Abstract
Background:
Black individuals have high incident diabetes risk, despite having paradoxically lower triglyceride (TG) and higher high-density lipoprotein cholesterol (HDL-C) levels. The basis of this is poorly understood. We evaluated the participants of the Systolic Blood Pressure Intervention Trial (SPRINT) to assess the association of estimated European genetic ancestry with the risk of incident diabetes in self-identified Black individuals.
Methods:
Self-identified non-Hispanic Black SPRINT participants free of diabetes at baseline were included. Black participants were stratified into tertiles (T1-T3) of European ancestry proportions estimated using 106 biallelic ancestry informative genetic markers. The multivariable-adjusted association of European ancestry proportion with indices of baseline metabolic syndrome (i.e., fasting plasma glucose [FPG], triglycerides, high-density lipoprotein-cholesterol [HDL-C], body mass index [BMI], and blood pressure [BP]) was assessed. Multivariable-adjusted Cox regression determined the risk of incident diabetes (FPG ≥126 mg/dL or self-reported diabetes treatment) across tertiles of European ancestry proportion.
Results:
Among 2,466 Black SPRINT participants, a higher European ancestry proportion was independently associated with higher baseline TG and lower HDL-C levels (p<0.001 for both). European ancestry proportion was not associated with baseline FPG, BMI, and BP (p>0.05). Compared with the first tertile, those in the second (HR:0.64 [95%CI:0.45–0.90]) and third tertiles (HR:0.61 [95%CI:0.44–0.89]) of European ancestry proportion had a lower risk of incident diabetes. A 5 percentage point higher European ancestry was associated with a 29% lower risk of incident diabetes (HR:0.71 [95%CI:0.55–0.93]). There was no evidence of a differential association between European ancestry proportion tertiles and incident diabetes between those randomized to intensive vs. standard BP treatment.
Conclusions:
The higher risk of incident diabetes in Black individuals may have genetic determinants in addition to adverse social factors. Further research may help understand the interplay between biological and social determinants of cardiometabolic health in Black individuals.
Clinical Trial Registration:
NCT01206062 registered at Clinicaltrials.gov.
Keywords: Blood Pressure, Diabetes Mellitus, Genetics, Health Disparities, Metabolic Syndrome, Race
Introduction
Non-Hispanic Black individuals are at a higher risk of incident diabetes, which is primarily believed to be driven by the differences in lived experience and social determinants of health.1 Non-Hispanic Black individuals also have a disproportionately higher prevalence of insulin resistance, diabetes, and diabetes-associated cardiovascular mortality.2–10 However, the prevalence of metabolic syndrome, which is defined by abdominal obesity, elevated triglyceride levels, reduced high-density lipoprotein cholesterol (HDL-C), elevated blood pressure, and elevated plasma glucose,11, 12 is relatively lower among non-Hispanic Black individuals.13 This incongruence is attributed to lower triglyceride levels, higher HDL-C, and metabolic paradox in Black individuals.14–16
While the social determinants of health explain much of the racial disparity for incident diabetes risk, the biological basis has also previously been explored.17–19 Self-reported non-Hispanic Black individuals have a wide variation of European admixture ranging from ~1–70%.20, 21 Lower European genetic ancestry in Black Americans has been associated with higher glycated hemoglobin, decreased adiponectin, increased C-reactive protein, and a lower resting metabolic rate, all of which are predictors of incident diabetes.17–19, 22 Genetic ancestry proportions have been previously noted to be associated with poor cardiometabolic phenotype and with adverse cardiovascular events.23, 24 Importantly, the relationship between the genetic ancestry proportion and incident diabetes among high-risk, hypertensive African-American individuals has not been previously evaluated.
We sought to evaluate the association between genetic European ancestry proportion and 1) metabolic syndrome components at baseline, and 2) incident diabetes, in self-identified Black participants of the Systolic Blood Pressure Intervention Trial (SPRINT), who were free of diabetes at baseline. We also assessed the risk of incident diabetes in non-Hispanic Black individuals relative to non-Hispanic White individuals in SPRINT.
Methods
The anonymized study data used for this analysis are publicly available at the National Heart, Lung, and Blood Institute’s BioLINCC data repository and can be accessed at https://biolincc.nhlbi.nih.gov/home/. The SPRINT study protocol was approved by the local Institutional Review Boards at the respective trial sites. Written informed consent was obtained from all SPRINT participants. The study complied with principles detailed in the Declaration of Helsinki. This analysis was approved by the University of Alabama at Birmingham Institutional Review Board. The full study methods are available as Supplementary Methods.
Results
There were 5,256 non-Hispanic White and 2,673 non-Hispanic Black individuals free of diabetes at baseline. Supplementary Table II depicts the baseline characteristics of the study participants stratified by race. The distribution of social determinants of health (education status, employment status, and health insurance status) in Black and White individuals are depicted in Supplementary Figure II.
Risk of Incident Diabetes Mellitus: Stratified by Race
The incidence of diabetes mellitus was 2.0 (95% CI: 1.8–2.3) per 1000-person years (event frequency: 10.0%) among non-Hispanic Black participants and 1.5 (95% CI: 1.3–1.6) per 1000-person years (event frequency: 7.5%) among non-Hispanic White participants. Non-Hispanic Black participants in SPRINT had a higher risk of incident diabetes (HR: 1.43 [95% CI: 1.14–1.80]) compared with non-Hispanic White participants after accounting for various clinical and socioeconomic factors (Figure 1). The relative strength of the association of various clinical and socioeconomic factors with incident diabetes is depicted in Supplementary Figure III. Baseline blood glucose, BMI, age, presence of clinical or subclinical cardiovascular disease, health insurance status, education status, employment status, and race were the strongest correlates of incident diabetes. The estimates from individual model variables are described in Supplementary Figure IV and Supplementary Table III. There was a significant interaction between sex and race on the risk of incident diabetes mellitus (p=0.01). In the sex-stratified analyses, among females, the hazard for incident diabetes was 1.67 (95% CI: 1.07–2.60) for non-Hispanic Black participants. Among males, the hazard for incident diabetes was 1.35 (95% CI: 1.03–1.78) for non-Hispanic Black participants (Supplementary Figure V).
Figure 1. Risk of Incident Diabetes Mellitus in the SPRINT Trial: Stratified by Race.
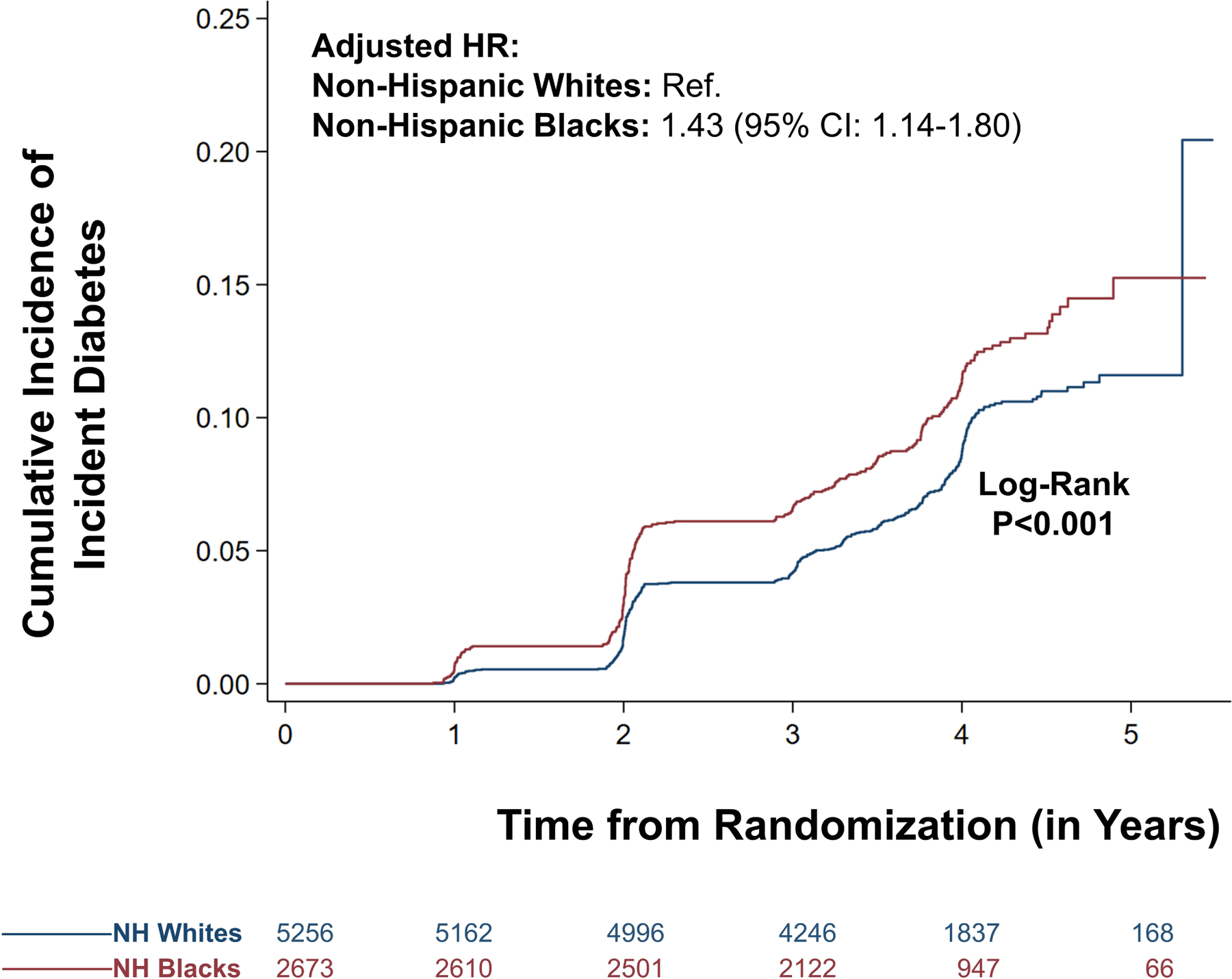
The curve in blue represents the non-Hispanic (NH) White population. The curve in red represents the NH Black population.
In the competing risk analysis, the hazard for incident diabetes among non-Hispanic Black participants was 1.50 (95% CI: 1.23–1.84) (Supplementary Table IV). In the sensitivity analysis, taking age as the time-scale, the hazard for non-Hispanic Black participants was 2.65 (95% CI: 2.19–3.21) compared with non-Hispanic White participants (Supplementary Table V).
Baseline Characteristics Across European Ancestry Tertiles
There were 2,466 self-identified Black participants without baseline diabetes who provided consent for genetic analysis, had their DNA analyzed, and for whom European ancestry admixture data was available. Supplementary Table VI depicts the differences in baseline characteristics of these individuals with the rest of the SPRINT participants. The participants were categorized into three tertiles of European ancestry (T1: ≤15%; T2: 16–24%; T3: ≥25%). Participants in the first tertile (T1) had a median European ancestry proportion of 11%, those in the second tertile (T2) had a median ancestry proportion of 20%, and those in the third tertile (T3) had a median ancestry proportion of 32%. The baseline characteristics of the participants, grouped by tertiles of European ancestry, are presented in Table 1. The distribution of social determinants of health (education status, employment status, and health insurance status) across tertiles of European ancestry proportion are depicted in Figure 2.
Table 1.
Baseline Characteristics of Self-Reported Black Individuals, Stratified by Tertile of European Admixture Proportion
| Characteristics | Tertile 1 (n=867) |
Tertile 2 (n=777) |
Tertile 3 (n=822) |
P-value |
|---|---|---|---|---|
| European Admixture Proportion | 11% (8–13%) | 20% (17–22%) | 32% (27–38%) | <.001 |
| Age | 62 (57–71) | 62 (57–69) | 64 (58–72) | .01 |
| Age ≥75 years | 139 (16.0) | 109 (14.0) | 155 (18.9) | .13 |
| Age ≥65 years | 362 (41.8) | 281 (36.2) | 383 (46.6) | <.001 |
| Female | 406 (46.8) | 352 (45.3) | 366 (44.5) | .63 |
| Framingham 10-year CHD Risk Score | 18.2 (12.6–26.8) | 18.9 (13.3–27.5) | 19.6 (13.7–28.6) | .003 |
| Framingham 10-year CHD risk ≥15% | 541 (62.4) | 506 (65.1) | 583 (70.9) | <.001 |
| Body Mass Index (kg/m2) | 30.0 (26.3–34.3) | 30.5 (26.7–34.6) | 29.6 (25.4–34.1) | .69 |
| Systolic Blood Pressure (mmHg) | 138 (129–150) | 139 (130–150) | 139 (129–149) | .44 |
| Diastolic Blood Pressure (mmHg) | 82 (73–90) | 82 (74–90) | 81 (72–89) | .13 |
| Fasting Plasma Glucose (mg/dL) | 94 (88–102) | 95 (88–102) | 96 (89–103) | .03 |
| Total Cholesterol (mg/dL) | 193 (170–220) | 194 (173–222) | 194 (166–219) | .39 |
| LDL Cholesterol (mg/dL) | 116 (95–140) | 119 (98–144) | 117 (93–139) | .87 |
| HDL Cholesterol (mg/dL) | 55 (46–65) | 53 (45–62) | 52 (44–62) | <.001 |
| Triglycerides (mg/dL) | 89 (67–120) | 93 (69–126) | 94 (70–131) | .02 |
| Serum Creatinine (mg/dL) | 1.08 (0.91–1.32) | 1.06 (0.88–1.27) | 1.04 (0.87–1.25) | |
| Urine Albumin to Creatinine Ratio (mg/gm) | 9.5 (5.0–22.5) | 9.1 (5.1–25.0) | 8.9 (5.1–20.0) | .26 |
| Estimated GFR (mL/min/1.73m2) | 73.3 (59.5–87.9) | 76.4 (62.1–91.5) | 78.3 (63.1–91.7) | <.001 |
| CKD (eGFR <60 mL/min/1.73m2) | 27.1 | 22.1 | 21.2 | .002 |
| CKD Stage II (eGFR 45–59 mL/min/1.73m2) | 15.5 | 14.9 | 13.9 | |
| CKD Stage III (eGFR 30–59 mL/min/1.73m2) | 8.8 | 4.9 | 5.7 | |
| CKD Stage IV (eGFR 15–29 mL/min/1.73m2) | 2.9 | 2.5 | 1.5 | |
| Aspirin Use | 40.9 | 39.6 | 33.3 | .29 |
| Statin Use | 31.7 | 32.6 | 37.0 | .02 |
| Antihypertensive Medications | 2 (1–3) | 2 (1–3) | 2 (1–3) | .63 |
| Smoking Status | ||||
| Never | 47.3 | 41.2 | 41.6 | |
| Former | 29.4 | 33.7 | 37.2 | |
| Current | 23.2 | 25.1 | 20.9 | |
| Education | ||||
| Less than High School | 348 (40.1) | 274 (35.3) | 244 (29.7) | .001 |
| High School or GED | 92 (10.6) | 73 (9.4) | 85 (10.3) | |
| Business or Vocational Training | 189 (21.8) | 194 (25.0) | 200 (24.3) | |
| Some College or Associate Degree | 128 (14.8) | 137 (17.6) | 150 (18.3) | |
| College Graduate | 83 (9.6) | 80 (10.3) | 116 (14.1) | |
| Doctoral or Masters Degree | 27 (3.1) | 19 (2.4) | 27 (3.3) | |
| Employment | ||||
| Full Time | 176 (21.1) | 179 (23.8) | 163 (20.4) | .17 |
| Part-Time | 84 (10.1) | 60 (8.0) | 73 (9.2) | |
| Retired | 432 (51.8) | 374 (49.7) | 446 (55.9) | |
| Looking for Employment | 40 (4.8) | 44 (5.8) | 34 (4.3) | |
| Unemployed | 102 (12.2) | 96 (12.8) | 82 (10.3) | |
| Lack of Health Insurance | 155 (17.9) | 160 (20.6) | 127 (15.5) | .22 |
Categorical variables are represented as counts with proportions, and continuous variables are represented as medians with interquartile ranges. Abbreviations: GFR: Glomerular Filtration Rate; CKD: Chronic Kidney Disease; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; CHD: Coronary Heart Disease. Continuous variables are compared using the Jonckheere-Terpstra test. Categorical variables are compared using the Cochran-Armitage test.
Figure 2. Social Determinants of Health Among Black Individuals Across Ancestry Proportion Tertiles.
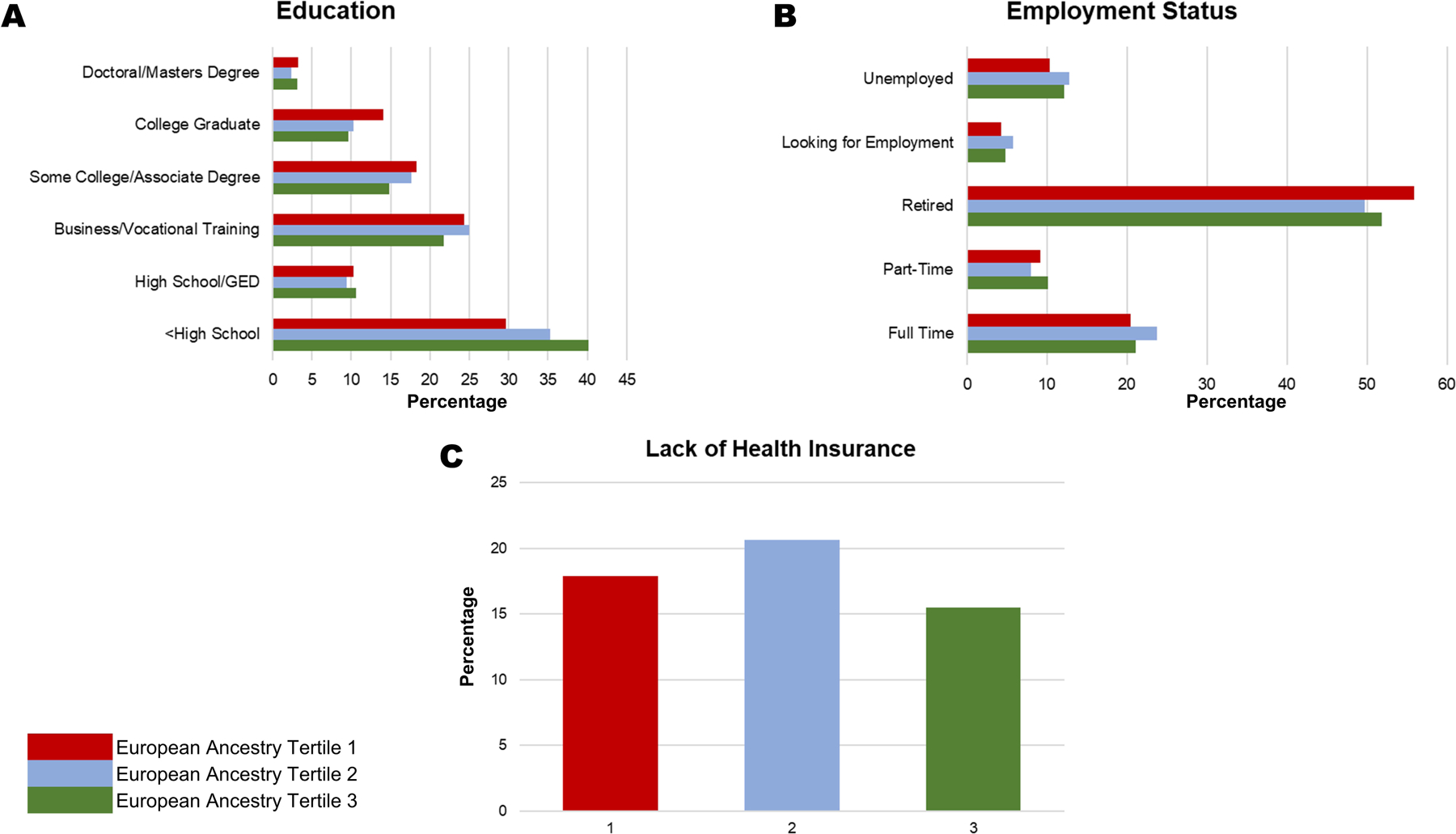
This figure depicts the distribution of the education status (Panel A), employment status (Panel B), and health insurance status (Panel C). P for education status=0.001. P for employment status=0.17. P for health insurance status=0.22.
Participants in the first tertile had lower baseline fasting triglyceride levels (T1: 89 [IQR: 67–120] mg/dL, T2: 93 [IQR: 69–126] mg/dL, T3: 94 [IQR: 70–131] mg/dL), and higher HDL cholesterol levels (T1: 55 [IQR: 46–65] mg/dL, T2: 53 [IQR: 45–62] mg/dL, T3: 52 [IQR: 44–62] mg/dL). Fasting total cholesterol, systolic and diastolic blood pressure, and BMI were not different between the tertiles. There was a trend for higher fasting plasma glucose across the European ancestry proportion tertiles (p=0.03). In multivariable-adjusted models, an increase in European ancestry proportion among non-Hispanic Black participants was associated with higher triglyceride levels (β=0.32, SE: 0.08; p<0.001) and lower HDL-C levels (β=−0.18, SE: 0.04; p<0.001)(Figure 3). The remaining metabolic syndrome parameters (blood glucose, systolic blood pressure, diastolic blood pressure, BMI) were not significantly associated with increasing European ancestry proportion (p>0.05 for all)(Supplementary Figure VI).
Figure 3.
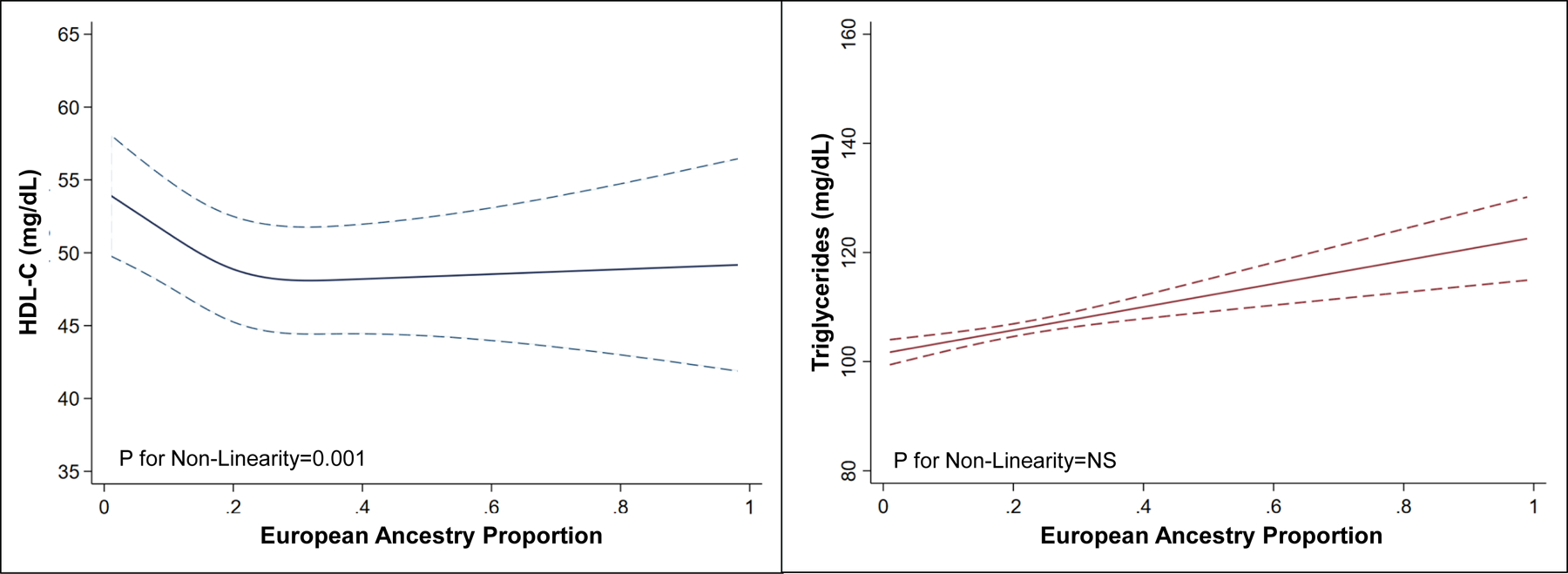
Multivariable-Adjusted Relationship of Baseline High-Density Lipoprotein-Cholesterol and Triglycerides with European Ancestry Proportions
Risk of Incident Diabetes Mellitus Among Non-Hispanic Black Participants Across European Ancestry Tertiles
The incidence of diabetes mellitus in the first, second, and third tertiles was 3.12 (95% CI: 26.6–38.8) per 1000-person years (event frequency: 12.1%), 22.7 (95% CI: 17.9–28.7) per 1000-person years (event frequency: 8.8%), and 23.8 (95% CI: 19.1–29.7) per 1000-person years (event frequency: 9.3%), respectively (Figure 4). After multivariable adjustment, participants in the second (HR: 0.64 [95% CI: 0.45–0.90]) and third (HR: 0.61 [95% CI: 0.44–0.89]) tertiles of European ancestry proportion had a lower risk of incident diabetes mellitus compared with those in the first tertile (Supplementary Table VII, Supplementary Figure VII). Each 5% increment in European ancestry proportion in non-Hispanic Black participants was independently associated with a 29% lower hazard of incident diabetes (HR: 0.71 [95% CI: 0.55–0.93]). There was no interaction between blood pressure control strategies and European ancestry proportion on incident diabetes (p=0.69). There was no interaction between sex and European ancestry proportion in incident diabetes (p=0.88). In the competing risk analysis, Black participants in second (HR: 0.69 [95% CI:0.49–0.96]) and third tertiles of European ancestry proportion (HR: 0.67 [95% CI:0.49–0.93]) have a lower risk of incident diabetes compared with those in the first tertile (Supplementary Table IV). Similar results were obtained in sensitivity analysis taking age as the time-scale (Supplementary Table V).
Figure 4. European Ancestry Proportion and the Risk of Incident Diabetes Among Black Individuals.
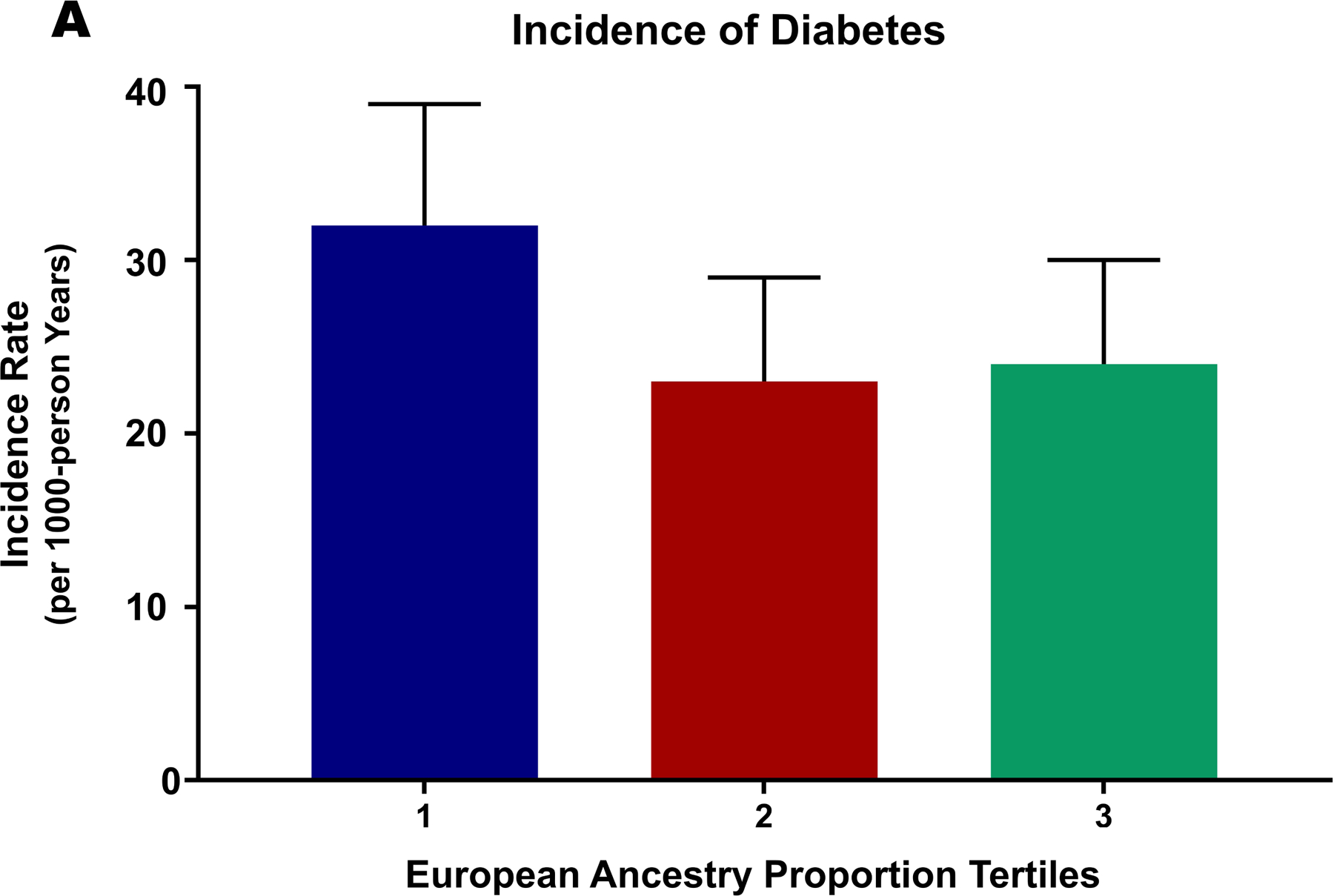
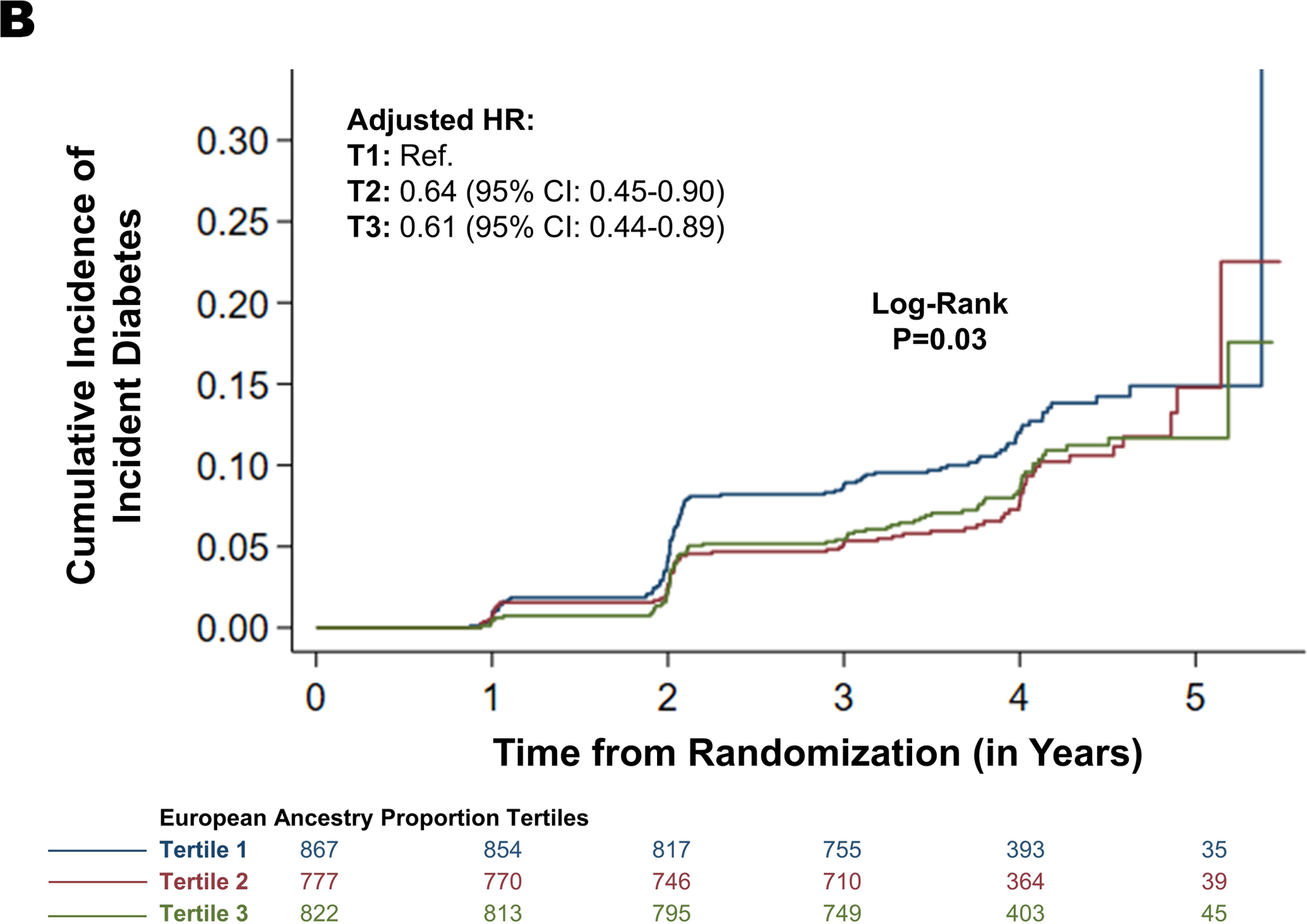
This figure depicts the incidence rate of diabetes mellitus across increasing European ancestry proportion as tertiles (Panel A and Panel B).
Discussion
In this study, we observed that among high-risk, hypertensive non-Hispanic Black individuals from the United States, a higher European ancestry proportion was associated with a lower risk of incident diabetes after accounting for numerous clinical and social factors. The lower incidence of diabetes, which was associated with higher European ancestry, was not different by sex or blood pressure control strategy. Among non-Hispanic Black individuals, a higher European ancestry proportion was associated with higher triglyceride levels and lower HDL-C levels. The distribution of social determinants of health is well understood to influence the higher risk of incident diabetes among Black individuals. However, our findings suggest that genetic factors may also contribute to a higher risk of incident diabetes and a paradoxically favorable baseline lipid profile (lower triglycerides and higher HDL-C) among non-Hispanic Black individuals.
While both biological and social factors clearly contribute to the development of cardiometabolic diseases, the relative contribution is difficult to evaluate on an individual level. Race is a social construct. However, there are many genetic variants with large differences in allele frequency by ethnic ancestry, with some contributing to disease and drug response.1, 5, 9, 24–27 We reiterate that apart from clinical factors (age, BMI, blood glucose, clinical or subclinical cardiovascular disease), social factors such as all of the downstream effects of structural and interpersonal racism on education status, health insurance status, and employment status are key correlates of incident diabetes in our study population. While our analyses accounted for the common social factors, our analyses cannot completely capture the adverse life experience of Black Americans attributed to systemic structural and interpersonal racism in healthcare and society.1, 7–10, 24 While these factors are important in driving the cardiometabolic disease burden, the higher risk of incident diabetes among non-Hispanic Black individuals may have genetic contributions that require evaluation. Given the strong association between European ancestry, metabolic profiles, and incident diabetes that we demonstrated, our investigation advocates for comprehensive genetic assessment to explore the various individual-level genetic determinants of incident diabetes among racial minorities while simultaneously mitigating the numerous extrinsic factors that are disproportionately impacting Black Americans.9 Large-scale genetic association studies in Black individuals will help improve our understanding of the biological determinants contributing to cardiometabolic disease. Such investigations coupled with an examination of approaches for mitigating the social determinants of health may help improve the cardiometabolic disease burden disproportionately impacting Black individuals.1, 7–9, 11, 2
Prior studies have noted the paradoxical finding that Black individuals have a more favorable lipid profile, a marker that is associated with a lower risk of cardiometabolic disease but is still predisposed to a higher risk of incident diabetes.28–31 Previous studies have shown that triglyceride and HDL-C levels to be predictive of insulin resistance in self-identified White individuals, but not in self-identified Black individuals.32, 33 We found that lower triglyceride and higher HDL-C levels track independently with the increasing genetic European ancestry proportion. Several genetic factors have been previously associated with the lower triglycerides, and higher HDL-C noted with lower European ancestry.18, 31, 34–36 Prior investigations indicate that variants in the LPL gene (lipoprotein lipase gene) such as rs12678919 (occurring in tight linkage disequilibrium with rs328) result in a favorable lipid profile (higher HDL-C, lower triglycerides), and these genetic findings have been validated among Black Americans.18, 36 Similarly, genetic variants in NRXN3, TTC7B and, GCKR have been suggested as potential contributors to racial differences in triglyceride levels.11, 18 Further research is needed to understand the contribution of these and other genetic variants to the eventual higher risk of incident diabetes in Black individuals. Our findings add to the growing body of evidence that the current definition of metabolic syndrome inadequately characterizes the burden of cardiometabolic disease among non-Hispanic Black individuals because it relies on HDL-C and triglyceride levels.15, 37–39
The higher incidence of diabetes mellitus among Black Americans has been previously noted in community cohort-based studies and was shown to be driven by socioeconomic, psychosocial, behavioral, and neighborhood factors alongside the clinical factors.1 The higher risk of incident diabetes among Black individuals is attenuated by ~96% on the inclusion of biological factors and further attenuated by 17% after accounting for the neighborhood, psychosocial, socioeconomic, and behavioral factors.1 We observed that Black participants have a greater risk of incident diabetes, and this may be driven primarily by their clinical profile and social factors. Older individuals are at a greater risk of development of diabetes.40 Age was one of the strongest correlates of incident diabetes in our study population. Our sensitivity analyses taking age as the time-scale yielded similar results of a higher risk of incident diabetes among non-Hispanic Black participants. Epidemiological evidence from American population cohorts suggests that a lower proportion of European ancestry is associated with higher odds of having diabetes mellitus after accounting for age, sex, BMI, and socioeconomic factors.27 Our study advances this finding by noting a strikingly higher risk of incident diabetes with lower European ancestry proportions among non-Hispanic Black individuals after accounting for numerous clinical, biological, and social factors. This suggests that there may be genetic variants that contribute to the higher risk of developing diabetes mellitus among non-Hispanic Black individuals, which have not yet been discovered. Prior genome-wide association studies (GWASs) have identified several genetic loci for diabetes.41–45 These findings are derived primarily from European populations and have not been validated in those with African ancestry. This may be due to smaller sample sizes in African ancestry studies, racial/ethnic differences in allele frequency or linkage disequilibrium, or smaller effect sizes in certain populations.31 Further research is needed to improve the understanding of the various genetic determinants of incident diabetes in Black individuals, alongside addressing the numerous social determinants of health driving the disproportionate cardiometabolic disease burden.
There are several limitations to our study. Our study was performed in the SPRINT population, which included high-risk hypertensive Americans, and this may introduce a collider bias in our investigation. We evaluated a subset of the SPRINT participants who represent a high-risk and hypertensive American population. Hence, these findings require validation in larger, more generalized populations.39 Our study included only Black Americans from the SPRINT and did not include Hispanic participants due to lack of data on ancestry proportion in the Hispanic participants of the study. Recently, higher African and Native American ancestry and lower European ancestry have been found to be associated with a higher genetic risk of diabetes mellitus in Hispanic/Latino populations.46 These findings strengthen our observed association of ancestry proportion with incident diabetes. While we used the available data regarding possible socioeconomic confounders, there are likely other unmeasured residual confounders, either extrinsic societal or biological, that may affect the observed results. Furthermore, though we utilized 106 biallelic AIMs for this analysis,38 prior studies have shown a strong correlation (correlation coefficient>0.9) between true individual ancestry and the estimated ancestry computed using at least 100 AIMs.47, 48 We also lacked data regarding the place of birth, acculturation status, and the impact of time spent in the United States on the development of incident diabetes.21, 49–52 We did not have access to the geographical location of the randomization site, and the geographical heterogeneity in the genetic ancestry proportion, socioeconomic, cultural, dietary, and cardiometabolic risk factors of the study population may have also contributed to the observed results.7, 8, 10, 21, 49–52 Due to the lack of availability of genetic data, the current investigation did not account for genetic variants that may be associated with the development of diabetes. The lack of interaction may result from inadequate power for the given sample size to detect a significant interaction. Notwithstanding these limitations, the randomized clinical trial setting provided a rigorous assessment of the clinical measures and study outcome in this study.
Conclusions
Among non-Hispanic Black SPRINT participants, higher levels of European genetic ancestry were associated with a less favorable lipid profile at baseline but with a lower risk of incident diabetes mellitus. Large-scale genetic association studies in Black Americans will help improve our understanding of the biological determinants contributing to the development of cardiometabolic diseases.
Supplementary Material
Acknowledgments:
We would like to thank the SPRINT trial investigators for making the study data available for public use through the NHLBI Biologic Specimen and Data Repository.
Source of Funding:
This work was supported by the National Institutes of Health Mentored Patient-Oriented Research Award [5K23HL146887-03] to Dr. Pankaj Arora.
Non-Standard Abbreviations and Acronyms
- AIM
Ancestry Informative Markers
- BMI
Body Mass Index
- HDL-C
High-Density Lipoprotein Cholesterol
- LDL-C
Low-Density Lipoprotein Cholesterol
- SPRINT
Systolic Blood Pressure Intervention Trial
Footnotes
Disclosures: None of the authors had any conflicts of interest or financial disclosures to declare.
Supplementary Material:
References
- 1.Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ and Carnethon MR. Association of Modifiable Risk Factors in Young Adulthood With Racial Disparity in Incident Type 2 Diabetes During Middle Adulthood. JAMA. 2017;318:2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L and Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- 3.McBean AM, Li S, Gilbertson DT and Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, hispanics, and asians. Diabetes Care. 2004;27:2317–24. [DOI] [PubMed] [Google Scholar]
- 4.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL and Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–26. [DOI] [PubMed] [Google Scholar]
- 5.Brancati FL, Kao WH, Folsom AR, Watson RL and Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–9. [DOI] [PubMed] [Google Scholar]
- 6.Gillum RF, Mussolino ME and Madans JH. Diabetes mellitus, coronary heart disease incidence, and death from all causes in African American and European American women: The NHANES I epidemiologic follow-up study. J Clin Epidemiol. 2000;53:511–8. [DOI] [PubMed] [Google Scholar]
- 7.Parcha V, Kalra R, Best AF, Patel N, Suri SS, Wang TJ, Arora G and Arora P. Geographic Inequalities in Cardiovascular Mortality in the United States: 1999 to 2018. Mayo Clin Proc. 2021;96(5):1218–1228. [DOI] [PubMed] [Google Scholar]
- 8.Parcha V, Kalra R, Suri SS, Malla G, Wang TJ, Arora G and Arora P. Geographic Variation in Cardiovascular Health Among American Adults. Mayo Clin Proc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parcha V, Patel N, Kalra R, Arora G and Arora P. Prevalence, Awareness, Treatment, and Poor Control of Hypertension Among Young American Adults: Race-Stratified Analysis of the National Health and Nutrition Examination Survey. Mayo Clin Proc. 2020;95:1390–1403. [DOI] [PubMed] [Google Scholar]
- 10.Parcha V, Malla G, Suri SS, Kalra R, Heindl B, Berra L, Fouad MN, Arora G and Arora P. Geographic Variation in Racial Disparities in Health and Coronavirus Disease-2019 (COVID-19) Mortality. Mayo Clin Proc Innov Qual Outcomes. 2020;4:703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, Rangarajan S, Gerstein HC, Anand SS and Investigators I. Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–8. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Hansen B, Smith SC Jr., Cleeman JI, Kahn RA, AHA, NHLBI and ADA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–6. [DOI] [PubMed] [Google Scholar]
- 13.Moore JX, Chaudhary N and Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR and Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu SS, Castillo DC, Courville AB and Sumner AE. The triglyceride paradox in people of African descent. Metab Syndr Relat Disord. 2012;10:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard T, Schuster D and Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis. 2009;19:S2–1–7. [PubMed] [Google Scholar]
- 17.Manini TM, Patel KV, Bauer DC, Ziv E, Schoeller DA, Mackey DC, Li R, Newman AB, Nalls M, Zmuda JM, et al. European ancestry and resting metabolic rate in older African Americans. Eur J Clin Nutr. 2011;65:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deo RC, Reich D, Tandon A, Akylbekova E, Patterson N, Waliszewska A, Kathiresan S, Sarpong D, Taylor HA Jr. and Wilson JG. Genetic differences between the determinants of lipid profile phenotypes in African and European Americans: the Jackson Heart Study. PLoS Genet. 2009;5:e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassel Fyr CL, Kanaya AM, Cummings SR, Reich D, Hsueh WC, Reiner AP, Harris TB, Moffett S, Li R, Ding J, et al. Genetic admixture, adipocytokines, and adiposity in Black Americans: the Health, Aging, and Body Composition study. Hum Genet. 2007;121:615–24. [DOI] [PubMed] [Google Scholar]
- 20.Shriver MD and Kittles RA. Genetic ancestry and the search for personalized genetic histories. Nat Rev Genet. 2004;5:611–8. [DOI] [PubMed] [Google Scholar]
- 21.Bryc K, Durand EY, Macpherson JM, Reich D and Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruthur NM, Kao WH, Clark JM, Brancati FL, Cheng CY, Pankow JS and Selvin E. Does genetic ancestry explain higher values of glycated hemoglobin in African Americans? Diabetes. 2011;60:2434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alame AJ, Garg S, Kozlitina J, Ayers C, Peshock RM, Matulevicius SA and Drazner MH. Association of African Ancestry With Electrocardiographic Voltage and Concentric Left Ventricular Hypertrophy: The Dallas Heart Study. JAMA Cardiol. 2018;3:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao S, Segar MW, Bress AP, Arora P, Vongpatanasin W, Agusala V, Essien UR, Correa A, Morris AA, de Lemos JA et al. Association of Genetic West African Ancestry, Blood Pressure Response to Therapy, and Cardiovascular Risk Among Self-Reported Black Individuals in the Systolic Blood Pressure Reduction Intervention Trial (SPRINT). JAMA Cardiol. 2020; 6(4):388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel N, Russell GK, Musunuru K, Gutierrez OM, Halade G, Kain V, Lv W, Prabhu SD, Margulies KB, Cappola TP, Arora G, Wang TJ and Arora P. Race, Natriuretic Peptides, and High-Carbohydrate Challenge: A Clinical Trial. Circ Res. 2019;125:957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parcha V, Patel N, Kalra R, Arora G, Januzzi JL Jr., Felker GM, Wang TJ and Arora P. Racial Differences in Serial NT-proBNP Levels in Heart Failure Management: Insights From the GUIDE-IT Trial. Circulation. 2020;142:1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CY, Reich D, Haiman CA, Tandon A, Patterson N, Selvin E, Akylbekova EL, Brancati FL, Coresh J, Boerwinkle E, et al. African ancestry and its correlation to type 2 diabetes in African Americans: a genetic admixture analysis in three U.S. population cohorts. PLoS One. 2012;7:e32840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Adamo E, Northrup V, Weiss R, Santoro N, Pierpont B, Savoye M, O’Malley G and Caprio S. Ethnic differences in lipoprotein subclasses in obese adolescents: importance of liver and intraabdominal fat accretion. Am J Clin Nutr. 2010;92:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA and Kraus WE. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–7. [DOI] [PubMed] [Google Scholar]
- 30.Miljkovic-Gacic I, Bunker CH, Ferrell RE, Kammerer CM, Evans RW, Patrick AL and Kuller LH. Lipoprotein subclass and particle size differences in Afro-Caribbeans, African Americans, and white Americans: associations with hepatic lipase gene variation. Metabolism. 2006;55:96–102. [DOI] [PubMed] [Google Scholar]
- 31.Bentley AR and Rotimi CN. Interethnic Differences in Serum Lipids and Implications for Cardiometabolic Disease Risk in African Ancestry Populations. Glob Heart. 2017;12:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumner AE, Finley KB, Genovese DJ, Criqui MH and Boston RC. Fasting triglyceride and the triglyceride–HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165:1395–1400. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C and Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. [DOI] [PubMed] [Google Scholar]
- 34.Bentley AR and Rotimi CN. Interethnic Variation in Lipid Profiles: Implications for Underidentification of African-Americans at risk for Metabolic Disorders. Expert Rev Endocrinol Metab. 2012;7:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coram MA, Duan Q, Hoffmann TJ, Thornton T, Knowles JW, Johnson NA, Ochs-Balcom HM, Donlon TA, Martin LW, Eaton CB, et al. Genome-wide characterization of shared and distinct genetic components that influence blood lipid levels in ethnically diverse human populations. Am J Hum Genet. 2013;92:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty PB, Tang H, Feng T, Tayo B, Morrison AC, Kardia SL, Hanis CL, Arnett DK, Hunt SC, Boerwinkle E, et al. Variants for HDL-C, LDL-C, and triglycerides identified from admixture mapping and fine-mapping analysis in African American families. Circ Cardiovasc Genet. 2015;8:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumner AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, Oli J, Johnson T, Adebamowo C and Rotimi CN. Low HDL-Cholesterol with Normal Triglyceride Levels is the Most Common Lipid Pattern in West Africans and African Americans with Metabolic Syndrome: Implications for Cardiovascular Disease Prevention. CVD Prev Control. 2010;5:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman BI, Divers J and Palmer ND. Population ancestry and genetic risk for diabetes and kidney, cardiovascular, and bone disease: modifiable environmental factors may produce the cures. Am J Kidney Dis. 2013;62:1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, Agler C, Love SM, Okello S, Petty LE, et al. Importance of Genetic Studies of Cardiometabolic Disease in Diverse Populations. Circ Res. 2020;126:1816–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng MC. Genetics of Type 2 Diabetes in African Americans. Curr Diab Rep. 2015;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessel J, Chu AY, Willems SM, Wang S, Yaghootkar H, Brody JA, Dauriz M, Hivert MF, Raghavan S, Lipovich L, et al. Low-frequency and rare exome chip variants associate with fasting glucose and type 2 diabetes susceptibility. Nat Commun. 2015;6:5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng MC, Shriner D, Chen BH, Li J, Chen WM, Guo X, Liu J, Bielinski SJ, Yanek LR, Nalls MA, et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet. 2014;10:e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos E, Chen G, Shriner D, Doumatey A, Gerry NP, Herbert A, Huang H, Zhou J, Christman MF, Adeyemo A, et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia. 2011;54:783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chande AT, Rishishwar L, Conley AB, Valderrama-Aguirre A, Medina-Rivas MA and Jordan IK. Ancestry effects on type 2 diabetes genetic risk inference in Hispanic/Latino populations. BMC Med Genet. 2020;21:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai HJ, Choudhry S, Naqvi M, Rodriguez-Cintron W, Burchard EG and Ziv E. Comparison of three methods to estimate genetic ancestry and control for stratification in genetic association studies among admixed populations. Hum Genet. 2005;118:424–33. [DOI] [PubMed] [Google Scholar]
- 49.Han E, Carbonetto P, Curtis RE, Wang Y, Granka JM, Byrnes J, Noto K, Kermany AR, Myres NM, Barber MJ, et al. Clustering of 770,000 genomes reveals post-colonial population structure of North America. Nat Commun. 2017;8:14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathias RA, Taub MA, Gignoux CR, Fu W, Musharoff S, O’Connor TD, Vergara C, Torgerson DG, Pino-Yanes M, Shringarpure SS, et al. A continuum of admixture in the Western Hemisphere revealed by the African Diaspora genome. Nat Commun. 2016;7:12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baharian S, Barakatt M, Gignoux CR, Shringarpure S, Errington J, Blot WJ, Bustamante CD, Kenny EE, Williams SM, Aldrich MC, et al. The Great Migration and African-American Genomic Diversity. PLoS Genet. 2016;12:e1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, Daly MJ, Bustamante CD and Kenny EE. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am J Hum Genet. 2017;100:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freedman BI, Rocco MV, Bates JT, Chonchol M, Hawfield AT, Lash JP, Papademetriou V, Sedor JR, Servilla K, et al. APOL1 renal-risk variants do not associate with incident cardiovascular disease or mortality in the Systolic Blood Pressure Intervention Trial. Kidney Int Rep. 2017;2:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, Kaysen GA, Kimmel PL, Raj DS, Ricardo AC, et al. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, Iribarren C, Knowles JW, Li J, Narasimhan B, et al. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Nyunoya T, Leng S, Belinsky SA, Tesfaigzi Y and Bruse S. Softwares and methods for estimating genetic ancestry in human populations. Hum Genomics. 2013;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang H, Peng J, Wang P and Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005;28:289–301. [DOI] [PubMed] [Google Scholar]
- 60.Lawson DJ, van Dorp L and Falush D. A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots. Nat Commun. 2018;9:3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roumie CL, Hung AM, Russell GB, Basile J, Kreider KE, Nord J, Ramsey TM, Rastogi A, Sweeney ME, Tamariz L, et al. Blood Pressure Control and the Association With Diabetes Mellitus Incidence: Results From SPRINT Randomized Trial. Hypertension. 2020;75:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parcha V, Patel N, Kalra R, Kim J, Gutierrez OM, Arora G and Arora P. Incidence and Implications of Atrial Fibrillation/Flutter in Hypertension: Insights From the SPRINT Trial. Hypertension. 2020;75:1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel N, Cushman M, Gutierrez OM, Howard G, Safford MM, Muntner P, Durant RW, Prabhu SD, Arora G, Levitan EB et al. Racial differences in the association of NT-proBNP with risk of incident heart failure in REGARDS. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel N, Gutierrez OM, Arora G, Howard G, Howard VJ, Judd SE, Prabhu SD, Levitan EB, Cushman M and Arora P. Race-based demographic, anthropometric and clinical correlates of N-terminal-pro B-type natriuretic peptide. Int J Cardiol. 2019;286:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


