Abstract
The baculovirus expression system has proved to be a useful tool for the production of recombinant proteins. Here we have characterized the Neospora caninum surface protein NcSRS2 produced by two types of the recombinant virus and also have developed an enzyme-linked immunosorbent assay (ELISA) using recombinant NcSRS2 for the serologic diagnosis of Neospora infection. Western blot analysis showed two major protein bands that were detectable in insect cells infected with each recombinant baculovirus, and a lower-molecular-weight protein was detected in culture supernatants from a cell infected with the recombinant virus lacking the hydrophobic C-terminal tail. Analysis of the N-terminal amino acids showed that the secreted NcSRS2 lacked 6 kDa of the N-terminal signal peptide. Moreover, the detergent-soluble protein of insect cells infected with the recombinant baculovirus expressing the full-length NcSRS2 gene was used to develop an ELISA system based on specificity and reactivity to antisera against Toxoplasma gondii, Hammondia heydorni, or N. caninum. Anti-N. caninum mouse, dog, and bovine sera recognized the recombinant NcSRS2 on Western blots. Furthermore, we have shown that the developed ELISA system consistently discriminates indirect fluorescent-antibody test (IFAT)-positive bovine sera against N. caninum from IFAT-negative sera. These results indicate that the ELISA using baculovirus-expressed NcSRS2 can be useful for effective and reliable serodiagnosis of N. caninum infection.
Neospora caninum was originally identified as a Toxoplasma gondii-like parasite (7). Infection with N. caninum causes paralysis and death in livestock and companion animals (5). The predicted coccidian nature of the parasite was recently confirmed when its oocysts were found in dog feces (19). Thus, dogs can serve as definitive hosts.
Significant economic and reproductive losses to the livestock industry have been shown to be caused by N. caninum (4), mostly by bovine abortion. A wide range of antibody titers was found in blood samples obtained from cattle during an N. caninum-induced abortion outbreak (24). Therefore, the global importance of N. caninum has prompted the development of a number of serodiagnostic tests (for example, the indirect fluorescent-antibody test [IFAT], immunoblotting, enzyme-linked immunosorbent assays [ELISAs], and the direct agglutination test) based on tachyzoites or parasite antigens (1, 2, 6, 16, 24). Based on serodiagnostic tests using total parasite protein, there is a risk of false-positive results due to cross-reaction with other closely related parasites, for example, T. gondii. Therefore, the development of reliable reagents for identification of N. caninum and diagnosis of neosporosis depends on the characterization of N. caninum-specific antigens.
The surface proteins of all obligatory intracellular parasites are believed to play critical roles in infection. These proteins may collectively serve a number of important functions because they represent the initial interaction with the host cell and components of the host immune system. It is likely that the surface protein of N. caninum termed NcSRS2 is functionally involved in the process of adhesion and invasion (9–11, 23). Previous studies have shown that vaccination with recombinant vaccinia virus carrying the NcSRS2 gene prevents infection with and vertical transmission of N. caninum in BALB/c mice (20–22). In addition, NcSRS2 is a predominant antigen recognized by antisera from N. caninum-infected animals and is conserved in all isolates of the parasite (12).
Serologic tests based on an immunodominant surface antigen may be superior in terms of repeatability, reproducibility, and specificity to assays based on antigen mixtures. Therefore, we established a highly specific and sensitive ELISA method using recombinant NcSRS2 expressed in insect cells by a baculovirus. Our data indicate that recombinant baculovirus-expressed NcSRS2 can be a useful reagent for the serodiagnosis of N. caninum infection.
MATERIALS AND METHODS
Parasites.
N. caninum tachyzoites of the Nc-1 strain were maintained in human foreskin fibroblast cells (Hs68) cultured in Dulbecco modified Eagle medium (Sigma, St Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (FBS). For the purification of tachyzoites, the parasites and host cell debris were washed in cold phosphate-buffered saline (PBS) and the final pellet was resuspended in cold PBS and passed through a 27-gauge needle and a 5.0-μm-pore-size filter (Millipore, Bedford, Mass.).
Cells and virus.
The Autographa californica nuclear polyhedrosis virus and its recombinant viruses were grown in Spodoptera frugiperda (Sf9) cells in TC-100 insect medium (Gibco BRL, Grand Island, N.Y.) supplemented with 10% FBS and 0.26% Bacto tryptose broth (Difco, Detroit, Mich.).
Cloning of full-length and truncated NcSRS2 genes.
To clone full-length and truncated NcSRS2 genes into a baculovirus, the two primer sets indicated below were used. The primers were NT/SRS2 (5′-ACG AAT TCA TGG CGA CGC ATG CTT-3′), CT44 (5′-GCG TCG ACT CAG TAC GCA AAG ATT-3′), and CT41 (5′-ATG TCG ACC TCC TCT TAA CAC GG-3′). The template used in the PCRs was N. caninum tachyzoite cDNA produced by a ZAP-cDNA synthesis kit (Toyobo, Osaka, Japan). The resulting PCR fragments were blunted with Klenow fragment and were then ligated with the baculovirus transfer vector pBacPAK8 (Clontech, Palo Alto, Calif.), which had been previously digested with SmaI. The resulting two plasmids, which were constructed using the same NT/SRS2 primer and two different primers, CT44 and CT41, were designated pBac/SRS2p44 and pBac/SRS2p41, respectively. Plasmid pBac/SRS2p44 encoded the preprocessed translation product (401 amino acids) of the NcSRS2 gene from the start codon to the C-terminal stop codon. Plasmid pBac/SRS2p41 encoded the C-terminally truncated proteins from the first methionine to amino acid 375. An open reading frame of NcSRS2 encodes a 401-amino-acid protein that contains a potential 53-amino-acid signal peptide and a potential 25-amino-acid hydrophobic C-terminal tail (12).
Construction of recombinant baculoviruses that express NcSRS2.
Sf9 cells were cotransfected with a transfer vector and BaculoGold baculovirus DNA (PharMingen, San Diego, Calif.) using Lipofectin reagent (Gibco BRL). After 4 days of incubation at 27°C, the culture supernatant containing recombinant viruses expressing NcSRS2 genes was harvested and subjected to plaque purification. After three cycles of purification, recombinant viruses expressing NcSRS2 were obtained. The recombinant baculoviruses constructed using the plasmids pBac/SRS2p44 and pBac/SRS2p41 were designated Ba/SRS2p44 and Ba/SRS2p41, respectively.
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were carried out as described previously by Nishikawa et al. (23). Purified N. caninum tachyzoites (2 × 107) and Sf9 cells (1 × 106) infected with the recombinant baculovirus at 5 PFU per cell for 4 days were suspended in 100 μl of PBS, sonicated, and mixed with 100 μl of 2× SDS gel-loading buffer (100 mM Tris-HCl [pH 6.8], 100 mM 2-mercaptoethnol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) under reducing conditions. The samples were heated at 95°C for 5 min, and 10-μl samples were subjected to SDS-PAGE. For gel analysis, the gel was stained with Coomassie brilliant blue. After SDS-PAGE, the protein bands in the gel were electrically transferred to a membrane (Immobilon transfer membrane; Millipore). The membrane was blocked with PBS containing 3% skim milk (PBS-SM) and then incubated with anti-N. caninum mouse, dog, and cattle sera or with an anti-NcSRS2 monoclonal antibody (MAb) diluted 1:200 with PBS-SM at 37°C for 60 min. The membrane was washed three times with PBS for 5 min each and then incubated with horseradish peroxidase-conjugated mouse (Amersham Pharmacia Biotech, Piscataway, N.J.), dog (Bethyl, Montgomery, Tex.), and bovine (Bethyl) immunoglobulin G (IgG) antibodies diluted 1:1,000 with PBS-SM at 37°C for 60 min. The membrane was washed three times with PBS for 5 min each, incubated with enhanced chemiluminescence detection regents (Amersham Pharmacia Biotech) for 1 min, and exposed to a film.
IFAT.
For screening of dog and bovine sera, N. caninum or T. gondii tachyzoites (5 × 104) were mounted on glass slides, dried, and fixed with acetone before use. Bound bovine antibodies were detected with fluorescein isothiocyanate-conjugated anti-bovine IgG (Rockland, Gilbertsville, Pa.) diluted 1:200 with PBS supplemented with 3% FBS.
To investigate the antigenic properties of recombinant NcSRS2, Sf9 cells infected with baculovirus at 5 PFU/cell were fixed with acetone and incubated with antibodies or MAbs against N. caninum tachyzoites, NcSRS2, or NcSAG1 diluted 1:100 with PBS containing 3% FBS. The cells were then stained with fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Southern Biotechnology, Birmingham, Ala.) diluted 1:100 with PBS containing 3% FBS and examined by fluorescence microscopy (Nikon, Tokyo, Japan).
ELISA.
ELISA plates coated with an N. caninum soluble antigen (NLA) were prepared as described previously (22). For the preparation of ELISA plates coated with the recombinant NcSRS2, monolayers of Sf9 cells were grown in a 75-cm2 flask and infected with 10 ml of Ba/SRS2p44 or Ba/SRS2p41 at 5 PFU/cell in Sf-900II SFM medium (Gibco BRL). After 4 days, the culture medium was harvested from the infected cells and the virus particles were removed from the medium by centrifugation at 100,000 × g for 120 min at 4°C. The infected cells were sonicated, and Triton X-100 was added to give a final concentration of 1%. The suspension was left at room temperature for 2 h and then centrifuged at 10,000 × g for 10 min. The supernatant from each sample was dialyzed against PBS, and the protein concentration was then determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.). For investigation of serum samples, wells containing 60 ng of antigens were coated with a carbonate-bicarbonate buffer (pH 9.6) and left overnight at 4°C. After blocking with PBS-SM for 1 h at 37°C, the plates were washed twice with PBS containing 0.05% Tween 20, and 100-μl portions of serum samples diluted 1:250 (mouse or dog sera) or 1:500 (bovine sera) with PBS-SM were added to duplicate wells. The plates were incubated at 37°C for 1 h. After being washed five times with PBS–0.05% Tween 20, the plates were incubated with horseradish peroxidase-conjugated mouse, dog, and bovine IgG antibodies at 37°C for 1 h. Colorimetric reactions were performed by adding substrate after washing five times. The absorbance at 415 nm in each well was measured using an MTP-120 microplate reader (Corona Electric, Ibaraki, Japan).
Sera and MAbs.
To produce MAbs against NcSRS2 or NcSAG1, immunization of BALB/c mice (Clea Japan, Tokyo, Japan), cell fusion, and selection of fused cells were performed by methods described previously (23). Antibodies against NcSRS2 or NcSAG1 were produced by immunization of BALB/c mice with recombinant proteins expressed in Escherichia coli as described previously (23). Bovine serum samples, which were a gift from Nemuro Livestock Hygiene Service Center, Hokkaido, Japan, were obtained from dairy cows in Hokkaido. The sera were tested by IFAT and kept frozen at −20°C until used for ELISA. Sera with antibody titers of >200 in IFAT were judged positive. To produce antisera, 10-week-old female BALB/c mice were infected by intraperitoneal injection with 106 Nc-1 strain N. caninum tachyzoites or 103 PLK strain T. gondii tachyzoites. Sera were collected at 4-day intervals up to 40 days after the infection. Twelve-week-old beagle dogs were intravenously infected with 2 × 106 Nc-1 strain N. caninum tachyzoites. Sera were collected every week until 6 weeks after infection. Prior to experiments, the dogs were proven to be free of N. caninum and T. gondii infections by IFAT. Anti-Hammondia heydorni dog sera were a gift from T. Matsui of the Department of Parasitology, Kyorin University School of Medicine, Tokyo, Japan (18).
RESULTS
Antigenic properties of recombinant NcSRS2 in a baculovirus expression system.
To characterize the antigenicity of recombinant NcSRS2 expressed in Sf9 cells by a baculovirus, IFAT analysis using MAbs and antibodies to NcSRS2 or NcSAG1 was carried out. The MAb and antibody to NcSAG1, which is another surface protein of N. caninum, were used as negative controls. As shown in Table 1, all three MAbs (1B8, 2C8, and 2G2) and antibody to NcSRS2 recognized the recombinant proteins expressed by each recombinant baculovirus on IFAT. On the other hand, MAb and antibody to NcSAG1 did not react with the recombinant NcSRS2. These results indicate that the antigenic structures of the recombinant proteins were similar to that of the authentic parasite protein.
TABLE 1.
Antigenic properties of recombinant baculoviruses and their parent strain on IFAT
Western blot analysis for recombinant NcSRS2.
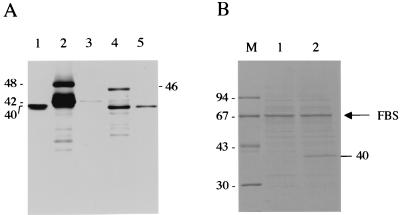
Two major bands were detected in the cell lysates of each group of recombinant baculovirus-infected Sf9 cells by Western blotting using anti-NcSRS2 MAb (Fig. 1A, lanes 2 [48 and 42 kDa] and 4 [46 and 40 kDa]). The lower-molecular-mass species may be due to some proteolytic degradation of the recombinant NcSRS2. In all cell lysates, the difference in molecular masses between the two bands was 6 kDa. Moreover, the lower-molecular-mass proteins were detectable in the culture supernatants of each group of recombinant baculovirus-infected Sf9 cells (Fig. 1A, lanes 3 [42 kDa] and 5 [40 kDa]), although the reactivity of the 42-kDa protein in the culture supernatants of Ba/SRS2p44-infected Sf9 cells was faint. The stained-gel analysis indicated that the 40-kDa protein was detectable in the culture supernatants of Ba/SRS2p41-infected Sf9 cells but not in the culture supernatants of Ba/SRS2p44-infected Sf9 cells (Fig. 1B). The 67-kDa protein seems to be FBS that remained during the cultivation of the Sf9 cells. Analysis of the N-terminal portion of the secreted protein from the culture supernatants of Ba/SRS2p41-infected Sf9 cells showed that a 53-amino-acid peptide from the N terminus was cleaved in the recombinant NcSRS2 (data not shown). These data indicate that the higher-molecular-mass proteins (48 kDa in Ba/SRS2p44 and 46 kDa in Ba/SRS2p41) are precursors of NcSRS2 that contain an N-terminal signal peptide and that the lower-molecular-mass proteins (42 kDa in Ba/SRS2p44 and 40 kDa in Ba/SRS2p41) are mature proteins. The apparent molecular mass of the mature form of the recombinant NcSRS2 encoded by the full-length gene (42 kDa) was higher than that of the authentic NcSRS2 (40 kDa). On the other hand, a protein of the same molecular mass estimated for authentic NcSRS2 (40 kDa) was detectable in the cell lysates and the culture supernatant of the Ba/SRS2p41-infected Sf9 cells.
FIG. 1.
(A) Western blot analysis for recombinant baculoviruses using a MAb to NcSRS2 (2C8) under reducing conditions. Cell lysates (lanes 2 and 4) and culture supernatants (lanes 3 and 5) of Sf9 cells infected with recombinant baculoviruses were analyzed. Lane 1, N. caninum tachyzoites; lanes 2 and 3, Ba/SRS2p44-infected cells; lanes 4 and 5, Ba/SRS2p41-infected cells. Molecular masses are given in kilodaltons. (B) Gel analysis of secreted proteins. The culture supernatants of Sf9 cells infected with recombinant baculoviruses were examined. The samples were separated by SDS-PAGE under reducing conditions, and the gel was then stained with Coomassie brilliant blue. Lane 1, Ba/SRS2p44-infected cells; lane 2, Ba/SRS2p41-infected cells; lane M, molecular mass markers. Molecular masses are given in kilodaltons. The arrow indicates the remaining FBS in each sample.
Specificity and sensitivity of recombinant NcSRS2.
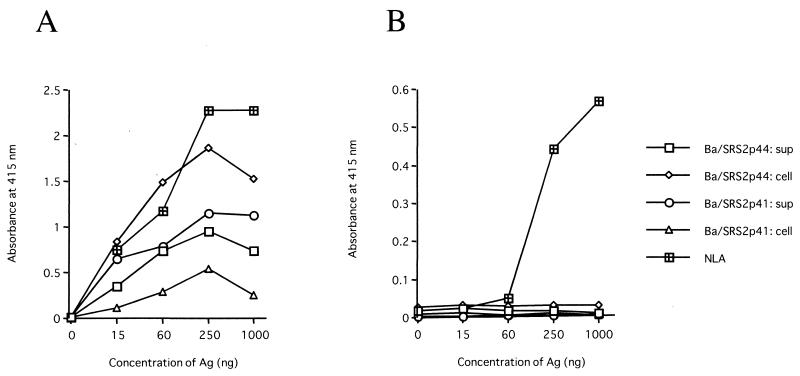
To develop a serodiagnostic system for N. caninum infection based on recombinant NcSRS2, we examined the specificity and reactivity to anti-N. caninum or T. gondii mouse serum by ELISA (Fig. 2). Although all tested antigens showed reactivity to antiserum against N. caninum, the ELISA using the detergent-soluble protein of the Ba/SRS2p44-infected Sf9 cells showed high reactivity to the serum (Fig. 2A). As shown in Fig. 2B, the recombinant NcSRS2 showed no cross-reactivity against anti-T. gondii mouse serum. On the other hand, antiserum against T. gondii recognized NLA in a dose-dependent manner. Moreover, neither the recombinant NcSRS2 nor the NLA reacted with the anti-H. heydorni dog sera (data not shown). Furthermore, we screened anti-N. caninum sera from mouse, dog, and cow by Western blotting using the cell lysates of Ba/SRS2p44-infected Sf9 cells. All anti-N. caninum sera recognized 48- and 42-kDa proteins in the cell lysates of Ba/SRS2p44-infected Sf9 cells, but sera from noninfected controls did not (data not shown). To investigate the immunogenicity of recombinant NcSRS2 prepared from Ba/SRS2p44-infected Sf9 cells during N. caninum infection, sera from mice and dogs experimentally infected with the parasites were examined by ELISA using recombinant NcSRS2 expressed by Ba/SRS2p44. The ELISA values obtained using recombinant NcSRS2 increased after N. caninum infection in mice and dogs as did those obtained using the NLA (data not shown). These data indicate that the antigen prepared from Ba/SRS2p44-infected Sf9 cells could be a highly specific and sensitive reagent to establish the ELISA method for serodiagnosis against N. caninum infection.
FIG. 2.
Specificity of recombinant NcSRS2 against anti-N. caninum serum. ELISA using NLA or the recombinant NcSRS2 expressed by Ba/SRS2p44 or Ba/SRS2p41 was carried out using anti-N. caninum (A) or anti-T. gondii (B) mouse serum (1:250). The antigens (Ag) from the culture supernatants (sup) and the cell lysates (cell) of recombinant baculovirus-infected Sf9 cells were prepared as described in Materials and Methods.
Examination of bovine sera.
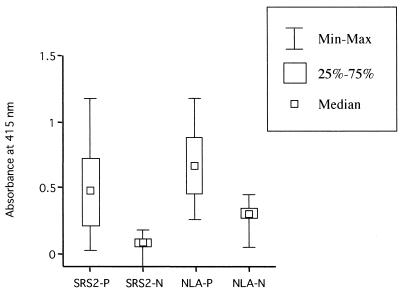
We tested the ELISA using recombinant NcSRS2 prepared from Ba/SRS2p44-infected Sf9 cells for bovine sera (Fig. 3). Sera from cattle that were N. caninum IFAT positive (n = 40) yielded high ELISA indices compared to sera that were IFAT negative (n = 40) in an ELISA using recombinant NcSRS2 and NLA. The mean ELISA index using recombinant NcSRS2 in sera that were N. caninum IFAT negative was four times lower than that using NLA. In addition, the cutoff value in the recombinant NcSRS2 ELISA (optical density = 0.189) was low compared to that in the NLA ELISA (optical density = 0.371). Based on the determined cutoff value, 10 and 7 samples of sera that were N. caninum IFAT positive were judged negative in the ELISA using recombinant NcSRS2 and NLA, respectively. However, these samples were also judged negative in Western blotting using crude tachyzoite extract (data not shown), indicating that the ELISA is a specific method compared to IFAT. These data show that the ELISA system using recombinant NcSRS2 can be useful for serodiagnosis of N. caninum infection in cattle.
FIG. 3.
Examination of bovine sera. Minimum and maximum values (Min-Max), 25 and 75% percentiles (25%–75%), and median values of ELISA using the NLA or the recombinant NcSRS2 prepared from Ba/SRS2p44-infected cells (SRS2) are shown. Bovine sera that were N. caninum IFAT positive (P) (n = 40) and negative (N) (n = 40), which were randomly selected, were examined by ELISA. The cutoff values were determined by the mean value plus two standard deviations from bovine sera that were IFAT negative (recombinant NcSRS2 ELISA, optical density = 0.189; NLA ELISA, optical density = 0.371).
DISCUSSION
An immunogenic surface protein of N. caninum designated NcSRS2 has been previously described (9–12). These previous studies have shown that NcSRS2 could be found in dense granules as well as in rhoptries, as determined by immunoelectron microscopy. In IFAT, the recombinant NcSRS2 expressed by Ba/SRS2p44 was observed on the surface of the infected cells (data not shown). This suggests that NcSRS2 contains signal sequences for directing proteins to the membrane. Previous studies have revealed an open reading frame of 1,203 nucleotides that encodes a 401-amino-acid protein from authentic NcSRS2 (12). Based on the N-terminal peptide sequence of purified NcSRS2, these studies showed that the protein contained a 53-amino-acid signal peptide. The present study indicates that the proprotein of recombinant NcSRS2 was detected in infected cells and that the mature truncated protein was secreted in the culture supernatants. N-terminal peptide sequence analysis of secreted NcSRS2 produced by a baculovirus suggests that the signal peptide is cleaved at the same cleavage site in both insect cells and parasites. The predicted protein sequence also has a hydrophobic C-terminal tail (12). The most likely site for addition of the glycosylphospatidylinositol (GPI) anchor is 25 amino acids from the termination codon (12). Although Sf9 cells are capable of recognizing GPI attachment sites on several human proteins, such as CD14 (8), CD59 (3), and the Schistosoma mansoni protein Sm32 (15), it has been shown that the GPI linkage attachment site in the SAG1 gene of T. gondii is not efficiently used in insect cells (13). Since the recombinant NcSRS2 was effectively secreted in the culture supernatant by treatment with phosphatidylinositol phospholipase C, it is possible that Sf9 cells may recognize the signal used for the attachment of GPI in NcSRS2 (data not shown). Western blot analysis for recombinant baculoviruses indicated that the molecular mass of the mature protein of truncated NcSRS2 that lacked 25 amino acids from the termination codon was 40 kDa, consistent with the molecular mass of authentic NcSRS2. The results of our present study suggest that the deletion of a hydrophobic C-terminal tail may provide molecules with a more native conformation of parasite protein in the baculovirus-Sf9 cell expression system.
As a serodiagnostic test for the detection of N. caninum-specific antibodies, the recombinant ELISA, which focuses on defined N. caninum antigens, appears to offer several distinct advantages over use of a lysate mixture of antigens. To improve the sensitivity and specificity of the ELISA system, the use of the recombinant antigens Nc4.1 and Nc14.1 (14, 16), N54 and N57 (17), and NCDG1 and NCDG2 (26) makes this assay easier to produce and standardize to achieve a reliable diagnostic test for N. caninum infection. The NcSRS2 antigen is consistently recognized as immunodominant by antisera from N. caninum-infected animals and is identified in diverse isolates of N. caninum (12). In previous reports, affinity-purified NcSRS2 prepared from the tachyzoites was shown to be useful for the development of an ELISA to diagnose N. caninum infection in cattle (25). Here we have reported the production of the NcSRS2 protein in the baculovirus expression system. This system is extensively applied to synthesize many kinds of important heterologous proteins for a wide variety of scientific purposes, such as diagnostic, therapeutic, structural, and functional studies. Moreover, this system is suitable for large-scale development of a protein. Following the successful production of the recombinant baculovirus-expressed NcSRS2 with antigenic properties of the authentic protein, we developed an ELISA system for the detection of N. caninum-positive sera. The ELISA based on recombinant NcSRS2 prepared from the Ba/SRS2p44-infected cells showed reactivity to antisera from mice and dogs that were experimentally infected with N. caninum. In addition, the recombinant NcSRS2 showed no cross-reactivity to antiserum against H. heydorni. In contrast to the ELISA using NLA, anti-T. gondii mouse serum did not react with the recombinant NcSRS2 antigen, demonstrating that the developed ELISA system has specificity and sensitivity for anti-N. caninum sera. Moreover, this system differentiated between N. caninum IFAT-positive and -negative bovine sera from field areas and showed low indices in IFAT-negative bovine sera compared to the ELISA using NLA. In addition, the ELISA method shows high specificity to N. caninum-positive bovine sera compared to the IFAT method. The present study indicates that the ELISA using recombinant NcSRS2 can be a useful diagnostic method for the serodiagnosis of N. caninum infection.
ACKNOWLEDGMENTS
We thank J. P. Dubey (Livestock and Poultry Sciences Institute and Parasite Biology and Epidemiology Laboratory, U.S. Department of Agriculture Agricultural Research Service) for supplying the N. caninum NC-1 isolate, Nemuro Livestock Hygiene Service Center for supplying bovine sera, and T. Matsui (Department of Parasitology, Kyorin University School of Medicine) for supplying dog sera against H. heydorni.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y.N. is supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Atkinson R, Harper P A, Reichel M P, Ellis J T. Progress in the serodiagnosis of Neospora caninum infections of cattle. Parasitol Today. 2000;16:110–114. doi: 10.1016/s0169-4758(99)01604-x. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkman C, Lunden A, Holmdahl J, Barber J, Trees A J, Uggla A. Neospora caninum in dogs: detection of antibodies by ELISA using an iscom antigen. Parasite Immunol. 1994;16:643–648. doi: 10.1111/j.1365-3024.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies A, Morgan B P. Expression of the glycosylphosphatidylinositol-linked complement-inhibiting protein CD59 antigen in insect cells using a baculovirus vector. Biochem J. 1993;295:889–896. doi: 10.1042/bj2950889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey J P. Recent advances in Neospora and neosporosis. Vet Parasitol. 1999;84:349–367. doi: 10.1016/s0304-4017(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 5.Dubey J P, Lindsay D S. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J P, Lindsay D S, Adams D S, Gay J M, Baszler T V, Blagburn B L, Thulliez P. Serologic responses of cattle and other animals infected with Neospora caninum. Am J Vet Res. 1996;57:329–336. [PubMed] [Google Scholar]
- 7.Dubey J P, Carpenter J L, Speer A, Topper M J, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;198:1269–1285. [PubMed] [Google Scholar]
- 8.Haziot A, Rong G W, Bazil V, Silver J, Goyert S M. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-alpha production by cells in whole blood. J Immunol. 1994;152:5868–5876. [PubMed] [Google Scholar]
- 9.Hemphill A, Felleisen R, Connolly B, Gottstein B, Hentrich B, Muller N. Characterization of a cDNA-clone encoding Nc-p43, a major Neospora caninum tachyzoite surface protein. Parasitology. 1997;115:581–590. doi: 10.1017/s0031182097001650. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill A. Subcellular localization and functional characterization of Nc-p43, a major Neospora caninum tachyzoite surface protein. Infect Immun. 1996;64:4279–4287. doi: 10.1128/iai.64.10.4279-4287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemphill A, Gottstein B. Identification of a major surface protein on Neospora caninum tachyzoites. Parasitol Res. 1996;82:497–504. doi: 10.1007/s004360050152. [DOI] [PubMed] [Google Scholar]
- 12.Howe D K, Crawford A C, Lindsay D, Sibley L D. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect Immun. 1998;66:5322–5328. doi: 10.1128/iai.66.11.5322-5328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter S, Ashbaugh L, Hair P, Bozic C M, Milhausen M. Baculovirus-directed expression and secretion of a truncated version of Toxoplasma SAG1. Mol Biochem Parasitol. 1999;103:267–272. doi: 10.1016/s0166-6851(99)00119-x. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins M C, Wouda W, Dubey J P. Serological response over time to recombinant Neospora caninum antigens in cattle after a neosporosis-induced abortion. Clin Diagn Lab Immunol. 1997;4:270–274. doi: 10.1128/cdli.4.3.270-274.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koster B, Strand M. Schistosoma mansoni: Sm23 is a transmembrane protein that also contains a glycosylphosphatidylinositol anchor. Arch Biochem Biophys. 1994;310:108–117. doi: 10.1006/abbi.1994.1146. [DOI] [PubMed] [Google Scholar]
- 16.Lally N C, Jenkins M C, Dubey J P. Evaluation of two Neospora caninum recombinant antigens for use in an enzyme-linked immunosorbent assay for the diagnosis of bovine neosporosis. Clin Diagn Lab Immunol. 1996;3:275–279. doi: 10.1128/cdli.3.3.275-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie K, Sverlow K W, Barr B C, Anderson M L, Conrad P A. Cloning and characterization of two recombinant Neospora protein fragments and their use in serodiagnosis of bovine neosporosis. Clin Diagn Lab Immunol. 1997;4:692–699. doi: 10.1128/cdli.4.6.692-699.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui T, Morii T, Iijima T, Ito S, Tsunoda K, Kobayashi F, Fujino T. Isospora heydorni isolated in Brazil: endogenous stage in dogs. Jpn J Parasitol. 1986;35:215–222. [Google Scholar]
- 19.McAllister M M, Dubey J P, Lindsay D S, Jolley W R, Wills R A, MuGuire A M. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- 20.Nishikawa Y, Inoue N, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine. 2001;19:1381–1390. doi: 10.1016/s0264-410x(00)00389-3. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa Y, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine. 2001;19:1710–1716. doi: 10.1016/s0264-410x(00)00407-2. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa Y, Kousaka Y, Fukumoto S, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Delivery of Neospora caninum surface protein, NcSRS2 (Nc-p43), to mouse using recombinant vaccinia virus. Parasitol Res. 2000;86:934–939. doi: 10.1007/s004360000267. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa Y, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Monoclonal antibody inhibition of Neospora caninum tachyzoite invasion into host cells. Int J Parasitol. 2000;30:51–58. doi: 10.1016/s0020-7519(99)00162-9. [DOI] [PubMed] [Google Scholar]
- 24.Romand S, Thulliez P, Dubey J P. Direct agglutination test for serologic diagnosis of Neospora caninum infection. Parasitol Res. 1998;84:50–53. doi: 10.1007/s004360050355. [DOI] [PubMed] [Google Scholar]
- 25.Schares G, Rauser M, Sondgen P, Rehberg P, Barwald A, Dubey J P, Edelhofer R, Conraths F J. Use of purified tachyzoite surface antigen p38 in an ELISA to diagnose bovine neosporosis. Int J Parasitol. 2000;30:1123–1130. doi: 10.1016/s0020-7519(00)00092-8. [DOI] [PubMed] [Google Scholar]
- 26.Venturini M C, Venturini L, Bacigalupe D, Machuca M, Echaide I, Basso W, Unzaga J M, Di Lorenzo C, Guglielmone A, Jenkins M C, Dubey J P. Neospora caninum infections in bovine foetuses and dairy cows with abortions in Argentina. Int J Parasitol. 1999;29:1705–1708. doi: 10.1016/s0020-7519(99)00143-5. [DOI] [PubMed] [Google Scholar]