Abstract
Introduction.
Postmenopausal women (PMW) display exaggerated increases in blood pressure during exercise, yet the mechanism(s) involved remain unclear. Moreover, research on the impact of menopausal changes in estradiol on cardiovascular control during exercise are limited.
Methods.
Herein, we tested the hypothesis that sympathetic responses during exercise are augmented in PMW compared to young women (YW), and estradiol administration attenuates these responses. Muscle sympathetic nerve activity (MSNA) and mean arterial pressure (MAP) were measured in 13 PMW (58±1 yrs) and 17 YW (22±1 yrs) during 2-min of isometric handgrip. Separately, MSNA and BP responses were measured during isometric handgrip in 6 PMW (53±1 yrs) before and after 1 month of transdermal estradiol (100 μg/day). A period of post-exercise ischemia (PEI) to isolate muscle metaboreflex activation followed all handgrip bouts.
Results.
Resting MAP was similar between PMW and YW whereas MSNA was greater in PMW (23±3 vs 8±1 bursts/min; P<0.05). During handgrip, the increases in MSNA (PMW Δ16±2 vs YW Δ6±1 bursts/min; P<0.05) and MAP (PMW Δ18±2 vs YW Δ12±2 mmHg; P<0.05) were greater in PMW and remained augmented during PEI. Estradiol administration decreased resting MAP but not MSNA in PMW. Moreover, MSNA (PMW (-E2) Δ27±8 bursts/min vs. PMW (+E2) Δ12±5 bursts/min; P<0.05) and MAP (Δ31±8 mmHg vs. Δ20±6 mmHg; P<0.05) responses during handgrip were attenuated in PMW following estradiol administration. Likewise, MAP responses during PEI were lower after estradiol.
Conclusion.
These data suggest that PMW exhibit an exaggerated MSNA and BP response to isometric exercise, due in part to heightened metaboreflex activation. Furthermore, estradiol administration attenuated BP and MSNA responses to exercise in PMW.
Keywords: Menopause, Handgrip Exercise, Muscle Sympathetic Nerve Activity, Muscle Metaboreflex
INTRODUCTION
Age-related increases in resting blood pressure (BP) are higher in women after menopause compared to men, and the prevalence of hypertension is also greater in women after menopause (1, 2). Exaggerated increases in BP during stressors such as exercise are associated with a greater risk for developing hypertension (3–5). This augmented increase in BP during exercise is also linked to a heightened risk of cardiovascular and cerebrovascular events during and after physical activity in both women and men (6–8). However, given the greater prevalence of hypertension and cardiovascular disease in women after menopause, understanding the mechanisms contributing to heightened BP responses to exercise are clinically relevant and can offer insight into strategies to improve exercise prescription for women.
Postmenopausal women (PMW) have recently been shown to experience greater increases in BP during exercise compared to young women (YW) (9) as well as age-matched men (10). Although the underlying mechanisms remain to be elucidated, greater increases in total peripheral resistance (TPR) have been reported during both isometric (9) and dynamic (10) exercise in PMW compared to YW, suggestive of heightened sympathetic activation. However, to date, no direct assessment of the sympathetic nervous system during exercise in PMW has been made. Thus, the extent to which sympathetic nerve activity contributes to heightened BP responses during exercise in PMW is currently unknown.
Elevations in resting muscle sympathetic nerve activity (MSNA) have been documented in PMW compared to YW (11, 12). While aging is likely a primary driver of the increase in resting MSNA (13), changes in estradiol may also be a contributing factor. For example, in YW, changes in resting MSNA throughout the menstrual cycle were negatively correlated with changes in plasma estradiol (14). Furthermore, age-related changes in resting MSNA are much greater in women compared to men, which is driven by menopause (15) and likely the associated changes in sex hormones like estradiol. In that regard, transdermal estrogen administration has been shown to decrease resting MSNA in PMW (16, 17). However, others have found no effect of estrogen replacement on resting MSNA (18), which may be dependent on route of administration (transdermal vs. oral). Although not consistent, there is ample data demonstrating estradiol directly impacts neural cardiovascular control in humans (11, 14, 19, 20). Likewise, numerous rodent studies have demonstrated direct effects of estradiol on neural cardiovascular control (21–25). Indeed, estradiol administration has been shown to attenuate BP responses to static muscle contraction in cats (26, 27). However, whether estradiol administration attenuates neural control of BP during exercise in PMW remains unclear. Given the controversy surrounding hormone therapy for PMW over the past ~20 years following the Women’s Health Initiative (28), it is important to understand the impact of estradiol on BP and sympathetic responses to exercise in PMW.
Accordingly, the purpose of this study was to examine the impact of aging and estradiol on BP and MSNA responses to exercise in women. We hypothesized that a) exaggerated BP responses during exercise in PMW would be accompanied by larger increases in MSNA, and b) that estradiol administration to PMW would attenuate BP and MSNA responses to exercise. Furthermore, since activation of metabolically sensitive skeletal muscle afferents (i.e., the muscle metaboreflex) is a primary driver of increases in MSNA and BP during exercise (29), we also examined the impact of age and estradiol on BP and MSNA responses during a period of post-exercise ischemia (PEI) used to isolate muscle metaboreflex activation.
METHODS
We recruited normotensive, non-obese YW and PMW that did not have a history of cardiovascular, neurological, renal, or metabolic disease, and not taking medications at the time of study. Two experimental protocols were conducted: one at the University of Delaware (Protocol 1 below), and one at the University of Texas Southwestern Medical Center (Protocol 2 below). The experimental procedures for each protocol were approved by the respective Institutional Review Board, and the studies conformed to the standards outlined in the Declaration of Helsinki. Verbal and written consent were obtained from all women prior to participation.
Protocol 1: Influence of Aging on Sympathetic Neural Responses to Exercise
YW (n=17) were tested either during the early follicular phase of their menstrual cycle (n=8) or placebo phase if using oral contraceptives (n=9). PMW (n=13) were at least 1 year since their last self-reported menstrual cycle (9±2 yrs since menopause), went through natural menopause, and had not used any form of hormone replacement therapy.
Women reported to the laboratory at least 4 hours postprandial and having abstained from alcohol, caffeine, and strenuous physical activity for the preceding 24 hours. Women were tested in the supine position and laboratory temperature was maintained between 20–22°C. Heart rate (HR) was monitored using a lead II electrocardiogram (ECG; Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI). Beat-by-beat arterial BP was measured noninvasively by a servo-controlled finger photoplethysmograph (Finometer; Finapres Medical Systems, Amsterdam, Netherlands) placed on the middle finger of the non-dominant hand and calibrated per manufacturers guidelines. Finometer-derived total peripheral resistance and cardiac output were estimated from the BP waveform (Modelflow method). Automated brachial artery BP (Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI) was used to verify absolute Finometer-derived BP measurements. Respiratory movements were monitored using a strain-gauge pneumograph (Pneumotrace; UFI, Morro Bay, CA) placed in a stable position over the abdomen, and used to ensure that subjects did not inadvertently perform Valsalva maneuvers or breath-holds during the protocol. Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as previously described in detail (30). Briefly, these recordings were obtained by inserting a unipolar tungsten microelectrode into the peroneal nerve. Neural signals were amplified (70,000-fold), bandpass filtered (700–2,000 Hz), rectified, and integrated (0.1 s time constant) to obtain mean voltage neurograms (Nerve Traffic Analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA). MSNA recordings were identified and verified by the presence of spontaneously occurring bursts with characteristic pulse synchronicity, responsiveness to an end-expiratory breath hold or Valsalva maneuver, and lack of response to arousal stimuli or skin stroking.
The maximal voluntary contraction (MVC) of the dominant hand was tested by having subjects squeeze a commercially available handgrip device (ADInstruments, Bella Vista, NSW, Australia) at maximal effort three to five times. The highest force production was subsequently used as the MVC, which was then used to calculate the relative work rate of 30% for the experimental protocol. After subject instrumentation and a satisfactory MSNA recording was obtained (15 YW and 12 PMW), women rested quietly for at least 5 min. BP, HR, and MSNA were measured continuously throughout the experimental protocol.
Women performed isometric handgrip (HG) exercise at 30% of their MVC for 2 minutes and were provided with continuous visual feedback of force production. Ratings of perceived exertion (RPE) were obtained using the standard 6–20 Borg scale. With 5 seconds remaining in HG, an occlusion cuff placed on the upper arm of the exercising limb was rapidly inflated to suprasystolic BP (≥ 200 mmHg) and remained inflated for 3 min and 15 sec following the completion of exercise (post-exercise ischemia; PEI). PEI was used to isolate activation of the skeletal muscle metaboreflex (29, 31).
Protocol 2: Influence of Estradiol on Sympathetic Neural Responses to Exercise in PMW
Six PMW were studied 6±1 yrs since their last menstrual cycle. PMW were normotensive, had no history of cardiovascular disease, and were not taking any medications at the time of the study. Two PMW who were taking hormone replacement prior to enrollment were asked to discontinue usage for 4 weeks consistent with previous studies (16, 32), and serum estradiol levels were confirmed at <40 pg ml−1 at the start of the study.
On experimental days, PMW reported to the laboratory at least 4 hours postprandial and were asked to abstain from caffeinated beverages for at least 8 hours and from strenuous activity for 24 hours. Women were studied in the supine position. Blood samples were taken for 17β-estradiol measures. HR was recorded continuously from lead II electrocardiogram, and BP was measured by automated oscillometric sphygmomanometry (CE0050, Welch Allyn, Skaneateles Falls, NY, USA). Respiration and MSNA were measured as described above in Protocol 1. Isometric HG exercise was performed for 2 minutes in the dominant hand at 35% MVC, followed by 2 minutes and 15 seconds of PEI. This protocol was performed before and after 4 weeks of transdermal estradiol at a dose of 100 μg day−1, which would be expected to restore circulating estradiol to premenopausal levels.
Data Analysis & Statistics
Data were recorded using Powerlab (ADInstruments, Bella Vista, NSW, Australia). MSNA bursts were identified from the mean voltage neurogram using a customized LabVIEW program with fixed criteria (33, 34), which generated synchronized beat-by-beat data of all recorded variables accounting for the latency from the R wave of the ECG and incorporated a signal-to-noise ratio of at least 3:1. MSNA was quantified as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Cardiovascular and MSNA variables were calculated as mean values over an initial resting baseline period. For the HG/PEI trial, MSNA and cardiovascular variables were calculated as mean values during the baseline preceding handgrip, during the peak responsiveness to HG (the last 30s for BP and HR, the last 60s for MSNA), and during the full PEI period (excluding the initial 15 seconds). As increases in BP and MSNA are fairly stable during PEI, the entire period was used for comparisons. Because the main focus was on sympathetic and BP reactivity to exercise, the change in MSNA and cardiovascular variables were determined by calculating the difference between baseline and peak responses as defined above. For protocol 1, unpaired t-tests were used to compare baseline subject characteristics and the changes in MSNA and BP during HG and PEI between YW and PMW. For protocol 2, paired t-tests were used to compare baseline subject characteristics and the changes in MSNA and BP during HG and PEI within PMW before and after estradiol administration. Results are reported as mean±SE and the alpha level was set at P<0.05.
RESULTS
Protocol 1:
Baseline demographics in YW and PMW are shown in Table 1. By design, PMW were older than YW. Resting systolic (P=0.08), diastolic (P=0.10) and mean arterial BP (P=0.06) tended to be higher in PMW. BMI was slightly higher in PMW, and resting MSNA (burst frequency and burst incidence) was greater in PMW. Model-flow derived cardiac output (YW 4.88±0.25 vs PMW 4.75±0.34 L/min, P=0.76) and total peripheral resistance (YW 1608±61 vs PMW 1797±108 mmHg/L/min, P=0.12) were similar between groups.
Table 1.
Participant characteristics Protocol 1.
| YW (n = 17) | PMW (n = 13) | |
|---|---|---|
|
| ||
| Age, yrs | 22 ± 1 | 58 ± 1 * |
| BMI, kg/m2 | 22 ± 1 | 24 ± 1 * |
| SBP, mmHg | 109 ± 2 | 115 ± 3 |
| DBP, mmHg | 65 ± 2 | 70 ± 2 |
| MAP, mmHg | 80 ± 2 | 85 ± 2 |
| HR, bpm | 62 ± 2 | 54 ± 2 * |
| MSNA, bursts/min | 8 ± 1 | 23 ± 3 * |
| MSNA, bursts/100 hb | 12 ± 2 | 42 ± 6 * |
Values are mean±SE. YW, young women; PMW, postmenopausal women; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity.
P<0.05 vs. YW.
Representative neurograms are shown in Figure 1. Changes in mean arterial BP (MAP) during HG exercise were greater in PMW (Fig 2A; P=0.03) and associated with greater increases in MSNA burst frequency (Fig 2B; P<0.001). The increase in systolic BP during HG was greater in PMW (YW Δ14±2 vs PMW Δ24±4 mmHg, P=0.01), whereas changes in diastolic BP (YW Δ13±2 vs PMW Δ15±2 mmHg, P=0.34) and HR (YW Δ15±2 vs PMW Δ12±1 bpm, P=0.29) were similar between groups. Changes in MSNA burst incidence (YW Δ6±3 vs PMW Δ16±4 bursts/100 heart beats, P<0.01) were also higher in PMW during HG. The change in total peripheral resistance was greater in PMW during HG (YW Δ−89±71 vs PMW Δ177±76 mmHg/L/min, P=0.02), whereas cardiac output was not different between groups (YW Δ1.14±0.28 vs PMW Δ0.53±0.17 L/min, P=0.10).
Figure 1.
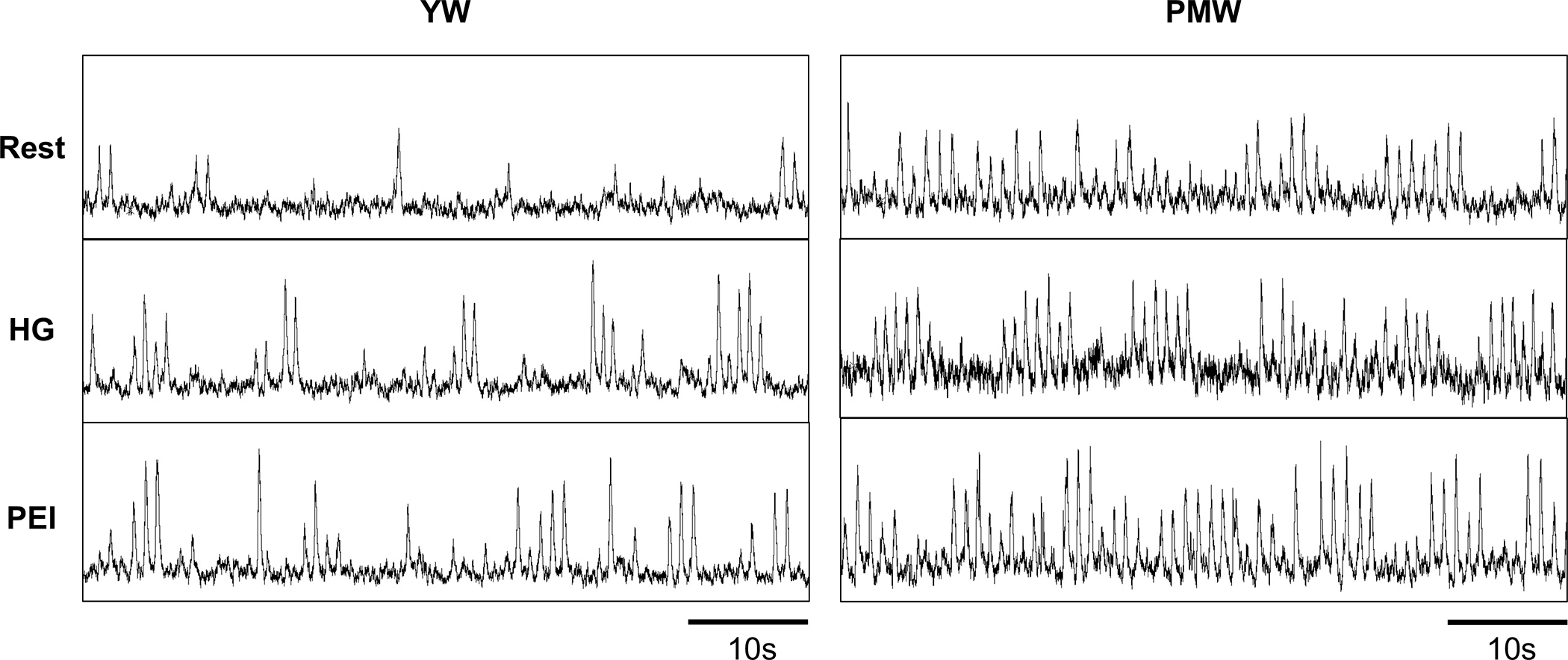
Original recordings of muscle sympathetic nerve activity in one young woman (YW) and one postmenopausal woman (PMW) at rest, during the last minute of isometric handgrip exercise (HG), and post-exercise ischemia (PEI).
Figure 2.
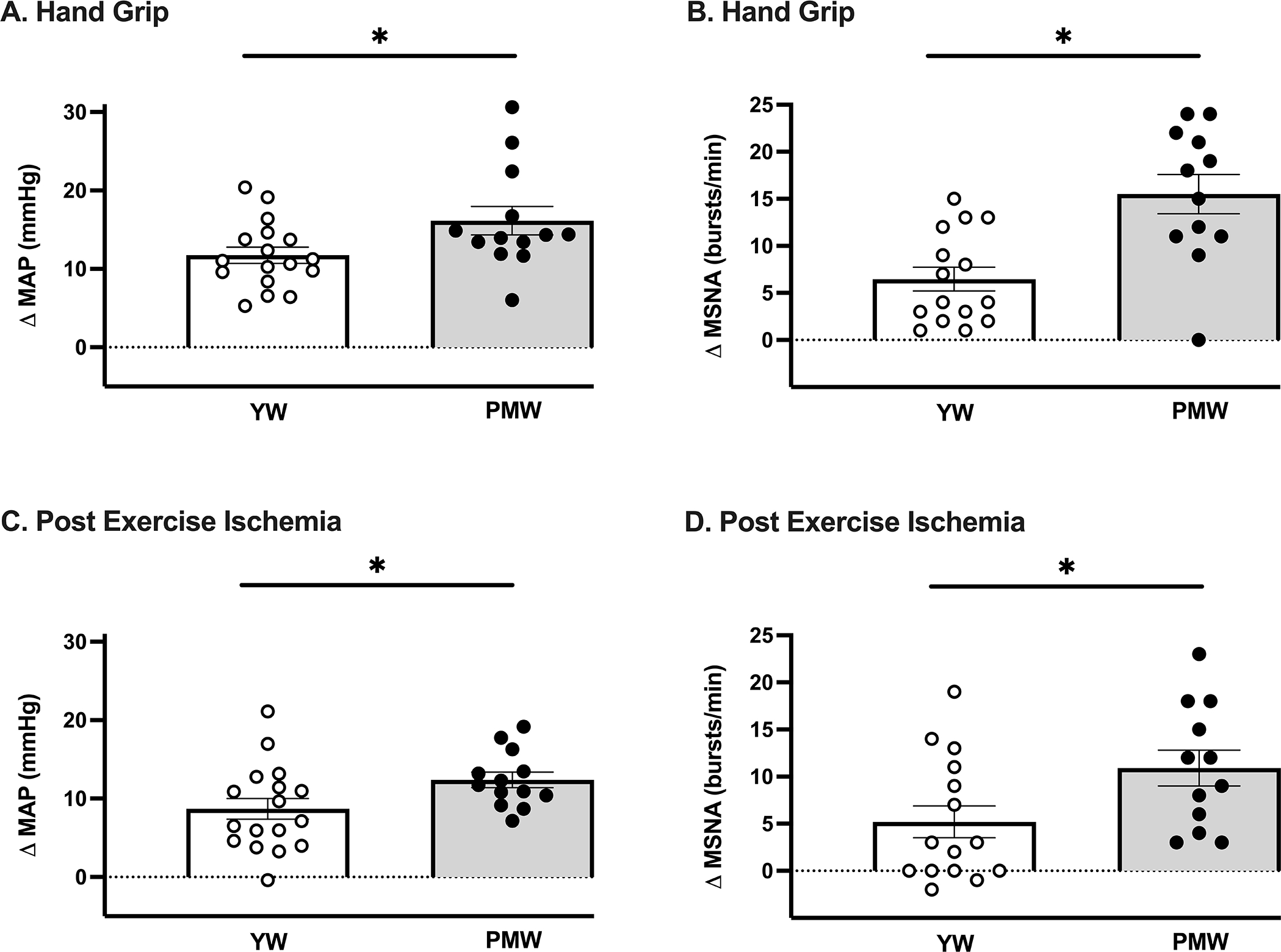
Peak changes in (A) mean arterial blood pressure (MAP, P=0.03; n=17) and (B) muscle sympathetic nerve activity (MSNA, P<0.001; n=15) during isometric handgrip exercise were greater in postmenopausal women (PMW) compared to young women (YW). Changes in (C) MAP (P=0.04; n=17) and (D) MSNA (P=0.03; n=15) during post-exercise ischemia were also greater in PMW. * P<0.05 between groups. Data expressed as mean±SE.
The elevated MAP and MSNA observed during HG exercise in PMW were also noted during PEI (Fig 2C&D). Systolic BP was also higher during PEI (YW Δ10±2 vs PMW Δ19±2 mmHg, P<0.01) whereas diastolic BP was not different (YW Δ8±1 vs PMW Δ9±1 mmHg, P=0.55) between groups. MSNA burst incidence also trended higher in PMW during PEI (YW Δ8±4 vs PMW Δ16±4 bursts/100 heart beats, P=0.06). The change in total peripheral resistance was higher in PMW during PEI (YW Δ−3±23 vs PMW Δ255±75 mmHg/L/min, P<0.01), whereas cardiac output was greater in young women (YW Δ0.46±0.10 vs PMW Δ0.04±0.15 L/min, P=0.02). Maximal voluntary contraction (YW 208±17 vs PMW 224±23 Newtons, P=0.58) and RPE (YW 13±1 vs PMW 14±1, P=0.14) were not different between YW and PMW.
Protocol 2:
Subject characteristics are shown in Table 2. One month of estradiol therapy (E2) increased serum estradiol concentrations from 17±6 to 105±23 pg ml-1. Estradiol therapy had no effect on resting HR, systolic blood pressure, or MSNA, whereas diastolic blood pressure, and MAP were reduced (Table 2). Original records for MSNA are shown in Figure 3. During HG, the increases in MSNA burst frequency and MAP were attenuated in PMW after estradiol administration (Figure 4A&B). Comparable MSNA results were found for burst incidence (-E2 Δ19±7 vs +E2 Δ5±4 burst/100 heart beats, P<0.05). Likewise, HG-induced increases in diastolic blood pressure (-E2 Δ29±8 vs +E2 Δ17±4 mmHg, P<0.05) and systolic blood pressure (-E2 Δ34±8 vs +E2 Δ24±11 mmHg, P<0.05) were significantly reduced following estradiol therapy. In contrast, HR responses to HG were not different after estradiol therapy (-E2 Δ23±4 vs +E2 Δ20±5 bpm, P>0.05). Similarly, RPE values during HG were not affected by estradiol administration (15 ± 1 vs. 14 ± 1; P > 0.05). During PEI, estradiol administration also attenuated the increase in MAP (Figure 4C); however, systolic (-E2 Δ31±10 vs +E2 Δ25±11 mmHg, P=0.07) and diastolic (-E2 Δ17±5 vs +E2 Δ14±5 mmHg, P=0.20) blood pressure were not impacted. Likewise, increases in MSNA burst frequency (Figure 4D) and MSNA burst incidence (-E2 Δ21±6 vs +E2 Δ20±6 burst/100 heart beats, P>0.05) during PEI were not affected by estradiol therapy.
Table 2.
Participant characteristics Protocol 2.
| PMW −E2 (n = 6) | PMW +E2 (n=6) | |
|---|---|---|
|
| ||
| Age, yrs | 53 ± 1 | -- |
| BMI, kg/m2 | 26 ± 1 | 26 ± 1 |
| SBP, mmHg | 122 ± 4 | 120 ± 4 |
| DBP, mmHg | 78 ± 3 | 74 ± 2 * |
| MAP, mmHg | 93 ± 3 | 89 ± 3 * |
| HR, bpm | 62 ± 2 | 59 ± 2 |
| MSNA, bursts/min | 28 ± 4 | 23 ± 3 |
| MSNA, bursts/100 hb | 38 ± 7 | 33 ± 5 |
Values are mean ± SE. PMW, postmenopausal women; −E2, baseline before initiation of estradiol; +E2, 1month post estradiol; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity.
P<0.05 vs. −E2.
Figure 3.
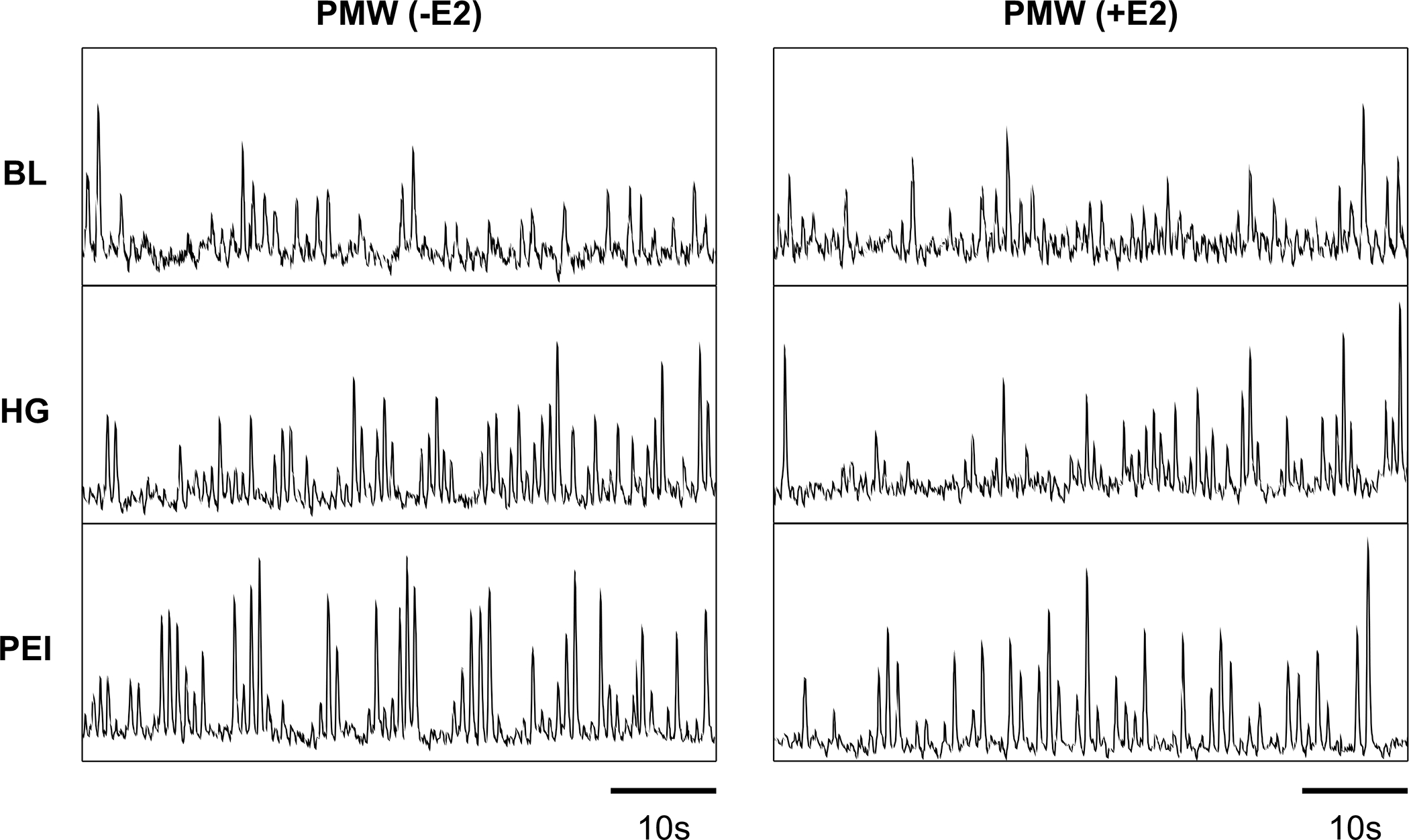
Original recordings of muscle sympathetic nerve activity in one postmenopausal woman (PMW) at rest, during the last minute of isometric handgrip exercise (HG), and post-exercise ischemia (PEI) before (-E2) and following 1-month of transdermal estradiol therapy (+E2).
Figure 4.
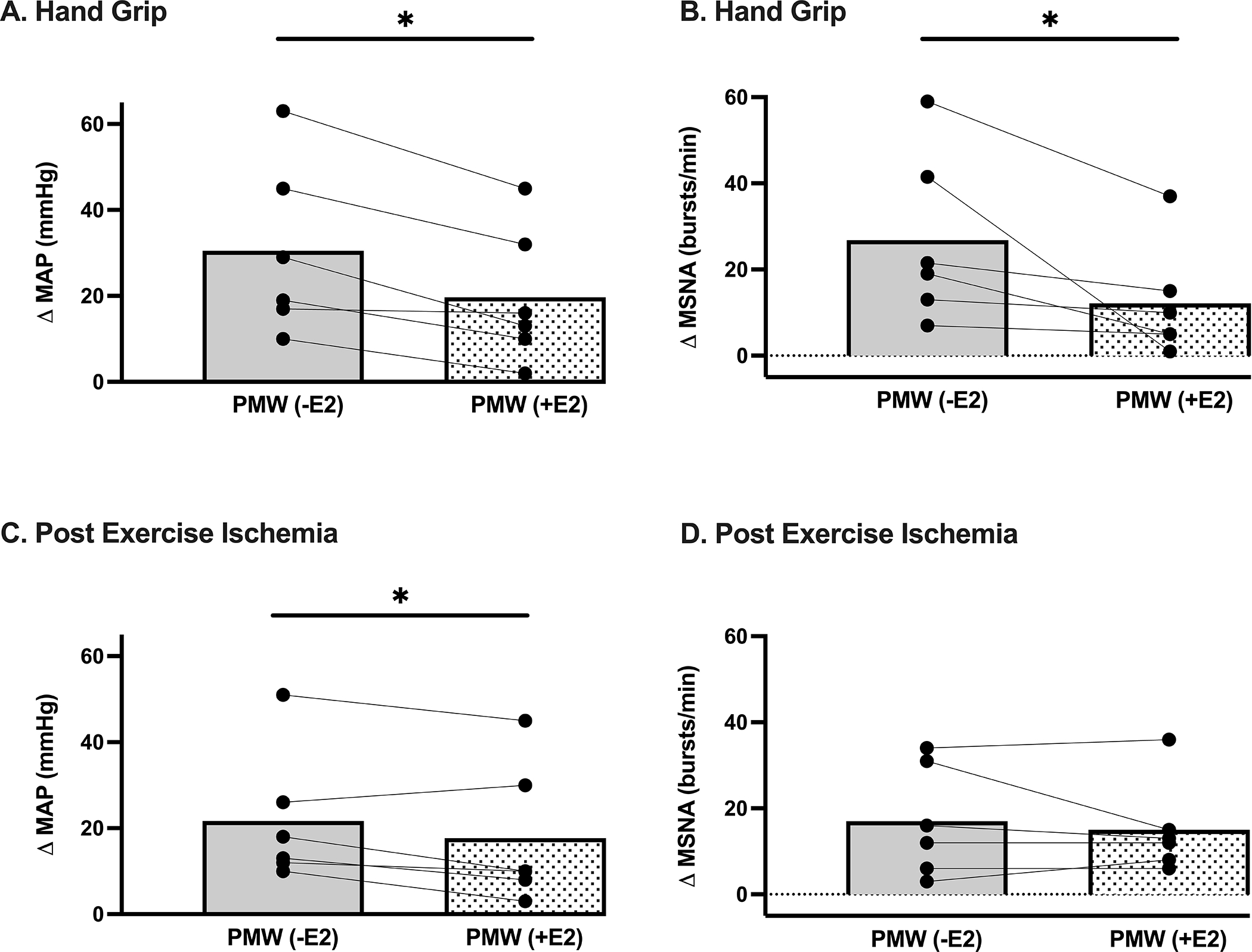
Peak changes in (A) mean arterial blood pressure (MAP, P<0.001; n=6) and (B) muscle sympathetic nerve activity (MSNA, P=0.03; n=6) during isometric handgrip exercise were attenuated in postmenopausal women (PMW) following 1-month of transdermal estradiol (+E2) compared to baseline (-E2). Changes in (C) MAP (P=0.04; n=6) were also lower during post-exercise ischemia in PMW following 1-month of transdermal estradiol (+E2) compared to baseline (-E2), whereas (D) MSNA (P=0.27; n=6) was maintained. * P<0.05 between groups. Data expressed as mean±SE.
DISCUSSION
The main novel finding of the current study was that PMW displayed exaggerated increases in MSNA during isometric HG exercise compared to YW. Furthermore, estradiol administration attenuates MSNA and BP responses during HG exercise in PMW. These data extend previous findings by demonstrating that the sympathetic nervous system is a primary contributor to the greater increases in BP during isometric exercise in PMW. Moreover, this is the first study to examine the effect of estradiol administration on MSNA responses to isometric exercise and PEI in PMW, showing that estradiol attenuates MSNA and BP responses to isometric exercise. Finally, these exaggerated MSNA and BP responses were due in part to an over-active metaboreflex, as demonstrated by greater MSNA and BP responses during PEI in PMW. Taken together, these data suggest that PMW have exaggerated MSNA and BP responses to isometric exercise, mediated in part by heightened muscle metaboreflex activation. Estradiol administration attenuates the BP and MSNA responses to isometric exercise in PMW.
The influence of age and menopause on resting MSNA has been well documented (12, 35). Similar with prior findings, we observed significantly higher resting MSNA in PMW compared to young women. Moreover, data from the current study extend these findings demonstrating for the first time that MSNA increases more dramatically during isometric exercise in PMW compared to young women. These exaggerated increases in MSNA, coupled with increases in TPR, demonstrate a direct mechanism for the greater increases in BP observed in PMW during HG exercise.
MSNA and BP responses during exercise are mediated, in part, by the exercise pressor reflex, a peripheral feedback neural mechanism initiated in the working skeletal muscle (36). The exercise pressor reflex is comprised of group III (mechanoreceptors) and IV (metaboreceptors) afferent fibers, which reflexively increase BP during exercise via stimulation of efferent sympathetic nerve activity (37). Previous data suggest heightened muscle mechanoreflex function in PMW, although it is unclear as to whether these differences are related to aging, changes in sex hormones such as estrogen, or obesity (38). However, in young adults, women displayed a blunted increase in central hemodynamics compared to men during passive limb movement, suggesting an attenuated mechanoreflex in young women (39). In the current study, we did not focus on isolating mechanoreflex function since it has been well documented that the skeletal muscle metaboreflex contributes importantly to the magnitude of BP increases during isometric exercise and is augmented in cardiovascular diseases such as hypertension (40, 41). Choi and colleagues (9) previously demonstrated exaggerated BP responses in PMW during isometric exercise which remained elevated during isolation of the muscle metaboreflex with PEI. Our data are consistent with those of Choi et al (9), and extend these findings to show that these larger changes in BP are accompanied by exaggerated MSNA responses during both handgrip exercise and PEI. Collectively, these data indicate that PMW exhibit an overactive muscle metaboreflex, and this mechanism is contributing, in part, to the exaggerated BP responses observed.
After one month of estradiol administration in PMW, we observed reductions in BP and MSNA during HG exercise. Estradiol also lowered BP during metaboreflex isolation; however, this was not accompanied by a statistically significant reduction in MSNA. The reason for these discrepant effects of estradiol are not known but some possibilities warrant discussion. Estrogen receptors are located in the brain, and in particular, key regions of the brainstem that are involved in regulating neural cardiovascular control (42), thus potentially reducing sympathetic outflow and BP. During metaboreflex activation, information from the periphery is sent via afferent neural signals to the brain and primarily integrated in the nucleus tractus solitarius to change efferent sympathetic outflow and BP. However, a feed-forward mechanism originating in higher brain centers (central command) also regulates cardiovascular responses during exercise, contributing to increases in MSNA and BP. Central command is sensitive to estrogen; IV injections of 17B-estradiol attenuated BP, HR, and ventilatory responses to central command activation in cats (43). Thus, we cannot rule out a role of central command in the observed attenuated MSNA responses during exercise but not PEI in PMW following estradiol administration. However, changes in HR were not different and subjects reported similar ratings of perceived exertion during the exercise bout before and after estradiol administration suggesting central command is not affected. Perhaps estrogen is directly impacting nuclei in the RVLM to decrease MSNA and contribute to reductions in BP during exercise. Indeed, previous studies in both animals (21) and humans (16) have shown that estradiol administration decreases sympathetic nerve activity, and that hypertension induced in female rats via aldosterone infusion was only apparent when knocking out estrogen receptors in the RVLM (44). This is important to consider since skeletal muscle afferents directly project to the RVLM in addition to the nucleus tractus solitarius (45). Additional work understanding the central effects of estrogen on neural cardiovascular control during exercise in humans is warranted.
Other factors may also be involved in contributing to the reductions in BP noted with estradiol in the absence of a reduction in MSNA during metaboreflex isolation. Aging and changes in sex hormones impact peripheral vascular function, and in particular beta-adrenergic receptor function. Notably, young women exhibit beta-adrenergic mediated dilation that can offset sympathetically-mediated vasoconstriction, an effect that is lost in postmenopausal women (47). This may also have effects on sympathetic transduction (48, 49) and BP regulation during exercise. Moreover, there is cross-talk between estrogen receptors and beta-adrenergic receptors; estradiol increases vascular β-adrenergic receptor expression and NO-mediated vasodilation in female rats suggesting that estrogen’s cardioprotective role is accomplished partly through this mechanism which acts to blunt α-adrenergic mediated vasoconstriction (50). This is supported by research in humans which shows that estrogen supplementation attenuates the vasoconstrictor responses to arterial norepinephrine infusion in perimenopausal women (51) as well as reflex sympathetic activation in postmenopausal women (32). Thus, it is plausible that direct estradiol effects on the vasculature are also contributing to the reductions in BP during PEI in PMW without influencing MSNA. This also may explain the reductions in diastolic BP via estradiol administration during exercise, despite PMW not exhibiting exaggerated diastolic BP responses compared to young women (protocol 1). Overall, further investigation is warranted.
It is also important to consider that estradiol is not the only hormone that changes with menopause. Progesterone also declines, and there can be an increase in androgens or testosterone. Although it is well documented that estradiol is cardioprotective and reduces sympathetic outflow, more recent data suggest that the ratio of estradiol to progesterone is a stronger regulator of MSNA in young women (14). This has yet to be studied as women age or transition through menopause. The increased concentration of androgens relative to estrogen that can occur with menopause has also been proposed to contribute to cardiovascular disease risk (46). This is clinically relevant give that testosterone is given to PMW to improve libido. Likewise, studies examining the impact of progesterone and androgens on muscle metaboreflex activation in PMW are also needed.
Perspectives
Postmenopausal women are at an increased risk for developing hypertension, and one mechanism contributing to their higher risk may be heightened BP reactivity to stressors such as exercise. Herein, we demonstrate that augmented MSNA responses accompany the exaggerated BP during a submaximal isometric task in PMW compared to young women. Notably, these repeated episodic surges in both MSNA and BP from submaximal isometric workloads throughout the day may place postmenopausal women at a greater risk for developing hypertension and experiencing a cardiovascular or cerebrovascular event. Importantly, we found that one month of transdermal estradiol administration attenuated these responses. Although hormone therapy in PMW has been the focus of much controversy over the past 20 years, it is now accepted that hormone therapy use does not increase risk of cardiovascular disease in postmenopausal women when used within 10 years of menopause (28), and that formulation also matters; transdermal estradiol is preferred over oral estrogen for safety reasons (52, 53). Our data show that transdermal estradiol administration reduces BP and MSNA responses to isometric exercise, and therefore may have beneficial effects on overall cardiovascular function and responses to stressors in PMW. Finally, it is well known that engaging in regular exercise has numerous benefits for cardiovascular health, and we are not suggesting that older women should not engage in physical activity based on our findings. Importantly, exercise training can also attenuate exercise pressor reflex activity (54, 55), and may be one mechanism of the beneficial effects of training on BP. Thus, findings from this study are meant to inform exercise prescription guidelines for women and highlight the importance of monitoring BP.
In conclusion, PMW display exaggerated MSNA and BP responses during isometric exercise. Furthermore, the metabolic component of the exercise pressor reflex is augmented in normotensive PMW and appears to contribute to the abnormal pressor response to exercise in this population. Notably, transdermal estradiol administration attenuates MSNA and BP responses during exercise, suggesting that these heightened cardiovascular responses are modulated by estradiol.
ACKNOWLEDGMENTS
The authors wish to thank Kelly Sebzda, MS, Stephanie Mraz, and Jenna Azzolini for assistance with data collection and analysis, and the participants for their time.
Funding Support:
This research was supported in part by the University of Delaware Research Foundation and NIH P20 GM 113125. MMW is supported by NIH Grant R01 HL146558. JLG is supported by K99/R00 HL133414 and R21 MH123928. WV is supported by P30 DK079328. PJF is supported by NIH Grant R01 HL 127071.
Footnotes
DISCLOSURES
None. Results of this study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14(3):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24(7):740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger A, Grossman E, Katz M et al. Exercise blood pressure and the risk for future hypertension among normotensive middle-aged adults. J Am Heart Assoc. 2015;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J. 1983;106(2):316–20. [DOI] [PubMed] [Google Scholar]
- 5.Singh JP, Larson MG, Manolio TA et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99(14):1831–6. [DOI] [PubMed] [Google Scholar]
- 6.Hoberg E, Schuler G, Kunze B et al. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65(9):583–9. [DOI] [PubMed] [Google Scholar]
- 7.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14(2):263–70. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121(19):2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HM, Stebbins CL, Nho H, Kim KA, Kim C, Kim JK. Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur J Appl Physiol. 2012;112(7):2671–8. [DOI] [PubMed] [Google Scholar]
- 10.Trinity JD, Layec G, Hart CR, Richardson RS. Sex-specific impact of aging on the blood pressure response to exercise. Am J Physiol Heart Circ Physiol. 2018;314(1):H95–H104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charkoudian N Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin Auton Res. 2001;11(5):295–301. [DOI] [PubMed] [Google Scholar]
- 12.Keir DA, Badrov MB, Tomlinson G et al. Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults. Hypertension. 2020;76(3):997–1005. [DOI] [PubMed] [Google Scholar]
- 13.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21(4):498–503. [DOI] [PubMed] [Google Scholar]
- 14.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61(2):395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275(5):R1600–4. [DOI] [PubMed] [Google Scholar]
- 16.Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103(24):2903–8. [DOI] [PubMed] [Google Scholar]
- 17.Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab. 2001;86(1):344–8. [DOI] [PubMed] [Google Scholar]
- 18.Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation. 2001;103(24):2909–14. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger SM, Silber DH, Gray KS et al. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol (1985). 1998;85(6):2075–81. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–8. [DOI] [PubMed] [Google Scholar]
- 21.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879(1–2):105–14. [DOI] [PubMed] [Google Scholar]
- 22.Saleh TM, Connell BJ. Role of 17beta-estradiol in the modulation of baroreflex sensitivity in male rats. Am J Physiol. 1998;275(3 Pt 2):R770–8. [DOI] [PubMed] [Google Scholar]
- 23.Saleh TM, Connell BJ. Centrally mediated effect of 17beta-estradiol on parasympathetic tone in male rats. Am J Physiol. 1999;276(2 Pt 2):R474–81. [DOI] [PubMed] [Google Scholar]
- 24.Saleh TM, Connell BJ. 17beta-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J Auton Nerv Syst. 2000;80(3):148–61. [DOI] [PubMed] [Google Scholar]
- 25.Saleh TM, Connell BJ. Central nuclei mediating estrogen-induced changes in autonomic tone and baroreceptor reflex in male rats. Brain Res. 2003;961(2):190–200. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt PM, Kaufman MP. Estrogen attenuates the exercise pressor reflex in female cats. J Appl Physiol (1985). 2003;95(4):1418–24. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt PM, Kaufman MP. High concentrations of 17beta -estradiol attenuate the exercise pressor reflex in male cats. J Appl Physiol (1985). 2003;94(4):1431–6. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson JC, Hodis HN, Pickar JH, Lobo RA. Coronary heart disease and menopause management: the swinging pendulum of HRT. Atherosclerosis. 2009;207(2):336–40. [DOI] [PubMed] [Google Scholar]
- 29.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57(3):461–9. [DOI] [PubMed] [Google Scholar]
- 30.Greaney JL, Matthews EL, Wenner MM. Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol. 2015;308(8):H816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89(4):372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004;561(Pt 3):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol. 2013;304(5):H759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol. 2013;305(6):H867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45(4):522–5. [DOI] [PubMed] [Google Scholar]
- 36.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224(1):173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–42. [DOI] [PubMed] [Google Scholar]
- 38.Park SA, Kim JK. [Estrogen attenuates the pressor response mediated by the group III mechanoreflex]. J Korean Acad Nurs. 2011;41(2):191–6. [DOI] [PubMed] [Google Scholar]
- 39.Ives SJ, McDaniel J, Witman MA, Richardson RS. Passive limb movement: evidence of mechanoreflex sex specificity. Am J Physiol Heart Circ Physiol. 2013;304(1):H154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299(5):H1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens. 2015;29(7):402–8. [DOI] [PubMed] [Google Scholar]
- 42.Scott CJ, Rawson JA, Pereira AM, Clarke IJ. The distribution of estrogen receptors in the brainstem of female sheep. Neurosci Lett. 1998;241(1):29–32. [DOI] [PubMed] [Google Scholar]
- 43.Hayes SG, Moya Del Pino NB, Kaufman MP. Estrogen attenuates the cardiovascular and ventilatory responses to central command in cats. J Appl Physiol (1985). 2002;92(4):1635–41. [DOI] [PubMed] [Google Scholar]
- 44.Xue B, Zhang Z, Beltz TG et al. Estrogen receptor-beta in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013;61(6):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol. 2006;91(1):59–72. [DOI] [PubMed] [Google Scholar]
- 46.Phillips GB. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J Clin Endocrinol Metab. 2005;90(5):2708–11. [DOI] [PubMed] [Google Scholar]
- 47.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol. 2011;589(Pt 21):5285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briant LJ, Burchell AE, Ratcliffe LE et al. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol. 2016;594(17):4753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;302(11):H2419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riedel K, Deussen AJ, Tolkmitt J et al. Estrogen determines sex differences in adrenergic vessel tone by regulation of endothelial beta-adrenoceptor expression. Am J Physiol Heart Circ Physiol. 2019;317(2):H243–H54. [DOI] [PubMed] [Google Scholar]
- 51.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30(6):1538–43. [DOI] [PubMed] [Google Scholar]
- 52.Menon DV, Vongpatanasin W. Effects of transdermal estrogen replacement therapy on cardiovascular risk factors. Treat Endocrinol. 2006;5(1):37–51. [DOI] [PubMed] [Google Scholar]
- 53.The NHTPSAP. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–53. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno M, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA. Dynamic exercise training prevents exercise pressor reflex overactivity in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2015;309(5):H762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mostoufi-Moab S, Widmaier EJ, Cornett JA, Gray K, Sinoway LI. Forearm training reduces the exercise pressor reflex during ischemic rhythmic handgrip. J Appl Physiol (1985). 1998;84(1):277–83. [DOI] [PubMed] [Google Scholar]


