Abstract
Evidence exists that behavioral patterns only stabilize once mice reach adulthood. Detailed information about the course of behavioral patterns is of particular relevance for neuroscientific research and for the assessment of cumulative severity in genetically modified mice. The analysis considered five age groups focusing on behavioral assessments in the animals’ familiar home cage environment during the adolescence phase. We confirmed age- and sex-specific differences for several of the behavioral parameters and fecal corticosterone metabolites. Interestingly, an age-dependent decline in saccharin preference was detected in female mice. Regardless of sex, relevant levels of burrowing activity were only observed during later developmental phases. The development of nest complexity following the offer of new material was affected by age in female mice. In female and male mice, an age-dependency was evident for wheel running reaching a peak at P 50. A progressive increase with age was also observed for Open field activity. The data sets provide guidance for behavioral studies and for development of composite measure schemes for evidence-based severity assessment in young mice. Except for the burrowing test, the different behavioral tests can be applied in different age groups during post-weaning development. However, age- and sex-specific characteristics need to be considered.
Subject terms: Developmental biology, Neuroscience
Introduction
As a result of neuronal remodeling during the developmental trajectory of an individual, behavioral patterns as well as the response to potentially threatening situations and coping with distress can change tremendously during early development in mice1–3. Compelling evidence exists that behavioral patterns stabilize once the animals reach adulthood1. Various genetic mouse lines modeling neurodevelopmental and neuropsychiatric disorders display behavioral alterations during distinct developmental phases. These animal models can be characterized by peaks of distress during the ‘adolescence’ phase4. In neuroscientific preclinical research, genetically engineered mice provide powerful tools to investigate the linkages between genetic deficiencies and phenotypical alterations. In order to ensure a successful translation from preclinical model to clinical application, age- and sex-specific characteristics underlying the phenotypical profile of the genetic model have to be carefully considered. Thereby, robust and reproducible reference data5, rendering a ‘baseline behavioral profile’ in adolescent mice, can serve as a valuable basis to improve face validity in genetic animal models of neuropsychiatric disease.
Given the frequent use of the mouse as a model in animal-based research, it is of general interest and practical relevance to evaluate sensitive and conclusive parameters suitable for the assessment of severity in young mice. Parameters applied to assess the wellbeing of adult mice cannot merely be transferred as testing paradigms to mice undergoing different developmental stages. With a view to the current legal situation, the burden of genetically modified animals needs to be classified in three severity levels, in accordance with the EU Directive 2010/63/EU on the protection of animals used for scientific purpose6. As suggested by the German Centre for the Protection of Laboratory Animals (Bf3R), the classification is often based on two evaluations of the litter (newborn/weaning) including a clinical adspection of the offspring animals with assessment of the nutritional condition, body posture and body weight7. The next comprehensive clinical adspection and investigation of the individual animal is recommended when the animals reach an age of 2 months7. Thus, the phenotype of ‘adolescent’ animals is often not considered for the final assessment of genetically modified mouse lines. With regard to the cumulative burden experienced in the course of a lifetime, lacking information about the animal’s whole developmental period remains a particular cause of concern. Furthermore, the criteria currently applied for the assessment, based on clinical symptoms and alterations in activity7, might not comprehensively reflect the mouse’ wellbeing as a prey animal. In context of the 3R principle (Reduction, Replacement, Refinement), we aimed to develop sensitive approaches which can more precisely differentiate and grade different levels of distress, including more subtle alterations that remain undetectable with common clinical scoring systems. We assessed behavioral patterns that reflect the affective state of the animals, a range of non-essential activities as well as locomotor activity of the animals, to develop an adjusted composite measure scheme suitable for the assessment of severity in young mice. Aiming to reduce interference on the one hand, and to enable universal, standardized implementation of the respective parameters on the other hand, we focused on approaches applicable in the animal’s home cage environment. The applied candidate composite measure scheme was based on a comprehensive consortium data set for assessing and classifying severity in different adult rodent animal models8. Parameters with a high informative value were selected based on a bioinformatic approach among others comprising principal component analyses identifying the parameters best distinguishing between experimental and control animals and cross-correlation analyses guiding the selection of parameters with added value9,10.
We generated reference data in female and male C57BL/6JRj wildtype mice, one of the most commonly used background strains for genetically modified mice and the most widely used inbred mouse strain11, in order to assess the generalizability and robustness of selected parameters as a basis for candidate composite measure schemes in young mice. The comprehensive reference data are suitable for further subsequent testing in genetically modified mouse lines, and phenotypical characterization of the latter. Once validated in genetically altered strains, age-specific composite measure schemes provide a basis for evidence-based refinement recommendations in the sense of a reliable and comprehensive severity classification of genetic mouse models.
Results
Behavioral home cage assessment
Saccharin preference
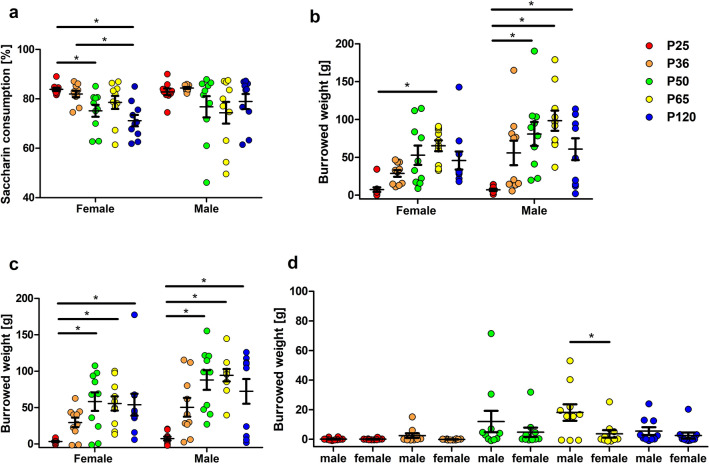
All animal groups displayed a preference for sweetened saccharin solution (Fig. 1a). The analysis of saccharin preference at different time points during the post-weaning phase revealed an age-dependency with young female animals exhibiting a stronger preference for the sweet solution (Fig. 1a). Both, prepubescent (P25) and pubescent (P36) female mice consumed a higher amount of saccharin solution in comparison to mature adult (P120) female mice (Fig. 1a, F(4,45) = 6.495, p = 0.0003). The statistical differences between prepubescent and sexually mature (P50) mice indicate a decline in saccharin preference, when female animals reach sexual maturity (Fig. 1a, F(4,45) = 6.495, p = 0.0003). In contrast to female mice, the respective analysis of saccharin preference in male mice did not identify significant age-dependent differences (Fig. 1a, F(4,44) = 1.694, p = 0.1686). However, in both, female and male mice the variance increased from P50 onwards (Fig. 1a), indicating more pronounced interindividual differences as compared to younger animals.
Figure 1.
Saccharin preference and burrowing performance. (a) Age-dependent differences in saccharin preference, shown as total saccharin intake, were only detected in female mice: preference for saccharin in prepubescent and pubescent mice exceeded that of the adult control group, and prepubescent mice showed a higher preference than the respective sexually mature mice (F(4,45) = 6.495, p = 0.0003). Regardless of sex, burrowing performance overnight (first test) increased significantly in mice reaching sexual maturity (b, first test; interaction p = 0.5912, age phase p < 0.0001, sex p = 0.0051) (c, second test; interaction p = 0.6044, age phase p < 0.0001, sex p < 0.0020). A difference between sexes was only detected in the second burrowing test during the light phase in young adult animals (d) (interaction p = 0.2394, age phase p = 0.0068, sex p = 0.0119). One-way ANOVA, followed by Bonferroni multiple comparison tests for (a), and two-way ANOVA, followed by Bonferroni multiple comparison tests for (b–d). *p < 0.05. Colored dots refer to the respective age groups. Error bars indicate the standard error of the mean (SEM).
Burrowing
The overnight burrowing performance developed with increasing age in female and male mice (Fig. 1b,c). Concerning the overnight (dark phase) burrowing activity, at P25 no relevant burrowing activity was detected in animals of both sexes during the two tests (Fig. 1b, interaction p = 0.5912, age phase p < 0.0001, sex p = 0.0051; Fig. 1c, interaction p = 0.6044, age phase p < 0.0001, sex p = 0.0020). At P36 interindividual differences were increasing with some male animals already exhibiting a higher level of burrowing (Fig. 1b,c). However, a significant increase as compared to P25 was only confirmed from P50 onwards. This increase became evident in both female and male animals. Comparison of the findings from both test days did not indicate pronounced differences (Fig. 1b,c).
While no sex differences were detected for overnight (dark phase) burrowing activity (Fig. 1b,c), young adult (P65) male mice displayed a higher burrowing performance than the respective female mice during the 120 min test session (light phase) (Fig. 1d, interaction p = 0.2394, age phase p = 0.0068, sex p = 0.0119). However, this difference became only evident during the second of the two burrowing test days. The analysis of burrowing activity during the 120 min test sessions did not reveal age-dependent differences with a relatively low mean performance in almost all age groups (Fig. 1d, Supplementary Fig. S1a).
Nest building
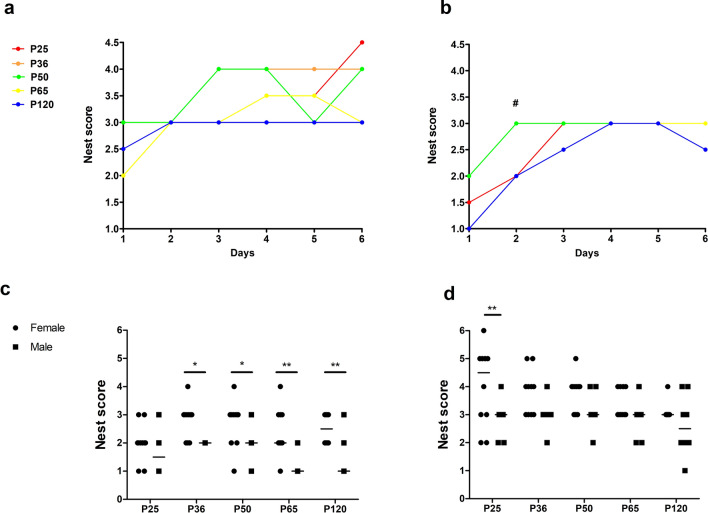
Concerning the development of the nest complexity scores over the total observation period of 6 days, age-dependent differences in the slope of the nest complexity curve were observed in female (Fig. 2a, F(4, 290) = 2.75991, p = 0.02804) but not in male mice (Fig. 2b, F(4, 290) = 1.07471, p = 0.3692). When directly comparing scores from different days of the observation phase among the age groups, we only identified a significant difference at day 2 with nest building performance of male mice aged 50 days exceeding that of adult mice (Fig. 2b, F(4, 45) = 2.885, p = 0.0329).
Figure 2.
Nest building performance and nest complexity. The development of nest complexity scores differed between age phases in (a) female animals (F(4, 290) = 2.75991, p = 0.02804), but not in (b) male animals (F(4, 290) = 1.07471, p = 0.3692). In male animals, the nest scores in P50 animals exceeded that of P120 animals on the second day (b, #) (F(4, 45) = 2.885, p = 0.0329). Sex differences were evident (c) on the first day in all age groups except for P25 animals (interaction p = 0.3161, age phase p = 0.0205, sex p < 0.0001). In contrast, sex differences in P25 mice became evident (d) only on the last day (interaction p = 0.6905, age phase p = 0.0504, sex p < 0.0001). Linear regression for (a) and (b); one-way ANOVA, followed by Bonferroni multiple comparison tests for #; two-way ANOVA followed by Bonferroni multiple comparison tests for (c) and (d), *p < 0.05, median. Colored dots/lines refer to the respective age groups.
From P36 onwards, sex differences were always evident at the first day of the observation period (Fig. 2c, interaction p = 0.3161, age phase p = 0.0205, sex p < 0.0001). These data indicate a longer latency to construct complex nests in male mice. Differences between female and male mice were also confirmed by mean sum scores from day 1 to 6, and on other selected observation days at different age levels (Fig. 2d, interaction p = 0.6905, age phase p = 0.0504, sex p < 0.0001, Supplementary Fig. S2a–d). Thereby, female mice exhibited a better nest building performance than male mice.
Voluntary wheel running
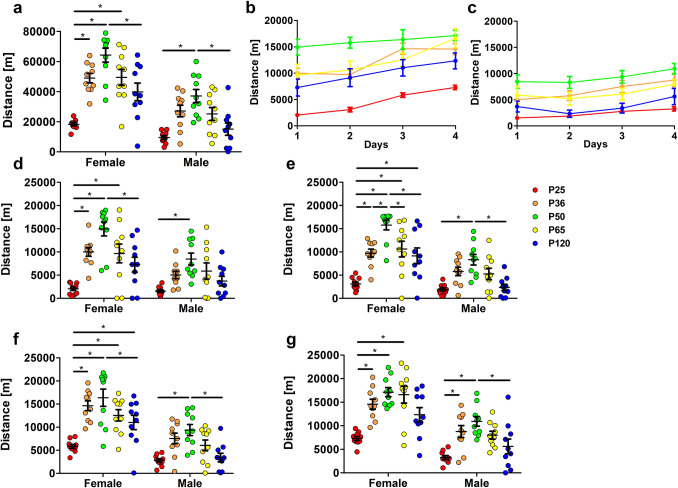
Considering activity over the course of the four testing days, animals of all age phases and of both sexes performed wheel running. In female and male mice, the total distance moved in the wheel during the 4 days of testing significantly increased during the weeks after weaning, reaching a peak at P50 (Fig. 3a, interaction p = 0.1802, age phase p < 0.0001, sex p < 0.0001).
Figure 3.
Voluntary wheel running. The total distance moved during the entire observation period (a) increased until animals reached sexual maturity (interaction p = 0.1802, age phase p < 0.0001, sex p < 0.0001). Analysis of the increase in activity during the testing phase did not reveal age-related differences in female (b) (F(4,190) = 1.02147, p = 0.3974) and male animals (c) (F(4,190) = 0.367026, p = 0.8319). Assessment of running activity at separate days (day 1–4) (d–g) demonstrated that running activity in P50 animals exceeded that of the sex-matched P25 group on all test days. Two-way ANOVA followed by Bonferroni multiple comparison tests for (a,d–g); linear regression for (b) and (c); *p < 0.05. Colored dots/lines refer to the respective age groups. Error bars indicate the standard error of the mean (SEM).
A decline became significantly evident in female and male mice with further development when comparing P50 and P120 data (Fig. 3a).
Sex differences were detected in all age groups except for the group of prepubescent mice (Fig. 3a). The distance moved in female mice exceeded that in male mice from pubescent age onwards (Fig. 3a). Considering the increase in activity over the course of the four testing days, we did not detect any differences in all groups of both sexes (Fig. 3b,c, females F(4,190) = 1.02147, p = 0.3974, males F(4,190) = 0.367026, p = 0.8319). Hence, the distance moved per day did not increase significantly during the test phase in all groups. A breakdown of running activity per day reveals that the activity level in P50 animals of both sexes was increased on each day of testing as compared to the respective P25 group (Fig. 3d,e–g).
Open field test
The first minutes in a novel environment can provide information about exploratory activity.
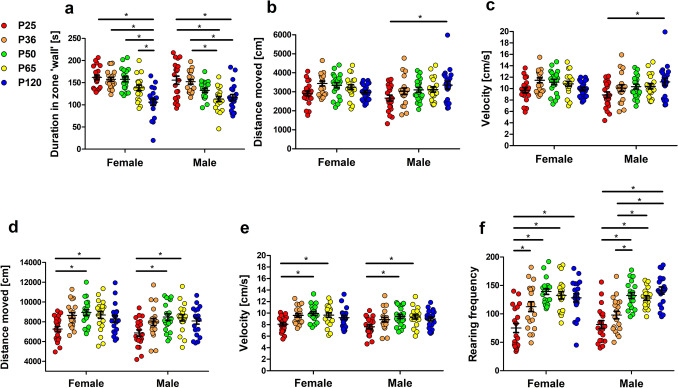
During the first 5 min in the Open field, prepubescent and pubescent animals of both sexes spent more time in the ‘wall’ zone than adult mice (Fig. 4a, interaction p = 0.0239, age phase p < 0.0001, sex p < 0.0095). Thereby, sex differences became evident in the groups of P50 and P65 mice with females spending longer times in the ‘wall’ zone than age-matched male mice (Fig. 4a). In this early phase, male prepubescent animals moved a shorter distance (Fig. 4b, interaction p = 0.0733, age phase p = 0.0122, sex p = 0.1613) and moved with lower speed (Fig. 4c, interaction p = 0.0734, age phase p = 0.0125, sex p = 0.1618) in comparison to male adult animals.
Figure 4.
Open field test. In the first 5 min of the Open field test, illustrated in (a–c), prepubescent and pubescent animals of both sexes spent more time in the ‘wall’ zone than adult mice (a) (interaction p = 0.0239, age phase p < 0.0001, sex p < 0.0095). Male prepubescent animals moved a shorter distance (b) (interaction p = 0.0733, age phase p = 0.0122, sex p = 0.1613) and moved more slowly (c) (interaction p = 0.0734, age phase p = 0.0125, sex p = 0.1618) than the sex-matched adult group. Analysis of activity during the total test duration of 15 min, illustrated in (d–f), revealed that the distance moved (d) and velocity (e) in P50 and P65 mice exceeded that of the respective prepubescent animals (d, interaction p = 0.9626, age phase p < 0.0001, sex p = 0.0441, e, interaction p = 0.9628, age phase p < 0.0001, sex p = 0.0440). Independent of the sex of the animals, rearing frequency (f) proved to be lower in prepubescent animals as compared to sexually mature, young adult, and adult animals (interaction p = 0.2458, age phase p < 0.0001, sex p = 0.7122). Two-way ANOVA, followed by Bonferroni multiple comparison tests, *p < 0.05. Colored dots refer to the respective age groups. Error bars indicate the standard error of the mean (SEM).
Data from the total monitoring phase of 15 min can inform about the overall activity status and locomotion. Distance moved and velocity in P50 and P65 mice exceeded that in respective prepubescent animals (Fig. 4d, interaction p = 0.9626, age phase p < 0.0001, sex p = 0.0441, Fig. 4e, interaction p = 0.9628, age phase p < 0.0001, sex p = 0.0440). Independent of the sex of the animals, rearing frequency proved to be lower in prepubescent animals as compared to sexually mature, young adult and adult animals (Fig. 4f, interaction p = 0.2458, age phase p < 0.0001, sex p = 0.7122). In female animals, the age-dependent increase was already evident at an earlier time point during development with a significant difference between prepubescent and pubescent animals (Fig. 4f). However, comparison of rearing data within the different age groups did not indicate relevant sex differences.
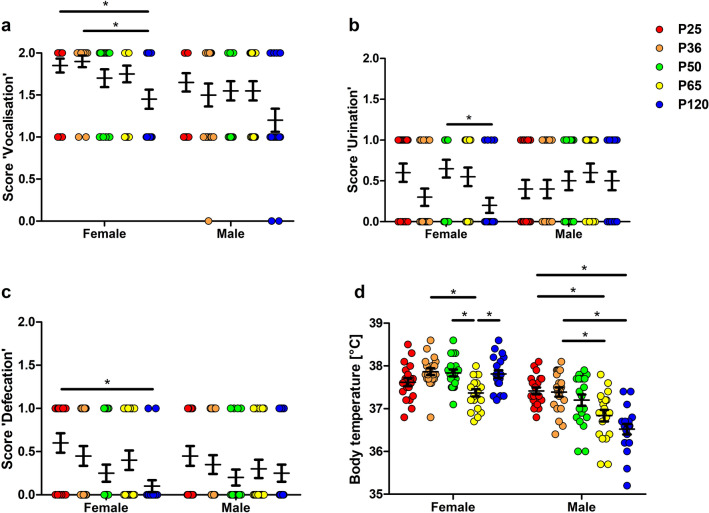
Irwin score
The analysis of the Irwin observation test, a widely applied neurobehavioral assay, revealed several age-specific characteristics of handling-associated parameters. While Irwin sum scores did not indicate sex- or age-dependent differences (Supplementary Fig. S3a), we detected an age-dependency of selected Irwin parameters in female mice (Fig. 5a–d). The scores for handling-associated vocalization were higher in prepubescent and pubescent female animals compared to female adult animals (Fig. 5a, F(4,95) = 3.387, p = 0.0123). Moreover, the scores for handling-associated urination in female sexually mature animals exceeded those in female adult animals (Fig. 5b, F(4,95) = 3.437, p = 0.0114). Handling-associated defecation was more pronounced in prepubescent female mice than in female adult mice (Fig. 5c, F(4,95) = 3.474, p = 0.0108). The comparison of Irwin data between female and male animals within the different age groups did not confirm relevant sex differences. The only exception were rectal body temperatures in female mice, which exceeded those in male mice in all age groups from P36 onwards (Fig. 5d, interaction p < 0.0001, age phase p < 0.0001, sex p < 0.0001). An age-dependency of body temperature was confirmed in both sexes with a higher temperature in female and male pubescent mice than in young adult mice (Fig. 5d). Moreover, the rectal temperature in prepubescent male mice exceeded that in young adult and adult animals (Fig. 5d).
Figure 5.
Irwin score. As compared to female adult animals, handling-associated vocalization (a) and defecation (c) scores were increased in prepubescent animals. Handling-associated urination (b) in sexually mature female animals exceeded that of female adult animals (a, F(4,95) = 3.387, p = 0.0123; b, F(4,95) = 3.437, p = 0.0114; c, F(4,95) = 3.474, p = 0.0108). Rectal body temperatures (d) in female mice exceeded those in male mice in all age groups except for P25, and body temperatures in pubescent animals of both sexes were higher as compared to those in young adult animals (interaction p < 0.0001, age phase p < 0.0001, sex p < 0.0001). One-way ANOVA, followed by Bonferroni multiple comparison tests for (a–c), and two-way ANOVA, followed by Bonferroni multiple comparison tests for (d); *p < 0.05. Colored dots refer to the respective age groups. Error bars indicate the standard error of the mean (SEM).
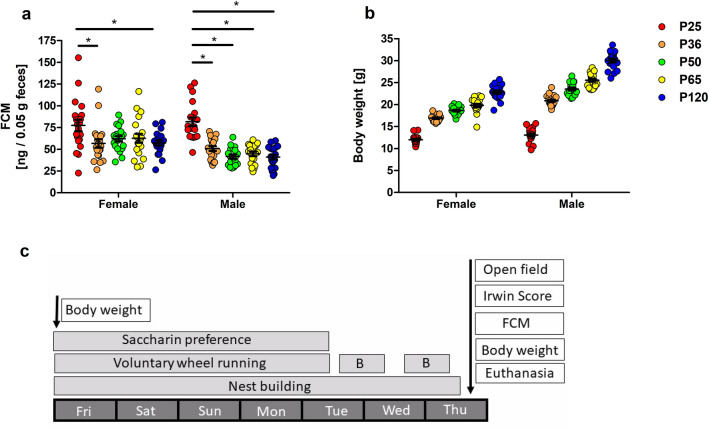
Fecal corticosterone metabolites
In prepubescent male animals, the concentration of fecal corticosterone metabolites was significantly higher as compared to all other age groups (Fig. 6a, interaction p = 0.0130, age phase p < 0.0001, sex p < 0.0001). In female animals, the concentrations during prepubescence exceeded those from pubescent and adult animals (Fig. 6a, interaction p = 0.0130, age phase p < 0.0001, sex p < 0.0001). We did not detect sex differences within the age groups.
Figure 6.
Fecal corticosterone metabolite concentrations (FCM) (a) were higher in prepubescence than in pubescence and adulthood in both female and male animals (interaction p = 0.0130, age phase p < 0.0001, sex p < 0.0001). Moreover, concentrations in prepubescent male animals were significantly higher than in all other age groups. Two-way ANOVA, followed by Bonferroni multiple comparison tests, *p < 0.05. Error bars indicate the standard error of the mean (SEM). The age-dependent body weight increase in the different age groups is illustrated in (b). Colored dots refer to the respective age groups. (c) Illustrates an overview of the weekly experimental scheme. B burrowing.
Body weight
When comparing data from the beginning and end of the different testing phases in all age groups, a pronounced body weight gain was only evident for the groups of prepubescent female and male mice (Supplementary Fig. S3b). As expected an age-dependent body weight increase was confirmed when comparing the different age groups (Fig. 6b).
Discussion
In line with our aim, this study identified significant age-dependent differences in the development of behavioral patterns in the early developmental phase following weaning. Moreover, sex was confirmed as an influencing factor, with differences evident for the development of selected parameters. The data sets provide comprehensive information for behavioral studies in neuroscience and for the development of age-specific composite measure schemes for evidence-based severity assessment approaches.
The parameters of interest have been selected based on consortium data, which were obtained in adult rodent disease models, and which supported an informative value for the purpose of severity assessment (e.g.8,9,12–20). Information about model-specific characteristics provide key elements for an evidence-based grading of severity, and allow filling of gaps in model-specific refinement recommendations8. Based on the promising candidate parameters, fully validated in adult rodent models (e.g.9,12–15,17), we subsequently tested for applicability in young mice. Moreover, the compilation of tests considered aspects of animal welfare such as the ethical necessity of group housing, and also allows practical implication in the majority of conventional animal facilities. Behavioral tests conducted in the home cage under standardized housing conditions allow phenotypical profiling throughout the circadian rhythm, thereby minimizing external influences and factors of distress experienced by the prey animals21,22. Sensitive home cage measures allow depiction of even subtle and spontaneous changes of behavioral patterns, and standardized housing strengthens reproducibility and inter-facility comparability of preclinical data5.
To our knowledge, a study in the rat kindling paradigm was the first exploring saccharin preference as a potential parameter for severity assessment15. In subsequent studies, we have detected an influence of different chronic models of epilepsy13,14,23. Assessment of the preference for a sweet solution has originally been integrated as a behavioral test in research focusing on psychiatric disorder and depression-associated alterations in laboratory rodents (e.g.24–26). Thereby, reduction or loss of the preference for a sweet solution is interpreted as sign of anhedonia-associated behavior, i.e. the reduction or loss of the ability to experience pleasure. A recent study evaluating the depression-like phenotype of different mouse strains in animals aged 22–26 days and 32–36 days reported significant differences between the strains in the preference for sucrose during adolescence27. An age-dependency of saccharin preference has been previously described in groups of female and male rabbits. Surprisingly, younger animals aged between 4 and 6 months preferred water, whereas older rabbits aged between 31 and 69 months preferred the sweet solution28. In contrast, we demonstrated an age-dependency of saccharin preference in female mice with higher levels of saccharin consumption in prepubescent and pubescent mice, and a significant decline already evident at P50, when animals reach sexual maturity. Interestingly, there was a lack of differences between age groups in male mice. The fact that we confirmed a relevant saccharin preference in all age groups in both sexes suggests that saccharin preference can be applied in young animals even in the early phase following weaning. Interestingly, we confirmed lower interindividual variance of the preference for sweetness in young female and male wildtypes. Considering the typically increased behavioral variability displayed by young animals during their post-weaning development, these findings might reinforce the particular suitability of the saccharin preference test, even suggesting that lower animal numbers might be necessary to reach a sufficient test-specific power. Concerning the value as a severity assessment parameter in young mice, future studies need to address the impact of intervention- or model-associated distress on the consumption ratios.
Burrowing and nest building activity have been suggested as valuable indicators of distress, pain, suffering and a compromised well-being in mice29. Both behavioral patterns are considered non-essential but evolutionary preserved29. Burrowing activity proved to serve as a sensitive indicator of well-being compromised by painful procedures, sickness behavior associated with immune system activation and models of psychiatric or neurological disorders29–32. Overnight burrowing performance developed with increasing age in young mice reaching a relevant activity at P50 in both sexes. The 120 min assessment towards the end of the light phase did not confirm a relevant burrowing performance during this time phase. This might be related to the fact that the exposure was limited to two test days without a longer habituation phase in advance. In contrast, data from the overnight phase suggest that burrowing activity can be applied as a severity assessment parameter in female and male mice aged 50 days and beyond, i.e. when animals reach sexual maturity. However, one needs to pay attention to the high level of variance, which needs to be considered when determining the necessary animal number. Moreover, follow-up studies are necessary—as for all other parameters of interest—to determine the sensitivity to experimental distress. In this context, two studies are of interest, which reported a higher sensitivity of burrowing performance in 19-months-old or 21-months-old mice to the influence of exposure to a viral mimetic or to lipopolysaccharide, when compared to the response of 4-months-old mice33,34. However, these results might rather be related to a more pronounced sickness response in aged animals than to a difference in the sensitivity of burrowing activity towards stressors.
To our knowledge the only other study that evaluated burrowing in young mice focused on mice aged 31 days. Regardless of the strain the authors reported that mice at this age exhibit burrowing activity35. In line with our data, the authors did not report sex differences.
Concerning our findings in prepubescent and pubescent mice, it might be of future interest to assess the influence of the burrowing material, as the body size in relation to the particle size of the burrowing material might matter in very young animals. In this context, it is of interest that the choice of the burrowing material affected the sensitivity of burrowing as a parameter for pain assessment in a multicenter study in rats36.
The pronounced sensitivity of nest building to detrimental influences has for instance been demonstrated as a consequence of abdominal surgery, transmitter implants, systemic inflammation, hippocampal scrapie infection, models of psychiatric disorders, and Alzheimer models30,37–43. Based on studies exploring the influence of pain on nest building performance, Turner and colleagues44 suggested analysis of nest building behavior as an important parameter for pain assessment. Earlier reports from adult mice reported that nest building performance in female mice exceeds that in male mice45. In line with these findings from adult animals, the data sets from young female mice also confirm a higher rate of nest building with a reduced latency to construct complex nests.
An impact of age on nest building performance has previously been described in a study focused on the impact of ageing reporting a decline in nest building performance in 25-month-old mice as compared to 7-month-old mice45. This study raises the question, if there is also an impact of age in phases of early post-weaning development. While only minor age-dependent differences were detected in male mice in our study, the analysis of the slope of nest complexity curves from female mice revealed age-dependent differences in the latency to construct complex nests. Thereby, the steeper curve in prepubescent female mice suggests a longer latency in younger animals. The findings confirm that a relevant nest building activity can already be observed in young female and male mice. This is in line with an earlier report by Moy and colleagues46 describing relevant nest building in different mouse strains at an age of 3–4 weeks.
The development of nest complexity during the 6-day observation phase in our study suggests that an influence of experiment-associated distress should rather be evaluated towards end of the week before replacing the nest material by a new batch. It is likely that the application of nest complexity as a severity assessment parameter needs to consider various influencing factors including the mouse strain, ambient temperature, and nest material as these have been reported to exert tremendous effects on the performance in adult47–49 and young mice35,46. Respective factors might have also contributed to the very low interest to construct complex nests observed in P30 and P40 mice in an earlier study35.
Häger and colleagues50 have analyzed the informative value of voluntary wheel running as a basis for observer-independent fine-scale grading of severity levels. In their initial study, the group confirmed a negative influence of subchronic restraint stress and a colitis model50. In subsequent studies, the sensitivity of voluntary wheel running to recurrent stress exposure and a dextran sodium sulphate-induced colitis model was further confirmed17,18. Based on these findings, it is of particular interest to assess the development of voluntary wheel running performance in young mice as this might provide a basis for the application of this sensitive severity assessment parameter in earlier developmental phases. Comparison between age groups of young mice revealed a progressive increase during the early post-weaning phase reaching a peak at P50, i.e. a time point at which animals are considered sexually mature. Interestingly from P36 onwards a pronounced sex difference was evident with voluntary wheel running activity in female mice exceeding that in male mice. Nevertheless, in both sexes a relevant activity was observed at different age levels. The development on subsequent days suggests that habituation is of relevance in very young animals and that assessment should focus on activity following at least three habituation days. However, at the earliest age assessed in the present study, we cannot exclude that the development of the performance at subsequent testing days was also influenced by the growth curves and development of muscle strength of the young animals. At later age levels, we did not detect a relevant habituation impact, so that assessment can be based on data from the first days of wheel exposure.
The age-dependent development of activity patterns has already been assessed in mice in various previous studies1,35,51–54. These studies demonstrated a progressive increase of activity levels during the developmental phase that corresponds to adolescence in humans. In this context, some groups have also applied the Open field paradigm, which can provide information about exploratory and locomotor behavior and other activity patterns such as rearing24. In line with available data, we confirmed a progressive increase of velocity and distance moved as well as rearing frequency reaching a plateau from P50 onwards without any further age-dependent differences from this time point on. The analysis of exploratory behavior during the first 5 min of the paradigm revealed a lower locomotion level in prepubescent female and male mice in comparison with selected other age groups. More importantly, these very young animals exhibited a higher level of thigmotaxis spending more time in the wall zone of the Open field. Thigmotaxis proved to be more pronounced in female mice than male mice. While we did not design the Open field as a test for anxiety-associated behavior in this study and applied rather dim light conditions allowing to focus on locomotion and activity patterns, these data suggest a higher level of anxiety-associated behavior in this early post-weaning phase. In previous studies focusing on the development of anxiety-associated behavior in young mice, controversial data have been reported1,35,51–53. This might be related to the influence of various factors including environmental conditions, maternal care, handling procedures, habituation to the laboratory and to the person handling the animal during the experiment.
Our findings from the Open field suggest that the paradigm might be applied at different age levels in young mice. However, the data emphasize the need to consider the characteristic behavioral patterns at different age levels. Moreover, it seems to be of particular relevance to pay attention to the course of behavioral patterns during the test time with a separate analysis of different time bins.
Irwin scores have been developed for the assessment of the adverse effect potential of central nervous system (CNS) drug candidates55. The parameters have been selected to provide information about the autonomic, peripheral and central nervous system and behavioral patterns. We have recently developed an adjusted Irwin score excluding all parameters that intensely interfere with the animal in order to avoid any stress bias related to the assessment. This adjusted Irwin score was successfully applied in different models. It proved to provide valuable information about the well-being and general condition in the early post-surgical phase and following the induction of a status epilepticus13,14,56. While the Irwin sum scores were in a comparable range, the analysis of selected parameters revealed an age-dependency. These parameters comprised vocalization and handling-associated defecation reaching higher scores in younger female mice as well as handling-associated urination with a peak in female mice at an age of 50 days. As expected higher rectal body temperatures were confirmed in younger mice of both sexes. In this context, we cannot exclude a bias by an age-dependent difference in the response to the handling procedure necessary to determine rectal body temperature. Taken together, application of an Irwin scoring system for the purpose of severity assessment in young mice needs to consider the age-specific characteristics in these selected parameters.
The measurement of fecal corticosterone metabolites serves as a non-invasive parameter for assessing adrenocortical activity (4–12 h prior to feces sampling in mice) and has been applied in a range of species including laboratory rodents57,58. To our knowledge, age-related differences have been rarely studied and reported in mice. One longitudinal study in females of two mouse strains reported age- and strain-related differences59. The data obtained in our experiments revealed an age-dependency of the fecal corticosterone metabolite levels with prepubescent animals of both sexes showing higher baseline levels as compared to the mature adult control group. However, external influences such as the more recently experienced procedure of weaning, separation from the litter, and new housing conditions, have to be considered as factors in the youngest age group, which may contribute to increased levels of distress60. Although sex differences in excreted corticosterone metabolites (lower levels in males) have been reported58,60,61, they were not significant in our study.
The analysis of baseline performance in the different age phases benefits from behavioral assessments in the animals’ home cages since respective alterations in the phenotypical profile during post-weaning development draw a subtler and more precise picture of observer-independent and spontaneous characteristics. As already emphasized follow-up studies are necessary to assess the sensitivity of the parameters to distress in the different age levels. Moreover, the impact of various factors needs to be explored in mice of different age. Among others, these include handling procedures, habituation procedures, environmental factors, and time point of weaning. In this context, the sequence and parallel analysis of different parameters also needs to be taken into account, when drawing conclusions and when designing future studies further exploring the generalizability and robustness of the findings from this study.
In conclusion, the data sets provide valuable guidance for behavioral studies in young mice and for the development of composite measure schemes for evidence-based severity assessment in young mice. The majority of the behavioral tests can be applied in different age groups during early post-weaning development. However, age- and sex-specific characteristics need to be considered. As an exception the burrowing paradigm should only be used in mice with an age of at least 50 days.
Materials and methods
Ethical statement
All animal experiments were conducted and reported in accordance with the EU Directive 2010/63/EU and the German Animal Welfare Act. All investigations were carried out in line with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the Basel declaration (http://www.basel.declaration.org) including the 3R principle. All animal experiments were approved by the government of Upper Bavaria (Munich, Germany, license number ROB-55.2-2532.Vet_02-19-157). As requested by the government of Upper Bavaria, the required sample size had been calculated by a power analysis before the start of the study.
Animals
Pregnant C57BL/6JRj mice (n = 51), obtained at embryonic stages (E) 10, E11 and E13 (Janvier Labs, Le Genest-Saint-Isle, France), were single-housed under controlled environmental conditions (22–24 °C, 45–60% humidity) in individually ventilated cages (Tecniplast Deutschland GmbH, Hohenpeißenberg, Germany) in a 12-h dark–light cycle with ad libitum access to food (Ssniff Spezialdiäten GmbH, Soest, Germany) and tap water. Each cage was supplemented with bedding material (Lignocel Select, J. Rettenmaier & Söhne GmbH & Co. KG, Rosenberg, Germany), 14 g of nesting material (Enviro Dri, Claus GmbH, Limburgerhof, Germany), a wood brick (Labodia AG, Niederglatt, Switzerland), and a triangular mouse house (Zoonlab GmbH, Castrop-Rauxel, Germany). Mother animals with their litters obtained a fresh cage at postnatal day (P) 6 and 14. From weaning at P21 until the start of the experiments, experimental animals (n = 200, 50% female/male), C57BL/6JRj offspring mice, were randomly divided (http://www.randomizer.org) in experimental units of same-sex siblings (n = 2) and each experimental unit was housed in a Makrolon cage type III (Ehret GmbH & Co. KG, Emmendingen, Germany), providing bedding material (Lignocel Select, J. Rettenmaier & Söhne GmbH & Co. KG, Rosenberg, Germany), two nestlets (Ancare, Bellmore, New York, USA), and one square animal house (Zoonlab GmbH, Castrop-Rauxel, Germany). The order of cages was randomized (http://www.randomizer.org). Animals received a fresh cage once per week on Tuesdays. During the observation period, animals were housed in groups of two as the respective experimental unit in a home cage system with continuous video recordings (PhenoTyper, Noldus, Wageningen, the Netherlands). Each PhenoTyper was supplemented with 200 g bedding material (Lignocel Select, J. Rettenmaier & Söhne GmbH & Co. KG, Rosenberg, Germany), two nestlets (Ancare, Bellmore, New York, USA), an infrared translucent shelter (Noldus, Wageningen, the Netherlands) and two drinking bottles (Noldus, Wageningen, the Netherlands). The home cage system provided a removable wall for the insertion of a running wheel (PhenoWheel, Noldus, Wageningen, the Netherlands).
Experimental design
In the present study, offspring animals were randomly (http://www.randomizer.org) distributed to the following four postnatal time phases (Table 1), representing the different developmental stages in murine lifetime1: The ‘adolescence’ phase: (1) prepubescent, experiments starting at P25 (no age range), (2) pubescent, experiments starting at P36 (age range: P35–36), and (3) sexually mature mice, experiments starting at P50 (no age range); followed by (4) young adult mice, experiments starting at P65 (no age range). A fifth group of mature adult mice from P120 onwards (experiments starting at P120, age range: P117–120), representing a frequently used age in experimental mice, was additionally tested. Experimental animals (n = 200) of all five age phases (n = 40 per age phase) and of either sex (n = 20 per age phase and sex) were investigated. For the tests conducted in the PhenoTyper home cage, the analysis was performed per experimental unit (n = 2). All behavioral tests carried out in the PhenoTyper home cage were video recorded. As a measure against risk of bias and with regard to the weekly workflow in the animal facility and its possible impact on animal’s behavior on certain days, experimental testing for all groups was based on a weekly routine (Fig. 6c). Experiments were executed in four batches per age phase, resulting in a total duration of 20 weeks.
Table 1.
Group allocation and age of experimental animals undergoing the behavioral test battery. SP saccharin preference, NEST nest building performance, VWR voluntary wheel running, BUR burrowing, OF open field, IRW Irwin score, FCM fecal corticosterone metabolites, BW body weight.
| Group | Represented age phase | n female/male |
Behavioral tests (observation period) | Age range in days |
|---|---|---|---|---|
| P25 | Prepubescence | 20/20 | SP, NEST, VWR (P25-P29) | ± 0 |
| BUR (P29 and P30) | ||||
| OF, IRW, FCM (P31) | ||||
| BW (P25 and P31) | ||||
| P36 | Pubescence | 20/20 | SP, NEST, VWR (P36-P40) | − 1 |
| BUR (P40 and P41) | ||||
| OF, IRW, FCM (P42) | ||||
| BW (P36 and P42) | ||||
| P50 | Sexual maturity | 20/20 | SP, NEST, VWR (P50-P54) | ± 0 |
| BUR (P54 and P55) | ||||
| OF, IRW, FCM (P56) | ||||
| BW (P50 and P56) | ||||
| P65 | Young adulthood | 20/20 | SP, NEST, VWR (P65-P69) | ± 0 |
| BUR (P69 and P70) | ||||
| OF, IRW, FCM (P71) | ||||
| BW (P65 and P71) | ||||
| P120 | Mature adulthood | 20/20 | SP, NEST, VWR (P120-P124) | − 3 |
| BUR (P124 and P125) | ||||
| OF, IRW, FCM (P126) | ||||
| BW (P120 and P126) |
Behavioral home cage assessment
Saccharin preference
In the PhenoTyper home cage, we assessed anhedonia-associated behavior successively over 4 days with the saccharin preference test. The PhenoTyper home cage provided a wall with two water bottles, which were filled with 200 g tap water on the first and third day. On day 2 and 4, animals had access to a water bottle containing 200 g of a 0.1% saccharin solution (Aldrich Saccharin ≥ 98%, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and a water bottle filled with 200 g tap water. To exclude a possible side preference of the animals, the side of the bottle containing the saccharin solution on the cage wall was switched from the right on day 2 to the left on day 4. The respective consumption over 24 h was measured and analysis was carried out as defined in a protocol by Klein et al.62. Data from one male prepubescent experimental unit (n = 2) was excluded in the analysis as one of the water bottles leaked on one of the testing days.
Burrowing
Burrowing performance was evaluated on two consecutive days, and analysis was carried out for the respective light and dark phases. In the afternoon, 2 h prior to the dark phase, an empty water bottle (length: 20 cm, diameter of the bottleneck: 3.5 cm; Zoonlab GmbH, Castrop-Rauxel, Germany) filled with 200 g ± 1 g food pellets (Ssniff Spezialdiäten GmbH, Soest, Germany), was placed in the PhenoTyper home cage. After 120 min, the weight of the bottle with the remaining pellets was measured. After removal of the distributed pellets in the animals’ home cage, the bottle was replaced overnight. On the next day, immediately after the dark phase, the weight of the bottle with the remaining pellets was measured again.
Nest building
We investigated nest building performance in the PhenoTyper home cage, assessing the complexity and shape of the nest over 6 days. On the first testing day, animals were offered two pressed cotton squares per PhenoTyper home cage. Pictures of the nest were taken on a daily basis in the morning, including a top-down view and two side views at an angle of 90 and approximately 45 degrees. Several protocols for the assessment of nestbuilding performance in mice have been established37,63,64. For the image-based evaluation of the nest complexity, we applied a slightly modified version of a protocol reported by Jirkof et al.37. For detailed information about the applied scoring scheme, see Supplementary information. The scoring of the images was carried out by a person who was blinded for the group allocation.
Voluntary wheel running
We assessed the development of voluntary wheel running activity in the PhenoTyper home cage over four consecutive days. Thereby, we evaluated the time necessary to adapt to the running wheel and the progressive increase in running activity. Animals were exposed to the PhenoTyper home cage providing a 24 h per day freely accessible running wheel (diameter: 15 cm, width: 7 cm; PhenoWheel, Noldus, Wageningen, the Netherlands). Analysis of voluntary wheel running activity was performed with the integrated software EthoVision XT 15 (EthoVision XT, RRID:SCR_000441), based on the counts registered per minute.
Open field test
At the end of each testing week, exploratory behavior and locomotor activity were analyzed in the open field test. For habituation, animals were transferred to the testing room at least 30 min prior to the experiment. The animal was placed in a circular shaped open field (diameter: 60 cm; lighting condition: 20 lx), with its head facing the wall, 10 cm apart from the wall. The open field tests for each experimental unit (n = 2) were conducted in parallel, placing the two animals in its respective arenas simultaneously. Tracking duration lasted 15 min. Open fields were cleaned with 0.1% acetic acid after each trial. Male animals were tested prior to female animals. Analysis was carried out using the tracking software EthoVision XT 8.5 (EthoVision XT, RRID:SCR_000441). Additionally, rearing frequency was counted manually by a person unaware of the group allocation.
Body weight
The development of the body weight of the animals was measured on the first day on Fridays and the last day on Tuesdays of the testing week.
Irwin score
General condition of the mice was evaluated on the sixth day of the testing week, based on the traditional Irwin scoring system55, which provides information about general behavioral, neurological, and vegetative changes. We applied a slightly modified Irwin scale, divided into three consecutive parts (home cage, Open field, fresh single cage), including rectal body temperature measurement. Detailed information on the applied scoring system is provided in the Supplementary file.
Fecal corticosterone metabolites
Samples for the assessment of fecal corticosterone metabolite concentrations were obtained from animals (n = 200) after the Open field test on the last testing day. For detailed information on sampling, processing and analysis, see the Supplementary file.
Statistics
Statistical analysis of group differences was carried out using GraphPad Prism 5.04 for Windows (GraphPad Prism Software, San Diego, USA). We evaluated group differences by two-way analysis of variance (ANOVA), focusing on the two factors age phase and sex, followed by Bonferroni multiple comparison test, or one-way ANOVA, followed by Bonferroni multiple comparison test. Where indicated we determined group differences based on a model of linear regression. A p value < 0.05 was considered statistically significant. For nest complexity scores the median is shown. All other data are expressed as mean ± SEM.
Supplementary Information
Acknowledgements
This project was supported by a grant of the Deutsche Forschungsgemeinschaft, Germany (FOR 2591, GZ: PO681/9-2). The authors thank Sarah Glisic, Claudia Siegl, Helen Stirling, Sabine Vican and Edith Klobetz-Rassam for their excellent technical assistance, and Helen Stirling for language revision of the manuscript.
Author contributions
M.R.: study design, study execution, data analysis, statistics, writing of the manuscript; I.K.: Study design; C.P.: Breeding management; K.S.: Data analysis; L.S.: Data analysis; R.P.: Analysis of fecal corticosterone metabolites; H.P.: Study concept, study design, supervision, writing of the manuscript. All authors have carefully read the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All datasets used for the analysis will be made available in the severity assessment repository of the DFG research unit 2591: https://for.severity-assessment.de.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06395-1.
References
- 1.Brust V, Schindler PM, Lewejohann L. Lifetime development of behavioural phenotype in the house mouse (Musmusculus) Front. Zool. 2015;12:S17. doi: 10.1186/1742-9994-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattwell SS, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun. 2016;7:11475. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey BJ, Heller AS, Gee DG, Cohen AO. Development of the emotional brain. Neurosci. Lett. 2019;693:29–34. doi: 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukoff Rizzo SJ, Crawley JN. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annu. Rev. Anim. Biosci. 2017;5:371–389. doi: 10.1146/annurev-animal-022516-022754. [DOI] [PubMed] [Google Scholar]
- 5.Percie du Sert N, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EU. EU Directive 2010/63/EU on the protection of animals used for scientific purposes. http://www.eur-lex.europa.eu/legal-content/de/ALL/?uri=CELEX%3A32010L0063 (2010).
- 7.BfR. Severity Assessment of genetically altered mice and rats. http://www.bfr.bund.de/cm/349/severity-assessment-of-genetically-altered-mice-and-rats-version-2.pdf (2016).
- 8.Bleich A, Bankstahl M, Jirkof P, Prins JB, Tolba RH. Severity assessment in animal based research. Lab. Anim. 2020;54:16. doi: 10.1177/0023677219898105. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk RM, et al. Design of composite measure schemes for comparative severity assessment in animal-based neuroscience research: A case study focussed on rat epilepsy models. PLoS One. 2020;15:e0230141. doi: 10.1371/journal.pone.0230141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keubler LM, et al. Where are we heading? Challenges in evidence-based severity assessment. Lab. Anim. 2020;54:50–62. doi: 10.1177/0023677219877216. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo N, et al. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldt L, et al. Toward evidence-based severity assessment in mouse models with repeated seizures: I. Electrical kindling. Epilepsy Behav. 2021;115:107689. doi: 10.1016/j.yebeh.2020.107689. [DOI] [PubMed] [Google Scholar]
- 13.Koska I, et al. Toward evidence-based severity assessment in rat models with repeated seizures: II. Chemical post-status epilepticus model. Epilepsia. 2019;60:2114–2127. doi: 10.1111/epi.16330. [DOI] [PubMed] [Google Scholar]
- 14.Seiffert I, et al. Toward evidence-based severity assessment in rat models with repeated seizures: III. Electrical post-status epilepticus model. Epilepsia. 2019;60:1539–1551. doi: 10.1111/epi.16095. [DOI] [PubMed] [Google Scholar]
- 15.Möller C, et al. Impact of repeated kindled seizures on heart rate rhythms, heart rate variability, and locomotor activity in rats. Epilepsy Behav. 2019;92:36–44. doi: 10.1016/j.yebeh.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Schwabe K, et al. Nest-building performance in rats: Impact of vendor, experience, and sex. Lab. Anim. 2020;54:17–25. doi: 10.1177/0023677219862004. [DOI] [PubMed] [Google Scholar]
- 17.Weegh N, et al. Wheel running behaviour in group-housed female mice indicates disturbed wellbeing due to DSS colitis. Lab. Anim. 2020;54:63–72. doi: 10.1177/0023677219879455. [DOI] [PubMed] [Google Scholar]
- 18.Mallien AS, et al. Systematic analysis of severity in a widely used cognitive depression model for mice. Lab. Anim. 2020;54:40–49. doi: 10.1177/0023677219874831. [DOI] [PubMed] [Google Scholar]
- 19.Zieglowski L, et al. Severity assessment using three common behavioral or locomotor tests after laparotomy in rats: A pilot study. Lab. Anim. 2020;54:525–535. doi: 10.1177/0023677220911680. [DOI] [PubMed] [Google Scholar]
- 20.Tang G, et al. Comparing distress of mouse models for liver damage. Sci. Rep. 2020;10:19814. doi: 10.1038/s41598-020-76391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat. Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- 22.Tappe-Theodor A, King T, Morgan MM. Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci. Biobehav. Rev. 2019;100:335–343. doi: 10.1016/j.neubiorev.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot SR, et al. Defining body-weight reduction as a humane endpoint: A critical appraisal. Lab. Anim. 2020;54:99–110. doi: 10.1177/0023677219883319. [DOI] [PubMed] [Google Scholar]
- 24.Crawley, J. What's Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd edn, 1–523. 10.1002/9780470119051 (2006).
- 25.Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin. Exp. Res. 2010;34:1353–1362. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austen JM, Sprengel R, Sanderson DJ. GluA1 AMPAR subunit deletion reduces the hedonic response to sucrose but leaves satiety and conditioned responses intact. Sci. Rep. 2017;7:7424. doi: 10.1038/s41598-017-07542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eltokhi A, Kurpiers B, Pitzer C. Baseline depression-like behaviors in wild-type adolescent mice are strain and age but not sex dependent. Front. Behav. Neurosci. 2021;15:759574. doi: 10.3389/fnbeh.2021.759574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreshfield LJ, Berry SD. Age-dependent taste preferences in rabbits for a .025% sodium saccharin solution: Preliminary findings. Exp. Aging Res. 1994;20:145–154. doi: 10.1080/03610739408253960. [DOI] [PubMed] [Google Scholar]
- 29.Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods. 2014;234:139–146. doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Deacon RM, et al. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behav. Brain Res. 2008;189:126–138. doi: 10.1016/j.bbr.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Deacon RM. Burrowing: A sensitive behavioural assay, tested in five species of laboratory rodents. Behav. Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Aubert A. Sickness and behaviour in animals: A motivational perspective. Neurosci. Biobehav. Rev. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 33.Hart AD, Wyttenbach A, Perry VH, Teeling JL. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain Behav. Immun. 2012;26:754–765. doi: 10.1016/j.bbi.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLinden KA, et al. Age exacerbates sickness behavior following exposure to a viral mimetic. Physiol. Behav. 2012;105:1219–1225. doi: 10.1016/j.physbeh.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Eltokhi A, Kurpiers B, Pitzer C. Behavioral tests assessing neuropsychiatric phenotypes in adolescent mice reveal strain- and sex-specific effects. Sci. Rep. 2020;10:11263. doi: 10.1038/s41598-020-67758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wodarski R, et al. Cross-centre replication of suppressed burrowing behaviour as an ethologically relevant pain outcome measure in the rat: A prospective multicentre study. Pain. 2016;157:2350–2365. doi: 10.1097/j.pain.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 37.Jirkof P, et al. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab. Anim. 2013;47:153–161. doi: 10.1177/0023677213475603. [DOI] [PubMed] [Google Scholar]
- 38.Jirkof P, Cesarovic N, Rettich A, Fleischmann T, Arras M. Individual housing of female mice: Influence on postsurgical behaviour and recovery. Lab. Anim. 2012;46:325–334. doi: 10.1258/la.2012.012027. [DOI] [PubMed] [Google Scholar]
- 39.Arras M. Improvement of pain therapy in laboratory mice. Altex. 2007;24 Spec No:6–8. [PubMed] [Google Scholar]
- 40.Takao K, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38:1409–1425. doi: 10.1038/npp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filali M, Lalonde R, Rivest S. Cognitive and non-cognitive behaviors in an APPswe/PS1 bigenic model of Alzheimer's disease. Genes Brain Behav. 2009;8:143–148. doi: 10.1111/j.1601-183X.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 42.Filali M, Lalonde R. Age-related cognitive decline and nesting behavior in an APPswe/PS1 bigenic model of Alzheimer's disease. Brain Res. 2009;1292:93–99. doi: 10.1016/j.brainres.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 43.Neely CLC, Pedemonte KA, Boggs KN, Flinn JM. Nest building behavior as an early indicator of behavioral deficits in mice. J. Vis. Exp. 2019 doi: 10.3791/60139. [DOI] [PubMed] [Google Scholar]
- 44.Turner PV, Pang DS, Lofgren JL. A review of pain assessment methods in laboratory rodents. Comp. Med. 2019;69:451–467. doi: 10.30802/aalas-cm-19-000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong XD, Xiong WD, Xiong SS, Chen GH. Age- and gender-based differences in nest-building behavior and learning and memory performance measured using a radial six-armed water maze in C57BL/6 mice. Behav. Neurol. 2018;2018:8728415. doi: 10.1155/2018/8728415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moy SS, et al. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 47.Bult A, Lynch CB. Nesting and fitness: Lifetime reproductive success in house mice bidirectionally selected for thermoregulatory nest-building behavior. Behav. Genet. 1997;27:231–240. doi: 10.1023/a:1025610130282. [DOI] [PubMed] [Google Scholar]
- 48.Robinson-Junker A, Morin A, Pritchett-Corning K, Gaskill BN. Sorting it out: bedding particle size and nesting material processing method affect nest complexity. Lab. Anim. 2017;51:170–180. doi: 10.1177/0023677216652384. [DOI] [PubMed] [Google Scholar]
- 49.Martin TL, Balser SR, Young GS, Lewis SD. Cost and effectiveness of commercially available nesting substrates for deer mice (Peromyscus maniculatus) J. Am. Assoc. Lab. Anim. Sci. 2016;55:412–418. [PMC free article] [PubMed] [Google Scholar]
- 50.Häger C, et al. Running in the wheel: Defining individual severity levels in mice. PLoS Biol. 2018;16:e2006159. doi: 10.1371/journal.pbio.2006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav. Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macrì S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim. Behav. 2002;64:541–546. doi: 10.1006/anbe.2002.4004. [DOI] [Google Scholar]
- 53.Moore EM, Linsenbardt DN, Melon LC, Boehm SL., II Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: Anxiety-like, locomotor, and consummatory behaviors. Dev. Psychobiol. 2011;53:141–156. doi: 10.1002/dev.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Jackson Laboratory. Phenome Database. https://phenome.jax.org/ (2021).
- 55.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/bf00401402. [DOI] [PubMed] [Google Scholar]
- 56.Buchecker, V. et al. Toward evidence-based severity assessment in mouse models with repeated seizures: (II). Impact of surgery and intrahippocampal kainate. Eur Surg Res. 10.1159/000522156 (2022). [DOI] [PMC free article] [PubMed]
- 57.Palme R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019;199:229–243. doi: 10.1016/j.physbeh.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Kolbe T, Palme R, Tichy A, Rülicke T. Lifetime dependent variation of stress hormone metabolites in feces of two laboratory mouse strains. PLoS One. 2015;10:e0136112. doi: 10.1371/journal.pone.0136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailoo JD, et al. Effects of weaning age and housing conditions on phenotypic differences in mice. Sci. Rep. 2020;10:11684. doi: 10.1038/s41598-020-68549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Touma C, Sachser N, Möstl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- 62.Klein S, Bankstahl JP, Löscher W, Bankstahl M. Sucrose consumption test reveals pharmacoresistant depression-associated behavior in two mouse models of temporal lobe epilepsy. Exp. Neurol. 2015;263:263–271. doi: 10.1016/j.expneurol.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Deacon RM. Assessing nest building in mice. Nat. Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 64.Kraeuter AK, Guest PC, Sarnyai Z. The nest building test in mice for assessment of general well-being. Methods Mol. Biol. 2019;1916:87–91. doi: 10.1007/978-1-4939-8994-2_7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets used for the analysis will be made available in the severity assessment repository of the DFG research unit 2591: https://for.severity-assessment.de.