Abstract
Pregnancy might impact immunity after SARS‐CoV‐2 infection and/or vaccination. We describe the first case of reinfection with SARS‐CoV‐2 during a pregnancy. While the mother lacked detectable antibodies 2 months after the first infection, both mother and baby had IgG antibodies at delivery. Infection did not cause any adverse pregnancy outcome.
Keywords: case report, COVID‐19, pregnancy, reinfection, SARS‐CoV‐2
1. BACKGROUND
The new coronavirus SARS‐CoV‐2 has been associated with adverse pregnancy outcome such as preeclampsia, both spontaneous and iatrogenic preterm delivery, and fetal growth restriction. Pregnant women with COVID‐19 are at increased risk for severe disease, thrombosis, and maternal mortality. 1 , 2 An increasing number of cases of vertical transmission during pregnancy have been reported. 3 While neonates born to women testing positive for SARS‐CoV‐2 during pregnancy generally do not seem to be at increased risk of adverse neonatal outcome due to the virus itself, 4 , 5 neonatal health may be affected by obstetric complications such as preterm delivery. Further, placenta pathology after maternal infection with SARS‐CoV‐2 raises concern that SARS‐CoV‐2 might lead to miscarriage, restricted fetal growth, or still‐birth in certain cases. 6
The longevity and quality of the immune response to SARS‐CoV‐2 and how well it protects the host from reinfection is not yet fully understood. 7 , 8 Studies on convalescent patients are contradictory; some indicate that 10% of patients with mild COVID‐19 never develop detectable IgG antibodies, 9 while others report that up to 50% are seronegative after 2 months 10 or that more than 90% remain seropositive at least four months. 7 , 11 A population‐based study from Denmark showed that a natural infection with SARS‐CoV‐2 led to observed protection against reinfection of approximately 80% after 6 months. 12 Now almost 2 years into the pandemic, several hundred cases of reinfection with SARS‐CoV‐2 have been described; these episodes have predominantly been mild, but in some cases the second infection has been more severe. 13
Reinfection with SARS‐CoV‐2 could be due to insufficient immune response to the primary infection, to a decline in neutralizing antibodies (NAbs) or to infection with new genetic viral variants escaping the immune response. 8 , 14 A study among healthcare workers and individuals living in care homes in England found that risk for reinfection was associated with a lack of NAbs at the time of reinfection. 15 Pregnancy is a state of altered immune state. 16 Recent data suggest that the antibody response to SARS‐CoV‐2 infection and vaccination may be less efficient during pregnancy. 15 , 17 To the best of our knowledge, no case of reinfection during pregnancy has been described and it is unknown whether the altered immune state during pregnancy is associated with increased risk of reinfection.
In summary, data on duration of immunity against SARS‐CoV‐2 after both asymptomatic and symptomatic infection, as well as after vaccination, are of especial interest for pregnant women and their caregivers. If immunity lasts shorter after infection or vaccination during pregnancy or if immunity lasts for a shorter period than the course of a normal pregnancy, women, and caregivers must be aware of the risk of reinfection or infection despite previous vaccination. We do not yet know how infection with SARS‐CoV‐2 at different time‐points during pregnancy affects clinical outcome, nor how several infectious episodes during a pregnancy might impact maternal and child health.
To the best of our knowledge, this is the first reported case of a woman reinfected with SARS‐CoV‐2 during a pregnancy, with viral sequencing confirming two different strains, as well as reports on obstetric and neonatal follow‐up, including maternal and neonatal antibody status.
2. METHODS
2.1. Case presentation
A 32‐year‐old previously healthy Caucasian woman (gravida 3, para 1), employed in the healthcare sector, developed symptoms of COVID‐19 at gestational week 10+2, in June 2020 (Figure 1).
FIGURE 1.
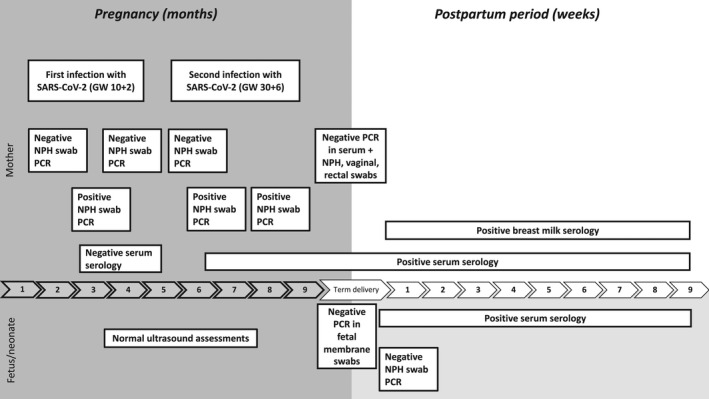
Timeline of the pregnancy and postpartum period
She had no risk factors for severe COVID‐19 except pregnancy, and had only mild symptoms (cough and runny nose). No vital signs are available for this episode as the patient did not seek care for her symptoms. At gestational week 30+6, she developed symptoms again after being exposed to a COVID‐19 patient at her workplace. This time symptoms were more pronounced: cough, runny nose, fever peaking at 38.2°C for 2 days, fatigue, and persisting dry cough for a month. However, the disease course was mild and she did not require hospitalization. The Modified early obstetric warning score (MEOWS) was 0 when she presented at gestational week 31+5 for screening regarding thrombosis risk which was clinical standard for pregnant patients with COVID‐19 at that time. The patient did not experience decreased fetal movements. According to local routines at that stage of the pandemic, the pregnancy was monitored with ultrasound scans, starting at gestational week 24+5 and subsequently every 3–4 weeks until delivery. Scans included assessment of movements and amniotic fluid, as well as Doppler assessment of flow in the umbilical artery, ductus venosus, and middle cerebral artery. Thrombosis risk was also assessed, based on medical history and coagulation tests (Table 1), and anticoagulation was not deemed necessary. An external cephalic version was performed to correct a breech presentation at gestational week 36+5. Otherwise, the pregnancy proceeded without complications.
TABLE 1.
Laboratory findings and vital signs, mother
| Time‐point | qRT‐PCR | Serology a | Other laboratory findings and vital signs |
|---|---|---|---|
| GW 8+5 | SARS‐CoV−2 IgG negative | ||
| GW 10+2 | CT 20.09 (NPH swab) | ||
| GW 17+4 |
SARS‐CoV−2 IgG negative NAbs negative |
||
| GW 21+1 | Negative (NPH swab) | ||
| GW 26+4 | Negative (NPH swab) | ||
| GW 30+6 | CT 28.52 (NPH swab) | ||
| GW 31+4 |
B‐Hb 116 g/L (117–153) B‐PLT 177 × 10*9/L (165–387) Antithrombin 0.95 kU/L (0.80–1.20) APTT 25 s (24–32) PC (INR) <0.9 (0.9–1.2) Fibrinogen 4.7 g/L (1.8–3.8) D‐Dimer (FEU) 0.70 mg/L FEU (<0.50) |
||
| GW 31+5 |
CT 32.32 (NPH swab) CT 35.81 (saliva) |
SARS‐CoV−2 IgG 102 AU/ml (≥10) |
CRP 1.3 (<3) B‐Leucocytes 9.8 × 10*9/L (3.5–8.8) B‐Neutrophils 7.6 × 10*9/L (1.8–7.5) B‐Eosinophils 0.04 × 10*9/L (0.04–0.4) B‐Basophils 0.0 × 10*9/L (0–0.1) B‐Lymphocytes 1.6 × 10*9/L (0.8–4.5) B‐Monocytes 0.6 × 10*9/L (0.1–1.0) Vital signs with MEOWS =0 Blood pressure 114/56 Heart rate 74/min Respiratory rate 15/min Oxygen saturations (without supplemental oxygen) 99% Temperature 35.5°C |
| GW 32+2 | Negative (NPH swab +saliva) | SARS‐CoV−2 IgG 60 AU/ml (≥10) |
CRP 0.5 (<3) B‐Leucocytes 8.9 × 10*9/L (3.5–8.8) B‐Neutrophils 6.2 × 10*9/L (1.8–7.5) B‐Eosinophils 0.05 × 10*9/L (0.04–0.4) B‐Basophils 0.0 × 10*9/L (0–0.1) B‐Lymphocytes 2.1 × 10*9/L (0.8–4.5) B‐Monocytes 0.6 × 10*9/L (0.1–1.0) CD4+ T‐cell reactivity against the S1 domain of SARS‐CoV−2; see Table S1 |
| GW 35+5 |
SARS‐CoV−2 IgG 76 AU/ml (≥10) NAb titer >256 |
CD4+ T‐cell reactivity against the S1 domain of SARS‐CoV−2; see Table S1 Lymphocyte composition in blood: see Table S2 |
|
| Delivery GW 37+5 | Negative (urine and NPH, vaginal, rectal, and fetal membrane swab) | SARS‐CoV−2 IgG 101 AU/ml (≥10) |
Serum SARS ‐CoV−2 S IgG and IgA positive b Serum SARS ‐CoV−2 RBD IgG and IgA positive b Serum SARS ‐CoV−2 N IgG positive and IgA negative b |
| 4 days postpartum | Negative (breast milk) | CD4+ T‐cell reactivity against the S1 domain of SARS‐CoV−2; see Table S1 | |
| 2 months postpartum | Negative (breast milk) | SARS ‐CoV−2 IgG 145 AU/ml (≥10) |
Breast milk b : SARS ‐CoV−2 S IgA positive SARS ‐CoV−2 RBD IgA positive SARS ‐CoV−2 N IgA negative Serum b : SARS ‐CoV−2 S IgA and IgG positive SARS ‐CoV−2 RBD IgA and IgG positive SARS ‐CoV−2 N IgA negative, IgG positive |
Abbreviations: APTT, activated partial thromboplastin time; B, blood; CRP, C‐reactive protein; CT, cycle threshold (<20 high viral load, >30 low viral load); GW, gestational week; Hb, hemoglobin; MEOWS, Modified early obstetric warning score; NPH, nasopharynx; PC(INR), prothrombin complex (international normalized ratio); PLT, platelet count.
All samples were analyzed using the qualitative IgG‐assay on the Architect platform. Positive samples were confirmed on the quantitative iFlash 1800 platform and reactivity is expressed as AU/ml (cut‐off 10 AU/ml). All positive samples were reactive in both assays. For some sera, neutralization was performed and expressed as antibody titer (NAb).
Serum or breast milk antibodies analyzed by the Meso Scale Discovery multiplex platform.
At gestational week 37+5, the woman presented with spontaneous contractions and gave birth to a healthy girl 5 h after admission to the delivery ward. Apgar score was 9/10/10 and blood acid‐base status normal. The birth weight was 3320 g, appropriate for gestational age, and on a higher percentile than the baby's older sibling. The third stage of labor was uncomplicated, with 165 ml blood loss. Mother and baby were discharged 24 h after delivery in good health.
At routine follow‐ups at age 90 h and 2 months, the neonate was in good health. For information regarding sample collection and laboratory methods, see appendix.
3. RESULTS
A SARS‐CoV‐2 NPH swab was positive at gestational week 10+2 with a cycle threshold (CT) value of 20.09, which is on the verge of being classified as high viral load (<20) and hence very infectious. The first SARS‐CoV‐2 antibody test (Architect), almost 2 months after the first infection, was negative, as was the NAbs test. NPH swabs at gestational weeks 21+1 and 26+4 were negative. A repeat qRT‐PCR from a NPH swab at gestational week 30+6 was positive, with a CT value of 32.32 (low viral load). One week after the second infection, a significant level of IgG antibodies was found in maternal serum with both the qualitative assay on the Architect platform, and on the quantitative iFlash platform (Table 1), as well as CD4+ T‐cell reactivity against the S1 domain of the S protein (Table S1). CD4+ T‐cell reactivity persisted during the pregnancy, and lymphocyte subsets in blood did not reveal any signs of immunodeficiency, although levels of certain CD4+ subsets and natural killer (NK) cells were slightly lowered (Table 1 and Table S2).
Sequencing of the viral genomes revealed 17 nucleotide differences between the SARS‐CoV‐2 sequences, respectively, obtained during the first and second episodes of infection. The total number of changes fulfilled the CDC criteria for reinfection (https://www.cdc.gov/coronavirus/2019‐ncov/php/invest‐criteria.html). Phylogenetic analysis showed that the first strain belonged to Pangolin clade B1.1.254 and the second to B.1.1159. Both belonged to 20B, according to the Nextclade method. The sequence of the second infection was not complete, lacking 6781 nt due to low coverage of certain areas of the genome, but it was sufficient for clade typing and comparison with the first sequence Figure 2.
FIGURE 2.
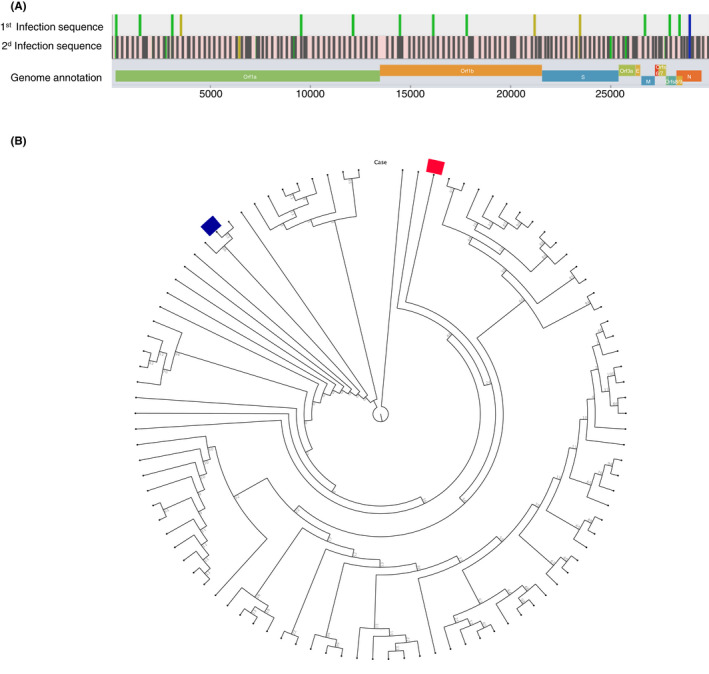
SARS‐CoV‐2 sequence analysis. (A) Differences between sequences from first and second infections. (B) The blue bar indicates the first episode and the red bar indicates the second episode of COVID‐19 in a circular cladogram tree depicting SARS‐CoV‐2 strains circulating in Sweden during 2020. The strains from the two episodes cluster with disparate strains, strongly supporting infection with two different viral strains
All ultrasound examinations showed normal fetal growth and reassuring fetal blood flow curves (Table 2).
TABLE 2.
Follow‐up of the fetus with ultrasound scans
| Expected weight a | Blood flow velocity umbilical artery | Amniotic fluid (single deepest pocket) | Biophysical profile | Blood flow velocity ductus venosus | Blood flow velocity middle cerebral artery | Blood flow velocity, uterine artery | |
|---|---|---|---|---|---|---|---|
| 24+5 | +3.1% | PI 1.3, BFC 0 | 47 | Normal | |||
| 28+6 | +4.2% | PI 1.07, BFC 0 | 70 | Normal | |||
| 33+2 | −1.7% | PI 0.93, BFC 0 | 69 | Normal | Positive A‐wave (PI 0.26) | 0.76 MoM |
Left PI 0.98, no notch Right PI 0.46, no notch |
| 35+6 | −3.8% | PI 0.81, BFC 0 | 37 | Normal | Positive A‐wave (PI 0.32) |
Abbreviations: BFC, blood flow class; MoM, multiple of the median; PI, pulsatility index.
According to Swedish fetal growth standards. 33
At delivery, qRT‐PCR in maternal serum and urine was negative, as were NPH, vagina, rectum, and fetal membrane swabs. Maternal serology at delivery and 2 months after delivery was positive for SARS‐CoV‐2 IgG and S1‐reactive CD4 T cells were still found at the latter time‐point (Table 1 and Table S1). Breast milk collected 4 days after delivery contained higher concentrations of SARS‐CoV‐2 IgA against S and RBD, compared with samples from women with negative IgG serology and no history of SARS‐CoV‐2 infection (Table 1 and Table S3). SARS‐CoV‐2 S and RBD IgA levels in breast milk and serum had declined 2 months after delivery but remained higher than in the controls. Concentrations of IgA against N were low in both breast milk and maternal serum at birth and 2 months after delivery, whereas high levels of IgG antibodies against all three antigens were detected in maternal serum at both time‐points.
Gross examination of the placenta revealed normal size and weight (416 g). The umbilical cord and membranes appeared normal. Normal placental parenchyma without inflammatory infiltrates or other focal changes were found at histological examination.
qRT‐PCR from a neonatal NPH swab at age 2 h was negative. A significant level of IgG antibodies against SARS‐CoV‐2 and high titers of Nabs were found in umbilical cord blood and in the baby's serum 90 h after delivery and at follow‐up 2 months postpartum (Table 3). Levels of IgG against SARS‐CoV‐2 S, RBD, and N in serum from the baby were comparable to those detected in maternal serum at delivery, but the S and RBD antibodies declined faster in the baby than in the mother up to 2 months postpartum. As expected, the baby's serum contained very low levels of IgA antibodies against S, RBD, and N (Table 3 and Table S3).
TABLE 3.
Laboratory findings, neonate
| Time‐point | qRT‐PCR | Serology a | Other laboratory findings |
|---|---|---|---|
| Delivery |
Negative (NPH swab) |
SARS‐CoV−2 IgG 116 AU/ml (≥10) NAb titer 256 |
Umbilical artery pH 7.28 pO2, kPa 2.78 pCO2, kPa 7.67 BE, mmol/L −1.80 Umbilical vein pH 7.29 pO2, kPa 2.84 pCO2, kPa 7.03 BE, mmol/L −2.20 Serum Sars‐CoV−2 S, RBD, N IgG positive b Serum Sars‐CoV−2 S, RBD, N IgA negative b |
| 4 days postpartum |
SARS ‐CoV−2 IgG 71 AU/ml (≥10) NAb titer >256 |
Bilirubin, conjugated 8.1 µmol/L P‐Bilirubin 92 µmol/L (<25) Serum Sars‐CoV−2 S, RBD, N IgG positive b Serum Sars‐CoV−2 S, RBD, N IgA negative b |
|
| 2 months postpartum | SARS ‐CoV−2 IgG 145 AU/ml (≥10) | Serum Sars‐CoV−2 S, RBD, N IgG positive b Serum SARS‐CoV−2 S, RBD, N IgA negative b |
Abbreviations: BE, base excess; NPH, nasopharynx.
Positive samples were confirmed on the quantitative iFlash 1800 platform and reactivity is expressed as AU/ml (cut‐off 10 AU/ml). All positive samples were reactive in both assays. For some sera, neutralization was performed and expressed as antibody titer (NAb).
Serum antibodies analyzed by the Meso Scale Discovery multiplex platform.
4. DISCUSSION
We are reporting a case of reinfection with SARS‐CoV‐2 during a pregnancy in a healthy, immunocompetent woman. The woman had mild symptoms in both episodes, but milder symptoms during the first episode, as reported in most, but not all, previous case reports on reinfection with SARS‐CoV‐2. 13 Unfortunately, the first serology test was not done earlier than 2 months after the first infection. However, the lack of NAbs at this time‐point suggests that the woman did not develop any class of detectable antibodies after the first infection. Both mother and baby had significant levels of serum IgG antibodies at delivery and at 2‐month follow‐up, and the maternal T cells reactive to the S1 SARS‐CoV‐2 domain persisted. The woman was not immunocompromised other than by the pregnancy, a well‐known state of immunomodulation entailing a switch from the Th1 cytokine profile to the Th2 profile. 16 Indeed, she had slightly decreased Th1 cell levels after the second infection, compared with healthy non‐pregnant blood donors, but that was also true for her Th2 cell levels. Two recent publications suggest that the antibody response to SARS‐CoV‐2 infection and vaccination may be less efficient during pregnancy. 17 , 18 In the current case report, there were no detectable IgG antibodies against SARS‐CoV‐2 or NAbs 2 months after the first episode of infection, which might explain the reinfection. We did not examine T‐cell activity against SARS‐CoV‐2 after the first infection, and for this reason we do not know whether memory cells formed in the absence of an antibody response. Several predisposing factors may influence the risk of reinfection. 19 Low levels of NAbs after an initial infection were associated with an increased risk of reinfection in health workers and individuals living in care homes. 15 Another potential hypothesis is that a high viral dose might penetrate the immune defense, even in the presence of adequate anti‐SARS‐CoV‐2 IgG concentrations. 20 This has previously been reported in cases of measles, where vaccinated hospital staff developed mild infections after exposure to patients with very high viral loads. 21 Non‐synonymous mutations in the S region probably impact the risk of reinfection, as they might lead to immune evasion, but studies have shown that acquired immunity most often also protects against other variants. 11 , 22 In contrast, one study reported a lack of neutralizing effect of COVID‐19 convalescence sera against viruses harboring the E484K S mutation. 23 In the case presented in this article, none of the mutations found in the SARS‐CoV‐2 genomes have been reported as immune escape variants and it is rather the absence of NAbs after the first infection that explains the susceptibility to reinfection. Notably, the mild symptoms and high levels of antibodies detected after reinfection here may indicate that while the initial infectious episode did not trigger an efficient antibody response, memory cells still formed and were able to respond with a boost response to a secondary infection, as described in non‐pregnant individuals. 15
While the majority of pregnant women with SARS‐CoV‐2 have mild symptoms without any impact on pregnancy outcome, pregnancy is a risk factor for severe COVID‐19. 1 In this patient, none of the reported complications, such as preeclampsia or thrombosis, 1 , 2 were observed.
Fetal development was normal and the baby was born healthy and with appropriate birth weight for gestational age, in a spontaneous, uncomplicated delivery when the mother was in remission. A recent systematic review by Musa et al summarized that more than 70% of the mother‐to‐child SARS‐CoV‐2 infections was likely due to environmental exposure; however, a possible vertical transmission during pregnancy was found in about 20% of the cases where the infant was infected. 3 In most cases, COVID‐19 in the pregnant woman, with or without transmission to the fetus, does not lead to severe disease in the offspring, but there are reports of increased rates of admission to the neonatal ward, need for respiratory support, phototherapy, and preterm birth. 24 A recent Swedish population‐based study found no direct risks for the neonate in cases of maternal SARS‐CoV‐2 infection during pregnancy, but neonatal outcome was impaired due to obstetric complications, mainly increased rates of preterm delivery. 5 A review by Bwire et al. reported IgG/IgM in 90% of all infants with intrauterine exposure to COVID‐19 and negative SARS‐CoV‐2 tests at birth. 25 In this case, significant amounts of IgG antibodies were also isolated from umbilical cord blood upon delivery and from blood samples at age 2 months.
In the reported case, analysis of the placenta revealed no significant histopathological abnormalities. Placentas from women with COVID‐19 during pregnancy do not display any characteristic histopathology and placental infection seems rare. 26 However, significantly more cases of fetal or maternal vascular malperfusion, delayed villous maturation, chorangiosis, and intervillous thrombi have been reported, including in women without hypertensive disease. 27 In cases of infected neonates, chronic histiocytic intervillositis and trophoblast necrosis are typical features and have been suggested to be risk factors for placental infection and vertical transmission. 28 So far, most reported cases are women with severe COVID‐19 at delivery or during the second or third trimester and several studies do not report the gestational age at infection at all.
Analysis of breast milk samples from our patient showed high levels of IgA antibodies against S and RBD, but not N, corresponding to IgA antibodies in serum collected at the same time‐points. In contrast, IgG antibodies against all three antigens were detected in serum. Several studies have found antibodies, mainly IgA, against N, S, and RBD in breast milk. 29 , 30 SARS‐CoV‐2 RNA has also been found in breast milk samples from women infected with SARS‐CoV‐2, although contamination of the samples could not be ruled out in all studies. 31 , 32 To the best of our knowledge, only one study published so far has reported an attempt, that failed, to isolate replication‐competent virus. 32
4.1. Strength and limitations
To the best of our knowledge, this is the first case of maternal reinfection with SARS‐CoV‐2 during a pregnancy. We describe a thorough clinical and immunological follow‐up of both mother and baby, including real‐time PCR analyses for SARS‐CoV‐2 in different body compartments, T‐cell reactivity in maternal blood and repeated positive serology in maternal and neonatal serum and breast milk, until 2 months postpartum. Virus whole genome sequencing was performed, verifying reinfection with a different strain.
A limitation is that antibody testing after the first episode was only performed after 2 months, which means that we cannot exclude that the woman developed low levels of antibodies after her first infection that subsequently disappeared. Moreover, we were unable to examine T‐cell activity against SARS‐CoV‐2 immediately after the first infection.
5. CONCLUSION
While symptoms were mild in both COVID‐19 episodes described in this report, pregnancy is an established risk factor for severe COVID‐19. Our case shows that reinfection with SARS‐CoV‐2 after an initial symptomatic infection with high viral load in an immunocompetent patient is possible within the course of a pregnancy. We hypothesize that the altered immune response caused by pregnancy might have impacted the risk for reinfection in this case. There are still little data on long‐term duration of immunity after infection during pregnancy or vaccination and the risk for vaccine breakthrough infection is not known. Pregnant women and their caregivers must be aware that a previous infection or vaccination might not guarantee immunity to SARS‐CoV‐2 throughout the course of a pregnancy. Further research is needed to assess who will and will not acquire persisting immunity to SARS‐CoV‐2, how pregnancy affects the risk for reinfection, how maternal immunity affects pregnancy outcome, and to what extent maternal antibodies are transferred to the baby via the placenta or breast milk.
CONFLICT OF INTERESTS
MB has received materials, for testing of reagents for SARS‐CoV‐2 reactive T cells from Miltenyi. The reagents have not been used in the work of this manuscript. All other authors have nothing to declare.
AUTHOR CONTRIBUTION
VS conceived the idea for the study. VS, YC, AE, and MG contributed with clinical data and undertook clinical care. YC performed the ultrasound scans. IMF analyzed the placenta. AE took samples from the baby at the follow‐up visits. MB and AL performed additional immunological analyses. JL developed the routine serological assays for IgG. KN performed the NAbs assay. JR performed the bioinformatical analyses. VS and AE conducted the literature search with the aid of the medical library at Sahlgrenska University Hospital. VS wrote the first draft of the manuscript. All authors contributed to writing the manuscript and approved the final version.
ETHICAL APPROVAL
The mother and the baby's other parent participated in the COPE study (approved by the Swedish Ethical Review Authority; permit numbers 2020–02189, 2020–02848, 2020–05016, 2020–06696, and 2021–00870) and they both provided written consent to publish this case report.
CONSENT
Both the mother and the other parent have provided written consent to publish this case report.
Supporting information
Tab S1‐S3
ACKNOWLEDGEMENTS
We thank the family described in this report, for permitting us to publish their case, and the midwives at the delivery ward of Sahlgrenska University Hospital, for their help with sampling of the woman and baby. We are grateful to the Swedish government, SciLifeLab Sweden, and Hjärt‐Lungfonden for funding.
1. PROCEDURES
1.1. Sampling
Maternal NPH swabs were obtained during both COVID‐19 episodes, as well as on additional occasions due to symptoms or exposure at work. At delivery, a urine sample and NPH, rectal, vaginal, and fetal membrane swabs were taken. A NPH swab was taken from the baby 2 h after delivery. At 2 months after the first infection and at several time‐points after the second infection, maternal blood samples were drawn to test for serology and complete blood count. Breast milk samples were taken on day four and 2 months after delivery.
Blood samples were collected from the umbilical cord 90 h and 2 months after delivery.
All samples were immediately sent to the Department of Clinical Microbiology, Sahlgrenska University Hospital, Gothenburg, Sweden and were stored at +4°C (max 2 days) or frozen at −20°C until analysis.
1.2. Real‐time PCR
NPH swabs were tested using the COBAS SARS‐CoV‐2 assay (Roche), as part of routine diagnostics at the Department of Clinical Microbiology, Sahlgrenska University Hospital. Additional confirmative NPH samples, breast milk, and urine, as well as fetal membrane, rectal, and vaginal swabs, were analyzed for SARS‐CoV‐2 RNA with an in‐house one‐step real‐time PCR after RNA extraction, using the total nucleic acid extraction kit on the MagnaPure LC 2.0 instrument (Roche Life Sciences).
1.3. Antibodies
IgG against SARS‐CoV‐2 nucleocapsid (N) antigen in serum samples was first analyzed with the qualitative assay on the Architect platform (Abbott), and quantification of IgG against N and spike (S) antigens, expressed as AU/ml, was performed on the iFlash 1800 platform (YHLO), both according to manufacturers' instructions.
Neutralizing antibodies titres were determined after inactivation of serum samples, diluted in serum‐free medium, with 100 TCID50 SARS‐CoV‐2 added to each well. Virus and serum dilutions were added to Vero cells in duplicate after 2‐h incubation at 37°C. After 72 h, cytopathic effect was determined, as previously described. 9 A titer ≥4 was defined as positive.
Concentrations of IgA antibodies in breast milk and serum and serum IgG against SARS‐CoV‐2 S, N and the receptor‐binding domain (RBD) of S were determined, using a multiplex electrochemiluminescence assay (Meso Scale Discovery), and analyzed on a Meso Quickplex SQ 120 reader (Meso Scale Discovery). Serum samples were analyzed at 1/5000 dilution, according to manufacturers' instructions. Breast milk samples were centrifuged at 800 g for 3 × 15 min before analysis to remove fat and analyzed at 1/5000 (anti‐S and RBD IgA) or 1/100 (anti‐N IgA) dilution. Antibody concentrations in maternal and neonatal serum and in breast milk were compared to levels in corresponding samples collected from three control women with negative SARS‐CoV‐2 IgG serology and no history of SARS‐CoV‐2 infection. Samples were defined as positive if the antibody concentration was more than fourfold higher than the highest concentrations measured in any of the control samples.
1.4. Lymphocyte characterisation
A detailed flow cytometric analysis of the blood lymphocyte concentration was performed on a FACSLyric (BD Biosciences) at the Department of Clinical Immunology and Transfusion Medicine, Sahlgrenska University Hospital. CD4 T‐cell reactivity against SARS‐CoV‐2 was tested three times by whole‐blood stimulation with peptides covering the S1 domain of the S protein of SARS‐CoV‐2 (Miltenyi Biotech), essentially as previously described. 34
1.5. Sequencing
RNA from NPH samples was extracted using the total nucleic acid extraction kit on the MagnaPure LC 2.0 instrument (Roche Life Sciences). Libraries for sequencing of RNA were prepared for the Ion AmpliSeq SARS‐CoV‐2 Research Panel, in accordance with the manufacturers' protocol (Thermo Fisher). The SuperScript VILO cDNA synthesis kit was used for RNA, reverse‐transcribed on an IonCode 96‐well PCR Plate (Thermo Fisher). Downstream library preparation was performed using the Ion AmpliSeq Kit for Chef DL8 on the Ion Chef platform (Thermo Fisher). Quantification of libraries was performed with the Ion Library TaqMan Quantification kit (Thermo Fisher). Sequence length was estimated on the Agilent 4200 TapeStation system with the High Sensitivity D1000 DNA Kit (Agilent Technologies).
Libraries for template preparation were pooled to a final concentration of 30 pM. Libraries were ligated onto spheres using the Ion 510, 520, 530 Kit‐Chef on the Ion Chef Platform (Thermo Fisher). Libraries were loaded onto the Ion 530 Chip, following clonal amplification. Sequencing was performed on the Ion Torrent S5 System (XL, Prime; Thermo Fisher) according to the manufacturer's protocol for 200‐bp read length.
1.6. Bioinformatics
Raw data were exported from the S5 System (Thermo Fisher) as fastq files. Reads were initially mapped against MN.908947 (GenBank acquisition) using the Torrent Server aligner (Thermo Fisher), BAM files were created and a consensus sequence was obtained from the IRMA report (Thermo Fisher). Consensus sequences were extracted from mappings using IRMAreport (Thermo Fischer). Nextclade, Pangolin and GISAID methods were used to assign SARS‐CoV‐2 clades for consensus sequences, respectively. Alignments and phylogenetic trees were created using the CLC Genomics Workbench (Qiagen). A selection of sequences from nationally and internationally circulating strains was downloaded from GISAID (gisaid.org) and used for phylogenetic comparison. If equal to or more than two nucleotide changes per month, the SARS‐CoV‐2 sequence of the second sample was considered to contain a separate strain (https://www.cdc.gov/coronavirus/2019‐ncov/php/invest‐criteria.html).
Sengpiel V, Carlsson Y, Liljeqvist J‐Å, et al. Confirmed reinfection with SARS‐CoV‐2 during a pregnancy: A case report. Clin Case Rep. 2022;10:e05400. doi: 10.1002/ccr3.5400
Funding information
The work was supported by ALF grants from the Swedish government and county regions [ALFGBG‐77860 and 75710 to YC, ALFGBG‐717531 to MG]; SciLifeLab Sweden [KAW 2020.0182 to MG and KN, and 2020.0015 to JL]; and Hjärt‐Lungfonden [20200411]. The funders had no role in study design, data collection, data analysis, data interpretation, or writing the report. All authors had full access to all data in the study and accept responsibility for submitting for publication. The corresponding author had the final responsibility for the decision to submit for publication
Key Clinical Message
Pregnant women and care givers need to be aware that reinfection with SARS‐CoV‐2 is possible within the course of a pregnancy. Further research is needed on the impact of pregnancy on immunity after SARS‐CoV‐2 infection and/or vaccination, as well as on antibody transfer from mother to child.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status ‐ United States, January 22‐October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musa SS, Bello UM, Zhao S, Abdullahi ZU, Lawan MA, He D. Vertical transmission of SARS‐CoV‐2: a systematic review of systematic reviews. Viruses. 2021;13(9):1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker KF, O'Donoghue K, Grace N, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG. 2020;127(11):1324‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norman M, Navér L, Söderling J. Association of maternal SARS‐CoV‐2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325(20):2076‐2086. https://pubmed.ncbi.nlm.nih.gov/33914014/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rad HS, Rohl J, Stylianou N, et al. The effects of COVID‐19 on the placenta during pregnancy. Front Immunol. 2021;12:743022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383(18):1724‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS‐CoV‐2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marklund E, Leach S, Axelsson H, et al. Serum‐IgG responses to SARS‐CoV‐2 after mild and severe COVID‐19 infection and analysis of IgG non‐responders. PLoS One. 2020;15(10):e0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel MM, Thornburg NJ, Stubblefield WB, et al. Change in antibodies to SARS‐CoV‐2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324(17):1781‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with SARS‐CoV‐2 among 4 million PCR‐tested individuals in Denmark in 2020: a population‐level observational study. Lancet. 2021;397(10280):1204‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrukh L, Mumtaz A, Sana MK. How strong is the evidence that it is possible to get SARS‐CoV‐2 twice? A systematic review. Rev Med Virol. 2021;31(5):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ringlander J, Olausson J, Nystrom K, Harnqvist T, Jakobsson HE, Lindh M. Recurrent and persistent infection with SARS‐CoV‐2 ‐ epidemiological data and case reports from Western Sweden. Infect Dis (Lond). 2020;2021:1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Jeffery‐Smith A, Rowland TAJ, Patel M, et al. Reinfection with new variants of SARS‐CoV‐2 after natural infection: a prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. 2021;2(12):e811‐e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orefice R. Immunology and the immunological response to pregnancy. Best Pract Res Clin Obstet Gynaecol. 2020;76:3‐12. https://pubmed.ncbi.nlm.nih.gov/33191116/ [DOI] [PubMed] [Google Scholar]
- 17. Atyeo C, DeRiso EA, Davis C, et al. COVID‐19 mRNA vaccines drive differential antibody Fc‐functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13(617):eabi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sherer ML, Lei J, Creisher PS, et al. Pregnancy alters interleukin‐1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol. 2021;225(3):301 e301‐301 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murillo‐Zamora E, Trujillo X, Huerta M, Rios‐Silva M, Aguilar‐Sollano F, Mendoza‐Cano O. Symptomatic SARS‐COV‐2 reinfection: healthcare workers and immunosuppressed individuals at high risk. BMC Infect Dis. 2021;21(1):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Damme W, Dahake R, van de Pas R, Vanham G, Assefa Y. COVID‐19: does the infectious inoculum dose‐response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses. 2021;146:110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundell N, Dotevall L, Sansone M, et al. Measles outbreak in Gothenburg urban area, Sweden, 2017 to 2018: low viral load in breakthrough infections. Euro Surveill. 2019;24(17):1900114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature. 2020;584(7819):115‐119. [DOI] [PubMed] [Google Scholar]
- 23. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS‐CoV‐2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130‐135. [DOI] [PubMed] [Google Scholar]
- 24. Naz S, Rahat T, Memon FN. Vertical transmission of SARS‐CoV‐2 from COVID‐19 infected pregnant women: a review on intrauterine transmission. Fetal Pediatr Pathol. 2020;40(1):80‐92. doi: 10.1080/15513815.2020.1865491. Epub 2020 Dec 27. [DOI] [PubMed] [Google Scholar]
- 25. Bwire GM, Njiro BJ, Mwakawanga DL, Sabas D, Sunguya BF. Possible vertical transmission and antibodies against SARS‐CoV‐2 among infants born to mothers with COVID‐19: a living systematic review. J Med Virol. 2020;93(3):1361‐1369. [DOI] [PubMed] [Google Scholar]
- 26. Hecht JL, Quade B, Deshpande V, et al. SARS‐CoV‐2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID‐19‐positive mothers. Mod Pathol. 2020;33(11):2092‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154(1):23‐32. doi: 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwartz DA, Morotti D. Placental pathology of COVID‐19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS‐CoV‐2. Viruses. 2020;12(11):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA against SARS‐CoV‐2 detected in human milk. iScience. 2020;23(11):101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu F, Zozaya C, Zhou Q, De Castro C, Shah PS. SARS‐CoV‐2 genome and antibodies in breastmilk: a systematic review and meta‐analysis. Arch Dis Child Fetal Neonatal Ed. 2021;106(5):514‐521. [DOI] [PubMed] [Google Scholar]
- 31. Tam PCK, Ly KM, Kernich ML, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2020;72(1):128‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chambers CD, Krogstad P, Bertrand K, et al. Evaluation of SARS‐CoV‐2 in breastmilk from 18 infected women. JAMA. 2020;324(13):1347‐1348. doi: 10.1001/jama.2020.15580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843‐848. [DOI] [PubMed] [Google Scholar]
- 34. Zaunders JJ, Munier ML, Seddiki N, et al. High levels of human antigen‐specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol. 2009;183(4):2827‐2836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1‐S3
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


