Abstract
Laboratory diagnosis of human ehrlichioses is routinely made by an indirect immunofluorescence assay (IFA) using cultured ehrlichia-infected whole cells as antigen. Concern has been raised that incorrect diagnoses of human monocytic ehrlichiosis (HME) or human granulocytic ehrlichiosis (HGE) may be made on the basis of serologic cross-reactivity between Ehrlichia chaffeensis and the agent of HGE. The present study examined whether two recombinant major outer membrane proteins, rP30 and rP44, that were previously shown to be sensitive and specific serodiagnostic antigens for HME and HGE, respectively, could be used to discriminate IFA dually reacting sera. Thirteen dually IFA-reactive sera, three sera that were IFA positive only with E. chaffeensis, and three sera that were IFA positive only with the HGE agent were examined by Western immunoblot analysis using purified whole organisms and recombinant proteins as antigens. All 16 E. chaffeensis IFA-positive sera reacted with rP30. However, none of these sera reacted with rP44, regardless of IFA reactivity with the HGE agent. The three HGE-agent-only IFA-positive sera reacted only with rP44, not with rP30. Western immunoblotting using purified E. chaffeensis and the HGE agent as antigens suggested that heat shock and other proteins, but not major outer membrane proteins, cross-react between the two organisms. Therefore, Western immunoblot analysis using rP44 and rP30 may be useful in discriminating dually HME and HGE IFA-reactive sera.
Human ehrlichioses are emerging tick-borne zoonoses caused by small, gram-negative, obligatory intracellular bacteria that are members of the genus Ehrlichia (12, 13). Since the first case of human ehrlichiosis was reported in the United States in 1987 (8), three Ehrlichia species have been demonstrated to cause human disease in the United States. Ehrlichia chaffeensis is the etiologic agent of human monocytic ehrlichiosis (HME) and infects monocytes-macrophages. The agent of human granulocytic ehrlichiosis (HGE), which was identified by a molecular method as a strain of Ehrlichia phagocytophila in 1994 (2), infects granulocytes. Human infection with Ehrlichia ewingii, another granulocytotropic ehrlichia, was first described in 1999 (1).
Most cases of HGE are reported from the northeast and upper midwestern United States, whereas HME has been reported predominantly in the south-central and southeastern quarter of the country (21). The distribution corresponds approximately to the distribution of the vector ticks, Ixodes scapularis and Amblyomma americanum, respectively. Ehrlichiae are transmitted to humans through the bite of infected ticks, which acquire the agents after feeding on infected animals. Despite the different etiologies, clinical and laboratory manifestations of the human ehrlichioses can be quite similar. The human ehrlichioses are moderate to severe illnesses but may be life threatening in some cases. Clinical manifestations include fever, malaise, headache, myalgia, rigors, arthralgia, nausea, vomiting, and diaphoresis. Rashes occur infrequently. Most patients have hematological abnormalities, such as leukopenia, thrombocytopenia, and anemia, as well as mild elevations in levels of transaminases in serum (9, 21).
The reference standard for diagnosis of HME and HGE is isolation of the organism in cell culture (culture isolation of E. ewingii has not been reported). However, few laboratories are equipped to perform routine culture isolation. Examination of Wright- or Giemsa-stained peripheral blood smears for clusters of intraleukocytic bacteria (morulae) may also assist in diagnosis. However, morulae may be sparse and extremely difficult to detect, even by experienced observers. Therefore, a negative blood smear cannot rule out ehrlichiosis. False-positive interpretations may also occur due to toxic granulations, Dohle bodies, or superimposed platelets or contaminant particles, which may be mistaken for organisms (21). PCR is another important diagnostic technique. This assay generally requires whole blood collected during the acute phase of the illness. PCR can yield false-positive results due to DNA contamination (21).
Most human ehrlichiosis cases have been diagnosed by tests that detect antibodies to the causative agents in patient serum or plasma. Serodiagnosis is sensitive, but it is often useful only for retrospective confirmation of the clinical diagnosis. Sixty percent of samples taken from patients when they first visit their physicians are serologically nondiagnostic. However, more than 80% of such patients eventually develop diagnostic levels of antiehrlichial antibodies (4, 21). Serologic methods for ehrlichiosis include indirect immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), and immunoblotting techniques using whole organisms or recombinant protein antigens as the primary or screening assays for antibodies to the HGE agent or E. chaffeensis (4–6, 10, 11, 15, 19, 20, 22, 23, 26). IFA is currently the most frequently used test (4, 21).
The concern that diagnoses made by using IFA may be incorrect because of serologic cross-reactivity between E. chaffeensis and the HGE agent has been raised (4, 21, 22). In a large study conducted by the Centers for Disease Control and Prevention (CDC, Atlanta, Ga.), of 207 patients with confirmed or probable HME, 34 (16.4%) had one or more sera reactive with the HGE agent antigen by IFA (4). Conversely, 10 (12.8%) of 78 patients with confirmed or probable HGE had one or more serum specimens reactive with E. chaffeensis antigen by IFA (4). Overall, in this series, 98 of 346 (28%) samples reactive to E. chaffeensis were also positive for the HGE agent (4). One-third of the serum samples from HGE patients in New York State have been reported to have low-titer antibodies to E. chaffeensis by IFA (22). IFA cross-reactions were more frequently detected in sera with high HGE antibody titers (22). Western immunoblot profiles typical for E. chaffeensis, HGE agent, and E. ewingii human infection have been determined in several laboratories using purified E. chaffeensis or HGE agent or recombinant outer membrane proteins as antigens (1, 3, 5, 6, 11, 15, 19, 20, 23, 25, 26).
However, sera that are highly reactive to both HME and HGE by IFA have not been examined by Western immunoblotting using both agents as antigens. The present study was carried out to investigate whether two recombinant major outer membrane proteins, rP30 and rP44, which were previously shown to be sensitive and specific serodiagnostic antigens for HME (20) and HGE (19, 25), respectively, can be used to discriminate dually IFA-reactive sera. Western immunoblotting using purified E. chaffeensis and the HGE agent as antigens was also carried out to analyze cross-reacting proteins of the two organisms.
MATERIALS AND METHODS
Organisms and antigen preparation.
E. chaffeensis (Arkansas isolate) was cultivated in DH82 cells (dog macrophage cell line) in Dulbecco's minimal essential medium (GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, Ga.) and 2 mM l-glutamine and in 10 mM N-(2-hydroxyethyl-piperazine)-N′-(4-butanesulfonic acid) buffer (GIBCO-BRL) in a humidified 37°C incubator with 5% CO2–95% air. The HGE agent (HZ isolate [14]) was cultivated in HL60 cells (human promyelocytic leukemia cell line) in RPMI 1640 medium (GIBCO-BRL) supplemented with 5% heat-inactivated fetal bovine serum (Atlanta Biologicals), 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acid mixture (GIBCO-BRL) in a humidified 37°C incubator with 5% CO2–95% air. Ehrlichial organisms were purified by Sephacryl S-1000 column chromatography as described elsewhere (16). Protein concentrations of purified E. chaffeensis, the HGE agent, and DH82 and HL60 cells were determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.), using bovine serum albumin as the standard.
Serum specimens and IFA.
A total of 47 blood specimens from MRL Reference Laboratory, Cypress, Calif., and CDC were initially screened by IFA for both HME and HGE, and 13 highly dually reactive (10 from MRL and 3 from CDC) and 3 HME-only-positive (all from CDC) IFA specimens were selected for this study. An additional 3 serum specimens were randomly selected for this study from 24 HGE IFA-positive specimens all from New York Medical College, Valhalla, N.Y. All serum samples were preabsorbed three times with pET29a-transformed Escherichia coli at 4°C overnight before use.
IFA was performed as previously described (16). E. chaffeensis-infected DH82 cells and HGE agent-infected HL60 cells were used as antigens, and fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (IgG) (Organon Teknika Co., Durham, N.C.) was used at a 1:200 dilution as a secondary antibody. Serum titrations started at a 1:20 dilution, with subsequent twofold serial dilutions.
Purification of rP30 and rP44.
rP30 and rP44 were affinity purified by using the His-Bind buffer kit (Novagen Inc., Madison, Wis.) as previously described (20, 25). The protein concentrations of rP30, rP44, and pET29a-transformed E. coli lysate control were determined by the bicinchoninic acid protein assay.
Western immunoblotting.
Western immunoblotting was performed as previously described (20). Fifteen micrograms of uninfected DH82 cell, HL-60 cell, and E. coli lysates (negative controls) and purified E. chaffeensis and HGE agent and 0.3 μg of purified rP44 and rP30 protein each were used per lane for Western immunoblot analysis. Serum were diluted at 1:100 to 1:1,000. Peroxidase-conjugated, affinity-purified anti-human IgG+IgM+IgA (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used at a 1:2,000 dilution.
RESULTS
IFA titers of 19 sera from patients with antibodies reacting with E. chaffeensis and/or the HGE agent are shown in Table 1. Geometric means of titers of 13 dually IFA-positive sera were 2,360 for E. chaffeensis and 614 for the HGE agent. Geometric means of IFA titers of 3 E. chaffeensis-positive and 3 HGE agent-only-positive sera were 266 and 213, respectively. PCR and culture results and state of residence or presumed tick exposure are also shown in Table 1. Distinct geographical distributions of HME and HGE are well established (9, 21). Overlapping of HME and HGE distribution has been seen in only a few states. Of 16 E. chaffeensis IFA-positive sera, four specimens were derived from patients with PCR- and/or culture-confirmed HME and six specimens were from locations where HME but not HGE is considered endemic. Although PCR results, culture results, and residence information are not available for eight specimens, these specimens were submitted to MRL Reference Laboratory for HME serodiagnosis, since the patients were suspected of having HME. All three HGE-only IFA-positive sera, including two sera from patients with PCR- or culture-confirmed HGE, were from a region where HGE but not HME is endemic.
TABLE 1.
IFA, Western blot, and available PCR and culture results of the samples from human ehrlichiosis patients and their states of residence
| Sample | IFA titer
|
Western immunoblotting
|
PCR (culture) result
|
State of residence or presumed tick exposure | |||||
|---|---|---|---|---|---|---|---|---|---|
| E. chaffeensis | HGE agent | Approximate molecular size(s) (kDa) of reacting antigen(s)
|
rP30 | rP44 | E. chaffeensis | HGE agent | |||
| Purified E. chaffeensis | Purified HGE agent | ||||||||
| 1 | 5,120 | 320 | 40, 46, 55, 58, 110 | 42 | +a | − | NAb | NA | NA |
| 2 | 2,560 | 1,280 | 28, 47 | 30, 45, 72 | +a | − | NA | NA | NA |
| 3 | 5,120 | 2,560 | 26, 28, 34, 42, 52 | 47, 49, 55 | +a | − | NA | NA | NA |
| 4 | 1,280 | 640 | 30, 44, 47, 110 | 49, 55, 72 | +a | − | NA | NA | NA |
| 5 | 1,280 | 640 | 44, 55 | 45, 47, 72 | +a | − | NA | NA | NA |
| 6 | 2,560 | 1,280 | 27, 28, 55, 74 | 55 | +a | − | NA | NA | NA |
| 7 | 5,120 | 320 | 23, 26, 30, 45, 55, 58, 90 | +a | − | NA | NA | NA | |
| 8 | 5,120 | 320 | 30, 40, 45, 55 | 72 | +a | − | NA | NA | NA |
| 9 | 1,280 | 640 | 45, 48, 58, 110 | 42, 47, 55 | +a | − | +c | NA | NA |
| 10 | 640 | 320 | 28, 45, 55 | +a | − | +c | NA | NA | |
| 11 | 640 | 1,280 | 45, 58 | 49, 55 | + | − | NA | NA | Arkansas |
| 12 | 2,560 | 320 | 28, 40, 44 | + | − | NA | NA | Missouri | |
| 13 | 5,120 | 320 | 44 58, 74, 90, 110 | + | − | NA | NA | Missouri | |
| 14 | 640 | 28, 32, 45, 58, 110 | + | − | NA | NA | Texas | ||
| 15 | 160 | 30, 40, 58 | + | − | +d | NA | Washington, D.C. | ||
| 16 | 160 | 40, 55, 110 | + | − | +(+)d | NA | Florida | ||
| 17 | 160 | 47 | − | + | NA | −e | New York | ||
| 18 | 320 | 49 | − | + | NA | +e | New York | ||
| 19 | 160 | 44, 47, 49 | − | + | NA | − (+)e | New York | ||
Data from reference (20).
NA, not available.
Performed at MRL Reference Laboratory.
Performed at CDC.
Performed at New York Medical College.
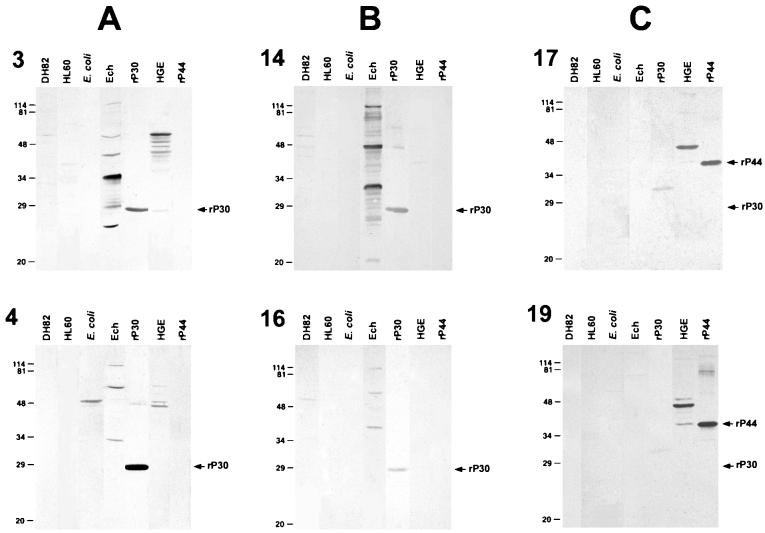
Western blot results for two representative serum samples from each of the three groups (dually positive, HME-only positive, and HGE-only positive) are shown in Fig. 1. Western immunoblot analysis showed that all 16 E. chaffeensis IFA-positive sera had the reaction profile typical of HME (3, 15, 20) using purified E. chaffeensis and rP30 (27-kDa fusion protein) as antigens (Table 1 and Fig. 1). All three HGE-only IFA-positive sera had the reaction profile typical of HGE (5, 11, 19, 25, 26) using purified HGE agent and rP44 (35-kDa fusion protein) as antigens (Table 1 and Fig. 1). These data combined with the background information indicate that all 13 dually IFA-reactive sera in this study were from HME patients.
FIG. 1.
Western immunoblot analysis of representative serum samples. (A) dually E. chaffeensis and HGE agent IFA-positive sera 3 and 4 (Table 1); (B) E. chaffeensis IFA-positive, HGE agent IFA-negative sera 14 and 16; (C) E. chaffeensis IFA-negative, HGE agent IFA-positive sera 17 and 19. DH82 cells, HL60 cells, and pET29-transformed E. coli were negative controls. Ech, purified E. chaffeensis; HGE, purified HGE agent; rP30, affinity-purified recombinant fusion protein of E. canis; rP44, affinity-purified recombinant fusion protein of the HGE agent. Antigens (15 μg of DH82 cells, E. coli, purified E. chaffeensis, and the HGE agent and 0.3 μg of rP30 and rP44) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Numbers on the left are molecular masses (in kilodaltons) based on broad-range prestained standards (Bio-Rad Laboratories, Richmond, Calif.).
Nine of 13 dually E. chaffeensis and HGE agent IFA-positive sera reacted with one to three bands of the purified HGE agent antigen by Western blot analysis (Table 1; Fig. 1). Seven of these nine (78%) reacted with the 42- to 49-kDa HGE agent antigen, and 8 of 13 (89%) reacted with 55-kDa and/or 72-kDa proteins of the HGE agent. Although molecular masses of 42 to 47 kDa correspond to the P44s major outer membrane proteins of the HGE agent (5, 11, 19, 25, 26), none of 16 E. chaffeensis IFA-positive sera reacted with rP44, regardless of HGE agent IFA reactivity. Although none of the sera reactive with only the HGE agent by IFA reacted to rP30 (27-kDa fusion protein), the two sera weakly reacted to an ∼32-kDa protein contaminating the rP30 preparation (Fig. 1).
Reactivities of 16 sera to uninfected DH82 and HL60 cells and pET29a-transformed E. coli as the antigen were insignificant. This suggests that Western blot cross-reactivity of HME sera with HGE agent is to HGE agent proteins and not to host cells used for cultivation of these antigens, E. coli, or the plasmid vector. In conclusion, serologic cross-reactivities of HME sera to the HGE agent were directed to 42- to 49-kDa, 55-kDa, and 72-kDa proteins of the HGE agent as determined by immunoblot analysis.
DISCUSSION
Because as many as 28% of serum samples from patients with human ehrlichioses cross-react with the HGE agent and E. chaffeensis by IFA (4, 10, 22), an improved serologic test that can discriminate between these two infections is desirable, particularly in regions where vectors overlap and epidemiologic information indicates that such infections are likely (21). Western immunoblot analysis is generally thought to be more specific than IFA, since it provides information about specific reactive antigens. Western blot studies performed with rabbit, dog, and human antisera to purified E. chaffeensis organisms revealed more than 20 bands ranging from 20 to 200 kDa (3, 15). Western immunoblot studies identified multiple bands of 23- to 30-kDa major outer membrane proteins encoded by a multigene family and a 120-kDa protein as candidate antigens for sensitive and specific serodiagnosis (20, 21, 23). In the present study, 10 (63%) and 6 (38%) of 16 (4 confirmed and 12 suspected) HME sera reacted with native 23- to 30-kDa and 110-kDa antigens of E. chaffeensis. The 110-kDa antigen may correspond to the 120-kDa antigen found by Yu et al. (23). Serologic tests using the recombinant 120-kDa or rp30 protein as the antigen have been developed (20, 23). Western immunoblot analysis of 27 E. chaffeensis IFA-reactive sera showed high sensitivity and specificity using rP30 as antigen (20). The present study found that all 16 E. chaffeensis IFA-positive sera were reactive to rP30 but not to rP44. Based on these data combined with epidemiologic and available PCR and culture isolation data, most likely all 13 dually IFA-reactive sera in this study were from HME patients. The problem of IFA cross-reacting antigens has been previously observed with E. ewingii and E. chaffeensis or Ehrlichia canis. Western blot analysis with purified whole organisms or rP30 has been also used to distinguish between these infections (1, 15, 16).
The 42- to 49-kDa and 72-kDa major antigen profiles of the outer membrane protein fractions of whole organisms for six HGE agent isolates have been found to be similar (26). Consistent reactivity of HGE patients' sera with the 42- to 49-kDa major outer membrane proteins of the multigene family of the HGE agent suggests that this protein is useful for serodiagnosis (5, 11, 25, 26). Western immunoblot analysis using rP44 that includes the N-terminal regions conserved among all members of the P44 protein family (25) showed that 100% of 20 HGE IFA-positive sera originating from a region where HGE but not HME is endemic were positive. Five of these sera were derived from culture isolation, and eight were from PCR-positive HGE patients (25). Sera from 14 patients with culture- and/or PCR-confirmed HGE were positive by the rP44-based ELISA (19). IFA-negative sera examined in many of these studies confirmed that these Western blot or ELISA reactions using native and recombinant antigens are specific to sera from patients infected with either HME or HGE agent but control specimens from noninfected patients do not react.
When both E. chaffeensis and HGE agent were used as antigens, it became evident that some specimens from patients with one infection appeared to react to antigens from the other by IFA (4, 14, 21, 22). However, samples with dual reactivity with E. chaffeensis and the HGE agent have not previously been studied by Western immunoblot analysis using both ehrlichia agents as antigens. In the present study, 69% (9 of 13) of dually IFA-reactive sera reacted with 42- to 49-kDa and 55- or 72-kDa proteins of the HGE agent. These molecular masses correspond to the 44-kDa major outer membrane protein of the HGE agent and heat shock proteins (HSP), respectively (24–26). The lack of reactivity of these sera to rP44 by Western blotting indicates that there are other proteins with sizes similar to that of the P44s major outer membrane protein in the HGE agent that react to some HME sera. The identities of these proteins are unknown.
Bacterial infections often induce production of cross-reactive antibodies to bacterial HSP. The 55-kDa HSP60 homologue appears to be a highly immunoreactive antigen common among Ehrlichia spp., including Ehrlichia sennetsu, E. chaffeensis, the HGE agent, Ehrlichia risticii, and E. canis (24). It was suggested that HSP60 and its homologous antigens might function as a common protective antigen in the immune response against bacterial infections because of their high immunoreactivity (7). The E. coli GroEL homolog in the 55- to 65-kDa range has been called a common bacterial antigen and is related to the eukaryotic HSP60 family (17, 18). GroELs with 76.4% amino acid sequence identity were found in the HGE agent and E. chaffeensis (18). We previously reported a patient with HGE (culture isolation and PCR positive) whose serum was highly IFA cross-reactive with E. chaffeensis antigen and had a strong reaction to a 55-kDa antigen by Western immunoblot analysis (26). A recent study investigating serologic cross-reactions among Ehrlichia equi, E. phagocytophila, and the HGE agent showed that most sera reacted with antigens between approximately 56 and 75 kDa, presumably to HSP (5). Therefore, cross-reactivity between E. chaffeensis and HGE agent is probably due to HSP and 42- to 49-kDa proteins of the HGE agent other than the major outer membrane protein P44.
Although the use of purified antigens provides information on molecular sizes of reacting proteins and the typical patterns of reaction are distinct between HME and HGE, cross-reactivity of patient sera with antigens of E. chaffeensis and the HGE agent by Western blotting may preclude definitive determination of the specific ehrlichial agent. Previous studies showed that anti-E. chaffeensis sera do not react with rP44. However, sera from patients with HGE have antibodies that specifically recognize rP44 (25). In the present study, 16 sera (including 13 cross-reacting sera that reacted with rP30) did not react with rP44. Samples reactive only with the HGE agent by IFA reacted only with rP44, not with rP30. Therefore, the use of rP44 as an antigen would provide better differentiation of HME from HGE than purified whole HGE organisms. Although dually IFA-reactive HGE-confirmed sera such as the one previously described by us (14) were not included in the present study, use of rP44 and rP30 as antigens is expected to help in ascertaining the true identity of dually HME and HGE IFA-reactive sera. In this context, Western blotting and the use of recombinant major outer membrane proteins can be helpful in discriminating etiologic agents of ehrlichioses. For clinical application, a Western blot strip containing both rP30 and rP44 can be used to facilitate a time- and cost-effective analysis. Although the serologic test would not replace the PCR or culture isolation, it is the most convenient test for HME and HGE diagnosis. An improved serologic test using recombinant antigens would have enhanced utility.
ACKNOWLEDGMENTS
This study was supported by R01 AI40934 and R01AI47407 from the National Institutes of Health. A. Unver is the recipient of Turkish government fellowship.
REFERENCES
- 1.Buller R S, Arens M, Hmiel S P, Paddock C D, Sumner J W, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian F A, Liddell A M, Schmulewitz N, Storch G A. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 2.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S M, Dumler J S, Feng H M, Walker D H. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–58. [PubMed] [Google Scholar]
- 4.Comer J A, Nicholson W L, Olson J G, Childs J E. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumler J S, Asanovich K M, Bakken J S, Richter P, Kimsey R, Madigan J E. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic Ehrlichia. J Clin Microbiol. 1995;33:1098–1103. doi: 10.1128/jcm.33.5.1098-1103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijdo J W, Wu C, Magnarelli L A, Fikrig E. Serodiagnosis of human granulocytic ehrlichiosis by a recombinant HGE-44-based enzyme-linked immunosorbent assay. J Clin Microbiol. 1999;37:3540–3544. doi: 10.1128/jcm.37.11.3540-3544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann S H E. Heat shock proteins and immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 8.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–857. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 9.McQuiston J H, Paddock C D, Holman R C, Childs J E. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson W L, Comer J A, Sumner J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravyn M D, Goodman J L, Kodner C B, Westad D K, Coleman L A, Engstrom S M, Nelson C M, Johnson R C. Immunodiagnosis of human granulocytic ehrlichiosis by using culture-derived human isolates. J Clin Microbiol. 1998;36:1480–1488. doi: 10.1128/jcm.36.6.1480-1488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rikihisa Y. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1999;1:367–376. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 14.Rikihisa Y, Zhi N, Wormser G P, Wen B, Horowitz H W, Hechemy K E. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York State. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 15.Rikihisa Y, Ewing S A, Fox J C. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rikihisa Y, Ewing S A, Fox J C, Siregar A G, Pasaribu F H, Malole M B. Enzyme-linked immunosorbent assay and Western immunoblot analysis of Ehrlichia canis and canine granulocytic Ehrlichia. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumner J W, Sims K G, Jones D C, Anderson B E. Ehrlichia chaffeensis expresses an immunoreactive protein homologous to the Escherichia coli GroEL protein. Infect Immun. 1993;61:3536–3539. doi: 10.1128/iai.61.8.3536-3539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima T, Zhi N, Lin Q, Rikihisa Y, Horowitz H W, Raffalli J, Wormser G P, Hechemy K. Enzyme-linked immunosorbent assay using major outer membrane protein of the human granulocytic ehrlichiosis agent. Clin Diagn Lab Immunol. 2000;7:652–657. doi: 10.1128/cdli.7.4.652-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unver A, Rikihisa Y, Ohashi N, Cullman L C, Buller R, Storch G A. Western and dot blotting assay analysis of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J Clin Microbiol. 1999;37:3888–3895. doi: 10.1128/jcm.37.12.3888-3895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker D H the Task Force on Consensus Approach for Ehrlichiosis. Diagnosing human ehrlichioses: current status and recommendations. ASM News. 2000;66:287–290. [Google Scholar]
- 22.Wong S J, Brady G S, Dumler J S. Serological responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York State. J Clin Microbiol. 1997;35:2198–2205. doi: 10.1128/jcm.35.9.2198-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X-J, Crocquet-Valdes P, Cullman L C, Walker D H. The recombinant 120-kDa protein of E. chaffeensis, a potential diagnostic tool. J Clin Microbiol. 1996;34:2853–2855. doi: 10.1128/jcm.34.11.2853-2855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Ohashi N, Lee E H, Tamura A, Rikihisa Y. Ehrlichia sennetsu groE operon and antigenic properties of the GroEL homolog. FEMS Immunol Med Microbiol. 1997;18:39–46. doi: 10.1111/j.1574-695X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhi N, Rikihisa Y, Kim H-Y, Wormser G P, Horowitz H W. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J Clin Microbiol. 1997;35:2606–2611. doi: 10.1128/jcm.35.10.2606-2611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]