Abstract
Background.
Chlamydia trachomatis (CT) infection remains highly prevalent and young women are disproportionately affected. Most CT-infected women are asymptomatic and their infection often goes unrecognized and untreated. We hypothesized that testing for active CT infection with molecular diagnostics and obtaining a reported history of CT infection underestimates the prevalence of current and past CT infection, and incorporating serum CT antibody testing in addition to these other prevalence measures would generate more accurate estimates of the prevalence of CT infection in asymptomatic young women.
Methods.
We enrolled 362 asymptomatic women ages 16–29 years at four different clinical settings in Birmingham, AL, between August 2016 to January 2020 and determined the prevalence of CT infection based on having one or more of the following prevalence measures: an active urogenital CT infection based on molecular testing, reported prior CT infection, and/or being CT seropositive. Multivariable regression analysis was used to determine predictors of the prevalence of CT infection after adjustment for participant characteristics.
Results.
The prevalence of CT infection was 67.7% (95% CI, 62.6%–72.5%). Addition of CT antibody testing to the other individual prevalence measures more than doubled the CT infection prevalence. Non-Hispanic black race, reported prior gonorrhea, and reported prior trichomoniasis predicted a higher prevalence of CT infection.
Conclusions.
More than half of women were unaware of ever having CT infection, suggesting many were at risk for CT-associated reproductive complications. These data reinforce the need to adhere to chlamydia screening guidelines and to increase screening coverage in those at risk.
Keywords: chlamydia, prevalence, predictors, women, antibody
Short Summary
An estimate of chlamydia prevalence based on self-report and antibody and molecular testing revealed that over half of women were unaware of ever having chlamydia.
INTRODUCTION
Chlamydia trachomatis (CT) urogenital infection remains the most prevalent bacterial sexually transmitted infection (STI) worldwide and in the U.S.1,2 In the U.S. in 2018, there were over 1.75 million CT cases reported to the Centers for Disease Control and Prevention (CDC),2 although estimates suggest the actual case number is about 2-fold higher.3 The CT infection rate in women is more than double the rate in men, and the majority of CT infections reported to the CDC are among women under the age of 30 years.2 Women with untreated CT infections are at risk for reproductive complications (e.g., infertility) and perinatal complications (e.g., preterm birth).4 Among treated CT-infected women, reinfection occurs in about 10%–20% within 6 months after treatment.5,6
CT infection control efforts in the U.S. focus primarily on early detection of CT infection and prompt, effective treatment to prevent CT-associated reproductive morbidity and transmission of CT to others. Because most CT-infected women are asymptomatic, CT detection relies primarily on voluntary screening, which is recommended by the CDC annually for sexually active women age <25 years and older women with risk factors; highly sensitive CT nucleic acid amplification tests (NAATs) are the recommended tests for screening.7 For CT-infected women, repeat screening at 3 months after treatment is recommended.7 Despite control efforts, reported CT cases in the U.S. are at an all-time high,2 which suggests a large number of unidentified CT infections being transmitted to others.
The overall burden of CT infection among young women is unknown because cross sectional surveys and voluntary annual CT screening using CT NAAT only capture some active CT infections at a single point in time. These strategies likely underestimate the real prevalence of CT infection as a result of insufficient provider adherence with annual CT screening recommendations,8 lack of healthcare access to obtain screening,9 suboptimal adherence with repeat screening after CT infection treatment (to detect reinfections),10 and incident infections spontaneously resolving in between annual screenings,11–13 which could be detected by more frequent screening. In order to inform policy, recommendations, and guidelines, it is important to have an accurate estimate of the prevalence of CT infection and associated predictors among women. In a cross-sectional study of CT infection prevalence in asymptomatic young women seen in four different clinical settings in Birmingham, AL, we incorporated serum CT antibody testing in addition to current CT NAAT results and self-reported history of CT infection in an effort to determine the prevalence of CT infection in young women and estimate the proportion of women in whom one or more CT infections have been previously missed.
MATERIALS AND METHODS
Study Design, Participants, and Procedures
Between August 2016 to January 2020, we enrolled women ages 16–29 years without urogenital symptoms who presented to the following types of university affiliated clinical sites in Birmingham, Alabama: primary care internal medicine, adolescent health, student health, and emergency medicine. This age range was chosen based on 2018 CDC surveillance data showing most reported CT infections in women occurred in this age group.2 Exclusion criteria were: reported urinary, genital, or pelvic symptoms; presence of pregnancy, HIV infection, diabetes mellitus, or an immunocompromised state (e.g. autoimmune disease, malignancy, immunosuppressive therapy); exposure to antibiotics with anti-CT activity in the preceding one month; or prior hysterectomy.
Women were consecutively recruited on days when research staff were available to recruit at the clinical site(s) and were enrolled after written informed consent was obtained. Participants were seen for a single study visit at enrollment in which they were interviewed by trained research staff and self-reported clinical data on demographics, medical history, symptoms, medications (including hormonal contraceptive therapy and current or recent antibiotics), sexual history and history of prior STIs were collected onto a case report form and scanned electronically into a database using TeleForm software. Participants self-collected a vaginal swab for CT and Neisseria gonorrhoeae (NG) NAAT (Aptima Combo 2; Hologic, Marlborough, MA) and provided blood, from which serum was separated for CT serological testing. Clinical and laboratory data were linked by a unique study number. Participants did not complete the study until their NAAT results were available and any participant with a positive NAAT was contacted and treated with a CDC recommended treatment regimen.7 The study was approved by the University of Alabama at Birmingham Institutional Review Board.
NAAT and Serological Testing
A NAAT for CT and NG was performed on all vaginal swab specimens per the manufacturer’s protocol (Hologic). A CT-specific IgG1 response was measured in serum using a CT elementary body (EB) enzyme-linked immunosorbent assay (ELISA) that has been previously shown to be highly sensitive, without specificity concerns, and capable of detecting recently resolved CT infections and remote CT infections.14–16 EB ELISA methods have been previously reported.14 Briefly, ELISA was performed using formalin-fixed CT EBs pooled from serovars D, F, and J. IgG1 response was detected using alkaline phosphatase–labeled mouse antihuman IgG1 (a pool of clones 4E3, Southern Biotech, Birmingham, Alabama; and HP6069, Cal Biochem, San Diego, California) at an optical density of 405 nm (OD405). The cutoff OD405 value for a positive IgG1 was >0.35. Each participant’s serum was run in triplicate at a 1:32 dilution.
Statistical Analyses
We determined the prevalence of CT infection to be the proportion of women positive for at least one of the following three prevalence measures: active urogenital CT infection based on a positive CT NAAT, prior CT infection based on participant self-report, and CT seropositivity (which can detect missed CT infection in those with a negative CT NAAT and absence of reported prior CT infection). Prevalence measures are presented in proportion and 95% confidence intervals (CIs). Differences in participant characteristics across the four clinical sites were evaluated using the Pearson’s chi-squared, Fisher’s exact, analysis of variance, or Kruskal-Wallis tests as appropriate. We evaluated the univariate association of participant characteristics with CT infection prevalence measures using the Pearson’s chi-squared test, Fisher’s exact, t-test, or Wilcoxon rank-sum test as appropriate. We conducted a multivariable regression analysis of participant characteristics associated with the prevalence of CT infection that included covariates with a P value of <0.05 on univariate analysis. Multivariable analysis findings are presented in adjusted odds-ratios (aORs), 95% CIs, and P values. All P values presented are 2-sided. Analyses were conducted on Stata (version 14.0, StataCorp, College Station, Texas).
RESULTS
Participant Characteristics
There were 362 participants enrolled at the four clinical sites: adolescent health (N = 44), emergency medicine (N = 195), primary care (N = 20) and student health (N = 103). Most were of non-Hispanic black (62.4%) or non-Hispanic white (27.6%) race, and 5.5% were of Hispanic ethnicity (Table 1). The mean age was 23 years. Most participants (89%) reported being sexually active in the prior 3 months. Current genital CT infection and gonorrhea were detected (by NAAT) in 5.0% and 1.7%, respectively.
Table 1.
Characteristics of 362 Asymptomatic Women Enrolled in Different Clinical Settings in Birmingham, Alabama, 2016–2020
| Characteristic | No. (%) |
|---|---|
| Clinical Settings | |
| Adolescent Health | 44 (12.15) |
| Emergency medicine | 195 (53.87) |
| Primary care internal medicine | 20 (5.52) |
| Student health | 103 (28.45) |
| Mean age (SD) | 23 (3.8) |
| Race | |
| Non-Hispanic black | 226 (62.4) |
| Non-Hispanic white | 100 (27.6) |
| Other | 36 (9.9) |
| Hispanic ethnicity | 20 (5.5) |
| Mean years sexually active (SD) | 6.5 (4.2) |
| Median lifetime sexual partners (range) | 5 (1–40) |
| Proportion sexually active in last 3 months | 322 (89.0) |
| Median sex partners 3 months (range)* | 1 (1–4) |
| Sexual partner type in last 3 months* | |
| Male | 295 (91.6) |
| Female | 18 (5.6) |
| Both | 9 (2.8) |
| New sexual partner in last 3 months* | 90 (28.0) |
| Hormonal contraception use | 159 (43.9) |
| Current genital chlamydia | 18 (5.0) |
| Current genital gonorrhea | 6 (1.7) |
| Reported prior STIs or vaginal infections | |
| Chlamydia | 102 (28.2) |
| Gonorrhea | 36 (9.9) |
| Trichomoniasis | 65 (18.0) |
| Candidiasis | 219 (60.5) |
| Syphilis | 1 (0.3) |
| Genital herpes | 11 (3.0) |
| Bacterial vaginosis | 86 (23.8) |
| Genital warts | 3 (0.8) |
Abbreviations: STIs, sexually transmitted infections; SD, standard deviation.
Denominator for these characteristics is 322, since they only include participants that were sexually active in the last 3 months.
Upon stratification of participant characteristics by site of enrollment, we found several characteristics differed significantly between the clinical sites (Table 2). Regarding race distribution, participants from the adolescent health and emergency medicine sites were predominantly non-Hispanic black (81.8% and 78.0%, respectively), whereas at the primary care and student health sites, the most common racial category was non-Hispanic white (75.0% and 50.0%, respectively). The average age of participants at the adolescent health site (17.9 years) was lower than other sites (>21 years). In terms of sexual history, participants at the emergency medicine site had been sexually active for longer (mean 8.5 years) and had the highest lifetime number of sexual partners (median 5) compared to the other clinical sites. In the 3 months prior to the study, participants at the student health site had the highest reported frequency of being sexually active (95.2%) and having a new sexual partner (41.8%). Participants at the adolescent health and emergency medicine sites more often reported prior CT infection, gonorrhea, and trichomoniasis, compared to the primary care and student health sites.
Table 2.
Characteristics of 362 Asymptomatic Women that Differed by Clinical Setting, Birmingham, AL, 2016–2020
| Characteristic | Adolescent Health (N = 44) | Emergency Medicine (N = 195) | Primary Care Internal Medicine (N = 20) | Student Health (N = 103) | P value* |
|---|---|---|---|---|---|
| Non-Hispanic black race | 36 (81.8) | 152 (78.0) | 3 (15.0) | 35 (34.0) | <0.001 |
| Mean age (SD) | 17.9 (1.5) | 24.7 (3.3) | 23.7 (3.2) | 21.6 (2.9) | <0.001 |
| Sexually active in last 3 months | 31 (70.5) | 176 (90.3) | 17 (85.0) | 98 (95.2) | <0.001 |
| Median days since last sex (range)† | 14 (1–90) | 5(1–90) | 5 (1–51) | 9 (1–60) | 0.002 |
| New sexual partner in last 3 months† | 8 (25.8) | 36 (20.4) | 5 (29.4) | 41 (41.8) | 0.002 |
| Mean years sexually active (SD) | 2.6 (1.9) | 8.5 (3.8) | 6.5 (3.9) | 4.3 (3.5) | <0.001 |
| Median lifetime sexual partners (range) | 2 (1–30) | 5 (1–100) | 4 (1–30) | 4 (1–27) | <0.001 |
| Hormonal contraception use | 29 (65.9) | 52 (26.7) | 9 (45.0) | 69 (66.9) | <0.001 |
| Reported prior STIs or vaginal infections | |||||
| Chlamydia | 16 (36.4) | 66 (33.8) | 2 (10) | 18 (17.5) | 0.003 |
| Gonorrhea | 7 (15.9) | 27 (13.9) | 0 (0) | 2 (1.9) | 0.002 |
| Trichomoniasis | 10 (22.7) | 50 (25.6) | 0 (0) | 5 (4.9) | <0.001 |
| Candidiasis | 19 (43.2) | 138 (70.8) | 9 (45.0) | 53 (51.5) | <0.001 |
| Bacterial vaginosis | 4 (9.1) | 60 (30.8) | 1 (5.0) | 21 (20.4) | 0.002 |
Abbreviations: STI, sexually transmitted infection; SD, standard deviation. Data are no. (%) unless otherwise noted.
Determined using the Pearson’s chi-squared test, ANOVA, or Kruskal-Wallis test as appropriate.
Denominator for these characteristics is 322, since they only include participants that were sexually active in the last 3 months.
Prevalence of CT Infection
Considering CT infection prevalence measures, 102 participants (28.2%; 95% CI, 23.6%–33.1%) reported a prior CT infection (with 73.1% of all participants reporting having prior CT testing) and 18 participants (5.0%; 95% CI, 3.0%–7.7%) had a positive CT NAAT at enrollment; there were 109 participants (30.1%; 95% CI, 25.4%–35.1%) who reported a history of prior CT infection and/or had a positive CT NAAT (Fig. 1). Upon incorporating CT seropositivity status as an additional CT prevalence measure, the CT infection prevalence increased to 67.7% (95% CI, 62.6%–72.5%) (N = 245), with 230 participants (63.5%; 95% CI, 58.3%–68.5%) being CT seropositive.
Figure 1.
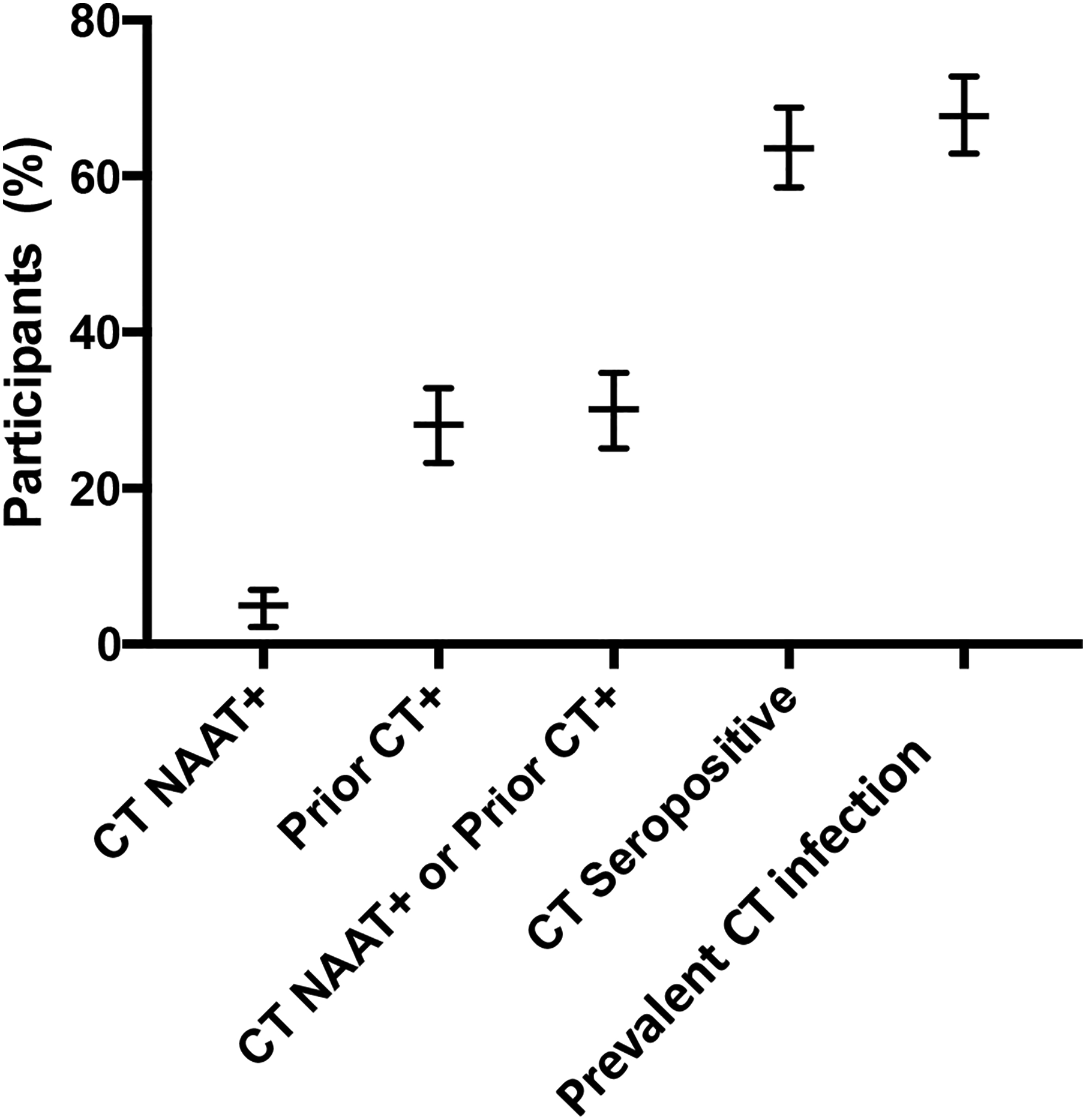
Different Chlamydia trachomatis (CT) infection prevalence measures and 95% confidence intervals (CIs) among 362 asymptomatic women enrolled in Birmingham, Alabama, 2016–2020: 1) CT nucleic acid amplification tests (NAAT) positive (+) (N = 18; 5.0%; 95% CI, 3.0%–7.7%), 2) reported prior CT infection (N = 102; 28.2%; 95% CI, 23.6%–33.1%), 3) CT NAAT+ and/or reported prior CT infection (N = 109; 30.1%; 95% CI, 25.4%–35.1%), 4) CT seropositive based on detection of CT-specific antibody by a CT elementary body ELISA (N = 230; 63.5%; 95% CI, 58.3%–68.5%), and 5) CT infection prevalence defined as the percentage of subjects with CT NAAT+, reported prior CT infection, and/or CT seropositive (N = 245; 67.7%; 95% CI, 62.6%–72.5%).
Associations of Participant Characteristics with CT infection Prevalence Measures
We evaluated the univariate associations of participant characteristics with the following CT infection prevalence measures: CT NAAT positivity alone (reflecting current CT infection), CT NAAT positivity or reported prior CT infection (reflecting one or more CT infections now known to the participant), CT seropositivity alone (reflecting current and/or previous CT infections based on serum CT antibody testing), and CT infection prevalence based on the presence of at least one of the three prevalence measures (reflecting one or more CT infections based on CT NAAT positivity, reported prior CT infection, and/or CT seropositivity) (Table 3). Non-Hispanic black race was associated with a higher CT infection prevalence for all prevalence measures. Younger age was associated with a higher CT infection prevalence by CT NAAT positivity alone and CT seropositivity alone. Hormonal contraception use was associated with a lower prevalence of CT infection by all measures except CT NAAT positivity alone. More years of sexual activity and reported prior gonorrhea, trichomoniasis, and bacterial vaginosis were associated with a higher CT infection prevalence by all measures except CT NAAT positivity alone. There was also a significant difference in the CT infection prevalence across the four clinical sites for all prevalence measures except CT NAAT positivity alone, with prevalence being highest in the adolescent health and emergency medicine sites, followed by the student health site and then the primary care site.
Table 3.
Univariate Analyses of Predictors of Chlamydia trachomatis Infection Prevalence Measures among 362 Asymptomatic Women Enrolled in Birmingham, Alabama, 2016–2020
| Participant characteristics | CT NAAT | P value | NAAT and/or Reported Prior CT Infection | P value | CT Seropositivity | P value | CT Infection Prevalence* | P value† | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + (N = 18) |
− (N = 344) |
+ (N = 109) |
− (N = 253) |
+ (N= 230) |
− (N= 132) |
+ (N = 245) |
− (N = 117) |
|||||
| Mean age (SD) | 20.2(3.1) | 23.1(3.8) | 0.002 | 23 (3.8) | 22.9 (3.8) | 0.937 | 23.3 (3.9) | 22.3 (3.5) | 0.017 | 23.2 (3.9) | 22.4 (3.6) | 0.074 |
| Non-Hispanic black race | 16 (88.9) | 210(61.1) | 0.017 | 88 (80.7) | 138(54.6) | <0.001 | 179(77.8) | 47(35.6) | <0.001 | 185(75.5) | 41 (35.0) | <0.001 |
| Hispanic ethnicity | 1 (5.6) | 19 (5.5) | 0.995 | 5 (4.6) | 15 (5.9) | 0.608 | 8 (3.5) | 12 (9.1) | 0.024 | 10 (4.1) | 10 (8.5) | 0.082 |
| Clinical Settings | 0.613 | 0.003 | <0.001 | <0.001 | ||||||||
| Adolescent Health | 3 (16.7) | 41 (11.9) | 16(14.7) | 28 (11.1) | 33 (14.4) | 11 (8.3) | 35 (14.3) | 9 (7.7) | ||||
| Emergency medicine | 11 (61.1) | 184(53.5) | 71 (65.1) | 124 (49.0) | 148(64.4) | 47(35.6) | 153(62.4) | 42 (35.9) | ||||
| Primary care internal medicine | 0 (0) | 20 (5.8) | 2(1.8) | 18 (7.1) | 5 (2.2) | 15 (11.4) | 5 (2.0) | 15 (12.8) | ||||
| Student health | 4 (22.2) | 99 (28.8) | 20 (18.4) | 83 (32.8) | 44 (19.1) | 59 (44.7) | 52 (21.2) | 51 (43.6) | ||||
| Sexually active last 3 months | 16 (88.9) | 306(88.9) | 0.993 | 98 (89.9) | 224 (88.5) | 0.703 | 205(89.1) | 117(88.6) | 0.885 | 218(89.0) | 104(88.9) | 0.979 |
| Median no. of partners (range)†† | 1 (1–4) | 1 (1–2) | 0.122 | 1 (1–3) | 1 (1–4) | 0.184 | 1 (1–3) | 1 (1–4) | 0.017 | 1 (1–3) | 1 (1–4) | 0.420 |
| Partner type†† | 0.434 | 0.050 | 0.395 | 0.176 | ||||||||
| Male | 15(93.7) | 280(91.5) | 95 (96.9) | 200 (89.3) | 191(93.2) | 104(88.9) | 204(93.6) | 91 (87.5) | ||||
| Female | 0 (0) | 18 (5.9) | 1 (1.02) | 17 (7.6) | 9 (4.4) | 9 (7.8) | 9 (4.1) | 9 (8.6) | ||||
| Both | 1 (6.2) | 8 (2.6) | 2 (2.0) | 7 (3.1) | 5 (2.4) | 4 (3.4) | 5 (2.3) | 4 (3.8) | ||||
| Median days of last sex (range)†† | 5.5(1–21) | 7 (0–90) | 0.368 | 4 (0–72) | 7 (0–90) | 0.001 | 7 (1–90) | 7 (1–60) | 0.119 | 7 (0–90) | 7 (1–60) | 0.232 |
| New partner†† | 7(43.7) | 83 (27.1) | 0.149 | 27 (27.5) | 63 (28.1) | 0.916 | 46 (22.4) | 44 (37.6) | 0.004 | 54 (24.8) | 36 (34.6) | 0.066 |
| Mean years sexually active (SD) | 4.4 (3.9) | 6.6 (4.2) | 0.035 | 7.1 (4.0) | 6.2 (4.3) | 0.069 | 7.0 (4.2) | 5.6 (4.1) | 0.001 | 6.9 (4.2) | 5.5 (4.2) | 0.003 |
| Median lifetime sexual partners | 3.5 (1–10) | 5 (1–40) | 0.508 | 5 (1–60) | 4 (1–40) | 0.001 | 5 (1–60) | 4 (1–40) | 0.581 | 5 (1–60) | 4 (1–40) | 0.145 |
| (range) | ||||||||||||
| Hormonal contraception use | 6 (33.3) | 153(44.5) | 0.353 | 35 (32.1) | 124 (49.0) | 0.003 | 87 (37.8) | 72(54.5) | 0.002 | 94 (38.4) | 65 (55.6) | 0.002 |
| Current genital gonorrhea | 3 (16.7) | 3 (0.9) | 0.002 | 5 (4.6) | 1 (0.4) | 0.011 | 5 (2.2) | 1 (0.8) | 0.422 | 6 (2.5) | 0 (0) | 0.183 |
| Reported prior STIs‡ or vaginal infections | ||||||||||||
| Gonorrhea | 2 (11.1) | 34 (9.9) | 0.865 | 26 (23.9) | 10 (4.0) | <0.001 | 33(14.4) | 3 (2.3) | <0.001 | 35(14.3) | 1 (0.9) | <0.001 |
| Trichomoniasis | 3 (16.7) | 62 (18.0) | 0.884 | 33 (30.3) | 32 (12.7) | <0.001 | 56 (24.4) | 9 (6.8) | <0.001 | 59 (24.1) | 6 (5.1) | <0.001 |
| Candidiasis | 5 (27.8) | 214(62.2) | 0.004 | 67 (61.5) | 152 (60.1) | 0.804 | 139(60.4) | 80(60.6) | 0.974 | 147(60.0) | 72(61.5) | 0.779 |
| Syphilis | 0 (0) | 1 (0.3) | 0.819 | 1 (0.9) | 0 (0) | 0.127 | 1 (0.4) | 0 (0) | 0.448 | 1 (0.4) | 0 (0) | 0.489 |
| Herpes | 2 (11.1) | 9 (2.6) | 0.041 | 8 (7.3) | 3 (1.2) | 0.002 | 10 (4.4) | 1 (0.8) | 0.055 | 10 (4.1) | 1 (0.9) | 0.094 |
| BV | 4 (22.2) | 82 (23.8) | 0.875 | 41 (37.6) | 45 (17.8) | <0.001 | 66 (28.7) | 20 (15.2) | 0.004 | 71 (29.0) | 15 (12.8) | 0.001 |
| Genital Warts | 0 (0) | 3 (0.9) | 0.691 | 1 (0.9) | 2 (0.8) | 0.903 | 1 (0.4) | 2 (1.5) | 0.275 | 1 (0.4) | 2 (1.7) | 0.202 |
Abbreviations: CT, Chlamydia trachomatis; STI, sexually transmitted infection; SD, standard deviation. Data are no. (%) unless otherwise noted.
Defined as the percentage of subjects with CT NAAT+, reported prior CT infection, and/or CT seropositive.
Determined using the Pearson’s chi-squared test, t-test, or Wilcoxon rank-sum test as appropriate.
Denominator for these characteristics is 322, since they only include participants that were sexually active in the last 3 months.
Reported prior CT infection not included.
In a multivariable analysis that evaluated predictors of prevalence of CT infection, only non-Hispanic black race (aOR 3.6; 95% CI, 2.1–6.3; P < 0.001), reported prior gonorrhea (aOR 8.9; 95% CI: 1.2–68.3; P = 0.036), and reported prior trichomoniasis (aOR 3.1; 95% CI: 1.2–7.9; P = 0.018) remained significantly associated with prevalence of CT infection (Table 4). The other variables that were no longer significantly associated with prevalence of CT infection were all significantly associated with non-Hispanic black race and thus, race was a confounding factor in univariate analyses. Compared with women of other races, non-Hispanic black women less often reported hormonal contraceptive use (34.5% vs. 59.6%), reported more years of sexual activity (median 7 years vs. 5 years), and more often reported prior bacterial vaginosis (32.3% vs. 9.6%) (all P ≤ 0.002)
Table 4.
Multivariable Analysis of Predictors of the Prevalence of Chlamydia trachomatis Infection among 362 Asymptomatic Women Enrolled in Birmingham, Alabama, 2016–2020
| Characteristic | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Non-Hispanic black race | 3.6 | 2.1–6.3 | <0.001 |
| Mean years sexually active | 1.0 | 0.96–1.1 | 0.534 |
| Hormonal contraception use | 0.8 | 0.50–1.4 | 0.502 |
| Reported prior gonorrhea | 8.9 | 1.2–68.3 | 0.036 |
| Reported prior trichomoniasis | 3.1 | 1.2–7.9 | 0.018 |
| Reported prior bacterial vaginosis | 1.3 | 0.63–2.6 | 0.489 |
| Clinical setting | 0.83 | 0.64–1.1 | 0.177 |
Abbreviations: CI, confidence interval; OR, odds ratio
DISCUSSION
Asymptomatic young women is a population in whom CT infection is common, often unrecognized, and can be associated with reproductive morbidity. An accurate CT infection prevalence measure is important for guiding recommendations on CT screening strategies. This is the first study to our knowledge to incorporate serum CT antibody testing with CT NAAT and reported CT infection history to determine the prevalence of CT infection in asymptomatic women and estimate the proportion of women in whom one or more CT infections have been previously missed. Our estimate of CT infection prevalence based on one of more of these three prevalence measures was 68%, which was more than double the prevalence when only based on having a positive CT NAAT or self-reported history of prior CT infection (30% total). Thus, CT serological testing was critical for identifying more than half of young women who had one or more previous CT infections go undetected. Previous population-based CT seroprevalence studies in England and New Zealand that used a CT Pgp3 serology assay also demonstrated that more than half of seropositive women did not report a previous diagnosis of CT infection;17,18 these studies did not determine the prevalence of CT infection based on having one or more of the three prevalence measures we evaluated. Together, these findings suggest that despite having CT screening guidelines and availability of highly sensitive CT NAATs, the majority of young women with CT infections do not have their infection detected and thus are at risk for silent reproductive complications. A previous study in women with PID by Dize et al. reported that one-third of the women had no reported prior STI and no current CT infection but had an antibody response to CT Pgp3,19 thus prior CT infections also often go undetected in women who later develop PID.
Among the reasons that CT infections are missed in young women is insufficient adherence to CDC recommended annual CT screening.8 Based on the reported 2018 data for the Healthcare Effectiveness Data and Information Set (HEDIS) measure of CT testing done at least once in women 16–24 years of age, only 48%–58% of women had testing performed (lowest for commercial Preferred Provider Organization providers and highest for Medicaid Health Maintenance Organization providers);20 73% of our participants reported prior CT testing, however the accuracy of this could not be confirmed. Another reason CT infections are missed is lack of healthcare access to obtain screening. We did not collect data on measures of access to care, including health insurance coverage, type of primary care provider, and healthcare seeking behaviors (all which influence opportunities for CT screening and treatment), which we showed in a previous study were associated with CT infection prevalance (based on CT NAAT positivity).9 CT infections are also missed due to suboptimal adherence to CDC recommended repeat screening after CT infection treatment to detect CT reinfections. A study by Hoover et al. that evaluated CT test data from a large US laboratory found that only about one-third of CT-infected women had repeat CT testing within 6 months of their initial positive CT test and 15% had CT detected again.10 Finally, CT infections are missed when incident infections occur and then spontaneously resolve (due to immune-medicated clearance) in between annual screenings. It has been reported that CT infections spontaneously resolve in up to 50% of women within a year of detection.11,12 Thus, assuming young women have CT screening conducted only on an annual basis per CDC recommendations,7 then up to 50% of infections would be missed; screening more frequently than annually could detect more CT infections.
Determining predictors of CT infection prevalence also has potential implications for guiding CT screening strategies, such as more frequent CT screening in higher CT-prevalence populations. For example, incorporating CT screening as opt-out testing or as automatically-ordered testing in asymptomatic females 16–24 years of age in emergency room settings would increase screening in a higher-prevalence population without adding additional task burden to providers. The only participant characteristic in our cohort of young women that was significantly associated with all of the different CT prevalence measures we studied was race, with all CT infection prevalence measures being higher in non-Hispanic black women compared with women of other races. While there were several participant characteristics other than race associated with the prevalence of CT infection on univariate analysis, reported prior gonorrhea and reported prior trichomoniasis were the only characteristics other than race that remained significantly associated with the prevalence of CT infection on multivariable analysis; this was due to race confounding the univariate associations with these other participant characteristics. The 2018 CDC surveillance data showed that CT infection rates based on positive CT NAAT were about 6-fold higher in black compared to white persons (including both Hispanic and non-Hispanic),2 which supports corresponding data from our study population in which there was about a 7-fold higher CT NAAT positivity frequency in black vs. white women (6.8% vs. 0.94%; data not shown in the Results section as this data includes both Hispanic and non-Hispanic women). The prevalence of CT infection was 2.3-fold higher in black vs. white women (81.3% vs. 34.9%; data not shown in the Results section per above reason), which still supports the data that black women have higher rates of CT infection. The reason for the higher CT infection prevalence in persons of black race remains unknown. Some studies have attributed racial disparities in CT infection prevalence to various demographic or socioeconomic factors,9, 21–24 while another study reported an association of race with CT infection independent of these factors.25 Ultimately, our data suggest that CT screening may be needed on more than an annual basis in some women, such as those in high CT infection prevalence settings, to detect missed infections which could lead to reproductive complications.
Our study had some limitations. Most clinical data and all sexual history data were self-reported and therefore their accuracy could not be verified. This is particularly relevant for our CT prevalence measure of reported prior CT infection. Among women reporting prior CT infection, 87% were seropositive and 13% were seronegative. Some of these seronegative women who reported prior CT infection may have been truly infected previously but tested seronegative while others may have inaccurately reported prior CT infection. While there may have been some misclassification of reported prior CT infection, it was critical to still include this outcome in our study for determining the proportion of women in whom one or more CT infections may have been previously missed if CT serological testing had not been incorporated into determining the prevalence of CT infection. We did not have data on the timing and frequency of prior CT testing, which could have been useful to further understand why some CT infections were not detected. We cannot rule out the possibility that some previously CT-infected individuals became seronegative based on our EB ELISA OD cutoff for anti-CT IgG1 positivity. However, we have previously shown that EB ELISA IgG1 OD readings in CT seropositive persons are stable at 6 months after an initial measurement14 and that the frequency of CT seropositivity using EB ELISA is high in certain infertile female populations,26 which suggests the anti-CT IgG1 response is long-lived. Although we enrolled participants from different clinical sites with diversity in participant characteristics, there was lower enrollment at some sites such as primary care, which may have underrepresented certain participant characteristics being evaluated; however the site of enrollment was not associated with prevalence of CT infection on the multivariable analysis. Additionally, we only studied clinic-based patient populations in Birmingham, AL, and thus it is unclear whether findings could be generalizable to other populations and to other geographic areas in the U.S.
In summary, our study demonstrated that the prevalence of CT infection in young women based on an CT seropositivity, CT NAAT positivity, and reported prior CT infection was more than 2-fold higher than the CT infection prevalence determined based on CT NAAT positivity and reported prior CT infection alone. The addition of CT serological testing to the other prevalence measures enabled identification of women in whom one or more CT infections were missed by the other measures. Thus, the majority of asymptomatic women were unaware of having a prior CT infection and may have been at risk for silent CT-associated reproductive complications. The majority of women had prior CT testing done, but the testing interval may not have been frequent enough to capture infections before they spontaneously resolved. Our study findings enforce the need for stricter adherence with recommendations for annual CT screening and partner notification and repeat screening after treatment of CT infection, and they also suggest CT screening in young women may be warranted more often than on an annual basis, especially in those in high CT infection prevalence settings, to detect missed infections which could lead to reproductive complications. Thus, our findings have the potential to impact CT testing recommendations and thereby contribute to improving current CT infection prevention and control efforts.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health [1K24AI125685 to W.M.G.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health.
We thank Meghan Whitfield, Peggy Fogg, Saralyn Richter, Kiana Wilson, Christen Press, Jasmine Walker McQueen, Erin Boyd, Lauren Walter, Joel Rodgers, and Kristal Aaron for their valuable contributions to the study.
Footnotes
Potential conflicts of interest: WMG and BVDP report receiving research funding from Hologic, Inc. outside the range of the current work. The other authors report no potential conflicts of interest.
REFERENCES
- 1.Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10:e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sexually Transmitted Diseases Surveillance 2018. Centers for Disease Control and Prevention, Atlanta, GA: 2019. [Google Scholar]
- 3.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40:187–193. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell CM, Ferone ME. Chlamydia trachomatis genital infections. Microb Cell 2016; 3:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 2009; 36:478–489. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol 2011; 118:231–239. [DOI] [PubMed] [Google Scholar]
- 7.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 8.Hoover KW, Leichliter JS, Torrone EA, et al. Chlamydia screening among females aged 15–21 years--multiple data sources, United States, 1999–2010. MMWR Suppl 2014; 63:80–88. [PubMed] [Google Scholar]
- 9.Geisler WM, Chyu L, Kusunoki Y, et al. Health insurance coverage, health care-seeking behaviors, and genital chlamydial infection prevalence in sexually active young adults. Sex Transm Dis 2006; 33:389–396. [DOI] [PubMed] [Google Scholar]
- 10.Hoover KW, Tao G, Nye MB, Body BA. Suboptimal adherence to repeat testing recommendations for men and women with positive Chlamydia tests in the United States, 2008–2010. Clin Infect Dis 2013; 56:51–57. [DOI] [PubMed] [Google Scholar]
- 11.Molano M, Meijer CJ, Weiderpass E, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 2005; 191:907–916. [DOI] [PubMed] [Google Scholar]
- 12.Morre SA, van den Brule AJ, Rozendaal L, et al. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS 2002; 13 Suppl 2:12–18. [DOI] [PubMed] [Google Scholar]
- 13.Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 2010; 201 Suppl 2:S104–113. [DOI] [PubMed] [Google Scholar]
- 14.Geisler WM, Morrison SG, Doemland ML, et al. Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis 2012; 206:1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K, Brown L, Bakshi RK, et al. Performance of Chlamydia trachomatis OmcB enzyme-linked immunosorbent assay in serodiagnosis of Chlamydia trachomatis infection in women. J Clin Microbiol 2018; 56:e00275–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakshi R, Gupta K, Jordan SJ, et al. Immunoglobulin-based investigation of spontaneous resolution of Chlamydia trachomatis infection. J Infect Dis 2017; 215:1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodhall SC, Wills GS, Horner PJ, et al. Chlamydia trachomatis Pgp3 antibody population seroprevalence before and during an era of widespread opportunistic chlamydia screening in England (1994–2012). PLoS One 2017; 12:e0152810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horner PJ, Wills GS, Righarts A, Vet al. Chlamydia trachomatis Pgp3 antibody persists and correlates with self-reported infection and behavioural risks in a blinded cohort study. PLoS One 2016; 11:e0151497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dize L, Martin D, Gwyn S, et al. Comparison of three serological assays to measure antibody response to Chlamydia antigen Pgp3 in adolescent and young adults with pelvic inflammatory disease. Int J STD AIDS 2018; 29:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Quality Assurance. State of Health Care Quality Report. https://www.ncqa.org/hedis/measures/chlamydia-screening-in-women/. Accessed May 9, 2020.
- 21.Learner ER, Torrone EA, Fine JP, et al. Chlamydia prevalence trends among women and men entering the National Job Training Program from 1990 through 2012. Sex Transm Dis 2018; 45:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen TQ, Ford CA, Kaufman JS, et al. Infrequent chlamydial testing among young adults: financial and regional differences. Sex Transm Dis 2008; 35:725–730. [DOI] [PubMed] [Google Scholar]
- 23.Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis 2012; 39:92–96. [DOI] [PubMed] [Google Scholar]
- 24.Sales JM, Smearman EL, Swartzendruber A, et al. Socioeconomic-related risk and sexually transmitted infection among African-American adolescent females. J Adolesc Health 2014; 55:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton DT, Morris M. The racial disparities in STI in the U.S.: Concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics 2015; 11:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorwitz RJ, Wiesenfeld HC, Chen PL, et al. Population-attributable fraction of tubal factor infertility associated with chlamydia. Am J Obstet Gynecol 2017; 217:336.e1–336.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]


