Case Presentation
A 66-year-old man with dyspnea reported symptom onset 5 years ago, triggered by prolonged standing or walking, and relieved immediately with sitting. Dyspnea progressed over recent 6 months. He could not walk 50 feet nor stand greater than 20 minutes, limiting his work as a pastor. He denied palpitations, orthopnea, or wheezing.
Serial vital signs were normal with orthostatic changes with standing. Jugular venous pressure was estimated 6 cm H2O with normal heart and lung sounds, and no hepatojugular reflex. Extensive cardiovascular and pulmonary evaluation was unremarkable (Table). A supine invasive cardiopulmonary exercise testing (iCPET) was performed. Resting right atrial pressure (7 mmHg), mean pulmonary artery pressure (mPAP, 18 mmHg), pulmonary capillary wedge pressure (PCWP, 12 mmHg), and cardiac output (CO) of 7.3 L/min were normal. With leg raise, mPAP rose to 23 mmHg and PCWP to 20 mmHg. At peak exercise, mPAP increased to 40 mmHg and PCWP to 28 mmHg alongside CO (15.3 L/min); ΔPCWP/ΔCO was 2.14 mmHg/L/min. He achieved maximal exercise (RER 1.18) and maximal aerobic capacity (pVO2) of 77% predicted. However, this study failed to reproduce the patient’s dyspnea.
Table:
Summary of Patient Characteristics and Cardiopulmonary Diagnostic Imaging Results in the Workup of Exertional and Positional Dyspnea
| Diagnostic Test | Results | |
|---|---|---|
| Supine diagnostic imaging tests | ||
| Orthostatic vital signs measured 3 minutes apart | Supine: blood pressure 140/89 mmHg, heart rate 65 bpm Sitting: blood pressure 147/92, heart rate 65 bpm Standing: blood pressure 129/73, heart rate 87 bpm |
|
| N-terminal pro-brain natriuretic peptide (serial checks) | 67 pg/mL or less (normal ref ≤ 225 pg/mL) | |
| Transthoracic echocardiography | Normal right and left ventricular cardiac function, ejection fraction ≥ 60%, normal wall thickness, normal valvular function and morphology, and no abnormal diastology | |
| Invasive coronary angiography | No obstructive coronary disease with mild luminal irregularities in the proximal LAD. Normal left ventricular end diastolic pressure of 17 mmHg | |
| Pulmonary function test | Adequate quality study. No obstructive or restrictive lung disease (FVC 86%, FEV1 81%, FEV1/FVC 94%, VC 86% predicted, respectively); diffusing capacity for carbon monoxide normal (73% predicted). | |
| Ventilation-perfusion scan | Very low probability lung scan for pulmonary embolism (absent) | |
| Resting right heart catheterization | Right atrial pressure | 7 mmHg |
| Pulmonary artery pressure | 29/12 (mean 18) mmHg; increased to mean 23 mmHg with leg raise | |
| Pulmonary capillary wedge pressure | 10 mmHg; increased to 20 mmHg with leg raise | |
| Cardiac output | 6.9 L/min (index 3.5 L/min-m2) | |
| Arteriovenous oxygen difference | 3.5 vol%;hemoglobin 13.6 g/dL | |
| Pulmonary vascular resistance | 1.2 Wood units | |
| Mean arterial pressure | 109 mmHg | |
| Heart rate | 63 bpm | |
| Exercise right heart catheterization | Pulmonary artery pressure | 60/25 (mean 40) mmHg |
| Pulmonary capillary wedge pressure | 28 mmHg | |
| Cardiac output | 15.3 L/min (index 7.6 L/min-m2) | |
| Arteriovenous oxygen difference | 10.5 vol%; hemoglobin 14.3 g/dL | |
| Pulmonary vascular resistance | 0.8 wood units | |
| Mean arterial pressure | 117 mmHg | |
| Heart rate | 116 bpm | |
| ΔmPAP/ΔCO | 2.62 mmHg/L/min | |
| ΔPCWP/ΔCO | 2.14 mmHg/L/min | |
| Cardiopulmonary exercise test | Peak VO2 | 18.6 mL/kg/min (77% predicted) |
| Peak RER | 1.18 (maximal effort) | |
| VE-VCO2 | 32 | |
| Cardiac magnetic resonance imaging with stress | Normal left ventricular and right ventricular cavity size, wall thickness, ejection fraction 69%, and no regional wall motion abnormalities. Atrial sizes normal. Normal valvular morphologies and functions. No evidence of myocardial infarction, scar, or infiltrative disease. Adenosine stress perfusion imaging demonstrated no inducible myocardial ischemia. Wall thickness were anteroseptal 1.0 cm and inferolateral 0.8 cm with left ventricular end-diastolic diameter 4.8 cm and end-systolic diameter 2.8 cm. Left atrial diameter 3.1 cm, area 19 cm2, and volume 74 mL (normal). Ascending aorta diameter 3.2 cm, and pulmonary artery 2.5 cm. | |
| Computed tomography arteriography | No evidence of aortic aneurysm or dissection. Mild coronary atherosclerosis. Prominent patent ductus arteriosus measuring 9 mm. No significant chamber enlargements. | |
| Upright diagnostic imaging | ||
| Resting right heart catheterization | Right atrial pressure | 5 mmHg |
| Pulmonary artery pressure | 22/13 (mean 16) mmHg | |
| Pulmonary capillary wedge pressure | 6 mmHg (mean) | |
| Cardiac output | 5.5 L/min (index 2.7 L/min-m2) | |
| Arteriovenous oxygen difference | 6.4 vol%; hemoglobin 13.9 g/dL | |
| Pulmonary vascular resistance | 1.8 wood units | |
| Mean arterial pressure | 123 mmHg | |
| Heart rate | 75 bpm | |
| Exercise right heart catheterization | Right atrial pressure | 7 mmHg |
| Pulmonary artery pressure | 40/17 (mean 23) mmHg | |
| Pulmonary capillary wedge pressure | 9 mmHg (mean) | |
| Cardiac output | 10.4 L/min (index 5.2 L/min-m2) | |
| Arteriovenous oxygen difference | 12.5 vol%; hemoglobin 14.2 g/dL | |
| Pulmonary vascular resistance | 1.3 wood units | |
| Mean arterial pressure | 94 mmHg | |
| Heart rate | 110 bpm | |
| ΔmPAP/ΔCO | 1.43 mmHg/L/min | |
| ΔPCWP/ΔCO | 0.61 mmHg/L/min | |
| Cardiopulmonary exercise test | Peak VO2 | 15 mL/kg/min (62% predicted) |
| Peak RER | 1.31 (maximal effort) | |
| VE-VCO2 | 32.6 | |
Abbreviations: CO, cardiac output; FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity; mPAP, Mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RER, Respiratory exchange ratio; VC, Vital capacity; VE-VCO2, Minute ventilation/carbon dioxide production slope; VO2, Maximal aerobic capacity.
The patient was diagnosed with exercise-induced heart failure with preserved ejection fraction (HFpEF) based on changes in invasive cardiac pressures with supine exercise. He was briefly trialed on spironolactone and furosemide for his exertional dyspnea. At follow-up, he unfortunately described worsened dyspnea, most apparent while upright. Given ongoing symptoms and postural nature of his dyspnea, he was evaluated with repeat iCPET off diuretics in the upright position. Repeat resting supine invasive pressures were largely unchanged. Unlike the supine study, upright iCPET revealed minimal changes in resting to peak exercise mPAP and PCWP (Figure). Profound dyspnea recurred at peak upright exertion within 6 minutes. He demonstrated a drop from resting to peak mean arterial systemic pressures (MAP; 123 to 94 mmHg) with suggestive signs of Bezold-Jarisch reflex including paradoxical bradycardic response (pulse 110 to 51 bpm) and hypotension that persisted into recovery (BP 83/38 [MAP 53] mmHg requiring treatment). Despite achieving maximal exercise (RER 1.31), he surprisingly had blunted increase in CO (5.5 to 10.4 L/min), only 62% predicted pVO2, yet normal ΔPCWP/ΔCO of 0.61 mmHg/L/min. Diagnosis was reclassified from HFpEF to cardiac preload failure. The patient was advised to increase fluid and salt intake, and utilize compression garments which remarkably improved upright and exercise dyspnea through follow-up.
Figure. Resting and Peak Exercise Pressure Changes by Position in the Workup of Exertional and Positional Dyspnea.
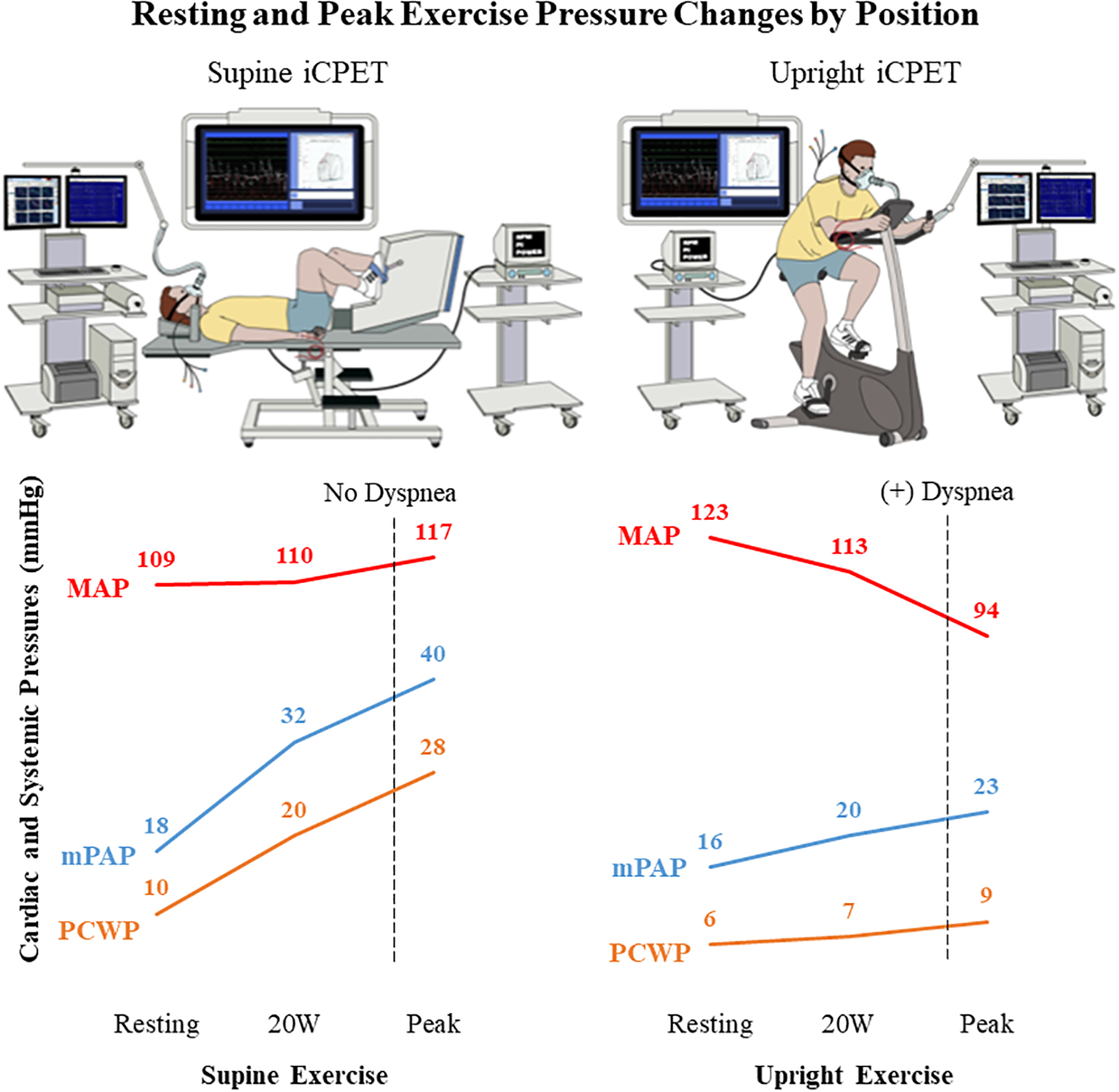
Use of supine versus upright invasive cardiopulmonary exercise testing (iCPET) in a patient with unexplained exertional and positional dyspnea to reclassify a diagnosis from heart failure with preserved ejection fraction to cardiac preload failure. Unlike the supine study, the upright iCPET revealed an inability to augment cardiac preload with exercise despite having normal resting volume. (Abbreviations: MAP, mean arterial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure)
Discussion:
Cardiac preload failure is an unusual but important cause of unexplained dyspnea on exertion. In this condition, right-sided filling does not adequately increase in response to activity despite normal resting volume, resulting in a blunted CO and inability to meet circulatory demands.1 Cardiac preload failure differs from normal exercise conditions in which preload increases with exercise from augmented venous return from abdominal and lower extremity compartments through peripheral muscle and respiratory pump contractions and constriction of capacitance vessels.1, 2 Though the mechanism is unknown, preload failure is thought to be related to either dysregulation of autonomic central venous volume recruitment or peripheral muscle and venous insufficiency, resulting in failed active and passive augmentation in CO, insufficient pulmonary perfusion, and inadequate blood delivery to active muscles.2–5 Cardiac preload failure may evade diagnosis by standard resting and supine exercise diagnostics as in the present case with unusual but important clues of postural and exertional nature to dyspnea.
Data on the prevalence of cardiac preload failure are few.1, 2 Among a single-center cohort of patients presenting with unexplained dyspnea and without structural heart disease, ~10% had preload failure.1 Upright exercise CO and pVO2 on average increased after a 2-liter saline infusion, yet a subset did not respond to volume loading,1 underscoring the importance of broadly identifying the prevalence of preload failure with impaired activation of venous reserves during exercise despite euvolemia. The proportion of patients with paradoxical dyspnea despite low pulmonary filling pressures is unclear.
This case highlights the value of upright versus supine hemodynamic testing in patients with symptoms that are limited to upright position. Our patient’s initial supine iCPET suggested exercise-induced HFpEF, characterized by peak PCWP of ≥25 mmHg with activity despite normal natriuretic peptide levels, euvolemic exam, and resting invasive cardiac pressures.6 However, the upright iCPET revealed paradoxically blunted changes in cardiac pressures during exercise, opposite to the supine iCPET results. As suggested by his postural nature of dyspnea that exacerbated with diuretics, only the upright iCPET unmasked venous insufficiency and/or impaired preload activation during exercise that ultimately led to the diagnosis of cardiac preload failure, although their relative roles cannot be ascertained with available data.
Preload failure, an important cause of exertional dyspnea, may evade diagnosis on resting and supine studies. While data on this condition is limited, clinicians must maintain a high level of suspicion for cardiac preload failure in the workup of unexplained dyspnea. Future investigations are needed to determine treatments for various physiologic phenotypes.
Sources of Funding:
VNR and MDK are supported by a National Institutes of Health (NIH) Training Grant (NIH 5T32HL069749-18). Dr Fudim was supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer, Bodyport and BTG Specialty Pharmaceuticals. He receives consulting fees from Abbott, Audicor, AxonTherapies, Bodyguide, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, NXT Biomedical, Pharmacosmos, PreHealth, Splendo, Vironix, Viscardia, Zoll.
Footnotes
Disclosures: The authors do not have conflicts of interest relevant to the submitted material.
References:
- 1.Oldham WM, Lewis GD, Opotowsky AR, Waxman AB and Systrom DM. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm Circ. 2016;6:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fudim M, Sobotka PA and Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail. 2021;14:e007308. [DOI] [PubMed] [Google Scholar]
- 3.Nobrega AC, O’Leary D, Silva BM, Marongiu E, Piepoli MF and Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int. 2014;2014:478965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S and Alpert N. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation. 1990;81:1550–9. [DOI] [PubMed] [Google Scholar]
- 5.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE and Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–91. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Nishimura RA, Sorajja P, Lam CS and Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


