Abstract
Introduction
Extensively drug-resistant (XDR) Pseudomonas aeruginosa (PA) infections are difficult to treat. We aimed to compare aminoglycosides or polymyxin monotherapy versus other antibiotic regimens (carbapenems, aztreonam, ceftazidime, cefepime, ceftolozane-tazobactam, or ceftazidime-avibactam) in complicated urinary tract infections (cUTI) caused by XDR-PA.
Methods
Study performed at a tertiary-care hospital from 2010 to 2019. All consecutive adult patients with XDR-PA urine cultures and diagnosed with cUTI were retrospectively reviewed. XDR phenotype was defined according to Magiorakos et al. A propensity score was used as a covariate in multivariate analyses and for matching. Primary outcome was early clinical failure and at end of treatment (EOT). Main secondary outcomes were 30- and 90-day mortality, microbiological clearance, and antibiotic-related side effects.
Results
Of the 465 episodes screened, 101 were included, 48% were treated with aminoglycoside or colistin monotherapy. Most XDR-PA were susceptible to colistin (100%) and amikacin (43%). Patients treated with antibiotic regimens other than aminoglycosides or polymyxin monotherapy were more likely to have hematologic malignancy (p < 0.001), higher SOFA score (p = 0.048), and bacteremia (p = 0.003). In multivariate models adjusted by propensity score, aminoglycoside or colistin monotherapy was not associated with worse outcomes. After propensity score matching, 28 episodes in each treatment group were matched. Adjusted ORs (95% CI) for early clinical failure and at EOT with aminoglycosides or polymyxin monotherapy were 0.53 (0.18–1.58) and 1.29 (0.34–4.83), respectively. Aminoglycoside or colistin monotherapy was not associated with higher 30-day (HR 0.93, 95% CI 0.17–5.08) or 90-day mortality (HR 0.68, 95% CI 0.20–2.31), nor with absence of microbiological clearance (OR 0.72, 95% CI 0.33–1.58). No statistically significant differences were found in terms of nephrotoxicity. Clostridioides difficile infection was observed only in the “other antibiotic regimens” group (n = 6, 11.3%).
Conclusions
Aminoglycosides or polymyxin monotherapy showed good efficacy and safety profile in treating cUTI caused by XDR-PA. These results may be useful for antibiotic stewardship activities.
Electronic Supplementary Material
The online version of this article (10.1007/s40121-021-00570-z) contains supplementary material, which is available to authorized users.
Keywords: Extensively drug-resistant Pseudomonas aeruginosa, Urinary tract infections, Amikacin, Colistin, Antimicrobial stewardship
Key Summary Points
| Aminoglycosides or polymyxin monotherapy might be an alternative for urinary tract infections (UTIs) caused by extensively drug-resistant (XDR) P. aeruginosa. |
| Aminoglycosides or polymyxin monotherapy was not associated with poor outcomes compared to other antibiotic regimens. |
| Patients treated with antibiotic regimens other than aminoglycosides or polymyxin monotherapy were more likely to have Clostridioides difficile infection |
| These results may be useful for antimicrobial stewardship activities. |
Introduction
The increasing incidence of multidrug-resistant gram-negative bacteria (GNB) is a worldwide problem. Pseudomonas aeruginosa is particularly worrisome because of its extraordinary capacity to develop resistance [1]. The emergence of extensively drug-resistant (XDR) strains in recent years has increased the concern [2]. P. aeruginosa is frequently isolated in complicated urinary tract infection (UTI), mainly those of nosocomial or healthcare-related acquisition [3]. Aminoglycosides and colistin are usually active against GNB, including many XDR P. aeruginosa isolates [4]. Both agents have favorable pharmacokinetics characteristics, which theoretically makes them suitable molecules for the treatment of complicated UTIs. Aminoglycosides are excreted in high concentrations in the urine, exceeding plasma concentrations by up to 100-fold, and remain above therapeutic levels for 72 h or longer [5]. Approximately 60–70% of colistimethate sodium (CMS) is eliminated in the urine. Furthermore, the conversion of CMS into colistin can occur in the renal tubular cells and in the bladder, suggesting that concentrations of formed colistin may be higher than those in plasma [6, 7]. However, as a result of concern about their nephrotoxicity [8, 9], clinical effectiveness [10, 11], and the risk of emergence of resistance in vivo [11, 12], combined antimicrobial therapy or broad-spectrum antibiotics such as carbapenems, ceftolozane-tazobactam, or ceftazidime-avibactam are frequently used to treat complicated UTIs caused by XDR P. aeruginosa.
On the other hand, previous studies [13, 14] have shown that in P. aeruginosa infections, UTI is associated with lower mortality rates and is therefore considered a low-risk source of infection. Thus, antibiotic monotherapy with aminoglycosides or colistin could be explored as an alternative therapeutic strategy, even in complicated UTIs. Furthermore, the prescription of broad-spectrum or combined antimicrobial therapy can also have deleterious effects, such us development and persistence of antimicrobial resistance [15], higher risk of Clostridioides difficile infection [16], and higher pharmacy costs [17].
We hypothesized that aminoglycosides or polymyxin monotherapy could be an alternative effective option for the treatment of complicated UTIs caused by XDR P. aeruginosa.
The aim of the present study was to evaluate the efficacy and safety of aminoglycosides or polymyxin monotherapy in comparison to other antibiotic regimens, including combined antimicrobial therapy, in complicated UTIs due to XDR P. aeruginosa.
Methods
Hospital Setting, Study Design, and Participants
This study was conducted from January 2010 to June 2019 at the Hospital del Mar, a tertiary-care university hospital in Barcelona (Spain), within the framework of an antimicrobial stewardship (AMS) program.
All consecutive positive urinary cultures for XDR P. aeruginosa during the study period were retrospectively reviewed. XDR P. aeruginosa was defined as non-susceptible to one or more agent in all but no more than two antipseudomonal antimicrobial categories, according to Magiorakos et al. [18].
The inclusion criteria were patients aged at least 18 years old, diagnosed with acute pyelonephritis or complicated UTI and with a monomicrobial urine culture positive for XDR P. aeruginosa. Non-complicated UTIs and asymptomatic bacteriuria were excluded. All episodes were retrospectively reviewed by two authors (I.L.M. and S.G.-Z.). Patients treated with aminoglycosides or colistin in the form of CMS monotherapy were compared to those treated with other antibiotic regimens including carbapenems, aztreonam, ceftazidime, cefepime, ceftolozane-tazobactam, or ceftazidime-avibactam, alone or in combination (including also combinations with aminoglycosides or CMS). Dose selection was at the discretion of the responsible clinicians and was adjusted according to glomerular filtration rate (GFR).
Patients were followed for up to 90 days from the date of the urine culture. In cases of more than one episode of P. aeruginosa UTI, the second and following episodes were assessed if they occurred at least 90 days after the prior one. Patients who died within the first 48 h or did not complete follow-up were not included in the analysis.
Ethics
The study was approved by the Clinical Research Ethics Committee of the Parc de Salut Mar (register no. 2020/9321). The need for written informed consent was waived because of the observational nature of the study and retrospective analysis. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guideline and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Clinical Variables, Data Source, and Definitions
The main outcome variable was clinical failure assessed early (day 7) and at end of treatment (EOT). Secondary outcomes were crude 30- and 90-day mortality; recurrence, reinfection, microbiological clearance, and readmission rates within 90 days. The incidence of acute kidney injury (AKI), C. difficile infection, rash, hematological toxicity, hepatotoxicity, and neurological symptoms were also evaluated as secondary outcomes to study antibiotic-related side effects.
Demographic, clinical, and microbiological data were collected from hospital medical charts. Recorded data included the following: age and sex; comorbidities and severity of underlying diseases, assessed using the Charlson comorbidity index [19], and immunosuppression state, defined as neutropenia (absolute neutrophil count of 500 cells/mm3 or less), chemotherapy or other immunosuppressant drugs, HIV infection, and/or congenital immunosuppression. Prior history of benign prostatic hypertrophy, urologic malignancy, obstructive nephropathy, recurrent UTI, and urological devices in the last 14 days were also recorded.
Severity of illness was calculated using the Sequential Organ Failure Assessment (SOFA) score [20], the need for intense care unit (ICU) admission, and the presence of septic shock [21]. The Pitt score [22] was applied in the case of bacteremia.
Acute pyelonephritis was considered if the patient had at least two of the following criteria: temperature above 37.7 °C, UTI symptoms (dysuria, urgency, suprapubic pain, and/or pollakiuria), local pain (lumbar back pain, costovertebral angle tenderness, and/or pelvic or perineal pain in men), and/or altered mental status in people up to 70 years. Those with the same criteria and a prior history of benign prostatic hyperplasia, intermittent or permanent indwelling urinary catheter (or withdrawal within 48–72 h before infection onset), or underlying urologic abnormalities such us nephrolithiasis, strictures, stents, history of renal transplant or urinary diversions or neurogenic bladder were classified as complicated UTI. The site of infection acquisition was defined according to Friedman et al. [23].
Appropriate empiric antibiotic therapy was considered when at least one antipseudomonal antibiotic with in vitro activity was administered during the first 24 h after urine cultures were taken. Appropriate definite antibiotic therapy was treatment based on the results of antibiotic susceptibility testing. Combination therapy was defined as two or more antipseudomonal drugs used for at least 48 h.
Adequate source control was defined as removal or insertion of indwelling urinary catheters, percutaneous drainage of the urinary tract (double-J stent, nephrostomy), or surgical intervention, as appropriate.
Clinical failure was considered if there was persistence or worsening signs and/or symptoms of UTI, the need to modify antibiotic therapy because of antibiotic side effects, the emergence of resistance to the study drug, and/or death.
Recurrence was defined as recurrent signs or symptoms of UTI and a urinary isolate of XDR P. aeruginosa with the same susceptibility profile as the index infection. Reinfection was defined as recurrent signs or symptoms of UTI with isolation of a P. aeruginosa strain with a different phenotypic profile from the prior one and/or a urinary isolate different from P. aeruginosa. Microbiological clearance was considered if there was no growth of P. aeruginosa in the final urine culture, if available. Episodes with missing urine samples during follow-up were classified as indeterminate. All microbiological assessments referred to up to 90 days following onset of the index UTI.
Antibiotic side effects (i.e., nephrotoxicity, C. difficile infection, rash) were also recorded. GFR, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), was registered at baseline and at EOT. In case of AKI, the RIFLE score [24] was applied.
Microbiological Studies
Bacterial isolates were identified as P. aeruginosa following standard procedures. Antibiotic susceptibility testing of isolates was performed by broth microdilution using MicroScan® panels [Beckman-Coulter] in the automated MicroScan® WalkAway system [Beckman-Coulter]. The following antimicrobials were tested: ciprofloxacin, piperacillin-tazobactam, ceftazidime, cefepime, imipenem, meropenem, aztreonam, gentamicin, tobramycin, amikacin, and colistin. Ceftolozane-tazobactam was not in routine use for a large part of the study; it was tested by Etest® gradient diffusion (bioMérieux, Marcy-l'Etoile, France) from 2017 onwards. Antibiotic susceptibility testing results were categorized according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [25] in force at the time of urine culture.
Statistical Analysis
The required sample size (100 patients) was determined from the results of a previous study [26] to detect a 20% difference in early clinical failure between an aminoglycoside-based or colistin group vs. “other regimens” group for infections caused by drug-resistant P. aeruginosa; statistical power was set at 80%, alpha error at 0.05, and 0.2 estimated losses to follow-up.
Categorical variables were compared by the χ2 test or Fisher exact test and continuous variables by Student’s t test or Mann–Whitney U test, as appropriate. A logistic regression model examined associations between exposures and clinical failure and microbiological clearance whereas Cox proportional hazards regression was applied to assess mortality until day 30 and 90. Variables with a p value of at most 0.1 in univariate analysis and those clinically relevant were included in the multivariate models and selected manually using backward stepwise regression.
A propensity score for receiving monotherapy with aminoglycosides or colistin was calculated. Variables used for calculating propensity score were age, sex, Charlson comorbidity index, hematologic malignancy, positive blood cultures, SOFA score, and presentation with sepsis/septic shock. Its predictive ability was estimated by calculating the area under the receiver operating characteristic curve (AUC) with 95% confidence interval (CI). The variance inflation factor value was calculated for every variable included to control for the potential occurrence of collinearity between the propensity score and other potential confounders. We selected the best model according to the likelihood ratio test. The final model showed a p value of 0.71 for the Hosmer–Lemeshow goodness-of-fit test and an AUC of 0.8 (95% CI 0.71–0.88). The propensity score was used in two different ways, as a covariate of control for residual confounding in multivariate models, and to perform a matched cohort analysis in which patients receiving amikacin or CSM were matched 1:1 according to their propensity score with those receiving other antibiotic regimens. The caliper was set to a width equal to 0.2 of the standard deviation of the logit of the propensity score [27]. Clinical failure in the matched pairs was compared by conditional logistic regression whereas Cox regression was used to compared mortality. Sensitivity analyses for all the studied outcomes were performed excluding patients receiving amikacin or CMS as part of a combination therapy from the control group. All p values were two-tailed and those less than 0.05 indicated statistical significance. The STROBE recommendations were used to ensure the reporting of the study (Supplementary Material). Statistical analyses were performed using STATA 15.1.
Results
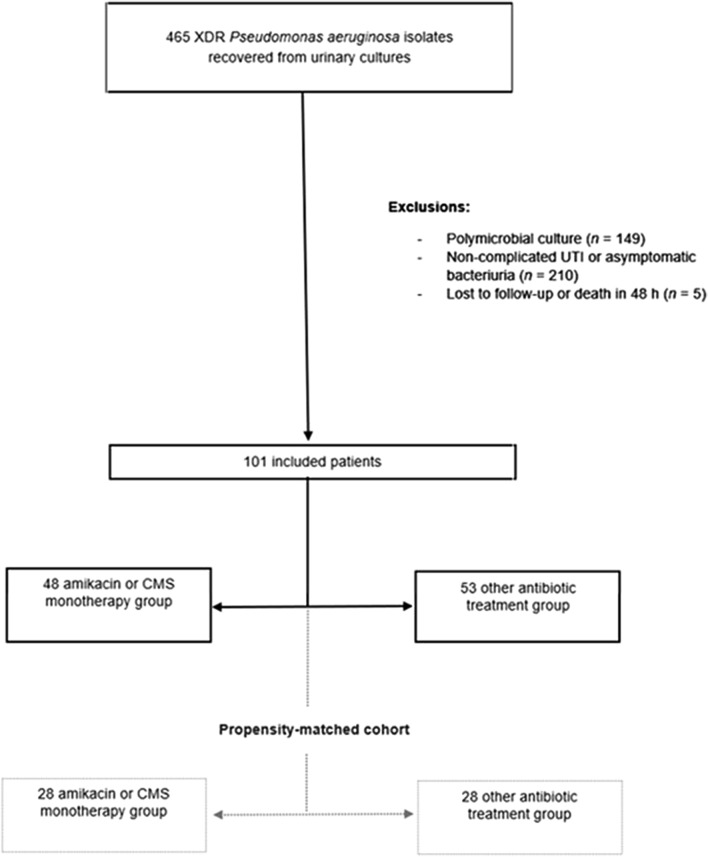
Of the 465 cases with urine cultures positive for XDR P. aeruginosa screened, 101 episodes met the inclusion criteria and were included in the final analysis (Fig. 1). Only four patients had two episodes of UTI, the rest had a single episode. Most XDR P. aeruginosa were susceptible to colistin (100%) and amikacin (42.6%, n = 43/101). Complete antimicrobial susceptibility phenotypes are shown in the Supplementary Material.
Fig. 1.
Flowchart of the patients included in the study. XDR extensively drug-resistant, UTI urinary tract infection, CMS colistimethate sodium
In the aminoglycoside or CMS monotherapy group (n = 48), 27 episodes were treated with CMS and 21 with aminoglycosides. Among those with other antibiotic therapies (n = 53), the most frequent antibiotic regimens were amikacin and/or CMS plus carbapenem (n = 24), CMS plus ceftazidime or cefepime (n = 7), and amikacin or CMS plus aztreonam (n = 6). Only 14 episodes were treated with amikacin- or colistin-free antibiotic regimens: ceftazidime (n = 5), ceftolozane-tazobactam (n = 5), aztreonam (n = 2), and carbapenems (n = 2). All patients treated with an aminoglycoside (n = 35; 21 in the monotherapy group vs. 14 in the “other therapies” group) received amikacin in a once-daily strategy, with the most frequent regimen being 1 g every 24 h [n = 22, 15/21 (71.4%) in the monotherapy group vs. 7/14 (50%) in other regimens]. In the case of CMS (n = 52; 27 in the monotherapy group vs. 25 in the “other treatments group”), the most frequent doses were 2 million international units (IU) three times a day in 9 (33.3%), 3 million IU twice daily in 8 (29.6%), 1 million IU twice daily in 8 (29.6%), and 1 million IU once a day in 8 (29.6%) episodes.
Overall, 80% were men and the median age was 76 years. Most cases were considered complicated UTI (n = 93), whereas acute pyelonephritis was observed in only eight patients. The 20% of episodes were bacteremic UTI. Bloodstream infection was more frequently observed among patients treated with amikacin or CMS monotherapy than those who received other antibiotic regimens (32.1% vs 8.3%, p = 0.003).
After propensity score matching, 56 (55.4%) patients were matched, with 28 in each treatment group. Baseline epidemiological and clinical characteristics between treatment groups before and after propensity score analysis are shown in Table 1. No significant differences were observed in the baseline demographic or clinical characteristics after propensity score matching, apart from prior residence in long-term care facility (p = 0.026).
Table.1.
Baseline characteristics of patients in overall and propensity-matched cohorts
| Variable | Overall cohort (n = 101) | Propensity score matched cohort (n = 56) | ||||
|---|---|---|---|---|---|---|
| Amikacin or CMS treatment (n = 48) | Other treatments (n = 53) | p value | Amikacin or CMS treatment (n = 28) | Other treatments (n = 28) | p value | |
| Demographic information | ||||||
| Age (years), m (IQR) | 74.5 (67–84.5) | 77 (67.5–82) | 0.796 | 77 (69.5–87) | 77 (66–82) | 0.640 |
| Male sex | 40 (83.3) | 40 (75.5) | 0.331 | 23 (79.3) | 21 (77.8) | 0.899 |
| Underlying condition | ||||||
| Charlson comorbidity index, m (IQR) | 4 (2–5.75) | 4 (2–6) | 0.961 | 3 (2–5) | 4 (2–6) | 0.337 |
| Diabetes mellitus | 13 (27.1) | 17 (32.1) | 0.583 | 8 (27.6) | 12 (44.4) | 0.188 |
| COPD | 15 (31.2) | 16 (30.2) | 0.908 | 5 (17.2) | 8 (29.6) | 0.273 |
| Cirrhosis | 2 (4.2) | 2 (3.8) | 1 | – | 1 (3.7) | 0.482 |
| Hematologic malignancy | 1 (2.1) | 16 (30.2) | < 0.001* | 1 (3.4) | 2 (7.4) | 0.605 |
| Solid tumor malignancy | 24 (50) | 25 (47.2) | 0.776 | 15 (51.7) | 11 (40.7) | 0.410 |
| Nephro-urological history | ||||||
| Baseline GFR (ml/min), m (IQR) | 58.1 (35–83) | 50 (25.5–83.5) | 0.835 | 43 (27–68.25) | 48 (27–82) | 0.476 |
| Chronic kidney disease | 10 (20.8) | 15 (28.3) | 0.385 | 8 (27.6) | 9 (33.3) | 0.640 |
| Dialysis | 1 (2.1) | 5 (9.4) | 0.208 | 1 (3.4) | 2 (7.4) | 0.605 |
| Renal transplant | 1 (2.1) | 4 (7.6) | 0.365 | – | 1 (3.7) | 0.482 |
| Benign prostatic hypertrophy | 14 (29.2) | 16 (30.2) | 0.911 | 7 (24.1) | 10 (37) | 0.386 |
| Obstructive urinary disease | 6 (12.5) | 6 (11.3) | 1 | 3 (10.3) | 1 (3.7) | 0.612 |
| Recurrent UTI | 20 (41.7) | 29 (54.7) | 0.19 | 15 (51.7) | 14 (51.9) | 0.992 |
| Indwelling urinary catheter in last 14 days | 36 (75) | 33 (62.3) | 0.202 | 23 (79.3) | 20 (74.1) | 0.643 |
| Other urological devices in last 14 days | 6 (12.5) | 12 (22.6) | 0.205 | 2 (6.9) | 1 (3.7) | 1 |
| Acquisition | ||||||
| Healthcare-related | 23 (51) | 28 (52.8) | 0.622 | 20 (69) | 13 (48.1) | 0.114 |
| Nosocomial | 25 (52.1) | 25 (47.2) | 0.622 | 9 (31) | 14 (51.9) | 0.114 |
| HCA risk factors | ||||||
| Hospital stay in last 3 months | 24 (50) | 33 (62.3) | 0.234 | 13 (48.1) | 17 (58.6) | 0.432 |
| Surgery in last 3 months | 22 (45.8) | 16 (30.2) | 0.150 | 12 (41.4) | 8 (29.6) | 0.359 |
| ICU admission in last 3 months | 13 (27.1) | 9 (17) | 0.238 | 7 (24.1) | 6 (22.2) | 0.865 |
| Residence in long-term care | 8 (16.7) | 6 (11.3) | 0.567 | 8 (27.6) | 1 (3.7) | 0.026* |
| Antibiotic exposure in last 3 months | 38 (79.2) | 49 (92.4) | 0.082 | 25 (86.2) | 24 (88.9) | 0.762 |
| Baseline illness severity | ||||||
| SOFA score, m (IQR) | 1 (0–2.7) | 2 (1–4) | 0.048* | 2 (0.5–3) | 2 (0–4) | 0.973 |
| Sepsis or septic shock | 11 (22.9) | 21 (39.6) | 0.072 | 10 (34.5) | 6 (22.2) | 0.310 |
| ICU admission | 5 (10.4) | 7 (13.2) | 0.764 | 5 (17.2) | 2 (7.4) | 0.424 |
| Bacteremia | 4 (8.3) | 17 (32.1) | 0.003* | 4 (13.8) | 4 (14.8) | 0.913 |
| Pitt score, m (IQR) | 2 (0.5–2.7) | 1 (0–1.5) | 0.282 | 2 (0.5–2.7) | 0.5 (0–1) | 0.134 |
| Management | ||||||
| Appropriate empirical treatment | 8 (16.7) | 13 (24.5) | 0.331 | 6 (20.7) | 5 (18.5) | 0.838 |
| Appropriate definitive treatment | 48 (100) | 49 (92.5) | 0.119 | 29 (100) | 24 (88.4) | 0.106 |
| 72 h delay to start appropriate antibiotic treatment | 24 (50) | 30 (56.6) | 0.506 | 15 (51.7) | 18 (66.7) | 0.256 |
| Adequate source control | 44 (91.7) | 46 (86.8) | 0.432 | 27 (93.1) | 24 (88.9) | 0.580 |
Data are presented as n (%), unless otherwise specified
CMS colistimethate sodium, COPD chronic obstructive pulmonary disease, GFR glomerular filtration rate, UTI urinary tract infection, HCA healthcare acquired, ICU intensive care unit, SOFA Sequential Organ Failure Assessment, m median, IQR interquartile range
*Statistical significance at p < 0.05
Primary Outcome: Clinical Failure
Early clinical failure rate was 28.7% (29/101): 18.7% (9/48) in the amikacin or CMS monotherapy group vs. 37.7% (20/53) in other antibiotic regimens (p = 0.035). Reasons for failure were persistence or worsening signs and/or symptom, 26 cases (7/29, 24.1% in amikacin or CMS monotherapy vs. 19/29, 65.5% in other antibiotic regimens); death, two patients (1/29, 3.5% in each group); and need to modify therapy because of antibiotic side effects, one patient (3.5%) in the amikacin or CMS monotherapy group.
The rate of clinical failure at EOT was 19.8% (20/101): 20.8% (10/48) in amikacin or CMS monotherapy vs. 18.9% (10/53) in other antibiotic regimens (p = 0.805). Reasons for failure were (amikacin or CMS monotherapy vs. other antibiotic regimens) persistence or worsening signs and/or symptoms, nine patients (4/20, 20% vs. 5/20, 25%); need to modify therapy because of antibiotic side effects, four cases (3/20, 15% vs. 1/20, 5%); death, four patients (1/20, 5% vs. 3/20, 15%); and emergence of resistance, three isolates (2/20, 10% and 1/20, 5%). In all cases, nephrotoxicity was the reason for switching antibiotic treatment because of antibiotic side effects.
Table 2 shows crude and adjusted analyses of variables involved in early clinical failure and at EOT. Monotherapy with amikacin or CMS was not associated with higher rates of clinical failure.
Table.2.
Crude and adjusted associations between different variables and clinical failure at day 7 and at end of treatment in overall and propensity-matched cohorts
| Overall cohort | Propensity-matched cohorts | ||||
|---|---|---|---|---|---|
| Crude OR (95% CI) | aOR (95% CI) | p value | aOR (95% CI) | p value | |
| Day 7 | |||||
| Age (years), m (IQR) | 1.01 (1–1.09) | 1.05 (1.01–1.1) | 0.041 | 1.01 (0.96–1.06) | 0.725 |
| Charlson comorbidity index, m (IQR) | 1.06 (0.89–1.25) | 1.09 (0.9–1.32) | 0.356 | 1.05 (0.81–1.35) | 0.717 |
| SOFA score, m (IQR) | 1.12 (0.9–1.38) | 1.01 (0.82–1.31) | 0.770 | 1.13 (0.82–1.55) | 0.460 |
| Amikacin or CMS treatment | 0.38 (0.15–0.95) | 0.5 (0.17–1.44) | 0.198 | 0.53 (0.18–1.58) | 0.251 |
| Propensity score | 0.16 (0.03–0.86) | 0.34 (0.04–2.74) | 0.311 | ||
| End of treatment | |||||
| Age (years), m (IQR) | 1.03 (0.98–1.08) | 1.05 (0.99–1.11) | 0.101 | 1.04 (0.97–1.19) | 0.301 |
| Charlson comorbidity index, m (IQR) | 1.18 (0.98–1.43) | 1.24 (1.01–1.53) | 0.047 | 1.39 (0.97–1.97) | 0.071 |
| SOFA score, m (IQR) | 1.05 (0.82–1.34) | 1 (0.76–1.31) | 0.980 | 0.88 (0.57–1.36) | 0.552 |
| Amikacin or CMS treatment | 1.13 (0.42–3.01) | 1.58 (0.47–5.32) | 0.462 | 1.29 (0.34–4.83) | 0.707 |
| Propensity score | 0.48 (0.07–3.2) | 0.35 (0.31–4) | 0.401 | ||
SOFA Sequential Organ Failure Assessment, ICU intensive care unit, CMS colistimethate sodium, m median, IQR interquartile range, OR odds ratio, aOR adjusted odds ratio, CI confidence interval
The estimations of the associations of CMS or amikacin in monotherapy with clinical failure at day 7 and at EOT in sensitivity analyses were consistent with the analysis in the whole cohort (Supplementary Material).
Secondary Outcomes: Mortality and Microbiological Clearance
The 30-day mortality rate was 8.3% (4/48 patients) among patients treated with CMS or amikacin in monotherapy and 11.3% (6/53 patients) among those who received other antibiotic regimens (p = 0.744). The 90-day mortality was 18.8% (9/48 patients) and 30.2% (16/53 patients), respectively (p = 0.183). In multivariate analysis, receipt of amikacin or CMS monotherapy was not associated with either crude 30- or 90-day mortality (Table 3). Sensitivity analyses for mortality did not show different trends (Supplementary Material).
Table.3.
Crude and adjusted associations between different variables and 30- and 90-day mortality in overall and propensity-matched cohorts
| Overall cohort | Propensity-matched cohorts | ||||
|---|---|---|---|---|---|
| Crude HR (95% CI) | aHR (95% CI) | p value | aHR (95% CI) | p value | |
| 30-day mortality | |||||
| Age (years), m (IQR) | 1.05 (0.99–1.12) | 1.09 (1.01–1.19) | 0.033* | 1.12 (1.01–1.25) | 0.046* |
| Charlson comorbidity index, m (IQR) | 1.21 (0.99–1.49) | 1.36 (1.07–1.73) | 0.012* | 1.73 (1.01–2.99) | 0.049* |
| SOFA score, m (IQR) | 1.36 (1.05–1.78) | 1.37 (1.02–1.83) | 0.036* | 1.24 (0.75–2.06) | 0.398 |
| Amikacin or CMS treatment | 0.73 (0.2–2.57) | 1.25 (0.29–5.45) | 0.763 | 0.93 (0.17–5.08) | 0.937 |
| Propensity score | 0.16 (0.02–1.67) | 0.27 (0.01–7) | 0.438 | ||
| 90-day mortality | |||||
| Age (years), m (IQR) | 1.01 (0.98–1.05) | 1.04 (0.99–1.09) | 0.113 | 1.07 (0.99–1.15) | 0.065 |
| Charlson comorbidity index, m (IQR) | 1.3 (1.14–1.49) | 1.37 (1.17–1.59) | < 0.001* | 1.59 (1.13–2.22) | 0.007* |
| SOFA score, m (IQR) | 1.22 (1.03–1.45) | 1.22 (1.01–1.48) | 0.037* | 1.32 (0.88–1.98) | 0.177 |
| Amikacin or CMS treatment | 0.59 (0.26–1.34) | 0.96 (0.36–2.54) | 0.933 | 0.68 (0.20–2.31) | 0.534 |
| Propensity score | 0.2 (0.48–0.82) | 0.34 (0.06–2.03) | 0.236 | ||
SOFA Sequential Organ Failure Assessment, CMS colistimethate sodium, m median, IQR interquartile range, HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval
*Statistical significance at p < 0.05
Regarding the microbiological assessment, 51 patients had a follow-up urine culture within 90 days. No statistically significant differences were found between treatment groups after adjusting for confounders (Supplementary Material).
Adverse Events
Antibiotic-related side effects are shown in Supplementary Material. No statistically significant differences were found in terms of nephrotoxicity between groups. C. difficile was only observed in patients in the group treated with other antibiotic regimens (11.3%).
Discussion
In the present study, we were unable to demonstrate that amikacin or CMS monotherapy was associated with worse outcomes in terms of mortality, clinical failure, or microbiological clearance than combination or other antibiotic therapies in cases of complicated UTI caused by XDR P. aeruginosa isolates, after controlling for confounders. Although these results cannot be interpreted as that amikacin or CMS monotherapy is equally effective as combination or other antibiotic therapies, they reinforce the message that alternative narrow-spectrum antibiotic use should be considered in some scenarios despite that we are facing a difficult-to-treat bacteria.
The challenge of treating XDR P. aeruginosa has been thoroughly discussed in the literature. Many clinicians favor combination treatment even though the clinical evidence of the superiority of combination therapy over monotherapy is scarce and of low quality [28, 29]. Although the use of combination therapy may be tempting in this type of infection, combination therapies increase antibiotic pressure in the hospital ecosystem and the selection of multidrug-resistant bacteria [15]. In this setting, the World Health Organization, not surprisingly, has urged the implementation of AMS programs to optimize antibiotic use and control increased multidrug resistance worldwide [30]. Further, although the new antipseudomonal agents ceftolozane-tazobactam and ceftazidime-avibactam have recently become available in daily clinical practice, the emergence of resistant mutants has been already reported [31, 32], suggesting that the “old drugs” still have a place.
The effectiveness of aminoglycosides and/or polymyxins for treating XDR P. aeruginosa infections has already been assessed in previous studies. However, most of these included different sources of infection, with few UTI episodes, or had no control group, which makes interpretation difficult. Pogue et al. [26] compared ceftolozane-tazobactam vs. polymyxin or aminoglycoside-based therapy for the treatment of drug-resistant P. aeruginosa infections in a multicenter retrospective study. A total of 200 patients were assessed, but only 27 of these had UTI. The authors reported statistical differences in clinical success rate (81% in the ceftolozane-tazobactam group vs. 61% in the comparative group), but not in mortality. Other authors have described their clinical experience of ceftolozane-tazobactam in the treatment of drug-resistant P. aeruginosa with large cohorts (more than 100 patients assessed) [33–35], with successful clinical outcome rates ranging from 63% to 83%. However, the limited number of UTIs included (n < 30) makes interpretation difficult.
In a systematic review of polymyxins in monotherapy or in combination for the treatment of carbapenem-resistant GNB, Zusman et al. [36] suggested a less than optimal outcome in patients who received colistin monotherapy, although most studies did not include P. aeruginosa infections, and UTI was not a frequent source of infection. Our group has previously assessed the performance of CMS in XDR P. aeruginosa infections [7, 37] and detected no differences between monotherapy and combination therapy or in clinical failure or mortality. One of those studies was specifically focused on UTIs [37]. In that prospective cohort of 33 patients, more than half of whom received CMS monotherapy, clinical cure was achieved in 89.5% of patients treated with CMS monotherapy.
Regarding the effectiveness of aminoglycosides, the evidence on monotherapy for treating UTIs caused by drug-resistant P. aeruginosa was extrapolated from carbapenem-resistant Enterobacterales [38–40], with response rates ranging from 61% to 100%. In a systematic review [11], Vidal et al. demonstrated that aminoglycosides as single agents were as effective as beta-lactams or quinolones for achieving clinical improvement in patients with UTI, including those caused by P. aeruginosa. However, the impact of the new antipseudomonal agents was not assessed as a result of the date of publication.
Our data show that patients treated with other antibiotic regimens had more underlying comorbidities and severe disease compared to those in the amikacin or CMS group. It may be inferred that clinicians were reluctant to administer amikacin or CMS monotherapy in more complicated patients. To overcome this indication bias, a double propensity score analyses was performed and no differences between groups were found for the studied outcomes.
One of the main concerns in treatment with amikacin or CMS is nephrotoxicity. However, since many patients in the “other antibiotic regimens” group were also treated with combination therapies that included amikacin or CMS, this side effect was not properly assessed. In our study the rate of renal toxicity was in fact lower in the amikacin or CMS monotherapy group. There are several possible reasons for this, apart from the antibiotic treatment received: patients in the “other antibiotic regimens” group were more severely ill and some of the cases were probably sepsis-related; second, the kidney infection itself; third, the concomitant use of nephrotoxic drugs; and finally, a cautious attitude to using amikacin or CMS in patients with abnormal GFR baselines.
Another worrisome antibiotic-related side effect is the incidence of C. difficile infection. Aminoglycosides and polymyxins are not among the “high-risk” drugs for the development of C. difficile infection [16], in accordance with our findings. Reducing the risk of C. difficile infection could be another reason for using them in the treatment of XDR P. aeruginosa infections.
Perhaps the greatest challenge associated with XDR P. aeruginosa is achieving the appropriate balance between efficacy, security, and ecology. Strategies aimed at safeguarding broad-spectrum drugs should be approached with caution, particularly in less severe patients with a low-risk source of infection such us UTI, where the favorable pharmacokinetics characteristics of aminoglycosides and colistin could provide an excellent opportunity to use more ecological agents.
Our study has the inherent limitations of a retrospective design and a single-center study. As a result of imbalances in the baseline characteristics of the treatment groups, a double propensity-based approach was performed to reduce potential biases. Although the initial analysis included 101 patients, the matched cohort resulted in a smaller sample which reduces the statistical power of the study. It could have been of interest to study monotherapy with CMS or amikacin in more severe patients, but groups were too small for specific analyses to be performed. Another limitation is that many patients in the control group used aminoglycosides or colistin combined with other drugs. Although a sensitivity analysis was performed excluding those patients, as a result of the limited number of episodes treated with amikacin- or colistin-free antibiotic regimens (n = 14), results should be cautiously interpreted. In addition, not all patients had a urine control culture to assess microbiological clearance. Another potential limitation is that patient comorbidities were not determined using disease codes. Even though all clinical records were cautiously reviewed for two infectious diseases clinicians, there is a risk of misclassification or measurement error, particularly in a retrospective study. Finally, it would have been interesting to conduct genotypic studies. Prior studies have shown that the major XDR clone involved in our hospital is the less virulent ST-175 clone [4], which is widespread in our country and in Europe [1, 2]. Thus, our results might not be transferable to other settings with a different epidemiology. As strengths, a propensity score approach was used for controlling confounders at baseline. This is one of the recommended strategies to emulate the random assignment of clinical trials [41]. Finally, it explores more ecological agents in a difficult-to-treat bacteria, such as XDR P. aeruginosa, in a “real life” situation.
Conclusions
Our findings might reinforce that amikacin or CMS monotherapy does not have a detrimental impact on outcomes of complicated UTIs caused by XDR P. aeruginosa when compared with combination or other antibiotic therapies. These results may be useful for antibiotic stewardship activities given their clinical and ecological impact. However, further studies are needed to confirm these findings, particularly in more severely ill patients.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Janet Dawson for English language editing and the Department of Medicine of Universitat Autònoma de Barcelona for their support of I.L.M. PhD studies. The authors thank the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) which supports the research of Silvia Gómez-Zorrilla. We also thank Dr Estela Membrilla and Professor Francisco Álvarez Lerma for their contribution with PROA PSMAR group, and Professor Jesús Rodríguez Baño for his support in the study methodology. Finally, we thank the participants of the study.
Funding
This work was supported by the Ministerio de Economia y competitividad of Spain, Instituto de Salud Carlos III [grant number PI17/00251 to J.P.H.], co-financed by the European Development Regional Fund ‘A way to achieve Europe’ (ERDF), Operative program Intelligent Growth 2014–2020. Silvia Gómez-Zorrilla has received a research grant from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) to support her research. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Fundacio IMIM funded the Journal’s Rapid Service Fee.
Author Contributions
ILM and SG-Z designed the study, collected the data, and wrote the initial manuscript. DEE, SG, NP, and EP performed microbiological and PK/PD tests. ILM, SG-Z, ZRPB, and XDJ performed the statistical analysis. ZRPB, DEE, MPG, ES, LS, MM, RG, SG, and JPH reviewed and edited the final manuscript.
Prior Presentation
These data were previously presented, in part, in the abstract book at the 30th European Congress of Clinical Microbiology and Infectious Diseases (2020).
Disclosures
Juan Pablo Horcajada has received fees from Angelini, Pfizer, MSD, Menarini, and Zanbom as a speaker and participant in advisory board meetings, and a research grant from MSD. Santiago Grau has received fees as a speaker for Pfizer, Angellini, Kern, and MSD and research grants from Astellas Pharma, Pfizer. Inmaculada López Montesinos, Silvia Gómez-Zorrilla, Zaira Raquel Palacios-Baena, Nuria Prim, Daniel Echeverria-Esnal, María Pilar Gracia, María Milagro Montero, Xavier Durán-Jordà, Elena Sendra, Luisa Sorli, Roberto Guerri-Fernandez and Eduardo Padilla, declare that they have no conflict of interest.
Compliance with Ethics Guidelines
The study was approved by the Clinical Research Ethics Committee of the Parc de Salut Mar (register no. 2020/9321). The need for written informed consent was waived because of the observational nature of the study and retrospective analysis. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guideline and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Inmaculada López Montesinos, Email: ilopezmontesinos@psmar.cat.
Silvia Gómez-Zorrilla, Email: sgomezzorrilla@psmar.cat.
References
- 1.Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32:e00031–19. http://cmr.asm.org/content/32/4/e00031-19.abstract. [DOI] [PMC free article] [PubMed]
- 2.Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat. 2015;21–22:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Lamas Ferreiro JL, Álvarez Otero J, González González L, et al. Pseudomonas aeruginosa urinary tract infections in hospitalized patients: mortality and prognostic factors. PLoS ONE. 2017;12:e0178178. doi: 10.1371/journal.pone.0178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Barrio-Tofiño E, Zamorano L, Cortes-Lara S, et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J Antimicrob Chemother. 2019;74:1825–1835. doi: 10.1093/jac/dkz147. [DOI] [PubMed] [Google Scholar]
- 5.Goodlet KJ, Benhalima FZ, Nailor MD. A systematic review of single-dose aminoglycoside therapy for urinary tract infection: is it time to resurrect an old strategy? Antimicrob Agents Chemother. 2018;63. https://aac.asm.org/content/63/1/e02165-18. [DOI] [PMC free article] [PubMed]
- 6.Vogel-González M, Talló-Parra M, Herrera-Fernández V, et al. Low zinc levels at admission associates with poor clinical outcomes in SARS-CoV-2 infection. Nutrients. 2021;13:562. https://www.mdpi.com/2072-6643/13/2/562. [DOI] [PMC free article] [PubMed]
- 7.Luque S, Escaño C, Sorli L, et al. Urinary concentrations of colistimethate and formed colistin after intravenous administration in patients with multidrug-resistant gram-negative bacterial infections. Antimicrob Agents Chemother. 2017;61. 10.1128/AAC.02595-16. [DOI] [PMC free article] [PubMed]
- 8.Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48:1724–1728. https://academic.oup.com/cid/article-lookup/doi/s. [DOI] [PubMed]
- 9.Destache CJ. Aminoglycoside-induced nephrotoxicity—a focus on monitoring. J Pharm Pract 2014; 27:562–566. 10.1177/0897190014546102. [DOI] [PubMed]
- 10.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. https://linkinghub.elsevier.com/retrieve/pii/S1473309906705801. [DOI] [PubMed]
- 11.Vidal L, Gafter-Gvili A, Borok S, Fraser A, Leibovici L, Paul M. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2007;60:247–257. doi: 10.1093/jac/dkm193. [DOI] [PubMed] [Google Scholar]
- 12.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–642. 10.1093/jac/dkm511. [DOI] [PubMed]
- 13.Joo E-J, Kang C-I, Ha YE, et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia: clinical impact of antimicrobial resistance on outcome. Microb Drug Resist. 2011;17:305–312. doi: 10.1089/mdr.2010.0170. [DOI] [PubMed] [Google Scholar]
- 14.Britt NS, Ritchie DJ, Kollef MH, et al. Importance of site of infection and antibiotic selection in the treatment of carbapenem-resistant Pseudomonas aeruginosa sepsis. Antimicrob Agents Chemother. 2018;62. https://aac.asm.org/content/62/4/e02400-17. [DOI] [PMC free article] [PubMed]
- 15.Karam G, Chastre J, Wilcox MH, Vincent J-L. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016;20:136. 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed]
- 16.Kazakova S V, Baggs J, McDonald LC, et al. Association between antibiotic use and hospital-onset clostridioides difficile infection in US Acute Care Hospitals, 2006–2012: an ecologic analysis. Clin Infect Dis. 2020;70:11–18. http://www.ncbi.nlm.nih.gov/pubmed/30820545. [DOI] [PubMed]
- 17.Huang W, Qiao F, Zhang Y, et al. In-hospital medical costs of infections caused by carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis. 2018;67:S225–S230. https://academic.oup.com/cid/article/67/suppl_2/S225/5181281. [DOI] [PubMed]
- 18.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Jones AE, Trzeciak SKJ. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2010;37:1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the PITT bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock. 2009;31:146–150. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 23.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. https://linkinghub.elsevier.com/retrieve/pii/S0085253815530445. [DOI] [PubMed]
- 25.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone. https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/. Accessed 15 Oct 2021.
- 26.Pogue JM, Kaye KS, Veve MP, et al. Ceftolozane/tazobactam vs polymyxin or aminoglycoside-based regimens for the treatment of drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2020;71:304–310. https://academic.oup.com/cid/article/71/2/304/5572677. [DOI] [PubMed]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. 10.1002/pst.433. [DOI] [PMC free article] [PubMed]
- 28.Karaiskos I, Antoniadou A, Giamarellou H. Combination therapy for extensively-drug resistant gram-negative bacteria. Expert Rev Anti Infect Ther. 2017;15:1123–1140. http://www.ncbi.nlm.nih.gov/pubmed/29172792. [DOI] [PubMed]
- 29.Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDS. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:10–39. 10.1002/phar.2209. [DOI] [PMC free article] [PubMed]
- 30.WHO. Antimicrobial stewardship programmes in low- and middle-income countries. A practical toolkit. Geneva: World Health Organization; Licence CC BY-NC-SA 30.
- 31.Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis. 2017;65:110–120. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanz-García F, Hernando-Amado S, Martínez JL. Mutation-driven evolution of Pseudomonas aeruginosa in the presence of either ceftazidime or ceftazidime-avibactam. Antimicrob Agents Chemother. 2018;62. https://aac.asm.org/content/62/10/e01379-18. [DOI] [PMC free article] [PubMed]
- 33.Bassetti M, Castaldo N, Cattelan A, et al. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents. 2019;53:408–415. https://linkinghub.elsevier.com/retrieve/pii/S0924857918303261. [DOI] [PubMed]
- 34.Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis. 2018;5. 10.1093/ofid/ofy280/5149696. [DOI] [PMC free article] [PubMed]
- 35.Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-world experience with ceftolozane-tazobactam for multidrug-resistant gram-negative bacterial infections. Antimicrob Agents Chemother. 2020;64. https://aac.asm.org/content/64/4/e02291-19. [DOI] [PMC free article] [PubMed]
- 36.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 37.Sorlí L, Luque S, Li J, et al. Colistin for the treatment of urinary tract infections caused by extremely drug-resistant Pseudomonas aeruginosa: Dose is critical. J Infect. 2019;79:253–261. https://linkinghub.elsevier.com/retrieve/pii/S0163445319301926. [DOI] [PMC free article] [PubMed]
- 38.Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother. 2011;55:5893–5899. https://aac.asm.org/content/55/12/5893. [DOI] [PMC free article] [PubMed]
- 39.Zavascki AP, Klee BO, Bulitta JB. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther. 2017;15:519–526. 10.1080/14787210.2017.1316193. [DOI] [PubMed]
- 40.van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed]
- 41.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available: Table 1. Am J Epidemiol. 2016;183:758–764. 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.