Abstract
Gallium nitride (GaN) is a key material when it comes to light-emitting diodes (LEDs) and has pushed the LED revolution in lighting and displays. The concept of down-conversion of a GaN-based blue LED offers the possibility to provide efficient generation of monochromatic, high-color purity light resulting in a highly efficient warm-white all-nitride phosphor-converted light emitting diode (pc-LED). Although the down conversion of blue light from InGaN LEDs has become a dominant technique for producing white light, there are still some technical challenges, e.g. the immiscibility of GaN and InN and the lattice mismatch between the substrate and InGaN, that have to be overcome. Here we demonstrate the doping of bulk GaN with europium, terbium and the combination of both resulting in intriguing luminescence properties, pushing the role of GaN:Eu,Tb as a chief component in future light emitting diodes. This colour tuning proves that one luminescence host can provide three colours (red, green and orange) and that even the so called “yellow gap” could be closed with a III-nitride. By using one material for all colours, it will be possible to overcome the technical challenges in building up LED devices, which will open up new capabilities for modern highly efficient phosphors.
Subject terms: Chemistry; Materials chemistry; Materials for optics; Lasers, LEDs and light sources
Introduction
Gallium nitride (GaN) has set the benchmark in the last decades when it comes to light-emitting diodes (LEDs). As a key material, it has driven the LED revolution in lighting and displays. The lighting industry with its various market segments, including automotive lighting, indoor and outdoor lighting, medical applications, lifestyle products and vertical farming has arisen from these fundamental discoveries enabled by the exploration of light's interaction with matter1,2. The first light emission from a solid-state material driven by an electric current was reported in 1907 by H.J. Round3 and this outreaching discovery paved the way of electroluminescence and highly efficient phosphors in modern LED technology. This investigation triggered further studies on the optoelectronic processes taking place in semiconductors based on the recombination of electric charges4,5. The first GaAsP LEDs was reported by Craford et al.6,7 in 1971 and 1972, but many technical challenges still were to overcome. The development of metal-organic vapour phase epitaxy (MOVPE) technique led the foundation for all future milestones to come. The final breakthrough came from the investigations of Nakamura and coworkers8–10, making p-doping of GaN and its ternary alloys (InGaN, AlGaN) easily accessible by a post-growth thermal annealing treatment and launched the invention of highly efficient blue-emitting diodes.
In 2014, the Nobel Prize in Physics was awarded to Isamu Akasaki, Hiroshi Amano and Shuji Nakamura for “the invention of efficient blue light-emitting diodes which has enabled bright and energy-saving white light sources”11. White light from LEDs can be build up combining a red, green and blue emitting semiconductor or by phosphors down-converting the emission of short wavelength emitting GaN/InGaN LEDs12–14.
One of the challenges here is the comparatively low external quantum efficiency (EQE) of the green-emitting semiconductors, because of the lattice mismatch between the substrate and InGaN, causing high defect density, immiscibility of GaN and InN15 or chemical interaction with packaging materials. As such, there is an urgent necessity of developing a solid-state material with a very narrow emission bandwidth in the green spectral region14. The concept of down conversion of a GaN-based blue LED offers also the possibility to provide efficient generation of monochromatic, high-color purity light resulting in a highly efficient warm-white all-nitride phosphor-converted light emitting diode (pc-LED). Eu2+-doped nitridosilicates and oxonitridosilicates emerged as important host lattices for phosphor-converted light-emitting diodes (pc-LEDs) due to their very high chemical and thermal stability, their very high quantum efficiency of the luminescence process and their very low thermal quenching13,16–26. Namely M2Si5N8:Eu2+ and MSi2O2N2:Eu2+ (M = alkaline earth) have been employed as highly effective red–orange (2-5-8) and yellow–green (1-2-2-2) phosphors, respectively. Even the so called “yellow gap”, neither III-nitrides nor III-phosphides had been able to close, could be bridged with (Ba,Sr)2Si5N8:Eu2+14. Just recently, new Mg-nitridosilicates emerged as next generation red phosphor materials with superior luminescence properties and exceptionally narrow red emission27–29.
Many investigations concerning MOVPE-grown GaN, GaN nanowires/ nanocrystals, epilayers/thin layers, GaN quantum dots doped with Eu or in some cases with Tb, can be found in literature27–40. (and references herein) But definitely Eu was not combined together with Tb. And as we know that there exist so many epitaxial layers and thin film studies we consciously wanted to differentiate and have used bulk material of GaN for our studies. However investigations about Eu doped GaN and several co-dopants have been made. Photoluminescence properties of Er, Eu, Tm-doped GaN thin-films prepared by solid-source molecular beam epitaxy were studied by Hömmerich et al.44. Three-color integration on Tm, Er, and Eu doped GaN electroluminescent thin films was realized by Wang and Steckl45. But here it is important to notice as well that the dopants do not have been combined and it refers about electroluminescence and not photoluminescence. Mitchell et al.46 have demonstrated that the emission from a GaN:Eu LED can be tuned from red to yellow under current injection. They describe as well the co-doping of GaN with Si and Mg where new Si-Mg related Eu complexes were observed with a significantly enhanced energy transfer efficiency. But here again it´s not about photoluminescence. Mg doping in relation with GaN:Eu has been investigated by several other groups as well47–51, as the Mg co-doping is supposed to increase the photoluminescence (PL) as well as the electroluminescence intensity. Hoang52 investigated defect physics of Eu-doped GaN using first-principles hybrid density-functional. His calculations are taking into account as well the interaction between Eu and other ions as O, Si, C, H, and Mg. But here as well Eu and Tb are not incorporated into the host material.
To cover the colour range from blue, over green and orange to red (ca. 450 to 650 nm), a combination of InGaN und AlGa(In)P is necessary. Several challenges have to be addressed here. The decrease of the external quantum efficiency versus emission wavelength around 560 nm, is termed the “yellow gap”14. The immiscibility of GaN and InN leads to a reduction in performance of InGaN-based LEDs with higher InN mole fractions14. The lattice mismatch between GaN, InN, GaAlN, AlGaP, different thermal expansion coefficients and the variations of the In/Al content decrease the luminescence performance tremendously. Down conversion of blue light from InGaN LEDs by suitable color converters, especially phosphors, has become a dominant technique for producing white light, but still some technical challenges have to be overcome. One point here are structural misfits of thin films because of the different host materials. Using one bulk material (GaN) for all colours (blue, red, green, orange) could enable therefore the next step of modern high performance GaN LEDs.
Here, we demonstrate the doping of bulk GaN with europium and terbium and the combination of both results in intriguing luminescence properties of all three doped compounds. This renders GaN:Eu,Tb53 as a prospective chief component in future light emitting diodes (LEDs).
Results and discussion
Synthesis
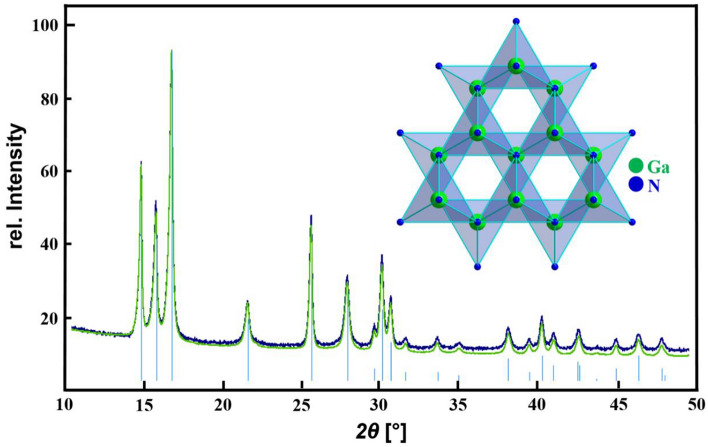
By using various synthetic approaches (e.g. low temperature urea-based method) we succeeded to co-dope GaN (Chempur 99,999%) with MCl3 × 6 H2O or M(NO3)3 × 5H2O (M = Eu, Tb) as substitutes (see Fig. 1). For a comparison of the powder diffraction pattern of GaN:Eu3+ and GaN:Tb3+ see supplement Figure S1. SEM EDX measurements of GaN confirmed the atomic ratio of Ga:N of 1:2 and an europium and terbium content of 3–5% was found, but no oxygen within the detection limit.
Figure 1.
X-ray powder diffraction patterns of undoped GaN (blue), GaN:Tb (green) and GaN ICSD [50-07920] (bright blue), (λ = 0.709026 Å), the inset shows the characteristic motif of the underlying GaN structure.
Luminescence properties
Eu3+ is considered as one of the most important activator ions with red emission corresponding to the transition 5D0–7FJ (J = 1–6). The green emission of Tb3+ is due to the transition between the emitting states of 5DJ and the excited states of 7FJ. The main intense green emission is attributed to the transition of 5D4–7F5, which is located at approx. 544 nm. A relevant point here is that normally the doping ion is inserted during the main synthesis and not afterwards. In general, doping is performed with Eu2+ and only one activator ion is used. Consequently, one host can only provide one colour. Since different aspects come into play when we think about mixing colours within one host, such an approach for colour tuning offers tremendous opportunities for highly efficient phosphors.
In this work, we doped GaN with Eu3+ and Tb3+ as activator ions, with each individually and with both simultaneously. Figure 2c shows the CIE 1931 diagram and the colours of doped GaN, proving that a colour tuning of one host with different activator ions and their combination is possible. Having a closer look at the CIE and the basics of colour mixing, it is clear that the combination of green and red leads to orange. (see Fig. 2d) This effect could be proven in the meantime for other doped nitrides and carbodiimides as well53,54.
Figure 2.
Excitation (black) and emission spectra (coloured) of GaN doped with (a) Eu3+ (red, filled hexagonal), (b) Tb3+ (green, filled star) and (d) Eu3+ and Tb3+ (orange, filled circle), (c) CIE 1931 diagram of GaN doped with Tb3+, Eu3+,Tb3+ and Eu3+.
GaN: Eu3+ shows strong characteristic emission peaks of Eu3+ within the region of 570–700 nm, indicating an energy transfer between the GaN host and the Eu3+ ions. The peaks associated with the intra-4f shell transitions (5D0–7FJ) of the Eu3+ ions, are the peaks at 576, 595, 613 and 645 nm, wherein the one at 613 nm is the strongest (see Fig. 2a). Peng et al.37assumed according to extended x-ray absorption fine structure measurements, that the Eu3+ ion substitutes the Ga site, which is in accordance with our assumption.
The excitation (monitored at 544 nm) and emission spectra of Tb3+ doped GaN are represented in Fig. 2b. The excitation spectrum exhibits a broad and intense band in the range from 290 to 380 nm with a peak at around 322 nm. This broad band is attributed to 4f8–4f75d1 transition of the Tb3+ ions. The strongest emission peak is at 544 nm with a Full Width at Half Maximum (FWHM) ~ 12 nm corresponding to the 5D4 → 7F5 transition, while the peaks at 488 nm, 584 nm and 620 nm, respectively, originate from the 5D4 → 7F6, 5D4 → 7F4 and 5D4 → 7F3 transitions of the Tb3+ ions. Until now, green emitting phospors could only be realized as oxynitrides (e.g. β-SiAlON:Eu2+55) or oxonitridosilicates (e.g. SrSi2O2N2:Eu2+56,57). The here presented luminescence behavior of GaN:Tb3+ shows that green emitting phosphors53 can be also achieved with purely nitridic compounds.
The energy level distributions of Tb3+ and Eu3+ have a large overlap and their energy transfer has been proven to be very effective58. The blue-green light of the Tb3+ transition (5D4 → 7F6,5) is emitted by polychromatic relaxation and the energy is transferred to the 5D1 and 5D0 levels of the Eu3+ by cross relaxation. The Eu3+ ions absorb the energy from Tb3+ and emit therefore orange light.
Figure 2d proves that it is possible to insert two different activator ions in one host showing the typical bands of Eu3+ as well as the ones of Tb3+ in one spectrum resulting in a saturated orange body colour. However a close look reveals that this is not only a superposition of the Eu3+- and the Tb3+-spectrum of GaN, because this orange spectrum differs clearly concerning peak form, intensity and wavelength. Here it becomes evident, that this orange colour is only possible by mixing the ions on an atomic scale and cannot be realized by a mixture of particles of the red and the green doped GaN. To prove this GaN:Eu and GaN:Tb (same molar ratio Eu:Tb as in GaN:Eu,Tb) have been mixed in a mortar. It becomes obvious that this does not result in an orange luminescent GaN. Instead the red GaN:Eu is dominant in the mixture, resulting in the same red colour coordinates of just GaN:Eu. See Figure S10. The FWHM point out the same values as in the single dopings. The line widths of the emission spectra (FWHM) of very narrow banded nitridosilicate phopshors range between 35 and 50 nm2, of nitride-based LEDs they vary typically between 20 and 35 nm and of phosphide-based ones between 15 and 25 nm12. Here we range from 8 to 12 nm for GaN:Eu3+,Tb3+ which fits perfectly to the respecting GaN spectra in literature. The CIE values of amber emitting Phosphor GaN:Eu3+/Tb3+ x,y = 0.528, 0.454 (see Fig. 2c) are very similar to those of (Ba,Sr)2Si5N8:Eu2+14(x,y = 0.579, 0.416) and are lying therefore well within the “amber box” of the SAE specifications14. This 2-5-8 nitridosilicate phosphor has been considered as an important breakthrough for bridging the “yellow gap”14, and is established nowadays in numerous optoelectronic applications.
To elucidate if the Eu,Tb ions really have been inserted into the GaN structure and the luminescence does not result from the respective doping compounds a comparison of the luminescence spectra of GaN:Eu3+ and EuCl3 × 6 H2O is shown in Figure S1. As optical and luminescence spectra are highly sensitive to structural deformation of the nearest environment of rare-earth (RE) ions, it is clearly evident that the spectrum has changed due to the insertion of the Eu cation into the structure of GaN. Furthermore, very detailed comparisons of several Eu- and Tb-doped GaN thin films and possible side products have been carried out (see supplement Figures S2-9). Here it becomes obvious that some luminescence spectra seem very similar, but when taking a closer look, they clearly differ. According to De Boer et al.31, sample growth and doping conditions play as well an important role in influencing the PL (photoluminescence) spectra.
Mott–Schottky (MS) measurements
Electrochemical Impedance Spectroscopy (EIS) is an appropriate tool to study ion diffusion and to resolve the chemical identity of the charge carriers by the use of blocking electrodes. Mott–Schottky (MS) measurements are a very sensitive technique to probe changes in the electronic band structure, i.e. charge carrier density, type of semiconducting behaviour and band edge positions, upon doping. For the elucidation of doping effect on the electronic properties of GaN, Mott-Schottky (MS) measurements were conducted for the bare and RE-doped samples. They were performed in a 0.1 M potassium phosphate electrolyte at an applied frequency of 10 Hz. As shown in Fig. 3, all of the acquired curves show a positive slope corresponding to the characteristic of n-type semiconductors59. In addition, a smaller slope for all curves can be attributed to increased charge carrier density for GaN upon doping with the rare earth metals60,61. The extrapolation of the curves allows to estimate the flat band potential as the corresponding conduction band edge of n-type semiconductor. The obtained conduction band edges reveal that RE-doped GaN yield a significant negative shift, indicating RE doping can be applied to modify the (photo)electrochemical properties of GaN61–63.
Figure 3.

Mott-Schottky (MS) analysis of Electrochemical Impedance Spectroscopy (EIS) measurements of (a) undoped GaN (blue), GaN:Tb3+ (green), GaN:Eu3+ (red) and GaN:Eu3+,Tb3+ (orange); (Measurements were performed in a 0.1 M potassium phosphate electrolyte at an applied frequency of 10 Hz). The extrapolated curves at y = 0 correspond to the conduction band edges, because the materials exhibit a positive slope that is characteristic for an n-type semiconductor. The determined flatband potentials are provided in the inset.
Conclusion
We demonstrated the doping of bulk GaN with europium and terbium and the combination of both resulting in intriguing luminescence properties of all three doped compounds (red, green, orange), rendering GaN:Eu,Tb as an prospective component in future light emitting diodes (LEDs). Our results highlight the opportunities for controlling functionality and luminescence properties of modern energy-efficient white light-emitting diodes and energy-efficient power electronic devices. Especially the closing of the “yellow gap” is a big step foreward.
The here presented methodology of doping bulk GaN applied may be generalized to create different dimensions of device architectures for LEDs, as the possibility to modify their charge transport properties by introducing dopant atoms has turned out for some time to be crucial for the performance of inorganic LEDs.
Experimental section
Doping of GaN
The doping of GaN (Chempur 99,999%) was realised by a successive combustion synthesis.
Briefly, the respective metal chlorides (MCl3·6 H2O or M(NO3)3·5H2O (M = Eu, Tb), NH4NO3, Urea and H2O were added to the sample. This mixture was put into an oven at 400–600° for 10 min. The doping, which is about 3–5%, has been checked via EDX measurements.
X-ray diffraction
Powder Diffraction
X-ray diffraction experiments on powder samples of GaN were performed on a STOE STADI P powder diffractometer in Debye–Scherrer geometry with Ge(111)-monochromatized Mo-Kα1 radiation (λ = 0.709026 Å). The sample was enclosed in a glass capillary of 0.3 mm diameter.
EDX measurements
SEM was performed on a Zeiss Merlin microscope and for EDX we used a Quantax 400 system from Bruker.
Mott–Schottky (MS) measurements
Electrodes fabrication
GaN and the corresponding rare earth metal (RE, RE = Eu3+, Tb3+ and Eu3+/Tb3+) doped semiconductor electrodes were prepared by electrophoretic deposition. Fluorine doped tin oxide (FTO) glass (2.2 mm thick, Sigma-Aldrich) was used as the substrate after sequentially ultrasonic cleaning with dilute nitric acid, acetone and ethanol for 15 min. The dispersion was prepared by mixing 5 mg iodine and 20 mg sample with 20 ml acetone, followed by treatment with ultrasounds. The electrodes were obtained after depositing the dispersed powder at 30 V and drying under ambient atmosphere.
Mott–Schottky (MS) measurements
The MS measurements were performed in an electrochemical cell using a potentiostat (Gamry instruments) operating in a three-electrode setup. The deposited samples on FTO, a 1 M Ag/AgCl electrode and a platinum wire were used as a working electrode, a reference electrode and a counter electrode, respectively. All MS data were recorded vs. E1 M Ag/AgCl (V), which was subsequently converted with respect to ERHE (V) according to the formula: ERHE (V) = 0.235 + E1 M Ag/AgCl + [0.059 × pH] (V) at 25 °C.
Luminescence
The luminescence spectra and quantum yield measurements were performed on a Fluorolog®-3 Horiba Jobin Yvon equipped with a TBX detector picosecond photon detection device and a 450 W xenon lamp.
Supplementary Information
Acknowledgements
The authors gratefully acknowledge Udo Geckle (IAM-ESS KIT Karlsruhe) for the EDX and Sabine Schlabach (INT KIT Karlsruhe) for luminescence measurements.
Author contributions
C.B. concept, investigations, writing L.M. technical help Z.C. and A.S Mott-Schottky (MS) measurements.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06148-0.
References
- 1.Ball P. Let there be light. Nat. Mater. 2015;14:453–453. [Google Scholar]
- 2.Pust P, Schmidt PJ, Schnick W. A revolution in lighting. Nat. Mater. 2015;14:454–458. doi: 10.1038/nmat4270. [DOI] [PubMed] [Google Scholar]
- 3.Round HJ. Light-emitting diodes hit the centenary milestone. Electr. World. 1907;19:309–310. [Google Scholar]
- 4.Wolff GA, Hebert RA, Broder JD. Electroluminescence of GaP. Phys. Rev. 1955;100:1144–1145. [Google Scholar]
- 5.Braunstein R. Radiative transitions in semiconductors. Phys. Rev. 1955;99:1892–1893. [Google Scholar]
- 6.Craford MG, Shaw RW, Herzog AH, Groves WO. Radiative recombination mechanisms in GaAsP diodes with and without nitrogen doping. J. Appl. Phys. 1972;43:4075–4083. [Google Scholar]
- 7.Groves WO, Herzog AH, Craford MG. The effect of nitrogen doping on GaAs1–xPx electroluminescent diodes. Appl. Phys. Lett. 1971;19:184–186. [Google Scholar]
- 8.Nakamura S, Mukai T, Senoh M, Iwasa N. Thermal annealing effects on P-type Mg-doped GaN films. Jpn. J. Appl. Phys. 1992;31:L139–L142. [Google Scholar]
- 9.Nakamura S, Mukai T. High-quality InGaN films grown on GaN films. Jpn. J. Appl. Phys. 1992;31:L1457–L1459. [Google Scholar]
- 10.Nakamura S, Mukai T, Senoh M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994;64:1687–1689. [Google Scholar]
- 11.The Nobel Prize in Physics 2014—NobelPrize.org. https://www.nobelprize.org/prizes/physics/2014/summary/.
- 12.Mueller-Mach R, Mueller GO. White-Light-Emitting Diodes for Illumination. In: Yao HW, Ferguson IT, Schubert EF, editors. Light-Emitting Diodes: Research, Manufacturing, and Applications. International Society for Optics and Photonics; 2000. pp. 30–41. [Google Scholar]
- 13.Mueller-Mach R, et al. Highly efficient all-nitride phosphor-converted white light emitting diode. Phys. Status Solidi A. 2005;202:1727–1732. [Google Scholar]
- 14.Mueller-Mach R, et al. All-nitride monochromatic amber-emitting phosphor-converted light-emitting diodes. Phys. Status Solidi - Rapid Res. Lett. 2009;3:215–217. [Google Scholar]
- 15.El-Masry NA, Piner EL, Liu SX, Bedair SM. Phase separation in InGaN grown by metalorganic chemical vapor deposition. Appl. Phys. Lett. 1998;72:40–42. [Google Scholar]
- 16.Schnick W. Solid-state chemistry with nonmetal nitrides. Angew. Chem. Int. Ed. Engl. 1993;32:806–818. [Google Scholar]
- 17.Li YQ, de With G, Hintzen HT. Luminescence of a new class of UV–blue-emitting phosphors MSi2O2–δN2+2/3δ:Ce3+(M = Ca, Sr, Ba) J. Mater. Chem. 2005;15:4492–4496. [Google Scholar]
- 18.Piao X, Horikawa T, Hanzawa H, Machida K. Characterization and luminescence properties of Sr2Si5N8:Eu2+ phosphor for white light-emitting-diode illumination. Appl. Phys. Lett. 2006;88:161908-1–161908-3. [Google Scholar]
- 19.Xie RJ, Hirosaki N. Silicon-based oxynitride and nitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 2007;8:588–600. [Google Scholar]
- 20.Xie RJ, Hirosaki N, Li Y, Takeda T. Rare-earth activated nitride phosphors: Synthesis, luminescence and applications. Materials (Basel) 2010;3:3777–3793. [Google Scholar]
- 21.Jüstel T, Nikol H, Ronda C. Neue Entwicklungen auf dem Gebiet lumineszierender Materialien für Beleuchtungs- und Displayanwendungen. Angew. Chem. 1998;110:3250–3271. [Google Scholar]
- 22.Ronda C. Luminescence. Wiley-VCH Verlag GmbH & Co. KGaA; 2007. [Google Scholar]
- 23.Schnick W. Shine a light with nitrides. Phys. Status Solidi - Rapid Res. Lett. 2009;3:1–2. [Google Scholar]
- 24.Schmidt, P. et al. Materials design and properties of nitride phosphors for LEDs—Art. no. 66690P, in Seventh International Conference on Solid State Lighting, vol. 6669, pp. P6690–P6690 (2007).
- 25.Uheda K, Shimooka S, Mikami M, Imura H, Kijima N. Synthesis and characterization of new green phosphor for white LED. Sci. Technol. 2008;2007:899–902. [Google Scholar]
- 26.Zeuner M, Pagano S, Schnick W. Nitridosilicates and oxonitridosilicates: From ceramic materials to structural and functional diversity. Angew. Chem. Int. Ed. Engl. 2011;50:7754–7775. doi: 10.1002/anie.201005755. [DOI] [PubMed] [Google Scholar]
- 27.Pust P, et al. Group (III) nitrides M[Mg2Al2N4] (M = Ca, Sr, Ba, Eu) and Ba[Mg2Ga2N4]—Structural relation and nontypical luminescence properties of Eu2+ doped samples. Chem. Mater. 2014;26:6113–6119. [Google Scholar]
- 28.Pust P, et al. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 2014;13(9):891–896. doi: 10.1038/nmat4012. [DOI] [PubMed] [Google Scholar]
- 29.Elzer E, Strobel P, Weiler V, Schmidt PJ, Schnick W. Illuminating nitridoberylloaluminates: The highly efficient red-emitting phosphor Sr2[BeAl3N5 ]:Eu2+ Chem. Mater. 2020;32:6611–6617. [Google Scholar]
- 30.Hu X, et al. Mild preparation and high fluorescence emission efficiency of europium-doped gallium nitride nanocrystals and first-principles density functional theoretical analysis of optical properties. J. Mater. Chem. C. 2017;5:7904–7910. [Google Scholar]
- 31.de Boer WDAM, et al. Optical excitation and external photoluminescence quantum efficiency of Eu3+ in GaN. Sci. Rep. 2015;4:5235. doi: 10.1038/srep05235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodiou L, et al. Optically active centers in Eu implanted, Eu in situ doped GaN, and Eu doped GaN quantum dots. J. Appl. Phys. 2009;105:043104. [Google Scholar]
- 33.Lozykowski HJ, Jadwisienczak WM, Han J, Brown IG. Luminescence properties of GaN and Al0.14Ga0.86N/GaN superlattice doped with europium. Appl. Phys. Lett. 2000;77:767–769. [Google Scholar]
- 34.Nishikawa A, Kawasaki T, Furukawa N, Terai Y, Fujiwara Y. Room-temperature red emission from a p-type/Europium-doped/n-type gallium nitride light-emitting diode under current injection. Appl. Phys. Express. 2009;2:071004. [Google Scholar]
- 35.Nakanishi Y, et al. Improvement of luminescence capability of Tb3+-related emission by AlxGa1−xN. Phys. Status Solidi. 2003;240:372–375. [Google Scholar]
- 36.Hara K, Ohtake N, Ishii K. Green emission from Tb-doped GaN grown by MOVPE. Phys. Status Solidi. 1999;216:625–628. [Google Scholar]
- 37.Peng HY, et al. Effect of optical excitation energy on the red luminescence of Eu3+ in GaN. Appl. Phys. Lett. 2005;86:051110. [Google Scholar]
- 38.Wakahara A, et al. Investigation of Tb-related green emission in group-III nitrides by time-resolved photoluminescence measurement. Phys. Status Solidi. 2008;205:56–59. [Google Scholar]
- 39.Lozykowski HJ, Jadwisienczak WM, Brown I. Visible cathodoluminescence of GaN doped with Dy, Er, and Tm. Appl. Phys. Lett. 1999;74:1129. [Google Scholar]
- 40.Lozykowski HJ, Jadwisienczak WM, Brown I. Photoluminescence and cathodoluminescence of GaN doped with Tb. Appl. Phys. Lett. 2000;76:861–863. [Google Scholar]
- 41.Mitchell B, et al. The role of donor-acceptor pairs in the excitation of Eu-ions in GaN:Eu epitaxial layers. J. Appl. Phys. 2014;115:204501. [Google Scholar]
- 42.Bang H, Morisima S, Nomura M, Yagi E. MBE growth of Eu or Tb doped GaN and its optical properties radial distance (Å-1) Jpn. J. Appl. Phys. 1998;73:378. [Google Scholar]
- 43.Nyk M, Kudrawiec R, Strek W, Misiewicz J. Synthesis and optical properties of Eu3+ and Tb3+ doped GaN nanocrystallite powders. Opt. Mater. 2006;28:767–770. [Google Scholar]
- 44.Hömmerich U, et al. Photoluminescence studies of rare earth (Er, Eu, Tm) in situ doped GaN. Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol. 2003;105:91–96. [Google Scholar]
- 45.Wang YQ, Steckl AJ. Three-color integration on rare-earth-doped GaN electroluminescent thin films. Appl. Phys. Lett. 2003;82:502–504. [Google Scholar]
- 46.Mitchell B, et al. Color-tunablility in GaN LEDs based on atomic emission manipulation under current injection. ACS Photonics. 2019;6:1153–1161. [Google Scholar]
- 47.Sekiguchi H, et al. Red-light-emitting diodes with site-selective Eu-doped GaN active layer. Jpn. J. Appl. Phys. 2013;52:08JH01. [Google Scholar]
- 48.Edwards PR, et al. Hysteretic photochromic switching (HPS) in doubly doped GaN(Mg): Eu-a summary of recent results. Materials. 2018;11:1800. doi: 10.3390/ma11101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirukawa K, et al. Isochronal annealing study of Mg-implanted p-type GaN activated by ultra-high-pressure annealing. Appl. Phys. Express. 2021;14:056501. [Google Scholar]
- 50.Matys M, et al. Mg-implanted bevel edge termination structure for GaN power device applications. Appl. Phys. Lett. 2021;118:093502. [Google Scholar]
- 51.Breckenridge MH, et al. High Mg activation in implanted GaN by high temperature and ultrahigh pressure annealing. Appl. Phys. Lett. 2021;118:022101. [Google Scholar]
- 52.Hoang K. Tuning the valence and concentration of europium and luminescence centers in GaN through co-doping and defect association. Phys. Rev. Mater. 2021;5:034601. [Google Scholar]
- 53.Braun, C. Bulk Materials of Doped Multinary Nitrides and Nitridosilicates, Their Production Method and Uses (2020).
- 54.Braun C, et al. SnCN 2: A carbodiimide with an innovative approach for energy storage systems and phosphors in modern LED technology. ChemElectroChem. 2020;7:4550–4561. [Google Scholar]
- 55.Kim DH, Ryu JH, Cho SY. Light emitting properties of SiAlON:Eu2+ green phosphor. Appl. Phys. A Mater. Sci. Process. 2011;102:79–83. [Google Scholar]
- 56.Li YQ, Delsing ACA, De With G, Hintzen HT. Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride MSi2O2-δN2+2/3δ (M = Ca, Sr, Ba): A promising class of novel LED conversion phosphors. Chem. Mater. 2005;17:3242–3248. [Google Scholar]
- 57.Bachmann V, Jüstel T, Meijerink A, Ronda C, Schmidt PJ. Luminescence properties of SrSi2O2N2 doped with divalent rare earth ions. J. Lumin. 2006;121:441–449. [Google Scholar]
- 58.Halmurat D, Yusufu T, Wang Q, He J, Sidike A. Rare earth ion Tb3+ doped natural sodium feldspar (NaAlSi3O8) luminescent properties and energy transfer. Sci. Rep. 2019;9:14637. doi: 10.1038/s41598-019-51171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lasia A. Semiconductors and Mott–Schottky Plots. In: Lasia A, editor. Electrochemical Impedance Spectroscopy and Its Applications. Springer; 2014. pp. 251–255. [Google Scholar]
- 60.O’Hayre R, Nanu M, Schoonman J, Goossens A. Mott–Schottky and charge-transport analysis of nanoporous titanium dioxide films in air. J. Phys. Chem. C. 2007;111:4809–4814. [Google Scholar]
- 61.Gelderman K, Lee L, Donne SW. Flat-band potential of a semiconductor: Using the Mott–Schottky equation. J. Chem. Educ. 2007;84:685–688. [Google Scholar]
- 62.Iandolo B, et al. Correlating flat band and onset potentials for solar water splitting on model hematite photoanodes. RSC Adv. 2015;5:61021–61030. [Google Scholar]
- 63.Hwang DW, Kim J, Park TJ, Lee JS. Mg-doped WO3 as a novel photocatalyst for visible light-induced water splitting. Catal. Lett. 2002;80:53–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.