Abstract
Paraquat (PQ) causes oxidative stress, the main source of damage in plants subjected to adverse environmental factors. Sodium nitroprusside (SNP), a signaling molecule, alleviates oxidative damage. The present study was carried out to investigate the role of exogenous SNP in the amelioration of PQ-mediated oxidative stress effects on Eruca sativa plantlets cultured in MS basal media. Firstly, MS medium supplemented with 6-BA was found to be the best basal medium for seed germination. Then, a rapid micropropagation protocol was designed to produce E. sativa plantlets by using nodal segments as explants, and 0.25 mg/L 6-BA in combination with 0.1 mg/L IBA was found to be the most favorable for shoot proliferation of E. sativa. Four weeks old plants were applied with or without SNP (100 μM) and exposed to oxidative stress induced by 2.5 μM PQ. The SNP application decreased membrane damage, hydrogen peroxide, and proline contents, and increased relative water, pigments, ascorbate and total phenolic contents, and some antioxidant enzyme activities in seedlings under PQ stress compared to PQ stress alone. These results suggested that exogenous SNP could protect E. sativa plantlets propagated in vitro with PQ stress through modulation of proline and phenolics biosynthesis and antioxidant defense system.
Keywords: Eruca sativa, Micropropagation, Sodium nitroprusside, Paraquat stress, Antioxidant defense system
Introduction
The paraquat, one of the most widely used herbicides, which catalyzes the production of superoxide radicals (O2•−) is a strong electron acceptor in photosystem I, are converted to hydrogen peroxide (H2O2), leading to oxidative stress (Varadi et al. 2000; Moustakas et al. 2016). Paraquat can also cause rapid chlorophyll loss, inhibition of photosynthesis, necrosis, lipid peroxidation, protein denaturation, and electrolyte leakage (Beligni and Lamattina 1999; Verma et al. 2013). Plants have tolerance mechanisms comprising of cellular mechanisms including osmoprotectants, enzymatic and non-enzymatic antioxidant defense systems to protect from the harmful effects of oxidative stress. The enzymatic antioxidant defense system consists of superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPOD), while non-enzymatic defense system consists of compounds such as ascorbate (AsA), carotenoid, and total phenolic (Sharma et al. 2012). Osmotic regulation relates to the synthesis of osmoprotectants such as proline, soluble sugars, sugar alcohols, organic acid, and some protective compounds, thus ensuring the maintenance of water potential in plant cells. Paraquat stress extremely increases the proline level, and this increase in proline is one of the widespread stress responses in plants (Hasanuzzaman et al. 2018). Moreover, secondary metabolites such as phenolics, which are regarded as potent nonenzymatic antioxidants are accumulated in response to paraquat-mediated oxidative stress and contribute significantly to oxidative stress tolerance (Cvetković et al. 2015).
Sodium nitroprusside (SNP) is used to produce nitric oxide (NO), which is a signaling molecule that plays a role in a variety of physiological responses to biotic and abiotic stresses, can function as a growth (Kováčik et al. 2019). In previous studies, it has been well reported that NO acts a role in improving plant tolerance against salt and metal-induced oxidative stresses (Khator and Shekhawat 2020a, b).
E. sativa is an important industrial oil-yielding crop that can grow in the arid and semiarid regions. The oil content of E. sativa ranges from 32 to 37% percent, and it is used as a protein meal supplement (Fagbenro 2004). The leaves of this plant have been found to contain substantial amounts of phytochemicals with strong anti-oxidant activities (Grami et al. 2018). Also, E. sativa production has continuously increased, because of the high demand for volatile oils in the pharmaceutical industry (El-Fadaly et al. 2017). Seed oil is used as hair oil, massage oil, lubricant, vesicant, illuminant, hair oil, and massage oil. It is also used as cow feed, and powdered seeds have antimicrobial properties (Jakhar et al. 2002).
Production of plants with economic value by the micropropagation method has gained a great acceleration in recent years. In addition to the used basal media, the plant growth regulators and concentrations are also important in the micropropagation of these plants. Micropropagation of E. sativa plantlets by using cotyledonary segments as explant growing in the India flora was studied by Sharma et al. (2012). Although, some morphological, physiological, and biochemical responses of E. sativa exposed to various stresses have been well-documented (Ozdener et al. 2010; Mahawar et al. 2018; Santiago et al. 2020), no attempts have not yet been made on its stress tolerance mechanisms from E. sativa plantlets propagated in vitro. It has been well reported that NO has a role in improving plant tolerance against salt and metal-induced oxidative stresses (Khator and Shekhawat 2020a, b). There were few studies on the function of NO in alleviating PQ toxicity in various plants (Morán et al. 2010; Hasanuzzaman et al. 2018), while the role of SNP in tolerance to paraquat stress of micropropagated E. sativa plantlets has not been clarified yet. The aim of the present study is to determine an influential procedure for the micropropagation of the seedlings of E. sativa by using nodal segments as explants, and to clarify the role of SNP in tolerance mechanisms of E. sativa seedlings under PQ-mediated oxidative stress.
Materials and methods
Plant material, growth conditions, and treatments
E. sativa seeds were subjected to a pre-washing process with fresh tap water for nearly 30 min, followed by the 70% ethanol treatment, the most common pre-disinfection process, were applied to these seeds for 30 s. Following removal of ethanol, seeds were disinfected with 20% commercial bleach (Domestos) for 10 min to prevent fungal and bacterial contamination. Finally, bleach residues left on the surface of the seeds were removed with autoclaved sterile distilled water. This process was repeated 3 times and each repetition took 15 min. The sterilized seeds were transferred to pre-prepared glass magenta culture vessels with approximately 30 mL of fresh nutrient medium. Cultured seeds were placed in an optimum plant growth chamber until the beginning of germination.
For seed germination, the most preferred two different basal media MS (Murashige and Skoog 1962) and B5 (Gamborg et al. 1968) each supported with 1.0 mg/L 6-BA and without PGR (control) were tested to determine the best effective basal medium. After four weeks of incubation, two important parameters such as the percentage of seed germination and shoot length of germinating seeds were evaluated for each treatment.
For shoot proliferation, nodal segments were excised from the shoots of seedlings at the end of the third subculture and cultured on MS basal medium. Sucrose (Duchefa Biochemie, Haarlem, The Netherlands), and agar (Duchefa Biochemie, Haarlem, The Netherlands), ratios of this components were 2% (w/v) sucrose 0.8% (w/v), respectively. Different types of plant growth regulators (PGRs) such as N6-[2-isopentenil] adenine (2iP), 6- Benzyladenine (6-BA), kinetin (KIN), and thidiazuron (TDZ) (1.0 mg/L, each) individually in combination with 0.1 mg/L indole-3- butyric acid (IBA) were added in this medium. Filter sterilization was applied to all tested PGRs with 0.22 µm filters except for 6-BA, and they were added to the cooled basal media after autoclaving. 1 N NaOH and HCl were preferred to adjust the pH of the media to 5.8. All cultures were maintained at 24 ± 2 °C under a 16 h light and 8 h photoperiod. To provide these conditions, daylight fluorescent lamps with 50 μmol m−2 s−1 photosynthetic flux were quite ideal. The ideal subculturing procedure was done according to the reaction of the explants under the culture conditions, and this period was usually four weeks. The shoot proliferation performance of the different media was assessed by calculating the number of shoots per explant, length of shoots, number of leaves, leaf width and biomass yield based on the fresh and dry weight on each shoot. Different 6-BA concentrations (0.25, 0.5 and 2.0 mg/L) in combination with 0.1 mg/L IBA or without PGR were tested in MS basal media for determining the best PGR concentration.
After the most suitable basal medium was determined, at the age of four weeks, a set of plants were pretreated with 100 μM SNP (Sigma Aldrich, USA) for 12 h and then exposed to oxidative stress induced by 2.5 μM PQ (Sigma Aldrich, USA) for 5 days. Another set of plants was applied with 2.5 μM PQ only for 5 days. Plants cultured only in MS media containing 6-BA/IBA (0.25/0.1 mg/L) were planned as control. In our study, the lowest membrane damage and the best water status were determined at an SNP concentration of 100 μM. Plant tissues were harvested to determine various stress parameters, changes in protective compound contents and antioxidant enzymes activities. The experiments were carried out in three replicates.
Determination of thiobarbituric acid reactive substances (TBARS) content
TBARS contents were measured according to method of Heath and Packer (1968). 0.1 g of fresh leaf tissues were homogenized in trichloroacetic acid (0.1%) by a homogenizer. The homogenate was centrifuged at 15,000 × g for 5 min. The mixture of thiobarbituric acid and 20% TCA was added to the supernatant and was heated at 95 °C for 30 min and then quickly cooled. The absorbance of the supernatant was measured at 532 and 600 nm after 10 min of centrifugation at 10,000 × g.
Determination of hydrogen peroxide (H2O2) content
The extract obtained from fresh leaf samples crushed with activated charcoal in TCA was centrifuged, then 1 mL of supernatant was taken and potassium phosphate (10 mM) buffer and potassium iodide (1 M) were added. The absorbance was then read at 390 nm (Velikova et al. 2000).
Determination of total chlorophyll and carotenoid contents
Fresh leaf tissues weighing 0.1 g were ground with liquid nitrogen and homogenized in a cold acetone solution (80%). At room temperature, the homogenate was centrifuged at 5000 rpm for 10 min and read at 450, 645, and 663 nm. The total chlorophyll and carotenoid contents were measured According to Arnon (1949) and Witham et al. (1971), respectively.
Determination of proline and total phenolic contents
Dried leaves were homogenized with sulfosalicylic acid (3%) for proline content then filtered. The filtrate was centrifuged at 4000 × g for 5 min. Acetic acid and ninhydrin were added to the supernatant. The mixture was then put in tubes and heated at 100 °C for 1 h. Toluene (3 mL) was added to the cooled samples. Then absorbance was read at 520 nm (Bates et al. 1973).
According to Singleton et al. (1999), total phenolic content was determined. Methanol (95%) was used to homogenize fresh leaf samples (0.1 g). After homogenate was centrifuged, Na2CO3 (7.5%) and 2 N Folin–Phenol Ciocalteu's Reagent were added to the diluted supernatant. The samples were held in the dark for 30 min before being measured at 765 nm using a gallic acid standard curve.
Determination of relative water content (RWC)
The plant leaves (0.1 g) were weighed to determine the fresh weight (FW), and then the leaves were placed in water and rehydrated for 12 h to estimate water saturated weight (SW). The leaves were dried at 75 °C for 48 h to determine their dry weight (DW).
Determination of ascorbate (AsA) content
Ascorbate concentration was determined by Liso et al. (1984). Fresh leaves (0.1 g) were homogenized with 5% (w/v) m-phosphoric acid. The extract was centrifuged at 10,000 × g for 4 min. The sample 70 (µL) was added to 3 mL of reaction medium containing 0.1 M citrate-0.2 M phosphate buffer (pH 6.2). Initial absorbance to be read at 265 nm, ascorbate concentration was determined by reading the reduction occurring 5 min after 2 units the ascorbate oxidase were added to the reaction medium. Then, 2.5 mM DTT was added to the mix. Following reduction (3 min) with DTT, the absorbance was read again at 265 nm.
Determination of changes in antioxidant enzymes
Fresh plant tissues (0.1 g) were ground to a fine powder in liquid nitrogen and were extracted in 5 mL of extraction buffer (50 mM K2HPO4, 1 mM EDTA, 1% PVPP, pH 7.0). The extracts were centrifuged at 20,000 × g for 20 min at 4 °C. Ascorbic acid (5 mM) was added to the buffer for APX. The activity SOD was measured using the Beauchamp and Fridovich (1971) method. Riboflavin (2 mM) was added to the reaction mix involving potassium phosphate buffer (50 mM, pH 7.8), EDTA (0.1 mM), L-methionine (13 mM), nitro blue tetrazolium, and 50 mL extract to start the reaction. After 10 min of exposure to white light at 375 µmol m−2 s−1, the absorbance values at 560 nm were determined. The activity CAT was determined according to Aebi (1983) method. The enzyme activity was measured at 240 nm in a reaction mixture (1 mM) containing 50 mM potassium phosphate buffer (pH 7.0), 30 mM H2O2, and 20 µL enzyme extract. The activity of APX was assayed by the decrease in absorbance at 290 nm (Nakano and Asada 1981).
Protein determination
Protein content was measured by method of Bradford (1976). Bovine serum albumin standards were prepared and protein complex with Coomassie Brillant Blue G250 dye was measured at 595 nm. Protein concentration was calculated in mg and used to express enzyme activities.
Statistical analysis
Each treatment was performed in triplicates and included four magenta culture vessels (each containing four explants) for shoot multiplication. All data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). For shoot multiplication, mean shoot length, mean number of shoots, mean number of leaves, mean number of leaf width, mean number of fresh and dry weight, were analyzed using analysis of variance (ANOVA) with Duncan’s multiple range test (DMRT, 95% confidence level). Values are means ± standard deviation (SD). Shoot-forming capacity (SFC) (Lambardi et al. 1993) was also calculated to determine the percentage of shoots regeneration as follows:
The obtained data were evaluated with one-way ANOVA variance analysis tests (Duncan Multiple Comparison Test, P < 0.05).
Results
Seed germination
E. sativa seeds were separately germinated in MS and B5 media supplemented with 1.0 mg/L 6-BA or cytokinin-free medium for 30 days. The most effective germination percentage was obtained in the MS containing 1.0 mg/L 6-BA with 90.00 ± 3.33%. These percentages were determined as 88.89 ± 1.92% in B5 medium supplemented with 1.0 mg/L 6-BA and also 76.67 ± 3.33% in cytokinin-free MS medium. The lowest germination percentage was also in cytokinin-free B5 medium with 73.33 ± 3.34% (Table. 1). Among all tested basal germination media, significant statistical differences were performed in terms of germination performance (P < 0.05).
Table 1.
The effects of different basal media supplemented with the same cytokinin concentration and without cytokinin on seed germination of E. sativa
| MS | GB-5 | |||
|---|---|---|---|---|
| Control | 1.0 mg/L 6-BA | Control | 1.0 mg/L 6-BA | |
| Shoot length (mm) | 15.72c ± 1.33 | 29.80a ± 2.22 | 14.87d ± 1.07 | 24.36b ± 1.32 |
| Germination percentage (%) | 76.67b ± 3.33 | 90.00a ± 3.33 | 73.33b ± 3.34 | 88.89a ± 1.92 |
Data were recorded 4 weeks after the culture with a total of 3 replicates of 30 seeds per treatment
a,b,c,dValues having the same letter(s) in the same line are not significantly different according to Duncan’s multiple range test at P < 0.05
The average maximum shoot length obtained from germinated seeds was 29.80 ± 2.22 mm in MS medium supplemented with 1.0 mg/L 6-BA followed by 24.36 ± 1.32 mm in B5 medium supplemented with 1.0 mg/L 6-BA. These values were calculated as 15.72 ± 1.33 and 14.87 ± 1.07 mm in cytokinin-free MS and B5 media, respectively. These values also showed that there were significant statistical differences between the other tested media and MS containing 1.0 mg/L 6-BA in terms of shoot length (Table 1). Due to lower germination percentage and weak shoot efficiency B5 medium was not preferred for subsequent shoot proliferation studies.
Shoot proliferation
When the four different cytokinins mentioned above were combined individually with IBA (0.1 mg/L) and without PGR (control) in MS basal medium, 6-BA was found to be most suitable than the cytokinin/auxin combinations (Fig. 1). Therefore, several concentrations of 6-BA (0.25, 0.5 and 2.0 mg/L) were tested to determine the optimal cytokinin concentration together with IBA (0.1 mg/L) again. Among all these tested combinations and concentrations, the highest shoot number and leaf number was calculated in MS basal media fortified 1.0/0.1 mg/L 6-BA/IBA with 3.50 ± 0.38 and 24.83 ± 2.06 per explant, respectively. Although MS medium supplemented with 1.0/0.1 mg/L 2iP/IBA gave the highest shoot length and leaf width compared to the other tested media with 39.29 ± 2.84 mm and 7.08 ± 0.55 mm per explant, respectively, it was not considered to be the most suitable cytokinin due to its low values for other tested parameters (Table 2). Because the number of leaves of the shoots obtained from MS media supplemented with this growth regulator was low and the shoots were weak. This situation was an undesirable result for the E. sativa whose leaf was edible. These data were a strong indicator of significant statistical differences between 2iP and the other tested cytokinins (P < 0.05). In micropropagation studies, fresh and dry weight obtained per explant are the two important parameters in the production of plants whose leaves are consumed or evaluated. In this context, the highest fresh and dry weights were calculated from MS basal medium containing 0.25 mg/L 6-BA with 1.152 ± 0.180 g and 0.061 ± 0.009 g per explant, respectively. As a result, MS medium supplemented with 0.25 mg/L 6-BA together with 0.1 mg/L IBA was found to be the most suitable for shoot proliferation of E. sativa.
Fig. 1.
A In vitro micropropagation of E. sativa. B Shoot proliferation after 4 weeks; B1 on culture medium from nodal explant on MS free-medium. B2 supplemented with 1.0/0.1 mg/L TDZ/IBA. B3 with 1.0/0.1 mg/L 6-BA/IBA. B4 with 1.0/0.1 mg/L. 2iP/IBA (B5) with 1.0/0.1 mg/L KIN/IBA Bar = 1.57 cm
Table 2.
The effects of different cytokinin in the presence of IBA (0.1 mg/L) combination and control group on shoot proliferation of E. sativa
| Control | TDZ | 2iP | KIN | 6-BA | IBA | Shoot number/ explant |
Shoot length/ explant (mm) |
Leaf number/ explant |
Leaf width/ explant (mm) |
Fresh weight/ explant (g) |
Dry weight/ explant (g) |
Shoot forming capacity (SFC) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokinin (mg/L) | ||||||||||||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.37 ± 0.29e | 36.05 ± 4.02bc | 5.31 ± 0.8f | 4.63 ± 0.72e | 0.395 ± 0.051e | 0.032 ± 0.005d | 1.28 |
| 1.0 | 0.1 | 3.02 ± 0.43bc | 32.27 ± 2.15d | 22.88 ± 1.44b | 5.19 ± 0.58d | 0.844 ± 0.066c | 0.058 ± 0.007ab | 2.64 | ||||
| 1.0 | 0.1 | 1.83 ± 0.42d | 39.29 ± 2.84a | 10.92 ± 1.83e | 7.08 ± 0.55a | 0.458 ± 0.075e | 0.034 ± 0.005d | 1.83 | ||||
| 1.0 | 0.1 | 2.96 ± 0.51bc | 36.52 ± 2.17bc | 21.25 ± 1.78d | 6.44 ± 0.53b | 0.730 ± 0.099d | 0.053 ± 0.005c | 2.96 | ||||
| 0.25 | 0.1 | 3.38 ± 0.89ab | 37.89 ± 2.84ab | 19.56 ± 1.21d | 6.50 ± 0.82b | 1.152 ± 0.180a | 0.061 ± 0.009a | 3.38 | ||||
| 0.5 | 0.1 | 3.06 ± 0.68bc | 35.90 ± 1.59c | 21.38 ± 1.54c | 5.06 ± 0.77de | 0.996 ± 0.099b | 0.057 ± 0.008bc | 3.06 | ||||
| 1.0 | 0.1 | 3.50 ± 0.38a | 35.86 ± 2.63c | 24.83 ± 2.06a | 5.94 ± 0.52c | 1.144 ± 0.121a | 0.060 ± 0.008ab | 3.50 | ||||
| 2.0 | 0.1 | 2.88 ± 0.62c | 27.25 ± 1.29e | 21.50 ± 1.51c | 5.00 ± 0.82de | 0.927 ± 0.099b | 0.053 ± 0.006c | 2.88 | ||||
Data were recorded 4 weeks after the culture and represents a total of 3 replicates of 16 plants per treatment on MS. Values having the same letter(s) in the same column are not significantly different according to Duncan’s multiple range test at P < 0.05
TBARS content
In the case of PQ stress, TBARS content increased in E. sativa, but SNP application prevented this increase. Namely, E. sativa seedlings treated with PQ stress alone (3.83 ± 0.04 nmol g−1 FW) resulted in rising TBARS content as compared to the control group (2.11 ± 0.09 nmol g−1 FW). However, the treatment of SNP decreased TBARS content in seedlings exposed to PQ stress (2.50 ± 0.03 nmol g−1 FW). Moreover, TBARS content (1.42 ± 0.05 nmol g−1 FW) was low in the SNP alone treatment (Fig. 2A).
Fig. 2.
Thiobarbituric acid reactive substances (TBARS) content (A), Hydrogen peroxide (H2O2) content (B), Total chlorophyll content (C), Total carotenoid content (D) in SNP-treated E. sativa seedlings under PQ stress. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
H2O2 content
It was found that H2O2 content (0.38 ± 0.009 mM g−1 FW) in E. sativa seedlings was induced by PQ stress compared to control (0.29 ± 0.01 mM g−1 FW). SNP application decreased H2O2 content (0.35 ± 0.009 mM g−1 FW) in seedlings exposed to PQ stress. Moreover, H2O2 content did not differ between control plants (0.29 ± 0.01 mM g−1 FW) and those treated with SNP (0.28 ± 0.009 mM g−1 FW) (P < 0.05) (Fig. 2B).
Total chlorophyll and carotenoid contents
As present in Fig. 2C, D, total chlorophyll and carotenoid contents declined in PQ-treated E. sativa seedlings than that of control seedlings. The inhibitory effects of PQ stress on the contents of total chlorophyll and carotenoid were reduced by the treatment of SNP. The total chlorophyll and carotenoid contents were 1.12 and 1.06-fold higher than that of the PQ alone treatments.
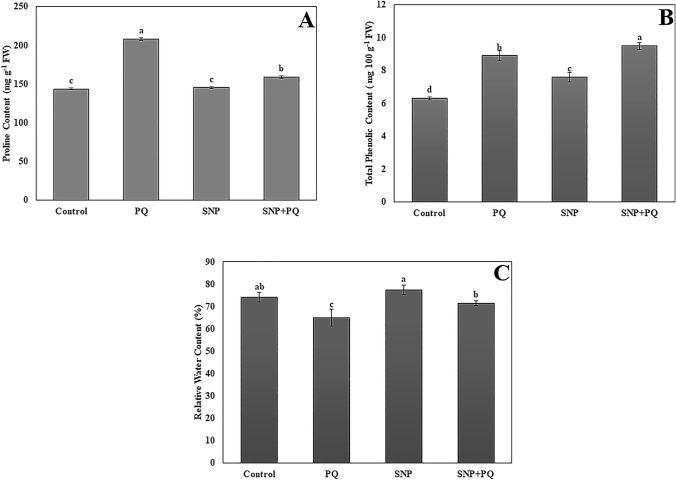
Proline and total phenolic contents
The changes in proline and total phenolic contents in E. sativa seedlings were illustrated in Fig. 3. PQ stress triggered the accumulation of proline content (207.85 ± 1.43 mg g−1 FW) as compared to control (143.54 ± 1.21 mg g−1 FW). Proline content did not have a statistically significant difference between control (143.54 ± 1.21 mg g−1 FW) and SNP treatment (145.45 ± 1.30 mg g−1 FW) (P < 0.05). Also, under PQ stress, SNP application decreased proline content (159.11 ± 1.53 mg g−1 FW) as compared to PQ alone treatment (207.85 ± 1.43 mg g−1 FW) (Fig. 3A).
Fig. 3.
Proline content (A), Total phenolic content (B), Relative water content (RWC) (C) in SNP-treated E. sativa seedlings under PQ stress. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments at P < 0.05
Total phenolic content increased in E. sativa seedlings under PQ stress (8.90 ± 0.30 mg 100 g−1 FW) t in comparison to the control (6.30 ± 0.10 mg 100 g−1 FW). Also, it was found that total phenolic content increased in E. sativa seedlings treated with the combination of SNP and PQ (9.50 ± 0.20 mg 100 g−1 FW) in comparison to PQ stress (8.90 ± 0.30 mg 100 g−1 FW). Moreover, total phenolic content in the SNP-treated seedlings (7.60 ± 0.30 mg 100 g−1 FW) was higher than the control (6.30 ± 0.10 mg 100 g−1 FW) (Fig. 3B).
RWC
Paraquat stress decreased the leaf RWC (65.13%) in comparison to control (74.20%). However, SNP treatment increased the leaf RWC (71.36%) in seedlings under PQ stress. There was no statistically significant difference between control and SNP alone treatment (77.36%) (P < 0.05) (Fig. 3C).
AsA content
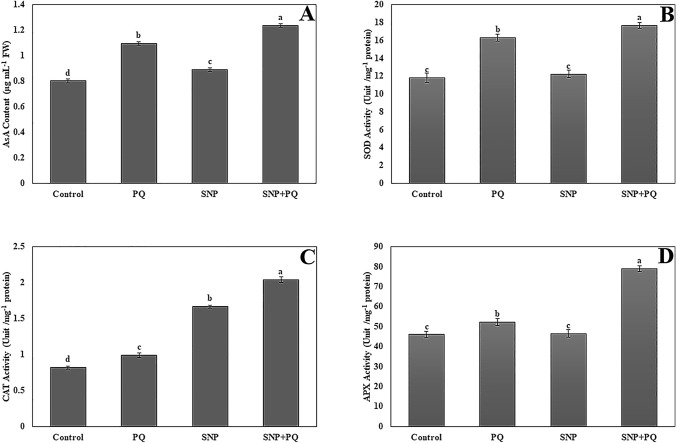
It was found that the AsA content (1.09 ± 0.014 µg mL−1 FW) increased under PQ stress in comparison to the control group 0.80 ± 0.015 µg mL−1 FW). Moreover, AsA content increased in SNP-applied seedlings under unstressed (0.89 ± 0.012 µg mL−1 FW) and stressed (1.23 ± 0.014 µg mL−1 FW) conditions (Fig. 4A).
Fig. 4.
Ascorbate (AsA) content (A), Superoxide dismutase (SOD) activity (B), Catalase (CAT) (C), Ascorbate peroxidase (APX) activity (D) in SNP-treated E. sativa seedlings under PQ stress. Vertical bars represent standard deviations of the means of three replicates. Different letters denote significant differences among all treatments P < 0.05
Changes in antioxidant enzymes
The activity of SOD was induced by PQ stress (16.29 ± 0.40 unit mg−1 protein) compared to control (11.81 ± 0.50 unit mg−1 protein) (Fig. 4B). The SNP application increased the activity (17.65 ± 0.30 unit mg−1 protein) in seedlings under PQ stress as compared to PQ stress (16.29 ± 0.40 unit mg−1 protein). On the other hand, SOD activity was not a statistically significant difference between control (11.81 ± 0.50 unit mg−1 protein) and SNP alone treatment (12.21 ± 0.40 unit mg−1 protein) (P < 0.05) (Fig. 4B).
The activity of CAT increased under PQ stress (0.98 ± 0.03 unit mg−1 protein) as compared to control (0.81 ± 0.02 unit mg−1 protein). CAT activity increased in SNP-treated seedlings under PQ stress as compared to PQ stress. The activity increased from 0.98 ± 0.03 unit mg−1 protein (PQ) to 2.04 ± 0.04 unit mg−1 protein (SNP + PQ). SNP treatment also increased the enzyme activity (1.66 ± 0.02 unit mg−1 protein) in comparison to control (Fig. 4C).
The activity of APX (52.23 ± 1.7 unit mg−1 protein) was enhanced by PQ stress compared to control (45.99 ± 1.5 unit mg−1). The highest APX activity was measured in SNP- treated seedlings under PQ stress (78.86 ± 1.5 unit mg−1 protein) (Fig. 4D).
Discussion
Depending on the rapid population growth, the need for food is also increasing rapidly nowadays. To meet this demand, researchers generally focus on higher quality, faster and higher production per unit area. The determination of the most suitable and fast production method is very crucial in the production of plants. Biotechnological production methods offer many advantages such as rapid and mass commercial production regardless of time and place on a year round basis, shortening the growth cycle, well-adapted clonal multiplication, controlled environment, or production of healthy and stress-resistant plant materials (Suman et al. 2012; Suman 2017). All these positive factors have led researchers to create effective micropropagation methods of different plant species that have medicinal, aromatic, and also economic value nowadays (Zhang et al. 2005; Cüce et al. 2017; Bekircan et al. 2018). The researchers were preferred seeds or cotyledonary nodes as explant in their studies on E. sativa. They tested the different concentrations of BAP and kinetin alone or in combination with different concentrations of IAA, IBA and NAA and were found BAP combination with IAA superior to other applications. These researchers have reported that kinetin applications alone were not suitable for micropropagation of E. sativa (Sharma et al. 2012). Although there were little differences between this report and our data, these results support our findings in general terms. The occurrence of these differences is based on many factors such as explant source, explant type, explant collection time, environmental conditions of the explant, media types, and culture conditions in micropropagation studies (Ružić et al. 2012; Cüce and Sökmen 2017; Cüce et al. 2019).
At the present time, many plant species are cultivated in vitro and regenerated into full plants. Plant tissue culture including various techniques like micropropagation offers grand opportunities for plant propagation and the growth of plants with desirable agronomic traits such as increased yield, environmental stress (salt, frost, drought, herbicide) tolerance, and plant engineering (Jan et al. 2018). Recently, changes in some physiological, biochemical and molecular parameters of plantlets grown in vitro in tolerance to various stresses have been reported (Arshad et al. 2014; Jan et al. 2018; Simsek et al. 2018; Muchate et al. 2019). However, to our knowledge there is no study about stress tolerance of E. sativa plantlets grown in vitro in the literature. Therefore, the role of SNP in PQ-mediated oxidative stress tolerance in E. sativa plantlets grown in vitro was investigated in the current study.
After the most suitable basal medium was determined, to understand how PQ-mediated oxidative stress was damaging E. sativa plantlets propagated in vitro, we analyzed the TBARS, H2O2, RWC, and total chlorophyll contents. The contents of TBARS and H2O2 increased in E. sativa under PQ stress, possibly due to cell membrane damage caused by ROS-mediated oxidative damage. The previous study conducted by Khator and Shekhawat et al. (2020b) in B. juncea seedling revealed that the cytoprotective roles of SNP against cadmium stress to lipid membrane by reducing the production of free radicals. In the present study, the decrease in TBARS and H2O2 levels in SNP-treated E. sativa may have a role in the induction of antioxidant defense system that scavenges H2O2. Similar results from other PQ studies corroborate the current study's oxidative harm trend in seedlings (Moustaka et al. 2015; Takeda et al. 2016). Paraquat stress decreased total chlorophyll and carotenoids contents in E. sativa, which may be due to oxidative damage to photosynthetic pigments. Moreover, it was found that exogenous SNP inhibited the degradation of photosynthetic pigments in E. sativa seedlings under PQ stress. The application of SNP was documented to be enhanced the level of photosynthetic pigments in plants under salt induced oxidative stress (Khator and Shekhawat 2020a).
In order to reduce the negative effect of PQ stress, plants trigger to increase production of proline level. In the present study, similar trend of proline accumulation was determined in E. sativa treated with PQ stress. The proline level decreased when SNP was supplemented with PQ application, indicating that stress was alleviated. The findings of this study also support the findings of Hasanuzzaman et al. (2018). The application of SNP (NO donor) increased the accumulation of proline in salt stressed plants (Ahmad et al. 2016). In our study on E. sativa plants showed SNP application had a useful effect under PQ stress: SNP application arranged the proline level. The total phenolic contents of E. sativa seedlings subjected to PQ was higher than that of control. In comparison to the regulation, exogenous SNP application increased the phenolic contents. In comparison to PQ treatment alone, combining SNP with PQ improved phenolics contents even more. Previous research has shown that salt stress increased the total phenolic compounds and increase even further when SNP is applied to the salt stress treatment (Mohsenzadeh and Zohrabi 2018).
Paraquat-affected E. sativa seedlings showed a decrease in RWC in comparison to control, however, RWC increased after SNP application. The improved RWC may be due to a variety of factors. In plants, SNP reduces solute potential while rising water potential, assisting in the increase of RWC under osmotic stress (Ke et al. 2013).
AsA, the most plentiful antioxidant in plants, protects plants against oxidative stress by scavenging a wide variety of ROS (Liu et al. 2009). In the present study, similar to the findings of Hasanuzzaman et al. (2018), increased AsA content under PQ stress was increased by SNP application. The upregulation of the antioxidant defense system is an important tolerance mechanism to minimize the ROS-induced toxicity in stress-affected plants. In the current study, the activities of antioxidant enzymes were determined to investigate the role of SNP in the tolerance of PQ-mediated oxidative stress in E. sativa seedlings. The activities of SOD, CAT and APX were induced by PQ stress compared to control. Moreover, exogenous SNP increased the activities of these enzymes in seedlings under PQ stress as compared to PQ alone treatment, showing an increase in the H2O2 scavenging process. The activities of antioxidant enzymes varied under PQ stress have been reported by Hasanuzzaman et al. (2018) in B. napus seedlings. Similarly, SNP application improved the activity of CAT in salt treated B. Juncea (Khator and Shekhawat 2020b). Given these results, the study demonstrated that SNP regulated antioxidant defense system in E. sativa plants during PQ stress and provided cellular protection against PQ induced oxidative stress by detoxification of ROS, like H2O2.
Conclusion
It was determined that E. sativa plantlets by using nodal segments as explants showed high micropropagation performance in MS media containing 6-BA/IBA (0.25/0.1 mg/L) in the present study. Moreover, the role of SNP in micropropagated plantlets of E. sativa under PQ stress was investigated for the first time in optimum in vitro culture conditions. As a result, it was revealed that the role of SNP in alleviating PQ-induced oxidative stress in E. sativa. The application of SNP (100 μM) decreased TBARS level and it had a beneficial effect on E. sativa under PQ stress: SNP was able to adjust the proline level and rise the RWC in plants. The study also demonstrated that SNP could act as a ROS scavenger, like H2O2, provide cellular protection against PQ-induced stress either directly or through enhancing antioxidant protection system components like AsA; raised the activities of SOD, CAT, and APX in E. sativa. Here, we suggested for the first time that exogenous SNP could protect E. sativa plantlets propagated in vitro from PQ stress through modulation of proline and phenolics biosynthesis and antioxidant defense system.
Acknowledgements
We thank to native speaker Professor Joseph Arditti for English language improvement.
Author contributions
MC designed the research. MC and ASM conducted the experiments, analyzed all data and wrote manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mustafa Cüce, Email: mustafacuce@windowslive.com.
Asiye Sezgin Muslu, Email: asiyeszgn@outlook.com.
References
- Aebi HE (1983) Catalase methods of enzymatic analysis. Bergmeyer, H.U., Editör, Verlag Chemie, Weinhern, pp 273–286
- Ahmad P, Abdel Latef AA, Hashem A, AbdAllah EF, Gucel S, Tran LSP. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci. 2016;7:347–357. doi: 10.3389/fpls.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad NA, Kadhimi AA, Ibrahim AR, Anizan I, Azhar M, Doni F, Che R. Salinity tolerant enhancement, tissue culture in vitro biochemical procedures. J Plant Bio Res. 2014;3:51–64. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bekircan T, Yaşar A, Yıldırım S, Sökmen M, Sökmen A. Effect of cytokinins on in vitro multiplication, volatiles composition and rosmarinic acid content of Thymus leucotrichus Hal. shoots. 3 Biotech. 2018;8:1–9. doi: 10.1007/s13205-018-1206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide. 1999;3:199–208. doi: 10.1006/niox.1999.0222. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cüce M, Sökmen A. In vitro production protocol of Vaccinium uliginosum L. (bog bilberry) growing in the Turkish flora. Turk J Agrıc For. 2017;41:294–304. [Google Scholar]
- Cüce M, Bekircan T, Laghari AH, Sökmen M, Sökmen A, Önay Uçar E, Kılıç AO. Antioxidant phenolic constituents, antimicrobial and cytotoxic properties of Stachys annua L. from both natural resources and micropropagated plantlets. Indian J Tradit Knowl. 2017;16:407–416. [Google Scholar]
- Cüce M, Bekircan T, Laghari AH, Sökmen M, Sökmen A, Önay Uçar E, Kılıç AO. Phenolic profiles, antimicrobial and cytotoxic properties of both micropropagated and naturally growing plantlets of Calamintha sylvatica subsp. sylvatica Bromf. Not Bot Horti Agrobot Cluj Napoca. 2019;47:1145–1152. [Google Scholar]
- Cvetković J, Milutinović M, Boljević J, Aničić N, Nestorović Živković J, Živković S, Mišić D. Paraquat-mediated oxidative stress in Nepetapannonica L. Bot Serb. 2015;39:121–128. [Google Scholar]
- El-Fadaly HA, El-Kadi SM, El-Moghazy MM, Soliman AAM, El-Haysha MS. Antioxidant activity studies on extracts of Eruca sativa seed meal and oil, detoxification, the role of antioxidants in the resistant microbes. IJSRM Human J. 2017;6:31–51. [Google Scholar]
- Fagbenro OA. Soybean meal replacement by roquette (Eruca sativa Miller) seed meal as protein feedstuff in diets for African catfish, Clarias gariepinus (Burchell 1822), fingerlings. Aquac Res. 2004;35:917–923. [Google Scholar]
- Gamborg OL, Miller R, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Grami D, Rtibi K, Selmi S, Jridi M, Sebai H, Marzouki L, Sabovic I, Foresta C, De Toni L. Aqueous extract of Eruca Sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod Toxicol. 2018;82:103–110. doi: 10.1016/j.reprotox.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Bhuyan MB, Oku H, Fujita M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2018;126:173–186. doi: 10.1016/j.plaphy.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast. I. kinetics and stoichiometry of fatty acid peroxidation. Arch Bio Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jakhar SS, Duhan JC, Chauhan MS. Effect of soil amendment with some oil cakes on root rot of cotton caused by Rhizoctoniasp. Plant Dis. 2002;17:16–20. [Google Scholar]
- Jan N, Qazi HA, Ramzan S, John R (2018) Developing stress-tolerant plants through in vitro tissue culture: family Brassicaceae. In: Biotechnologies of crop improvement, vol 1, pp 327–372
- Ke X, Cheng Z, Ma W, Gong M. Nitric oxide enhances osmoregulation of tobacco (Nicotiana tobacum L.) cultured cells under phenylethanoid glycosides (PEG) 6000 stress by regulating proline metabolism. Afr J Biotechnol. 2013;12:1257–1266. [Google Scholar]
- Khator K, Shekhawat GS. Cd-and Cu-induced phytotoxicity on 2–3 leaf stage of Cyamopsis tetragonoloba and its regulation by nitrate reductase and ROS quenching enzyme. Acta Physiol Plant. 2020;42:1–14. [Google Scholar]
- Khator K, Shekhawat GS. Nitric oxide mitigates salt-induced oxidative stress in Brassica juncea seedlings by regulating ROS metabolism and antioxidant defense system. 3 Biotech. 2020;10:1–12. doi: 10.1007/s13205-020-02493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik J, Dresler S, Micalizzi G, Babula P, Hladký J, Mondello L. Nitric oxide affects cadmium-induced changes in the lichen Ramalina farinacea. Nitric Oxide. 2019;83:11–18. doi: 10.1016/j.niox.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Lambardi M, Sharma KK, Thorpe TA. Optimization of in vitro bud induction and plantlet formation from mature embryos of Aleppo pine (Pinus halepensis Mill.) In Vitro Cell Dev Biol Plant. 1993;29:189–199. [Google Scholar]
- Liso R, Calabrese G, Bitonti MB, Arrigoni O. Relationship between ascorbic acid and cell division. Exp Cell Res. 1984;150:314–320. doi: 10.1016/0014-4827(84)90574-3. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Zhang XL, Bai JG, Suo BX, Xu PL, Wang L. Exogenous paraquat changes antioxidant enzyme activities and lipid peroxidation in drought-stressed cucumber leaves. Sci Hortic. 2009;121:138–143. [Google Scholar]
- Mahawar L, Khator K, Shekhawat GS. Role of proline in mitigating NaCl induced oxidative stress in Eruca sativa Miller: an important oil yielding crop of Indian Thar Desert. Vegetos Int J Plant Res Biotechnol. 2018;31:55–63. [Google Scholar]
- Mohsenzadeh S, Zohrabi M. Auxin and sodium nitroprusside effects on wheat antioxidants in salinity. Russ J Plant Physl. 2018;65:651–657. [Google Scholar]
- Morán JM, Ortiz-Ortiz MA, Ruiz-Mesa LM, Fuentes JM. Nitric oxide in paraquat-mediated toxicity: a review. J Biochem Mol Toxicol. 2010;24:402–409. doi: 10.1002/jbt.20348. [DOI] [PubMed] [Google Scholar]
- Moustaka J, Tanou G, Adamakis ID, Elefteriou EP, Moustakas M. Leaf age dependentmphotoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int J Mol Sci. 2015;16:13989–14006. doi: 10.3390/ijms160613989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas M, Malea P, Zafeirakoglou A, Sperdouli I. Photochemical changes and oxidative damage in the aquatic macrophyte Cymodocea nodosa exposed to paraquat-induced oxidative stress. Pestic Biochem Phys. 2016;126:28–34. doi: 10.1016/j.pestbp.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Muchate NS, Rajurkar NS, Suprasanna P, Nikam TD. NaCl induced salt adaptive changes and enhanced accumulation of 20-hydroxyecdysone in the in vitro shoot cultures of Spinaciaoleracea (L.) Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-48737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nakano Y, Asada Y. Hydrogen peroxide is scavenged by ascorbate-spesific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Ozdener Y, Aydin BK. The effect of zinc on the growth and physiological and biochemical parameters in seedlings of Eruca sativa (L.) (Rocket) Acta Physiol Plant. 2010;32:469–476. [Google Scholar]
- Ružić D, Vujović T, Libiakova G, Cerović R, Gajdošova A. Micropropagation in vitro of highbush blueberry (Vaccinium corymbosum L.) J Berry Res. 2012;2:97–103. [Google Scholar]
- Santiago FE, Silva ML, Cardoso AA, Duan Y, Guilherme LR, Liu J, Li L. Biochemical basis of differential selenium tolerance in arugula (Eruca sativa Mill.) and lettuce (Lactuca sativa L.) Plant Physiol Bioch. 2020;157:328–338. doi: 10.1016/j.plaphy.2020.11.001. [DOI] [PubMed] [Google Scholar]
- Sharma MM, Dhingra M, Dave A, Batra A. Plant regeneration and stimulation of in vitro flowering in Erucasativa Mill. Afr J Biotechnol. 2012;11:7906–7911. [Google Scholar]
- Simsek O. Effect of drought stress in in vitro and drought related gene expression in Carrizo citrange. Fresenius Environ Bull. 2018;27:9167–9171. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Suman S. Plant tissue culture: a promising tool of quality material production with special reference to micropropagation of banana. Biochem Cell Arch. 2017;17:1–26. [Google Scholar]
- Suman S, Rajak KK, Kumar H. Diversity of genome and ploidy in banana and their effect on tissue culture responses. Res Environ Life Sci. 2012;5:181–183. [Google Scholar]
- Takeda T, Kondo K, Ueda K, Iida A. Antioxidant responses of selenium-enriched broccoli sprout (Brassica oleracea) to paraquat exposure. Biomed Res Trace Elem. 2016;27:8–14. [Google Scholar]
- Varadi G, Darko E, Lehoczki E. Changes in the xanthophyll cycle and fluorescence quenching indicate light-dependent early events in the action of paraquat and the mechanism of resistance to paraquat in Erigeron canadensis (L.) Cronq. Plant Physiol. 2000;123:1459–1469. doi: 10.1104/pp.123.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective roles of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- Verma K, Mehta SK, Shekhawat GS. Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: Cross-talk between ROS, NO and antioxidant responses. Biometals. 2013;26:255–269. doi: 10.1007/s10534-013-9608-4. [DOI] [PubMed] [Google Scholar]
- Witham FH, Blaydes BF, Devlin RM. Experiments in plant physiology. New York: Van Nostrand Reinhold; 1971. [Google Scholar]
- Zhang T, Cao ZY, Wang XY. Induction of somatic embryogenesis and plant regeneration from cotyledon and hypocotyl explants of Eruca sativa Mill. In Vitro Cell Dev Biol Plant. 2005;41:655–657. [Google Scholar]