analysis
| Biguanide | DPP4 inhibitor | SGLT2 inhibitor | Sulfonylurea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR† | 95% CI | OR† | 95% CI | OR† | 95% CI | OR† | 95% CI | |||||
| Fiscal year (ref: FY2014 second half) | ||||||||||||
| FY 2015 | 1.07 | 1.06 | 1.09 | 0.96 | 0.95 | 0.97 | 2.51 | 2.43 | 2.61 | 0.79 | 0.77 | 0.81 |
| FY 2016 | 1.12 | 1.11 | 1.14 | 0.90 | 0.89 | 0.92 | 3.91 | 3.77 | 4.05 | 0.63 | 0.61 | 0.64 |
| FY 2017 | 1.21 | 1.18 | 1.23 | 0.83 | 0.82 | 0.84 | 5.34 | 5.16 | 5.53 | 0.48 | 0.47 | 0.49 |
| Sex (ref: male) | ||||||||||||
| Female | 1.18 | 1.17 | 1.19 | 0.87 | 0.87 | 0.88 | 1.18 | 1.16 | 1.19 | 0.87 | 0.86 | 0.89 |
| Antihypertensives (yes vs no) | 0.91 | 0.89 | 0.92 | 1.01 | 1.01 | 1.02 | 1.36 | 1.33 | 1.38 | 0.98 | 0.96 | 1.00 |
| Lipid‐lowering agents (yes vs no) | 0.93 | 0.92 | 0.94 | 1.00 | 0.99 | 1.01 | 1.35 | 1.33 | 1.37 | 0.74 | 0.73 | 0.76 |
| Ischemic heart disease (yes vs no) | 0.84 | 0.82 | 0.85 | 1.04 | 1.02 | 1.05 | 1.29 | 1.26 | 1.32 | 0.86 | 0.84 | 0.89 |
| Chronic kidney disease (yes vs no) | 0.99 | 0.97 | 1.01 | 1.06 | 1.04 | 1.07 | 1.03 | 1.00 | 1.06 | 0.71 | 0.68 | 0.74 |
| Facility (JDS certified vs non‐certified) | 1.30 | 1.28 | 1.33 | 0.85 | 0.84 | 0.87 | 1.10 | 1.06 | 1.14 | 0.78 | 0.74 | 0.81 |
| Category of hospital beds (ref: <19) | ||||||||||||
| 20–99 | 1.05 | 1.03 | 1.07 | 1.03 | 1.01 | 1.05 | 0.84 | 0.81 | 0.86 | 0.89 | 0.85 | 0.92 |
| 100–199 | 1.14 | 1.12 | 1.17 | 1.02 | 1.01 | 1.04 | 0.77 | 0.75 | 0.79 | 0.90 | 0.87 | 0.93 |
| ≥200 | 1.14 | 1.12 | 1.16 | 1.04 | 1.03 | 1.06 | 0.70 | 0.68 | 0.72 | 0.85 | 0.82 | 0.88 |
| Age category × type of insurance (ref: 20’s × National Health Insurance and others) | ||||||||||||
| 20's × Health Insurance Association of Japan | 1.29 | 1.16 | 1.44 | 0.79 | 0.71 | 0.89 | 0.95 | 0.83 | 1.10 | 0.74 | 0.55 | 1.01 |
| 20’s × Social Health Insurance Unions | 1.34 | 1.20 | 1.50 | 0.79 | 0.70 | 0.89 | 0.99 | 0.84 | 1.15 | 0.55 | 0.39 | 0.79 |
| 20’s × Mutual Aid Association | 1.58 | 1.34 | 1.86 | 0.58 | 0.47 | 0.70 | 0.81 | 0.64 | 1.03 | 0.55 | 0.31 | 0.96 |
| 30’s × National Health Insurance and others | 0.74 | 0.67 | 0.82 | 1.45 | 1.31 | 1.60 | 0.94 | 0.83 | 1.07 | 1.25 | 0.97 | 1.61 |
| 30’s × Health Insurance Association of Japan | 0.94 | 0.85 | 1.03 | 1.21 | 1.10 | 1.34 | 0.91 | 0.80 | 1.03 | 1.06 | 0.82 | 1.35 |
| 30’s × Social Health Insurance Unions | 1.04 | 0.95 | 1.15 | 1.13 | 1.03 | 1.25 | 0.92 | 0.81 | 1.04 | 0.80 | 0.62 | 1.03 |
| 30’s × Mutual Aid Association | 1.23 | 1.09 | 1.38 | 0.93 | 0.83 | 1.05 | 0.91 | 0.78 | 1.06 | 0.65 | 0.46 | 0.91 |
| 40’s × National Health Insurance and others | 0.52 | 0.47 | 0.56 | 2.17 | 1.98 | 2.38 | 0.73 | 0.65 | 0.83 | 1.48 | 1.16 | 1.87 |
| 40’s × Health Insurance Association of Japan | 0.59 | 0.54 | 0.64 | 1.95 | 1.78 | 2.14 | 0.80 | 0.71 | 0.90 | 1.14 | 0.90 | 1.44 |
| 40’s × Social Health Insurance Unions | 0.65 | 0.59 | 0.71 | 1.85 | 1.69 | 2.03 | 0.82 | 0.73 | 0.93 | 0.88 | 0.69 | 1.12 |
| 40’s × Mutual Aid Association | 0.66 | 0.60 | 0.72 | 1.69 | 1.53 | 1.87 | 0.87 | 0.76 | 0.99 | 0.96 | 0.74 | 1.25 |
| 50's × National Health Insurance and others | 0.42 | 0.38 | 0.46 | 2.87 | 2.62 | 3.15 | 0.50 | 0.45 | 0.57 | 1.50 | 1.18 | 1.90 |
| 50’s × Health Insurance Association of Japan | 0.45 | 0.41 | 0.49 | 2.74 | 2.50 | 3.00 | 0.53 | 0.47 | 0.60 | 1.20 | 0.95 | 1.52 |
| 50’s × Social Health Insurance Unions | 0.49 | 0.45 | 0.54 | 2.56 | 2.33 | 2.80 | 0.57 | 0.51 | 0.65 | 0.98 | 0.77 | 1.24 |
| 50’s × Mutual Aid Association | 0.48 | 0.44 | 0.53 | 2.48 | 2.26 | 2.73 | 0.60 | 0.53 | 0.67 | 1.02 | 0.80 | 1.31 |
| 60's × National Health Insurance and others | 0.33 | 0.30 | 0.36 | 3.99 | 3.65 | 4.37 | 0.26 | 0.23 | 0.29 | 1.41 | 1.12 | 1.78 |
| 60’s × Health Insurance Association of Japan | 0.36 | 0.33 | 0.39 | 3.74 | 3.42 | 4.10 | 0.32 | 0.28 | 0.36 | 1.20 | 0.95 | 1.52 |
| 60’s × Social Health Insurance Unions | 0.39 | 0.36 | 0.43 | 3.54 | 3.22 | 3.89 | 0.33 | 0.29 | 0.37 | 1.05 | 0.83 | 1.34 |
| 60’s × Mutual Aid Association | 0.40 | 0.36 | 0.45 | 3.26 | 2.94 | 3.61 | 0.37 | 0.32 | 0.43 | 1.08 | 0.83 | 1.41 |
| 60’s × Medical insurance for the elderly | 0.20 | 0.17 | 0.23 | 5.76 | 5.08 | 6.53 | 0.17 | 0.13 | 0.21 | 1.24 | 0.91 | 1.70 |
| 70’s × National Health Insurance and others | 0.22 | 0.20 | 0.24 | 5.49 | 5.01 | 6.02 | 0.17 | 0.15 | 0.19 | 1.36 | 1.07 | 1.72 |
| 70’s × Health Insurance Association of Japan | 0.23 | 0.21 | 0.25 | 5.30 | 4.81 | 5.83 | 0.21 | 0.18 | 0.24 | 1.32 | 1.03 | 1.68 |
| 70’s × Social Health Insurance Unions | 0.22 | 0.19 | 0.25 | 5.64 | 5.02 | 6.33 | 0.18 | 0.15 | 0.22 | 1.29 | 0.97 | 1.71 |
| 70s × Mutual Aid Association | 0.26 | 0.22 | 0.32 | 5.01 | 4.28 | 5.87 | 0.18 | 0.13 | 0.25 | 1.08 | 0.72 | 1.62 |
| 70s × Medical insurance for the elderly | 0.15 | 0.14 | 0.16 | 6.92 | 6.32 | 7.59 | 0.13 | 0.11 | 0.15 | 1.36 | 1.08 | 1.72 |
| 80s × Medical insurance for the elderly | 0.09 | 0.09 | 0.10 | 8.57 | 7.82 | 9.39 | 0.09 | 0.08 | 0.10 | 1.59 | 1.26 | 2.01 |
| >90s × Medical insurance for the elderly | 0.05 | 0.05 | 0.06 | 9.85 | 8.92 | 10.88 | 0.07 | 0.06 | 0.08 | 2.19 | 1.72 | 2.79 |
†The multivariate models include prefecture as a covariate in addition to covariates above listed. CI, confidence interval; DPP4; dipeptidyl peptidase‐4; FY, fiscal year; JDS, Japan Diabetes Society; OR, odds ratio; SGLT2, sodium–glucose cotransporter 2.
Meanwhile, DPP4 inhibitors and sulfonylureas were less likely to be prescribed in the JDS‐certified facilities. The number of beds was positively associated with a biguanide prescription, and was negatively associated with SGLT2 inhibitor and sulfonylurea prescriptions. Regarding the age category and type of insurance, the older the patients, the higher the number of DPP4 inhibitor and sulfonylurea prescriptions, and the lower the number of biguanide and SGLT2 inhibitor prescriptions. In the same age group, patients belonging to Health Insurance Association of Japan, Social Health Insurance Unions and Mutual Aid Association were more likely to be treated with biguanides and SGLT2 inhibitors, and were less likely to be treated with DPP4 inhibitors and sulfonylureas than those belonging to NHI. We obtained the predicted proportions by prefectures from the regression models (Table S1). Compared with the crude proportions, the regional differences were slightly attenuated, but they remained unchanged. The absolute differences between maximum and minimum among prefectures remained approximately 5% in sulfonylureas and SGLT2 inhibitors.
Total medical costs for 1 year after administration of antidiabetic agents
In a subset of patients with at least 12 months of follow up (Table 3), the median of the total medical costs was the lowest in biguanides, followed by those in sulfonylureas and thiazolidinediones. GLP‐1 receptor agonists were shown to be the most expensive. After adjusting for covariates, biguanides remained the cheapest, followed by thiazolidinediones and α‐glucosidase inhibitors. GLP‐1 receptor agonists remained to be the most expensive drug. Table S3 shows factors associated with total medical costs in the first year after administering antidiabetic agents in the multiple regression analysis. Compared with patients with biguanides, patients with antidiabetic agents other than thiazolidinediones incurred significantly higher total medical costs. Despite the increase in prescriptions of newer class drugs, such as SGLT2 inhibitors (Figure 2 and Table S2), the more the FY progressed, the lower the total medical costs became. Patients belonging to the medical insurance for the elderly in the later stages of life had significantly higher medical costs than those belonging to the other insurances. In the same age group, patients belonging to the NHI generally required higher medical costs than those belonging to other insurances. The presence of both IHD and CKD significantly increased total medical costs by approximately 30% and 60%, respectively. The difference in total medical costs between prefectures remained <10%. Patients in large hospitals or the JDS‐certified facilities incurred higher medical costs than those who visited the clinics or non‐certified facilities. Compared with the vast differences in the use of antidiabetic agents between prefectures (Table S1), regional differences for the total medical costs were smaller (Table S3).
Table 3.
Total medical costs in the first year after administration of antidiabetic agents
| Antidiabetic agents | n | Mean | 10th percentile | 25th percentile | Median | 75th percentile | 90th percentile | Point estimates with 95% CI† |
|---|---|---|---|---|---|---|---|---|
| Biguanides | 97,549 | 393,441 | 104,860 | 153,480 | 225,970 | 360,850 | 678,850 | 480,323 (475,398−485,248) |
| Sulfonylureas | 30,016 | 534,593 | 111,090 | 165,175 | 255,630 | 464,235 | 1,052,100 | 545,580 (535,703−555,456) |
| α‐Glucosidase inhibitors | 35,289 | 551,680 | 123,290 | 185,590 | 284,000 | 484,430 | 1,031,470 | 523,887 (515,288−532,487) |
| Thiazolidinediones | 11,438 | 452,043 | 115,9.0 | 170,990 | 258,025 | 425,0.0 | 816,930 | 483,632 (470,768−496,496) |
| DPP4 inhibitors | 428,041 | 608,414 | 155,290 | 214,460 | 313,760 | 529,790 | 1,162,160 | 578,220 (574,768−581,673) |
| SGLT2 inhibitors | 37,556 | 435,535 | 145,480 | 210,480 | 292,030 | 433,515 | 713,820 | 557,434 (548,587−566,280) |
| Glinides | 4,651 | 715,460 | 147,840 | 211,720 | 323,740 | 592,830 | 1,531,090 | 650,230 (617,595−682,865) |
| GLP‐1 receptor agonists | 953 | 968,503 | 228,430 | 349,100 | 520,150 | 793,250 | 1,982,170 | 890,252 (780,013−1,000,491) |
The total medical costs are shown in Japanese yen. Covariates: fiscal year, sex, the combination of age categories and type of insurance, use of antihypertensives, use of lipid‐lowering agents, ischemic heart disease, chronic kidney disease, prefecture, the Japan Diabetes Society‐certified facility, category of beds. †Point estimates were calculated adjusting for covariates listed below. CI, confidence interval; DPP4; dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; SGLT2, sodium–glucose cotransporter 2.
DISCUSSION
We showed trends in first‐line antidiabetic agents and subsequent medical costs in the first year in Japanese patients with type 2 diabetes using the NDB. The main findings are as follows: (i) DPP4 inhibitors were the most prescribed; (ii) total medical costs were lowest in patients who started biguanide; and (iii) there were clear differences in the initial choice of antidiabetic agents, especially biguanides and DPP4 inhibitors, by prefecture and by type of facilities in addition to age and comorbidities.
In the present study involving >1,000,000 Japanese patients with type 2 diabetes, DPP4 inhibitors were most prescribed, followed by biguanides and SGLT2 inhibitors. There are some possible reasons for the extremely high prescription rate of DPP4 inhibitors in Japan. DPP4 inhibitors can reduce glycated hemoglobin (HbA1C) levels more effectively in East Asian people, including Japanese people, than in people in European and North American countries 15 , 21 , 22 . Lower insulin secretion and adiposity (less insulin resistance) in Japanese paople 16 , and different genetic backgrounds between Japanese and European people 17 might also influence the choice of DPP4 inhibitors. Furthermore, the risk for severe hypoglycemia is extremely low when patients receive DPP4 inhibitors alone. As more than half of Japanese patients with type 2 diabetes are aged ≥65 years 23 , the JDS has attached great importance to the safety of antidiabetic agents, and the proper use of biguanides and SGLT2 inhibitors, including special attention for the elderly, is noted in their recommendations 24 , 25 . The inverse association of age with biguanides and SGLT2 inhibitors in the present study suggests that these agents were appropriately used from the safety point of view according to the JDS recommendations, and instead, starting DPP4 inhibitors might have been selected especially for the elderly. For the efficacy of DPP4 inhibitors in Japan, Torii et al. reported that the hazard of treatment intensification and risk for developing HbA1C >7% were lower in DPP4 inhibitors than in biguanides for treatment‐naïve type 2 diabetes patients 26 . Thus, the aging of the population of diabetes patients, insufficient insulin secretion and low adiposity, genetic background in Japanese people, and the efficacy and safety of DPP4 inhibitors for Asian people might have affected the physicians’ decision to select DPP4 inhibitors as the first‐line for Japanese patients with type 2 diabetes.
It is also an important finding that biguanide prescription has steadily increased during the study period. In Japan, starting biguanides had not been recommended for patients aged ≥75 years until 2014, but the JDS allowed the administration of metformin with careful consideration for these patients in 2014, which might have reassured physicians to start metformin for elderly patients with type 2 diabetes, presumably increasing the biguanide prescription.
We found a clear difference in biguanide and DPP4 inhibitor prescriptions by facility type. As many board‐certified diabetologists belong to JDS‐certified facilities, biguanides could be selected as a candidate for the first‐line therapy, with careful attention to their side‐effects, including the risk for lactic acidosis and the patient characteristics, including age and renal function in the JDS‐certified facilities. In contrast, physicians in the other facilities were less likely to select biguanides, probably due to the aforementioned burden of biguanides and the necessity of taking them twice or three times a day (extended‐release tablets were not available in Japan during the study period). Furthermore, some might not have had biguanides in mind as candidates for initiating antidiabetic agents, and instead selected DPP4 inhibitors due to their ease‐of‐use (efficacy and safety) relative to biguanides.
Metformin, the most prescribed biguanide in the present study, is effective, inexpensive and might reduce the risk of cardiovascular events and death; however, evidence has not been fully established regarding the effects of metformin on patient‐important outcomes 27 . Ideally, head‐to‐head large‐scale, long‐term randomized controlled trials should be carried out to clarify which medication is efficacious for glycemic control and to prevent complications, especially among East Asian populations.
Furthermore, it is expected that real‐world data to confirm evidence obtained from the randomized controlled trials will be accumulated by the long‐term follow up of the NDB and other databases 28 . The results of these studies will lead to improved guidelines for personalized diabetes care. To close the gap in the prescription patterns between the JDS‐certified and non‐certified facilities, it is of utmost importance to disseminate the “tailored therapy” concept described in the guidelines 18 . To facilitate the tailored therapy, an easy‐to‐understand guide might be warranted.
The remarkable increase in SGLT2 inhibitor prescription regardless of the presence or absence of IHD is an intriguing finding. A recent meta‐analysis of randomized controlled trials showed robust benefits of SGLT2 inhibitors in reducing risks for heart failure and kidney disease regardless of existing CVD 29 . Furthermore, in a multinational cohort study including Japanese participants, CKD was reported to be the most frequent first cardiorenal event followed by heart failure in type 2 diabetes patients 30 . These data would support the remarkable increase in SGLT2 inhibitor prescription regardless of IHD for preventing CKD and heart failure in the present study. In contrast, GLP‐1 receptor agonists remained the least prescribed regardless of IHD. The main reason is that only GLP‐1 receptor agonists for subcutaneous injection were available in Japan during the study period. Therefore, most physicians might have considered GLP‐1 receptor agonists as a second‐line or later‐line therapy after the initial choice of biguanides or SGLT2 inhibitors, or as an alternative drug to DPP4 inhibitors in case adequate glycemic control was not achieved.
Regarding geographical variation, there were significant regional differences in the prescriptions of biguanides and DPP4 inhibitors. In particular, prescriptions in Okinawa were distinctive (biguanide was more frequently prescribed, whereas DPP4 inhibitor was less frequently prescribed than that in other prefectures). These findings are consistent with a previous report from England 31 . The high prevalence of obesity 32 and the low wage 33 in Okinawa compared with those in other prefectures might influence physicians’ choice of antidiabetic drugs, leading to the high prescription rate of biguanides.
We also found that patients belonging to NHI were more likely to be treated with DPP4 inhibitors and sulfonylureas, and were less likely to be treated with biguanides and SGLT2 inhibitors than those belonging to other insurances. As NHI covers the self‐employed, unemployed and retired persons aged ≤75 years, the percentage of healthy workers who are considered as good candidates for metformin and SGLT2 inhibitors enrolled in the NHI might be low compared with that in other insurances, presumably affecting the drug selection.
A previous retrospective study from the USA found evidence of increasing comorbidities paralleled by large increases in costs for medical services, but less for prescriptions in patients with type 2 diabetes 34 . We found that patients with biguanides had the lowest total medical expenses, even after adjusting for age and covariates, including IHD and CKD, which might show the superiority of biguanides over other drugs from a health economics perspective. Patients taking newer antidiabetic drugs (DPP4 inhibitors, SGLT2 inhibitors and GLP‐1 receptor agonists) incurred higher costs than patients taking biguanides. Considering the present data and the limited evidence in cardiorenal protection for these drugs in Asian patients to date, it would be reasonable to consider biguanides' use in favor of the expected benefits, safety and total medical costs.
We evaluated almost all people who had received antidiabetic agents in Japan during the study period. As a result, the number of patients receiving antidiabetic medication counted in the NDB was approximately 7.5 million in 2015 35 , which is larger than the number of patients receiving antidiabetic medication estimated from the National Health and Nutrition Survey (~5.2 million) 36 . Therefore, whereas the National Health and Nutrition Survey has been used as the official data source of diabetes prevalence in Japan, we suppose that the NDB might be a more accurate data source than the National Health and Nutrition Survey in terms of antidiabetic medication prevalence, owing to its comprehensive data collection and supposedly accurate prescription information, although the problem of patient identification technique should be addressed.
The present study also had some limitations. First, the definition of diabetes was based on diagnosis codes and medication on the claims. Second, the NDB did not include any laboratory data, including HbA1C. Third, we were unable to determine the occurrence of CVD and include its cost in the total medical expenses. Fourth, data regarding safety (i.e., episodes of hypoglycemia) were unavailable. Fifth, information on obesity was unavailable. Finally, the target HbA1C levels, patient preference and comorbidities could have affected the physicians’ decision for multidrug diabetes treatment.
In conclusion, DPP4 inhibitor is the most commonly prescribed first‐line medication for type 2 diabetes patients in Japan, although there is a wide variation in the drug choice by facility type and prefecture. Further studies are required to clarify which medication is efficacious for glycemic control and the prevention of individual Japanese patients' complications, and improving the guideline for personalized diabetes care.
DISCLOSURE
The authors declare that they have no conflict of interest concerning this manuscript.
Supporting information
Appendix S1 | Supporting information on methods
Table S1 | Initial prescriptions of biguanides, dipeptidyl peptidase‐4 inhibitors, sodium–glucose cotransporter 2 inhibitors and sulfonylureas by prefecture
Table S2 | Prescriptions of sodium–glucose cotransporter 2 inhibitors and glucagon‐like peptide‐1 receptor agonists among patients with and without ischemic heart disease
Table S3 | Factors associated with total medical costs in the first year after the administration of antidiabetic agents
Figure S1 | Comparison of the dipeptidyl peptidase‐4 inhibitor prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society certified facilities and others.
Figure S2 | Comparison of the biguanide prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S3 | Comparison of the sodium–glucose cotransporter 2 inhibitor prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S4 | Comparison of the sulfonylurea prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S5 | Comparison of the distribution of prescription rates as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others with ≥10 patients, including only patients aged <65 years who neither had chronic kidney disease nor underwent dialysis.
Acknowledgments
We thank Dr Yasuyuki Okumura at the Initiative for Clinical Epidemiological Research, Tokyo, Japan, for his contribution to designing this study. This study was supported by Health and Labor Sciences Research Grants (Comprehensive Research on Life‐Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, 20FA1016). The funding agency had no role in the design or conduct of the study; collection, management, analysis and interpretation of data, preparation, review or approval of the manuscript, and the decision to submit the manuscript for publication.
J Diabetes Investig 2022; 13: 280–291
Contributor Information
Ryotaro Bouchi, Email: rybouchi@hosp.ncgm.go.jp.
Takehiro Sugiyama, Email: tsugiyama@hosp.ncgm.go.jp.
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas; 157. 9th. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 2. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008; 26: 77–82. [Google Scholar]
- 3. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the cross‐national Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013; 36: 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61: 2461–2498. [DOI] [PubMed] [Google Scholar]
- 6. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 12. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 13. Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buse JB, Wexler DJ, Tsapas A, et al. Update to: Management of hyperglycemia in Type 2 diabetes, 2019. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020; 63: 221–228. [DOI] [PubMed] [Google Scholar]
- 15. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huxley R, James WP, Barzi F, et al. Obesity in Asia Collaboration. Ethnic comparisons of the cross‐sectional relationships between measures of body size with diabetes and hypertension. Obes Rev 2008; 9(Suppl 1): 53–61. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki K, Akiyama M, Ishigaki K, et al. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet 2019; 51: 379–386. [DOI] [PubMed] [Google Scholar]
- 18. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int 2018; 9: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohro T, Yamazaki T, Sato H, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J 2013; 54: 93–97. [DOI] [PubMed] [Google Scholar]
- 20. Ihana‐Sugiyama N, Sugiyama T, Tanaka H, et al. Comparison of effectiveness and drug cost between dipeptidyl peptidase‐4 inhibitor and biguanide as the first‐line anti‐hyperglycaemic medication among Japanese working generation with type 2 diabetes. J Eval Clin Pract 2020; 26: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: A systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 22. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: Focus on East Asian perspectives. J Diabetes Investig 2016; 7(Suppl 1): 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.[Cited April 23, 2021] Available from: http://jddm.jp/data/index‐2018/.
- 24.[Cited April 23, 2021] Available from: http://www.fa.kyorin.co.jp/jds/uploads/recommendation_metformin.pdf.
- 25. The Committee on the Proper Use of SGLT2 Inhibitors . Recommendations on the proper use of SGLT2 inhibitors. Diabetol Int 2020; 11: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horii T, Iwasawa M, Shimizu J, et al. Comparing treatment intensification and clinical outcomes of metformin and dipeptidyl peptidase‐4 inhibitors in treatment‐naïve patients with type 2 diabetes in Japan. J Diabetes Investig 2020; 11: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gnesin F, Thuesen ACB, Kähler LKA, et al. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2020; 6: CD012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugiyama T, Miyo K, Tsujimoto T, et al. Design of and rationale for the Japan Diabetes compREhensive database project based on an Advanced electronic Medical record System (J‐DREAMS). Diabetol Int 2017; 8: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 30. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab 2020; 22: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curtis HJ, Dennis JM, Shields BM, et al. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes Metab 2018; 20: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willcox DC, Willcox BJ, Yasura S, et al. Gender gap in healthspan and life expectancy in Okinawa: health behaviours. Asian J Gerontol Geriatr 2012; 7: 49–58. [Google Scholar]
- 33.[Cited April 23, 2021] Available from: https://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2018/dl/08.pdf.
- 34. Weng W, Liang Y, Kimball ES, et al. Longitudinal changes in Medical Services and related costs in a single cohort of patients newly diagnosed with Type 2 diabetes, 2006 to 2012. Clin Ther 2016; 38: 1314–1326. [DOI] [PubMed] [Google Scholar]
- 35. Sugiyama T, Imai K, Ihana‐Sugiyama N, et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015–2016: An observational study of nationwide claims data. Diabetes Res Clin Pract 2019; 155: 107750. [DOI] [PubMed] [Google Scholar]
- 36.[Cited April 23, 2021] Available from: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28‐houkoku‐05.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Supporting information on methods
Table S1 | Initial prescriptions of biguanides, dipeptidyl peptidase‐4 inhibitors, sodium–glucose cotransporter 2 inhibitors and sulfonylureas by prefecture
Table S2 | Prescriptions of sodium–glucose cotransporter 2 inhibitors and glucagon‐like peptide‐1 receptor agonists among patients with and without ischemic heart disease
Table S3 | Factors associated with total medical costs in the first year after the administration of antidiabetic agents
Figure S1 | Comparison of the dipeptidyl peptidase‐4 inhibitor prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society certified facilities and others.
Figure S2 | Comparison of the biguanide prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S3 | Comparison of the sodium–glucose cotransporter 2 inhibitor prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S4 | Comparison of the sulfonylurea prescription rate distribution as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others.
Figure S5 | Comparison of the distribution of prescription rates as the first‐line antidiabetic medication by facilities between the Japan Diabetes Society‐certified facilities and others with ≥10 patients, including only patients aged <65 years who neither had chronic kidney disease nor underwent dialysis.


