Abstract
Aims/Introduction
Diabetic peripheral neuropathy is a common diabetes‐related microvascular complication. The relationship between peripheral nerve function and glucose variability is unclear. We investigated the association of glucose variability with subclinical diabetic polyneuropathy in a large‐scale sample of patients with type 2 diabetes.
Materials and Methods
We enrolled 509 individuals with type 2 diabetes who were screened for diabetic peripheral neuropathy and monitored using a continuous glucose monitoring system. Multiple glycemic variability parameters, including the mean amplitude of glycemic excursions, glucose standard deviation (SDgluc) and glucose coefficient of variation were calculated from 3‐day glucose profiles obtained from continuous glucose monitoring. All participants underwent nerve conduction studies, and the composite Z‐scores for nerve conduction parameters were calculated.
Results
Multivariate logistic regression analyses showed that SDgluc and the conventional risk factor hemoglobin A1c (HbA1c) were independently associated with abnormal nerve function, and the corresponding odds ratios (95% confidence interval) were 1.198 (1.027–1.397, SDgluc) and 1.182 (1.061–1.316, HbA1c), respectively. The composite Z‐score of nerve conduction velocity and response amplitude obviously decreased with greater SDgluc, and the composite Z‐score of distal latency significantly increased with increasing tertiles of SDgluc (all P trend <0.05). After adjusting for age, sex, body mass index, diabetes duration and HbA1c, SDgluc was independently associated with nerve conduction velocity (β = −0.124, P = 0.021).
Conclusions
The SDgluc is a significant independent contributor to subclinical diabetic polyneuropathy, in addition to conventional risk factors including diabetes duration and HbA1c.
Keywords: Continuous glucose monitoring, Diabetic peripheral neuropathy, Glycemic variability
We carried out a cross‐sectional study to investigate the association of glycemic variability assessed by continuous glucose monitoring with subclinical diabetic polyneuropathy in type 2 diabetes patients. Our study found that increased glycemic variability shown as glucose standard deviation is a significant independent contributor to subclinical diabetic polyneuropathy.
![]()
INTRODUCTION
Diabetes mellitus is a metabolic disorder with a significantly increasing prevalence in China in recent decades, which has contributed to the increase in diabetes‐related complications, including chronic kidney disease, diabetic retinopathy, diabetic peripheral neuropathy (DPN), diabetic foot and cardiovascular disease in China 1 , 2 . Typical DPN is a chronic, symmetrical, distant sensorimotor polyneuropathy and is considered the most common type of heterogeneity 3 . It might be silent, asymptomatic, undetectable or present with clinical symptoms. DPN is usually associated with a loss or diminished sensation in the foot and leads to an increased incidence of foot ulcers 4 , 5 , resulting in amputation in patients with a long duration of diabetes 6 . Up to 50% of DPN might be asymptomatic 7 . Subclinical diabetic neuropathy frequently occurrs at the early stage of DPN with abnormal electrophysiological features and no typical clinical manifestations of diabetic neuropathy 8 . As the early diagnosis and timely interventions are essential to prevent the progression of diabetic neuropathy, the reliance on clinical symptoms or signs alone might lead to decreased diagnostic accuracy for subclinical diabetic polyneuropathy. Therefore, as a surrogate measurement, nerve conduction study is widely used as an evaluation method of DPN. Of all the feasible evaluation methods to date, nerve conduction studies are considered the most objective, sensitive and reproducible method of early detection and quantification of DPN, especially appropriate for detecting patients without classic DPN symptoms.
Glycemic dysregulation is a risk factor for the onset or progression of DPN. Glycemic disorders are not only confined to traditional markers reflecting glycemic levels, such as plasma glucose levels, glycated albumin and hemoglobin A1c (HbA1c), but also contain markers of glycemic variability (GV), such as standard deviation of glucose levels (SDgluc), coefficient of variation of glucose levels (CVgluc) and mean amplitude of glycemic excursions (MAGE). Most GV parameters can be examined and presented in detail by using a continuous glucose monitoring (CGM) system 9 , 10 . In recent years, a few studies have reported a positive association between GV and diabetic macrovascular and microvascular complications 11 . Hu et al. 12 found that MAGE, as an indicator of glycemic variability, independently contributed to the presence of diabetic peripheral neuropathy. In contrast, Lachin et al. 13 found that GV within a day, as determined from daily glucose levels by point‐of‐care testing, did not have a significant effect on the development of diabetic microvascular complications. Thus, the uncertain association between GV and DPN requires further investigation. In the present study, we designed a cross‐sectional study to determine the correlation of GV, assessed using CGM, with nerve conduction function in patients with type 2 diabetes mellitus and subclinical diabetic neuropathy.
MATERIALS AND METHODS
Study population
All participants were admitted to the Endocrinology Department of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China, for screening of diabetic chronic complications and to optimize their antidiabetic regimens. A flow diagram of patient selection is shown in Figure 1. We enrolled 509 individuals with type 2 diabetes mellitus who had undergone a nerve conduction study for DPN screening and 3‐day CGM from April 2013 to August 2014. The individuals satisfied the following inclusion criteria: (i) were aged 25–75 years; (ii) had valid and available data of nerve conduction studies; and (iii) had received the current antidiabetic medication regimen for at least 3 months. Individuals were excluded if they had any of the following criteria: (i) no complete data of sex, age, HbA1c and diabetes duration; (ii) history of neurological diseases that could influence nerve conducting function; (iii) history of severe kidney or liver disease; (iv) history of mental disorder or malignancy; (v) presence of symmetrical distant neuropathy symptoms or signs; (vi) use of vitamin B1, vitamin B12 or folic acid in the previous 6 months; and (vii) acute diabetic complications. Diabetes was diagnosed according to the 1999 World Health Organization criteria. The diagnostic criteria of subclinical diabetic polyneuropathy are defined as the presence of no neuropathy signs or symptoms and abnormal nerve conduction 14 . The present study protocol was approved by the Institutional Review Board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from all participants.
Figure 1.
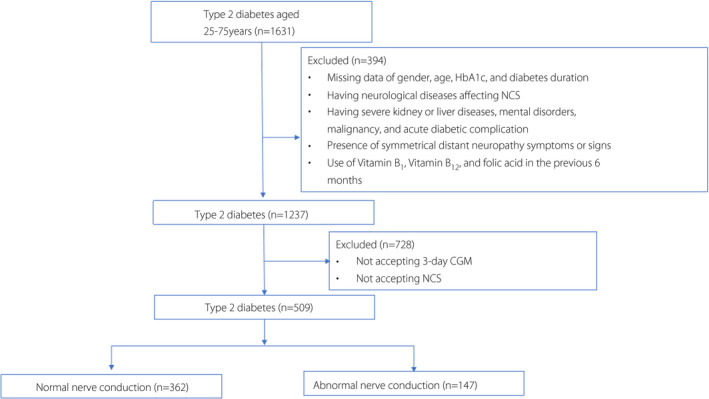
Flow diagram of patient selection. CGM, continuous glucose monitoring; NCS, nerve conduction study.
Anthropometric measurements
During recruitment, all participants were surveyed, and their age, sex, lifestyle behaviors, past medical history and somatometric parameters were recorded. Weight (kg) divided by height squared (m2) was calculated to determine body mass index (BMI). Blood pressure was calculated as the average of three measurements.
Biochemical measurements
Blood samples from all participants were collected for laboratory biochemical measurements. HbA1c was measured using an analyzer (Tosoh HLC‐723 G7, Yamaguchi, Japan) using high‐performance liquid chromatography. Fasting lipid profiles were measured using an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan). The Modification of Diet in Renal Disease equation was used to obtain the estimated glomerular filtration rate. More than two 24‐h urinary samples were collected to determine urinary albumin excretion (UAE) as the mean 24‐h urinary albumin level using a turbidimetric immunoassay.
The participants were defined as having cardiovascular disease if they had at least one cardiovascular event. We used standardized non‐mydriatic fundus photography to determine diabetic retinopathy. Diagnosis of diabetic retinopathy was determined according to the International Classification of Diabetic Retinopathy criteria by an ophthalmologist. At least two 24‐h UAE measurements were made, and the mean level was recorded for each participant. The participants were diagnosed with diabetic nephropathy if the mean UAE was ≥30 mg/24 h.
Nerve conduction study
All enrolled patients underwent an electrophysiological evaluation system using the Dantec Keypoint Focus EMG System (Natus, Taastrup, Denmark). Both the motor and sensory nerves on the non‐dominant side were detected. Motor nerve studies were carried out on the ulnar, median and tibial nerves. Sensory nerve studies were carried out in the ulnar, median and sural nerves. Distal latency, response amplitude and nerve conduction velocity (NCV) were measured. Upper body temperature was maintained at ≥35°C, and lower body temperature was maintained at ≥32°C 15 .
All measurements were collected and compared with laboratory reference values. Nerve conduction function was regarded as abnormal if one or more parameters in more than one of the tested motor or sensory nerves were beyond the reference values 16 .
Additionally, the composite Z‐scores of the tested nerve conduction parameters, including NCV, response amplitude and distal latency, were estimated. Z‐score = (individual value of patient − mean value of control group) / SD of the control group 1 . The composite NCV Z‐score was then calculated as [(Z‐score motor median NCV) + (Z‐score sensory median NCV) + (Z‐score motor ulnar NCV) + (Z‐score sensory ulnar NCV) + (Z‐score tibial NCV) + (Z‐score sural NCV)] / 6. The composite Z‐scores for distal latency and response amplitude were then determined using a similar equation 17 .
CGM examination
All participants underwent retrospective 3‐day CGM (Medtronic Inc., Northridge, CA, USA) beginning on the first day of admission in accordance with clinical indications for retrospective CGM technology 18 . The sensor of the CGM system was inserted on day 0 and removed 72 h later, generating a daily record of 288 continuous sensor values. All participants adhered to the preadmission therapy regimen during the 3‐day CGM and followed a standard dietary pattern at the same time. The CGM system was managed and operated by specialized staff according to standard operation procedures to guarantee the accuracy and validity of the results. The CGM system was calibrated four times daily using capillary blood glucose measurements, including fasting, 2 h after breakfast, before dinner and at 09.00 hours using a SureStep blood glucose meter (LifeScan, Milpitas, CA, USA). If three or more pairs of sensor glucose values and capillary blood glucose values matched per day, the CGM data were considered accurate. We applied three parameters to assess GV: SDgluc, CVgluc and MAGE to reflect intraday GV. All parameters were calculated using specific computer software after the 3‐day CGM. CVgluc was determined as SDgluc divided by the mean glucose level. In addition, the arithmetic mean of the differences between consecutive nadirs and peaks was computed to determine the MAGE value.
Statistical analysis
Data of normally distributed variables are presented as the mean ± SD. Skewed data are expressed as the median (25 and 75% interquartile range). Student’s t‐test was used to test the differences in continuous variables, and the χ2‐test was used to compare the proportions of categorical variables. The differences in composite Z‐score of nerve conduction parameters among tertiles of SDgluc were analyzed by a test for trend. One‐way analysis of variance was used to compare nerve conduction parameters among the tertiles of SDgluc. Taking into account potential confounders, binary logistic regression was carried out to investigate associations between GV parameters and nerve conduction function, and the corresponding odds ratios (ORs) and 95% confidence intervals are provided. Five binary logistic regression models were constructed for HbA1c (model 1), SDgluc (model 2), MAGE (model 3), CVgluc (model 4) and average glucose (model 5) after controlling for clinical risk factors including age, sex, BMI and diabetes duration. Additionally, a multiple linear regression analysis was applied to investigate associations between GV parameters and the continuous composite Z‐score of nerve conduction parameters, including NCV, distal latency and response amplitude as dependent variables. Five multivariate linear regression models were carried out for HbA1c (model 1), HbA1c and SDgluc (model 2), HbA1c and MAGE (model 3), HbA1c and CVgluc (model 4), and HbA1c and average glucose (model 5) after controlling for clinical risk factors. Covariates in the models included age, BMI, diabetes duration, HbA1c, CVgluc, SDgluc, MAGE and average glucose as continuous variables, and sex as a categorical variable. All statistical analyses were carried out using SPSS software (version 25.0). Statistical differences were considered significant if the two‐tailed P‐value was <0.05.
RESULTS
A total of 509 patients with type 2 diabetes mellitus, including 278 men and 231 women aged 43–71 years, were enrolled. All participants were classified into two groups according to whether they had normal or abnormal nerve conduction functions. We have presented the reference values and mean values of nerve conduction study parameters in Table 1. As shown in Table 1, 147 patients (28.9%) had abnormal nerve conduction results, and they were relatively older than the normal nerve conduction group (P = 0.003). In addition, the abnormal nerve conduction group had higher HbA1c values (P = 0.021), longer diabetes duration (P = 0.002), and higher average glucose (P = 0.041) and SDgluc (P = 0.008) values than the normal nerve conduction group. The lipid profile, MAGE, CVgluc, UAE, estimated glomerular filtration rate and blood pressure were not significantly different between the normal and abnormal nerve conduction groups. Diabetic retinopathy, diabetic nephropathy, cardiovascular disease, smoking status and alcohol consumption were similar between the two groups.
Table 1.
Characteristics of patients in the study
| Variable | Total | Normal nerve conduction | Abnormal nerve conduction | P‐value |
|---|---|---|---|---|
| Samples (n) | 509 | 362 | 147 | |
| Age (years) | 57.68 ± 13.88 | 56.52 ± 13.83 | 60.52 ± 13.65 | 0.003 |
| Sex (male/female) | 278/231 | 203/159 | 75/72 | 0.299 |
| BMI (kg/m2) | 24.35 ± 3.65 | 24.40 ± 3.74 | 24.24 ± 3.44 | 0.662 |
| Duration of diabetes (years) | 9.02 ± 7.65 | 8.33 ± 7.33 | 10.70 ± 8.18 | 0.002 |
| Alcohol consumers, n (%) | 69 (13.56%) | 56 (15.50%) | 13 (8.84%) | 0.062 |
| Current smokers, n (%) | 167 (13.56%) | 122 (33.70%) | 45 (30.61%) | 0.533 |
| Systolic blood pressure (mmHg) | 130.60 ± 17.86 | 129.83 ± 17.84 | 132.49 ± 17.83 | 0.194 |
| Diastolic blood pressure (mmHg) | 78.36 ± 10.37 | 78.21 ± 10.77 | 78.74 ± 9.35 | 0.640 |
| HbA1c (%) | 8.61 ± 2.04 | 8.47 ± 2.03 | 8.97 ± 2.01 | 0.021 |
| HDL‐C (mmol/L) | 1.08 ± 0.31 | 1.09 ± 0.30 | 1.07 ± 0.33 | 0.665 |
| LDL‐C (mmol/L) | 2.74 ± 0.85 | 2.74 ± 0.80 | 2.74 ± 0.97 | 0.999 |
| Triglyceride (mmol/L) | 1.65 ± 1.49 | 1.64 ± 1.39 | 1.68 ± 1.72 | 0.776 |
| Total cholesterol (mmol/L) | 4.59 ± 1.02 | 4.58 ± 0.96 | 4.62 ± 1.16 | 0.738 |
| Average glucose (mmol/L) | 9.37 ± 1.90 | 9.26 ± 1.78 | 9.64 ± 2.13 | 0.041 |
| SDgluc (mmol/L) | 5.96 ± 1.27 | 5.86 ± 1.18 | 6.19 ± 1.43 | 0.008 |
| MAGE (mmol/L) | 6.33 ± 2.48 | 6.23 ± 2.43 | 6.60 ± 2.59 | 0.129 |
| CVgluc (%) | 63.72 ± 6.00 | 63.47 ± 5.87 | 64.32 ± 6.29 | 0.150 |
| Diabetic retinopathy, n (%) | 79,15.5 | 50, 13.8 | 29,19.7 | 0.064 |
| Diabetic nephropathy, n (%) | 87, 17.1 | 56, 15.5 | 31, 21.1 | 0.083 |
| CVD (n, %) | 83, 16.3 | 53, 14.6 | 30, 20.4 | 0.073 |
| eGFR (mL/min/1.73 m2) | 80.86 ± 48.96 | 80.33 ± 49.14 | 82.18 ± 48.64 | 0.607 |
| UAE (mg/24 h) | 21.00 (15.00, 44.00) | 20.44 (15.00, 40.00) | 22.50 (16.00, 57.00) | 0.097 |
| Nerve conduction parameters (reference values) | ||||
| Motor median conduction velocity, m/s (>50.0) | 54.65 ± 5.22 | 55.30 ± 4.63 | 53.05 ± 6.18 | <0.001 |
| Motor median latency, m/s (<4.2) | 3.50 ± 0.57 | 3.36 ± 0.46 | 3.83 ± 0.67 | <0.001 |
| Motor median amplitude, mv (>5.0) | 6.43 ± 3.33 | 7.17 ± 3.22 | 4.60 ± 2.88 | <0.001 |
| Motor ulnar conduction velocity, m/s (>50.0) | 58.21 ± 6.39 | 58.51 ± 6.02 | 57.46 ± 7.16 | 0.095 |
| Motor ulnar latency, ms (<3.1) | 2.44 ± 0.36 | 2.39 ± 0.33 | 2.58 ± 0.44 | <0.001 |
| Motor ulnar amplitude, mv (>5.0) | 5.09 ± 2.33 | 5.42 ± 2.52 | 4.27 ± 1.48 | <0.001 |
| Motor tibial conduction velocity, m/s (>37.0) | 44.74 ± 5.43 | 45.11 ± 5.189 | 43.83 ± 5.90 | 0.016 |
| Motor tibial latency, ms (<5.8) | 3.72 ± 0.78 | 3.73 ± 0.77 | 3.70 ± 0.79 | 0.674 |
| Motor tibial amplitude, mv (>4.8) | 6.38 ± 3.90 | 6.95 ± 4.09 | 4.95 ± 2.96 | <0.001 |
| Sensory median conduction velocity, m/s (>45.0) | 55.51 ± 8.58 | 57.16 ± 7.20 | 51.46 ± 10.24 | <0.001 |
| Sensory median latency, ms (<3.5) | 2.55 ± 0.40 | 2.48 ± 0.31 | 2.74 ± 0.50 | <0.001 |
| Sensory median amplitude, mv (>20.0) | 10.33 ± 5.85 | 11.68 ± 5.95 | 7.02 ± 3.99 | <0.001 |
| Sensory ulnar conduction velocity, m/s (>44.0) | 57.34 ± 7.65 | 57.47 ± 7.04 | 57.01 ± 9.00 | 0.573 |
| Sensory ulnar latency, ms (<2.8) | 2.17 ± 0.32 | 2.17 ± 0.31 | 2.17 ± 0.35 | 0.974 |
| Sensory ulnar amplitude, mv (>17.0) | 9.14 ± 4.83 | 5.42 ± 2.52 | 4.27 ± 1.48 | <0.001 |
| Sensory sural conduction velocity, m/s (>40.0) | 46.95 ± 8.54 | 47.81 ± 7.95 | 44.79 ± 9.57 | <0.001 |
| Sensory sural latency, ms (NA) | 2.04 ± 0.92 | 1.93 ± 1.00 | 2.31 ± 0.56 | <0.001 |
| Sensory sural amplitude, mv (NA) | 13.25 ± 9.57 | 14.94 ± 9.86 | 8.95 ± 7.18 | <0.001 |
Data are expressed as mean ± standard deviation (SD) for normal distribution variables. Skewed data are expressed as the medians (25 and 75% interquartile ranges). Categorical variables are expressed as numbers (percentage).
BMI, body mass index; CVD, cardiovascular disease; CVgluc, glucose coefficient of variation; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐c, high density lipoprotein cholesterol; LDL‐c, low density lipoprotein cholesterol; MAGE, mean amplitude of glycemic excursions; NA, not available; SDgluc, glucose standard deviation; UAE, urinary albumin excretion.
Logistic regression analysis was carried out to evaluate the relationship between GV parameters and abnormal nerve conduction (Table 2). HbA1c and SDgluc were associated with abnormal nerve conduction. The ORs of HbA1c and SDgluc were 1.182 (P = 0.002) and 1.198 (P = 0.021), respectively, after adjusting for age, sex, BMI and diabetes duration. Although MAGE, CVgluc and average glucose were considered potential risk factors, these parameters were not associated with abnormal nerve conduction (OR for MAGE = 1.057, P = 0.173; OR for CVgluc = 1.017, P = 0.311; OR for average glucose = 1.102, P = 0.06).
Table 2.
Association of potential risk factors with abnormal nerve conduction after adjustment
| Variables | Abnormal nerve conduction | |
|---|---|---|
| OR (95% CI) | P | |
| Model 1 | ||
| HbA1c | 1.182 (1.061–1.316) | 0.002 |
| Model 2 | ||
| SDgluc | 1.198 (1.027–1.397) | 0.021 |
| Model 3 | ||
| MAGE | 1.057 (0.976–1.144) | 0.173 |
| Model 4 | ||
| CVgluc | 1.017 (0.984–1.051) | 0.311 |
| Model 5 | ||
| Average glucose | 1.102 (0.996–1.219) | 0.060 |
Model 1: adjusted for age, sex, body mass index (BMI), diabetes duration and hemoglobin A1c (HbA1c); model 2: adjusted for age, sex, BMI, diabetes duration and glucose standard deviation (SDgluc); model 3: adjusted for age, sex, BMI, diabetes duration and mean amplitude of glycemic excursions (MAGE); model 4: adjusted for age, sex, BMI, diabetes duration and glucose coefficient of variation (CVgluc); model 5: adjusted for age, sex, BMI, diabetes duration and average glucose.
Next, to further evaluate the association of nerve conduction function and SDgluc, composite Z‐scores of NCV, distal latency and response amplitude were calculated. Figure 2 shows that the composite Z‐scores of NCV and response amplitude obviously decreased with increasing tertiles of SDgluc (P for trend <0.01), whereas the composite Z‐score of distal latency was significantly prolonged in the groups with higher SDgluc (P for trend <0.01).
Figure 2.
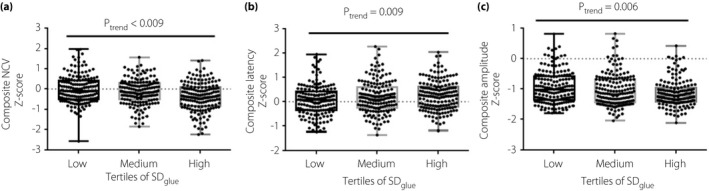
Dot plots for composite Z‐score of nerve conduction parameters, according to tertiles of glucose standard deviation (SDgluc). Results were represented as the mean ± standard error. (a) Composite Z‐score of nerve conduction velocity across SDgluc tertiles; (b) Composite Z‐score of latency across SDgluc tertiles; (c) Composite Z‐score of amplitude across SDgluc tertiles. NCV, nerve conduction velocity.
Finally, we carried out multivariate linear regression to assess the correlation between GV parameters and peripheral nerve conduction function by applying five different models. Model 1 showed that after adjusting for age, sex, BMI and duration of diabetes, HbA1c was associated with all nerve conduction parameters (NCV: β = −0.313, P < 0.001; latency: β = 0.229, P < 0.001; amplitude: β = −0.180, P < 0.001). Further adjustment for SDgluc, but not MAGE, CVgluc or average glucose, attenuated the relationship between HbA1c and NCV (SDgluc: β = −0.124, P = 0.02; HbA1c: β = −0.245, P < 0.001). Additionally, after controlling for age, BMI, sex, duration of diabetes and HbA1c, NCV, but not distal latency or amplitude, was associated with SDgluc (Table 3).
Table 3.
Association of glucose variability parameters with nerve conduction parameters after adjustments
| Variable | Composite Z‐score of NCV | Composite Z‐score of latency | Composite Z‐score of amplitude | |||
|---|---|---|---|---|---|---|
| β | P‐value | β | P‐value | β | P‐value | |
| Model 1 | ||||||
| HbA1c | −0.313 | <0.001 | 0.229 | <0.001 | −0.180 | <0.001 |
| Model 2 | ||||||
| SDgluc | −0.124 | 0.021 | 0.048 | 0.367 | −0.082 | 0.146 |
| HbA1c | −0.245 | <0.001 | 0.202 | <0.001 | −0.135 | 0.018 |
| Model 3 | ||||||
| MAGE | −0.066 | 0.181 | 0.045 | 0.354 | −0.030 | 0.555 |
| HbA1c | −0.291 | <0.001 | 0.213 | <0.001 | −0.170 | 0.001 |
| Model 4 | ||||||
| CVgluc | −0.040 | 0.384 | 0.049 | 0.284 | 0.012 | 0.806 |
| HbA1c | −0.310 | <0.001 | 0.225 | <0.001 | −0.181 | <0.001 |
| Model 5 | ||||||
| Average glucose | −0.100 | 0.058 | 0.017 | 0.747 | −0.099 | 0.074 |
| HbA1c | −0.258 | <0.001 | 0.220 | <0.001 | −0.126 | 0.026 |
Model 1: adjusted for age, sex, body mass index (BMI), diabetes duration and hemoglobin A1c (HbA1c); model 2: adjusted for age, sex, BMI, diabetes duration, HbA1c and glucose standard deviation (SDgluc); Model 3: adjusted for age, sex, BMI, diabetes duration and HbA1c, mean amplitude of glycemic excursions (MAGE); model 4: adjusted for age, sex, BMI, diabetes duration, HbA1c and glucose coefficient of variation (CVgluc); model 5: adjusted for age, sex, BMI, diabetes duration, HbA1c and average glucose.
NCV, nerve conduction velocity.
DISCUSSION
In the present study, we investigated the correlation of GV parameters in individuals with type 2 diabetes mellitus and subclinical diabetic polyneuropathy. The results showed that increased GV, revealed as SDgluc, was significantly associated with abnormal nerve conduction. The higher the SDgluc, the lower the composite Z‐scores of NCV and response amplitude, and the higher the composite Z‐score of latency. After controlling for potential confounders, elevated SDgluc was still significantly associated with a slower NCV.
DPN is a common disease with a complicated pathogenesis and diverse mechanisms, including hypertension, dyslipidemia, diabetes duration and alcohol consumption 19 , 20 , 21 . Unlike other microvascular complications of diabetic retinopathy and nephropathy, there is little information regarding the relationship between GV and DPN, and whether GV is an independent contributor to DPN remains controversial. A prospective study found that quarterly point‐of‐care glucose values, reflecting within‐day GV, did not contribute to the development of microvascular complications independent of the mean glucose level in type 1 diabetes 13 . Nevertheless, Pai showed that individuals with a higher CV of fasting plasma glucose had an obviously greater risk of DPN and no interaction effects between CV of fasting plasma glucose and HbA1c 22 , 23 . Hu et al. 12 found that GV, assessed as MAGE, had a strong relationship with DPN in individuals with type 2 diabetes mellitus, and the MAGE threshold of 4.60 mmol/L was considered the cut‐off point to identify DPN. In the present study, GV assessed by SDgluc was an independent factor correlated with nerve conduction function in patients with subclinical diabetic neuropathy.
Although the known influence of HbA1c on the onset and development of diabetic vascular complications is definite, GV, as a pattern of glycemic disorders, is complementary to the development of diabetic vascular complications. Several mechanisms, including altered peripheral blood flow, damage to small fibers and central sensitization, have been implicated in the psychophysiological processes of DPN 24 .
The lifetime incidence of diabetic neuropathy is approximately 45% in patients with type 2 diabetes mellitus, and even higher in patients with type 1 diabetes mellitus. Studies of nerve conduction tests carried out at the time of diabetes mellitus diagnosis showed that diabetic neuropathy was already present in patients with subclinical neuropathy; that is, without symmetrical distal numbness and pain 25 , 26 . Accordingly, a nerve conduction study is a commonly used method to detect nerve conduction abnormalities, even in patients with subclinical diabetic polyneuropathy. In the present study, electrophysiological assessments of peripheral nerves were carried out, which provided multiple markers of peripheral nerve function. Therefore, composite Z‐scores were constructed to reflect overall neurological function, because composite nerve conduction test Z‐scores have been shown to be sensitive and reproducible to correlate with neuropathic impairment 17 . Nerve conduction function has a positive correlation with the composite NCV and amplitude Z‐scores, and a negative correlation with the composite Z score for distal latency.
CVgluc and SDgluc are the most common parameters of GV because of their availability, simplicity and certainty. In general, CVgluc is correlated with a risk of hypoglycemia and has a weak association with the average glucose level. The SDgluc can reflect both within‐ and between‐day variability. Thus, SDgluc has a moderate correlation with the average glucose level, but a weak relationship with the risk of hypoglycemia. MAGE is another GV metric that is applied to access the intraday GV by computing the mean height of the glycemic fluctuations between consecutive nadirs and peaks that were >1 SDgluc. MAGE is estimated by computer programs, resulting in discrepancies due to differences in algorithms, definitions and the degree of initial smoothing of the blood glucose curve over time 27 . Hu et al. 12 found that increased GV was a significant independent risk factor for DPN in patients with type 2 diabetes mellitus. The enrolled participants presented with neuropathic symptoms and signs, an abnormal nerve conduction test, and a higher HbA1c level (10.18% in the DPN group). In the present study, we enrolled individuals with subclinical diabetic polyneuropathy and moderately elevated HbA1c levels (8.97% in the abnormal nerve conduction group). We hypothesized that SDgluc might reflect inter‐ and intraday GV, and have a close relationship with subclinical diabetic polyneuropathy, the early stage of DPN.
Several limitations to our research should be addressed. First, this was a cross‐sectional observational study to show the association between GV and DPN based on a large‐scale sample of individuals with type 2 diabetes mellitus. A prospective follow‐up study is required to further examine the effect of GV on DPN. Second, it has recently been shown that long‐term GV, as determined by the variability of HbA1c values, has an effect on micro‐ and macrovascular complications 28 . Although the present study assessed only short‐term GV through CGM, long‐term GV, such as HbA1c variability, could be assessed further. Third, this study was carried out in a single clinical center with a Chinese population, and investigation in other races and countries is required for further generalization. Finally, concomitant medication data were not available in the electronic medical records for this study. However, to our knowledge, there is a limited number of studies focusing on the direct effect of antidiabetic medication on DPN, and we excluded subjects using vitamin B1, vitamin B12 and folic acid, which can affect nerve conduction function.
In conclusion, increased GV showed that SDgluc was a significant independent contributor to subclinical diabetic polyneuropathy, in addition to conventional risk factors, including diabetes duration and HbA1c. It is suggested that blood SDgluc might be another potential target for the management of subclinical diabetic polyneuropathy.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The present study protocol was approved by the Institutional Review Board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital in accordance with the principles of the Helsinki Declaration (approval no. 2012–21).
Informed consent: Written informed consent was obtained from all participants.
Approval date of registry: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This work was supported by The National Natural Science Foundation of China (Grant No. 82070913), Shanghai Science and Technology Development Funds (Grant No. 20ZR1446000), Shanghai Professional and Technical Services Platform (18DZ2294100), and Research start‐up fund from Shanghai Fourth People's Hospital (sykyqd01801).
J Diabetes Investig 2022; 13: 328–335
REFERENCES
- 1. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 3. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824. [DOI] [PubMed] [Google Scholar]
- 4. Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014; 160: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Diabetes Care 2015; 38: 793–800. [DOI] [PubMed] [Google Scholar]
- 6. Janghorbani M, Rezvanian H, Kachooei A, et al. Peripheral neuropathy in type 2 diabetes mellitus in Isfahan, Iran: prevalence and risk factors. Acta Neurol Scand 2006; 114: 384–391. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Microvascular complications and foot care: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl 1): S124–S138. [DOI] [PubMed] [Google Scholar]
- 8. Bae JS, Kim BJ. Subclinical diabetic neuropathy with normal conventional electrophysiological study. J Neurol 2007; 254: 53–59. [DOI] [PubMed] [Google Scholar]
- 9. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019; 7: 221–230. [DOI] [PubMed] [Google Scholar]
- 10. Ceriello A, Ihnat MA. ‘Glycaemic variability’: a new therapeutic challenge in diabetes and the critical care setting. Diabet Med 2010; 27: 862–867. [DOI] [PubMed] [Google Scholar]
- 11. Frontoni S, Di Bartolo P, Avogaro A, et al. Glucose variability: an emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract 2013; 102: 86–95. [DOI] [PubMed] [Google Scholar]
- 12. Hu Y‐M, Zhao L‐H, Zhang X‐L, et al. Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine 2018; 60: 292–300. [DOI] [PubMed] [Google Scholar]
- 13. Lachin JM, Bebu I, Bergenstal RM, et al. Association of glyceamic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care 2017; 40: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 2011; 44: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Lu J, Lu W, et al. Evaluating peripheral nerve function in asymptomatic patients with type 2 diabetes or latent autoimmune diabetes of adults (LADA): results from nerve conduction studies. J Diabetes Complications 2015; 29: 265–269. [DOI] [PubMed] [Google Scholar]
- 17. Dyck PJ, O'Brien PC, Litchy WJ, et al. Monotonicity of nerve tests in diabetes: subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care 2005; 28: 2192–2200. [DOI] [PubMed] [Google Scholar]
- 18. Bao Y, Chen L, Chen L, et al. Chinese clinical guidelines for continuous glucose monitoring (2018 edition). Diabetes Metab Res Rev 2019; 35: e3152. [DOI] [PubMed] [Google Scholar]
- 19. Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013; 36: 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldman EL, Callaghan BC, Pop‐Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers 2019; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sone H, Mizuno S, Yamada N. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 1925–1927. [PubMed] [Google Scholar]
- 22. Pai Y‐W, Lin C‐H, Lee I‐T, et al. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab 2018; 44: 129–134. [DOI] [PubMed] [Google Scholar]
- 23. Yang CP, Li CI, Liu CS, et al. Variability of fasting plasma glucose increased risks of diabetic polyneuropathy in T2DM. Neurology 2017; 88: 944–951. [DOI] [PubMed] [Google Scholar]
- 24. Jin HY, Lee KA, Park TS. The impact of glycemic variability on diabetic peripheral neuropathy. Endocrine 2016; 53: 643–648. [DOI] [PubMed] [Google Scholar]
- 25. Zilliox L, Russell JW. Treatment of diabetic sensory polyneuropathy. Curr Treat Options Neurol 2011; 13: 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albers JW, Herman WH, Pop‐Busui R, et al. Subclinical neuropathy among diabetes control and complications trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care 2007; 30: 2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodbard D. Glucose variability: a review of clinical applications and research developments. Diabetes Technol Ther 2018; 20: s25–s215. [DOI] [PubMed] [Google Scholar]
- 28. Jang JY, Moon S, Cho S, et al. Visit‐to‐visit HbA1c and glucose variability and the risks of macrovascular and microvascular events in the general population. Sci Rep 2019; 9: 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]


