Abstract
Aims/Introduction
This randomized controlled trial aimed to determine whether frequent nutritional education improves the clinical parameters associated with the onset and progression of diabetic kidney disease in type 2 diabetes mellitus patients.
Materials and Methods
A total of 96 patients with type 2 diabetes and diabetic kidney disease were randomly assigned to the intensive intervention group that received nutritional education at every outpatient visit, and the usual intervention group that received nutritional education once a year. The anthropometric parameters, blood pressure, blood chemistry, albuminuria, protein and salt intake, and prescribed medications of 87 patients who completed the 2‐year follow up were analyzed.
Results
In the intensive intervention group, body mass index and salt intake significantly decreased over the study period. Hemoglobin A1c levels and body fat percentage were significantly lower in the intensive intervention group than in the usual intervention group. At the end of the 2‐year intervention period, the intensive intervention group had significantly lower salt intake (8.1 vs 9.4 g/day) than the usual intervention group. A significant positive correlation was found between salt intake and albuminuria in the overall group and intensive intervention group (r = 0.26, P = 0.02, and r = 0.36, P = 0.02, respectively). The intensive intervention group had a significantly lower insulin use rate than the usual intervention group after the 2‐year intervention period (18% vs 42%). No differences were found in estimated glomerular filtration rate and albuminuria.
Conclusion
Intensive nutritional education is useful for alleviating the risk factors associated with the onset and progression of diabetic kidney disease.
Keywords: Body composition, Hemoglobin A1c, Nutritional education
Frequent nutritional education were effective in decreasing body mass index, salt intake, hemoglobin A1c and body fat percentage. Salt intake was significantly associated with albuminuria.

Introduction
Type 2 diabetes is a global pandemic. In Japan, the estimated number of both patients with diabetes and prediabetes is approximately 10 million 1 . In addition, >35,000 patients with chronic kidney disease (CKD) undergo renal replacement therapy every year, and >40% of them have CKD caused by diabetes mellitus (diabetic nephropathy) 2 . Therefore, prevention of the onset and progression of DN is urgently required to reduce the number of newly introduced dialysis patients.
In patients with diabetes, the occurrence of microalbuminuria indicates the progression to early nephropathy, and when it progresses to overt proteinuria, renal function declines at an accelerated rate, leading to end‐stage renal failure within a short period of time 3 , 4 , 5 . In a previous cross‐sectional study of patients with type 2 diabetes, Asian and Hispanic patients had a higher incidence of microalbuminuria (44 and 46%, respectively) and overt proteinuria (10 and 12%, respectively) 6 . According to the results of the United Kingdom Prospective Diabetes Study (UKPDS 64), 2.0% of patients developed microalbuminuria, whereas 2.8% of patients with microalbuminuria developed overt proteinuria 1 year after the diagnosis of diabetes mellitus 7 . A total of 10 years after the diagnosis of diabetes mellitus, 24.9% and 5.3% of the patients developed microalbuminuria and overt proteinuria, respectively 7 . Given this background, it is important to determine the risk factors for developing microalbuminuria and overt proteinuria in patients with diabetes in Japan. We previously analyzed the nutritional factors associated with the onset and progression of diabetic kidney disease (DKD) in a retrospective study of 71 patients with type 2 diabetes 8 . The results showed that obesity, dyslipidemia, hyperuricemia, arteriosclerosis and excessive salt intake were significantly associated with the onset and progression of type 2 diabetic nephropathy. Arteriosclerosis, hyperuricemia and excessive salt intake are independent risk factors for the development and progression of nephropathy 8 .
The education of patients is an important step in achieving better control of chronic diseases, such as hypertension 9 , 10 . In terms of the efficacy of nutritional education on glycemic control, results of a previous meta‐analysis of randomized controlled trials (RCTs) showed that >11 h of behavioral therapy significantly improved the hemoglobin A1c (HbA1c) levels compared with a regular intervention 11 . The Frontier of Renal Outcome Modifications in Japan (From‐J) study 12 , an RCT carried out in Japan, compared the effects of an intensive intervention (in which family physicians and non‐nephrologists prescribed a regular diet therapy formulated by a dietitian to patients with additional support to promote consultation of interrupted patients) with those of a regular intervention (in which family physicians and non‐nephrologists provided medical care in accordance with the CKD Clinical Practice Guidelines without additional support). Intensive intervention significantly improved patients’ behavioral change indicators, such as continued consultation rates, and the physicians’ behavioral change indicators, such as the rate of co‐treatment based on referral to specialists and re‐referral back to general practitioners. Furthermore, the effect of encouraging patients to change their behavior was superior to that of changing the prescriptions of CKD stage G3 patients in the intensive intervention group, in terms of preventing an estimated glomerular filtration rate (eGFR) decline and significant weight loss, and improving glycemic and blood pressure control. In a 2‐year RCT of patients with type 2 diabetic nephropathy (CKD stages 3–4), Fogelfeld et al. 13 showed that a team‐based intervention involving endocrinologists, nephrologists, nurses and dieticians inhibited the progression of CKD stages 3–4 to end‐stage renal failure, and improved the HbA1c level and urinary albumin/creatinine ratio. To our knowledge, no study has evaluated the effect of nutritional interventions provided by dietitians on the nutritional factors in patients with type 2 diabetes or DKD.
Thus, we carried out a study of clinical parameters associated with diabetic nephropathy in patients with type 2 diabetes mellitus (SUCCEED). In the SUCCEED trial, we aimed to determine whether frequent nutritional education by dietitians can improve the clinical, physical and nutritional parameters associated with the development and worsening of DKD, and to identify which clinical parameters are associated with changes in nutritional factors.
Materials and methods
Patients
Patients with type 2 diabetes mellitus and DKD (CKD stage G1–3), aged ≥20 years, who were examined in the Division of Endocrinology and Metabolism and the Division of Nephrology in Jichi Medical University Hospital, Shimotsuke, Japan, between May 2013 and October 2016, and who did not receive nutritional education in the past 5 years were included in the present study. The clinical diagnosis of DKD was on the basis of measurement of eGFR and albuminuria. DKD was clinically defined by a persistently high urinary albumin‐to‐creatinine ratio ≥30 mg/g and/or sustained reduction in eGFR <60 mL/min/1.73 m2 2 , 14 . Of the 127 patients who met the enrollment criteria, and received an explanation regarding the purpose and nature of the study, just 102 provided informed consent.
After excluding five patients who withdrew their consent and one patient who had membranous nephropathy as a primary disease, 96 patients were finally enrolled in the study. The patients were randomly assigned into two groups: (i) an intensive intervention group that received nutritional education by a dietitian at each outpatient visit; and (ii) a control group that received nutritional education by a dietitian once a year. After excluding an additional nine patients (intensive intervention group [INT]: five patients who refused to receive nutritional education; control group [CON]: one patient who refused to receive nutritional education, one patient who did not visit the hospital, one patient who was transferred to another hospital and one patient who did not receive a request for nutritional education from a physician), only 44 patients in the INT group and 43 patients in the CON group who completed the 2‐year follow up were analyzed in an RCT (Figure 1).
Figure 1.
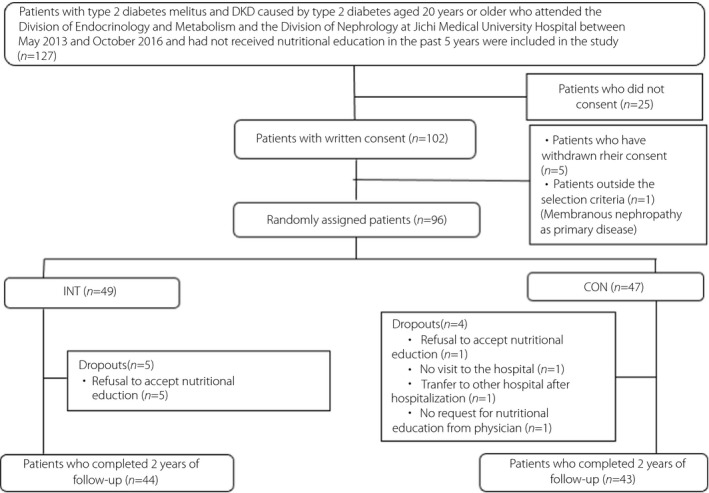
Process of patient selection in this study. Of the 127 patients who visited the Jichi Medical School Hospital, met the enrollment criteria and were provided with an explanation for obtaining consent, just 102 were able to provide consent. A total of 96 patients were enrolled in the study, excluding five patients who withdrew their consent and one patient who did not meet the selection criteria. The 96 patients were randomly divided into two groups: (i) those who received nutritional education by a dietitian at each outpatient visit; or (ii) those who received nutritional education by a dietitian once a year. After excluding nine patients (five patients who refused nutritional education in the intensive intervention group [INT], and one patient who refused nutritional education, one patient who did not come to the hospital, one patient who was transferred after hospitalization and one patient who did not receive a request for nutritional education from a physician in the control group [CON]), 44 patients in INT and 43 patients in CON who completed 2 years of follow‐up were included in the randomized controlled trial. DKD, diabetic kidney disease.
Study design
Of the two randomly assigned groups, the INT group received nutritional education on eating habits at each outpatient visit by a dietitian for 2 years. The CON group received nutritional education by a dietitian at the beginning of the study, and 1 and 2 years after the intervention. Nutritional education was provided according to the physician’s instructions. The physicians prescribed nutritional therapy (e.g., energy intake, PFC balance and salt intake) based on the 2012–2013 Diabetes Care Guidelines of the Japan Diabetes Society.
Variable measurements
In both the INT and CON groups, data on the presence or absence of diabetic retinopathy, the coefficients of variation of the R‐R interval using electrocardiography, waist circumference, body mass index (BMI), body fat percentage, systolic blood pressure, diastolic blood pressure, HbA1c level, eGFR, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol calculated using the Friedewald formula, non‐HDL cholesterol (non‐HDL‐C), and triglycerides were obtained at the beginning of the study and at 1 and 2 years after the intervention; then, the urinary protein or urinary albumin excretion volume, protein intake and salt intake measured from the 24‐h urine samples were determined. These data were examined and assessed as follows: (i) comparison of baseline data and the changes over time between the INT and CON groups; (2) comparison of changes over time between the two groups; and (iii) cross‐sectional comparison between the two groups at the beginning of the study and at 2 years after the intervention. We used salt intake measured from the 24‐h urine samples to accurately assess salt intake in the present study, as salt intake obtained through dietary intake surveys is more difficult to estimate than other nutrients, and the estimation of this parameter might cause errors 15 . Protein intake was calculated using Maroni’s formula, based on the 24‐h urine samples 16 . In addition, achievement of treatment goals for controlling weight, blood pressure, blood glucose and serum lipid levels, as well as adherence to the prescribed drugs, were assessed at the beginning and end of the study. The eGFR was calculated using the following formula, which was published by the Japanese Society of Nephrology: [eGFR [mL/min/1.73 m2] = 194 × Cr−1.094 × age−0.287 (× 0.739 for women)] 17 .
Non‐HDL‐C was calculated by subtracting the HDL‐C level from the total cholesterol level. Albuminuria was classified as A1 when the urine albumin level was <30 mg/day, A2 when the urine albumin level was ≥30 mg/day, but ≤300 mg/day, and A3 when the urine albumin level was ≥300 mg/day or when the urine protein level was ≥0.50 g/day. Blood pressure was measured in the clinic at any time using an automatic sphygmomanometer (BP‐203RVIII; Omron, Kyoto, Japan) according to the guidelines of the Japanese Society of Hypertension 18 . Blood biochemistry tests were carried out using an automated analyzer (LABOSPECT 008 a; Hitachi High‐Technologies Corp., Tokyo, Japan). Body fat percentage was measured using a multifrequency body composition analyzer (MC‐190; Tanita Corp., Tokyo, Japan), and waist circumference was measured according to the diagnostic criteria for metabolic syndrome 19 .
The participants were informed about the purpose and methods of the study, that their personal information will be adequately protected, and that they were free to refuse to participate in the study and they would not be disadvantaged if they refused; a written consent was obtained from all study participants.
Outcome measurements
The primary end‐points were ‘differences in clinical, physical, and nutritional factors over time between the INT and CON groups’ and ‘association of improved or worsening nutritional and clinical factors.’ The secondary end‐point was the achievement of treatment goals for controlling BMI, blood pressure, blood glucose and serum lipids. The treatment goals were a BMI of <25 kg/m2, a systolic blood pressure of <130 mmHg, a diastolic blood pressure of <80 mmHg, an HbA1c level of <7.0% and a non‐HDL‐C level of <150 mg/dL.
Statistical analysis
Values were presented as the mean ± standard deviation, median (interquartile range) or percentage. The Kolmogorov–Smirnov test was used for assessing the normality of data. The unpaired t‐test, Mann–Whitney U‐test or χ2‐test was used to compare the clinical, physical and nutritional factors between the two groups at the beginning and at 2 years after the study period. The changes in each group were analyzed by repeated measures analysis of variance or the Friedman test. Comparisons between the two groups, taking into account the changes over time during the study, were carried out using a two‐way analysis of variance or a mixed‐effects model. The association between improved nutritional factors and clinical parameters was analyzed using the Pearson’s product‐moment correlation coefficient. Comparison of the two groups in terms of achieving the therapeutic goal was carried out using the χ2‐test. The rates of medication use in the two groups at the beginning and end of the study were determined using the χ2‐test or Fisher’s exact test. A risk rate of <5% was considered significant, and SPSS® Statistics 21 (IBM Corp., Armonk, NY, USA) was used to carry out all statistical analyses.
Sample size calculation was based on the Japan Diabetes Optimal Integrated Treatment study for three major risk factors of cardiovascular diseases (J‐DOIT3 study) 20 ; the predicted HbA1c levels after a 2‐year intervention period were 7.2 ± 0.6% in the CON group and 6.8 ± 0.6% in the INT group; a total of 74 patients were included in the study, with each group comprising 37 participants, to detect a difference between groups <80% power and 5% α level.
Results
Comparison of clinical, physical, and nutritional factors between the INT group and the CON group at the beginning and the second year of the study
The comparison of clinical, physical, and nutritional factors between INT and CON at the beginning and the second year of the study are shown in Tables 1 and 2, respectively. The frequency of nutritional education (mean ± standard deviation) provided by the dietitian in the INT group was 12.6 ± 3.3 times. No differences were observed in sex, age, duration of diabetes mellitus, diabetic retinopathy complications, albuminuria category, DKD, coefficients of variation of the R‐R interval (at rest), blood pressure, blood test data, waist circumference, BMI, body fat percentage, protein intake and salt intake between the two groups at the beginning of the study. After 2 years, salt intake was significantly lower in the INT group than in the CON group; meanwhile, no differences were observed in other parameters, including eGFR and albuminuria categories.
Table 1.
Comparison of clinical, physical, and nutritional factors between the intensive intervention group and control group at the beginning of the study
| INT | CON | P | |
|---|---|---|---|
| n | 44 | 43 | |
| Male (%) | 68 | 56 | 0.235 † |
| Age (years) | 68.0 (62.2–71.0) | 65.0 (58.0–71.0) | 0.277 § |
| Duration of diabetes (years) | 13.0 (8.0–21.0) | 14.0 (9.0–24.0) | 0.377 § |
| Diabetic retinopathy (%) | 48 | 58 | 0.370 † |
| Albuminuria category (A1, A2 and A3) ¶ (%) | 63, 17, 20 | 51, 30, 19 | 0.352 † |
| Diabetic kidney disease (%) | 50 | 58 | 0.521 † |
| CVR‐R (at rest) (%) | 1.83 (1.46–3.16) | 1.96 (1.46–2.70) | 0.856 § |
| Systolic blood pressure (mmHg) | 134 (122–141) | 133 (124–142) | 0.538 § |
| Diastolic blood pressure (mmHg) | 73 ± 10 | 76 ± 14 | 0.319 ‡ |
| HbA1c (%) | 7.0 ± 0.7 | 7.1 ± 0.8 | 0.395 ‡ |
| eGFR (mL/min/1.73 m2) | 67.7 ± 18.7 | 70.2 ± 16.6 | 0.517 ‡ |
| Total cholesterol (mg/dL) | 180 (166–197) | 186 (163–201) | 0.468 § |
| HDL‐C (mg/dL) | 53 (48–64) | 53 (43–61) | 0.688 § |
| LDL‐C (mg/dL) | 97 ± 23 | 101 ± 33 | 0.548 ‡ |
| Triglycerides (mg/dL) | 100 (82–185) | 139 (78–199) | 0.521 § |
| Non‐HDL‐C (mg/dL) | 125 ± 23 | 131 ± 40 | 0.408 ‡ |
| Waist circumference (cm) | 90.0 (84.0–100.5) | 90.0 (86.0–95.0) | 0.566 § |
| BMI (kg/m2) | 24.7 ± 3.5 | 25.1 ± 3.3 | 0.605 ‡ |
| Body fat percentage (%) | 25.3 ± 8.9 | 27.0 ± 10.0 | 0.393 ‡ |
| Protein intake (g/kg/day) | 1.00 (0.86–1.23) | 0.98 (0.75–1.12) | 0.105 § |
| Salt intake (g/day) | 10.1 ± 3.5 | 10.2 ± 3.9 | 0.896 ‡ |
Values are expressed as the mean ± standard deviation, median (interquartile range) or percentage.
BMI, body mass index; CON, control group; CVR‐R, coefficients of variation of the R‐R interval; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; INT, intensive intervention group; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol; kg, ideal weight.
χ2‐test.
Unpaired t‐test.
Mann–Whitney U‐test.
A1, urine albumin level was <30 mg/day; A2, urine albumin level was ≥30 mg/day, but <300 mg/day; A3, urine albumin level was ≥300 mg/day.
Table 2.
Comparison of clinical, physical, and nutritional factors between the intensive intervention group and control group after a 2‐year intervention
| n | INT | CON | P |
|---|---|---|---|
| 44 | 43 | ||
| Diabetic retinopathy (%) | 49 | 61 | 0.267 † |
| Albuminuria category (A1, A2, A3) ¶ (%) | 57, 18, 25 | 58, 21, 21 | 0.884 † |
| Diabetic kidney disease (%) | 68 | 53 | 0.160 † |
| CVR‐R (at rest) (%) | 1.78 (1.25–2.49) | 2.09 (1.51–2.71) | 0.450 § |
| Systolic blood pressure (mmHg) | 133 ± 16 | 135 ± 14 | 0.526 ‡ |
| Diastolic blood pressure (mmHg) | 73 ± 13 | 76 ± 11 | 0.158 ‡ |
| HbA1c (%) | 7.0 ± 0.7 | 7.3 ± 0.8 | 0.125 ‡ |
| eGFR (mL/min/1.73 m2) | 64.9 ± 21.0 | 68.9 ± 18.0 | 0.247 ‡ |
| eGFR <60 mL/min/1.73 m2 (%) | 45 | 30 | 0.143 † |
| Total cholesterol (mg/dL) | 179 ± 26 | 183 ± 32 | 0.546 ‡ |
| HDL‐C (mg/dL) | 54 (45–63) | 53 (43–64) | 0.919 § |
| LDL‐C (mg/dL) | 97 ± 32 | 101 ± 25 | 0.452 ‡ |
| Triglycerides (mg/dL) | 119 (71–194) | 125 (83–186) | 0.757 § |
| Non‐HDL‐C (mg/dL) | 123 ± 26 | 127 ± 28 | 0.524 ‡ |
| Waist circumference (cm) | 87.8 ± 8.8 | 90.9 ± 11.7 | 0.174 ‡ |
| BMI (kg/m2) | 23.2 (21.8–26.0) | 24.7 (22.4–26.8) | 0.103 § |
| Body fat percentage (%) | 24.1 ± 7.5 | 27.6 ± 9.5 | 0.056 ‡ |
| Protein intake (g/kg/day) | 0.93 ± 0.20 | 1.02 ± 0.35 | 0.503 ‡ |
| Salt intake (g/day) | 8.1 (6.4–9.6) | 9.4 (8.3–12.8) | 0.028 § |
Values are expressed as the mean ± standard deviation, median (interquartile range) or percentage.
BMI, body mass index; CON, control group; CVR‐R, coefficients of variation of the R‐R interval; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; INT, intensive intervention group; LDL‐C, low‐density lipoprotein cholesterol; non‐HDL‐C, non‐high‐density lipoprotein cholesterol; kg, ideal weight.
χ2‐test.
Unpaired t‐test.
Mann–Whitney U‐test.
A1, urine albumin level was <30 mg/day; A2, urine albumin level was ≥30 mg/day, but <300 mg/day; A3, urine albumin level was ≥300 mg/day or urine protein level was ≥0.50 g/day.
Comparison of clinical, physical, and nutritional factors over time between the INT group and CON group
Regarding the changes over time in the clinical, physical, and nutritional factors listed in Tables 1 and 2, the items that were significantly different within the 2‐year study period are shown in Figure 2.
Figure 2.
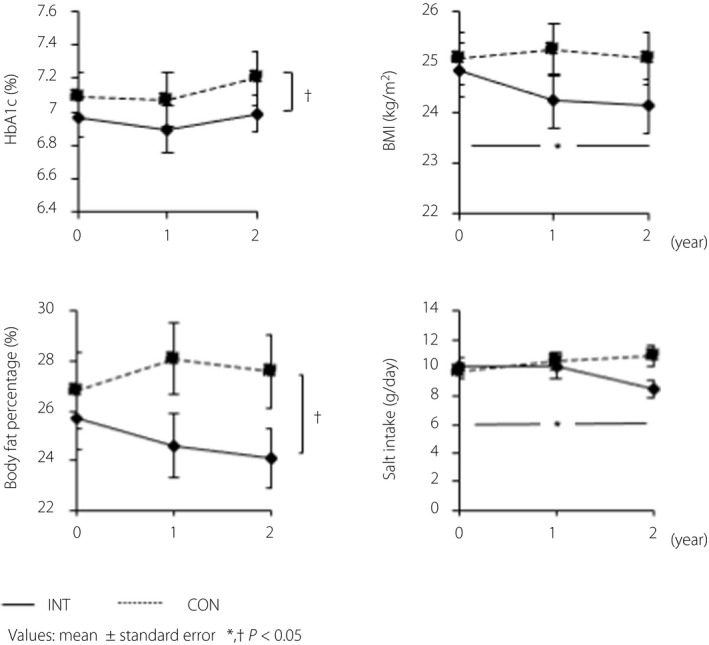
Changes of clinical, physical and nutritional factors over the 2‐year study period. In the intensive intervention group (INT), both body mass index (BMI) and salt intake decreased significantly over the study period (P = 0.020 and P =0.023, respectively). In addition, hemoglobin A1c (HbA1c) and body fat percentage were significantly lower in the INT group than in the control (CON) group in a comparison that took into account changes over time during the 2‐year study period. (P = 0.047 and P = 0.003, respectively). No differences were observed for the other parameters. Values are presented as the mean ± standard error. The horizontal axis indicates the study period, whereas the vertical axis indicates the HbA1c levels, BMI, body fat percentage and salt intake. Changes in each item were analyzed by repeated measures analysis of variance or the Friedman test, whereas comparisons between the two groups were carried out using the two‐way analysis of variance or mixed‐effects models. *Significant differences in the change over time in each group; †significant differences in the change over time between the two groups.
Both BMI and salt intake decreased significantly (P = 0.020 and P = 0.023, respectively) in the INT group within the 2‐year study period. In addition, the HbA1c levels and body fat percentage were significantly lower in the INT group than in the CON group in a comparison that considered the changes over time during the study period (P = 0.047 and P = 0.003, respectively). No differences were observed in the other parameters.
Association between improved nutritional factors and clinical parameters
In the second year, we examined the association between dietary salt intake and the clinical factors associated with the development and progression of nephropathy, such as blood pressure, HbA1c level, eGFR, albumin excretion and albuminuria category (Table 3).
Table 3.
Association between salt intake and clinical parameters after 2 years of intervention
| Total | INT | CON | ||||
|---|---|---|---|---|---|---|
| R | P | r | P | r | P | |
| Systolic blood pressure | −0.145 | 0.196 | −0.134 | 0.391 | −0.210 | 0.205 |
| Diastolic blood pressure | 0.022 | 0.843 | −0.126 | 0.420 | 0.117 | 0.485 |
| HbA1c | 0.128 | 0.258 | 0.129 | 0.414 | 0.047 | 0.780 |
| eGFR | 0.036 | 0.752 | 0.165 | 0.292 | −0.216 | 0.194 |
| Albumin excretion | 0.264 | 0.024 | 0.365 | 0.024 | 0.105 | 0.549 |
| Albuminuria category | 0.145 | 0.196 | 0.062 | 0.694 | 0.227 | 0.170 |
r, Pearson’s product moment correlation coefficient.
CON, control group; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; INT, intensive intervention group.
Salt intake in the second year showed a significant positive correlation with albumin excretion in the overall and INT groups (r = 0.264, P = 0.024 and r = 0.365, P = 0.024, respectively), whereas no correlation was found between these two parameters in the CON group.
Achievement of treatment goals for controlling BMI, blood pressure, blood glucose and serum lipids
The treatment goals for controlling BMI, blood pressure, blood glucose, and serum lipids achieved at the beginning and end of the study are shown in Table S1. No difference was observed between the INT group and CON group.
Use rates of medications for diabetes, dyslipidemia, and hypertension at the beginning and end of the study
We compared the use rates of medications for diabetes, dyslipidemia, and hypertension at the beginning and end of the study between the INT group and CON group. At the end of the study, the use of antidiabetic drugs tended to be lower in the INT group than in the CON group (84% in the INT group and 95% in the CON group, P = 0.084). No difference was observed in the use rates of medications for dyslipidemia (INT group: 52%; CON group: 49%) and hypertension (INT group: 64%; CON group: 65%) between the two treatment groups.
Therefore, we compared the use rates of sulfonylureas, biguanides, alpha‐glucosidase inhibitors, thiazolidinediones, rapid insulin secretagogues, dipeptidyl peptidase‐4 inhibitors, sodium–glucose cotransporter 2 inhibitors, glucagon‐like peptide‐1 receptor agonists and insulin as diabetes medications at the beginning and end of the study between the two groups. The results are presented in Table S2.
The use rate of alpha‐glucosidase inhibitors was significantly lower in the INT group than in the CON group at the beginning of the study, but it was not significantly different at the end of the study. The rate of insulin use was also significantly lower in the INT group than in the CON group at the end of the study, although no significant difference was observed at the beginning of the study.
No significant differences were also found in the other treatments.
Discussion
In the INT group, the BMI throughout the study period was significantly lower than that at baseline according to the Friedman test. The HbA1c level and body fat percentage of the INT group were also significantly lower than those of the CON group throughout the study period; meanwhile, no significant differences were observed in the eGFR and albuminuria category between the two groups at the different periods of the study. A previous observational study prospectively following DKD patients with type 2 diabetes for 3 years reported that weight, BMI and body fat mass were independently associated with worsening DKD, suggesting that the significant correlation between increased obesity and worsening DKD is due to the worsening glycemic control 21 . Therefore, it is plausible to speculate that the improvement of obesity and hyperglycemia observed in the INT group might be effective in preventing the development and progression of DKD.
In addition to BMI, salt intake was also significantly lower throughout the study period in the INT group. After 2 years of intervention, the salt intake was significantly lower in the INT group than in the CON group; it was also significantly and positively correlated with albumin excretion after 2 years in the overall and INT groups, whereas the use rate of antihypertensive drugs including renin–angiotensin system inhibitors was not different between the two groups. These results suggest that INT is effective in reducing salt intake.
Previously, we carried out a cross‐sectional study of nutritional factors as risk factors for albuminuria in patients with type 2 diabetes, mainly those with normal or mildly impaired renal function, and found that salt intake was a significant independent factor, suggesting that excessive salt intake is a risk factor for albuminuria 8 . A retrospective study by Cianciaruso et al. 22 showed that patients with CKD who consumed a low‐sodium diet at baseline achieved better renal outcomes during a 3‐year follow up compared with those who consumed a high‐sodium diet at baseline. Interestingly, the mean blood pressure during the study period was comparable between the two groups, suggesting that effective salt restriction in CKD patients improves the outcome of renal disease, independent of the effects of any antihypertensive medications 22 . In a recent cross‐over study of 46 patients with impaired glucose tolerance and type 2 diabetes mellitus, a salt restriction group (salt intake: 7.0 g/day) showed significantly lower blood pressure and reduced urinary albumin levels compared with an unrestricted group (salt intake: 10.0 g/day) 23 . This findings seemed to support our present results; that is, the salt intake in the INT group significantly decreased throughout the study period, and the salt intake after 2 years was significantly lower in the INT group than in the CON group and showed a significant positive correlation with urinary albumin excretion after 2 years. Thus, the present results suggest that salt restriction might be important in inhibiting the development and progression of DKD.
A systematic review by Pastors et al. 24 reported that a comprehensive dietary education provided by dieticians was effective in improving glycemic control, and a recent meta‐analysis reported that education provided by dieticians was associated with significant improvements in both weight loss and HbA1c levels compared with the education provided by physicians and other medical staff 25 . These reports might support our present results that frequent intervention by a dietitian was effective in decreasing BMI and improving glucose metabolism.
The present results also showed that the rate of insulin use at the end of the study was significantly lower in the INT group than in the CON group, suggesting that intensive intervention in this study promoted the patients’ behavioral changes and improved their dietary habits without intensifying drug therapy. In contrast, no differences were observed in the achievement rates of therapeutic goals for BMI, blood pressure, HbA1c levels, and non‐HDL‐C at the beginning and end of the study in each group. Thus, support by a dietitian might be important in reducing the use rate of glucose‐lowering medications.
The present study had several limitations. Two years after the intervention, no differences were observed in the eGFR and albuminuria. Because of the high rate of A1 albuminuria in both groups in this study, a longer follow‐up period might be required to clarify the effect of intensive nutrition education on the renal function of DKD patients. Previous studies have reported the so‐called legacy effects of pre‐dialysis psychological education interventions for CKD patients 26 , and multidisciplinary interventions with multiple medications and behavioral modifications for patients with diabetes 27 , with the effects of those interventions continuing over the long term. In the J‐DOIT3 trial, a 32% reduction in the occurrence of renal events was reported in the group receiving intensified therapy, with stricter targets for the blood glucose, blood pressure and lipid levels compared with guideline‐based treatments, after a median follow‐up period of 8.5 years 20 . In addition, a large epidemiological study in the UKPDS 64 showed that the transition rates from normoalbuminuria to microalbuminuria and from microalbuminuria to overt proteinuria were 2 and 2.8%, respectively 7 . However, the annual transition rate from normal and mild microalbuminuria to diabetic nephropathy in the Japan Diabetes Complications Study was 0.67%, which is lower than that in other countries. That study suggested that if patients with type 2 diabetes are treated for hyperglycemia and hypertension by a diabetologist in the early stages of nephropathy, the transition rate to proteinuria is considerably reduced, which might be similar to the results of the present study 28 .
The present study suggests that correcting excessive salt intake is important to prevent the development and progression of nephropathy when supporting dietary therapy in patients with diabetes, and that frequent interventions provided by dietitians contribute to a reduction in salt intake. In the future, it is necessary to establish a long‐term follow‐up period to clarify whether the rate of renal function decline is significantly moderated when DKD patients are subjected to strict dietary salt restriction compared with those who are not.
In patients with type 2 diabetes mellitus, frequent nutritional education by dieticians significantly reduced the body fat percentage, HbA1c level, salt intake and insulin usage compared with the usual nutritional education. However, frequent nutritional education did not significantly improve retinopathy, eGFR or albuminuria. A long‐term follow up is warranted to evaluate the effect of nutritional intervention on the renal outcome of patients with diabetic nephropathy.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study protocol was approved by Jichi Medical University Clinical Research Ethics Committee (no. A16‐28).
Informed consent: All the participants gave informed consent.
Approval date of registry and the registration no. of the study: The registry was approved by University Hospital Medical Information Network (UMIN) Clinical Trials Registry on the 17th of April, 2021 with the Registration No. UMIN000043955.
Animal studies: N/A.
Supporting information
Table S1 | Achievement of treatment goals regarding control levels of bodyweight, blood pressure, blood glucose and serum lipids.
Table S2 | Use rates of medications for diabetes at the beginning and end of the study.
ACKNOWLEDGMENTS
We express our deepest gratitude to the staff of the Division of Endocrinology and Metabolism and the Division of Renal Medicine, Jichi Medical University, for their cooperation in conducting this study. We also thank Y Arakawa, A Haga, A Kogure and M Chiba from the Department of Clinical Nutrition, Jichi Medical University Hospital, for their nutritional education. Part of this work was supported by a grant from The Kidney Foundation, Japan (JKF13‐3). We thank Editage (www.editage.com) for English language editing.
J Diabetes Investig 2022; 13: 271–279
REFERENCES
- 1. Ministry of Health, Labor and Welfare . The National Health and Nutrition Survey in Japan 2016 (in Japanese). Available from https://www.mhlw.go.jp/stf/houdou/0000177189.html Accessed September 13, 2021.
- 2. Nakai S, Iseki K, Itami N, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther Apher Dial 2012; 16: 11–53. [DOI] [PubMed] [Google Scholar]
- 3. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004; 27(Suppl 1): S79–S83. [DOI] [PubMed] [Google Scholar]
- 4. Gaede P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004; 19: 2784–2788. [DOI] [PubMed] [Google Scholar]
- 5. Yokoyama H, Tomonaga O, Hirayama M, et al. Predictors of the progression of diabetic nephropathy and the beneficial effect of angiotensin‐converting enzyme inhibitors in NIDDM patients. Diabetologia 1997; 40: 405–411. [DOI] [PubMed] [Google Scholar]
- 6. Parving H‐H, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 7. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 8. Kawabata N, Kawamura T, Utsunomiya K, et al. High salt intake is associated with renal involvement in Japanese patients with type 2 diabetes mellitus. Intern Med 2015; 54: 311–317. [DOI] [PubMed] [Google Scholar]
- 9. Kurtkulagi O, Aktas G, Bilgin S, et al. Combined antihypertensive treatment is better than mono‐therapy in hypertensive patients. Neth J Med 2020; 78: 239–243. [PubMed] [Google Scholar]
- 10. Atik F, Aktas G, Zahid Kocak M, et al. Analysis of the factors related to the blood pressure control in hypertension. J Coll Physicians Surg Pak 2018; 28: 423–426. [DOI] [PubMed] [Google Scholar]
- 11. Pillay J, Armstrong MJ, Butalia S, et al. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta‐analysis. Ann Intern Med 2015; 163: 848–860. [DOI] [PubMed] [Google Scholar]
- 12. Yamagata K, Makino H, Iseki K, et al. Effect of behavior modification on outcome in early‐ to moderate‐stage chronic kidney disease: a cluster‐randomized trial. PLoS One 2016; 11: e0151422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogelfeld L, Hart P, Miernik J, et al. Combined diabetes‐renal multifactorial intervention in patients with advanced diabetic nephropathy: proof‐of‐concept. J Diabetes Complications 2017; 31: 624–630. [DOI] [PubMed] [Google Scholar]
- 14. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Keyzer W, Dofkova M, Lillegaard IT, et al. Reporting accuracy of population dietary sodium intake using duplicate 24 h dietary recalls and a salt questionnaire. Br J Nutr 2015; 113: 488–497. [DOI] [PubMed] [Google Scholar]
- 16. Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 1985; 27: 58–65. [DOI] [PubMed] [Google Scholar]
- 17. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 18. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390. [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome, 2006. Available from: https://www.idf.org/e‐library/consensus‐statements/60‐idfconsensus‐worldwide‐definitionof‐the‐metabolic‐syndrome.html Accessed September 13, 2021.
- 20. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. [DOI] [PubMed] [Google Scholar]
- 21. Moh AMC, Wang J, Tan C, et al. Association between gain in adiposity and diabetic kidney disease worsening in type 2 diabetes is mediated by deteriorating glycaemic control: a 3‐year follow‐up analysis. Diabetes Res Clin Pract 2019; 157: 107812. [DOI] [PubMed] [Google Scholar]
- 22. Cianciaruso B, Bellizzi V, Minutolo R, et al. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab 1998; 24: 296–301. [DOI] [PubMed] [Google Scholar]
- 23. Suckling RJ, He FJ, Markandu ND, et al. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double‐blind trial. Hypertension 2016; 67: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 24. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002; 25: 608–613. [DOI] [PubMed] [Google Scholar]
- 25. Moller G, Andersen HK, Snorgaard O. A systematic review and meta‐analysis of nutrition therapy compared with dietary advice in patients with type 2 diabetes. Am J Clin Nutr 2017; 106: 1394–1400. [DOI] [PubMed] [Google Scholar]
- 26. Devins GM, Mendelssohn DC, Barré PE, et al. Predialysis psychoeducational intervention extends survival in CKD: a 20‐year follow‐up. Am J Kidney Dis 2005; 46: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 27. Gæde P, Lund‐Andersen H, Parving H‐H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 28. Katayama S, Moriya T, Tanaka S, et al. Low transition rate from normo‐ and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia 2011; 54: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Achievement of treatment goals regarding control levels of bodyweight, blood pressure, blood glucose and serum lipids.
Table S2 | Use rates of medications for diabetes at the beginning and end of the study.


