Abstract
Background:
We investigated weight changes following antiretroviral therapy (ART) initiation, the development of metabolic syndrome (MetS) and its association with all-cause mortality among Asian adults living with HIV.
Methods:
Participants enrolled in a regional Asian HIV cohort with weight and height measurements at ART initiation were eligible for analysis. Factors associated with weight changes and incident MetS (according to IDF definition) were analyzed using linear mixed models and Cox regression, respectively. Competing-risk regression models were used to investigate the association of MetS with all-cause mortality.
Results:
Among 4931 PLWH, 66% were male. At ART initiation, median age was 34 (interquartile range [IQR], 29-41) years, and median (IQR) weight and BMI were 55 (48-63) kg and 20.5 (18.4-22.9) kg/m2, respectively. At 1, 2 and 3 years of ART, overall mean(±SD) weight gain was 2.2(±5.3), 3.0(±6.2) and 3.7(±6.5) kg, respectively. Participants with baseline CD4≤200 cells/mm3 (weight difference[diff]=2.2kg, 95%CI, 1.9-2.5), and baseline HIV RNA ≥100,000 copies/mL (diff=0.6kg, 95%CI, 0.2-1.0), and those starting with integrase strand transfer inhibitor (INSTI)-based ART (diff=2.1kg, 95%CI, 0.7-3.5 vs. NNRTI) had greater weight gain. After excluding those with abnormal baseline levels of MetS components, 295/3503 had incident MetS (1.18 [95%CI, 1.05-1.32]/100 person-years [PYS]). The mortality rate was 0.7 (95%CI, 0.6-0.8)/100 PYS. MetS was not statistically associated with all-cause mortality in the adjusted model (p=0.236).
Conclusion:
Weight gain after ART initiation was significantly higher among those initiating ART with lower CD4, higher HIV RNA and INSTI-based regimen after controlling for baseline BMI. Greater efforts to identify and manage MetS among PLWH are needed.
Keywords: Weight gain, metabolic syndrome, all-cause mortality, HIV/AIDS, Asian PLWH
Introduction
There is an alarming trend of increased rates of obesity globally and in the Asia region (1, 2). Even though people living with HIV (PLWH) have largely benefited from advances in antiretroviral therapy (ART) with drastic reductions in AIDS-defining illness and deaths, and improvement in life expectancy, comorbidities remain a significant burden (3, 4). The risk for cardiovascular diseases (CVD) in PLWH is up to two times higher than among HIV-negative individuals (5). More importantly, obesity and weight gain contribute to chronic inflammation, which leads to metabolic and CVD complications in the population (6, 7). There are growing concerns regarding weight gain after ART initiation, especially among PLWH starting regimens using integrase strand transfer inhibitors (INSTIs) (8). Although post-ART weight gain may reflect a ‘return-to-health’ effect for those who are initially underweight, the effects of excessive weight gain and factors associated with it are less studied among Asian PLWH.
Metabolic syndrome (MetS) is characterized by central obesity and a cluster of risk factors such as (e.g., dyslipidemia, hypertension, insulin resistance, fat distribution changes), and has become a common finding in PLWH. A recent meta-analysis showed that the prevalence of MetS was higher in PLWH than the HIV-negative population (9). The presence of MetS also imposes higher risks for CVD complications (10), making it an important health concern among PLWH (11). In the general population, MetS has also been shown to be an important risk factor for all-cause mortality (12). However, it is unclear whether weight gain after ART initiation contributes to the development of MetS or whether MetS is a predictor of all-cause mortality in PLWH population.
Body weight changes after ART initiation, and the severity and risk factors for MetS are also poorly documented among Asian PLWH populations. Therefore, we evaluated longitudinal weight changes and associated factors among participants enrolled and starting ART, and treatment-emergent MetS and its association with all-cause mortality in a regional cohort in the Asia-Pacific.
Methods
Study design and population
This study is a longitudinal analysis of PLWH enrolled in the TREAT Asia HIV Observational Database (TAHOD) cohort of IeDEA Asia-Pacific. TAHOD is a regional observational cohort study which includes 21 sites from 12 countries in the Asia-Pacific region (13). Briefly, patients are in routine standard care at the sites, and all clinical parameters/measurements and interventions, including the choice of ART regimen, are implemented according to site standard practices and guidelines. PLWH aged ≥18 years enrolled between January 2003 and March 2020 and have been on ART for >6 months were included.
Ethical consideration
Ethics approvals were obtained from the respective local ethics committees of all TAHOD-participating sites, the Kirby Institute (data management and statistical analysis center) and TREAT Asia/amfAR (coordinating center). Participant informed consent was obtained or waived according to the regulations from the local institutional review boards of each participating site.
Study outcomes
We firstly evaluated the longitudinal weight changes, and factors associated with weight changes. Secondly, we investigated the incidence of MetS and the factors associated with incident MetS. Thirdly, we evaluated the association of MetS with all-cause mortality in the cohort.
Eligibility criteria
Participants with baseline body weight and height measurements and at least one weight measurement after 3 months of ART were included in the weight changes analysis. Baseline was defined latest pre-ART measurements within six months of treatment initiation. For the MetS analysis, participants with at least one BMI measurement and at least one measurement for any of two risk factors (i.e., elevated blood pressure, reduced HDL-cholesterol, abnormal TG-cholesterol, and fasting blood glucose) after 3 months of ART initiation were included. Those with baseline obese body mass index (BMI >27.5 kg/m2) or any known abnormal baseline values of blood pressure, HDL and triglycerides, and fasting blood glucose were excluded in the MetS analysis.
Statistical analysis
Weight changes
Body weight measurements were analyzed using 6-month intervals (with a window period of ±30 days). BMI was categorized as underweight (<18.5 kg/m2), normal (18.5-23 kg/m2), overweight (23-27.5 kg/m2) and obese (>27.5 kg/m2) according to the World Health Organization guidelines for Asian population (14). Weight changes at 3 years after ART initiation from baseline and the associated factors were analyzed using linear mixed effect models with random intercepts and slopes on patients and time since ART initiation. The multivariate model for weight changes was adjusted for age, sex, types of initial ART regimen, cumulative exposure of ART regimen, prior AIDS-defining events, year of ART initiation, baseline BMI, CD4 count and HIV-1 RNA level.
Metabolic syndrome and all-cause mortality
MetS was defined as central obesity AND two of any of the following factors, according to International Diabetic Federation (IDF) definition (10): 1) TG ≥150 mg/dL or on treatment for dyslipidemia, 2) HDL ≤40 mg/dL for males and ≤50 mg/dL for females or on treatment for dyslipidemia, 3) elevated blood pressure (systolic blood pressure [SBP] ≥130 mmHg and diastolic blood pressure [DBP] ≥85 mmHg) or on treatment for hypertension or 4) fasting blood glucose ≥100 mg/dL or diagnosis of type-2 diabetes mellitus (DM) or treatment with oral hypoglycemic agents. Of note, the IDF suggests the waist circumference does not need to be measured and central obesity can be assumed if the definition of obesity is met (10). In this analysis, the Asian obesity threshold of BMI >27.5 kg/m2 was used. Crude incidence rates of MetS were reported per 100 person-years of follow-up (PYS). Factors associated with incident MetS were analyzed using Cox regression model. The separate models were employed to evaluate the association of weight gain over 3 years of ART initiation from baseline with the incidence of MetS components (i.e., elevated blood pressure, low HDL cholesterol, high triglycerides and impaired fasting blood glucose).
Causes of death were based on review of the standardized Cause of Death (CoDe) form developed by the D:A:D study (15). Follow-up was censored at death, loss-to-follow-up (LTFU) or transferred to another clinic. LTFU was defined as no visit in the previous 12 months. LTFU were censored as competing risks for all-cause mortality in the regression analysis. Association of MetS with all-cause mortality was investigated using the Fine and Gray competing-risk regression model, adjusting for known risk factors for mortality.
The adjusted regression models for weight changes and incident MetS fitted the covariates that were significant (p<0.10) in the univariate models using backward stepwise selection process. The competing-risk regression for all-cause mortality included covariates that were selected a priori based on clinical relevance. Time-fixed covariates included pre-ART CD4, HIV-1 RNA, BMI and weight, family history of CVD, hepatitis B and C co-infection and initial ART regimen. Time-varying covariates were post-ART CD4 count, HIV-1 RNA, AIDS-defining events, and exposure to stavudine (D4T) and protease inhibitors (PI).
All analyses were stratified by site to account for the potential disparities in health care systems and differences in clinical practices across the participating sites (16). A two-sided level of 5% statistical significance was used for all inferences. The analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and STATA software version 14.2 (STATA Corp., College Station, TX, USA).
Results
Characteristics of participants
Of 9947 PLWH aged ≥18 years who enrolled in TAHOD between January 2003 and March 2020, 4931 were included in this analysis after excluding those without baseline weight and height measurements (Figure 1). Of 4931, 3252 (66%) were male, 834 (17%) acquired HIV through homosexual contact, and 410 (8.2%) through injecting drugs use (Table 1). Participants included in the study were from the participating sites in Cambodia (n=554), China (n=61), Hong Kong (n=244), India (n=520), Indonesia (n=655), Japan (n=111), Malaysia (n=282), Philippines (n=108), Singapore (n=163), South Korea (n=20), Taiwan (n=205), Thailand (n=1449) and Vietnam (n=559). Among them, 92%, 6.6% and 1.2% started NNRTI-based, PI-based and INSTI-based ART, respectively. Details of initial ART regimen including the third ART agent and NRTI backbone are summarized in Supplementary Table S1. Efavirenz and nevirapine were the commonly used NNRTIs, whereas atazanavir/ritonavir and lopinavir/ritonavir were the commonly used PIs at ART initiation. Majority of the participants had started with NRTI backbone of tenofovir disoproxil fumarate (20.6%), zidovudine (34.2%) and stavudine (39.7%). Less than 1% started with tenofovir alafenamide-containing ART. The median age at ART initiation was 34 (interquartile range [IQR], 29-41) years; most PLWH started ART during 2008-2012 (56%), and 42% had CD4 ≤100 cells/mm3 at ART initiation. The median follow-up time was 8.3 (IQR, 5.1-11.5) years. Twelve per cent and 9% had hepatitis B and C co-infection. The median pre-ART weight and BMI were 55 (IQR, 48-63) kg and 20.5 (IQR, 18.4-22.9) kg/m2, respectively. PLWH who started with INSTI-based ART had higher baseline body weight than those started with NNRTI-based regimen (mean 63.8 ±SD 16.6 vs. 55.7 ±11.4 kg, p=0.001). In addition, 5.1%, 8.6% and 5.5% had obese BMI, impaired FBG and elevated blood pressure at baseline, respectively.
Figure 1.
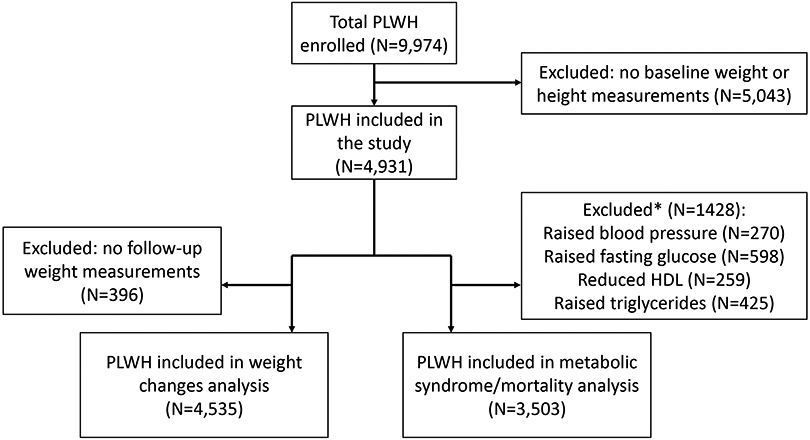
Study flow diagram for inclusion of participants
*Participants excluded had more than one risk factor for metabolic syndrome.
Table 1.
Participant characetristics
| Baseline characteristics | Total (N=4,931) | Males (N=3,252) | Females (N=1,679) | |||
|---|---|---|---|---|---|---|
| Frequency or Median |
Percentage or IQR |
Frequency or Median |
Percentage or IQR |
Frequency or Median |
Percentage or IQR |
|
|
Age at ART initiation
Median (IQR) |
34 | 29-41 | 35 | 29-42 | 34 | 29-40 |
| ≤30 | 1,569 | 31.8 | 980 | 30.1 | 589 | 35.1 |
| 31-40 | 2,072 | 42.0 | 1,362 | 41.9 | 710 | 42.3 |
| 41-50 | 904 | 18.3 | 623 | 19.2 | 281 | 16.7 |
| 51+ | 386 | 7.8 | 287 | 8.8 | 99 | 5.9 |
| Mode of HIV acquisition | ||||||
| Heterosexual contact | 3,446 | 69.9 | 1,868 | 57.4 | 1,578 | 94.0 |
| Homosexual contact | 831 | 16.9 | 823 | 25.3 | 8 | 0.5 |
| Injecting drug use | 410 | 8.2 | 382 | 11.8 | 28 | 1.7 |
| Other/unknown | 244 | 5.0 | 179 | 5.5 | 65 | 3.8 |
| Year of ART initiation | ||||||
| 2003-2007 | 1,755 | 35.6 | 1,125 | 34.6 | 630 | 37.5 |
| 2008-2012 | 2,749 | 55.8 | 1,840 | 56.6 | 909 | 54.1 |
| 2013-2019 | 427 | 8.6 | 287 | 8.8 | 140 | 8.3 |
|
Pre-ART CD4, cells/mm3
Median (IQR) |
125 | 40-228 | 111 | 33-223 | 148 | 56-233 |
| <=100 | 2,057 | 41.7 | 1,452 | 44.7 | 605 | 36.0 |
| 101-200 | 1,128 | 22.9 | 688 | 21.2 | 440 | 26.2 |
| 201-350 | 1,086 | 22.0 | 670 | 20.6 | 416 | 24.8 |
| >350 | 368 | 7.5 | 233 | 7.2 | 135 | 8.0 |
| Missing | 292 | 5.9 | 209 | 6.43 | 83 | 4.94 |
| HIV viral load at ART initiation, copies/mL | ||||||
| <100,000 | 1,215 | 24.6 | 819 | 25.2 | 396 | 23.6 |
| ≥100,000 | 1,190 | 24.2 | 876 | 26.9 | 314 | 18.7 |
| Missing | 2,526 | 51.2 | 1,557 | 47.9 | 969 | 57.7 |
|
Pre-ART BMI, kg/m2
Median (IQR) |
20.5 | 18.4-22.9 | 20.6 | 18.6-23.1 | 20.3 | 18.3-22.6 |
| Underweight, <18.5 | 1259 | 25.5 | 796 | 24.5 | 463 | 27.6 |
| Normal, 18.5-23.0 | 2458 | 49.9 | 1,615 | 49.7 | 843 | 50.2 |
| Overweight, 23-27.5 | 961 | 19.5 | 681 | 20.9 | 280 | 16.7 |
| Obese, >27.5 | 253 | 5.1 | 160 | 4.9 | 93 | 5.5 |
|
Pre-ART body weight, kg
Median (IQR) |
55 | 48-63 | 58 | 51.5-66 | 49 | 43.5-55 |
| Initial ART regimen | ||||||
| NNRTI-based | 4523 | 91.7 | 2,919 | 89.8 | 1,604 | 95.5 |
| PI-based | 327 | 6.63 | 272 | 8.4 | 55 | 3.3 |
| INSTI-based | 58 | 1.2 | 44 | 1.4 | 14 | 0.8 |
| Others | 23 | 0.47 | 17 | 0.5 | 6 | 0.4 |
| Prior AIDS diagnosis ¶ | ||||||
| No | 3061 | 62.1 | 1,880 | 57.8 | 1,181 | 70.3 |
| Yes | 1,870 | 37.9 | 1,372 | 42.2 | 498 | 29.7 |
| Country income level (World Bank) | ||||||
| Lower middle income | 2396 | 48.6 | 1,493 | 45.9 | 903 | 53.8 |
| Upper middle income | 1,792 | 36.3 | 1,093 | 33.6 | 699 | 41.6 |
| High income | 743 | 15.1 | 666 | 20.5 | 77 | 4.5 |
| Ever smoke | ||||||
| Yes | 1725 | 35.0 | 1577 | 48.5 | 148 | 8.8 |
| No | 2272 | 46.1 | 1020 | 31.4 | 1252 | 74.6 |
| Not reported | 934 | 18.9 | 655 | 20.1 | 279 | 16.6 |
| Hepatitis C co-infection | 588 | 11.9 | 501 | 15.4 | 87 | 5.2 |
| Hepatitis B co-infection | 424 | 8.6 | 315 | 9.7 | 109 | 6.5 |
|
Pre-ART fasting blood glucose (mg/dL)
Median (IQR) (N=1921) |
88 | 80-98 | 89.7 | 81-100 | 85 | 77-94 |
| Impaired fasting blood glucose* at baseline | 425 | 8.6 | 321 | 9.9 | 104 | 6.2 |
| Diabetes mellitus* at baseline | 111 | 2.2 | 87 | 2.7 | 24 | 1.4 |
|
Pre-ART triglycerides (mg/dL)
Median (IQR) (N=1590) |
128 | 93-177.1 | 133 | 97-182 | 121 | 88-168 |
|
Pre-ART HDL (mg/dL)
Median (IQR) (N=848) |
36 | 28.2-45.6 | 34 | 27-42 | 41 | 34-50 |
| Dyslipidemia* at baseline | 798 | 16.2 | 569 | 17.5 | 229 | 13.6 |
| Hypertension* at baseline | 270 | 5.5 | 201 | 6.2 | 69 | 4.1 |
Data in the table are presented in either number (percentage) or median (IQR).
Impaired fasting blood glucose (FBG) was defined as FBG ≥100 mg/dL. Diabetes melltius at baseline was defined as FBG ≥126 mg/dL. Dyslipidemia at baseline was defined as total cholestrol >200 mg/dL or HDL <35 mg/dL or triglycerides >150 mg/dL. Hypertension was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg or use.
Prior AIDS diagnosis was defined as having a condition from the modified CDC AIDS-indicator list, including tuberculosis or an AIDS-related malignancy.
Abbreviations: IQR, interquartile range; ART, antiretroviral therapy; NNRTI, non-nucelotide reverse transcriptase inhibitor; PI, protesase inhibitor; INSTI, integrase strand transfer inhibitor.
Longitudinal weight changes over 3 years after ART initiation
After excluding participants who did not have a follow-up weight measurement after ART initiation, 4535 PLWH were included in the weight change analysis. Overall mean weight gain (± standard deviation, SD) was 2.2 (±5.3) kg, 3.0 (±6.2) kg and 3.7 (±6.5) kg at 1, 2 and 3 years of ART, respectively. PLWH started with an INSTI-based ART had greater weight gain compared with NNRTI-based ART at 6, 12, 18, 24, 30 and 60 months (Supplementary Figure S1 and Supplementary Table S2, p-values <0.05). Males and PLWH with lower baseline BMI had greater weight gain at year 1, 2 and 3 after ART initiation (Supplementary Table S3). In the multivariate regression, those started with INSTI-based ART (weight difference=2.1 kg, 95%CI 0.7 to 3.5, p=0.003, compared to NNRTI-based), underweight BMI, 2.5 kg, 95%CI 2.1 to 2.8, p<0.001, compared to normal BMI), prior AIDS diagnosis (1.6 kg, 95%CI 1.2 to 1.8, p<0.001) had greater weight gain. Females (−1.2 kg, 95% CI −1.5 to −0.9, p<0.001), those with overweight BMI, −1.6 kg, 95% CI −1.9 to −1.2, p<0.001, and obese BMI, −2.7 kg, 95%CI −3.3 to −2.1, p<0.001) had lower weight gain.
We observed the similar weight gain in the adjusted model when ART regimen was analyzed as the cumulative exposure (INSTI-based ART, 2.2 kg, 95%CI 0.6 to 3.3, p=0.005 compared with NNRTI-based). In addition, lower CD4 (≤200 cells/mm3, 2.2 kg, 95%CI 1.9 to 2.5, p<0.001; vs. >200 cells/mm3) and higher HIV-1 RNA (≥100,000 copies/mL, 0.6 kg, 95%CI 0.2 to 1.0, p=0.004; vs. <100,000 copies/mL) at ART initiation were also associated with greater weight gain (Table 2). The pattern of weight change did not differ after controlling time-varying CD4 count or HIV-1 RNA level.
Table 2.
Factors associated with weight changes over 3 years after starting ART
| Total N=4,535 | Univariable model | Multivariable model | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean weight difference (kg) |
95% CI | P-value | Adjusted mean weight difference (kg) |
95% CI | P-value | |||
| Age at ART initiation | 0.354 | 0.172 | ||||||
| ≤30 | Ref | Ref | ||||||
| 31-40 | 0.2 | −0.1 | 0.6 | 0.188 | 0.2 | −0.1 | 0.6 | 0.093 |
| 41-50 | 0.1 | −0.4 | 0.5 | 0.686 | 0.2 | −0.2 | 0.6 | 0.343 |
| 51+ | −0.2 | −0.8 | 0.4 | 0.492 | −0.01 | −0.6 | 0.5 | 0.969 |
| Female (vs. male) | −1.6 | −2.0 | −1.3 | <0.001 | −1.2 | −1.5 | −0.9 | <0.001 |
| Mode of HIV acquisition | 0.662 | |||||||
| Heterosexual | ||||||||
| Homosexual | 0.1 | −0.6 | 0.7 | 0.796 | ||||
| Injecting drugs use | ||||||||
| Others | 0.2 | −0.6 | 0.9 | 0.663 | ||||
| Initial ART regimen | 0.112 | 0.029 | ||||||
| NNRTI-based | Ref | Ref | ||||||
| PI-based | −0.2 | −0.4 | 0.3 | 0.115 | 0.2 | −0.5 | 0.8 | 0.407 |
| INSTI-based | 1.3 | −0.7 | 2.4 | 0.084 | 2.1 | 0.7 | 3.5 | 0.003 |
| Other | −0.5 | −2.9 | 1.8 | 0.332 | −0.4 | −2.5 | 1.7 | 0.314 |
| Pre-ART BMI (kg/m2) | <0.001 | <0.001 | ||||||
| <18.5 | 3.0 | 2.7 | 3.4 | <0.001 | 2.5 | 2.1 | 2.8 | <0.001 |
| 18.5-23 | Ref | Ref | ||||||
| 23-27.5 | −1.9 | −2.3 | −1.5 | <0.001 | −1.6 | −1.9 | −1.2 | <0.001 |
| >27.5 | −3.5 | −4.1 | −2.8 | <0.001 | −2.7 | −3.3 | −2.1 | <0.001 |
| Prior AIDS diagnosis (yes vs. no) | 3.1 | 2.7 | 3.4 | <0.001 | 1.6 | 1.2 | 1.8 | <0.001 |
| HCV co-infection (yes vs. no) | −0.1 | −0.6 | 0.4 | 0.776 | ||||
| HBV co-infection (yes vs. no) | 0.2 | −0.3 | 0.7 | 0.481 | ||||
| Year of ART initiation | <0.001 | 0.001 | ||||||
| 2003-2007 | ||||||||
| 2008-2012 | −0.8 | −0.2 | −0.4 | <0.001 | 0.4 | 0.01 | 0.8 | 0.044 |
| 2013-2019 | −1.7 | −2.4 | −1.0 | <0.001 | 0.8 | 0.2 | 1.4 | 0.009 |
| Pre-ART CD4 cell count (cells/mm3) | 0.032 | <0.001 | ||||||
| ≤200 | 3.7 | 3.4 | 4.1 | <0.001 | 2.2 | 1.9 | 2.5 | <0.001 |
| >200 | Ref | Ref | ||||||
| Missing | 3.2 | 2.5 | 3.9 | <0.001 | ||||
| Pre-ART HIV-1 RNA, copies/mL | <0.001 | 0.006 | ||||||
| <100,000 | Ref | Ref | ||||||
| ≥100,000 | 2.0 | 1.5 | 2.4 | <0.001 | 0.6 | 0.2 | 1.0 | 0.004 |
| Missing | 1.7 | 1.3 | 2.2 | <0.001 | ||||
The linear mixed models were also adjusted for study sites and time from ART initiation.
Abbreviations: ART, antiretroviral therapy; NNRTI, non-nucelotide reverse transcriptase inhibitor; PI, protesase inhibitor; INSTI, integrase strand transfer inhibitor
Incident metabolic syndrome and its associated factors
After excluding those with abnormal levels of MetS components at baseline, 3503 participants remained in this analysis. Among them, 295 (8.4%) PLWH developed MetS during 25,085 PYS, an incidence rate of 1.18 (95%CI 1.05-1.32) per 100 PYS. Of 295, 93.2%, 70.4%, 27.6% and 90.9% had developed abnormal levels of BP, FBG, HDL-cholesterol and TG-cholesterol, respectively (Supplementary table S4). The Kaplan-Meier estimation showed the probability of developing MetS was 8.4% (95%CI, 7.5-9.4), 8.9% (95%CI, 7.9-10.1) and 9.0% (95%CI, 8.0-10.2) at 1, 3 and 5 years after ART initiation. The probability of MetS development was higher among PLWH with higher baseline BMI group (p <0.001). In multivariate Cox regression (Table 3), baseline overweight BMI (adjusted hazard ratio[aHR]=5.55, 95%CI 4.32-7.10, p<0.001; compared to baseline normal BMI), follow-up AIDS-defining events (aHR=1.40, 95%CI 1.09-1.79, p=0.08), lower CD4 count (≤200 cell/mm3, aHR=1.78, 95% CI 1.33-2.39, p<0.001; compared to >200 cells/mm3) and higher HIV-1 RNA (>400 copies/mL, aHR=4.72, 95%CI 2.13-10.47, p<0.001; compared to <400 copies/mL) and stavudine exposure (aHR=1.51, 95%CI 1.13-2.02, p=0.005) were positively associated with MetS development. The association of INSTI use with the MetS was not statistically significant in the adjusted analysis (aHR=0.75, 95%CI 0.21-5.51, p=0.777). There were also no significant associations of other ARVs (e.g., DDI, EFV or NVP) or hepatitis B or C co-infection with the incident MetS (all p-values >0.2).
Table 3.
Factors associated with incident metabolic syndrome
| No. of patients |
Follow- up years |
No. of MetS |
Incidence rate (per 100 PYS) |
95% CI (per 100 PYS) |
aHR | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | 3503 | 25,085 | 295 | 1.18 | 1.05-1.32 | ||||
| Age group * | 0.844 | ||||||||
| ≤30 | 1205 | 8035 | 79 | 0.98 | 0.79-1.23 | Ref | |||
| 31-40 | 1473 | 11,213 | 129 | 1.15 | 0.97-1.37 | 1.01 | 0.75 | 1.33 | 0.992 |
| 41-50 | 605 | 4337 | 58 | 1.34 | 1.03-1.73 | 0.97 | 0.68 | 1.38 | 0.852 |
| 51+ | 220 | 1500 | 29 | 1.93 | 1.34-2.78 | 1.18 | 0.76 | 1.84 | 0.461 |
| Sex | |||||||||
| Male | 2252 | 15,564 | 201 | 1.29 | 1.12-1.48 | Ref | |||
| Female | 1251 | 9521 | 94 | 0.99 | 0.81-1.21 | 1.07 | 0.82 | 1.39 | 0.637 |
| Pre-ART BMI (kg/m2) | <0.001 | ||||||||
| <18.5 | 1103 | 7821 | 10 | 0.13 | 0.07-0.24 | 0.15 | 0.08 | 0.29 | <0.001 |
| 18.5-23.0 | 1834 | 13,729 | 107 | 0.78 | 0.64-0.94 | Ref | |||
| 23.0-27.5 | 656 | 3535 | 178 | 5.04 | 4.35-5.83 | 5.55 | 4.32 | 7.10 | <0.001 |
| AIDS-defining events * | |||||||||
| No | ~ | 14,784 | 169 | 1.14 | 0.98-1.33 | Ref | |||
| Yes | ~ | 10,301 | 126 | 1.22 | 1.03-1.46 | 1.40 | 1.09 | 1.79 | 0.008 |
| CD4 cell count (cells/mm3) * | |||||||||
| ≤200 | ~ | 4016 | 199 | 4.96 | 4.31-5.69 | 1.78 | 1.33 | 2.39 | <0.001 |
| >200 | ~ | 20,830 | 80 | 0.38 | 0.31-0.48 | Ref | |||
| Missing | ~ | 239 | 16 | 6.69 | 4.10-10.91 | ||||
| HIV-1 RNA (copies/mL) * | |||||||||
| >400 | ~ | 3142 | 52 | 1.66 | 1.26-2.17 | 4.72 | 2.13 | 10.47 | <0.001 |
| ≤400 | ~ | 15,984 | 5 | 0.03 | 0.01-0.08 | Ref | |||
| Missing | ~ | 5959 | 238 | 3.99 | 3.52-4.53 | ||||
| Year of ART initiation | 0.129 | ||||||||
| 2003-2007 | 1220 | 11,604 | 132 | 1.14 | 0.96-1.35 | Ref | |||
| 2008-2012 | 1968 | 12,547 | 148 | 1.18 | 1.00-1.39 | 0.81 | 0.59 | 1.09 | 0.164 |
| 2013-2019 | 315 | 934 | 15 | 1.61 | 0.97-2.66 | 0.55 | 0.29 | 1.02 | 0.060 |
| Family history of CVD | |||||||||
| No | 1317 | 10,601 | 121 | 1.14 | 0.96-1.36 | Ref | |||
| Yes | 177 | 1380 | 26 | 1.88 | 1.28-2.77 | 1.36 | 0.85 | 2.16 | 0.199 |
| Unknown | 2009 | 13,104 | 148 | 1.13 | 0.96-1.33 | ||||
| Ever smoke | |||||||||
| No | 1197 | 12,787 | 160 | 1.25 | 1.07-1.46 | Ref | |||
| Yes | 1650 | 94,467 | 117 | 1.24 | 1.03-1.48 | 1.05 | 0.82 | 1.36 | 0.679 |
| Missing | 656 | 2851 | 18 | 0.63 | 0.40-1.00 | ||||
| INSTI exposure * | |||||||||
| No | ~ | 24,472 | 291 | 1.19 | 1.06-1.33 | 0.54 | 0.11 | 3.94 | 0.553 |
| Yes | ~ | 316 | 1 | 0.32 | 0.04-2.25 | Ref | |||
| Stavudine exposure * | |||||||||
| No | ~ | 19,953 | 157 | 0.79 | 0.67-0.92 | Ref | |||
| Yes | ~ | 5132 | 138 | 2.69 | 2.28-3.18 | 1.51 | 1.13 | 2.02 | 0.005 |
| Protease inhibitor exposure * | |||||||||
| No | ~ | 22,185 | 265 | 1.19 | 1.06-1.35 | Ref | |||
| Yes | ~ | 2900 | 30 | 1.03 | 0.72-1.48 | 1.12 | 0.68 | 1.84 | 0.646 |
These are time-varying variables.
In the time-to-event analysis of MetS, we excluded individuals with baseline BMI >27.5 kg/m2, elevated blood pressure (SBP ≥130 mmHg, DBP ≥85 mmHg), high trigylercides (≥150 mg/dL), low HDL (≤ 40 mg/dL for males and ≤ 50 mg/dL for females) and high fasting blood glucose (≥100 mg/dL) levels.
The multivariable model was stratified by study site. The multivariate model was adjusted for age, sex, baseline BMI, family CVD history, smoking, and follow-up AIDS events, CD4 count, HIV-1 RNA level and stavudine exposure. There were no significant associations of time-varying exposure of other ARVs (e.g., TDF, EFV or NVP), hepatitis B or C co-infection with the incident MetS (all p-values >0.2).
Abbreviations: MetS, metabolic syndrome; BMI, body mass index; ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; aHR, adjusted hazard ratio.
In the separate models, greater weight gain over 3 years of ART initiation from baseline was also significantly associated with the MetS components (p-values <0.001; Supplementary table S5).
All-cause mortality and its association with metabolic syndrome
There were 163 deaths among 3503 participants, resulted in the mortality rate of 0.72 (95%CI 0.61-0.82) per 100 PYS. Among 163 deaths, 79 (48%) were AIDS-related and 84 (52%) were non-AIDS related deaths or unknown cause. The adjusted model showed that older age (p<0.001), HCV co-infection (p<0.001), AIDS-defining events (p=0.001), current low CD4 count (p<0.001) and high HIV-1 RNA (p<0.001) had higher risks for all-cause mortality (Table 4). Females had lower hazard for all-cause mortality, compared to males (p=0.028). The association of MetS and all-cause mortality was not statistically significant after controlling the confounders (adjusted sub-hazard ratio=0.57, 95%CI 0.23-1.44, p=0.236). In a sensitivity analysis which limited to only non-AIDS deaths (n=84), MetS was also not statistically associated with non-AIDS-related mortality in the adjusted model (p=0.166).
Table 4.
Factors associated with all-cause mortality
| Total N=3503 (163 deaths) | aSHR | 95% CI | P-value | |
|---|---|---|---|---|
| Age at ART initiation | <0.001 | |||
| <30 | Ref | |||
| 31-40 | 1.10 | 0.74 | 1.63 | 0.633 |
| 41-50 | 1.49 | 0.93 | 2.39 | 0.094 |
| 51+ | 3.56 | 2.04 | 6.20 | <0.001 |
| Female (vs. male) | 0.64 | 0.44 | 0.93 | 0.028 |
| HCV co-infection (yes vs. no) | 2.42 | 1.52 | 3.83 | <0.001 |
| AIDS-defining events * (yes vs. no) | 1.79 | 1.26 | 2.55 | 0.001 |
| CD4 cell count (cells/mm3) * | <0.001 | |||
| ≤200 | 4.02 | 2.67 | 6.05 | <0.001 |
| >200 | Ref | |||
| Missing | 1.22 | 0.16 | 9.23 | 0.849 |
| HIV-1 RNA (copies/mL) * | <0.001 | |||
| ≤400 | Ref | |||
| >400 | 2.60 | 1.65 | 4.09 | <0.001 |
| Missing | 0.74 | 0.47 | 1.18 | 0.208 |
| Year of ART initiation | 0.008 | |||
| 2002-2008 | Ref | |||
| 2008-2012 | 0.57 | 0.36 | 0.83 | 0.004 |
| 2013-2019 | 0.34 | 0.11 | 1.20 | 0.094 |
| Metabolic syndrome * (yes vs. no) | 0.57 | 0.23 | 1.44 | 0.236 |
These are time-varying variables. The multivariable model was also adjusted by site to account for the differences in pracitices acrossing different study sites. HCV co-infection is defined by positive HCV anti-body. There were no significant associations of baseline BMI, types of ART regimen, HBV co-infection with all-cause mortality in the mutlivariable competing risk model.
Abbreviations: aSHR, adjusted subdistribution hazard ratio.
Discussion
In an Asia regional cohort of adults living with HIV on ART, several demographics, clinical, HIV-related, and ART-specific factors are associated with post-ART weight gain. Greater weight gain was seen among PLWH with low BMI and CD4, and high HIV-1 RNA at ART initiation, and those initiating ART with an INSTI-based regimen. Females and those with high BMI at baseline had lower weight gain. Anti-retroviral treatment-emergent MetS was common among Asian PLWH, with individuals treated with older ARV such as stavudine and those who had immunosuppression during follow-up (low CD4 and AIDS diagnosis) and high HIV-1 RNA at higher risks for MetS development. However, MetS was not associated with either all-cause mortality or non-AIDS deaths.
Following ART initiation, the absolute mean weight gain in this cohort of Asian PLWH (2.2 kg and 3 kg at year 1 and 2) was comparable to the results from the pooled analysis of the large phase-3 trials from different settings which reported a mean weight change of 2 kg and 3 kg at year 1 and 2, respectively (17). However, at year 3 of ART initiation, PLWH in our study had slightly greater mean weight gain than the pooled analysis (3.7 kg vs. 3 kg) despite majority of our study participants started with NNRTI-based ART. Greater weight gain was observed in PLWH started with baseline underweight BMI while lower weight gain was seen in those with overweight or obese BMI at baseline compared to the normal BMI. Previous studies have demonstrated the greater weight gain among individuals with low pre-ART CD4 counts and high pre-ART HIV RNA levels (18-21).
Consistent with previous research, our results showed the greater weight gain among PLWH with advanced HIV disease (reflected by low CD4 and AIDS diagnosis at baseline) or underweight BMI at ART initiation. This could reflect the contribution of immune reconstitution effects as the immune recovery of PLWH improved after ART initiation. In contrast to those studies with the participants predominantly from the U.S., Europe, and other settings which demonstrated sex and racial differences in weight gain (17, 18, 20), Asian male PLWH in our study had greater weight gain compared to female PLWH upon ART initiation. This suggests that the sex differences in weight gain are not similar across different races among PLWH populations. Of note, most PLWH in the pooled trials (17) had started with INSTI-based regimens while majority of our participants initiated with NNRTI-based ART.
We did not find the association of NNRTI or PI use with the weight change. Very small proportion (<1%) of PLWH started with TAF-based regimen among the included participants. However, our adjusted analysis demonstrated that PLWH started with an INSTI-based regimen had greater weight gain compared to those started with a NNRTI-based ART. Since INSTI-based ART is being scaled up rapidly in the Asia-Pacific region, close monitoring of weight gain and its related comorbidities should be done regularly, particularly for PLWH who are starting ART with overweight or obese BMI.
Despite the benefits of weight gain from the ‘return-to-health’ phenomenon with underweight or advanced HIV disease at ART initiation, the effects of excessive post-ART weight gain among PLWH who started ART with normal or obese BMI are unclear (19). MetS, which includes obesity as the central component and a clustering of other metabolic parameters, was known to be associated with higher CVD risk in both HIV-seronegative and PLWH populations (22, 23). Importantly, we also found that greater weight gain over 3 years of ART initiation was associated with the development of MetS components such as hypertension, impaired glucose and dyslipidemia, irrespective of baseline BMI levels.
Previous reports demonstrated that the specific factors for HIV infection such as immunosuppression and viremia were associated with metabolic abnormalities such as dyslipidemia (24) and insulin resistance (25). Consistent with other studies (26, 27), low CD4 count, unsuppressed HIV or AIDS events during the follow-up were associated with MetS development in the present study. Increased immune activation and inflammation caused by immunosuppression or HIV viremia likely contribute to the alterations in lipid profiles and other metabolic abnormalities, which in turn increase the risks of atherosclerosis and CVD (26, 28, 29). In addition, high BMI was also a known risk factor for the metabolic complications (e.g., DM and CVD events) (30-32). In our study, PLWH who were overweight had greater hazards of developing MetS compared to those who had normal BMI at ART initiation. This highlights the importance of routine and adequate monitoring of metabolic abnormalities for the obese or overweight individuals starting ART.
Despite several reports have demonstrated the links between PIs use and CVD risks, we did not find the use of PIs to be a predictive factor of the incident MetS. However, stavudine exposure, a known inducer for mitochondrial cytotoxicity, was independently associated with MetS. These results are consistent with previous research demonstrating the stavudine exposure but not PIs as the predictor of MetS (33). In addition, a recent analysis showed that individuals from a randomized controlled trial (ADVANCE) started with either TDF- or TAF-based INSTI-containing regimen had significantly higher proportions of incident MetS compared with those started with an NNRTI-based regimen (34), and increased risk of type-2 DM and higher CVD risks with the use of TAF/FTC+DTG (35). However, we did not find the statistical association of INSTI with treatment-emergent MetS in the present analysis. Due to the small number PLWH treated with INSTI in this cohort, the association of such regimens with the risk of developing MetS needs to be further explored with a larger sample size.
In some studies, MetS was associated with all-cause mortality or CVD-related deaths in the general population (36-38). To the best of our knowledge, only one previous study evaluated the longitudinal relationship between the MetS development with all-cause mortality, and the study in the U.S with over 3 years of follow-up demonstrated the link between the MetS and the increased risk of mortality among PLWH (39). In contrast, the current analysis did not find the association of the incident MetS with all-cause mortality in this Asian PLWH cohort. Furthermore, no significant association was seen between the MetS and non-AIDS deaths.
Our study has certain limitations. Firstly, the number of individuals who started with INSTI-based ART was small so the association with greater weight gain should be interpreted cautiously. Secondly, we did not evaluate inflammation or immune activation markers which could be associated with MetS and mortality as these data were not available. Thirdly, as the components of MetS were assessed sporadically according to local clinical practices, some cases might have been missed. Fourthly, we also were unable to investigate the effect of TAF on weight gain since only 4 PLWH included in the analysis started or switched to TAF-based regimen. Fifth, data on other known risk factors for MetS (e.g., dietary and lifestyle factors, physical activity) and interventions to possibly reduce MetS development risk (e.g., statin use) were not available. Finally, as an observational study, our estimates may be impacted by other uncontrolled confounders.
In conclusion, greater weight gain was seen in Asian PLWH started on first-line INSTI-based ART, and MetS was not uncommon in this population. Advanced HIV disease and immunosuppression were associated with both greater weight gain and treatment- emergent MetS. However, MetS was not associated with all-cause mortality or non-AIDS deaths. Further research to assess the effects of weight gain and the MetS on CVD risks are needed. Clinicians should also be encouraged to monitor weight changes more proactively among PLWH, especially those starting ART with low CD4 count and those with baseline and incident elevated BMI. Despite the effective ART, lifestyle modifications including healthy diet and physical activity, together with routine screening of hypertension, glucose intolerance and lipid abnormalities or preventive interventions should be encouraged for PLWH population, especially within a society with an increasing trend of obesity.
Supplementary Material
Funding
This work was supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International Epidemiology Databases to Evaluate AIDS [IeDEA; U01AI069907]. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Acknowledgements
TAHOD study members
PS Ly*, V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao, N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee*, PCK Li, TS Kwong, TH Li, Queen Elizabeth Hospital, Hong Kong SAR;
N Kumarasamy*, C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India;
S Pujari*, K Joshi, S Gaikwad, A Chitalikar, Institute of Infectious Diseases, Pune, India;
S Sangle*, V Mave, I Marbaniang, S Nimkar, BJ Government Medical College and Sassoon General Hospital, Pune, India;
TP Merati*, DN Wirawan, F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
E Yunihastuti*, A Widhani, S Maria, TH Karjadi, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
J Tanuma*, S Oka, T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S, JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
YM Gani*, NB Rudi, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
I Azwa*, A Kamarulzaman, SF Syed Omar, S Ponnampalavanar, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco*, MK Pasayan, ML Mationg, Research Institute for Tropical Medicine, Muntinlupa City, Philippines;
YJ Chan*, WW Ku, PC Wu, E Ke, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee, T Yap, Tan Tock Seng Hospital, National Centre for Infectious Diseases, Singapore (note: OT Ng is also supported by the Singapore Ministry of Health’s (MOH) National Medical Research Council (NMRC) Clinician Scientist Award (MOH-000276). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not reflect the views of MOH/NMRC.);
A Avihingsanon*, S Gatechompol, P Phanuphak, C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul*, A Phuphuakrat, L Chumla, N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith*, T Sirisanthana, J Praparattanapan, K Nuket, Research Institute for Health Sciences, Chiang Mai, Thailand;
S Khusuwan*, P Payoong, P Kantipong, P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
TN Pham*, KV Nguyen, DTH Nguyen, DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
CD Do*, AV Ngo, LT Nguyen, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn*, JL Ross*, B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
MG Law*, A Jiamsakul*, D Rupasinghe, The Kirby Institute, UNSW Sydney, NSW, Australia.
* TAHOD Steering Committee member
Footnotes
Conflict of interest
The authors declare no conflict of interest related to this work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London, England). 2017;390(10113):2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw Open. 2020;3(6):e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–66. [DOI] [PubMed] [Google Scholar]
- 5.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation. 2018;138(11):1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conley LJ, Bush TJ, Rupert AW, Sereti I, Patel P, Brooks JT, et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. AIDS. 2015;29(16). [DOI] [PubMed] [Google Scholar]
- 7.Mave V, Erlandson KM, Gupte N, Balagopal A, Asmuth DM, Campbell TB, et al. Inflammation and Change in Body Weight With Antiretroviral Therapy Initiation in a Multinational Cohort of HIV-Infected Adults. J Infect Dis. 2016;214(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S, Hill A. Risks of metabolic syndrome and diabetes with integrase inhibitor-based therapy: Republication. Current opinion in HIV and AIDS. 2021;16(2):106–14. [DOI] [PubMed] [Google Scholar]
- 9.Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa-a systematic review and meta-analysis. Systematic reviews. 2019;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti G ZP, Shaw J, Grundy SM,. The IDF consensus worldwidedefinition of the metabolic syndrome. Belgium: International Diabetes Federation; 2006. [Google Scholar]
- 11.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. European journal of epidemiology. 2010;25(6):375–84. [DOI] [PubMed] [Google Scholar]
- 13.A Decade of Combination Antiretroviral Treatment in Asia: The TREAT Asia HIV Observational Database Cohort. AIDS research and human retroviruses. 2016;32(8):772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 15.Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. [DOI] [PubMed] [Google Scholar]
- 16.Jiamsakul A, Kerr SJ, Chandrasekaran E, Huelgas A, Taecharoenkul S, Teeraananchai S, et al. The occurrence of Simpson's paradox if site-level effect was ignored in the TREAT Asia HIV Observational Database. Journal of clinical epidemiology. 2016;76:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020;71(6):1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakal DR, Coelho LE, Luz PM, Clark JL, De Boni RB, Cardoso SW, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. The Journal of antimicrobial chemotherapy. 2018;73(8):2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhagwat P, Ofotokun I, McComsey GA, Brown TT, Moser C, Sugar CA, et al. Changes in Waist Circumference in HIV-Infected Individuals Initiating a Raltegravir or Protease Inhibitor Regimen: Effects of Sex and Race. Open Forum Infect Dis. 2018;5(11):ofy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS research and human retroviruses. 2013;29(3):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–72. [DOI] [PubMed] [Google Scholar]
- 23.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes care. 2007;30(1):113–9. [DOI] [PubMed] [Google Scholar]
- 24.Levy ME, Greenberg AE, Magnus M, Younes N, Castel A. Immunosuppression and HIV Viremia Associated with More Atherogenic Lipid Profile in Older People with HIV. AIDS research and human retroviruses. 2019;35(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boufassa F, Goujard C, Viard JP, Carlier R, Lefebvre B, Yeni P, et al. Immune deficiency could be an early risk factor for altered insulin sensitivity in antiretroviral-naive HIV-1-infected patients: the ANRS COPANA cohort. Antivir Ther. 2012;17(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–8. [DOI] [PubMed] [Google Scholar]
- 27.David MH, Hornung R, Fichtenbaum CJ. Ischemic cardiovascular disease in persons with human immunodeficiency virus infection. Clin Infect Dis. 2002;34(1):98–102. [DOI] [PubMed] [Google Scholar]
- 28.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109(3):316–9. [DOI] [PubMed] [Google Scholar]
- 29.Funderburg NT, Mehta NN. Lipid Abnormalities and Inflammation in HIV Inflection. Current HIV/AIDS reports. 2016;13(4):218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isa SE, Oche AO, Kang'ombe AR, Okopi JA, Idoko JA, Cuevas LE, et al. Human Immunodeficiency Virus and Risk of Type 2 Diabetes in a Large Adult Cohort in Jos, Nigeria. Clin Infect Dis. 2016;63(6):830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han WM, Jiamsakul A, Kiertiburanakul S, Ng OT, Sim BL, Sun LP, et al. Diabetes mellitus burden among people living with HIV from the Asia-Pacific region. J Int AIDS Soc. 2019;22(1):e25236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijker R, Jiamsakul A, Uy E, Kumarasamy N, Ditango R, Chaiwarith R, et al. Cardiovascular disease-related mortality and factors associated with cardiovascular events in the TREAT Asia HIV Observational Database (TAHOD). HIV Med. 2019;20(3):183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondy K, Overton ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis. 2007;44(5):726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill A, McCann KM, Pilkington V, Moorhouse MA, Sokhela S, Serenata CM, et al. Risk of Metabolic Syndrome, Diabetes, and Cardiovascular Diseas in ADVANCED Trial. Conference on Retroviruses and Opportunistic Infections (CROI); March 8-11, 2020; Boston, Massachusetts, USA2020. [Google Scholar]
- 35.McCann K, Shah S, Hindley L, Hill A, Qavi A, Simmons B, et al. Implications of weight gain with newer antiretrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS. 2021;Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 36.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes care. 2005;28(2):391–7. [DOI] [PubMed] [Google Scholar]
- 37.Hess PL, Al-Khalidi HR, Friedman DJ, Mulder H, Kucharska-Newton A, Rosamond WR, et al. The Metabolic Syndrome and Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study. Journal of the American Heart Association. 2017;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. Journal of the American College of Cardiology. 2007;49(4):403–14. [DOI] [PubMed] [Google Scholar]
- 39.Jarrett OD, Wanke CA, Ruthazer R, Bica I, Isaac R, Knox TA. Metabolic syndrome predicts all-cause mortality in persons with human immunodeficiency virus. AIDS patient care and STDs. 2013;27(5):266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


