Abstract
There is a need for effective therapy for COVID-19 pneumonia. Convalescent plasma has antiviral activity and early observational studies suggested benefit in reducing COVID-19 severity. We investigated the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19 in a population with a high HIV prevalence and where few therapeutic options were available. We performed a double-blinded, multicenter, randomized controlled trial in one private and three public sector hospitals in South Africa. Adult participants with COVID-19 pneumonia requiring non-invasive oxygen were randomized 1:1 to receive a single transfusion of 200 mL of either convalescent plasma or 0.9% saline solution. The primary outcome measure was hospital discharge and/or improvement of ≥ 2 points on the World Health Organisation Blueprint Ordinal Scale for Clinical Improvement by day 28 of enrolment. The trial was stopped early for futility by the Data and Safety Monitoring Board. 103 participants, including 21 HIV positive individuals, were randomized at the time of premature trial termination: 52 in the convalescent plasma and 51 in the placebo group. The primary outcome occurred in 31 participants in the convalescent plasma group and and 32 participants in the placebo group (relative risk 1.03 (95% CI 0.77 to 1.38). Two grade 1 transfusion-related adverse events occurred. Participants who improved clinically received convalescent plasma with a higher median anti-SARS-CoV-2 neutralizing antibody titre compared with those who did not (298 versus 205 AU/mL). Our study contributes additional evidence for recommendations against the use of convalescent plasma for COVID-19 pneumonia. Safety and feasibility in this population supports future investigation for other indications.
Subject terms: Clinical trial design, Clinical trials, Randomized controlled trials
Introduction
There are limited drugs with convincing evidence of efficacy for hospitalized patients with COVID-19. Host-directed therapies including dexamethasone1, tocilizumab2,3, and baricitinib4, and the monoclonal antibody casirivimab/imdevimab cocktail5, and anakinra6 are associated with survival benefit. Remdesivir, an antiviral agent, has modest impact on shortening the time to recovery7. Of these, only dexamethasone is widely available in resource-limited settings and there is an urgent need for accessible therapeutic options for COVID-19 pneumonia.
Transfusion with convalescent plasma (CP) provides antiviral activity through transfer of neutralizing antibodies and possibly other immune components8. Observational studies suggested CP therapy may improve outcomes in severe viral infections9, including lower mortality and earlier hospital discharge in SARS-CoV10. There have been no safety signals associated with CP use in the treatment of severe viral infections11. CP is therefore an attractive potential therapy for COVID-19, particularly in resource-limited settings where access to other novel drugs is limited12, given its potential for rapid and relative low cost local production13.
In the absence of other therapeutic options, COVID-19 convalescent plasma (CCP) was widely deployed outside of clinical trials in the first year of the pandemic. Early experience from uncontrolled observational studies suggested efficacy for CCP, including improved survival, decreased viral load, and radiological improvement14,15. There was a dose–response relationship in an analysis of a large expanded access programme, with reduced 30-day mortality among patients receiving CCP with higher-titre anti-SARS-CoV-2 spike IgG compared with those who received lower titres, providing biological plausibility for clinical efficacy16. Encouraging results were also observed in a small randomised control trial (RCT) in India where CCP was associated with improvement in respiratory parameters and a shortened recovery time17. Another RCT from New York and Brazil found significantly improved survival at day 28 in participants treated with CCP18. By contrast, the RECOVERY trial, a large RCT with clinical endpoints, was halted prematurely as high titre CCP did not improve survival in hospitalized patients with COVID-19 in the UK19.
Prior to the announcement of the RECOVERY trial results and in the context of rapidly emerging observational data supporting potential efficacy and safety of CCP, we undertook a randomized controlled trial to test whether CCP improved clinical recovery for COVID-19 pneumonia in an African population with high HIV prevalence and limited available treatment options.
Methods
Study design and population
The PROTECT-Patient trial, A PROspective, randomized, placebo-controlled, double-blinded phase III clinical trial of the Therapeutic use of convalEsCenT plasma in the treatment of patients with moderate to severe COVID-19) evaluated the efficacy and safety of CCP for hospitalized patients with COVID-19 pneumonia. The trial took place at one private sector and three public sector hospitals in South Africa. The trial was first registered on 24/04/2020. The trial was sponsored by the South African National Blood Service (SANBS) and was conducted in accordance with the principles of the International Conference on Harmonisation–Good Clinical Practice guidelines, approved by the South African Health Products Regulatory Authority and the Human Research Ethics Committees of SANBS (2019/0524, 24/04/2020), University of Cape Town (312/2020, 14/07/2020), University of the Free State (UFS2020/1253/2710, 22/09/2020), University of Walter Sizulu (086/2020, 04/11/2020) and the Life Hospital group (07052020/1 07/07/2020). The trial is registered on clinicaltrials.gov (NCT04516811 (https://clinicaltrials.gov/ct2/show/NCT04516811), 18/08/2020) and the protocol is available as part of Supplementary Information.
Informed consent was obtained from hospitalized patients ≥ 18 years of age were eligible for inclusion if they had laboratory confirmation of SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction (RT-PCR) on any respiratory sample, radiologic evidence of pneumonia with pulmonary infiltrates on chest X-ray, oxygen saturation (SpO2) of < 94% on room air and requiring non-invasive oxygen therapy. We excluded patients who were mechanically ventilated or where survival for < 24 h was expected. Co-enrolment into another investigational therapy trial was not permitted.
Randomization, blinding, and intervention
Eligible and consenting participants were randomized (1:1) to receive either a single infusion of 200–250 mL CCP or 200 mL of placebo (0.9% normal saline), together with local standard of care. Random assignment was stratified by study site, age (≥ or < 65 years), and body mass index (BMI) (≥ or < 30 kg/m2). An electronic randomisation application (REDCap) hosted by SANBS20, was used to generate the treatment allocation. To mask treatment allocation, investigational product (IP) was covered in opaque paper wrapping prior to dispatch from the blood bank. Participants were transfused within 24 h of randomization. CCP or placebo was administered over 20–30 min; vital signs were monitored at 15-min intervals for one-hour post initiation of transfusion. Use of other treatments, including corticosteroids and anticoagulation, was at the discretion of treating clinicians; remdesivir was unavailable for routine use.
CCP was collected by SANBS and the Western Cape Blood Service (WCBS) from donors who had recovered from SARS-CoV-2 infection, confirmed by positive nasopharyngeal swab RT-PCR (Supplementary Information). SARS-CoV-2 antibody titre testing was performed by the National Institure of Communicable Diseases (NICD) using an in-house enzyme-linked immunosorbent assay (ELISA) assay based on the assay developed by Mount Sinai Hospital21, which detected the presence of anti-spike and -receptor binding domain antibodies. Testing for neutralizing antibodies (nAb) was performed on stored samples once the assay was developed and validated (methods described elsewhere)22. Initially we selected donor units with an anti-spike IgG optical density (OD450nm) of ≥ 0.4, which was considered to be positive21. As more information emerged regarding the importance of nAb titers, we aimed to transfuse CCP with nAb titres of 1:160 or higher based on findings from other clinical trials23. However, in line with Food and Drug Administration (FDA) guidance24 on the use of anti-spike binding antibody as a proxy for nAb, we moved to using CCP with anti-spike protein IgG OD450nm values greater than 2.0, which correlated well with nAb titres ≥ 1:16021.
Clinical and laboratory monitoring
We performed daily in-person or medical record assessments for adverse events, clinical status, and oxygen utilization during hospital admission. For participants discharged prior to day 28, clinical status and adverse events were determined via telephone. Concomitant medication was recorded at each clinical visit. Phlebotomy for safety and inflammatory markers was performed on the day of transfusion and when possible, on days 2, 5, 10, 14 and 28 during hospitalisation. A nasopharyngeal swab for SARS-CoV-2 genomic sequencing was collected at enrolment in a subset of patients and sent to the NICD for RT-PCR testing using the 3 Allplex™ 2019-nCoV assay (Seegene Inc). Retained RNA samples were processed using a commercially available version of the SARS CoV-2 ARTIC library preparation protocol (NEBNext ARTIC SARS-CoV-2 FS Library Prep Kit, cat# E7658 (Illumina, San Diego, California, USA)). Following library preparation, samples were sequenced on the NextSeq 500 platform (Illumina, San Diego, California, USA). A minimum of 1 million 2 × 150 bp PE reads were generated per sample. Analysis of sequence data was performed by the NICD. Whole genome assembly was conducted using the Exatype SARS-CoV-2 platform (https://sars-cov-2.exatype.com/).
Outcomes
The primary outcome measure was successful treatment at Day 28 post-randomization, defined as acute care hospital discharge or clinical improvement of ≥ 2 points on an ordinal scale recommended by the World Health Organization25: 0 -uninfected; (1) ambulatory with no limitation of activites; (2) ambulatory with limitation of activities; (3) hospitalized, not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, requiring high-flow oxygen therapy or non-invasive mechanical ventilation; (6) hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation (IMV), or both; (7) ventilation and additional organ support; (8) death. Secondary efficacy outcomes included clinical improvement and hospital discharge as separate categories; survival at Day 28 post-randomization; invasive mechanical ventilation (IMV); duration of hospitalisation; and time from randomization to clinical improvement and death. Safety outcomes were adverse events of special interest (transfusion associated circulatory overload, transfusion associated acute lung injury, allergic transfusion reactions); and serious and Grade 3 or 4 adverse events.
Sample size
We hypothesized that a single transfusion of CCP would be associated with improved treatment success compared with saline placebo in patients hospitalized with COVID-19 pneumonia. Based on observational studies at the time of protocol development26–28, we assumed that approximately 30% of patients with COVID-19 pneumonia would not reach the primary endpoint event of clinical improvement. We estimated a sample size of 600 participants (300 per treatment group) would provide 80% power at 2-sided alpha to detect a 33% difference in relative risk (10% absolute difference) in the primary outcome between the two arms.
Early trial termination and analysis
The trial management committee, in discussion with the trial DSMB, paused recruitment on 14 January 2021 in response to evidence that convalescent plasma, collected from people infected with ‘wild-type’ virus during the first wave in South Africa, had poor neutralizing activity against the Beta (B.1.351/501Y.V2) SARS-CoV-2 variant presumed to have become the dominant variant in trial participants29. The trial DSMB requested an unplanned interim analysis with futility calculations based on conditional power. The 103 participants who received IP at the time of pausing provided an information fraction of 0.17 with observed event probabilities of 0.63 and 0.61. Conditional power for a significance difference at the end of the trial based on the observed trend was 40%; if no effect was assumed from the time recruitment was paused to the end of the trial, conditional power was 3%. Based on these low conditional probabilities, and emerging data from the RECOVERY trial, the DSMB recommended that the trial be stopped for futility on 10 February 2021. To report findings, prevalence of endpoints in the intention to treat population were estimated at day 28 and/or date of discharge using risk ratios and 95% confidence intervals. Due to lack of power as a result of early trial cessation, no formal statistical comparisons were done. We used Kaplan–Meier estimates to compare and illustrate time to clinical improvement and death in the treatment groups. We used STATA V.17 (STATACORP, Texas) to perform the statistical analysis.
Results
107 participants were enrolled between 30 September 2020 and 14 Jan 2021; 103 were transfused with IP (two participants withdrew consent and two were transferred to alternative facilities prior to transfusion): 52 participants received CCP and 51 were given placebo (Fig. 1).
Figure 1.
Consort diagram illustrating numbers of participants with moderate-severe COVID-19 randomized (1:1) to treatment with convalescent plasma or placebo.
The trial groups were well balanced in terms of baseline characteristics, disease severity, and routine management (Tables 1 and 2). Twenty-one (25%) of those with known HIV status (n = 84) were HIV positive, 16 (76%) of whom were on antiretroviral therapy (ART). Median CD4 count for the HIV positive participants was 596 (interquartile range (IQR) 242–1029). Co-morbidities, including a high prevalence of diabetes and hypertension, were present in 42 (80.8%) participants. More than half of participants (n = 56, 54.4%) were classified as obese30. The median time from symptom onset to IP infusion was 9 (IQR 6–11) days. Corticosteroids were used in 97 (94.2%); 98 (95.1%) participants were prescribed heparin prophylaxis, mostly with therapeutic doses.
Table 1.
Participant characteristics at baseline in 103 participants with severe COVID-19 randomized to treatment with convalescent plasma versus placebo.
| CCP, n (%) | Placebo, n (%) | Total (%) | |
|---|---|---|---|
| Total | 52 | 51 | 103 |
| Age group (years) | |||
| < 40 | 9 (17.3) | 6 (11.8) | 15 (14.5) |
| 40–60 | 26 (50.0) | 26 (51.0) | 52 (50.5) |
| > 60 | 17 (32.7) | 19 (37.3) | 36 (35.0) |
| Age-median (IQR) | 54 (46–62) | 57 (47–64) | 56 (46–63) |
| Gender | |||
| Female | 31 (59.6) | 30 (58.8) | 61 (59.2) |
| Male | 21 (40.4) | 21 (41.2) | 42 (40.8) |
| HIV status | |||
| Positive | 6 (11.5) | 15 (28.8) | 21 (18.5) |
| On ART | 5 (83.3) | 11 (73.3) | 16 (76.2) |
| Not on ART | 1 (16.7) | 4 (26.7) | 5 (24.8) |
| Negative | 37 (71.2) | 26 (51.0) | 63 (62.1) |
| Unknown | 9 (17.3) | 10 (19.6) | 19 (19.4) |
| Smoking | |||
| Current smoker | 5 (9.6) | 6 (11.8) | 11 (10.7) |
| Ex-smoker | 11 (21.2) | 11 (21.6) | 22 (21.7) |
| BMI | |||
| BMI ≥ 30 kg/m2 | 29 (55.8) | 27 (52.9) | 56 (54.4) |
| BMI-Median (IQR) | 31.2 (26.8–37.6) | 31.0 (26.8–36.0) | |
| SARS-CoV-2 clade on sequencing | 34 | 32 | 66 |
| 19A | 2 (5.9) | 2 (6.3) | 4 (6.1) |
| 20A | 4 (11.8) | 6 (18.8) | 10 (15.2) |
| 20B | 5 (14.7) | 2 (6.3) | 7 (10.6) |
| 20H/501Y.V2 | 23 (67.6) | 22 (68.8) | 45 (68.2) |
Table 2.
Clinical and laboratory characteristics at baseline in 103 participants with severe COVID-19 randomized to treatment with convalescent plasma versus placebo.
| CCP | Placebo | Total | ||||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| Total | 52 (50.5%) | 51 (49.5%) | 103 (100%) | |||
| BOSCIa score at enrolment | ||||||
| 4 | 40 (76.9%) | 42 (82.4%) | 82 (79.6%) | |||
| 5 | 12 (23.1%) | 9 (17.6%) | 21 (20.4%) | |||
| Co-morbiditiesb | ||||||
| Chronic Kidney disease | 2 (3.8%) | 1 (2%) | 3 (2.9%) | |||
| Diabetes | 25 (48.1%) | 15 (29.4%) | 40 (38.8%) | |||
| Hypertension | 28 (53.8%) | 28 (54.9%) | 56 (54.4%) | |||
| Obesity | 24 (46.2%) | 25 (49%) | 49 (47.6%) | |||
| Cardiovascular disease | 1 (1.9%) | 2 (3.9%) | 3 (2.9%) | |||
| Cancer | 0 (0%) | 2 (3.9%) | 2 (1.9%) | |||
| Chronic pulmonary disease | 2 (3.8%) | 2 (3.9%) | 4 (3.9%) | |||
| Concomitant medication | ||||||
| Antibiotic | 14 (26.9%) | 11 (21.6%) | 25 (24.3%) | |||
| Enoxaparin sodium | 48 (92.3%) | 50 (98%) | 98 (95.1%) | |||
| Therapeutic dose | 38 (71.2%) | 36 (72%) | (%) | |||
| Prophylactic dose | 10 (20.8%) | 14 (28%) | (%) | |||
| Inotropes | 1 (1.9%) | 2 (3.9%) | 3 (2.9%) | |||
| Steroids (Dexamethasone/prednisone) | 50 (96.2%) | 47 (92.2%) | 97 (94.2%) | |||
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | |
|---|---|---|---|---|---|---|
| Average days from symptom onset to | ||||||
| Hospitalisation | 52 | 5 (3–7) | 51 | 5 (3.5–9.5) | 103 | 5 (3–8) |
| Enrolment | 52 | 8 (6–10.25) | 51 | 8 (6–11) | 103 | 8 (6–11) |
| Transfusion | 52 | 9 (6–11) | 51 | 9 (7–12) | 103 | 9 (6–11) |
| Baseline clinical measurements | ||||||
| Pulse (beats/min) | 52 | 85 (74–99) | 50 | 87 (77–95) | 102 | 86 (75–95) |
| Respiratory rate (breaths/min) | 52 | 26 (22–30) | 51 | 28 (22–32) | 103 | 28 (22–32) |
| Lowest SpO2 prior to starting O2 (%) | 50 | 85.5 (82–88) | 51 | 86 (78–89) | 101 | 86 (80–88) |
| PaO2 (kPa) | 36 | 10.3 (8.0–14.0) | 35 | 9.2 (6.0–13.9) | 71 | 9.3 (7.1–14.0) |
| Baseline laboratory measurements | ||||||
| Creatinine clearance (mL/min) | 43 | 86.6 (61.0–106.0) | 45 | 90.2 (82.3–104.8) | 88 | 88.9 (73.2–104.8) |
| Ferritin (μg/L) | 46 | 454.5 (161–776) | 45 | 495.0 (263–1177) | 91 | 495 (213–883) |
| d-Dimer (μg/mL) | 42 | 0.5 (0.3–1.7) | 43 | 0.5 (0.3–1.2) | 85 | 0.5 (0.3–1.4) |
| Haemoglobin (g/dL) | 45 | 12.9 (11.5–13.9) | 47 | 13.1 (12.1–14.6) | 92 | 13.1 (11.8–14.0) |
| Absolute white cell count (× 109/L) | 45 | 12.1 (10.4–15.5) | 48 | 9.8 (8.3–14.2) | 93 | 11.29 (8.4–14.7) |
| Absolute neutrophil count (× 109/L) | 44 | 10.0 (6.9–12.8) | 47 | 8.0 (6.3–11.1) | 91 | 9.13 (6.74–12.72) |
| Platelets (× 109/L) | 45 | 346 (261–423) | 47 | 322 (229–401) | 92 | 339 (257.5–405.5) |
| Alanine aminotransferase (ALT) (IU/L) | 41 | 30 (20–48) | 43 | 35 (26–57) | 84 | 31 (23–51) |
| C-reactive protein (μg/L) | 44 | 135 (56–202) | 43 | 111 (62–222) | 87 | 122 (57–207) |
| Troponin-T (ng/ml) | 41 | 7 (5–14) | 45 | 9 (6–18) | 86 | 9 (5–17) |
| Lactate dehydrogenase (LDH) (U/L) | 37 | 393 (298–459) | 34 | 456 (357–711) | 71 | 415 (314–571) |
aMinimum BOSCI score of 4 was required for eligibility. BOSCI 4: hospitalized, mild disease, on oxygen by mask or nasal prongs. BOSCI 5: hospitalized, severe disease, non-invasive ventilation or high flow oxygen.
bComorbidities are listed as number of comorbidities.
Information on the primary endpoint was unavailable for six participants who were uncontactable after initial discharge or transfer, however sensitivity analysis did not change the findings. The primary outcome was therefore assessed in 97/103 (94.2%) participants: 31 (66.0%) in the CCP group and 32 (64.0%) in the placebo group experienced ≥ 2 point BOSCI improvement or discharge by Day 28, relative risk 1.03 (95% CI 0.77 to 1.38) (Table 3). All participants who were discharged demonstrated a clinical improvement (BOSCI improvement ≥ 2) (Fig. 2). There were 11 and 13 deaths in the CCP and placebo groups, respectively. Four participants required invasive mechanical ventilation, one in the CCP group and three in the placebo group. There was no difference in time to death or clinical improvement between treatment groups (Fig. 3). Among HIV positive participants, the primary outcome was achieved in two (33%) and nine (60%) participants in the CCP and placebo groups, respectively.
Table 3.
Primary and secondary efficacy outcomes in 103 participants with severe COVID-19 randomized to treatment with convalescent plasma versus placebo.
| Primary outcome | CCP; n (%) | Placebo; n (%) | RR (95% CI) |
|---|---|---|---|
| Clinical improvement by D28 | 31/47 (66.0) | 32/50 (64.0) | 1.03 (0.77 to 1.38) |
| Secondary efficacy outcomes | |||
| Discharge from hospital by D28 | 28/46 (60.9) | 31/50 (62.0) | 1.21 (0.84 to 1.74) |
| BOSCI improvement: ≥ 2 by D28 | 31/47 (66.0) | 32/50 (64.0) | 1.03 (0.77 to 1.38) |
| Death by D28 | 11/52 (21.5) | 13/51 (25.5) | 0.83 (0.41 to 1.68) |
| Invasive mechanical ventilation | 1/52 (1.9) | 3/51 (5.9) | 0.33 (0.04 to 3.04) |
Six participants were discharged from primary acute care prior to Day 28 but were lost to follow up as they were uncontactable at Day 28. Clinical improvement is a composite of discharge from hospital and/or improvement in BOSCI score by ≥ 2 by Day 28.
Figure 2.
Proportion of clinical outcomes for 103 participants with moderate-severe COVID-19 randomized to treatment with convalescent plasma versus placebo at day 28 post recruitment.
Figure 3.
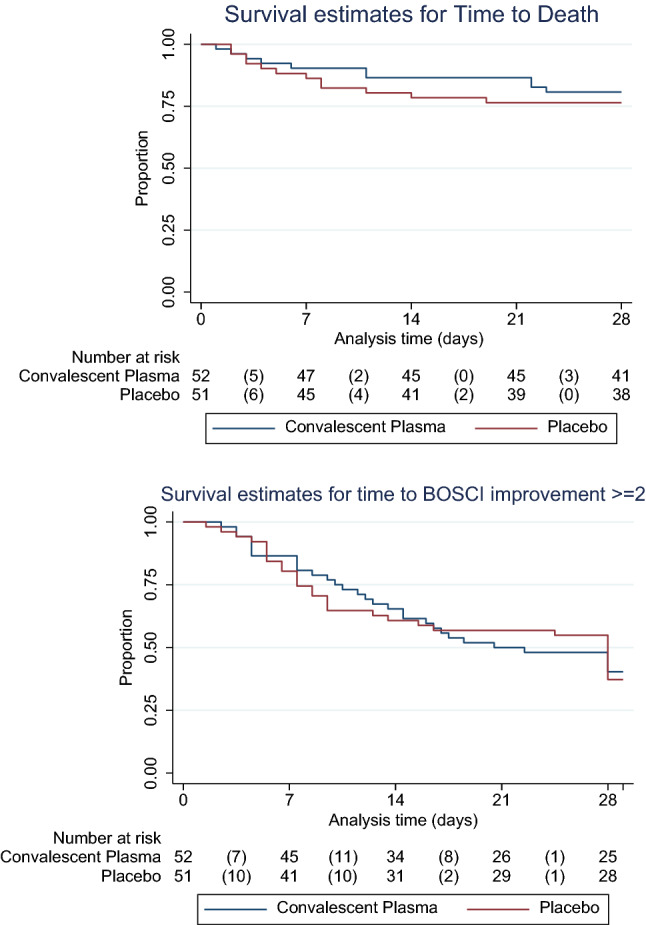
Kaplan–Meier survival analysis of time to death and separately for improvement by ≥ 2 BOSCI points in 103 participants with moderate-severe COVID-19 randomized treatment with convalescent plasma versus placebo. *BOSCI: World Health Organization Blueprint Ordinal Scale for Clinical Improvement.
The total number of adverse events, including serious adverse events, was similar across treatment groups (Table 4). Two transfusion-related adverse events occurred, both grade 1 allergic reactions. Six grade 3 or higher adverse events were recorded among HIV-positive participants who were transfused CCP, all assessed as unrelated to the study drug.
Table 4.
Number of adverse events in 103 participants with severe COVID-19 randomized to treatment with convalescent plasma versus placebo.
| Adverse events | CCP | Placebo | Total |
|---|---|---|---|
| Number of participants with adverse events | 23 | 17 | 40 |
| Number of adverse events | 32 | 27 | 59 |
| Number of serious adverse events | 18 | 18 | 36 |
| Number of deaths | 11 | 13 | 24 |
| Number of transfusion related AE | 1 | 1 | 2 |
In the transfused CCP, the median anti-spike protein IgG optical density (OD450nm) was 2.7 AU/mL (IQR 2.0 to 3.0); and the median anti-SARS-CoV-2 neutralizing antibody titre was an inhibitory dilution at which 50% neutralization is attained (ID50) of 1:234 AU/mL (IQR 194 to 304; range 71 to 1245). Participants who demonstrated clinical improvement received CCP transfusions with higher median anti-SARS-CoV-2 neutralizing antibody titres compared with those who did not : ID50 of 1:298 (IQR 212–374) versus 1:205 (IQR 181–254) (Fig. 4). Of those who showed clinical improvement, 21 (81%) had an antibody titre > 1:200 ID50 (Fig. 4).
Figure 4.
Correlation of neutralizing antibody titer and spike optical density, partitioned by clinical improvement status in patients who received CCP.
SARS-CoV-2 genomic sequencing was performed on available baseline nasopharyngeal samples from 66 participants. SARS-CoV-2 variants were similarly distributed across treatment groups. The beta variant was detected in 45 (68.2%) samples. In the CCP group, 6/22 (27.7%) participants with the beta variant died and 1/10 (10.0%) participants with other SARS-CoV-2 variants died. Clinical improvement by day 28 occurred in 11/18 (61.1%) of participants with beta variant infection who received CCP, and in 7/10 (70.0%) of those infected with other variants.
Discussion
Our trial showed that the use of therapeutic CCP for hospitalized patients with COVID-19 pneumonia was not associated with clinical improvement in a South African population with a high HIV-prevalence. Although our final sample size did not support formal hypothesis testing, the lack of any signal of benefit led to a conclusion of futility, and is consistent with findings from randomized controlled trials in other settings19,23,31. Notwithstanding early trial termination, our study contributes additional evidence for recommendations against use of CCP for established COVID-19 pneumonia, especially in a high HIV-prevalence setting, and may provide some insights into reasons for the lack of clinical efficacy.
Observational studies suggest that the clinical effect of CCP is related to neutralizing antibody titre16. There has been variability and lack of standardisation in transfused neutralizing titres across trials, which may explain some of the heterogeneity in outcomes with CCP. It is an operational challenge to perform neutralisation assays in real time, and similarly to other studies, we selected CCP units on anti-spike IgG antibodies that correlated with neutralizing titres. Consequently, we used a wide dosing range in transfused CCP in our trial, highlighting the challenge of CCP standardisation in future studies, or if implementation is contemplated. Informative subgroup analyses were not possible with our limited sample size, but we did observe higher neutralizing titres among participants with better outcomes. However, given the lack of clinical effect in the RECOVERY trial, which only transfused high anti-spike titre CCP, it is unlikely that selecting donor plasma with titres in accordance with current FDA guidelines24, would have altered our main finding.
The majority of CCP donations for the PROTECT trial were collected during the first COVID-19 wave in South Africa, during which donors were likely infected with the ‘wild type’ SARS-CoV-2. Due to delays in regulatory approvals, recruitment was unable to commence until the beginning of the second wave when the beta variant had become the predominant circulating SARS-CoV-2 strain32. This was subsequently confirmed by sequencing in over two-thirds of our participants with available samples. In vitro experiments indicated that SARS-CoV-2 antibodies in convalescent plasma provided for the PROTECT trial, obtained from donors infected before the emergence of beta, was significantly less effective at neutralizing pseudovirus expressing beta variant spike protein29. SARS-CoV-2 variants have also shown resistance to neutralisation by anti-receptor binding domain monoclonal antibodies33, and antigenic variability of SARS-CoV-2 with emergence of new variants will be a major obstacle to deployment of these therapeutic strategies. Although CCP may contribute some indirect antiviral effect through non-neutralizing antibody activity8, which is preserved in convalescent plasma against multiple variants29, this appears unlikely to translate into clinical efficacy.
Clinical studies suggest CCP16,34,35 and monoclonal antibody36 therapy may provide benefit early in the course of COVID-19 when SARS-CoV-2 viral load is highest at around 3 days post-diagnosis37, but this has been inconsistent38. The median time from symptom onset to transfusion in our trial was 9 days, which is comparable to other negative trials of CCP for inpatients with COVID-19 pneumonia18,19,39,40. The RECOVERY trial did not find benefit for earlier CCP administration on stratified analysis, and a trial evaluating monoclonal antibody therapy was stopped early for failing to demonstrate efficacy among hospitalized patients, even with low oxygen requirements41, suggesting that antibody based therapies are unlikely to be effective after activation of excessive host inflammation associated with severe disease.
CCP may have therapeutic potential in other contexts and patient groups and more likely as a prophylactic for severe COVID-19 in individuals with comorbidities. A randomized controlled trial showed that transfusion of CCP within three days of symptom onset reduced progression to severe COVID-19 among older people in Argentina34, and there are at least thirteen active trials evaluating CCP for treatment and prophylaxis of mild or moderate COVID-19 (https://covid-nma.com/dataviz). There is also accumulating evidence from case reports of good outcomes with CCP use in a heterogeneous group of patients with primary and secondary immunodeficiency, including those with haematological malignancy and solid organ transplantation42. HIV may be a risk factor for worse outcomes with COVID-1943, but data is scarce on the use of CCP in this patient group. Our trial included 21 HIV-positive participants, six of whom received CCP. Although this small number precludes definitive conclusions, absence of a major safety signal is somewhat reassuring. In line with experience in other settings11, CCP use was safe in our overall cohort, supporting future evaluation for different indications in high HIV burden populations.
The premature termination of the PROTECT trial illustrates the complexity of undertaking clinical research in a rapidly evolving global pandemic. Inconsistent case numbers, viral evolution, and rapidly changing evidence during the trial period contributed to this challenge. Despite this, our trial demonstrated the feasibility of deploying CCP in a resource-limited setting and contributed knowledge on the use of this therapeutic strategy for COVID-19 pneumonia. Our experience highlights the necessity of globally networked clinical trial sites and harmonised study protocols to more efficiently evaluate interventions among diverse populations during a pandemic.
Supplementary Information
Acknowledgements
We gratefully acknowledge the patients who agreed to participate in this study, the donors who donated the plasma and the staff at the two blood services, in particular those in the testing laboratories, blood banks, processing laboratories and collection teams without whom this study would not have been possible. We wish to acknowledge the following people for their hard work and dedication to this project: Sr Brenda Wright, our clinical trial monitor; our research nurses Jenna Ngxito, Simphiwe Mkhize, Laurette Pike and Bibi Makhanya; our study coordinators Mpumi Maxebengula, Letsie Strydom, Mary Isaacs, Senzeni Mahlangu; our SANBS donor and laboratory coordinators Jabulisile Jaza and Itumeleng Phayane; staff of the WCBS Specialised Donation Department (Sister Tania Paarman, Sister Natasha Brits, Sister Isabel Steenkamp, Kyle Mullins); staff of the WCBS Component Processing Department (Reginald Valensky, Notemba Msi); staff of the WCBS Transfusion Virology Department (Russell Cable, Nadia Pietersen, Carmen Addinall); staff of the WCBS Red Cross Blood Bank (special mention to Karen Seidel, Mpholisi Mbengo, Caren Overall) and Mignon du Plessis, Kedibone Ndlangisa and Maimuna Carrim (rRT-PCR), Catherine Scheepers (sequencing analysis) and Zanele Makhado, Frances Ayres and Mashudu Madzivhandila (serology and neutralization asays) of the NICD.
Author contributions
I confirm that the following authors have seen and approved the submitted manuscript, and that all have made substantial contributions to the work justifying authorship: K.B., T.G., M.V., F.L., R.S., C.B., R.C., M.B., C.N., A.S., C.M., T.C., G.K., J.N., P.S., J.C., T.M.-G., P.L.M., J.B., J.S., J.N.B., P.B., P.M., J.M., S.P., C.V., S.M., R.J.W., V.J.L., S.W.
Funding
This research was supported by The ELMA South Africa Foundation (20-ESA011), Allan & Gill Gray Philanthropy LIMITED, Wellcome, and the South African Medical Research Council with funds received from the Department of Science and Innovation. SW was supported by Wellcome (Grant number 203135/Z/16/Z) and National Institutes of Health (K43TW011421). KvdB and VL were also supported by NIH Fogarty International Center training grant 1D43-TW010345. PLM and TMG were supported by the South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation (Grant No 98341) and the MRC Strategic Health Innovations Program of the SA MRC. RJ was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC0010218), the UK Medical Research Council (FC0010218), and Wellcome (FC0010218); by Wellcome (203135, 222754) and by the Medical Research Council of South Africa. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Vernon J. Louw and Sean Wasserman.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06221-8.
References
- 1.Horby P, Lim WS, Emberson JR, et al. On behalf of the RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmers J, Abo-Leyah H, Loftus H, Spears M. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group* Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horby, P. W., Mafham, M., Peto, L., et al. On behalf of the RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542 (2021). [DOI] [PMC free article] [PubMed]
- 6.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27(10):1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas M, Rodriguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19(7):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2(9):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 10.Mair-Jenkins J, Saavedra-Campos M, Baillie JK. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 Convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch EM, Goel R, Wendel S, et al. Guidance for the procurement of COVID-19 convalescent plasma: Differences between high- and low-middle-income countries. Vox Sang. 2021;116(1):18–35. doi: 10.1111/vox.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N. Engl. J. Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajpai, M., Kumar, S., Maheshwari, A., et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: A pilot randomized controlled trial. medRxiv 2020: 20219337 [Preprint]. August 25, 2021 (accessed 12 Aug 2021). 10.1101/2020.10.25
- 18.O’Donnell MR, Grinsztejn B, Cummings MJ, et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J. Clin. Investig. 2021;131(13):e150646. doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horny P, Lim WS, Emberson JR, et al. On behalf of the RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19: A randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyo-Gwete T, Madzivhandila M, Makhado Z, et al. Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y.V2 (B.1.351) N. Engl. J. Med. 2021;384(22):2161–2163. doi: 10.1056/NEJMc2104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services Food and Drug Administration. Letter of Authorization, Reissuance of Convalescent Plasma EUA. 2021. https://www.fda.gov/media/141477/download (accessed 5 June 2021).
- 25.World Health Organization. WHO R&D Blueprint: COVID-19 Therapeutic Trial Synopsis. Geneva, 2020. http://www10.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdfp6. (accessed 13 May 2020).
- 26.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27(4):622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Defining Adult Overweight & Obesity. https://www.cdc.gov/obesity/adult/defining.html (accessed 23 July 2021).
- 31.Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–61 e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González, S. E., Regairaz, L., Salazar, M., et al. Timing of convalescent plasma administration and 28-day mortality for COVID-19 pneumonia. medRxiv 2021: 21250758 February 2, 2021. (Accessed 12 Aug 2021). 10.1101/2021.02.02.
- 36.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Infectious Diseases Society of America. Clarifying the Emergency Use Authorization framework for COVID-19 convalescent plasma: considerations for clinicians prepared jointly by the Infectious Diseases Society of America and AABB. November 18, 2020. https://www.idsociety.org/globalassets/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/aabb-idsa-convalescent-plasma-eua--final.pdf. (Accessed 31 Aug 2021).
- 38.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N. Engl. J. Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling, R. R., Sim, J. J. L., Tan, F. L., et al. Convalescent plasma for patients hospitalized with coronavirus disease 2019: A meta-analysis with trial sequential analysis of randomized controlled trials. Transfus Med Rev. S0887-7963(21)00056-0 (2021). [DOI] [PMC free article] [PubMed]
- 40.Axfors C, Janiaud P, Schmitt AM, et al. Association between convalescent plasma treatment and mortality in COVID-19: A collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect. Dis. 2021;21(1):1170. doi: 10.1186/s12879-021-06829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundgren JD, Grund B, Barkauskas CE, et al. On behalf of the Activ-Tico Ly- CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senefeld JW, Klassen SA, Ford SK, et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. medRxiv. 2020 doi: 10.1101/2020.11.08. [DOI] [Google Scholar]
- 43.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





