Abstract
Background:
Racial disparities in breast cancer mortality in the U.S. are well-documented. Non-Hispanic Black (NHB) women are more likely to die of their disease than their non-Hispanic White (NHW) counterparts. The disparity is most pronounced among women diagnosed with prognostically favorable tumors, which may be due—in part—to variations in receipt of guideline care. In this study, we sought to estimate the effect of guideline concordant care (GCC) on prognosis, and to evaluate whether receipt of GCC modified racial disparities in breast cancer mortality.
Methods:
Using the Georgia Cancer Registry, we identified 2784 NHB and 4262 NHW women diagnosed with a stage I–III first primary breast cancer in the Metropolitan Atlanta area (2010–2014). Women were included if they received surgery and information was available on breast tumor characteristics; all others were excluded. Receipt of recommended therapies (chemo-, radiation, endocrine, and anti-HER2 therapies) as indicated was considered GCC. We used Cox proportional hazards models to estimate the impact of receiving GCC on breast cancer mortality overall and by race, with multivariable adjusted hazard ratios (HR).
Results:
We found that NHB women were more likely to receive GCC (63% vs. 56%) compared with NHW women. Failure to receive GCC was associated with an increase in the hazard of breast cancer mortality (HR=1.34, 95%CI=1.06, 1.69). However, racial disparities in breast cancer mortality persisted whether or not GCC was received (HRGCC =2.08, 95%CI=1.54, 2.80; HRNon-GCC=1.95, 95%CI=1.38, 2.76 comparing NHB vs. NHW women).
Conclusion:
Although receipt of guideline-care is important for breast cancer outcomes, racial disparities in breast cancer mortality did not diminish with receipt of GCC; as the Black-White differences in mortality persisted across strata of GCC.
Keywords: Breast Cancer Outcomes, Health Disparities, Guideline Concordant Care
INTRODUCTION
In the U.S., racial disparities in breast cancer outcomes are well documented, wherein non-Hispanic Black (NHB) women are more likely to die from their disease than their non-Hispanic White (NHW) counterparts.1–3 The disparity is, in part, attributed to stage and subtype—as NHB women are more likely to be diagnosed with a metastatic cancer, and/or triple negative subtype; both of which have poor prognosis due to limited treatment options.4,5 However, we recently reported that the most pronounced racial disparities in breast cancer mortality were observed among women with non-metastatic, estrogen receptor (ER) positive tumors.6,7 Such tumors are known to have a favorable prognosis, given multiple highly effective biomarker-driven treatment regimens.8 One explanation for such paradoxical findings is that disparities in survival outcomes among women with early stage or ER+ disease may be due to factors downstream of their cancer diagnosis, such as failure to receive guideline-concordant care (GCC).9
Clinical guidelines for women diagnosed with stage I–III breast cancer are developed based on results from multiple clinical trials, and failure to receive guideline care has adverse effects on breast cancer outcomes.10–13 Adherence to guidelines could arise from multiple factors including structural racism, barriers to access, tumor and patient characteristics, or clinician and patient preferences.11 Therefore, non-adherence to clinical guidelines may be a contributing factor to the observed race disparity in breast cancer mortality.9,14 Observational studies assessing adherence to clinical guidelines are important in understanding patient outcomes; however, few studies have examined receipt of guideline care as a possible driver of disparate outcomes in a population-based setting.
To address this knowledge gap, we sought to evaluate how failure to receive GCC contributes to breast cancer mortality, overall, and to disparities in breast cancer mortality among NHB and NHW women diagnosed with a first primary stage I–III breast cancer in the Metropolitan Atlanta area.
METHODS
Study Population
Using the Georgia Cancer Registry (GCR), we identified women diagnosed with breast cancer between 2010 and 2014 while residing in Metropolitan Atlanta (i.e., Fulton, DeKalb, Gwinnett, Cobb or Clayton county). Breast cancer patients were included if they were diagnosed with an invasive stage I–III first primary breast tumor and were classified as being NHW or NHB. Race was based on U.S. Census Bureau definitions and Hispanic ethnicity was determined by the NAACR Hispanic Identification Algorithm.15,16 Additional criteria required that women had information available for assigning tumor subtype (ER, progesterone receptor [PR] used to define women as hormone receptor [HR]-positive or negative, and human epidermal growth factor receptor-2 [HER2] expression), and received surgery as part of their local therapy. HR and HER2 status were used to assign women as HR+/HER2−, HR+/HER2+, HR−/HER2+, or HR−/HER2−. Detailed information on the differences between women who received surgery (94.6%) compared to those who did not (5.4%) are reported in the online digital content (Supplemental Tables 1–3). Follow-up information was available on patients through December 31, 2016. The outcome of interest was breast cancer mortality (ICD10-C50), which was determined from death certificate data.
Exposure Assessment
Guideline Care
Determination of receipt of guideline care was defined based on the National Comprehensive Cancer Network’s (NCCN) 2011 Invasive Breast Cancer Clinical Practice Guidelines in Oncology (Figure 1).17 The NCCN guidelines were used to inform the indication for each type of breast cancer therapy for every patient in our dataset. Information regarding type of surgery received (mastectomy vs. breast conserving surgery), tumor receptor status (ER, PR, and HER2 expression), tumor size, lymph node involvement, and 21-gene recurrence score data (obtained via direct linkage with Genomic Health), were ascertained from the GCR (Supplemental Tables 4–7).
Figure 1:
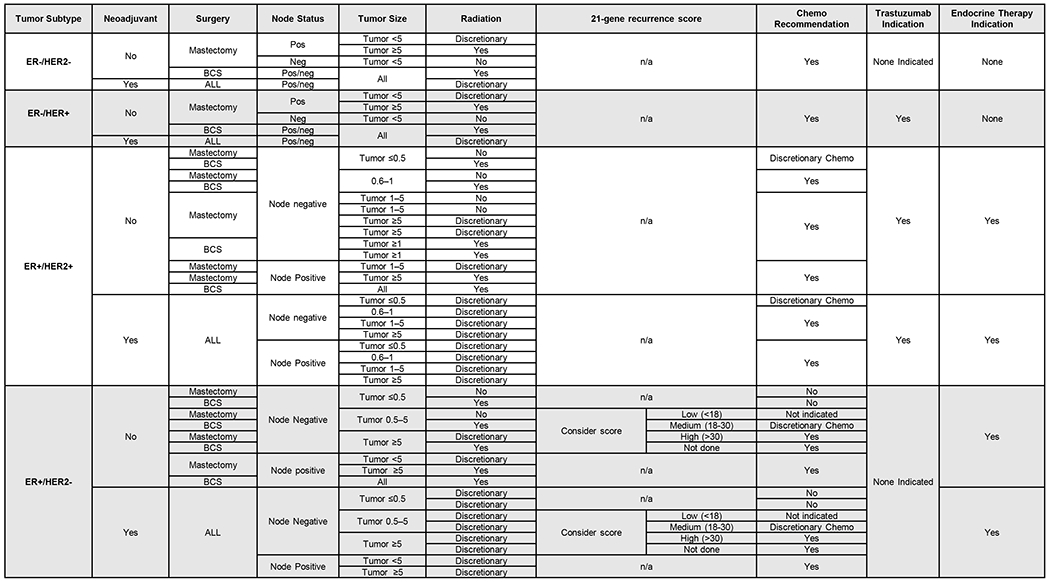
NCCN 2011 summary to determine treatment indication for chemotherapy, radiation, endocrine, and anti-HER2 therapies by type of surgery, tumor, and patient characteristics.
We first grouped women based on their breast cancer subtype: as HR+/HER2−, HR+/HER2+, HR−/HER2+, or HR−/HER2− as described above. These derived tumor subtypes inform the type of biomarker driven therapies (endocrine therapy and anti-HER2 therapy) a patient should receive. We then examined the possible combinations of surgery type, tumor size, lymph node status and 21-gene recurrence score to determine treatment indications. Based on these patient-level data in the GCR, each woman was classified as indicated, discretionary, not indicated, or indeterminate (missing) for each type of therapy. The latter occurred among 6.6% of NHB and 5.2% of NHW women.
Once the indication for each therapy was determined, we assessed whether the patient received therapy consistent with NCCN guidelines using GCR treatment data. The GCR regularly collects information on surgery type, and initiation of chemotherapy, radiation, and endocrine therapies. Anti-HER2 therapies were captured as chemotherapy in the GCR analytic database until 2013, and then coded as immunotherapy in subsequent years. Using natural language processing (NLP) we searched the treatment-related text fields of the GCR database for specific character strings related to an indication of trastuzumab receipt prior to 2013.18 Additionally, there were 271 (3.6%) women who did not have information available on initiation of endocrine therapy. For these women we used NLP to identify receipt of endocrine therapy in treatment-related text fields.
Patients who were indicated for a treatment regimen and did not receive it, or who received therapy without an indication, were classified as having not received guideline care for that treatment modality. Conversely, if a patient’s indication was consistent with receipt or non-receipt of a specific therapy, they were classified as guideline concordant. Among women for whom a therapy was discretionary (based on NCCN guidelines), any value (e.g., receipt, non-receipt, and missing/unknown value) resulted in classification of guideline concordant for that therapy.
Our primary exposure of interest was receipt of GCC across all treatment modalities. We assessed receipt of GCC for concordance of all treatment modalities and for each individual treatment modality. In a sensitivity analyses, we allowed for each patient to have any one therapy not meeting guideline care.
Covariates
We collected information on age at diagnosis (continuous), stage (I–III), derived breast cancer subtype (HR+/HER2−, HR+/HER2+, HR−/HER2+, or HR−/HER2−), a census-derived area-based measure of socioeconomic status [SES] (0%– <5%, 5%– <10%, 10%–<20%, 20%–100% below poverty), marital status (single, married, divorced/separated/other), and insurance status (private, Medicare, Medicaid, Military/other, and uninsured). SES status is based on census tract-level poverty data that is published annually from the American Community Survey,19 and has been used widely in population-based studies.20,21
Statistical Methods
Descriptive statistics were calculated as median values with interquartile ranges, or numbers (%) for covariates of interest across NHB and NHW population subgroups. We additionally report the frequency (%) of women who failed to receive GCC by treatment modality.
Follow-up was defined as time in months, from the date of surgery until the first of a) mortality event, b) last date of contact in registry, or c) December 31, 2016. We used multivariable-adjusted Cox proportional hazard models to calculate the hazard ratios (HR) and 95% confidence intervals (CIs) for the association between receipt of GCC for joint concordance of all treatment modalities and each treatment modality independently with breast cancer mortality.
To address our second aim, we estimated the association between race and breast cancer mortality, and whether this association was modified by receipt of GCC (yes/no). Interaction describes differences in the effect of one exposure across strata of another exposure, which depends on the scale.22,23 In this analysis, we assessed additive and multiplicative interaction for the effect of race on breast cancer mortality by receipt of GCC.24 The presence of interaction between race and GCC was estimated with the common referent approach to calculate the relative excess risk due to interaction (RERI), evaluating departure of the effect on the additive scale.24,25 We calculated the 95%CI for the RERI using the delta method.23,26,27 The presence of multiplicative interactions, indicating if the combined effect of race and GCC is greater than the product of the individual effects, was assessed by comparing stratum-specific effect estimates.25
We verified the proportional hazards assumption for all variables by checking the ln-ln survival curves for any gross violation.28 Potential confounders included in the models were based on a priori knowledge and graphical-based methods (DAG).29 For the association between receipt of GCC and breast cancer mortality, confounders included race, stage, age at diagnosis, SES, derived breast cancer subtype, and insurance status (Supplemental Figure 1). For the interaction model including race and GCC, age at diagnosis was the only confounder identified, based on our graphical assessment (Supplemental Figure 2). The other covariates (e.g., stage, SES, derived breast cancer subtype, and insurance status) are on the causal path between race and breast cancer mortality and including them in the model could potentially induce bias.30 However, to be consistent with prior studies of race disparities in breast cancer outcomes, we present additional results from analyses adjusting for stage, SES, derived breast cancer subtype, and insurance status.
The association between GCC and breast cancer mortality may be susceptible to immortal person-time bias.31,32 This is due to exposure assignment after the initiation of follow-up, which could lead to a mortality event occurring before the start of an indicated treatment. To evaluate the potential bias, we performed landmark analyses. We extended the initiation of follow-up in 3-month intervals, up until 12-months after the recorded date of surgery for each treatment modality and combination. All analyses were conducted using R version v3.5 (Vienna, Austria) and SAS v9.4 (Cary, NC).
RESULTS
We identified 7046 (2784 NHB and 4262 NHW) study eligible women treated with surgery, in the metropolitan Atlanta area. On average, NHB women were younger (median 56 vs. 60, years of age), less likely to have private health insurance (57% vs. 64%) and less likely to live in a high-SES neighborhood (6.2% vs. 32%) compared with NHW women (Table 1). NHB women were more likely to receive overall GCC (59% vs. 53%) (Table 2). Receipt of overall GCC was similar for NHB and NHW women with HR+/HER2− or HR+/HER2+ breast cancer (56% vs. 52% and 54% vs. 53% respectively). NHB women diagnosed with HR−/HER2+ or HR−/HER2− breast cancer were more likely to receive GCC compared with NHW women (59% vs. 53% and 92% vs. 67% respectively). Across individual treatment modalities, NHB and NHW women were nearly equally likely to receive guideline-concordant radiation (88% vs. and 91%), endocrine (83% vs. 80%), and HER2-targeted (94% vs. 95%) therapies. Yet, guideline-concordant receipt of chemotherapy differed by race (78% vs. 71% for NHB and NHW women, respectively) (Table 2).
Table 1.
Patient demographic, clinicopathological, and treatment characteristics among 7046 non-Hispanic White (NHW) and Black (NHB) women diagnosed with stage I–III breast cancer in metro-Atlanta between 2010–2014 and registered with the Georgia Cancer Registry.
| NHB (N=2784) | NHW (N=4262) | |||
|---|---|---|---|---|
|
| ||||
| Median | IQR | Median | IQR | |
| Age at Diagnosis (years) | 56 | 47, 64 | 60 | 50, 68 |
| Length of Follow-up (months) | 43 | 28, 59 | 44 | 29, 61 |
| Time to event (months) | 23 | 15, 37 | 28 | 14, 42 |
|
| ||||
| N | % | N | % | |
|
| ||||
| Breast Cancer-Specific Death Stage | 190 | 6.8 | 150 | 3.5 |
| I | 1230 | 44 | 2493 | 58 |
| II | 1151 | 41 | 1393 | 33 |
| III | 403 | 14 | 376 | 8.8 |
| Tumor Grade | ||||
| 1 | 418 | 15 | 1192 | 28 |
| 2 | 1029 | 37 | 1810 | 42 |
| 3+ | 1269 | 46 | 1150 | 27 |
| Unknown | 68 | 2.4 | 110 | 2.6 |
| Tumor size (cm) | ||||
| ≤0.5 | 231 | 8.3 | 471 | 11 |
| 0.6–1 | 382 | 14 | 813 | 19 |
| >1 to <5 | 1857 | 67 | 2724 | 64 |
| ≥5 | 306 | 11 | 242 | 5.7 |
| Unknown | 8 | 0.3 | 12 | 0.3 |
| Lymph Node | ||||
| Node negative | 1741 | 63 | 2986 | 70 |
| Node positive | 919 | 33 | 1122 | 26 |
| 1–3 | 617 | 22 | 830 | 19 |
| ≥4 | 216 | 7.8 | 238 | 5.6 |
| Unknown number | 86 | 3.1 | 54 | 1.3 |
| No nodes examined | 121 | 4.4 | 154 | 3.6 |
| Unknown node status | 3 | 0.1 | 0 | 0.0 |
| Estrogen Receptor Status | ||||
| ER− tumor | 718 | 26 | 540 | 13 |
| ER+ tumor | 2066 | 74 | 3722 | 87 |
| Derived Tumor Subtype | ||||
| HR+/HER2− | 1771 | 64 | 3272 | 77 |
| HR+/HER2+ | 341 | 12 | 482 | 11 |
| HR−/HER2+ | 141 | 5.1 | 143 | 3.4 |
| HR−/HER2− | 531 | 19 | 365 | 8.6 |
| Receipt of neoadjuvant therapy | ||||
| No | 2189 | 79 | 3783 | 89 |
| Yes | 595 | 21 | 478 | 11 |
| Surgery | ||||
| Breast Conserving Surgery | 1474 | 53 | 2327 | 55 |
| Mastectomy | 1309 | 47 | 1933 | 45 |
|
| ||||
| Receipt of Guideline Concordant Care | ||||
|
| ||||
| 21-gene recurrence score as indicated | ||||
| Not indicated not received | 1300 | 47 | 1520 | 36 |
| Not indicated and received | 414 | 15 | 621 | 18 |
| Indicated and received | ||||
| <18 | 340 | 12 | 759 | 18 |
| 18–30 | 217 | 7.8 | 453 | 11 |
| >30 | 59 | 2.1 | 93 | 2.8 |
| Indicated and not received | 391 | 14 | 697 | 16 |
| Unknown(nodes/ tumor size) | 63 | 2.3 | 119 | 2.8 |
| Chemotherapy as Indicated | ||||
| Not indicated not received | 465 | 17 | 1106 | 26 |
| Not indicated and received | 53 | 1.9 | 79 | 1.9 |
| Not indicated and unknown | 9 | 0.3 | 23 | 0.5 |
| Discretionary | ||||
| No | 124 | 4.5 | 290 | 6.8 |
| Yes | 73 | 2.6 | 110 | 2.6 |
| unknown | 3 | 0.1 | 12 | 0.3 |
| Indicated and not received | 459 | 16 | 1109 | 24 |
| Indicated and received | 1516 | 54 | 1508 | 35 |
| Indicated and unknown | 35 | 1.3 | 51 | 1.2 |
| Can’t determine indication | 47 | 1.7 | 74 | 1.7 |
| Radiation as Indicated | ||||
| Not indicated not received | 431 | 15 | 909 | 21 |
| Not indicated and received | 64 | 2.3 | 63 | 1.5 |
| Not indicated and unknown | 16 | 3.1 | 23 | 0.5 |
| Discretionary | ||||
| No | 281 | 10 | 440 | 10 |
| Yes | 550 | 20 | 509 | 12 |
| unknown | 61 | 2.2 | 61 | 1.4 |
| Indicated and not received | 136 | 4.9 | 178 | 4.2 |
| Indicated and received | 1131 | 41 | 1971 | 46 |
| Indicated and unknown | 65 | 2.3 | 50 | 1.2 |
| Can’t determine indication | 49 | 1.8 | 58 | 1.4 |
| Receipt of Endocrine Therapy | ||||
| Not indicated not received | 647 | 23 | 491 | 12 |
| Not indicated and received | 25 | 0.9 | 17 | 0.4 |
| Indicated and received | 1658 | 60 | 2899 | 68 |
| Indicated and not received | 454 | 16 | 855 | 20 |
| Receipt of Trastuzumab * | ||||
| Not indicated not received | 2263 | 81 | 3597 | 84 |
| Not indicated and received | 39 | 1.4 | 40 | 0.9 |
| Indicated and received | 361 | 13 | 434 | 10 |
| Indicated and not received | 121 | 4.4 | 191 | 4.5 |
|
| ||||
| Demographic Characteristics | ||||
|
| ||||
| Marital Status | ||||
| Single | 840 | 30 | 516 | 12 |
| Married (common law and unmarried domestic) | 1036 | 37 | 2674 | 63 |
| Other (divorced, widowed, separated | 784 | 28 | 966 | 23 |
| Unknown | 124 | 4.5 | 106 | 2.5 |
| Socioeconomic Index | ||||
| 0% – <5% poverty | 173 | 6.2 | 1344 | 31 |
| 5% – <10% poverty | 360 | 13 | 1265 | 30 |
| 10% – <20% poverty | 1030 | 37 | 1130 | 27 |
| 20% – 100% poverty | 1221 | 44 | 523 | 12 |
| Insurance Type | ||||
| Uninsured | 87 | 3.1 | 41 | 1.0 |
| Private | 1596 | 57 | 2735 | 64 |
| Medicaid | 376 | 14 | 111 | 2.6 |
| Medicare | 631 | 23 | 1289 | 30 |
| Military | 47 | 1.7 | 35 | 0.8 |
| Unknown | 47 | 1.7 | 51 | 1.2 |
Data abstracted from text fields of GCR database
Table 2.
Receipt of overall guideline concordant care and individual treatment modalities among non-Hispanic Black (NHB) and White (NHW) women diagnosed with stage I–III breast cancer in metro-Atlanta between 2010–2014 and registered with the Georgia Cancer Registry.
| NHB (N=2784) | NHW (N=4262) | |||
|---|---|---|---|---|
| Receipt of Guideline care | N | % | N | % |
| Derived Tumor Subtype | ||||
| HR+/HER2− | 987 | 56 | 1699 | 53 |
| HR+/HER2+ | 183 | 54 | 256 | 53 |
| HR−/HER2+ | 83 | 59 | 76 | 53 |
| HR−/HER2− | 382 | 72 | 246 | 67 |
|
| ||||
| Individual Treatment Modalities | ||||
|
| ||||
| All Modalities | ||||
| Guideline Concordant | 1635 | 59 | 2277 | 53 |
| Discordant | 965 | 35 | 1765 | 41 |
| Unknown | 184 | 6.6 | 220 | 5.2 |
| Radiation | ||||
| Guideline Concordant | 2454 | 88 | 3890 | 91 |
| Discordant | 200 | 7.2 | 241 | 5.7 |
| Unknown | 130 | 4.7 | 131 | 3.1 |
| Chemotherapy | ||||
| Guideline Concordant | 2181 | 78 | 3026 | 71 |
| Discordant | 512 | 18 | 1088 | 26 |
| Unknown | 91 | 3.3 | 148 | 3.5 |
| Endocrine Therapy | ||||
| Guideline Concordant | 2305 | 83 | 3390 | 80 |
| Discordant | 479 | 17 | 872 | 20 |
| HER2-targeted Therapy | ||||
| Guideline Concordant | 2624 | 94 | 4031 | 95 |
| Discordant | 160 | 5.8 | 231 | 5.4 |
Main Effects
We observed an increase in the hazard of breast cancer mortality among GCC-discordant women (HR=1.60, 95%CI 1.26, 2.04) compared with GCC-concordant women. We similarly found an increase in the hazard of breast cancer mortality among women who were discordant for chemotherapy (HR=1.39, 95%CI 1.02, 1.90), radiation therapy (HR=1.92, 95%CI 1.36, 2.71), endocrine therapy (HR=1.70, 95%CI 1.29, 2.24), anti-HER2 therapy (HR=1.79, 95%CI 1.20, 2.67) (Table 3). Mutual adjustment of the individual treatment modalities yielded similar results (data not shown).
Table 3:
Multivariable-adjusted association between receipt of guideline concordant care and breast cancer mortality among 7046 women diagnosed with stage I–III breast cancer in metro-Atlanta (2010–2014).
| Guideline Therapy | Events | HR (95% CI)* |
|---|---|---|
| Guideline Concordant Care | ||
| Discordant | 131 | 1.60 (1.26, 2.04) |
| Concordant | 178 | Reference |
| Chemotherapy | ||
| Discordant | 61 | 1.39 (1.02, 1.90) |
| Concordant | 261 | Reference |
| Radiation | ||
| Discordant | 38 | 1.92 (1.36, 2.71) |
| Concordant | 284 | Reference |
| Endocrine therapy | ||
| Discordant | 74 | 1.70 (1.29, 2.24) |
| Concordant | 266 | Reference |
| Anti-HER2 therapy | ||
| Discordant | 32 | 1.79 (1.20, 2.67) |
| Concordant | 308 | Reference |
| Any three modalities | ||
| Discordant | 62 | 2.23 (1.59, 3.12) |
| Concordant | 278 | Reference |
Adjusted for race, age, stage, SES, subtype, and insurance
In our sensitivity analysis, defining GCC as having received at least three treatment modalities consistent with guidelines, we observed a slightly more pronounced estimate of association (HR=2.23, 95%CI 1.59, 3.12). These results suggest that potential misclassification of GCC is not an explanation for our observed results. In our landmark analyses, we saw similar, HR estimates as we increased the time since surgery for receipt of GCC. Our findings are thus robust to any potential immortal person-time bias (Supplemental Table 8).
Race Disparities
The overall racial disparity in breast cancer mortality in our cohort was 1.98 (95%CI 1.59, 2.46) [Table 4],7 which persisted even within strata of receipt of overall GCC. Among women who received concordant care across all treatment modalities, we observed a two-fold increase in the NHB-NHW hazard of breast cancer mortality (HR=2.08, 95%CI 1.54, 2.64). Similarly, among those who did not receive GCC, the NHB-NHW HR was 1.95 (95%CI: 1.38, 2.76) (Table 4). In the common referent approach to assess departure on the additive scale, NHW women receiving discordant care had no greater risk of mortality compared with NHW women receiving concordant care. Conversely, NHB women had a 2-fold increase mortality rate regardless of whether they received overall concordant or discordant care. While NHB women were consistently more likely to die from their disease compared with NHW women, most pronounced race disparities were among those who received concordant care for most independent therapeutic regimens (Table 4). One exception was for endocrine therapy where NHB women classified as discordant had a 2.35-fold increased hazard of breast cancer mortality compared with discordant NHW women (95%CI: 1.48, 3.73). The disparity among women concordant for endocrine therapy was less pronounced (NHB vs. NHW HR=1.95 95%CI: 1.50, 2.45). There was no evidence of additive or multiplicative interaction between race and receipt of GCC in breast cancer mortality. In models additionally adjusting for stage, insurance, derived breast cancer subtype, and SES, we observed similar, although attenuated, associations in the disparities across treatment modalities (Table 4).
Table 4:
Association between receipt of guideline concordant care and breast cancer mortality among non-Hispanic black (NHB) and non-Hispanic white (NHW) women diagnosed with stage I–III breast cancer, who received surgery, in metro-Atlanta (2010–2014).
| No. Deaths | Common Referent HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| NHW | NHB | NHW | NHB | NHB vs. NHW Stratified Effects | |||
| Treatment Modality | N | N | HR (95% CI) | RERI (95% CI) | HR (95% CI)1 | HR (95% CI)2 | |
| Overall Race Disparity | 136 | 173 | -- | -- | 1.98 (1.59, 2.46) | -- | |
| Overall Guideline Care | |||||||
| Discordant | 63 | 68 | 1.06 (0.75, 1.49) | 2.07 (1.49, 2.88) | −0.06 (−0.74, 0.61) | 1.95 (1.38, 2.76) | 1.28 (0.89, 1.85) |
| Concordant | 73 | 105 | Reference | 2.08 (1.54, 2.80) | -- | 2.08 (1.54, 2.80) | 1.20 (0.87, 1.66) |
| Chemotherapy | |||||||
| Discordant | 34 | 27 | 0.89 (0.60, 1.31) | 1.53 (1.00, 2.34) | −0.52 (−1.31, 0.27) | 1.72 (1.03, 2.85) | 1.26 (0.74, 2.12) |
| Concordant | 104 | 157 | Reference | 2.13 (1.66, 2.73) | -- | 2.13 (1.67, 2.73) | 1.28 (0.97, 1.69) |
| Radiation | |||||||
| Discordant | 17 | 21 | 2.41 (1.45, 4.01) | 3.15 (1.98, 5.00) | −0.26 (−2.08, 1.55) | 1.31 (0.69, 2.49) | 0.95 (0.49, 1.83) |
| Concordant | 128 | 156 | Reference | 2.00 (1.58, 2.53) | -- | 2.00 (1.58, 2.53) | 1.21 (0.93, 1.58) |
| Endocrine therapy | |||||||
| Discordant | 33 | 41 | 0.95 (0.64, 1.40) | 2.24 (1.56, 3.20) | 0.36 (−0.47, 1.19) | 2.35 (1.48, 3.73) | 1.64 (1.01, 2.64) |
| Concordant | 117 | 149 | Reference | 1.92 (1.50, 2.45) | -- | 1.92 (1.50, 2.45) | 1.14 (0.87, 1.49) |
| HER2 Therapy | |||||||
| Discordant | 15 | 17 | 1.92 (1.13, 3.28) | 3.13 (1.89, 5.19) | 0.16 (−1.65, 1.97) | 1.63 (0.81, 3.26) | 1.16 (0.58, 2.34) |
| Concordant | 135 | 173 | Reference | 2.05 (1.63, 2.57) | -- | 2.05 (1.63, 2.57) | 1.24 (0.96, 1.60) |
| Any Three modalities | |||||||
| Discordant | 29 | 28 | 1.55 (1.03, 2.33) | 2.78 (1.84, 4.20) | 0.15 (−1.08, 1.38) | 1.80 (1.06, 3.03) | 1.51 (0.81, 2.79) |
| Concordant | 118 | 160 | Reference | 2.08 (1.64, 2.64) | -- | 2.08 (1.64, 2.64) | 1.21 (0.92, 1.59) |
Adjusted for age
Adjusted for age; stage; insurance; SES; subtype
DISCUSSION
We observed that, as clinical guidelines would suggest, failure to receive guideline concordant care was associated with an increased hazard of breast cancer mortality. This was observed for receipt of guideline care, overall, and across each treatment modality. Despite the importance of GCC on health outcomes, receipt of GCC did not appear to influence racial disparities in breast cancer mortality. While NHB women were more likely to receive care consistent with guidelines for all treatment modalities combined, they had nearly a 2-fold increase in breast cancer mortality compared with their NHW counterparts within strata of GCC receipt.
There are few previous population-based studies on receipt of GCC in relation to racial disparities in breast cancer mortality. The most recent, a study among women residing in rural Georgia, reported that NHB women were more likely to receive GCC compared with NHW women—which is consistent with our findings.33 However, authors did not evaluate treatment modalities in combination with racial disparities in breast cancer mortality. Early investigations (c. 1990–2005) have reported that minority women are less likely to receive appropriate adjuvant therapy, although findings appear to be mixed and few studies report survival disparities.34,35
Although our study captured guideline care based on indication and receipt of breast cancer therapies, we did not capture information on other aspects of the quality of care, such as treatment facility characteristics or healthcare access, that may have influenced the observed results.36 Higher quality care may lead to additional work-up by tumor boards or care coordination, leading to better treatment timelines—which may not be equitable across race/ethnic groups.37 The largest difference in receipt of guideline care was observed for chemotherapy, primarily driven by NHW women who were indicated for, but did not receive, chemotherapy. NHW women who were discordant for chemotherapy were more likely to have breast cancer diagnoses with limited lymph node involvement and slightly larger tumors. Non-receipt of chemotherapy may reflect structural inequities in barriers in access to care, or patient/provider preferences which are not captured in this study. Moreover, NHW women were more likely to be discretionary for chemotherapy (9.7% vs. 7.2%), which likely reflects the older age at diagnosis of NHW women compared with NHB women. Regardless, the race disparities in breast cancer mortality were strikingly similar among those who received concordant or discordant chemotherapy. In the CDC’s Patterns of Care Study, investigators likewise reported a greater proportion of NHB women received guideline care for chemotherapy.38
We acknowledge several limitations of this study. Our intent was to understand the association between receipt of guideline-concordant first-line therapy on breast cancer mortality and potential race disparities; however, we did not account for timing of treatment initiation, duration, or completion of adjuvant therapies—which have been associated with patient outcomes and may be differential by race.39–41 We excluded women who did not receive surgery, which likely represents a population with poor outcomes compared with women who did receive surgery. Further exploration of the decision to forego surgical treatment is important for future research. We similarly did not have information on adherence to endocrine therapy. In the US, poor adherence and early discontinuation of endocrine therapy have previously been reported, with some studies suggesting race differences.42–44 These may be important considerations for future investigations as we work to identify multi-level targets for intervention. We assumed that women who were discretionary for a treatment modality received GCC, but further exploration of the decision to forgo adjuvant therapy (and potential racial disparities within that decision) may be important for future research. Receipt of anti-HER2 therapy was determined using NLP from GCR treatment text fields among women diagnosed prior to 2013, which could result in misclassification of anti-HER2 therapy. To evaluate the ability of our NLP to correctly classify women as having received/not received anti-HER2 therapy, we compared results to the GCR treatment variable for women diagnosed after 2013. Results were largely consistent; we observed a 99% specificity and 97% sensitivity using the GCR treatment variable as the gold standard. While treatment-related data in cancer registries are often underreported, and may be subject to misclassification,45 our findings are similar to those of Guy et. al., which used chart abstraction to identify first-line therapy.33 Finally, we were unable to collect information on comorbid conditions, which may impact both treatment adherence and efficacy.32 Women with coronary artery disease or diabetes at diagnosis often have poor completion of taxane-based chemotherapy and are more likely to have adverse events from breast cancer treatments, which may affect prognosis.47 Such comorbidities are more likely to present among NHB women, which could—in part—contribute to the observed disparity.48
Race disparities in breast cancer survival outcomes are complex.49 In this study, we observed that although GCC was important for patient prognosis overall, we did not find evidence that differences in receipt of GCC contributed to disparate cancer outcomes between NHB and NHW breast cancer patients. Rather, NHB patients consistently had worse breast cancer survival outcomes than NHW, regardless of GCC status. Future studies may be strengthened from a multi-level approach to incorporate information on healthcare access, neighborhood characteristics, characteristics of the treating facilities, treatment duration and completion, as well as the presence and management of comorbid conditions.
Supplementary Material
REFERENCES
- 1.Ademuyiwa FO, Edge SB, Erwin DO, Orom H, Ambrosone CB, Underwood W. Breast Cancer Racial Disparities: Unanswered Questions. Cancer Res. 2011;71(3):640–644. doi: 10.1158/0008-5472.CAN-10-3021 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Sauer AG, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 3.Hershman D, McBride R, Jacobson JS, et al. Racial Disparities in Treatment and Survival Among Women With Early-Stage Breast Cancer. J Clin Oncol. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005;89(1):47–54. doi: 10.1007/s10549-004-1470-1 [DOI] [PubMed] [Google Scholar]
- 5.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–180. doi: 10.1002/cncr.23131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin LJ, Yan M, Jiang R, et al. Oncotype DX recurrence score implications for disparities in chemotherapy and breast cancer mortality in Georgia. Npj Breast Cancer. 2019;5(1):1–7. doi: 10.1038/s41523-019-0129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin LJ, Jiang R, Ward KC, et al. Racial Disparities in Breast Cancer Outcomes in the Metropolitan Atlanta Area: New Insights and Approaches for Health Equity. JNCI Cancer Spectr. 2019;3(3). doi: 10.1093/jncics/pkz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olopade OI, Grushko TA, Nanda R, Huo D. Advances in Breast Cancer: Pathways to Personalized Medicine. Clin Cancer Res. 2008;14(24):7988–7999. doi: 10.1158/1078-0432.CCR-08-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–238. doi: 10.3322/caac.21271 [DOI] [PubMed] [Google Scholar]
- 10.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433–451. doi: 10.6004/jnccn.2017.0044 [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Richardson LC, Kahn AR, et al. Survival Difference between Non-Hispanic Black and Non-Hispanic White Women with Localized Breast Cancer: The Impact of Guideline-Concordant Therapy. J Natl Med Assoc. 2008;100(5):490–499. doi: 10.1016/S0027-9684(15)31295-5 [DOI] [PubMed] [Google Scholar]
- 12.Inwald EC, Ortmann O, Zeman F, et al. Guideline Concordant Therapy Prolongs Survival in HER2-Positive Breast Cancer Patients: Results from a Large Population-Based Cohort of a Cancer Registry. BioMed Research International. doi: 10.1155/2014/137304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeMasters T, Madhavan SS, Sambamoorthi U, Hazard-Jenkins HW, Kelly KM, Long D. Receipt of Guideline-Concordant Care Among Older Women With Stage I–III Breast Cancer: A Population-Based Study. J Natl Compr Canc Netw. 2018;16(6):703–710. doi: 10.6004/jnccn.2018.7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griggs JJ, Culakova E, Sorbero MES, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(18):2522–2527. doi: 10.1200/JCO.2006.10.2749 [DOI] [PubMed] [Google Scholar]
- 15.NAACCR R. Ethnicity Work Group (2011) NAACCR guideline for enhancing Hispanic/Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2. 2.1]. North Am Assoc Cent Cancer Regist Springf. [Google Scholar]
- 16.Humes K, Jones N, Ramirez R. Overview of race and Hispanic origin: 2010. US Census Bureau. Published online 2011. [Google Scholar]
- 17.Carlson RW, Allred DC, Anderson BO, et al. Invasive Breast Cancer. J Natl Compr Canc Netw. 2011;9(2):136–222. doi: 10.6004/jnccn.2011.0016 [DOI] [PubMed] [Google Scholar]
- 18.Morrison FP, Li L, Lai AM, Hripcsak G. Repurposing the Clinical Record: Can an Existing Natural Language Processing System De-identify Clinical Notes? J Am Med Inform Assoc. 2009;16(1):37–39. doi: 10.1197/jamia.M2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. American Community Survey. Published online 2010.
- 20.Knoble NB, Alderfer MA, Hossain MJ. Socioeconomic status (SES) and childhood acute myeloid leukemia (AML) mortality risk: Analysis of SEER data. Cancer Epidemiol. 2016;44:101–108. doi: 10.1016/j.canep.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and Ethnic Disparities in Cancer Survival by Neighborhood Socioeconomic Status in Surveillance, Epidemiology, and End Results (SEER) Registries. JNCI Monogr. 2014;2014(49):236–243. doi: 10.1093/jncimonographs/lgu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele TJ, Knol MJ. A Tutorial on Interaction. Epidemiol Methods. 2014;3(1):33–72. doi: 10.1515/em-2013-0005 [DOI] [Google Scholar]
- 23.VanderWeele TJ, Vansteelandt S. Invited Commentary: Some Advantages of the Relative Excess Risk due to Interaction (RERI)—Towards Better Estimators of Additive Interaction. Am J Epidemiol. 2014;179(6):670–671. doi: 10.1093/aje/kwt316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–520. doi: 10.1093/ije/dyr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ. On the Distinction Between Interaction and Effect Modification. Epidemiology. 2009;20(6):863–871. doi: 10.1097/EDE.0b013e3181ba333c [DOI] [PubMed] [Google Scholar]
- 26.Richardson DB, Kaufman JS. Estimation of the Relative Excess Risk Due to Interaction and Associated Confidence Bounds. Am J Epidemiol. 2009;169(6):756–760. doi: 10.1093/aje/kwn411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Confidence Interval Estimation of Interaction. Epidemiology. 1992;3(5):452–456. [DOI] [PubMed] [Google Scholar]
- 28.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat Med. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510 [DOI] [PubMed] [Google Scholar]
- 29.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a Clearer Definition of Confounding” Revisited With Directed Acyclic Graphs. Am J Epidemiol. 2012;176(6):506–511. doi: 10.1093/aje/kws127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schisterman EF, Cole SR, Platt RW. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiol Camb Mass. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suissa S Immortal Time Bias in Pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 32.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340. doi: 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 33.Guy GP, Lipscomb J, Gillespie TW, Goodman M, Richardson LC, Ward KC. Variations in Guideline-Concordant Breast Cancer Adjuvant Therapy in Rural Georgia. Health Serv Res. 2015;50(4):1088–1108. doi: 10.1111/1475-6773.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 35.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799 [DOI] [PubMed] [Google Scholar]
- 36.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in Breast Cancer Treatment and Outcomes: Biological, Social, and Health System Determinants and Opportunities for Research. The Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–584. doi: 10.1056/NEJMsa040609 [DOI] [PubMed] [Google Scholar]
- 38.Wu X-C, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(2):142–150. doi: 10.1200/JCO.2011.36.8399 [DOI] [PubMed] [Google Scholar]
- 39.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016;2(3):330–339. doi: 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and Ethnicity are Associated with Delays in Breast Cancer Treatment (2003–2006). J Health Care Poor Underserved. 2011;22(1):128–141. [DOI] [PubMed] [Google Scholar]
- 41.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in Adjuvant Chemotherapy Treatment Among Patients With Breast Cancer Are More Likely in African American and Hispanic Populations: A National Cohort Study 2004–2006. J Clin Oncol. 2010;28(27):4135–4141. doi: 10.1200/JCO.2009.27.2427 [DOI] [PubMed] [Google Scholar]
- 42.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and Socioeconomic Disparities in Endocrine Therapy Adherence in Breast Cancer: A Systematic Review. Am J Public Health. 2015;105(Suppl 3):e4–e15. doi: 10.2105/AJPH.2014.302490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wigertz A, Ahlgren J, Holmqvist M, et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat. 2012;133(1):367–373. doi: 10.1007/s10549-012-1961-4 [DOI] [PubMed] [Google Scholar]
- 44.Hershman DL, Kushi LH, Shao T, et al. Early Discontinuation and Nonadherence to Adjuvant Hormonal Therapy in a Cohort of 8,769 Early-Stage Breast Cancer Patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noone A-M, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care. 2016;54(9):e55–e64. doi: 10.1097/MLR.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and Survival Disparities Among Black and White Patients With Breast Cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765 [DOI] [PubMed] [Google Scholar]
- 47.Hershman DL, Till C, Wright JD, et al. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34(25):3014–3022. doi: 10.1200/JCO.2015.66.2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cossrow N, Falkner B. Race/Ethnic Issues in Obesity and Obesity-Related Comorbidities. J Clin Endocrinol Metab. 2004;89(6):2590–2594. doi: 10.1210/jc.2004-0339 [DOI] [PubMed] [Google Scholar]
- 49.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. The Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


