Abstract
The present study reports the effect of different seasons on polyphenol content and antioxidant potential of ethanolic, methanolic, ethyl acetate, and aqueous extracts of leaves, stems, and roots of Premna integrifolia. Ethyl acetate extract of leaves (EAEPI) collected in the rainy season showed potent antioxidant activity with highest total phenol (74.33 ± 2.26 µg/mg, gallic acid equivalent), and flavonoid (98.83 ± 0.26 µg/mg, rutin equivalent) content. Therefore, EAEPI extract was subjected to characterization by UHPLC-Q-TOF-MS/MS and GC–MS analysis for the identification of active constituents. UHPLC-Q-TOF-MS/MS analysis in + ve ion mode revealed the presence of eight polyphenolic compounds namely quercetin-3-D-xyloside, kaempferol-3,7-O-bis-alpha-L-rhamnoside, isorhamnetin-3-Oglucoside, luteolin-3′,7-di-O-glucoside, eriodictyol-7-O-glucoside, syringetin-3-O-galactoside, petunidin-3-O-beta-glucopyranoside and vitexin-2″-O-rhamnoside. GC–MS analysis confirmed the presence of 26 compounds with six major compounds viz; citronellol, phytol acetate, campesterol, squalene, stigmasterol, and hexadecanoic acid. These compounds are reported for the first time from P. integrifolia except phytol and stigmasterol. Our previous study validates the hepatoprotective potential of P. integrifolia but there was no idea about the bioactive compound responsible for the activity. So, in present work, the major compounds identified in spectrometry analysis were subjected to in silico docking against an important liver enzyme alanine amino transaminase to confirm its hepatoprotective properties. Docking analysis validates the presence of two hepatoprotective lead compounds stigmasterol, and campesterol, which satisfy the drug-likeness criteria with good absorption, distribution, metabolism, and toxicity properties. Thus, present work gives a clear insight about the influence of season on the total polyphenolic constituent in different plant parts of P. integrifolia, their antioxidant potential and preclinical evaluation of hepatoprotective lead compounds.
Keywords: Antioxidants, Hepatoprotective, Lead compounds, Phenolics, Flavonoids
Introduction
Changing environmental conditions (Fokum et al. 2017), living style (McKillop and Schrum 2005) and food habit (Shin et al. 2016; Singh et al. 2019) of people caused several health complications. Several phytoconstituents available in medicinal plants contribute major role in scavenging reactive oxygen species (ROS), which provides protection against damages caused by the xenobiotics. Several factors viz; genetical set up of the plant, physiological factors, environmental conditions, and geographical variations influence the phytochemical constituent of plant (Yosr et al. 2018). Seasonal variations have a major influence on the phytochemical composition of a species (Lopez-Lázaro 2009). Premna integrifolia L. commonly known as Agnimantha is a member of family Lamiaceae (Group 2009). Traditionally, it is used as anticoagulant (Gopal and Purushothaman 1984), cardioprotective (Bose et al. 2012), anti-inflammatory and anti-arthritic (Rajendran and Krishnakumar 2010), antidiabetic (Majumder et al. 2014), and anti-hyperlipidaemic (Patel and Patel 2012). The present study reports the comparative analysis of seasonal influence on polyphenolic constituent and antioxidant activity of different plant parts of P. integrifolia. Stems, roots and leaves of P. integrifolia were extracted in different solvents like ethanol, methanol, ethyl acetate and water in different season and subjected to analysis. Among different extracts, ethyl acetate extract of leaves was showing maximum antioxidant activity with high polyphenolic constituent. Therefore, this extract was characterized by UHPLC-Q-TOF-MS/MS and GC–MS analysis to know the active ingredient present in the extract. Previous reports validates the antioxidant property of P. integrifolia root (Gokani et al. 2011), stem bark (Majumder et al. 2014), wood (Muthukumaran et al. 2013; Rajendran et al. 2009) and leaves (Selvam et al. 2012), but there is no report of the comparative study of different plant parts influenced by season of collection for their total phenol, flavonoid content and antioxidant activity. In previous study, we reported the hepatoprotective activity of P. integrifolia (Singh et al. 2018) but we could not find the bioactive constituent responsible for hepatoprotective activity. Therefore, in present study we have performed in silico analysis of hepatoprotective property of major compounds of P. integrifolia identified by GC–MS and LC–MS as ligand by using an important liver enzyme alanine amino transaminase. This is the first report of the in-silico docking analysis in P. integrifolia for hepatoprotective biomarkers from UHPLC-Q-TOF–MS/MS and GC–MS identified compounds.
Materials and methods
Chemicals 0.1,1-Diphenyl,2-picryl hydrazyl (DPPH), riboflavin, nitroblue tetrazolium (NBT), L-methionin, thio barbutiric acid (TBA), ethylenediaminetetraacetic acid (EDTA) gallic acid, rutin, ascorbic acid, potassium ferricynide [K3Fe(CN)6], trichloro acetic acid (TCA),and ferric chloride (FeCl3) were purchased from Hi- media Ltd. All reagents were of analytical grade.
Plant collection and extract preparation
Plant parts namely leaves, stem bark and root of P. integrifolia were collected from the ayurvedic garden of Banaras Hindu University, Varanasi during month of December for winter, May for summer and August for rainy season (Fig. 1). The plant was taxonomically identified by Prof. V.K. Joshi, Department of Dravyaguna, Faculty of Ayurveda, IMS, Banaras Hindu University, Varanasi, India. A voucher specimen BSI/CRC/2016–17 was deposited in the Botanical Survey of India. Collected plant sample leaves, stem bark and root were washed thoroughly under running tap water in order to avoid dust, and shade dried at room temperature and then powdered in a mechanical grinder. All extracts were prepared using protocol of Upadhyay et al. (2014).
Fig. 1.
Premna integrifolia a whole plant, b stem, c leaf, and d root
DPPH radical scavenging activity
The free radical scavenging capacity of the extracts were estimated using modified protocol of Upadhyay et al. (2014). Different concentrations of plant extracts were mixed with 5 ml of a 0.005% methanolic solution of DPPH and incubated for 15 min at room temperature. Absorbance was recorded at 517 nm.
Lipid peroxidation assay
Lipid peroxidation was measured using modified protocol of Upadhyay et al. (2014). 50 μl of each extract and egg homogenate (250 μl, 10% in distilled water, v/v) were mixed in a flask, and the final volume was made up to 500 ml by adding distilled water. Finally, 20 μl FeSO4 (0.07 M) was added to the above mixture and incubated for 40 min, to induce lipid peroxidation. This mixture was mixed with 750 μl of 20% acetic acid (pH 3.5) and 750 μl of 0.8% TBA (w/v) (prepared in 1.1% sodium dodecyl sulphate) and 25 μl 20% TCA and then heated in a boiling water bath for 60 min. After cooling, 3.0 ml of 1-butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured against 3 ml butanol at 532 nm. Distilled water was used as blank in place of the extract.
Superoxide radical scavenging property
A modified protocol of Upadhyay et al. (2018) was used for superoxide radical scavenging activity. In this assay, reaction mixture of 5 ml was made by adding 0.01 M a phosphate buffer solution (PBS) (pH 7.8), 130 mM methionine, 60 μM riboflavin, 0.5 mM EDTA, 0.75 mM NBT and 0.5 ml of test sample solution. The reaction mixture was kept in front of fluorescent light for 5 min, and absorbance was recorded at 560 nm. For control, identical tubes containing the reaction mixture was kept in the dark. The percentage inhibition was measured by comparing the absorbance of the control and the test sample. The blank was 0.01 M PBS.
Reducing power assay (RP). The reducing power of extracts was determined by using modified protocol of Mishra et al. (2016). Different concentrations (50–1000 µg ml−1) of extracts were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 37 °C for 15 min and after that 2.5 ml of trichloroacetic acid (TCA, 10%) was added to the mixture and centrifuged at 1000 rpm for 115 min. The upper organic layer of solution (2.5 ml) was taken and mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%), and then absorbance was recorded at 700 nm. High absorbance indicated high reducing power. Ascorbic acid was used as the standard in this assay.
Determination of total phenol content (TPC)
The TPC was determined by Folin-Ciocalteu method described by McDonald et al. (2001) with some modification. Briefly, 1 ml of distilled water, 0.1 ml of 1 mg ml−1 sample, and 0.3 ml of Folin-Ciocalteu reagent with distilled water (1:1) were mixed in a test tube, and kept for 5–8 min at room temperature. Then, 2 ml of 7% sodium carbonate solution was added, and reaction volume was maintained at 3 ml. Solutions were incubated for 1 h at room temperature, the absorbance was recorded at 750 nm. All determination was carried out in triplicate. Various concentrations of gallic acid used for the preparation of the standard curve and expressed as microgram per milligram of gallic acid equivalents (GAE).
Measurement of total flavonoid content (TFC)
Determination of TFC was done by AlCl3 colorimetric method with slight modification in protocol (Chang et al. 2002). Different extracts (0.1 ml of 1 mg ml−1) in ethanol were mixed with 0.1 ml of 2% AlCl3, 0.1 ml of 1 M potassium acetate, and 2.7 ml of ethanol. The reaction mixture was kept at room temperature for 30 min and absorbance was recorded at 415 nm. TF content was calculated using rutin as the standard and expressed as micrograms per milligram of rutin equivalents (RE).
Profiling of bioactive compounds using UHPLC-Q-TOF-MS/MS
Ethyl acetate extract of leaves collected from rainy season (EAEPI) was characterized using modified protocol of (Singh et al. 2018). The separation of EAEPI was done on an Acquity UPLC system (Waters, Milford, MA, USA) equipped with a BEH C18 column (100 mm × 2.1 mm, 1.7 μm). The column temperature was set at 25 °C. The mobile phase consisted of acetonitrile (B) and methanol (C) acidified with 0.1% formic acid (A). The flow rate of the mobile phase was set at 300 μl/min while 5 μl injection volumes were used. Analytes were eluted by using a gradient elution programme as follows: The initial composition of B:C was 90:10% and increased to 80:20% in 2 min, 50:50% for 1–3 min, 30:70% for 3–6 min, then 10:90% for 1 min and finally, decreased quickly to 90:10% for 7–10 min. The sample was analyzed by mass spectroscopic method (positive and negative ion modes equipped with an electrospray ionization (ESI) source) for the presence of any bioactive compounds. The following mass spectroscopy parameters were applied: The cone and desolation gas flows were 52 l/h and 647 l/h, respectively; the source and desolation temperatures were 40 °C and 450 °C, respectively. The capillary and cone voltage were set at 2.72 kV and 40 eV, respectively. The Q-TOF mass spectrometer was conducted in MSE mode with a low collision energy set at 6 eV in the first function and a collision energy ramp from 20 to 40 eV in the second function. Centroid mode data was collected over the m/z range 100–1000 in both functions, and the scan time was 1 s with an interscan delay of 0.024 s. The accurate mass and molecular formula denomination were acquired with the Mass Lynx 4.1 software (Waters MS Technologies).
Profiling of bioactive compounds using GC–MS analysis
The EAEPI was used for the GC–MS analysis using modified protocol of Tiwari et al. (2016). The bioactive constituents were identified by comparison of their retention indices (RI) relative to homologous alkane series (purchased from Sigma, St. Louis, USA) and by comparison of their mass spectral fragmentation patterns with those data provided in WILEY8.LIB and NIST11.LIB. Identification was assumed when a good match of mass spectrum and RI was achieved. EAEPI was subjected to GC–MS analysis on a GCMS-QP2010 Plus (Shimadzu, Kyoto, Japan) system with headspace sampler (AOC-20 s) and auto-injector (AOC-20i), equipped with the mass selective detector, having ion source temperature of 230 °C, interface temperature of 270 °C, a solvent cut time of 3.50 min threshold of 1000 eV and mass range of 40 to 650 m/z. Compounds were separated using a Rtx 5 MS capillary column (Restek Company, Bellefonte, USA: cross bond 5% diphenyl/95% dimethyl polysiloxane) having dimensions 30 m (length) × 0.25 mm (diameter) × 0.25 μm (film thickness). The split mode at a ratio of 10:1 was used. The temperature of the injector was initialized to 260 °C, having a split injection mode. The temperature was programmed from 80 °C (2 min), then further increased to 250 °C at a ramp rate of 10 °C/min (5 min hold) and finely increased to 280 °C at a ramp rate of 15 °C/min (24 min hold). Helium (> 99.999%) was used as the carrier gas at a linear flow velocity of 40.5 cm/s. The debit of gas (helium) vector with a total flow of 1.21 mL/min was fixed to 16.3 mL/min. The volume of the injected sample was 2 μL of methanol extract. Total MS running time was 46 min.
Proteins and ligands for in silico docking
The major compounds identified in UPLC-Q-TOF-MS/MS and GCMS compounds and a standard hepatoprotective drug known as silibinin were used as ligand for molecular docking analysis. The three-dimensional (3d) structure of the compounds was downloaded from Pubchem database (https://pubchem.ncbi.nlm.nih.gov) in sdf file then converted into Pdb file through Open Babel Gui. The protein structure (3d) of the enzyme alanine aminotransaminase with pdb id 3IHJ was downloaded from protein data bank (https://www.rcsb.org/) in pdb format.
Molecular docking analysis
The molecular docking studies of all selected compounds and proteins were performed with Auto doc tools 1.5.6 (Morris 2009). The interaction between ligand and protein were assessed on the basis of binding energies.
Evaluation of drug likeness and ADMET properties
The drug likeness properties of the compounds were evaluated by Lipinski’s rule of five (http://scfbio-iitd.res.in/software/drugdesign/lipinski.jsp#anchortag) (Lipinski et al. 1997). ADMET properties of compounds viz; adsorption, distribution, metabolism, excretion, and toxicity were evaluated using ADMETlab2 https://admetmesh.scbdd.com/service/evaluation/cal to know the pharmacologically potent hepatoprotective lead molecule (Xiong et al. 2021). For ADMET properties evaluation, different parameters viz; blood–brain absorption (BBB), human intestinal absorption (HIA), AMES toxicity, and carcinogenicity were used for scoring.
Statistical analysis
All experiments were performed in triplicate and data were expressed as means ± SE. Statistical comparisons were made by means of one-way ANOVA test followed by post hoc analysis with Dunnett's test by using SPSS (version 16). P < 0.05, P < 0.01 was considered a significant difference, while P > 0.05 was considered to be non-significant. EC50 values were calculated from linear regression analysis.
Results
DPPH radical scavenging assay
All three extracts of leaf, stem and root have significant free radical scavenging activity (Table 1). Lowest EC50 value was observed in leaf extract of ethyl acetate of rainy season (301.23 ± 1.25 µg/ml) compared to other extracts in their respective season. Antioxidant activity of rainy season ethyl acetate extract was higher compared to summer and winter extracts. Leaf sample of summer and winter season showed EC50 values 345.46 ± 1.35 and 369.12 ± 1.23 µg/ml respectively. EC50value of leaf sample extracted in ethanol during rainy season was 485.04 ± 1.32 µg/ml, while for summer and winter season extracts EC50 values was 498.14 ± 1.23 and 505.13 ± 1.23 µg/ml respectively. In case of methanol and aqueous extract of leaf, EC50 value of different seasonal extracts was in this order rainy < summer < winter. If we compare the EC50value of DPPH free radical scavenging activity of different extracts of stem, the trend was in this order ethyl acetate < ethanol < methanol < aqueous extract. On the basis of comparison of EC50 values of different root extracts the range was as follows: ethanol extract (EC50 = 489.16–490.16) < ethyl acetate (EC50 = 502.56–507.29) < methanol (EC50 = 998.12–999.89) < aqueous extract (EC50 = 1603.28–1608.19). Antioxidant activity of rainy season ethanol extract of root was higher (EC50 = 489.16 ± 0.29) compared to summer (EC50 = 490.18 ± 0.18) and winter (EC50 = 490.10 ± 1.67) extracts.
Table 1.
Comparative DPPH free radical scavenging activity of leaf, stem, and root extracts of P. integrifolia in different seasons
| Concentration (µg/ml) | Ethanol extract | Methanol extract | ||||
|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | |
| Inhibition Percentage (Leaf) | ||||||
| 50 | 1.50 ± 0.68** | 1.01 ± 0.35** | 0.93 ± 0.61** | 3.30 ± 0.39** | 2.85 ± 0.19** | 2.67 ± 1.23** |
| 100 | 12.83 ± 0.75** | 12.53 ± 0.83** | 11.56 ± 0.12** | 12.07 ± 0.87** | 11.54 ± 1.34** | 11.23 ± 0.56** |
| 200 | 22.10 ± 0.83** | 21.23 ± 0.25** | 21.63 ± 1.32** | 19.59 ± 0.50** | 19.01 ± 1.02** | 18.67 ± 0.34** |
| 300 | 27.31 ± 0.52** | 26.12 ± 1.23** | 26.10 ± 3.12** | 28.22 ± 0.66** | 27.12 ± 0.56** | 26.65 ± 0.19** |
| 400 | 39.53 ± 0.14** | 38.35 ± 0.14** | 37.15 ± 2.12** | 34.07 ± 0.84** | 32.83 ± 0.12** | 31.83 ± 1.32** |
| 500 | 51.82 ± 0.83** | 50.65 ± 1.32** | 49.48 ± 1.23** | 47.46 ± 1.33** | 46.45 ± 1.53** | 45.32 ± 2.12** |
| 600 | 59.23 ± 2.35** | 53.43 ± 0.75** | 51.67 ± 1.23** | 51.26 ± 1.12** | ||
| 700 | ||||||
| EC50 | 485.02 ± 1.32 | 498.14 ± 1.23 | 505.13 ± 1.26 | 578.34 ± 2.43 | 585.35 ± 2.12 | 588.59 ± 2.10 |
| Inhibition Percentage (Stem) | ||||||
| 50 | 11.50 ± 0.57* | 10.91 ± 2.65* | 10.34 ± 1.23** | 2.84 ± 0.36* | 1.94 ± 1.26** | 1.35 ± 1.25* |
| 100 | 17.88 ± 0.56** | 16.89 ± 0.19** | 16.43 ± 1.23 ns | 5.05 ± 0.68* | 4.85 ± 2.12** | 3.98 ± 1.23** |
| 200 | 23.24 ± 0.34* | 23.01 ± 0.34* | 22.01 ± 2.13* | 19.30 ± 0.74* | 18.21 ± 0.56* | 17.21 ± 0.56* |
| 300 | 31.56 ± 0.53** | 30.65 ± 0.19** | 29.83 ± 1.23** | 27.40 ± 0.57** | 26.15 ± 1.25** | 24.25 ± 1.35* |
| 400 | 41.65 ± 1.01* | 41.12 ± 1.01** | 38.96 ± 2.12 ns | 34.56 ± 0.95* | 32.85 ± 1.19** | 30.83 ± 1.23* |
| 500 | 53.31 ± 0.67* | 52.67 ± 1.26** | 50.12 ± 1.56** | 42.36 ± 1.15** | 39.12 ± 1.26* | 36.95 ± 0.35** |
| 600 | 52.19 ± 0.56** | 48.56 ± 1.36** | 46.35 ± 1.38** | |||
| 700 | 63.32 ± 0.27** | 58.45 ± 1.26** | 56.84 ± 0.83** | |||
| 800 | ||||||
| 1000 | ||||||
| 1200 | ||||||
| 1400 | ||||||
| 1600 | ||||||
| 1800 | ||||||
| 2000 | ||||||
| 2200 | ||||||
| EC50 | 485.15 ± 0.75 | 496.26 ± 1.23 | 503.12 ± 2.13 | 615.12 ± 1.26 | 645.15 ± 1.26 | 668.12 ± 2.01 |
| Inhibition Percentage (root) | ||||||
| 50 | 5.16 ± 1.26** | 5.05 ± 0.75* | 4.98 ± 0.19** | 11.48 ± 0.61* | 10.86 ± 0.27* | 10.87 ± 1.12** |
| 100 | 14.01 ± 1.03** | 13.67 ± 1.27** | 14.03 ± 1.19* | 12.53 ± 0.57* | 12.21 ± 0.47** | 13.01 ± 0.21* |
| 200 | 33.12 ± 0.12 ns | 32.18 ± 1.34 ns | 33.12 ± 1.45* | 16.53 ± 0.66* | 14.45 ± 1.27** | 14.26 ± 1.27* |
| 300 | 45.12 ± 1.23** | 44.72 ± 1.19* | 44.74 ± 0.18** | 20.12 ± 0.17* | 19.87 ± 0.26* | 18.76 ± 0.57* |
| 400 | 57.36 ± 1.17* | 57.12 ± 0.34** | 57.19 ± 1.19 ns | 25.66 ± 0.38* | 23.23 ± 2.01** | 24.27 ± 1.28** |
| 500 | 28.1 ± 0.51 ns | 28.41 ± 0.31* | 28.18 ± 0.57 ns | |||
| 600 | 31.17 ± 0.43* | 29.96 ± 1.29* | 29.65 ± 0.29* | |||
| 800 | 43.16 ± 0.34* | 43.12 ± 0.57** | 43.17 ± 0.17* | |||
| 1000 | 52.58 ± 0.51* | 51.12 ± 1.24** | 52.17 ± 0.56* | |||
| 1200 | ||||||
| 1400 | ||||||
| 1600 | ||||||
| EC50 | 489.16 ± 0.29 | 490.18 ± 0.18 | 490.10 ± 1.67 | 998.12 ± 2.18 | 1001.19 ± 0.19 | 999.89 ± 1.34 |
| Concentration (µg/ml) | Ethyl acetate extract | Aqueous extract | ||||
|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | |
| Inhibition Percentage (Leaf) | ||||||
| 50 | 22.00 ± 0.48** | 20.56 ± 0.81** | 19.31 ± 1.26** | 0.70 ± 0.22** | 0.60 ± 1.21** | 0.52 ± 1.12** |
| 100 | 24.86 ± 0.62** | 24.86 ± 1.23** | 23.12 ± 2.15** | 4.25 ± 1.05** | 3.65 ± 0.62** | 3.12 ± 1.23** |
| 200 | 40.14 ± 0.45** | 39.12 ± 0.41** | 37.12 ± 0.56** | 10.17 ± 0.60** | 9.64 ± 0.65** | 8.64 ± 0.12** |
| 300 | 50.25 ± 0.54** | 48.35 ± 0.54** | 46.95 ± 1.23** | 20.65 ± 0.87** | 19.46 ± 1.34** | 18.12 ± 2.15** |
| 400 | 61.34 ± 0.56** | 57.32 ± 1.35** | 54.12 ± 1.26** | 27.62 ± 0.46** | 25.12 ± 3.12** | 25.00 ± 3.12** |
| 500 | 36.84 ± 0.75** | 35.53 ± 1.23** | 35.12 ± 1.23** | |||
| 600 | 43.75 ± 0.98** | 42.12 ± 1.12** | 40.95 ± 1.12** | |||
| 700 | 52.47 ± 0.69** | 50.84 ± 1.26** | 50.12 ± 1.26** | |||
| EC50 | 301.23 ± 1.25 | 345.46 ± 1.35 | 369.12 ± 1.23 | 715 ± 1.26 | 765 ± 0.83 | 777.05 ± 1.23 |
| Inhibition Percentage (Stem) | ||||||
| 50 | 17.93 ± 0.87** | 16.38 ± 1.23** | 15.12 ± 1.23** | 0.47 ± 0.16** | 0.46 ± 0.23** | 0.43 ± 0.32** |
| 100 | 23.00 ± 0.50* | 22.56 ± 0.83** | 20.18 ± 0.83** | 1.02 ± 0.42* | 1.21 ± 0.31** | 1.31 ± 0.29** |
| 200 | 33.80 ± 0.66* | 31.45 ± 1.23** | 29.83 ± 1.23** | 2.99 ± 0.29** | 2.801 ± 1.21* | 2.58 ± 1.12** |
| 300 | 43.86 ± 1.21 ns | 40.95 ± 2.15 ns | 38.35 ± 0.34** | 4.21 ± 0.17 ns | 4.51 ± 0.42** | 3.83 ± 0.11** |
| 400 | 53.49 ± 0.66* | 49.85 ± 1.23* | 46.35 ± 2.15** | 5.07 ± 0.19* | 4.95 ± 0.34 * | 4.39 ± 0.38** |
| 500 | 63.23 ± 0.39 ns | 58.32 ± 0.86** | 56.38 ± 1.23 ns | 6.72 ± 0.45** | 6.42 ± 0.51 ns | 6.72 ± 0.39* |
| 600 | 8.52 ± 0.25** | 8.12 ± 0.21** | 8.02 ± 0.35** | |||
| 700 | 11.21 ± 0.29 ns | 10.79 ± 0.42** | 10.68 ± 0.39 ns | |||
| 800 | 14.82 ± 0.44** | 14.15 ± 0.23** | 14.12 ± 0.35** | |||
| 1000 | 19.57 ± 0.22* | 18.95 ± 1.12* | 18.05 ± 1.21** | |||
| 1200 | 27.98 ± 0.42 ns | 27.12 ± 1.12** | 26.83 ± 1.12* | |||
| 1400 | 32.65 ± 0.61** | 31.95 ± 0.12** | 30.98 ± 1.12** | |||
| 1600 | 38.86 ± 0.34** | 37.98 ± 1.25** | 35.12 ± 0.53** | |||
| 1800 | 43.72 ± 0.60* | 43.12 ± 1.12* | 42.12 ± 0.83* | |||
| 2000 | 51.94 ± 1.00** | 50.12 ± 1.21** | 49.32 ± 1.25** | |||
| 2200 | 61.61 ± 0.39** | 58.61 ± 0.39** | 56.23 ± 1.30** | |||
| EC50 | 398.12 ± 2.05 | 427.12 ± 1.35 | 445.23 ± 2.08 | 2015 ± 1.32 | 2041 ± 1.32 | 2105 ± 1.35 |
| Inhibition Percentage (root) | ||||||
| 50 | 8.81 ± 0.37* | 9.18 ± 0.34** | 9.20 ± 1.23** | 6.34 ± 0.38* | 6.32 ± 0.29** | 6.15 ± 0.17** |
| 100 | 12.54 ± 0.23** | 12.63 ± 0.12* | 12.67 ± 0.83** | 8.29 ± 0.51 ns | 7.94 ± 0.27* | 8.21 ± 0.41** |
| 200 | 24.66 ± 0.46* | 23.15 ± 1.26* | 23.25 ± 1.35 ns | 10.84 ± 0.32** | 10.89 ± 1.29** | 9.98 ± 0.18* |
| 300 | 31.40 ± 0.48** | 30.56 ± 1.21* | 30.12 ± 1.35** | 14.21 ± 0.52 ns | 13.21 ± 0.27 ns | 13.14 ± 0.57 ns |
| 400 | 44.77 ± 0.60 ns | 44.85 ± 1.26* | 44.01 ± 0.83 ns | 17.55 ± 0.99* | 17.49 ± 0.97** | 17.50 ± 1.45** |
| 500 | 51.78 ± 0.79* | 50.12 ± 1.38* | 50.12 ± 0.83* | 18.21 ± 0.21 ns | 17.44 ± 0.17 ns | 18.10 ± 0.26* |
| 600 | 20.33 ± 0.51** | 19.76 ± 0.28** | 19.67 ± 1.28** | |||
| 800 | 26.58 ± 0.10* | 26.87 ± 0.16* | 26.29 ± 1.29** | |||
| 1000 | 31.23 ± 0.51** | 31.00 ± 0.32** | 31.29 ± 1.21 ns | |||
| 1200 | 35.94 ± 0.40* | 35.87 ± 1.34* | 34.76 ± 0.19** | |||
| 1400 | 43.47 ± 0.61* | 42.35 ± 0.83* | 42.19 ± 0.29* | |||
| 1600 | 51.38 ± 0.46** | 51.19 ± 0.53* | 51.19 ± 0.18** | |||
| EC50 | 502.56 ± 1.23 | 505.53 ± 0.17 | 507.29 ± 0.19 | 1603.28 ± 1.26 | 1605.16 ± 0.29 | 1608.19 ± 0.28 |
EC50 value of ascorbic acid is 54.23 ± 2.35; *Significant P < 0.05, **P < 0.01 and P > 0.05 non-significant (ns)
Superoxide radical scavenging assay
Results revealed that leaf, stem and root exhibited potent scavenging activity for superoxide radicals in a concentration dependent manner (Table 2). Superoxide scavenging activity of leaf extract was higher compared to stem and root extracts as evident from EC50 values. Among leaf extracts, ethyl acetate extract has a lower range of EC50values (349.12 ± 1.26–383.19 ± 1.67) compared to ethanol (477.15 ± 0.12–495.18 ± 0.12), methanol (677.02 ± 1.26–697.25 ± 0.27) and aqueous (1096.12 ± 2.10–1112.45 ± 0.67) extracts. In conclusion, among all extracts, ethyl acetate extract of leaf of rainy season exhibited (EC50 = 349.12 ± 1.26) highest potential for superoxide radical scavenging activity compared to stem (EC50 = 707.12 ± 0.27) and root (EC50 = 733.91 ± 1.21) extracts.
Table 2.
Comparative superoxide radical scavenging activity of leaf, stem, and root extracts of P. integrifolia in different seasons
| Concentration (µg/mL) | Ethanol extract | Methanol extract | ||||
|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | |
| Inhibition Percentage (Leaf) | ||||||
| 50 | 10.55 ± 1.00* | 10.05 ± 0.23* | 9.79 ± 0.15** | 3.36 ± 0.48** | 3.12 ± 1.28** | 3.02 ± 1.16** |
| 100 | 27.55 ± 0.84** | 26.18 ± 0.45 ns | 24.96 ± 0.29 ns | 11.53 ± 0.48 ns | 11.21 ± 1.67* | 11.10 ± 1.27 ns |
| 200 | 38.55 ± 1.35* | 38.01 ± 1.23** | 37.09 ± 0.19** | 20.03 ± 0.55** | 18.98 ± 0.17* | 18.54 ± 0.28 ns |
| 300 | 47.84 ± 2.15 ns | 45.18 ± 0.34* | 45.12 ± 0.29 ns | 27.08 ± 0.73* | 26.45 ± 1.12 ns | 26.23 ± 0.38 ns |
| 400 | 58.33 ± 2.00* | 54.98 ± 0.13** | 54.01 ± 0.67** | 32.69 ± 0.96** | 31.90 ± 1.28** | 30.76 ± 0.86** |
| 500 | 38.46 ± 1.26** | 37.67 ± 1.67** | 36.89 ± 2.19 ns | |||
| 600 | 44.71 ± 0.10** | 43.18 ± 1.89 ns | 43.01 ± 0.16** | |||
| 700 | 52.24 ± 0.99** | 51.23 ± 1.28** | 50.98 ± 0.45** | |||
| 800 | ||||||
| 900 | ||||||
| 1000 | ||||||
| EC50 | 477.15 ± 0.12 | 487.35 ± 1.23 | 495.18 ± 0.12 | 677.02 ± 1.26 | 689.17 ± 0.18 | 697.25 ± 0.27 |
| Inhibition Percentage (Stem) | ||||||
| 50 | 2.52 ± 0.31** | 2.19 ± 0.45** | 2.10 ± 0.29* | 3.79 ± 1.02 ns | 3.17 ± 0.28* | 3.04 ± 0.28** |
| 100 | 7.20 ± 0.82* | 6.89 ± 0.18* | 6.36 ± 1.10* | 9.39 ± 2.01** | 9.28 ± 0.45 ns | 8.97 ± 1.70** |
| 200 | 12.97 ± 0.54* | 12.45 ± 0.28** | 12.10 ± 1.02* | 13.75 ± 1.20* | 13.34 ± 0.19** | 13.02 ± 0.34 ns |
| 300 | 21.44 ± 1.35* | 20.86 ± 0.18 ns | 20.18 ± 0.37 ns | 16.54 ± 0.77** | 15.98 ± 0.28* | 15.65 ± 0.28* |
| 400 | 30.26 ± 1.95** | 30.18 ± 1.07** | 29.67 ± 0.67* | 19.46 ± 0.67 ns | 18.97 ± 0.29** | 18.54 ± 0.19** |
| 500 | 38.19 ± 0.62** | 37.89 ± 0.29 ns | 37.84 ± 1.23** | 23.71 ± 0.77* | 23.65 ± 1.29 ns | 22.97 ± 1.89 ns |
| 600 | 45.07 ± 0.65* | 44.89 ± 0.18** | 44.18 ± 1.43* | 25.35 ± 0.36** | 25.19 ± 0.18** | 25.19 ± 0.29** |
| 700 | 50.62 ± 0.83** | 50.56 ± 0.18* | 49.19 ± 0.29** | 31.31 ± 1.54** | 30.97 ± 0.29** | 30.78 ± 0.34* |
| 800 | 58.08 ± 1.78* | 36.46 ± 3.20** | 35.34 ± 1.49 ns | 35.12 ± 0.56 ns | ||
| 900 | 44.63 ± 1.55* | 44.42 ± 1.45* | 44.12 ± 0.34** | |||
| 1000 | 51.22 ± 1.93* | 50.67 ± 0.28** | 50.23 ± 1.23** | |||
| 1200 | ||||||
| 1400 | ||||||
| 1600 | ||||||
| EC50 | 727.23 ± 0.26 | 738.18 ± 1.29 | 746.13 ± 1.91 | 1094.12 ± 1.23 | 1105.11 ± 0.18 | 1109.00 ± 0.10 |
| Inhibition Percentage (Root) | ||||||
| 50 | 3.82 ± 0.48** | 3.56 ± 0.18* | 3.45 ± 0.18* | 3.36 ± 0.48** | 3.31 ± 0.28* | 3.29 ± 0.28** |
| 100 | 14.36 ± 1.91 ns | 14.15 ± 0.38** | 13.98 ± 0.28** | 11.53 ± 0.48** | 11.20 ± 0.19** | 11.10 ± 0.19* |
| 200 | 21.36 ± 0.54* | 20.89 ± 0.18** | 20.29 ± 0.28** | 20.03 ± 0.55* | 19.89 ± 0.28** | 19.28 ± 0.19** |
| 300 | 33.17 ± 0.73* | 32.67 ± 0.49* | 32.78 ± 0.19* | 27.08 ± 0.73* | 26.98 ± 0.19 ns | 26.34 ± 0.49** |
| 400 | 41.62 ± 0.96 ns | 41.07 ± 0.39** | 40.19 ± 0.29** | 32.69 ± 0.96** | 32.65 ± 1.04* | 31.19 ± 0.29 ns |
| 500 | 52.26 ± 3.58* | 51.67 ± 0.38** | 51.29 ± 0.19* | 38.46 ± 1.26** | 37.19 ± 0.89* | 37.19 ± 0.39** |
| 600 | 44.71 ± 0.00* | 44.13 ± 0.29* | 43.96 ± 0.39** | |||
| 700 | 52.24 ± 0.99** | 51.01 ± 0.29* | 50.98 ± 0.59 ns | |||
| 800 | ||||||
| 900 | ||||||
| 1000 | ||||||
| 1200 | ||||||
| EC50 | 485.12 ± 0.67 | 496.10 ± 0.19 | 499.10 ± 0.14` | 677.02 ± 1.26 | 684.19 ± 0.29 | 686.02 ± 0.29 |
| Concentration (µg/mL) | Ethyl acetate extract | Aqueous extract | |||||
|---|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | ||
| Inhibition Percentage (Leaf) | |||||||
| 50 | 12.51 ± 2.58** | 11.23 ± 0.34 ns | 10.69 ± 0.19 ns | 0.87 ± 0.31* | 0.81 ± 1.26* | 0.80 ± 1.10** | |
| 100 | 18.53 ± 1.09 ns | 16.90 ± 1.13** | 16.78 ± 0.14** | 2.02 ± 0.21** | 1.98 ± 0.45** | 1.95 ± 0.38** | |
| 200 | 43.68 ± 0.68** | 43.12 ± 0.67* | 42.67 ± 1.17 ns | 6.36 ± 0.33* | 6.12 ± 1.34* | 6.28 ± 1.20** | |
| 300 | 52.32 ± 1.87** | 51.56 ± 2.13* | 50.29 ± 2.01** | 8.29 ± 0.27 ns | 8.01 ± 0.28* | 7.87 ± 0.18 ns | |
| 400 | 16.59 ± 1.13 ns | 15.98 ± 1.16 ns | 15.85 ± 0.18** | ||||
| 500 | 25.24 ± 1.45** | 25.21 ± 1.45** | 25.12 ± 0.29 ns | ||||
| 600 | 27.98 ± 0.13** | 25.95 ± 0.18** | 25.07 ± 1.20* | ||||
| 700 | 36.40 ± 0.17 ns | 34.38 ± 1.28** | 34.30 ± 1.10* | ||||
| 800 | 44.34 ± 0.33** | 43.18 ± 0.18** | 42.17 ± 0.28** | ||||
| 900 | 47.89 ± 0.45* | 45.98 ± 0.29 ns | 44.29 ± 0.19** | ||||
| 1000 | 59.55 ± 0.31** | 57.06 ± 1.17** | 56.89 ± 0.29** | ||||
| EC50 | 349.12 ± 1.26 | 376.45 ± 0.24 | 383.19 ± 1.67 | 1096.12 ± 2.10 | 1105.29 ± 1.34 | 1112.45 ± 0.67 | |
| Inhibition Percentage (Stem) | |||||||
| 50 | 6.32 ± 1.40* | 5.97 ± 0.56* | 5.24 ± 0.56* | 0.47 ± 0.64** | 0.42 ± 0.17* | 0.42 ± 0.6* | |
| 100 | 14.14 ± 1.16* | 13.76 ± 1.23 ns | 13.34 ± 0.56* | 2.5 ± 0.34** | 2.18 ± 0.45** | 2.10 ± 1.76* | |
| 200 | 19.92 ± 0.85** | 19.01 ± 0.56* | 18.89 ± 0.56 ns | 7.10 ± 0.12** | 7.10 ± 0.28 ns | 7.10 ± 0.28 ns | |
| 300 | 25.32 ± 0.85 ns | 24.98 ± 0.34* | 24.45 ± 1.79* | 10.12 ± 0.31 ns | 9.98 ± 1.45* | 9.76 ± 0.17* | |
| 400 | 32.40 ± 0.56* | 31.12 ± 0.34 ns | 31.01 ± 0.45* | 12.70 ± 0.55* | 11.96 ± 1.29** | 11.45 ± 0.13* | |
| 500 | 39.66 ± 1.48** | 38.56 ± 2.01* | 38.53 ± 0.34 ns | 15.50 ± 0.50** | 15.01 ± 0.29 ns | 14.89 ± 0.28* | |
| 600 | 47.41 ± 0.46** | 45.87 ± 1.03* | 45.54 ± 1.24** | 18.01 ± 0.19* | 17.86 ± 1.67 ns | 17.85 ± 0.56 ns | |
| 700 | 53.81 ± 0.31* | 52.02 ± 0.34 ns | 51.76 ± 0.78* | 21.43 ± 0.11 ns | 21.23 ± 0.54** | 21.21 ± 0.87** | |
| 800 | 24.56 ± 0.34** | 24.29 ± 0.18 ns | 23.79 ± 0.56* | ||||
| 900 | 28.50 ± 0.38** | 28.36 ± 0.28* | 28.01 ± 0.11** | ||||
| 1000 | 33.09 ± 0.62** | 32.19 ± 0.28* | 32.12 ± 0.45* | ||||
| 1200 | 37.01 ± 0.55** | 36.89 ± 0.24* | 36.56 ± 0.23** | ||||
| 1400 | 42.57 ± 0.44** | 42.10 ± 0.18* | 41.23 ± 0.34** | ||||
| 1600 | 52.48 ± 0.47* | 50.96 ± 0.29* | 50.23 ± 0.22** | ||||
| EC50 | 707.12 ± 0.27 | 719.32 ± 0.13 | 725.19 ± 0.17 | 1638.45 ± 0.18 | 1645.19 ± 1.27 | 1651.67 ± 0.23 | |
| Inhibition Percentage (Root) | |||||||
| 50 | 3.95 ± 0.69** | 3.85 ± 0.29* | 3.80 ± 1.29** | 2.86 ± 0.95* | 2.78 ± 0.29** | 2.67 ± 0.45* | |
| 100 | 8.82 ± 0.61** | 8.76 ± 0.29* | 8.67 ± 0.29* | 7.96 ± 2.21** | 7.94 ± 1.29** | 7.70 ± 0.34* | |
| 200 | 15.21 ± 0.80 ns | 15.10 ± 0.29** | 15.00 ± 0.29 ns | 13.07 ± 0.72** | 12.78 ± 0.19* | 12.70 ± 0.29* | |
| 300 | 23.43 ± 0.60 ns | 23.21 ± 0.39 ns | 23.01 ± 0.29 ns | 16.90 ± 0.72 ns | 16.87 ± 0.29* | 16.82 ± 0.19* | |
| 400 | 29.98 ± 0.15** | 29.39 ± 0.32* | 29.19 ± 0.21** | 22.32 ± 0.27** | 22.13 ± 0.29** | 22.10 ± 0.29 ns | |
| 500 | 36.52 ± 0.69* | 36.32 ± 0.39** | 36.29 ± 0.19* | 28.06 ± 0.27 ns | 27.45 ± 0.38 | 27.43 ± 0.45 ns | |
| 600 | 46.72 ± 0.94 ns | 46.29 ± 1.23 ns | 45.78 ± 0.29** | 32.37 ± 0.73** | 32.32 ± 0.34* | 31.76 ± 0.45** | |
| 700 | 56.31 ± 1.84** | 54.43 ± 0.19** | 54.89 ± 0.87* | 36.04 ± 0.73 ns | 35.01 ± 0.67* | 35.00 ± 0.67 ns | |
| 800 | 42.34 ± 0.42 ns | 42.02 ± 1.76* | 41.89 ± 0.45** | ||||
| 900 | 48.32 ± 1.26* | 48.23 ± 0.23 ns | 48.29 ± 0.29* | ||||
| 1000 | 51.99 ± 0.27** | 51.35 ± 0.45 ns | 51.46 ± 0.29 ns | ||||
| 1200 | 55.65 ± 0.99** | 55.12 ± 0.45* | 54.87 ± 0.38** | ||||
| EC50 | 733.91 ± 1.21 | 742.19 ± 0.38 | 743.19 ± 0.19 | 978.01 ± 0.62 | 987.12 ± 0.63 | 990.43 ± 0.19 | |
EC50 of copper sulphate is 109 ± 0.21; *Significance P < 0.05, **P < 0.01 and P > 0.05 non-significant (ns)
Lipid peroxidation assay
If we compare the EC50 values of lipid peroxidation of different extracts derived from different parts of plant, we can say that leaf extracts showed lower range of EC50 values (339.12 ± 0.23–599.19 ± 1.18) compared to stem (EC50 = 648.24 ± 0.38–1367.99 ± 0.54) and root (EC50 = 485.12 ± 0.67– 991.88 ± 0.65) extracts. It means leaf extract showed better inhibition of lipid peroxidation compared to stem and root extracts. Among all extracts, leaf ethyl acetate extracts exhibited lower range of EC50 value (339.12 ± 0.23–354.00 ± 0.13) compared to ethanol (503.15 ± 0.19–531.37 ± 1.12), methanol (579.56 ± 0.76–589.14 ± 0.56) and aqueous (585.23 ± 0.45–599.19 ± 1.18) extracts. EC50 values of ethyl acetate extracts obtained in different seasons are in the order rainy (339.12 ± 0.23) < summer (348.43 ± 0.25) < winter (354.00 ± 0.13). So, we can conclude that ethyl acetate extract of leaves derived during rainy season has highest lipid peroxidation inhibition (Table 3).
Table 3.
Comparative lipid peroxidation activity of leaf, stem, and root extracts of P. integrifolia in different seasons
| Concentration (µg/ml) | Ethanol extract | Methanol extract | ||||
|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | |
| Inhibition Percentage (Leaf) | ||||||
| 50 | 3.75 ± 1.35** | 3.43 ± 0.76** | 3.29 ± 1.01* | 14.57 ± 2.15* | 14.28 ± 0.29** | 14.12 ± 1.56* |
| 100 | 6.51 ± 1.21* | 6.29 ± 0.29** | 6.19 ± 0.12** | 21.78 ± 0.68** | 21.23 ± 0.18** | 20.89 ± 0.37* |
| 200 | 20.79 ± 0.21** | 19.89 ± 0.23* | 18.28 ± 1.29* | 24.80 ± 1.40** | 23.94 ± 0.18** | 22.45 ± 0.28** |
| 300 | 30.32 ± 1.92* | 29.97 ± 0.29* | 29.18 ± 2.19** | 31.24 ± 0.56* | 30.19 ± 0.17** | 30.10 ± 0.29** |
| 400 | 41.47 ± 2.06* | 40.49 ± 0.28** | 40.19 ± 1.28** | 37.30 ± 0.65** | 36.20 ± 0.53* | 35.95 ± 0.39** |
| 500 | 52.75 ± 1.89** | 51.02 ± 1.26* | 50.78 ± 0.18* | 41.47 ± 1.50** | 41.00 ± 0.65* | 41.00 ± 0.29* |
| 600 | 52.83 ± 1.13** | 51.26 ± 0.18** | 50.87 ± 0.27** | |||
| 700 | ||||||
| EC50 | 503.15 ± 0.19 | 527.54 ± 0.26 | 531.37 ± 1.12 | 579.56 ± 0.76 | 587.19 ± 0.19 | 589.14 ± 0.56 |
| Inhibition Percentage (Stem) | ||||||
| 50 | 1.67 ± 0.33* | 1.65 ± 1.02** | 1.47 ± 0.67* | 4.19 ± 0.17* | 4.10 ± 1.20** | 4.01 ± 0.36** |
| 100 | 4.39 ± 0.38** | 3.98 ± 0.28** | 3.37 ± 0.48** | 6.96 ± 0.19** | 6.46 ± 0.29* | 6.34 ± 0.28** |
| 200 | 8.31 ± 0.39** | 8.19 ± 0.17ns | 8.11 ± 0.34** | 11.47 ± 0.50ns | 11.17 ± 0.25* | 11.56 ± 0.45ns |
| 300 | 17.68 ± 0.57* | 17.28 ± 1.11ns | 16.57 ± 0.49* | 15.39 ± 0.57** | 15.32 ± 0.29** | 15.35 ± 0.56* |
| 400 | 28.03 ± 0.44** | 27.39 ± 0.26ns | 26.89 ± 0.45ns | 20.93 ± 0.43* | 19.59 ± 0.92ns | 18.79 ± 0.88ns |
| 500 | 38.13 ± 0.18** | 37.75 ± 0.38* | 37.27 ± 0.28ns | 26.69 ± 0.51** | 26.16 ± 1.27** | 26.19 ± 0.27** |
| 600 | 47.99 ± 0.59** | 47.10 ± 1.45* | 47.00 ± 2.01** | 33.34 ± 1.18* | 33.18 ± 0.22** | 33.00 ± 0.84** |
| 700 | 56.58 ± 1.67** | 54.87 ± 0.28** | 54.03 ± 1.56* | 41.39 ± 0.93** | 40.11 ± 0.33* | 39.79 ± 0.19** |
| 800 | 47.81 ± 0.71** | 46.28 ± 0.28** | 45.09 ± 0.98ns | |||
| 900 | 56.63 ± 1.64** | 55.35 ± 0.56* | 54.93 ± 0.29* | |||
| 1000 | ||||||
| 1200 | ||||||
| 1400 | ||||||
| EC50 | 684.23 ± 0.12 | 698.57 ± 0.34 | 704.34 ± 0.57 | 856.19 ± 0.39 | 875.12 ± 0.33 | 882.18 ± 1.22 |
| Inhibition Percentage ( Root) | ||||||
| 50 | 3.82 ± 0.48** | 3.32 ± 0.33** | 3.33 ± 0.98** | 3.36 ± 0.48** | 3.25 ± 0.19** | 3.18 ± 0.46* |
| 100 | 14.35 ± 1.91* | 14.10 ± 0.18ns | 14.01 ± 1.11ns | 11.53 ± 0.48** | 11.18 ± 0.57* | 11.01 ± 0.36* |
| 200 | 21.36 ± 0.54* | 20.96 ± 0.29* | 19.98 ± 0.28* | 20.03 ± 0.55** | 19.52 ± 0.19** | 19.16 ± 0.33** |
| 300 | 33.17 ± 0.73* | 32.11 ± 0.19* | 32.00 ± 0.55* | 27.08 ± 0.73* | 26.75 ± 0.15* | 25.89 ± 0.45* |
| 400 | 41.62 ± 0.96** | 40.29 ± 0.24** | 39.88 ± 0.19* | 32.69 ± 0.96** | 30.67 ± 0.16ns | 29.45 ± 0.67* |
| 500 | 53.26 ± 3.58** | 51.89 ± 1.28** | 50.89 ± 0.19* | 38.46 ± 1.23** | 38.18 ± 0.28ns | 37.49 ± 0.29* |
| 600 | 44.71 ± 0.10* | 43.11 ± 0.11** | 42.89 ± 1.19ns | |||
| 700 | 52.24 ± 0.99* | 50.78 ± 0.66** | 50.19 ± 0.88* | |||
| 800 | ||||||
| 900 | ||||||
| 1000 | ||||||
| 1100 | ||||||
| EC50 | 485.12 ± 0.67 | 499.67 ± 0.19 | 509.00 ± 0.35 | 677.02 ± 1.26 | 685.11 ± 0.33 | 692.11 ± 0.33 |
| Concentration (µg/ml) | Ethyl acetate extract | Aqueous extract | ||||
|---|---|---|---|---|---|---|
| Rainy | Summer | Winter | Rainy | Summer | Winter | |
| Inhibition Percentage (Leaf) | ||||||
| 50 | 1.61 ± 0.62** | 1.54 ± 0.29** | 1.38 ± 0.39** | 3.58 ± 2.35** | 3.34 ± 1.13** | 3.21 ± 0.61** |
| 100 | 10.20 ± 0.97* | 9.95 ± 0.49** | 9.85 ± 0.29** | 7.99 ± 1.06* | 7.67 ± 0.39ns | 7.65 ± 1.18ns |
| 200 | 24.97 ± 0.47** | 24.85 ± 0.13** | 24.28 ± 1.39* | 15.99 ± 0.61* | 15.75 ± 1.21** | 15.57 ± 1.02** |
| 300 | 40.30 ± 1.27** | 40.10 ± 0.29* | 40.10 ± 0.57* | 27.59 ± 1.38ns | 26.89 ± 0.49** | 26.82 ± 0.29** |
| 400 | 55.93 ± 1.61** | 54.89 ± 0.29** | 53.79 ± 0.48** | 37.75 ± 0.71** | 36.29 ± 0.23** | 36.01 ± 0.19* |
| 500 | 46.04 ± 1.38** | 46.00 ± 0.54** | 46.19 ± 0.25** | |||
| 600 | 50.55 ± 0.63** | 49.49 ± 0.53ns | 49.43 ± 0.28ns | |||
| 700 | 58.03 ± 0.34** | 57.31 ± 0.28** | 57.10 ± 0.29** | |||
| EC50 | 339.12 ± 0.23 | 348.43 ± 0.25 | 354.00 ± 0.13 | 585.23 ± 0.45 | 597.01 ± 0.32 | 599.19 ± 1.18 |
| Inhibition Percentage (Stem) | ||||||
| 50 | 8.03 ± 0.50* | 8.03 ± 0.44** | 7.76 ± 0.77** | 1.24 ± 0.19** | 1.12 ± 0.77** | 1.11 ± 0.22ns |
| 100 | 13.64 ± 0.61* | 13.13 ± 0.27* | 13.10 ± 0.29** | 2.31 ± 0.11* | 2.21 ± 0.39** | 2.19 ± 0.35** |
| 200 | 20.17 ± 0.13** | 20.17 ± 0.22** | 19.98 ± 0.57** | 6.63 ± 0.23** | 6.54 ± 0.32* | 6.47 ± 0.23* |
| 300 | 28.18 ± 0.04** | 27.87 ± 0.28** | 27.57 ± 0.29** | 10.36 ± 0.21** | 10.29 ± 0.39** | 9.97 ± 0.67ns |
| 400 | 35.48 ± 1.21* | 34.78 ± 0.29** | 32.90 ± 0.27* | 14.23 ± 0.11** | 14.28 ± 0.76* | 14.11 ± 0.22* |
| 500 | 43.09 ± 0.24* | 42.05 ± 0.24* | 41.00 ± 0.24** | 18.32 ± 0.18** | 18.10 ± 0.45** | 17.99 ± 0.55** |
| 600 | 53.54 ± 1.44** | 53.02 ± 0.55* | 52.19 ± 0.29* | 22.08 ± 0.29* | 21.89 ± 0.34ns | 21.08 ± 0.39** |
| 700 | 27.30 ± 0.54* | 27.01 ± 0.28* | 26.87 ± 0.29** | |||
| 800 | 30.45 ± 0.31* | 30.18 ± 0.29** | 30.19 ± 1.11** | |||
| 900 | 34.44 ± 0.37** | 34.28 ± 0.38** | 33.29 ± 0.29* | |||
| 1000 | 38.94 ± 0.40** | 38.38 ± 0.29* | 36.38 ± 0.39* | |||
| 1200 | 47.66 ± 0.62* | 47.10 ± 0.39** | 46.56 ± 0.24* | |||
| 1400 | 54.23 ± 0.87** | 54.10 ± 0.29ns | 53.00 ± 0.11** | |||
| EC50 | 648.24 ± 0.38 | 653.11 ± 0.22 | 664.11 ± 0.83 | 1345.45 ± 0.18 | 1358.09 ± 0.87 | 1367.99 ± 0.54 |
| Inhibition Percentage ( Root) | ||||||
| 50 | 3.95 ± 0.69* | 3.90 ± 0.44* | 3.54 ± 0.77** | 2.86 ± 0.95** | 2.32 ± 0.47* | 2.16 ± 0.29** |
| 100 | 8.82 ± 0.61** | 7.78 ± 0.22** | 7.23 ± 0.39* | 7.97 ± 2.21** | 7.28 ± 0.19* | 7.17 ± 0.22* |
| 200 | 15.21 ± 0.80** | 15.92 ± 0.44* | 15.88 ± 0.55* | 13.07 ± 0.72** | 12.49 ± 0.49* | 12.17 ± 0.33* |
| 300 | 23.43 ± 0.60* | 23.43 ± 0.47** | 23.42 ± 0.18* | 16.90 ± 0.72 | 16.59 ± 0.39* | 16.24 ± 0.44ns |
| 400 | 29.98 ± 0.15* | 28.88 ± 0.55** | 28.39 ± 1.66* | 22.32 ± 0.27** | 22.19 ± 0.29** | 21.89 ± 0.55* |
| 500 | 36.52 ± 0.69** | 36.22 ± 0.44* | 35.67 ± 0.59** | 28.06 ± 0.27** | 27.49 ± 0.42** | 27..11 ± 0.22** |
| 600 | 46.72 ± 0.94** | 45.89 ± 0.44** | 45.11 ± 1.89** | 32.37 ± 0.73** | 32.10 ± 0.29* | 31.98 ± 0.22* |
| 700 | 56.31 ± 1.84* | 54.98 ± 1.22** | 54.19 ± 1.66* | 36.04 ± 0.73** | 35.29 ± 0.39ns | 34.79 ± 0.27ns |
| 800 | 42.34 ± 0.42* | 42.10 ± 0.29** | 42.01 ± 0.44** | |||
| 900 | 48.32 ± 1.26* | 48.01 ± 1.01* | 47.01 ± 0.19* | |||
| 1000 | 51.99 ± 0.27** | 50.77 ± 0.22* | 50.56 ± 0.19** | |||
| 1100 | 55.65 ± 0.99** | 54.49 ± 0.49** | 53.89 ± 0.22** | |||
| EC50 | 733.91 ± 1.21 | 751.44 ± 0.33 | 757.99 ± 0.89 | 978.05 ± 0.62 | 988.11 ± 0.25 | 991.88 ± 0.65 |
EC50 value of ascorbic acid is 87.39 ± 0.61; *Significance P < 0.05, **P < 0.01 and P > 0.05 non-significant (ns)
Total phenolic content (TPC)
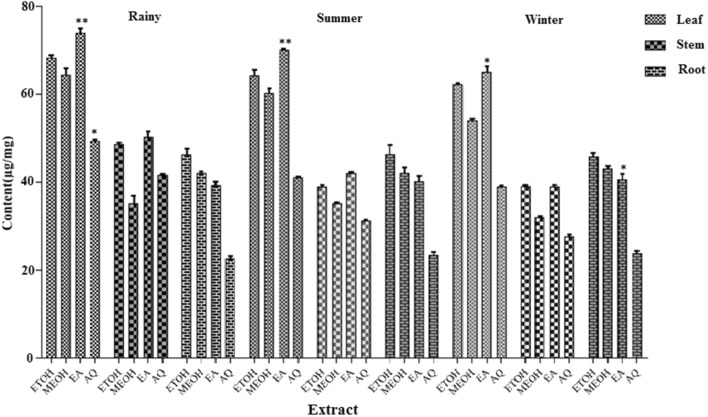
The phenolic compounds may contribute directly to antioxidant action (Awika et al. 2003). Total phenolic content was determined spectrophotometrically by Folin-ciocalteu method and reported as GAE in reference to standard curve (y = 0.003x–0.041, R2 = 0.945). In ethyl acetate extract of leaf, TPC was higher in rainy season which was 74.33 ± 2.26 mg g−1 followed by summer and winter having phenolic content 70.13 ± 0.26 mg g−1, 65.10 ± 1.26 mg g−1, respectively. In ethanolic extract of leaf the TPC was higher in rainy season which was 68.33 ± 0.56 mg g−1 while summer and winter had 64.3 ± 1.26 mg g−1, 62.32 ± 0.23 mg g−1, respectively. In methanol extract of leaf, TPC was higher in rainy season which was 64.43 ± 0.76 mg g−1 while by summer and winter had 60.3 ± 1.06 mg g−1, 54.06 ± 0.23 mg g−1, respectively. TPC in aqueous extract of leaf of rainy season was 49.0.35 ± 0.35 mg g−1 while by summer and winter season had 41.09 ± 0.20 mg g−1 and 39.00 ± 0.26 mg g−1. TPC in ethyl acetate extract of stem bark of rainy season was 50.33 ± 1.23 mg g−1 while summer and winter season had 42.09 ± 0.25 mg g−1 and 39.00 ± 0.36 mg g−1. TPC in ethanol extract of stem bark of rainy season was 48.66 ± 0.35 mg g−1 while summer and winter season had 39.09 ± 0.27 mg g−1 and 37.65 ± 0.44 mg g−1, respectively. TPC in methanolic extract of stem bark of rainy season was 46.33 ± 1.73 mg g−1 while summer and winter season had 35.19 ± 0.20 mg g−1 and 32.01 ± 0.26 mg g−1. TPC in aqueous extract of stem bark of rainy season was 41.66 ± 0.25 mg g−1 while summer and winter season had 31.29 ± 0.20 mg g−1 and 27.66 ± 0.46 mg g−1. Root extract, TPC was higher in ethyl acetate extract of summer season which was 46.35 ± 2.12 mg g−1 while rainy and winter season had 46.33 ± 1.32 mg g−1 and 45.84 ± 0.81 mg g−1, respectively, which indicates almost same value (Fig. 2).
Fig. 2.
Total phenol content in leaf, stem and root extracts of P. integrifolia in different seasons. Data are presented as mean ± SD. *P < 0.05; **P < 0.01 significantly different from the control
Total flavonoid content (TFC)
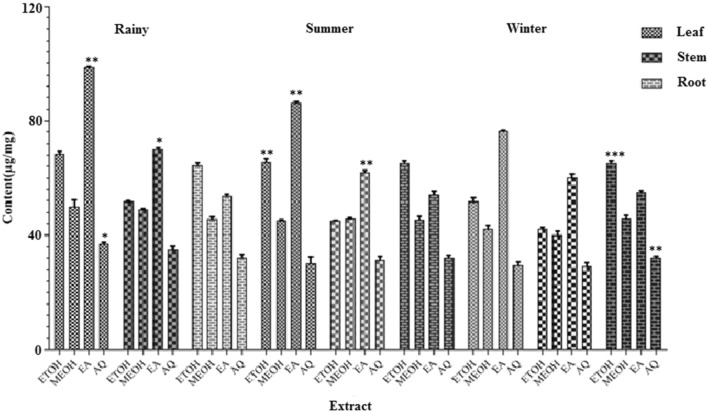
Total flavonoid content was determined by AlCl3 colorimetric method and rutin was used as standard for making curve (y = 0.006x–0.104, R2 = 0.966). Flavonoid content in different extracts of different parts during change in the season was represented in Fig. 3. Flavonoid content was higher in ethyl acetate extract of leaf of rainy season (98.83 ± 0.26 µg/mg) compared to summer (86.43 ± 0.43 µg/mg) and winter season (76.33 ± 0.23 µg/mg). In ethanol extract of leaf, flavonoid content in rainy season was observed higher (68.5 ± 0.19 µg/mg) compared to summer (65.5 ± 1.23 µg/mg) and winter (51.81 ± 0.82 µg/mg). In methanol extract of leaves of rainy season, flavonoid content was higher (50.12 ± 2.13 µg/mg) compared to summer (45.23 ± 0.56 µg/mg) and winter (42.12 ± 1.23 µg/mg). The flavonoid content in aqueous extracts of different seasons was in this order: rainy (37.16 ± 0.82 µg/mg) > summer (30.12 ± 2.31 µg/mg) > winter (29.43 ± µg/mg). Flavonoid content in different extracts of stem during rainy season was as follows: ethyl acetate (70.12 ± 0.56 µg/mg) > ethanol (51.81 ± 2.13 µg/mg) > methanol (49.16 ± 0.32 µg/mg) > aqueous extract (35.00 ± 0.32 µg/mg). Flavonoid content in ethanol, methanol, ethyl acetate and aqueous extracts of stem in summer season was 45.12 ± 0.12 µg/mg, 45.83 ± 0.53 µg/mg, 62.12 ± 0.73 µg/mg and 31.32 ± 1.32 µg/mg, respectively. Flavonoid content in ethanol extracts of roots of all three season was comparable. In rainy season, it was 64.33 ± 0.86 µg/mg, while in summer and winter flavonoid content was 65.12 ± 0.82 and 65.09 ± 0.82 µg/mg, respectively. TFC in methanol extract of rainy, winter and summer season was 45.66 ± 0.82, 45.45 ± 0.82, and 45.83 ± 1.21 µg/mg, respectively. Aqueous extract of winter contained lower flavonoid content (32.12 ± 0.39 µg/mg) compared to rainy (37.16 ± 0. 56 µg/mg) and summer season (32.15 ± 1.32 µg/mg). There was no significant difference in TFC of summer (65.12 ± 1.2 µg/mg) and winter (65.09.12 ± 0.13 µg/mg) extracts, while in rainy season extract it was 64.33 ± 0.43 µg/mg. TFC in ethyl acetate extract of root in rainy season it was 53.66 ± 1.13 µg/mg, while in summer and winter season it was 54.12 ± 0.41 and 55.01 ± 0.13 µg/mg, respectively. There was no significant difference in flavonoid content in methanol extract of root in rainy (45.66 ± 1.42 µg/mg), summer (45.45 ± 1.02 µg/mg) and winter (45.83 ± 0.73 µg/mg) seasons. Similarly, flavonoid content in aqueous extract of roots of all three season was almost similar and it was 32.16 ± 0.41, 32.15 ± 0.19 and 32.12 ± 0.57 µg/mg in rainy, winter and summer season, respectively (Fig. 3).
Fig. 3.
Total flavonoid content in leaf, stem and root extracts of P. integrifolia in different seasons. Data are presented as mean ± SD. *P < 0.05; **P < 0.01;***P < 0.001 significantly different from the control
Reducing power assay
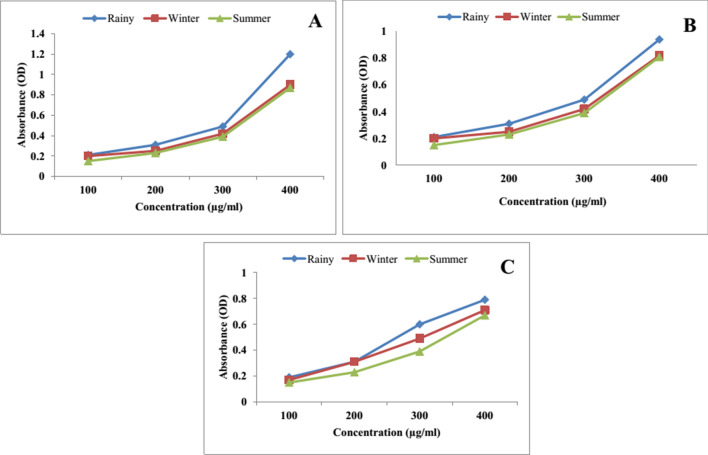
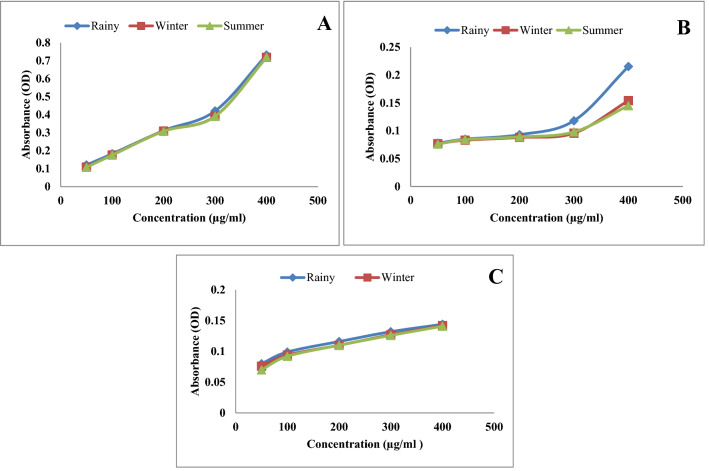
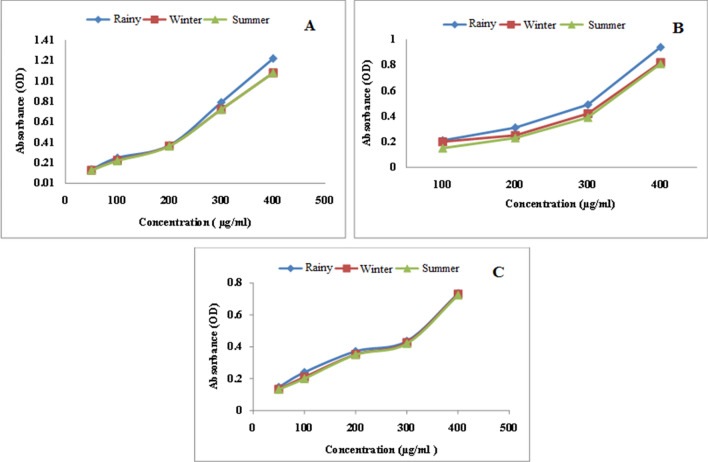
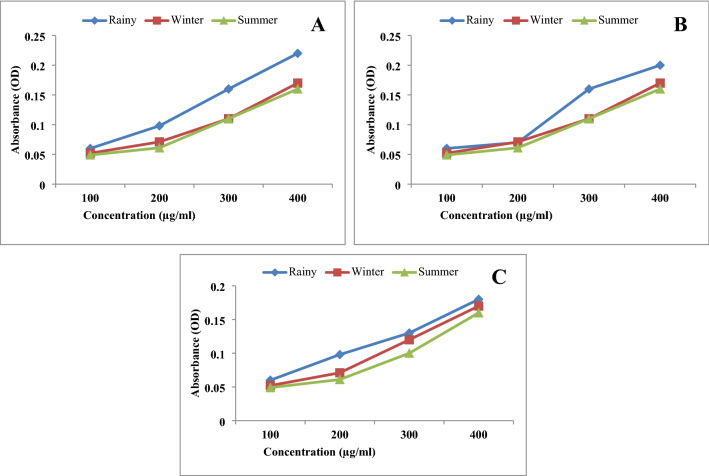
The reducing power of all the three extracts of leaf, stem and root in three different seasons like rainy, winter and summer, increased in concentration dependent manner as depicted in Figs. 3 and 4. Ethanol extract of leaf showed higher reducing potential in rainy season compared to summer and winter (Fig. 5a–c). Methanol extract of leaves of rainy season exhibited higher reducing potential compared to summer and winter season (Fig. 6a–c). Ethyl acetate extract of leaves of rainy season showed higher reducing potential compared to stem and root. Absorbance of rainy season's leaves extract was highest (OD = 1.21) (Fig. 6a–c). Aqueous extract of leaf showed higher reducing potential in rainy season compared to winter and summer season (Fig. 7a–c). On the basis of above results, it was observed that ethyl acetate extract of leaves during rainy season exhibited highest reducing potential.
Fig. 4.
Reducing power of ethyl acetate extract of a leaf, b stem and c root of P. integrifolia in different season
Fig. 5.
Reducing power of ethanol extract a leaf, b stem and c root of P. integrifolia in different season
Fig. 6.
Reducing power of methanol extract of a leaf, b stem and c root of P. integrifolia in different season
Fig. 7.
Reducing power of aqueous extract of a leaf, b stem and c root of P. integrifolia in different season
Correlation between total antioxidant activity and total polyphenol content
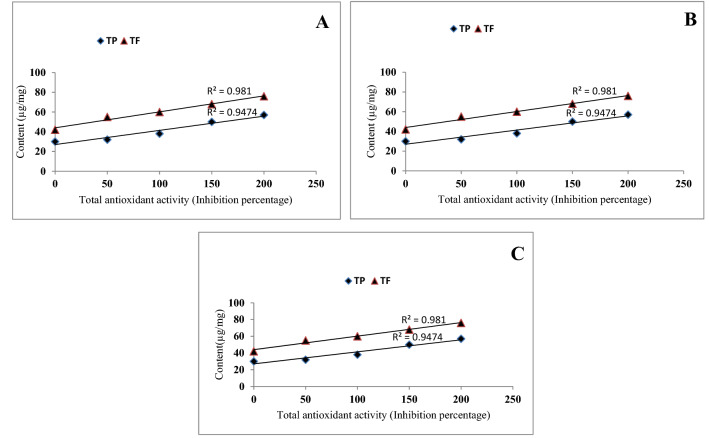
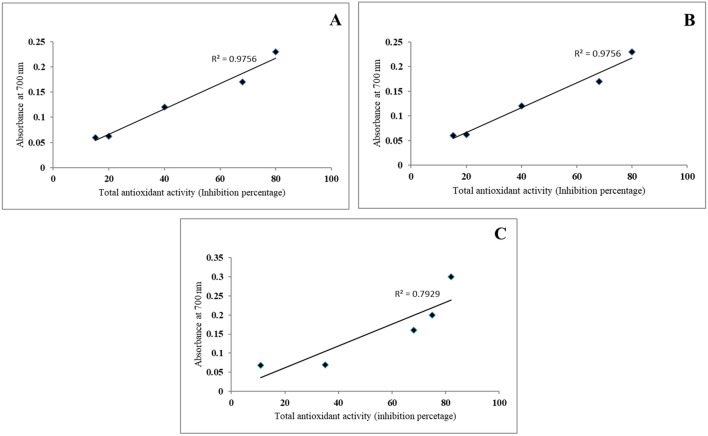
Ethyl acetate extracts of different parts of the plant during rainy season contained highest polyphenol content, so a correlation between the total phenol, flavonoid content and antioxidant activity in rainy season extracts was presented in Fig. 8. A positive correlation was observed between phenol and flavonoid content as well as total antioxidant activity in the ethyl acetate extract of leaf (R2 value ranges from 0.975 to 0.999). Similarly, the strong positive correlation was observed between antioxidant activity and phenol and flavonoid content in ethyl acetate extract of stem (R2 value ranges from 0.921 to 0.927) and root (R2 value ranges from 0.981 to 0.947), which is clear from the correlation coefficient (Fig. 8). A significant positive correlation was observed between ferric reducing antioxidant power (FRAP) and total antioxidant activity (R2 = 0.975) of rainy season leaf extract. Similarly, the ethyl acetate extract of stem (R2 = 0.948) and root (R2 = 0.792) of rainy season showed positive correlation with total antioxidant activity and ferric reducing power (Fig. 9). These results indicate that there was strong relationship between polyphenol content in leaf, stem and root with antioxidant activity. Therefore, in conclusion it was observed that presence of flavonoid and phenol in extracts contribute significant role for their antioxidant potential.
Fig. 8.
Correlation between total antioxidant activity and polyphenol content of rainy season ethyl acetate extract of leaf (a), stem (b) and root (c)
Fig. 9.
Correlation between total antioxidant activities and reducing power of rainy season ethyl acetate extract of leaf (a), stem (b) and root (c)
GC–MS analysis of EAEPI
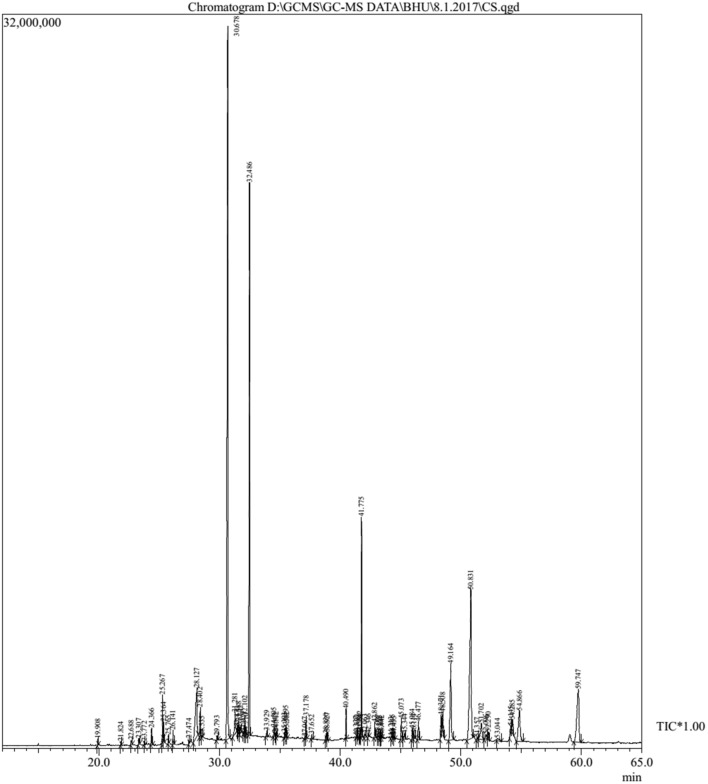
The result obtained from GC–MS analysis of rainy season ethyl acetate extract of leaves of P.integrifolia showed the presence of important phytoconstituents. The GC–MS chromatogram (Fig. 10) showed the presence of several peaks. It indicates about presence of bioactive compounds. The major compounds identified based on their retention time (RT), molecular formula, molecular weight, peak area percentage and bio activities as presented in Table 4. Among these identified compounds some major compounds with their percentage pick area (%) and retention times (min) were as follows: citronellol (29.1%, 30.67 min), phytol acetate (16%, 32.48 min), campesterol (12.5%, 50.83 min), phytol acetate (5.83%, 59.74 min), squalene (4.78%, 41.77%), stigmasterol (4.76%, 49.16 min) and hexadecanoic acid (4.52%, 28.12 min).
Fig. 10.
GC–MS total ion chromatogram of rainy season ethyl acetate extract of leaves of P. integrifolia
Table 4.
Bioactive compounds identified in ethyl acetate extract of leaves of P.integrifolia by GC–MS
| Peak | RT (min) | Name of the compounds | Area % | Chemical formula | Nature of the compounds | Bioactivity | References |
|---|---|---|---|---|---|---|---|
| 1 | 19.90 | 3-Octadecene, (E)- | 0.13 | C18H36 | Essential oil | Free radical scavenging activity | Adeosun et al. (2013) |
| 2 | 22.68 | 1-Tetradecanamine, N,N-dimethyl | 0.18 | C16H35N | Amine | Antibacterial activity | Idan et al. (2015) |
| 3 | 23.30 | 2(4H)-benzofuranone, 5,6,7,7a-tetrahydro-6-H | 0.20 | C11H16O3 | Furanone | Antimutagenic activity | Ganaie et al. (2016) |
| 4 | 23.77 | Myristic acid | 0.09 | C14H28O2 | Fatty acid | Antioxidant and cyclo oxygenase activity | Henry et al. (2002) |
| 5 | 24.36 | 1-Nonadecene | 0.47 | C19H38 | Long chain fatty acid | Anti-fungal activity | Poongulali and Sundararaman (2016) |
| 6 | 25.26 | 2,6,10-trimethyl,14-ethylene-14-pentadecne | 1.15 | C20H38 | Pentadecene | Antimutagenic activity | Ganaie et al. (2016) |
| 7 | 25.36 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.41 | C18H36O | Pentadecanone | Anti-asthmatic | Ogunlesi et al. (2009) |
| 8 | 25.76 | 2-hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R | 0.18 | C20H40O | Hexadecen-1-ol | Antimicrobial | Jananie et al. (2011) |
| 9 | 27.47 | 1-Hexadecen-3-ol, 3,5,11,15-tetramethyl- | 0.11 | C20H40O | Isophytol | Antimalarial and antifungal | Agnihotri et al. (2008) |
| 10 | 28.12 | Hexadecanoic acid | 4.52 | C16H32O2 | Palmitic acid | Antioxidant, Hypocholesterolemic Nematicide, Pesticide, Lubricant, Antiandrogenic, Hemolytic 5-Alpha reductase inhibitor | Sermakkani and Thangapandian (2012) |
| 11 | 28.40 | Palmitic acid | 0.87 | C24H48O2 | Fatty acid | Antitumor activity | Harada et al. (2002) |
| 12 | 30.67 | Citronellol | 29.1 | C20H40O | acyclic monoterpenoid | Anticancer, anti-inflammatory, promoting wound healing | Zhuang et al. (2009) |
| 13 | 31.28 | 9,12 Lienoleic acid | 1.30 | C18H32O2 | Fatty acid | Antioxidant | Leung and Liu (2000) |
| 14 | 31.74 | Stearic acid | 0.31 | C18H36O2 | Fatty acid | Control cholesterol | Bonanome and Grundy (1988) |
| 15 | 32.48 | Phytol, acetate | 16.4 | C22H42O2 | Phytol | Antimycobacterial | Rajab et al. (1998) |
| 16 | 40.49 | Celidoniol, deoxy- | 0.63 | C29H60 | straight-chain hydrocarbon | Antioxidant | Yulia et al. (2016) |
| 17 | 41.77 | Squalene | 4.78 | C30H50 | Triterpene | Hepatoprotective, Antibacterial, Antioxidant, Pesticide, Antitumor, Cancer preventive, Immunostimulant, Chemo preventive, Lipoxygenase-inhibitor | Sivakrishnan and Muthu (2014); Ko et al. (2002); Desai et al. (1996) |
| 18 | 45.07 | γ -Tocopherol | 0.73 | C28H48O2 | Vitamin | Traps mutagenic electrophiles | Leonetti et al. (2003) |
| 19 | 46.47 | dl-.alpha.-Tocopherol | 0.75 | C29H50O2 | Vitamine | Antioxidant, anti haemorrhage | Chiswick et al. (1983); Bottje et al. (1997) |
| 20 | 48.50 | Campestrol | 0.94 | C28H48O | Phytosterol | Antioxidant | Yoshida and Niki (2003); Safarpour et al. (2012) |
| 21 | 49.16 | Stigmasterol | 4.76 | C29H48O | Phytosterol | Antioxidant, Antitumor activity | Yoshida and Niki (2003); Ghosh et al. (2011) |
| 22 | 50.83 | Campesterol | 12.5 | C28H48O | Phytosterol | Prevention of cancer by antiangiogenic activity | Pandey (2016); Choi et al. (2007) |
| 23 | 51.70 | α Amyrin | 1.50 | C30H50O | Pentacyclic triterpenol | Antibacterial | Lajubutu et al. (1995); Saeed and Sabir (2001) |
| 24 | 52.09 | 5H-3,5a-Epoxynaphth [2,1-c]oxepin, dodecahydro-3,8,8,11a- | 0.27 | C18H30O2 | Antibacterial | Wang et al. (2011) | |
| 25 | 52.29 | Stigmasta-5, 22-dien-3-ol, acetate, (3.beta.)- | 0.40 | C31H50O2 | Ester | Antibacterial | Okeleye et al. (2013) |
| 26 | 59.74 | Phytol acetate | 5.83 | C22H42O2 | Ester | Antimycobacterial | Rajab et al. (1998) |
UPLC-Q-TOF–MS/MS analysis of EAEPI
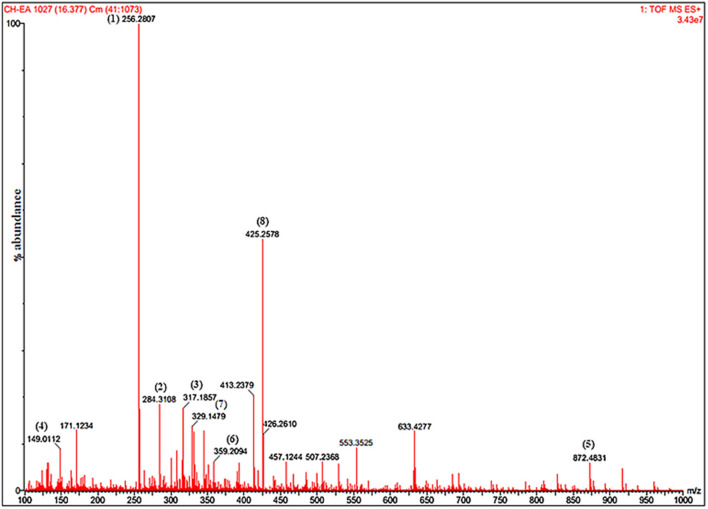
EAEPI was profiled for the presence of bioactive compounds by their mass through UPLC–Q-TOF–MS/MS (Fig. 10). The identified compounds with their biological activities were listed in Table 5. These compounds were tentatively identified based on their retention time, ESI [M-H-], MS/MS and m/z base ions. The compounds were identified by using the combination of mass spectrometry data and established database (http://spectra.psc.riken.jp/). Data revealed that EAEPI contained 8 polyphenolic compounds. These compounds with their m/z values were as follows: quercetin-3-Dxyloside (256), kaempferol-3,7-O-bis-alphaL-rhamnoside (284), isorhamnetin-3-O-glucoside (317), luteolin-3′,7-di-O-glucoside (149), eriodictyol-7-O-glucoside (872), syringetin-3-O-galactoside (359), petunidin-3-O-beta- glucopyranoside (329) and vitexin-2′′-O-rhamnoside (425). These compounds possess different pharmacological activities like antioxidant, hepatoprotective, antitumor, anticancer and antidiabetic, which is summarized in Table 5.
Table 5.
Major bioactive compounds identified in ethyl acetate extract of rainy season leaves of P. integrifolia using UPLC-MS/MS in + ve ion mode
| Peak No. | RT(min) | Tentative identification | Element Composition | M.W. (Da) | Ion (m/z) | Structure | Class | Reported activity | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.9 | Quercetin-3-D-xyloside | C20H18 | 434.35 | 256 |
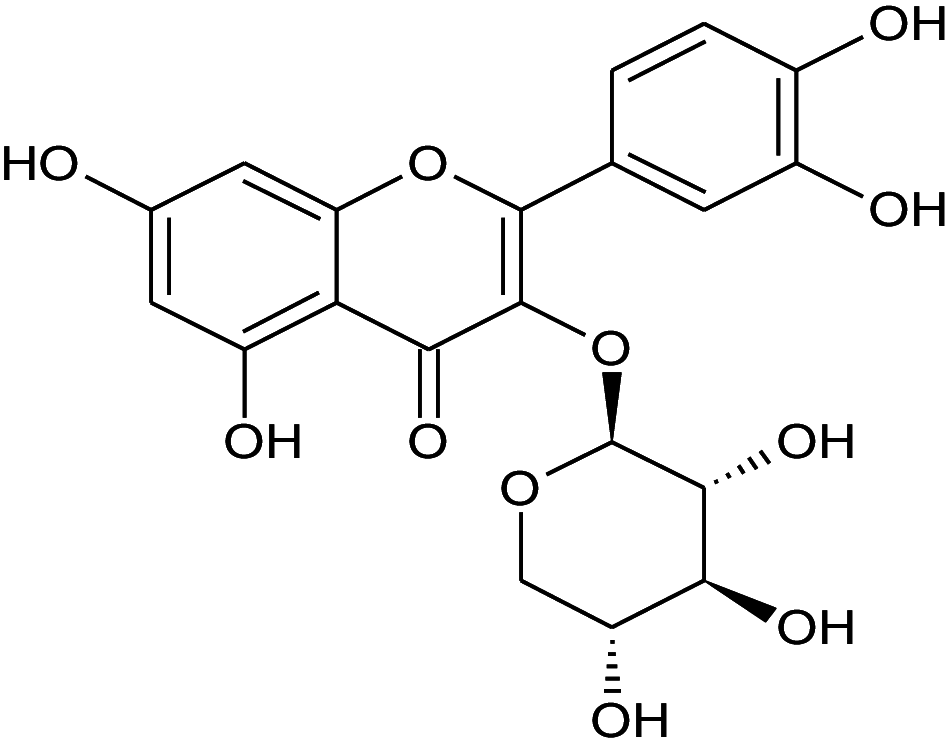
|
Flavonoid | Hepatoprotective | Choi et al. (2011) |
| 2 | 15.6 | Kaempferol-3,7-O-bis-alpha-L-rhamnoside | C27H30O14 | 578.51 | 284 |
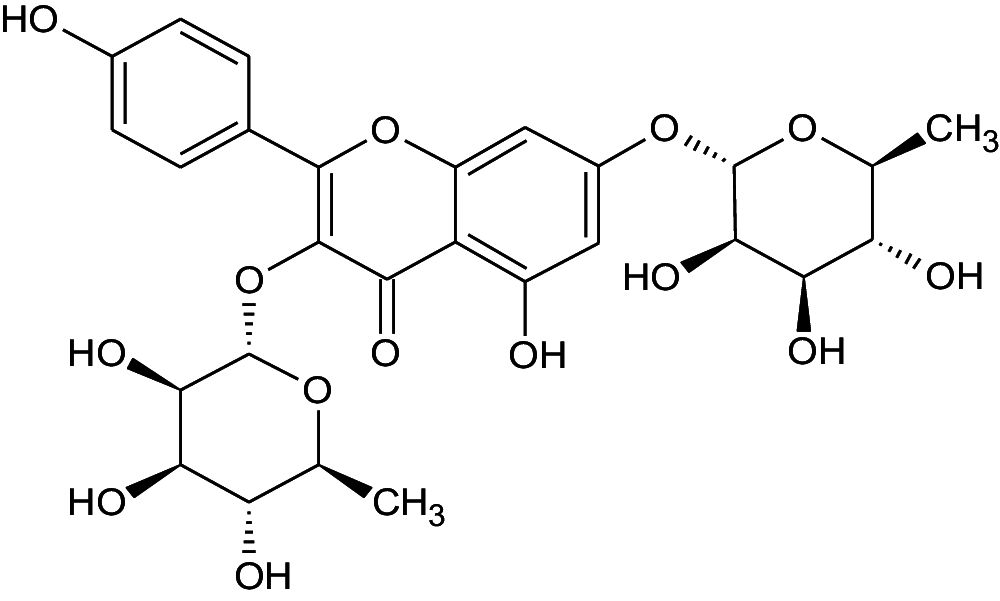
|
Flavonoid | Hepatoprotective | Wang et al. (2015) |
| 3 | 1.33 | Isorhamnetin-3-O-glucoside | C22H22 | 478.40 | 317 |
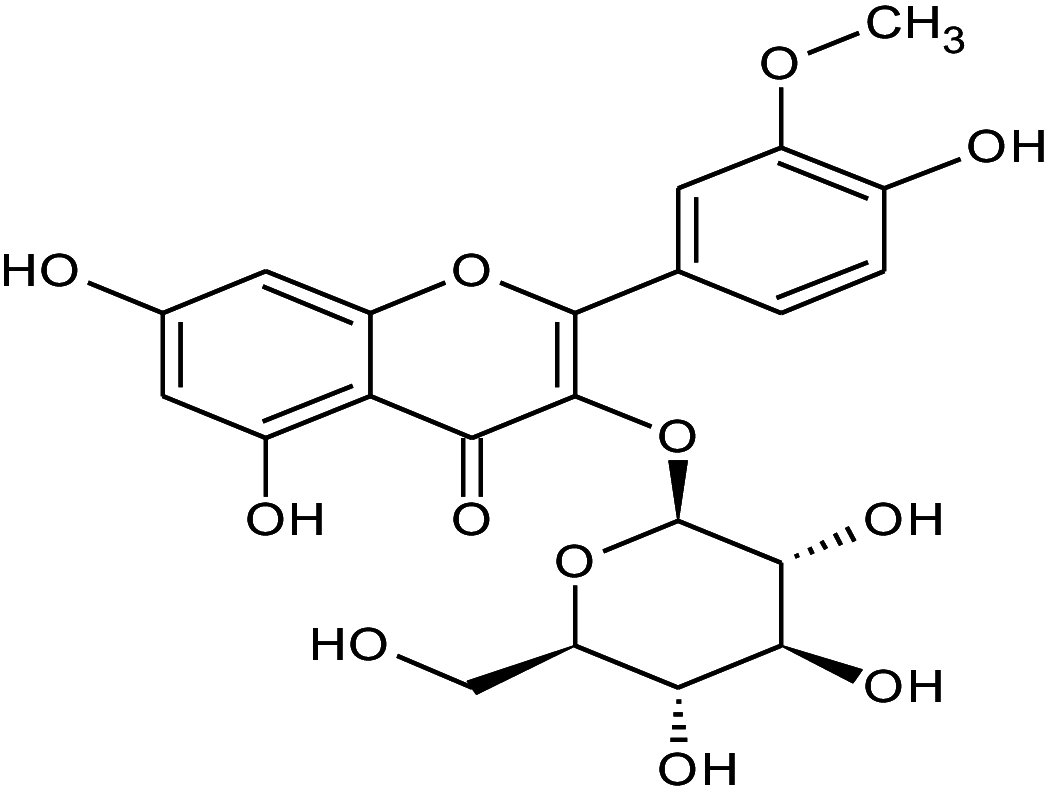
|
Flavonoid | Hepatoprotective, antiadipogenesis | Igarashi et al. (2008) |
| 4 | 4.34 | Luteolin-3',7-di-O-glucoside | C27H30 | 610.51 | 149 |
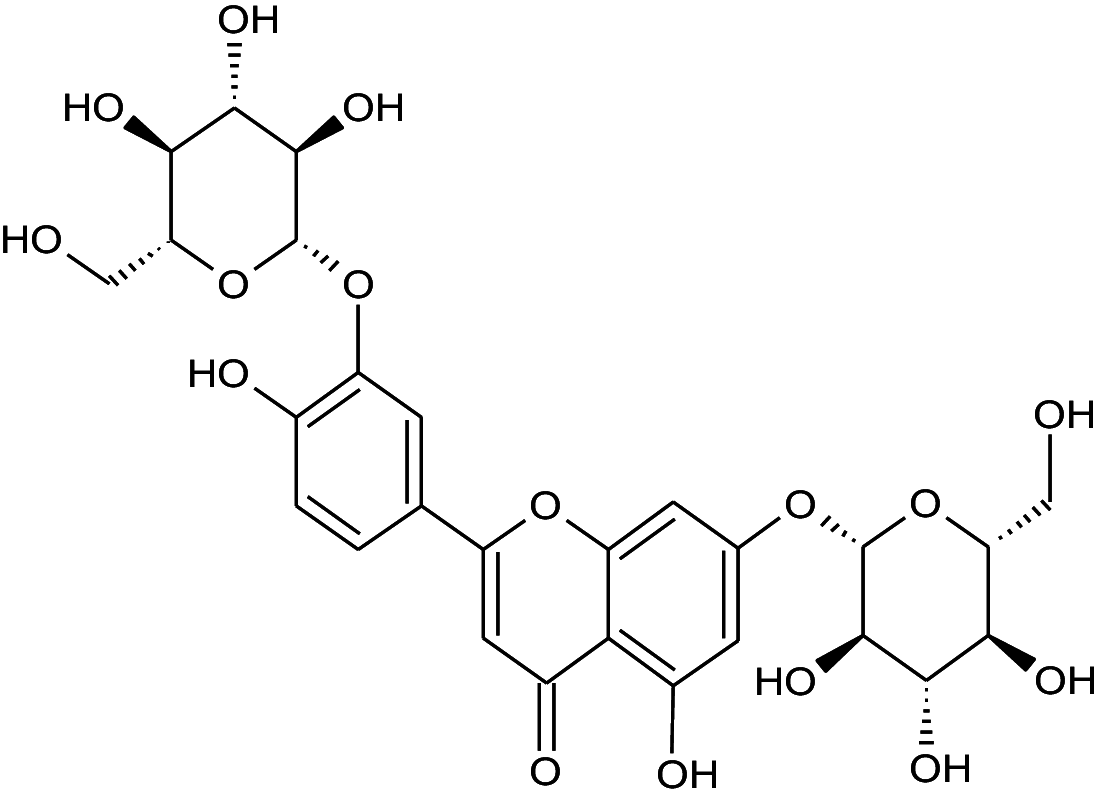
|
Flavonoid | Anticancer | López-Lázaro (2009) |
| 5 | 10.05 | Eriodictyol-7-O-glucoside | C21H22 | 450.39 | 872 |
|
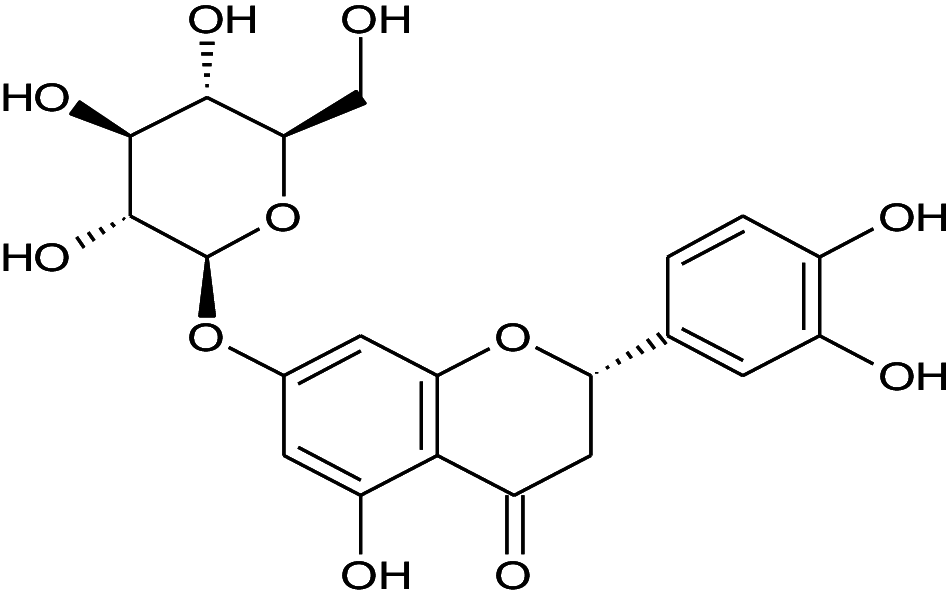
Flavonoid | Hepatoprotective and anticytotoxic | Hu et al. (2012) |
| 6 | 13.20 | Syringetin-3-O-galactoside | C23H24 | 508.42 | 359 |
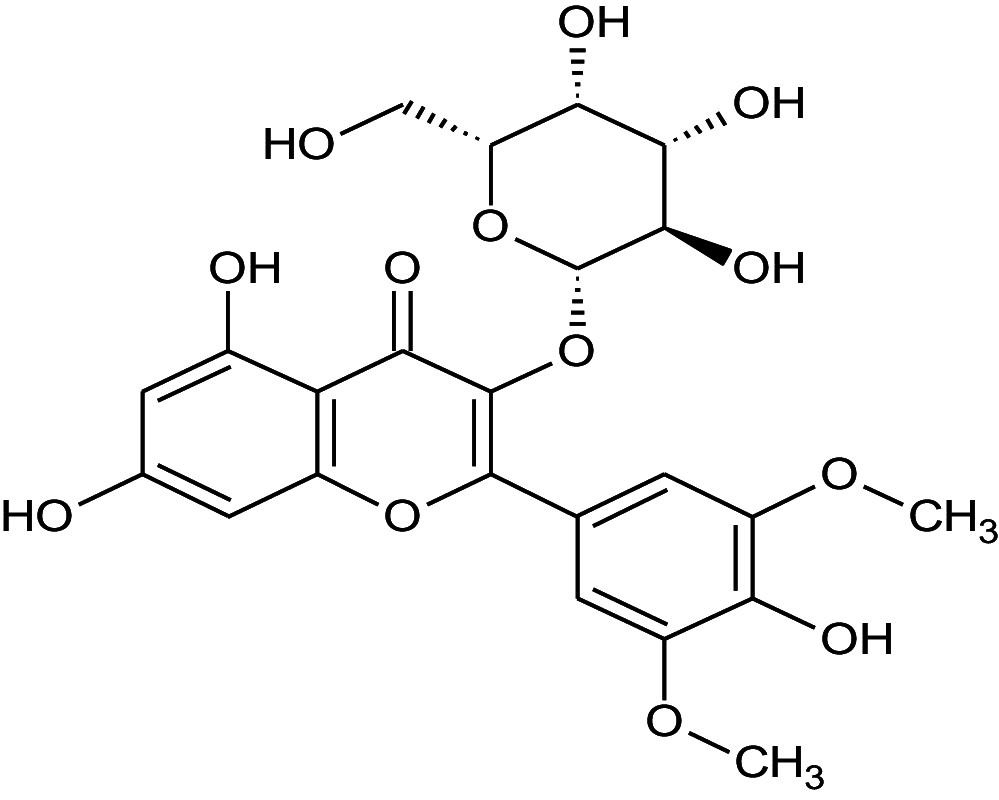
|
Flavonoid | Antiproliferative | Gómez-Alonso et al. (2012; Wu et al. (2012) |
| 7 | 15.89 | Petunidin-3-O-beta-glucopyranoside | C22H23 | 479.41 | 329 |
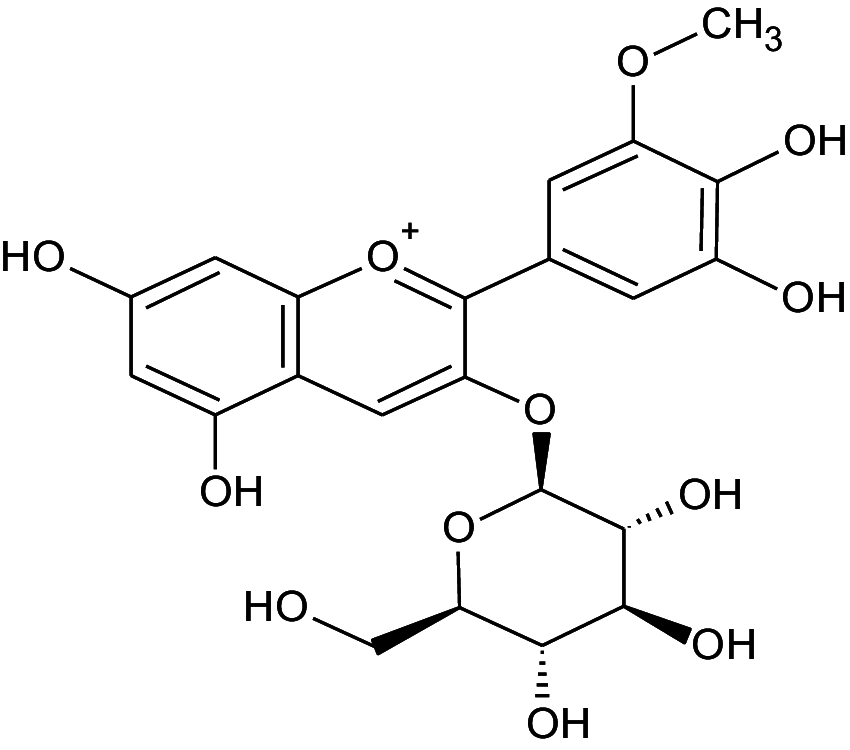
|
Flavonoid | Antioxidant | Kähkönen and Heinonen (2003) |
| 8 | 16.37 | Vitexin-2''-O-rhamnoside | C27H30 | 578.51 | 425 |
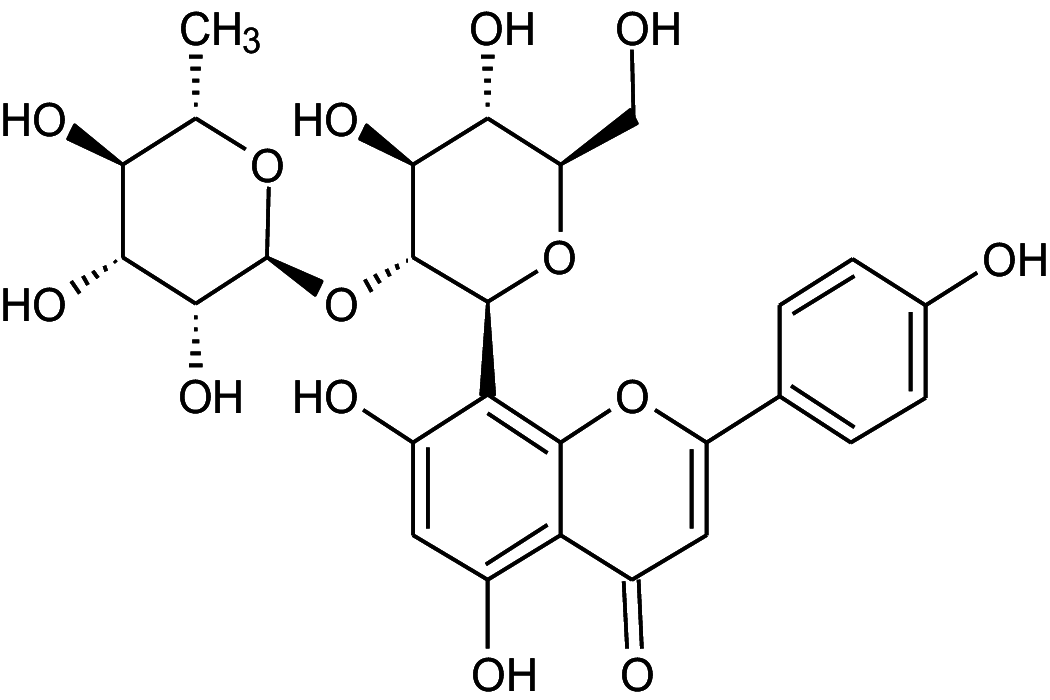
|
Flavonoid | Hepatoprotective,antiadipogenesis and antitumor | Igarashi et al. (2008) |
In silico docking analysis
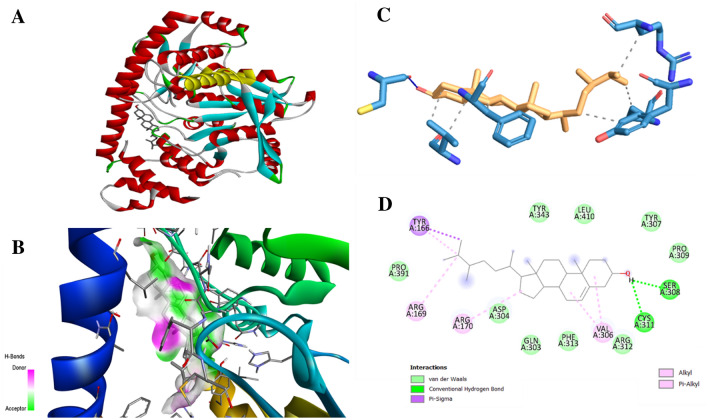
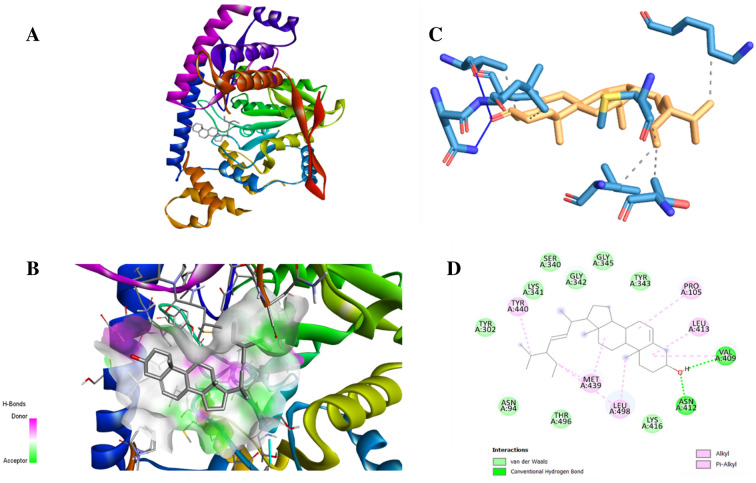
During in-silico docking analysis, three compounds stigmasterol, campesterol, and eriodictyol-7-O-glucoside were showing best binding potential with their binding energies − 8.98, − 8.67 and − 5.99 respectively (Table 6) (Fig. 11a and b). Their binding affinities were even higher than silibinin, the standard hepatoprotective compound.
Table 6.
Docking of Alanine amino transferase with major compounds identified in UPLC-MS/MS and GC–MS analysis
| S. No. | Comound name | ID | Binding energy | No. of hydrogen bonds involved in binding | Amino acids involved in bonding |
|---|---|---|---|---|---|
| 1 | Silibinin | CID31553 | − 5.63 | 3 | Glu 407 Asp 304 Ser 308 |
| 2 | Quercetin-3-D-xyloside | CID5320863 | − 4.36 | 4 | Tyr302, Ser340, Asp94, Tyr302 |
| 3 | Kaempferol-3,7-O-bis-alpha-L-rhamnoside | CID 5,323,562 | − 4.87 | 4 | Gln215, Tyr62, Ser220, Tyr62 |
| 4 | Isorhamnetin-3-O-glucoside | CID5318645 | − 4.10 | Nil | Nil |
| 5 | Eriodictyol-7-O-glucoside | CID13254473 | − 5.99 | 6 | Gly342, Asn412 |
| 6 | Vitexin-2''-O-rhamnoside | CID5282151 | − 3.96 | 4 | Gly345, Asn305, Asn94 |
| 7 | Citronellol | CID8842 | − 5.24 | 2 | Arg312, Arg370 |
| 8 | Phytol acetate | 6,428,538 | − 4.88 | 1 | Asn412 |
| 9 | Campestrol | 173,183 | − 8.67 | 2 | Ser 308, Cys311 |
| 10 | Squalene | 638,072 | − 5.69 | Nil | Nil |
| 11 | Stigmasterol | 5,280,794 | − 8.98 | 2 | Val 409, Asn412 |
| 12 | Hexadecanoic acid | − 3.25 | 2 | Asn412, Lys416 |
Fig. 11.
UPLC-MS/MS positive ion chromatogram of ethyl acetate extract of P. integrifolia leaves showing peaks of identified compounds with their m/z values. (1) Quercetin-3-Dxyloside (256), (2) Kaempferol-3,7-O-bis-alpha-L-rhamnoside (284), (3) Isorhamnetin-3-O-glucoside (317), (4) Luteolin-3′,7-di-O-glucoside (149), (5) Eriodictyol-7-O-glucoside (872), (6) Syringetin-3-O-galactoside (359), (7) Petunidin-3-O-beta-glucopyranoside (329) and (8) Vitexin-2′'-O-rhamnoside (425)
Drug likeness and ADMET Properties All the compounds showing best binding score with enzyme were also satistfying drug likeness criteria according to Lipinski’s rule of five except eriodictyol-7-O-glucoside (Table 7). The Lipinski’s rule differentiates between drug-like and non drug-like properties of compounds by compliance with 2 or more than two of five different parameters viz; molecular mass less than 500 Dalton, Log P value (indicator of lipophilic property) less than 5, hydrogen bond donor less than 5, hydrogen bond acceptor less than 10 and molar refractivity between 40 and 130. Among three compounds stigmasterol, campesterol, and eriodictyol-7-O-glucoside only two compounds stigmasterol, and campesterol were satisfying Lipinski’s rule of five while eriodictyol-7-O-glucoside was rejected by above criteria (Table 7). Besides drug likeness, any drug should fulfill ADMET parameters viz. absorption, distribution, metabolism, excretion and toxicity. It is evident from Table 8 that both stigmasterol, campesterol were showing best intestinal absorption and distribution. These two compounds were also non-ames toxic and non-carcinogenic. While the third compound eriodictyol-7-O-glucoside was toxic and carcinogenic with poor intestinal absorption (Table 8). Thus, it could not be recommended in pharmaceutical formulations.
Table 7.
Drug likeness properties of compounds
| Compound name | Molar mass | Hydrogen bond donor | Hydrogen bond acceptors | LOGP | Molar refractivity | Status |
|---|---|---|---|---|---|---|
| Eriodictyol-7-O-glucoside | 450.00 | 7 | 11 | 0.311 | 104.59 | Rejected |
| Campestrol | 400.00 | 1 | 1 | 7.63 | 123.59 | Accepted |
| Stigmasterol | 412.00 | 1 | 1 | 7.80 | 128.12 | Accepted |
Table 8.
ADMET properties of compounds
| Compound name | Blood brain barrier (BBB) | Human intestinal absorption (HIA) | AMES toxicity | Carcinogenicity | Status |
|---|---|---|---|---|---|
| Eriodictyol-7-O-glucoside | Average | Poor | Toxic | Carcinogenic | Rejected |
| Campestrol | Poor | Excellent | Nontoxic | Noncarcinogenic | Accepted |
| Stigmasterol | Poor | Excellent | Nontoxic | Noncarcinogenic | Accepted |
Discussion
Medicinal Plants are the rich sources of polyphenolic compounds. These polyphenolics act as antioxidants by scavenging free radicals and chelation of metal ions (Halliwell et al. 1995). Plant accumulates phenolic compounds under various stress conditions such as drought, heat, ultraviolet light exposure, air pollution and pathogens attack (Pasqualini et al. 2003). Changes in climatic conditions also influence the biosynthesis of polyphenol (Christie et al. 1994). The stresses enhance the production of reactive oxygen and nitrogen species in the body of the host plant. Host defends itself against ROS by escalating the biosynthesis of phenolic compounds (do Nascimento and Fett-Neto 2010). Temperature fluctuation from optimal level also cause alteration in physiology and biochemistry of several plants (Malhi et al. 1998). In present study, significant variation in total phenol and flavonoid content in different plant part extracts collected in different season have been recorded (Figs. 1 and 2). The polyphenol (TPC and TFC) content in all extracts derived from different plant parts varied with the seasonal variation. Phenolic content was highest in ethyl acetate extract leaf collected in rainy season, while it was lowest in winter season leaf extract. Since the microbial load enhances during the rainy season, so the biotic stress during rainy season might be responsible for higher polyphenol production in rainy season in P. integrifolia. Seasonal variation also affected the phenolic content in leaf exudates in Citrus species (Chaves et al. 1997). Plant parts extracted in different solvents showed steady increase in percentage inhibition by DPPH radicals with increasing concentration (Table 1). The degree of reduction in absorbance is the marker of the increased antioxidant capacity of the extract. Ethyl acetate extract of leaves and roots of rainy season depicted greater antioxidant activity compared to stem. Nearly similar variation pattern of EC50 value was obtained for stem and root sample extracted in ethanol methanol and aqueous extract. It was evident that season of collection of plant parts greatly affects the antioxidant activity towards DPPH free radical. Superoxide radical is very harmful species to cellular components because they are precursor of ROS (Buonocore et al. 2010). Current study revealed that all extracts are observed as effective scavenger of singlet oxygen generated in riboflavin-NBT-light system and their activity increased with increase in concentration of extracts (Table 2). In this assay ethyl acetate extract of leaves of rainy season has greater scavenging potential compared to other plant part extracts of different seasons. Ferric reducing antioxidant power (FRAP) is a useful assay for the measurement of antioxidant activity. FRAP was determined based on the colorimetric detection of the reaction mixture. Increase in the absorbance is the indication of the improved antioxidant activity (Mishra et al. 2016). In the present investigation, reducing potential of all extracts increased with increase in concentration, which was in close correlation with its observed antioxidant activity (Figs. 3, 4, 5, 6, 7, 8). Thus, this reducer might be responsible for antioxidant potential of extracts. Lipids are responsible for maintenance of cellular integrity. Results revealed that the rainy season ethyl acetate extract of leaves has higher inhibitory potential for lipid peroxidation compared to other plant parts (Table 3) The GC–MS analysis of ethyl acetate leaf extract of rainy season of P. integrifolia showed the presence of 26 compounds with seven major compounds (Table 4; Fig. 9). UPLC-Q-TOF–MS/MS analysis of above extract illustrated the presence of eight polyphenolic compounds whose antioxidant properties were reported earlier (Kähkönen and Heinonen 2003; Wu et al. 2012; Hu et al. 2012) (Table 5; Fig. 10).
Alanine amino transferase (ALT) test is normally used to check the hepatic damage such as hepatitis and cirrhosis. When the liver is damaged, the level of ALT rises in the liver and thereby in the bloodstream. Therefore, in present study, ALT was taken for the in-silico docking analysis against ligands. Among all compounds, three compounds stigmasterol, campesterol, and eriodictyol-7-O-glucoside were showing maximum binding energies against ALT (Table 6, Figs. 12 and 13). The residues were showing van der Walls, hydrophobic, hydrogen bond interactions which is presented in Figs. 12 and 13. Thus, it indicates that these three compounds have potential to protect hepatic damage. The two compounds stigmasterol and campesterol were satisfying the drug likeness properties according rule of the Lipinski’s five, while the eriodictyol-7-O-glucoside was rejected by Lipinski’s criteria (Table 7). Both compounds stigmasterol and campesterol were showing best absorption, distribution, and were non-toxic and non-carcinogenic (Table 8). While, eriodictyol-7-O-glucoside was showing poor intestinal absorption, and was observed as toxic and carcinogenic while ADMET scoring. This is the first report of the in-silico evaluation of hepatoprotective evaluation of stigmasterol and campesterol. There are reports supporting hepatoprotective activity of quercetin-3-D-xyloside, kaempferol-3,7-O-bis-alpha-L-rhamnoside, isorhamnetin-3-O-glucoside, vitexin-2''-O-rhamnoside, eriodictyol-7-O-glucoside (Table 5) but these compounds were not binding efficiently during in silico docking, which is evident from their binding energies (Table 6).
Fig. 12.
In silico interaction between campesterol and receptor alanine aminotransferase a docked complex, b complex showing the active site where ligand interacted, c 3D level interaction between receptor and campesterol, d 2D level interaction between receptor and campesterol
Fig. 13.
In silico interaction between stigmasterol and receptor alanine aminotransferase a docked complex, b complex showing the active site where ligand interacted, c 3D level interaction between receptor and stigmasterol, d 2D level interaction between receptor and stigmasterol
Conclusion
It can be concluded that polyphenol content and antioxidant activity was dependent on the season of collection of plant materials, parts of the plant used for extraction and nature of solvents used for extraction. Lipid peroxidation activity, superoxide radical scavenging activity and reducing power varied significantly based on season, solvent as well as plant parts. Results revealed that ethyl acetate extract of leaves of rainy season, contained highest total phenol and flavonoid content and showed highest antioxidant activity. UPLC-MS/MS analysis of EAEPI confirmed the presence of eight major bioactive compounds. GC–MS analysis reported the presence of twenty-six bioactive compounds with six major compounds. In silico docking analysis of major compounds against liver enzyme revealed the presence of two hepatoprotective lead compounds that can be used in hepatic drug formulations after clinical trials.
Acknowledgements
Authors CS and KNT would like to acknowledge University Grants Commission (UGC), Government of India, for financial assistance. Author KNT thankfully acknowledges to BHU Varanasi for providing financial assistance through incentive Grant under IoE scheme (6031) for research. Advance Instrumentation Research Facility (AIRF), Jawaharlal Nehru University, New Delhi acknowledged for providing GC-MS and UPLC/MS-MS facility.
Abbreviations
- EAEPI
Ethyl acetate extract of Premna integrifolia
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- NBT
Nitro blue tetrazolium
- TBARS
Thiobarbituric acid-reactive species
- TPC
Total phenolic content
- TFC
Total flavonoid content
- ALT
Alanine aminotransaminase
- ADMET
Absorption, Distribution, Metabolism, Excretion, Toxicity
Author contributions
CS designed the study, conducted the experiment, performs the analysis and wrote the paper. KNT supervisor of this study designed the study and reviewed drafts of the manuscript. RU performed the analysis and reviewed the final draft.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adeosun CB, Olaseinde S, Opeifa A, Atolani O. Essential oil from the stem bark of Cordia sebestena scavenges free radicals. JACME. 2013;3:138–141. [Google Scholar]
- Agnihotri VK, Elsohly HN, Khan SI, Jacob MR, Joshi VC, Smillie T, Khan IA, Walker LA. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem Lett. 2008;1:89–93. doi: 10.1016/j.phytol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51:6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med. 1988;318:1244–1248. doi: 10.1056/NEJM198805123181905. [DOI] [PubMed] [Google Scholar]
- Bose LV, George K, Iyer RS, Deepa T. Identification of novel cardiac principle in the roots of Premna serratifolia L. J Pharm Res. 2012;5:3261–3264. [Google Scholar]
- Bottje W, Erf G, Bersi T, Wang S, Barnes D, Beers K. Effect of dietary dl-alphatocopherol on tissue alpha-and gamma-tocopherol and pulmonary hypertension syndrome (ascites) in broilers. Poult Sci. 1997;76:1506–1512. doi: 10.1093/ps/76.11.1506. [DOI] [PubMed] [Google Scholar]
- Buonocore G, Perrone S, Tataranno ML. Seminars in fetal and neonatal medicine. Amsterdam: Elsevier; 2010. Oxygen toxicity: chemistry and biology of reactive oxygen species; pp. 186–190. [DOI] [PubMed] [Google Scholar]
- Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chaves N, Escudero JC, Gutierrez-Merino C. Role of ecological variables in the seasonal variation of flavonoid content of Cistus ladanifer exudate. J Chem Ecol. 1997;23:579–603. [Google Scholar]
- Chiswick ML, Johnson M, Woodhall C, Gowland M, Davies J, Toner N, Sims DG. Protective effect of vitamin E (DL-alpha-tocopherol) against intraventricular haemorrhage in premature babies. Br Med J (Clin Res Ed) 1983;287:81–84. doi: 10.1136/bmj.287.6385.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Lee EO, Lee HJ, Kim KH, Ahn KS, Shim BS, Kim NI, Song MC, Baek NI, Kim SH. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother Res. 2007;21:954–959. doi: 10.1002/ptr.2189. [DOI] [PubMed] [Google Scholar]
- Choi J-H, Kim D-W, Yun N, Choi J-S, Islam MN, Kim Y-S, Lee S-M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J Nat Prod. 2011;74:1055–1060. doi: 10.1021/np200001x. [DOI] [PubMed] [Google Scholar]
- Christie PJ, Alfenito MR, Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. [Google Scholar]
- Desai K, Wei H, Lamartiniere C. The preventive and therapeutic potential of the squalene-containing compound, Roidex, on tumor promotion and regression. Cancer Lett. 1996;101:93–96. doi: 10.1016/0304-3835(96)04122-5. [DOI] [PubMed] [Google Scholar]
- Do Nascimento NC, Fett-Neto AG. Plant secondary metabolism engineering. New York: Springer; 2010. Plant secondary metabolism and challenges in modifying its operation an overview; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Fokum FD, Shahidullah M, Jorgensen E, Binns H. Applied demography and public health in the 21st century. New Yoek: Springer; 2017. Prevalence and elimination of childhood lead poisoning in Illinois, 1996–2012; pp. 221–236. [Google Scholar]
- Ganaie H, Ali M, Ganai B, Kaur J, Ahmad M. GC–MS analysis and evaluation of mutagenic and antimutagenic activity of ethyl acetate extract of Ajuga bracteosa wall ex. Benth: an endemic medicinal plant of Kashmir Himalaya, India. J Clin Toxicol. 2016;6:0495–2161. [Google Scholar]
- Ghosh T, Maity TK, Singh J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient Pharm Exp Med. 2011;11:41–49. [Google Scholar]
- Gokani RH, Lahiri SK, Santani DD, Shah MB. Evaluation of anti-inflammatory and antioxidant activity of Premna integrifolia root. J Complement Integr Med. 2011 doi: 10.2202/1553-3840.1216. [DOI] [PubMed] [Google Scholar]
- Gómez-Alonso S, Collins VJ, Vauzour D, Rodríguez-Mateos A, Corona G, Spencer JP. Inhibition of colon adenocarcinoma cell proliferation by flavonols is linked to a G2/M cell cycle block and reduction in cyclin D1 expression. Food Chem. 2012;130:493–500. [Google Scholar]
- Gopal RH, Purushothaman K. Effect of plant isolates on coagulation of blood: an in vitro study. Bull Med Ethnobot Res. 1984;5:171–177. [Google Scholar]
- Group AP. An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009;161:105–121. [Google Scholar]
- Halliwell B, Aeschbach R, Löliger J, Aruoma O. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. Antitumoractivity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22:2587–2590. [PubMed] [Google Scholar]
- Henry GE, Momin RA, Nair MG, Dewitt DL. Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem. 2002;50:2231–2234. doi: 10.1021/jf0114381. [DOI] [PubMed] [Google Scholar]
- Hu Q, Zhang DD, Wang L, Lou H, Ren D. Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem Toxicol. 2012;50:1927–1932. doi: 10.1016/j.fct.2012.03.059. [DOI] [PubMed] [Google Scholar]
- Idan SA, Al-Marzoqi AH, Hameed IH. Spectral analysis and anti-bacterial activity of methanolic fruit extract of Citrullus colocynthis using gas chromatography-mass spectrometry. Afr J Biotech. 2015;14:3131–3158. [Google Scholar]
- Igarashi K, Mikami T, Takahashi Y, Sato H. Comparison of the preventive activity of isorhamnetin glycosides from atsumi-kabu (red turnip, Brassica campestris L.) Leaves on carbon tetrachloride-induced liver Injury in mice. Biosci Biotechnol Biochem. 2008;72:856–860. doi: 10.1271/bbb.70558. [DOI] [PubMed] [Google Scholar]
- Jananie R, Priya V, Vijayalakshmi K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. N Y Sci J. 2011;4:1–5. [Google Scholar]
- Kähkönen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51:628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- Ko T-F, Weng Y-M, Chiou RY-Y. Squalene content and antioxidant activity of Terminalia catappa leaves and seeds. J Agric Food Chem. 2002;50:5343–5348. doi: 10.1021/jf0203500. [DOI] [PubMed] [Google Scholar]
- Lajubutu B, Pinney R, Roberts M, Odelola H, Oso B. Antibacterial activity of diosquinone and plumbagin from the root of Diospyros mespiliformis (Hostch) (Ebenaceae) Phytother Res. 1995;9:346–350. [Google Scholar]
- Leonetti C, Biroccio A, Gabellini C, Scarsella M, Maresca V, Flori E, Bove L, Pace A, Toppacciaro A, Zupi G. α-tocopherol protects against cisplatin-induced toxicity without interfering with antitumor efficacy. Int J Cancer. 2003;104:243–250. doi: 10.1002/ijc.10933. [DOI] [PubMed] [Google Scholar]
- Leung YH, Liu RH. Trans-10, cis-12-conjugated linoleic acid isomer exhibits stronger oxyradical scavenging capacity than cis-9, trans-11-conjugated linoleic acid isomer. J Agric Food Chem. 2000;48:5469–5475. doi: 10.1021/jf991163d. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- Majumder R, Akter S, Naim Z, Al-Amin M, Alam MB. Antioxidant and anti-diabetic activities of the methanolic extract of Premna integrifolia bark. Adv Biol Res. 2014;8:29–36. [Google Scholar]
- Malhi Y, Nobre AD, Grace J, Kruijt B, Pereira MG, Culf A, Scott S. Carbon dioxidetransfer over a Central Amazonian rain forest. J Geophys Res Atmos. 1998;103:31593–31612. [Google Scholar]
- Mcdonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidantactivity of olive extracts. Food Chem. 2001;73:73–84. [Google Scholar]
- Mckillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Upadhyay R, Chaurasia JK, Tiwari KN. Comparative antioxidant study in different flower extracts of Nyctanthes arbor-tristis (L.)(Oleaceae): an important medicinal plant. Braz J Bot. 2016;39:813–820. [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaran P, Salomi S, Umamaheshwari R. In vitro antioxidant activity of Premna serratifolia Linn. Asian J Res Pharm Sci. 2013;3:15–18. [Google Scholar]
- Ogunlesi M, Okiei W, Ofor E, Osibote AE. Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn (Euphorbiaceae), a potential medication for asthma. Afr J Biotech. 2009;8:7042–7050. [Google Scholar]
- Okeleye BI, Mkwetshana NT, Ndip RN. Evaluation of the antibacterial and antifungal potential of Peltophorum africanum: toxicological effect on human chang liver cell line. Sci World J. 2013 doi: 10.1155/2013/878735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MP. Carpesterol-A novel phytosterol obtained from the plants of the family solanaceae with evaluation of antineoplastic activity. J Med Pharm Allied Sci. 2016;1:1–10. [Google Scholar]
- Pasqualini V, Robles C, Garzino S, Greff S, Bousquet-Mélou A, Bonin G. Phenolic compounds content in Pinus halepensis Mill. Needles: a bioindicator of air pollution. Chemosphere. 2003;52:239–248. doi: 10.1016/S0045-6535(03)00268-6. [DOI] [PubMed] [Google Scholar]
- Patel MJ, Patel J. Evaluation of the anti-hyperlipidaemic activity of Premna integrifolia on nicotine induced hyperlipidaemia in rats. Int J Pharm Biosci. 2012;3:226–232. [Google Scholar]
- Poongulali S, Sundararaman M. Antimycobacterial, anticandidal and antioxidant properties of Terminalia catappa and analysis of their bioactive chemicals. Int J Pharm Biol Sci. 2016;6:69–83. [Google Scholar]
- Rajab MS, Cantrell CL, Franzblau SG, Fischer NH. Antimycobacterial activity of (E)-phytol and derivatives: a preliminary structure-activity study. Planta Med. 1998;64:2–4. doi: 10.1055/s-2006-957354. [DOI] [PubMed] [Google Scholar]
- Rajendran R, Krishnakumar E. Anti-arthritic activity of Premna serratifolia Linn., wood against adjuvant induced arthritis. Avicenna J Med Biotechnol. 2010;2:101–106. [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Basha NS, Ruby S. Evaluation of in vitro Antioxidant Activity of stembark and stem-wood of Premna serratifolia Lin., (Verbenaceae) Phytochemistry. 2009;1:11–14. [Google Scholar]
- Saeed MA, Sabir A. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia. 2001;72:807–809. doi: 10.1016/s0367-326x(01)00292-1. [DOI] [PubMed] [Google Scholar]
- Safarpour S, Givianrad M, Beheshti P (2012) Detection and determination of antioxidant compounds of seed oil of capparis spinosa l. In iran
- Selvam TN, Venkatakrishnan V, Damodar KS, Elumalai P. Antioxidant and tumor cell suppression potential of Premna serratifolia Linn leaf. Toxicol Int. 2012;19:31. doi: 10.4103/0971-6580.94514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermakkani M, Thangapandian V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J Pharm Clin Res. 2012;5:90–94. [Google Scholar]
- Shin E, Hong H, Park J, Oh Y, Jung J, Lee Y. Characterization of Staphylococcus aureus faecal isolates associated with food-borne disease in Korea. J Appl Microbiol. 2016;121:277–286. doi: 10.1111/jam.13133. [DOI] [PubMed] [Google Scholar]
- Singh C, Prakash C, Tiwari KN, Mishra SK, Kumar V. Premna integrifolia ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and apoptosis. Biomed Pharmacother. 2018;107:634–643. doi: 10.1016/j.biopha.2018.08.039. [DOI] [PubMed] [Google Scholar]
- Singh C, Prakash C, Mishra P, Tiwari KN, Mishra SK, More RS, Kumar V, Singh J. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice. Toxicon. 2019;166:88–100. doi: 10.1016/j.toxicon.2019.05.014. [DOI] [PubMed] [Google Scholar]
- Sivakrishnan S, Muthu AK. Evaluation of hepatoprotective activity of squalene isolated from Albizia procera against paracetamol induced hepatotoxicity on Wistar rats. World J Pharm Pharm Sci. 2014;3:1351–1362. [Google Scholar]
- Tiwari S, Mishra S, Misra DR, Upadhyay R. Identification of new bioactive compounds from fruit of Abutilon indicum through GCMS analysis. Biol Forum. 2016;8:548–554. [Google Scholar]
- Upadhyay R, Tiwari KN. Phyllanthus fraternus a potent natural antioxidant as pharmaceutical supplement. Res J Biotech. 2018;13:55–62. [Google Scholar]
- Upadhyay R, Chaurasia JK, Tiwari KN, Singh K. Antioxidant property of aerial parts and root of Phyllanthus fraternus Webster, an important medicinal plant. Sci World J. 2014 doi: 10.1155/2014/692392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang Z, Wang S, Wang S, Liu J. Antioxidant and antibacterial activities of Camptotheca acuminate D. Seed oil. Afr J Microbiol Res. 2011;5:5854–5862. [Google Scholar]
- Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. On ccl4-induced oxidative liver injury in mice. J Food Drug Anal. 2015;23:310–317. doi: 10.1016/j.jfda.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-B, Zheng L-J, Wu J-G, Chen T-Q, Yi J, Wu J-Z. Antioxidant activities of extract and fractions from Receptaculum nelumbinis and related flavonol glycosides. Int J Mol Sci. 2012;13:7163–7173. doi: 10.3390/ijms13067163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G, Wu Z, Yi J, Fu L, Yang Z, Hsieh C, Yin M, Zeng X, Wu C, Chen X, Hou T, Cao D. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;49:5–14. doi: 10.1093/nar/gkab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol. 2003;49:277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- Yosr Z, Imen BHY, Rym J, Chokri M, Mohamed B. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. During seasons. Ind Crops Prod. 2018;121:151–159. [Google Scholar]
- Yulia R, Christyaningsih J, Kristina EM, Soegiono NK (2016) The antioxidant compounds in methanol extract of glycine max l. merr detam i and ii varieties by kinetic maceration method
- Zhuang SR, Chen SL, Tsai JH, Huang CC, Wu TC, Liu WS, Tseng HC, Lee HS, Huang MC, Shane GT. Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother Res Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2009;23:785–790. doi: 10.1002/ptr.2623. [DOI] [PubMed] [Google Scholar]