Abstract
Background:
Dementia with Lewy bodies (DLB) is one of the most common degenerative dementias. Clinical trials for individuals with DLB are increasing. We aimed to identify commonly used outcome measures for trials in DLB.
Methods:
A pragmatic literature search of PubMed and clinicaltrials.gov identified interventional studies including populations with DLB. Studies were included if they enrolled participants with DLB and met the National Institutes of Health criteria for a clinical trial. Data were collected using standardized forms. Outcome measures were categorized according to core and supportive features of DLB.
Results:
After de-duplication, 58 trials were identified. The most common cognitive outcome measures were the Mini Mental State Examination (n=24) and Cognitive Drug Research computerized Assessment System (n=5). The Clinician’s Assessment of Fluctuations was the most commonly employed measure for fluctuations (n=4). Over half of studies used the Neuropsychiatric Inventory to assess behavioral symptoms (n=31). The Unified Parkinson’s Disease Rating Scale was frequently used for motor assessment (n=23).
Conclusions and Relevance:
Clinical trial outcomes used in DLB are rarely validated in this population and some lack face validity. There is a need to validate existing scales in DLB and develop DLB-specific outcome measures.
Keywords: Dementia with Lewy bodies, Lewy body disease [MeSH term], Outcome Assessment – Health Care [MeSH term], outcome measure, clinical trials as topic [MeSH term]
INTRODUCTION
Lewy body dementia (LBD) is the second most common neurodegenerative dementia after Alzheimer disease (AD).1 LBD consists of dementia with Lewy bodies (DLB) and Parkinson disease dementia (PDD). There exist ongoing debates regarding the relationship of DLB, Parkinson disease (PD), and PDD.2,3 However, recent research found that ideal clinical trial outcomes are distinct between DLB and PDD, suggesting value to separating these two diagnoses in clinical trials.4 Outcome measures are rarely formally validated for use in DLB. It is possible that the lack of validated outcome measures is one contributor to recent trials showing no benefit (e.g. the HEADWAY-DLB study of RVT-101 [NCT02669433]). No treatments are Food and Drug Administration (FDA)-approved for individuals with DLB. Donepezil and zonisamide are approved for DLB treatment only in Japan. Developing symptomatic and disease-modifying therapies for LBD is a national clinical research priority established by the 2019 Alzheimer’s Disease–Related Dementias Summit,5 but the ideal outcome measures for such trials are yet to be established. In this setting, we aimed to identify the outcome measures used in prior DLB clinical trials in order to inform future trial planning.
METHODS
This review employed a pragmatic literature search. Pragmatic searches adapt conventional systematic review processes to take into consideration limited time or resources, typically by applying additional limits to search or eligibility criteria.6,7 In this review, we restricted the literature search to two sources (PubMed and clinicaltrials.gov) as these are likely to capture the majority of clinical trials in DLB (see Figure, Supplemental Digital Content 1, which demonstrates the selection process of publications and clinical trials in this review). Additionally, we limited key search terms to those directly related to dementia with Lewy bodies rather than using search terms related to dementia more broadly. In PubMed, the search included the terms “dementia with Lewy bodies” and “Lewy body dementia” combined with the clinical trial filter. The clinicaltrials.gov search used “dementia with Lewy bodies” as the condition/disease and study type was restricted to interventional studies. Studies were included if they (1) enrolled patients with DLB (with or without other populations) and (2) met the National Institutes of Health definition of a clinical trial (“a research study in which one or more human subjects are prospectively assigned to one or more interventions [which may include placebo or other control] to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes”).8 Open label and pilot studies were included if they met inclusion criteria. Excluded studies included case studies, case series, reviews, and studies using imaging modalities as an “intervention” or primary outcome. As this review focused on clinical trial outcome measures, biomarker outcomes (ie: imaging modalities) were not included. As this was a review of publicly available data, institutional review board approval was not required. The protocol was not registered.
Data extraction was performed by a research assistant and investigator (BP). Search results, study characteristics, and outcome measures were organized using data extraction forms in Microsoft Excel® 2016. For PubMed entries, data collected included the publication reference, trial registration, whether the manuscript reported a protocol or clinical trial results, population, study intervention, comparator, duration, and primary and secondary outcome measures. For clinicaltrials.gov entries, data extraction included the study title, registration, study status (and date assessed), population, intervention, comparator, study duration, and primary and secondary outcome measures. For studies with multiple publications, each publication was counted separately if there were different outcome measures.
Outcome measures were categorized according to the core and supportive features of DLB: dementia/cognitive impairment, other cognitive-behavioral features (e.g. fluctuations, hallucinations, depression), REM sleep behavior disorder (RBD), and parkinsonism.9 Other outcomes (e.g. quality of life, caregiver burden) were also categorized. As the review focused on identifying study outcomes, methodological quality of the clinical trials was not assessed.
RESULTS
Final searches were performed on February 27, 2020. The literature search identified 175 potentially relevant publications in PubMed and 67 potentially relevant studies on clinicaltrials.gov. After applying inclusion/exclusion criteria and removing duplicates, investigators included 58 studies in the review.
Study characteristics.
Twenty-three (40%) studies included individuals with PDD in addition to DLB. Eight (14%) included other populations such as AD, frontotemporal dementia, and Huntington disease dementia. No PubMed publications were protocol-only. Interventions (from both sources) included donepezil (8),10–17 memantine (8),18–25 rivastigmine (4),26–29 levodopa (4),30–33 nelotanserin (3),34–36 intepirdine (3),37–39 yokukansan (3),40–42 armodafinil (2),43,44 deep brain stimulation (2),45,46 nilotinib (2),47,48 and 1 each of galantamine,49 olanzapine,50 quetiapine,51 ramelton,52 zonisamide,53 tacrine,54 citalopram,55 feru-guard,56 tryptophan depletion,57 treadmill walking,58 cognitive rehabilitation,59 cognitive stimulation,60 and electroconvulsive therapy/transcranial magnetic therapy.61 The comparators were placebo (26), 8,14–18,20–22,25,26,32,33,35,36,45,48–51,55,60–65 treatment as usual,59,60, sham stimulation (2),45,46 relaxation therapy (1),59 risperidone,55 and different doses of donepezil (1),17 nelotanserin (1),36 and intepirdine (1)39. In all 4 levodopa studies, comparisons were made to patients with PD and/or PDD.30–33 One single-visit treadmill study lasted 20 minutes.58 For the remaining studies, duration ranged from 4 to 52 weeks. Outcome measures included in at least 2 studies either as primary or secondary outcomes are listed in Tables 1–4.
Table 1.
Cognitive scales used as primary or secondary outcomes in DLB clinical trials
| Primary outcome frequency | Ref. | Secondary outcome frequency | Ref. | |
|---|---|---|---|---|
| MMSE | 6 | 10,13,14,17,26,41 | 18 | 15,18,25,27,30,32,40,43,45,48–50,53,55,57,63,64,67 |
| COGDRAS | 3 | 12,27,49 | 2 | 18,32 |
| Verbal Fluency | 2 | 10,45 | 0 | n/a |
| COWAT or COWA | 1 | 54 | 4 | 24,27,43,57 |
| MoCA | 1 | 63 | 3 | 47,65,66 |
| Clinician’s Assessment of Fluctuations scale | 1 | 45 | 3 | 25,47,65 |
| ADAS-Cog (memory) | 0 | n/a | 6 | 11,24,43,47,49,65 |
| Trail Making test (executive) | 0 | n/a | 5 | 24,27,45,47,65 |
| Stroop test | 0 | n/a | 3 | 24,27,45 |
| Benton Judgement of Line Orientation (visuospatial) | 0 | n/a | 2 | 24,45 |
| Clock drawing 10 point (executive) | 0 | n/a | 2 | 24,25 |
| Digit span forward/backward | 0 | n/a | 2 | 43,57 |
| Cognitive Fluctuation Inventory | 0 | n/a | 2 | 15,63 |
| One day fluctuation assessment | 0 | n/a | 2 | 25,57 |
MMSE: Mini Mental State Examination, COGDRAS: Cognitive drug research computerised cognitive assessment system, COWAT or COWA: Controlled oral word association test measure of verbal fluency, MoCA: Montreal Cognitive Assessment, ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale
Table 4.
Additional scales used as primary or secondary outcomes in DLB clinical trials
| Outcome Measure Category | Scale | Primary outcome frequency | Ref. | Secondary outcome frequency | Ref. |
|---|---|---|---|---|---|
| ADL | UPDRS Part II | 1 | 35 | 6 | 11,34,47,48,59,65 |
| ADCS-ADL23 | 0 | N/A | 3 | 24,49,65 | |
| Caregiver burden | ZBI | 2 | 10,17 | 5 | 15,24,40,53,60 |
| NPI caregiver distress score | 0 | N/A | 2 | 13,63 | |
| Relative stress scale | 0 | N/A | 2 | 59,61 | |
| Motor symptoms | UPDRS-III | 11 | 10,13,14,21,26,30,31,35,37,53 | 12 | 11,18,24,25,32,34,47,48,52,59,63,65 |
| TUG | 0 | N/A | 4 | 47,58,65,67 | |
| Sleepiness | ESS | 2 | 33,43 | 2 | 18,66 |
ADL: Activities of Daily Living, UPDRS: Unified Parkinson’s Disease Rating Scale, ADCS-ADL23: Alzheimer’s Disease. Cooperative Study-Activities of Daily. Living, ZBI: Zarit Burden Interview, NPI: Neuropsychiatric Inventory, TUG: Timed up and go, ESS: Epworth Sleepiness Scale
Cognitive-behavioral outcome measures.
The most commonly used primary outcome measures for cognition were the Mini Mental State Examination (MMSE) and Cognitive Drug Research computerized Assessment System (COGDRAS) (Table 1). Frequently used secondary outcomes for cognition included the MMSE, Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), trail making test, and Controlled Oral Word Association Test (COWAT). Studies typically assessed cognitive fluctuations using the Clinician’s Assessment of Fluctuations scale (CAF), but the Cognitive Fluctuation Inventory (CFI) and One Day Fluctuation Assessment Scale (ODFAS) were each used in two studies (Table 1).
For behavioral symptom assessment, 53% of studies used the Neuropsychiatric Inventory (NPI) (Table 2). Studies used various NPI versions, including the original NPI with two subdomains added (sleep/nighttime behavior disorders, appetite/eating disorders) or the questionnaire form (NPI-Q). Some studies used specific domain scores as outcome measures (e.g. the “NPI-4,” including scores from the hallucinations, delusions, apathy, and agitation or dysphoria domains). Other measures of behavioral symptoms included the Unified Parkinson’s Disease Rating Scale (UPDRS) part 1, Irritability-Apathy Scale, Problem Behaviors Assessment-short form, and Hospital Anxiety and Depression Scale (Table 2).
Table 2.
Behavioral scales used as primary or secondary outcomes in DLB clinical trials
| Primary outcome frequency | Ref. | Secondary outcome frequency |
Ref. | |
|---|---|---|---|---|
| NPI (total, 12, 11, 4, plus, NH) | 9 | 10,13,14,17,26,27,41,49,56 | 22 | 11,15,18,24,27,30,32,40,43,45,47,50,53,55,60,63–67 |
| UPDRS part 1 | 0 | n/a | 3 | 47,48,65 |
| Irritability-Apathy Scale (IAS) | 0 | n/a | 2 | 47,65 |
| Problem behaviors assessment-short form | 0 | n/a | 2 | 47,65 |
| Hospital Anxiety and Depression Scale (HADS) - caregiver | 0 | n/a | 2 | 59,60 |
NPI: Neuropsychiatric Inventory, UPDRS: Unified Parkinson’s Disease Rating Scale
Sleep outcome measures.
The informant-completed Epworth Sleepiness Scale (ESS) was the most commonly used assessment of daytime sleepiness. Only two studies assessed changes in RBD frequency and severity based on a clinical evaluation (per clinicaltrials.gov; further details unavailable).34,36
Motor (parkinsonism) outcome measures.
Two studies used the newer Movement Disorder Society UPDRS (MDS-UPDRS), but analyzed the total score rather than the motor subscale independently (Table 3). The UPDRS motor subscale was almost always used for assessment of motor function in DLB clinical trials, followed by Timed Up and Go (TUG) test (Table 4).
Table 3.
Global scales used as primary or secondary outcomes in DLB clinical trials
| Primary outcome frequency | Ref | Secondary outcome frequency |
Ref | |
|---|---|---|---|---|
| Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC+) | 3 | 10,16,63 | 2 | 13,37 |
| ADCS-CGIC | 3 | 18,21,49 | 4 | 24,43,55,66 |
| UPDRS total | 0 | N/A | 3 | 27,48,53 |
| Clinician’s Global Impression of Change - In Dementia With Lewy Bodies (CGIC-DLB) Scale Score | 0 | N/A | 2 | 45,63 |
| MDS-UPDRS | 0 | N/A | 2 | 45,66 |
ADCS-CGIC: Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change, UPDRS: Unified Parkinson’s Disease Rating Scale, MDS-UPDRS: Movement Disorder Society Unified Parkinson’s Disease Rating Scale
Other outcome measures.
The most commonly used global measures of change included the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus) and Alzheimer’s Disease Assessment Scale Cognitive subscale – clinical global impression of change (ADCS-CGIC). Two studies used the Clinicians Global Impression of Change in DLB (CGIC-DLB).
Caregiver outcomes were assessed using Zarit Caregiver Burden Interview, NPI caregiver distress score, and the Relatives’ Stress Scale (Table 4).
DISCUSSION
Most outcome measures used in DLB clinical trials were developed for use in AD, PD, and/or general aging populations, with few efforts to validate these measures for DLB. Without disease-specific outcome measures, selecting optimal outcomes relies on face validity (in which a test measures the specific construct it is intended to measure) for use in DLB, test characteristics when the outcome measures are used in other populations (e.g. interrater reliability, test-retest reliability, sensitivity to change), and prior experience with use in DLB. There are advantages to using outcome measures common to existing DLB cohorts, such as the U.S.-based National Alzheimer Coordinating Center (NACC) database, DLB Consortium, and the European Union-based EU Joint Programme – Neurodegenerative Disease Research (JPND).68 The use of common measures was emphasized in the 2019 Alzheimer’s Disease–Related Dementias Summit research priorities.5 In this context, outcomes like the NPI and UPDRS may have particular benefits as their use allows comparison to existing DLB cohorts and comparison groups of AD and PD/PDD.
Cognitive outcome measures.
Cognitive outcomes are particularly challenging to select for DLB studies, due to the heterogeneity of cognitive impairment in DLB, impact of cognitive fluctuations, and outcome selection. The DLB clinical diagnostic criteria recommend neuropsychological testing covering the full range of potentially affected cognitive domains, with particular attention to the executive, attention, processing speed, and visuospatial/visuoperceptual impairments that are common in DLB.9 Current recommendations focus on measures used to assess cognition in PD, including screening tools and individual tests with executive, attention, and visuospatial tasks.69 The JPND report Level 1 recommendations for cognitive testing in Lewy body diseases included the Clinical Dementia Rating, MMSE, Montreal Cognitive Assessment (MoCA), and neuropsychological testing including the Consortium to Establish a Registry for Alzheimer’s Disease word list, degraded letter test, WAIS similarities, adaptive digit ordering, and animal fluency.68
MMSE use identified in this review is congruent with the JPND report, but it has substantial limitations given its limited coverage of commonly affected domains (i.e., lacking face validity) and mixed results in studies assessing validity and sensitivity to change in LBD.70 The MoCA, Mattis Dementia Rating Scale-2, and the Parkinson’s Disease‐Cognitive Rating Scale are recommended for cognitive screening in PD and have established reliability, validity, and sensitivity to change in PD populations.70 However, the use of screening tests to measure responsiveness to interventions has limitations based on measure design and intended use. Advantages of the COGDRAS include the use of an automated computerized approach to test attention, working memory, episodic memory, executive tasks, and motor abilities, however potential disadvantages include participant discomfort with computer testing and lack of visuospatial domain coverage (thus, lacking face validity for use in DLB).
The breadth of cognitive outcomes identified in DLB trials may reflect lack of consensus regarding optimal domains to assess when studying individuals with DLB. Attention, executive, processing speed, and visuospatial/visuoperceptual impairments are common in DLB, particularly in early disease.9 However, a recent study suggested that smaller sample sizes would be needed if using memory or language scores than visuospatial or executive scores for 2-year disease modification studies enrolling individuals with DLB.4 Furthermore, the presence of cognitive fluctuations can negatively affect performance – particularly during cognitive testing – but few trials incorporate this into study design.
Fluctuation Outcome Measures.
The relative rarity of fluctuation scale use is surprising given that fluctuations are a core DLB feature that can affect study result reliability. When last systematically reviewed, measures assessing cognitive fluctuations (the Mayo Fluctuations Scale, CAF, and ODFAS) lacked adequate testing of validity and reliability.71 Since that time, the Mayo Fluctuations Scale, previously shown to distinguish DLB from AD,72 was identified as having a sensitivity of 94% and a specificity of 71% for neurocognitive disorder with Lewy bodies in a Thai population when comparing it to blinded geriatric psychiatrists diagnoses based on the diagnostic and statistical manual of mental disorders (DSM)-5 criteria.73 The DLB Consortium, NACC LBD module, and suggested JPND protocols use the Mayo Fluctuations Scale. A study of the CAF identified near-perfect interrater reliability in the setting of severe fluctuation cognition and fair interrater reliability for presence of fluctuations. Physician ratings achieved a sensitivity for severe fluctuating cognition of 70% and specificity of 96% when comparing it with the Fluctuating Cognition item from the DLB diagnostic criteria form adapted from McKeith, et al 1996.74 Studies of these measures focus on screening rather than changes over time. The CAF, which includes frequency and duration scores, is likely better suited for assessing change over time than the 4-point Mayo Fluctuations Scale (which assesses the presence or absence of 4 symptoms). The CFI, developed in Japan and used in Japanese clinical trials, has good face/content validity and inter-rater reliability, but other validation is lacking.75
Behavior outcome measures.
The caregiver interview-based NPI is the most widely-used tool for assessing behavioral symptoms in DLB. It assesses the frequency and severity of 10–12 symptoms (depending on the version) and the degree of associated caregiver distress. The NPI-Q is a brief caregiver-completed questionnaire version that has acceptable reliability and correlation with the original NPI.76 The NPI has the advantages of: assessing a variety of behavioral symptoms, use in NACC, the DLB Consortium, and JPND protocols, and established validity, reliability, and sensitivity to change in populations outside DLB, including some studies in PDD.77 It is also a recommended scale for assessment of PD psychosis.78 However, the degree to which it is responsive to change remains unknown. Furthermore, the NPI total score does not necessarily reflect specific treatment effects, which may be diluted by little or no effect on other symptoms. There is limited evidence regarding the use of subscales as outcome measures, something commonly done in DLB studies.
Sleep outcome measures.
Even though RBD is a core feature of DLB, RBD symptoms were an outcome measure in only one trial. The measure used in that study was a clinical assessment and not a validated scale. The Mayo Sleep Questionnaire (participant & co-participant/caregiver versions) is used by the NACC LBD module and DLB Consortium, but it is designed more as a screening measure than an assessment of RBD symptoms over time. Most of the available scales for RBD are screening tools with the exception of REM Sleep Behavior Disorder Questionnaire Hong Kong (RBDQ-HK),79 which is sensitive to change over time in studies of individuals with RBD80 and PD.81 The REM Sleep Behavior Disorder Severity Scale has been studied in PD with good interrater reliability82 but requires polysomnography and longitudinal assessment is lacking. Additional scales are undergoing validation studies and may be useful for future trials.
Motor (parkinsonism) outcome measures.
The UPDRS (and more recently, the MDS-UPDRS) is the most commonly used measure of motor function in DLB, however there is limited information about the changes in motor function over time in DLB. The original UPDRS had strong clinimetric properties83 and assessment of clinically important differences 84 but also inadequate rating instructions, ambiguous text, and missing non-motor symptoms.83 These limitations led to MDS-UPDRS development.85 However, the NACC Lewy body dementia module, DLB Consortium, and JPND recommendations still reference the original UPDRS. The MDS-UPDRS may have limited precision in early/mild parkinsonism.86 A recent study using the UPDRS showed a significant difference between individuals with DLB treated with zonisamide as an adjunct to levodopa compared to those who received adjunctive placebo.53
Outcome measure selection.
This discussion focuses on which currently available measures are likely optimal, based on face validity in DLB, test characteristics in other populations, and prior experience with use in DLB. Even in the contexts for which they were designed, however, many of these outcome measures have limitations for use as clinical trial outcomes, including development as screening measures rather than longitudinal outcomes and lack of studies assessing clinically important changes. Currently there are insufficient data to support recommendations regarding the best outcome measures for DLB clinical trials, whether considering symptom-specific or global measures.
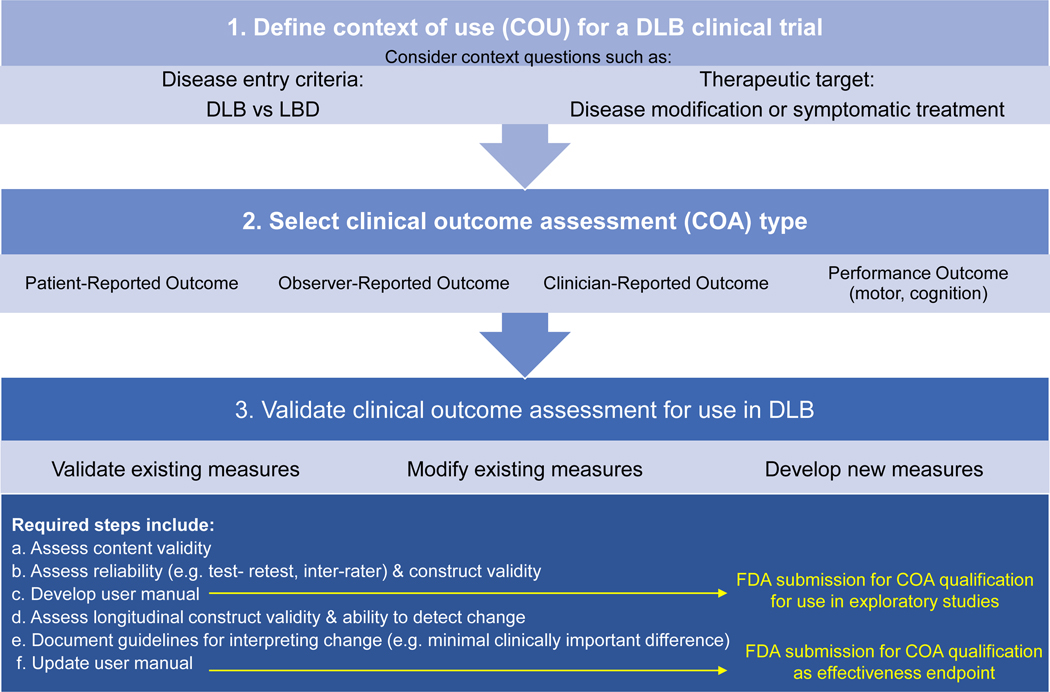
Ideal outcome measures would be validated specifically in DLB, including assessment of interrater reliability, test-retest reliability, means and standard deviations (to allow sample size calculations), and clinically important changes. It is worth considering whether existing scales truly fit the needs of DLB clinical trials or whether the best approach is to develop new DLB-specific outcome measures. The FDA has specific steps for developing and qualifying patient-focused outcome measures for use in clinical trials (Figure 1).87,88 To use, revise, or develop a clinical outcome assessment (COA), investigators must identify a context of use. In the context of DLB, investigators must decide on their population (DLB specifically or combined with PDD) and their question (e.g. disease modification vs symptomatic treatment), which impacts appropriate outcome selection or development. Investigators must decide on the type of COA desired: patient-reported, observer-reported, clinician-reported, or performance-based (e.g. motor findings, cognition). Observer (caregiver)-reported, clinician-reported, and performance-based outcomes are all represented in the reviewed studies.
Figure 1.
Steps to Clinical Outcome Measure Development in DLB.*
Legend: *Adapted and modified from the U.S. Food and Drug Administration Center for Drug Evaluation and Research Office of New Drugs
Once these key issues are decided, investigators must decide if the best approach is to validate an existing measure, modify an existing measure, or develop a new measure for use in DLB. Regardless of the approach chosen, investigators and outcome developers must assess key test characteristics both cross-sectionally and longitudinally (Figure 1). For pharmaceutical companies desiring to use clinical trials to support FDA approval, submission of COAs for FDA qualification is recommended (Figure 1). This roadmap highlights the limitations of current measures used in DLB trials, particularly with regard to longitudinal evaluation of measurement properties. For DLB clinical trials to be successful, funding agencies need to support research developing and validating DLB outcome measures including measurements of change over time.
Lacking clinical trial outcome measures are not the only clinical trial design limitation in DLB. While the DLB population is considered a single entity for current trials and this review, DLB is heterogeneous. Researchers in PD are advocating studying PD subtypes for disease modification.89 A similar approach in DLB could divide individuals with DLB into groups such as glucocerebrosidase (GBA) mutation carriers, individuals with AD co-pathology, or non-familial DLB without known co-pathology. Additionally, individuals with varying distributions of Lewy body pathology (e.g. diffuse vs. transitional, limbic vs. neocortical) have different trajectories of decline90, but biomarkers to identify these subtypes are lacking. Further studies are needed to assess the validity of the clinical diagnostic criteria for DLB and whether individuals with DLB should be studied alone or in combination with PDD. A recent study suggested that optimal trial design would split these populations.4
Another major confounding issue is cognitive fluctuations. Fluctuations affect study visit performance and obscure the ability to detect change in response to therapeutic agents, particularly on measures that require attention. The existence of fluctuations may require novel trial approaches, such as serial (or “burst”) testing over hours-days, using an average to adjust for fluctuations. This would require outcome measures with multiple versions or the ability to change the exact nature of the task (e.g. changing where the subject needs to tap on a screen). Such measures would likely need to be home-based, as many participants travel long distances to study centers. None of the trials reviewed adopted this approach.
This review identified the outcome measures used most commonly in DLB clinical trials to inform future trial planning. Authors used a pragmatic literature search of two databases with available filters, so it is possible that studies not contained in these resources were missed. Additionally, authors relied on publicly-available data. For studies posted on clinicaltrials.gov but not published in manuscript form, outcome measures (or details of outcome measures) not described on clinicaltrials.gov were not included in this review. For example, the Lewy Body Dementia Association helped modify the Scale for the Assessment of Positive Symptoms (SAPS) and SAPS-caregiver and develop of a sleep diary for use in nelotanserin clinical trials, but this information was not available in clinicaltrials.gov listings. Limitations of this review include the lack of formal assessment of content validity and assessing change within outcome measures, as this was outside the scope of this paper.
Developing symptomatic and disease-modifying therapies for LBD is a national research prioritiy.5 Most DLB clinical trials focus on treating symptoms, in contrast to AD, where more than half of studies are of potential disease-modifying interventions.91 DLB lacks any FDA-approved intervention. The ability to identify and test promising therapies, however, is constrained by a lack of outcome measures that reliably quantify symptoms in DLB and change in response to interventions. There is a need to validate existing scales for DLB-specific populations and develop DLB-specific outcome measures. Given the effort involved in measure development and validation, increased funding resources are needed to address this gap. Additionally, research is needed into other aspects of optimal DLB clinical trial design including population selection and how to mitigate the effects of cognitive fluctuations.
Supplementary Material
Acknowledgements:
The authors thank Slande Alliance (University of Florida, Department of Neurology) for her assistance in drafting the protocol and extraction of abstracts.
Conflicts of Interest and Source of Funding:
B. Patel: Dr. Patel has received a training grant from the American Brain Foundation. She received compensation for consultation with Medtronic.
D.J. Irwin: Dr. Irwin reports no disclosures.
D. Kaufer: Dr. Kaufer received research support from Acadia, Alector, Axovant, and EIP-Pharma, as well as NIH, AHRQ, the National Football League, and the Bryan Family Foundation. He has served as a consultant for Axovant and VeraSci, and receives royalties from UptoDate.
B. Boeve: Dr. Boeve has served as an investigator for clinical trials sponsored by Biogen, Alector, and EIP Pharma. He receives royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Turner Family Foundation.
A. Taylor: A. Taylor is an employee of the Lewy Body Dementia Association.
M.J. Armstrong: Dr. Armstrong is supported by an ARHQ K08 career development award (K08HS24159); receives compensation from the AAN for work as an evidence-based medicine methodology consultant and is on the level of evidence editorial board for Neurology® and related publications (uncompensated); receives publishing royalties for Parkinson’s Disease: Improving Patient Care (Oxford University Press, 2014); and has received honoraria from Medscape CME.
Funding: This project was performed in partnership with the Lewy Body Dementia Association with additional support from the University of Florida Mangurian Clinical-Research Center for Lewy Body Dementia and the Raymond E. Kassar Research Fund for Lewy Body Dementia.
References
- 1.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. Epub 2002. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, et al. Abolishing the 1-year rule: How much evidence will be enough? Mov Disord. Epub 2016. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Dickson DW, Duda JE, et al. Arguing against the proposed definition changes of PD. Mov. Disord. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smirnov D, Galasko D, Edland S, Filoteo V. Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology. Epub 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider J et al. ADRD Summit 2019 Report to the National Advisory Neurological Disorders and Stroke Council. [Google Scholar]
- 6.Martínez García L, Sanabria AJ, Araya I, et al. Efficiency of pragmatic search strategies to update clinical guidelines recommendations. BMC Med Res Methodol. Epub 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pragmatic Review. York Heal. Econ. Consort 2016. [Google Scholar]
- 8.NIH Clinical Trials Definition [online]. Accessed at: https://www.nidcd.nih.gov/research/clinical-studies/researchers-professionals/clinical-trials-definition. Accessed July 1, 2019.
- 9.McKeith IG, Boeve BF, DIckson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017. [DOI] [PubMed] [Google Scholar]
- 10.Mori E, Ikeda M, Kosaka K. Donepezil for dementia with Lewy bodies: A randomized, placebo-controlled trial. Ann Neurol. Epub 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: Preliminary findings from an open-label study. Psychiatry Clin Neurosci. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 12.Rowan E, McKeith IG, Saxby BK, et al. Effects of donepezil on central processing speed and attentional measures in Parkinson’s disease with dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord. Epub 2007. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AJ, Burn DJ, Rowan EN, et al. A comparison of the efficacy of donepezil in parkinson’s disease with dementia and dementia with lewy bodies. Int J Geriatr Psychiatry. Epub 2005. [DOI] [PubMed] [Google Scholar]
- 14.Minett TSC, Thomas A, Wilkinson LM, et al. What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson’s disease with dementia. Int J Geriatr Psychiatry. Epub 2003. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Mori E, Kosaka K, et al. Long-term safety and efficacy of donepezil in patients with dementia with lewy bodies: Results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord. Epub 2013. [DOI] [PubMed] [Google Scholar]
- 16.Eisai Co. L. A Post-Marketing Clinical Study of Aricept in Patients With Dementia With Lewy Bodies (DLB) [online]. p. clinicaltrials.gov Identifier NCT02345213. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02345213.
- 17.Mori E, Ikeda M, Nagai R, Matsuo K, Nakagawa M, Kosaka K. Long-term donepezil use for dementia with Lewy bodies: Results from an open-label extension of Phase III trial. Alzheimer’s Res Ther. Epub 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 19.Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 20.Larsson V, Engedal K, Aarsland D, Wattmo C, Minthon L, Londos E. Quality of life and the effect of memantine in dementia with Lewy bodies and Parkinson’s disease dementia. Dement Geriatr Cogn Disord. Epub 2012. [DOI] [PubMed] [Google Scholar]
- 21.Johansson C, Ballard C, Hansson O, et al. Efficacy of memantine in PDD and DLB: An extension study including washout and open-label treatment. Int J Geriatr Psychiatry. Epub 2011. [DOI] [PubMed] [Google Scholar]
- 22.Stubendorff K, Larsson V, Ballard C, Minthon L, Aarsland D, Londos E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: A prospective study. BMJ Open. Epub 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson’s disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry. Epub 2015. [DOI] [PubMed] [Google Scholar]
- 24.Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 25.Levin OS, Batukaeva LA, Smolentseva IG, Amosova NA. Efficacy and safety of memantine in Lewy body dementia. Neurosci Behav Physiol. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 26.McKeith IG, Grace JB, Walker Z, et al. Rivastigmine in the treatment of dementia with Lewy bodies: Preliminary findings from an open trial. Int J Geriatr Psychiatry. Epub 2000. [DOI] [PubMed] [Google Scholar]
- 27.McKeith I, Del Ser T, Spano PF, et al. Efficacy of rivastigmine in dementia with Lewy bodies: A randomised, double-blind, placebo-controlled international study. Lancet. Epub 2000. [DOI] [PubMed] [Google Scholar]
- 28.Wesnes KA, Mckeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with lewy bodies: A randomised placebo-controlled international study using the Cognitive Drug Research computerised assessment system. Dement Geriatr Cogn Disord. Epub 2002. [DOI] [PubMed] [Google Scholar]
- 29.Grace J, Daniel S, Stevens T, et al. Long-term use of rivastigmine in patients with dementia with Lewy bodies: An open-label trial. Int Psychogeriatrics. Epub 2001. [DOI] [PubMed] [Google Scholar]
- 30.Lucetti C, Logi C, Del Dotto P, et al. Levodopa response in dementia with lewy bodies: A 1-year follow-up study. Park Relat Disord. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 31.Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. Epub 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molloy SA, Rowan EN, O’Brien JT, McKeith IG, Wesnes K, Burn DJ. Effect of levodopa on cognitive function in Parkinson’s disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. Epub 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molloy S, Minett T, O’Brien JT, McKeith IG, Burn DJ. Levodopa use and sleep in patients with dementia with Lewy bodies. Mov Disord. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 34.A Phase 2, Double-blind, Randomized, Placebo-controlled Study of Nelotanserin Versus Placebo in Subjects With Dementia With Lewy Bodies (DLB) or Parkinson’s Disease Dementia (PDD) Who Have REM Sleep Behavior Disorder (RBD) [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02708186. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02708186. [Google Scholar]
- 35.A Phase 2, Double-blind, Randomized, Placebo-controlled Cross-over Study of Nelotanserin Versus Placebo in Lewy Body Dementia (LBD) Subjects Experiencing Visual Hallucinations (VH) [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02640729. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02640729. [Google Scholar]
- 36.An Open-label Study of Nelotanserin in Patients With Lewy Body Dementia Who Have Frequent Visual Hallucinations or REM Sleep Behavior Disorder [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02871427. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02871427. [Google Scholar]
- 37.A Phase 2b, Double-Blind, Randomized, Placebo-Controlled Study of RVT-101 in Subjects With Dementia With Lewy Bodies (DLB) [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02669433. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02669433. [Google Scholar]
- 38.A Phase 2, Double-blind, Randomized, Placebo-controlled Crossover Study Evaluating the Effect of RVT-101 on Gait and Balance in Subjects With Alzheimer’s Disease, Dementia With Lewy Bodies, or Parkinson’s Disease Dementia [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02910102. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02910102. [Google Scholar]
- 39.A Long-Term Extension Study of the Safety and Tolerability of RVT-101 in Subjects With Dementia With Lewy Bodies (DLB) [online]. Axovant Sci. Ltd. p. clinicaltrials.gov Identifier NCT02928445. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02928445. [Google Scholar]
- 40.Iwasaki K, Kosaka K, Mori H, et al. Open label trial to evaluate the efficacy and safety of yokukansan, A traditional Asian medicine, in dementia with Lewy bodies. J. Am. Geriatr. Soc. 2011. [DOI] [PubMed] [Google Scholar]
- 41.Mizukami K, Asada T, Kinoshita T, et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki K, Kosaka K, Mori H, et al. Improvement in delusions and hallucinations in patients with dementia with Lewy bodies upon administration of yokukansan, A traditional Japanese medicine. Psychogeriatrics. Epub 2012. [DOI] [PubMed] [Google Scholar]
- 43.Lapid MI, Kuntz KM, Mason SS, et al. Efficacy, Safety, and Tolerability of Armodafinil Therapy for Hypersomnia Associated with Dementia with Lewy Bodies: A Pilot Study. Dement Geriatr Cogn Disord. Epub 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attention Modulation for Treatment of Parkinson’s Disease and Dementia With Lewy Bodies [online]. New York Univ. p. clinicaltrials.gov Identifier NCT01256905. Accessed at: https://clinicaltrials.gov/ct2/show/NCT01256905. [Google Scholar]
- 45.Double Blind, Randomised, Dual Centre, Crossover, Pilot Trial of Bilateral Nucleus Basalis of Meynert Deep Brain Stimulation to Improve Cognitive Deficits in Patients With Dementia With Lewy Bodies. [online]. Univ. Coll. London; p. clinicaltrials.gov Identifier NCT02263937. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02263937. [Google Scholar]
- 46.Effects of Nucleus Basalis of Meynert Area Electrical Stimulation on Cognitive Behavioral Disorders in Dementia With Lewy Bodies : A Pilot Phase 1 Study [online]. Univ. Hosp. Rouen; p. clinicaltrials.gov Identifier NCT01340001. Accessed at: https://clinicaltrials.gov/ct2/show/NCT01340001. [Google Scholar]
- 47.Pagan F A Randomized, Double Blind, Placebo-controlled Study to Evaluate the Impact of Nilotinib Treatment on Safety, Tolerability, Pharmacokinetics and Biomarkers in Dementia With Lewy Bodies (DLB) [online]. Georg. Univ. p. clinicaltrials.gov Identifier NCT04002674. Accessed at: https://clinicaltrials.gov/ct2/show/NCT04002674. [Google Scholar]
- 48.Pagan F, Hebron M, Valadez EH, et al. Nilotinib effects in Parkinson’s disease and dementia with lewy bodies. J Parkinsons Dis. Epub 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: A 24-week open-label study. Dement Geriatr Cogn Disord. Epub 2007. [DOI] [PubMed] [Google Scholar]
- 50.Cummings JL, Street J, Masterman D, Clark WS. Efficacy of olanzapine in the treatment of psychosis in dementia with Lewy bodies. Dement Geriatr Cogn Disord. Epub 2002. [DOI] [PubMed] [Google Scholar]
- 51.Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. Epub 2007. [DOI] [PubMed] [Google Scholar]
- 52.NCT00907595. Treating Sleep/Wake Cycle Disturbances in Basal Ganglia Disorders With Ramelteon. Https://clinicaltrials.gov/show/nct00907595. Epub 2009.
- 53.Murata M, Odawara T, Hasegawa K, et al. Adjunct zonisamide to levodopa for DLB parkinsonism. Neurology. Epub 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Querfurth HW, Allam GJ, Geffroy MA, Schiff HB, Kaplan RF. Acetylcholinesterase inhibition in dementia with lewy bodies: Results of a prospective pilot trial. Dement Geriatr Cogn Disord. Epub 2000. [DOI] [PubMed] [Google Scholar]
- 55.Culo S, Mulsant BH, Rosen J, et al. Treating Neuropsychiatric Symptoms in Dementia With Lewy Bodies. Alzheimer Dis Assoc Disord. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 56.Kimura T, Hayashida H, Murata M, Takamatsu J. Effect of ferulic acid and Angelica archangelica extract on behavioral and psychological symptoms of dementia in frontotemporal lobar degeneration and dementia with Lewy bodies. Geriatr Gerontol Int. Epub 2011. [DOI] [PubMed] [Google Scholar]
- 57.Mace JL, Porter RJ, Dalrymple-Alford JC, Collins C, Anderson TJ. Acute tryptophan depletion and Lewy body dementias. Int Psychogeriatrics. Epub 2016. [DOI] [PubMed] [Google Scholar]
- 58.Immediate Effects of Treadmill Walking in Individuals With Dementia With Lewy Bodies and Huntington’s Disease [online]. Anne Kloos, Ohio State Univ. p. clinicaltrials.gov Identifier NCT02268617. Accessed at: https://clinicaltrials.gov/ct2/show/NCT02268617. [Google Scholar]
- 59.Hindle J V, Watermeyer TJ, Roberts J, et al. Goal-orientated cognitive rehabilitation for dementias associated with parkinson’s disease―a pilot randomised controlled trial. Int J Geriatr Psychiatry. Epub 2018. [DOI] [PubMed] [Google Scholar]
- 60.McCormick SA, Vatter S, Carter LA, et al. Parkinson’s-adapted cognitive stimulation therapy: feasibility and acceptability in Lewy body spectrum disorders. J Neurol. Epub 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi S, Mizukami K, Yasuno F, Asada T. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 62.A Randomized Double Blind , Placebo-controlled Study to Evaluate the Impact of Bosutinib on Safety, Tolerability, Biomarkers and Clinical Outcomes in Dementia With Lewy Bodies (DLB) [online]. Fernando Pagan MD, Georg. Univ. p. clinicaltrials.gov Identifier NCT03888222. Accessed at: https://clinicaltrials.gov/ct2/show/NCT03888222. [Google Scholar]
- 63.Placebo-Controlled A, Double-Blind, Parallel-Group, Randomized, Study To Evaluate the Efficacy, Safety and Tolerability of E2027 in Subjects With Dementia With Lewy Bodies [online]. Eisai Inc. p. clinicaltrials.gov Identifier NCT03467152. Accessed at: https://clinicaltrials.gov/ct2/show/NCT03467152. [Google Scholar]
- 64.A Randomized Double-blind, Controlled Placebo, Dose Ranging Study to Assess the Safety, Tolerability, and Efficacy of HTL0018318 in Patients With Dementia With Lewy Bodies. Heptares Ther. Ltd. p. clinicaltrials.gov Identifier NCT03592862. [Google Scholar]
- 65.Pagan F K0706 for Patients Diagnosed With Dementia With Lewy Bodies [online]. Georg. Univ. p. ClinicalTrials.gov Identifier NCT03996460. Accessed at: https://clinicaltrials.gov/ct2/show/study/NCT03996460. [Google Scholar]
- 66.Effect of LY3154207 on Cognition in Mild-to-Moderate Dementia Due to Lewy Body Dementia (LBD) Associated With Idiopathic Parkinson’s Disease (PD) or Dementia With Lewy Bodies (DLB) [online]. Eli Lilly Co. p. clinicaltrials.gov Identifier NCT03305809. Accessed at: https://clinicaltrials.gov/ct2/show/NCT03305809. [Google Scholar]
- 67.Double-Blind A, Placebo-Controlled 16-Week Study of the Cognitive Effects of Oral p38 Alpha Kinase Inhibitor Neflamapimod in Dementia With Lewy Bodies (DLB) [online]. EIP Pharma Inc; p. clinicaltrials.gov Identifier NCT04001517. Accessed at: https://clinicaltrials.gov/ct2/show/NCT04001517. [Google Scholar]
- 68.Aarsland D, Blanc F, Molllenhauer B et al. Multi-centre cohort studies in Lewy body dementia: Challenges in harmonizing different clinical and biomarker protocols. Epub 2015. [Google Scholar]
- 69.Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skorvanek M, Goldman JG, Jahanshahi M, et al. Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2018. [DOI] [PubMed] [Google Scholar]
- 71.Lee DR, Taylor JP, Thomas AJ. Assessment of cognitive fluctuation in dementia: A systematic review of the literature. Int. J. Geriatr. Psychiatry 2012. [DOI] [PubMed] [Google Scholar]
- 72.Ferman TJ, Smith GE, Boeve BF, et al. Specific features that reliably differentiate DLB from AD and normal aging. Neurology. Epub 2004. [DOI] [PubMed] [Google Scholar]
- 73.Thaipisuttikul P, Chittaropas P, Wisajun P, Jullagate S. Development and validation of a screening instrument for cognitive fluctuation in patients with neurocognitive disorder with Lewy bodies (NCDLB): The Mayo Fluctuations Scale-Thai version. Gen Psychiatry. Epub 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Dyk K, Towns S, Tatarina O, et al. Assessing Fluctuating Cognition in Dementia Diagnosis. Am J Alzheimers Dis Other Demen. Epub 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto M, Manabe Y, Mori E, Hirono N, Kosaka K, Ikeda M. Content validity and inter-rater reliability of the cognitive fluctuation inventory. Brain and Nerve. Epub 2014. [PubMed] [Google Scholar]
- 76.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. Epub 2000. [DOI] [PubMed] [Google Scholar]
- 77.Holden SK, Jones WE, Baker KA, Boersma IM, Kluger BM. Outcome Measures for Parkinson’s Disease Dementia: A Systematic Review. Mov. Disord. Clin. Pract. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez HH, Aarsland D, Fénelon G, et al. Scales to assess psychosis in Parkinson’s disease: Critique and recommendations. Mov Disord. Epub 2008. [DOI] [PubMed] [Google Scholar]
- 79.Skorvanek M, Feketeova E, Kurtis MM, Rusz J, Sonka K. Accuracy of rating scales and clinical measures for screening of rapid eye movement sleep behavior disorder and for predicting conversion to Parkinson’s disease and other synucleinopathies. Front. Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li SX, Lam SP, Zhang J, et al. A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med. Epub 2016. [DOI] [PubMed] [Google Scholar]
- 81.Kashihara K, Nomura T, Maeda T, et al. Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson’s disease - Results of a multicenter open trial. Intern Med. Epub 2016. [DOI] [PubMed] [Google Scholar]
- 82.Sixel-Döring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Intraindividual variability of REM sleep behavior disorder in Parkinson’s disease: A comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine. J Clin Sleep Med. Epub 2011. [PMC free article] [PubMed] [Google Scholar]
- 83.Goetz CC. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003. [DOI] [PubMed] [Google Scholar]
- 84.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified parkinson’s disease rating scale. Arch Neurol. Epub 2010. [DOI] [PubMed] [Google Scholar]
- 85.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. Epub 2008. [DOI] [PubMed] [Google Scholar]
- 86.Regnault A, Boroojerdi B, Meunier J, Bani M, Morel T, Cano S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J Neurol. Epub 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Food and Drug Administration. Wheel and Spokes Diagram: Clinical Outcome Assessments (text version) [online]. 2015. Accessed at: https://www.sciencedirect.com/science/article/pii/S1098301517302644?via%3Dihub. Accessed May 12, 2020.
- 88.Food and Drug Administration. Roadmap to Patient-Focused Outcome Measurement in Clinical Trials (text version) [online]. 2015. Accessed at: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/roadmap-patient-focused-outcome-measurement-clinical-trials-text-version. Accessed May 12, 2020.
- 89.Espay AJ, Kalia LV, Gan-Or Z, et al. Disease modification and biomarker development in Parkinson disease: Revision or reconstruction? Neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graff-Radford J, Aakre J, Savica R, et al. Duration and pathologic correlates of Lewy Body Disease. JAMA Neurol. Epub 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement Transl Res Clin Interv. Epub 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.