Abstract
Resistance to quaternary ammonium compounds (QAC) in staphylococci is common in hospital environments and has been described in the food industry. Little is known about staphylococcal QAC resistance associated with animal disease, although such disinfectants are widely used in veterinary medicine. In order to investigate the occurrence of QAC resistance in staphylococci isolated from QAC-exposed animals, 32 penicillin- and tetracycline-resistant and 23 penicillin- and tetracycline-susceptible Staphylococcus aureus isolates collected from milk from cows with mastitis during a 4-year period were selected for QAC susceptibility studies and genetic characterization. The isolates originated from four different herds that used a common pasture with a joint milking parlor in the summer. During the pasture season, a teat cream containing the QAC cetyltrimethylammonium bromide had been used daily for more than 10 years for mastitis control. Three of the penicillin- and tetracycline-resistant isolates, which were recovered from three different cows during a 20-month period, were resistant to QAC. Plasmid analysis, PCR, and DNA sequencing revealed a novel plasmid of 2,239 bp containing the smr gene. The plasmid, designated pNVH99, has similarities to small, smr-containing staphylococcal plasmids previously found in human and food isolates. pNVH99 is a new member of the pC194 family of rolling-circle replication plasmids. The three QAC-resistant isolates, as well as 28 of the 29 remaining penicillin- and tetracycline-resistant isolates, were indistinguishable by pulsed-field gel electrophoresis. The study indicates that the occurrence and spread of QAC-resistant S. aureus among dairy cows may be a problem that needs further investigation.
Antiseptics and disinfectants based on quaternary ammonium compounds (QAC) are used extensively in hospitals and other health care settings, as well as in the food industry (10, 18). Moreover, they have a wide range of veterinary applications and thus play an important role in the control of infectious diseases in animals (24). In the dairy industry, they are commonly used for disinfection of milking equipment and teat disinfection to prevent infectious mastitis (2). It has been shown that postmilking teat disinfection reduces the incidence of mastitis, especially new infections caused by Staphylococcus aureus (2), which is an important udder pathogen in many countries. Disinfection of teats has therefore been considered to be a key component in mastitis control (2, 20).
Increased attention is being paid to plasmid-encoded resistance to antiseptics and disinfectants in antibiotic-resistant staphylococci (13, 16, 25). Resistance to QAC among S. aureus and coagulase-negative staphylococci (CNS) was first studied and reported in human clinical isolates in which three QAC genes have been characterized: qacA (23, 33), qacB (21), and smr (formerly qacC) (4, 15, 22, 27). Studies on QAC-resistant staphylococci isolated from the food industry are comparatively recent (6, 7), and two additional resistance genes, qacG and qacH, have been characterized in such isolates (8, 9). Despite extensive use of QAC in veterinary medicine, studies on QAC-resistant staphylococcal strains associated with disease in animals have apparently not been carried out.
The present study involved a group of cooperating dairy herds in which QAC had been used for teat disinfection for more than 10 years in order to control mastitis caused by antibiotic-resistant S. aureus. The purpose of the study was to examine the susceptibility to QAC in S. aureus associated with mastitis, identify and describe QAC resistance plasmids and genes in isolates found to be resistant, and determine the genetic relatedness of such isolates.
MATERIALS AND METHODS
Herds, animals, and S. aureus isolates.
Surveillance of bovine mastitis in Norway involves frequent collection of mammary quarter milk samples (QMS) from dairy herds for microbiological examination at the mastitis laboratories of the National Veterinary Institute. QMS are occasionally collected by veterinary surgeons from cows with clinical mastitis. The four herds selected for this study used a common pasture and a common milking parlor during the summer. Previous microbiological examinations of QMS had shown a relatively frequent occurrence of penicillin- and tetracycline-resistant S. aureus in these herds over a 10-year period. Efforts to control mastitis in the herds included postmilking teat disinfection, use of a preparation containing the QAC cetyltrimethylammonium bromide (CTAB), and culling of chronically infected animals. From January 1996 to March 2000, a total of 32 penicillin- and tetracycline-resistant isolates were recovered from QMS collected from 22 cows in these herds. Additionally, 23 penicillin- and tetracycline-sensitive S. aureus isolates were obtained from QMS from the same herds during the final 6 months of the collection period. Isolates were stored at −70°C in heart infusion broth (Difco Laboratories, Detroit, Mich.) containing 15% (vol/vol) glycerol.
Examination of QMS.
Bacteriological examination of QMS was carried out at the National Veterinary Institute, Oslo, Norway, using methods (31) based on recommendations of the International Dairy Federation (12). Coagulase-positive staphylococci producing acetoin, but not β-galactosidase, were considered to be S. aureus.
Susceptibility testing.
All 55 S. aureus isolates were initially tested for susceptibility to QAC by studying their growth on Mueller-Hinton (MH) agar containing 12 different concentrations of CTAB or benzalkonium chloride (BC) ranging from 1 to 12 μg/ml. A control MH agar plate containing no drug was used for each isolate. Overnight MH broth cultures were diluted in 0.9% NaCl to an inoculum concentration of approximately 106 CFU/ml. Two hundred microliters of the diluted culture was transferred to the surface of an MH agar plate and incubated for 24 h at 37°C. Isolates showing confluent or semiconfluent growth on MH agar containing CTAB at ≥6.0 μg/ml and BC at ≥4 μg/ml, respectively, were considered resistant to QAC. A Staphylococcus haemolyticus strain containing the qacA gene served as a positive control. The MICs of CTAB and BC for the 32 penicillin- and tetracycline-resistant isolates were then determined in a microtiter assay at 0.5-μg/ml intervals from 0 to 12 μg/ml in MH broth as described by Sundheim et al. (32).
Plasmid analysis.
Plasmid DNA isolation was carried out by using a SNAP Miniprep Kit (Invitrogen BV, Groningen, The Netherlands). The procedure recommended by the manufacturer was modified by adding lysostaphin (Sigma Chemical Co., St. Louis, Mo.) to the resuspension buffer to a final concentration of 35 μg/ml, followed by incubation at 37°C for 90 min. Plasmid DNA was restricted with EcoRI and HaeIII (Life Technologies, Paisley, United Kingdom). The sizes of unrestricted plasmids and DNA fragments were estimated after agarose gel electrophoresis. Escherichia coli V517 (17) and Supercoiled DNA Ladder (Life Technologies) were used as molecular weight markers for the estimation of plasmid sizes.
PCR.
One of the QAC-resistant isolates was selected for PCR amplification. One strain that was sensitive to QAC was used as a negative control. smr-specific primers 5′-ATA-AGT-ACT-GAA-GTT-ATT-GGA-AGT-3′ and 5′-TTC-CGA-AAA-TGT-TTA-ACG-AAA-CTA-3′ were used in a PCR for amplification of an smr-specific amplicon (Life Technologies). Approximately 50 ng of plasmid DNA was added to 49 μl of a mixture of PCR reagents containing 1× PCR buffer (F-511 for Dynazyme DNA polymerase; Finnzymes Oy, Espoo, Finland) with 0.7 U of Taq polymerase (F501L Dynazyme DNA polymerase; Finnzymes), 10 pmol of each primer, and 200 μM each deoxynucleoside triphosphate (F-560L dNTP-mix; Finnzymes). PCR was performed in a thermal cycler, and the temperature profile was DNA denaturation at 95°C for 60 s and then 30 cycles at 95°C for 60 s, 48°C for 45 s, and 72°C for 60 s. The resulting PCR products were analyzed by agarose gel electrophoresis.
Cloning and DNA sequencing of pNVH99.
Plasmid pNVH99 was linearized with HaeIII and ligated into the pUC18 (SmaI/BAP) vector (Pharmacia, Uppsala, Sweden) and then transformed into competent E. coli DH5α (5). The nucleotide sequence of pNVH99 was determined (for both strands) by the dideoxy-chain termination method (26) using DNA Labstation 625, synthetic oligonucleotide primers, and a Labstation Thermo Sequenase labeled primer cycle sequencing kit (Vistra Systems, Amersham, Buckinghamshire, United Kingdom). Sequencing was done with an ALFexpress DNA Sequencer (APBiotech, Stockholm, Sweden). Nucleotide sequence files were analyzed by using ALFwin Sequence Analyser 2.00 (APBiotech), and the sequences were connected to a consensus by using the program GeneSkipper, version 1.1 (European Molecular Biology Laboratory). Nucleotide sequences of pNVH99 were compared with corresponding sequences in other plasmids from different sources by using a database search (Basic BLAST, version 2.1).
Genotyping.
Genotypes of S. aureus strains were analyzed by pulsed-field gel electrophoresis (PFGE) using the Gene Navigator system (Pharmacia Biotech, Uppsala, Sweden). SmaI-digested DNA was separated for 20 h at 14°C and 200 V with pulse times of 5 to 60 s at an angle of 120°. PFGE patterns were compared by visual examination and interpreted in accordance with recommended guidelines (34).
Nucleotide sequence accession number.
The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ296103).
RESULTS
Antimicrobial susceptibility.
According to the MH agar method used for the initial screening, 3 of the 32 penicillin- and tetracycline-resistant S. aureus isolates expressed resistance to QAC. None of the 23 penicillin- and tetracycline-susceptible S. aureus isolates showed QAC resistance. When the microtiter assay was used, the BC and CTAB MICs for the resistant isolates were 2.5 to 3.0 and 4.0 to 5.0 μg/ml, respectively. These isolates originated from three different cows in the same herd. The time interval between the first and last collections of samples from which these isolates were recovered was 20 months from the beginning of the collection period. The BC and CTAB MICs for the 29 QAC-susceptible isolates were 0.5 to 1.0 and 1.0 to 2.0 μg/ml, respectively.
Plasmid profiles and PCR results.
Plasmid analysis revealed the presence of a plasmid of approximately 20 kb in all of the 32 penicillin- and tetracycline-resistant S. aureus isolates. Endonuclease restriction analysis revealed indistinguishable restriction patterns for this plasmid in 31 of the 32 isolates. The three QAC-resistant isolates contained an additional plasmid of approximately 2.2 kb, designated pNVH99. PCR amplification with smr-specific primers generated amplicons of 285 bp with template DNA from the isolate that harbored the 2.2-kb plasmid; the PCR result was negative for the isolate that did not contain the 2.2-kb plasmid. The nucleotide sequence of the PCR product was found to be 99% homologous to the DNA sequence of the smr (qacC′) gene on pST827 published previously (6). None of the 23 penicillin- and tetracycline-susceptible S. aureus isolates contained plasmids.
DNA sequence analysis of pNVH99.
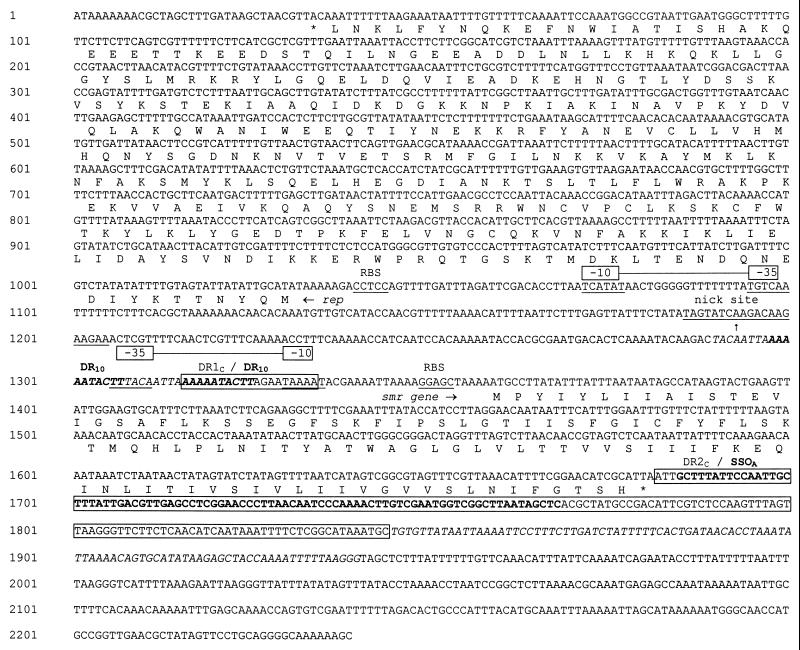
Sequence analysis of pNVH99 revealed 2,239 bp encoding two different open reading frames (ORFs). The 107-codon ORF (nucleotides [nt] 139 to 1682) is 99% similar at the nucleotide level with the previously sequenced smr genes in plasmids such as pST827, pSK41, pKH8, pSK89, pTZ22, and pSK108 (1, 11, 14, 15, 16, 28). The smr gene in pNVH99 is characterized by one single nucleotide alteration (nt 1439, A replaced by G), without involving an amino acid change. The smr region is preceded by a putative promoter region, TAAAAT (−10) and TTTACA (−35) (Fig. 1). The Pribnow box TAAAAT is identical to the corresponding sequence in plasmid pST827 (6). Upstream of the smr gene, transcribed in the opposite direction, is a 999-bp sequence (nt 31 to 1030) representing an ORF, designated repNVH99, that encodes a replication protein. The repNVH99 sequence is preceded by a putative promoter region, TCATAT (−10) and TGTCAA (−35) (Fig. 1). The deduced amino acid sequence encoded by repNVH99 is 95% similar to that encoded by rep827, which has been found in CNS isolated from the food industry in Norway (6). The smr sequence of pNVH99 is surrounded by flanking regions containing sequences identical to previously described repeat structures, DR1C (nt 1316 to 1334) and DR2C (nt 1681 to 1844), including a palindromic sequence termed SSOA (formerly palA) involved in lagging-strand replication of rolling-circle replication plasmids (1, 14). Nucleotide sequence comparisons revealed that pNVH99 is a new member of the pC194 family of rolling-circle replication plasmids. This is based on the presence of the cassette-like structure, comprising the smr gene, typically located between the nick site and the SSOA sequence (1, 14, 19) and the similarities between repNVH99 and other replication genes located on plasmids belonging to the pC194 family (6, 8, 11, 14, 15).
FIG. 1.
Nucleotide sequence of pNVH99 (GenBank accession no. AJ296103). Putative promoter sequences (−10 and −35) and ribosome binding sites (RBS) are indicated (underlined) for both smr and repNVH99. The DNA sequence resembling the pC194 plasmid family replication nick site is indicated by a vertical arrow and underlined. Direct repeat sequences previously described (14), viz., DR1C and DR2C, and the single-strand origin sequence SSOA (formerly designated palA) (1) are boxed. Upstream from the smr gene is a DNA-sequence of 18 bp (nt 1290 to 1308, italic letters) directly repeated downstream (DR18+18 in Fig. 2) and overlapping DR1C by 10 bp (DR10, bold letters). DR2C is truncated by a 96-bp sequence (italicized), followed by a 299-bp sequence showing a close relationship to pSK89 and other small staphylococcal plasmids (4, 15).
PFGE results.
Thirty-one of the 32 penicillin- and tetracycline-resistant S. aureus isolates were indistinguishable by PFGE, including the three QAC-resistant isolates. The one penicillin- and tetracycline-resistant isolate that had a different plasmid profile also had a different PFGE banding pattern. The 23 penicillin- and tetracycline-susceptible isolates showed five different PFGE banding patterns, and all differed from that of the QAC-resistant isolates.
DISCUSSION
No clear-cut definition exists for the classification of staphylococci as QAC-susceptible or QAC-resistant strains. The MICs of both BC and CTAB for S. aureus strains containing the smr gene have been reported to be between 4 and 6 μg/ml by some researchers (13). A previous study on S. aureus carrying the smr gene found the MICs of BC and CTAB to be >3 μg/ml and 6 μg/ml, respectively (18); in another study, the MIC of both BC and CTAB was found to be 6 μg/ml (16). We chose concentrations of 4 μg/ml for BC and 6 μg/ml for CTAB when carrying out the preliminary screening on MH agar for the detection of QAC-resistant staphylococcal strains. Further examinations using MH broth (32) revealed BC MICs between 2.5 and 3.0 μg/ml and CTAB MICs between 4.0 and 5.0 μg/ml for the strains that contained the smr gene, i.e., a somewhat lower tolerance to QAC compared with previous findings (13, 18). The MIC obtained for the susceptible isolates agrees more closely with previous reports (13, 16, 18). However, comparison between different MICs obtained at different laboratories requires a high degree of method standardization. The MICs found in this study indicate a three- to fourfold increase in QAC resistance for isolates that contain the smr gene.
Other studies have shown a tendency toward widespread dissemination of identical S. aureus strains within dairy herds (30). In this study, the PFGE results showed that 31 of the 32 penicillin- and tetracycline-resistant S. aureus isolates belonged to a single clone that had persisted within the common pasture for at least 4 years. QAC-resistant S. aureus was found in only three cows in one herd, and no further spread to the other herds that used the same pasture and milking parlor as the index herd was observed. During the first 2 years of the study, QMS were recovered mainly from this particular herd. Extensive culling of chronically infected animals is another possible explanation for the detection of QAC-resistant isolates in only one of these herds. The occurrence of a small QAC resistance plasmid in 3 out of 31 isolates belonging to the same clone suggests a lateral transfer of plasmid-borne QAC resistance.
In this study, the smr gene was located on a 2,239-bp plasmid carried by S. aureus. Previous studies have found that the smr gene was located either on large conjugative plasmids or on small nonconjugative plasmids of less than 3 kb (25). Among large conjugative S. aureus plasmids are pSK41 (1), pJE1 (3, 4), pTZ20 (27), and pTZ22 (28). Small nonconjugative plasmids are S. aureus plasmids pSK89 (15) and pKH8 (11) and CNS plasmids pSK108 (14), pST827 (6), and pST94 (8).
It has been proposed that the smr gene might be part of a gene cassette comprising the nick site and SSOA (the single-strand origin, formerly designated palA) (14, 19). Apparently, plasmid pNVH99 is a new member of the pC194 family of rolling-circle replication plasmids. This is indicated by the presence of the smr gene connected to the nick site of a plus strand replication origin and SSOA—the origin of lagging-strand synthesis—as well as the similarities between the putative replication protein encoded by repNVH and several other replication proteins located on plasmids belonging to the pC194 family (1, 14, 29). It has been reported that the pC194 family plasmids carry several different resistance genes, which are similarly located and arranged as cassette structures, encoding resistance to chloramphenicol, lincosamide, and fosfomycin (14).
It has been suggested that the smr gene cassette located on pSK41 is the precursor of other smr regions that have been described (14). The coding region of the smr gene on pSK41 is bounded by 240-bp direct repeats, DR1D and DR2D, thus forming palindromic structures (Fig. 1). A similar organization of the smr gene with corresponding flanking regions is present on the small CNS plasmid pST827 (6). At the amino acid level, the smr gene on pNVH99 is 100% homologous with the smr genes located on several other small plasmids, such as pSK89 (15), pKH8 (11), and pSK108 (14). The smr gene on pST827 (designated qacC′ by Heir et al.) (6), however, has one nucleotide substitution leading to an amino acid deviation in the protein product. The flanking regions of all of these plasmids have possibly been subjected to rearrangements and deletions. Comparison of pNVH99 with pSK89 and pSK108 shows that some regions of the flanking DNA are similar in the three plasmids: the DNA region downstream of the smr gene—DR2C—is truncated precisely at the end of the SSOA region in all three plasmids. Thus, this region is conserved in three different plasmids carried by different staphylococcal species that have been recovered from various sources (animals, humans, and foods) in different geographical areas. It is likely that this region has been conserved because of its role in replication and maintenance of the plasmid. The DR1C sequence (a 19-bp stretch of DR1D) is present in pNVH99, as well as in pSK89 and pSK108. The putative Pribnow box, TAAAAT (−10), is located within this region, and the putative −35 consensus (TTTACA) is located 18 bp upstream from the −10 consensus (TAAAAT). Conservation of specific flanking regions is apparently essential to ensure adequate expression of the smr gene.
The first 10 bp of DR1C, designated DR10, are duplicated upstream (nt 1298 to 1307) in pNVH99. DR1C is partly overlapped by a sequence of 18 bp directly repeated downstream (nt 1290 to 1308; italic letters in Fig. 1), marked DR18+18 in Fig. 2. This 18-bp duplication is not present in any of the other smr-containing plasmids that have been described. On pSK108, however, the 9 bp at the downstream end of DR1C are duplicated. It has been proposed that this is a result of a duplication of the smr gene and flanking regions on this plasmid (14). An evolutionary rearrangement of such an smr duplication could possibly result in only partial conservation of the gene and flanking regions present on pSK108. A similar duplication of the smr gene and flanking regions has probably occurred on pNVH99, followed by subsequent deletions, thus resulting in an 18-bp sequence as the only fragment being left from the flanking regions. The similarity between CNS plasmid pSK108 and pNVH99 indicates that CNS was a possible source for the smr gene found in S. aureus.
FIG. 2.
Genetic organization of pNVH99 in relation to other staphylococcal plasmids that contain a proposed smr gene cassette, extending from the putative replication nick site (indicated by a vertical arrow) to the end of the direct repeat region downstream from the smr gene. The directions of the marked ORFs are denoted by horizontal arrows within the boxes. Direct repeat sequences previously described, DR1C, ′DR1C, DR2C, DR1D, DR2D (14), and the 10-bp direct repeat sequence within DR1C in pNVH99, DR10, are denoted by solid boxes. The 18-bp direct repeat sequence DR18+18 is indicated by a bracket. The single-strand origin sequence SSOA (formerly designated palA) (1) within some of the DR sequences is shown as a white box.
Extensive use of antiseptics and disinfectants based on QAC has led to selection of staphylococcal strains resistant to such compounds in human hospital environments, as well as in the food industry (6, 13). The smr gene has previously been found in strains of S. aureus and CNS associated with clinical disease in humans (1, 13, 16) and in CNS recovered from foods (6, 7). In our study, resistance to QAC was detected in S. aureus isolates from dairy cows in a herd in which a teat cream containing QAC had been used for several years for mastitis control. As shown by the examination of available isolates, S. aureus containing pNVH99 had persisted for at least 20 months within the herd. Maintenance of QAC resistance might have been a consequence of the continuous use of preparations containing QAC. These findings support previous assumptions that the smr gene has a widespread distribution among different staphylococcal species.
ACKNOWLEDGMENTS
We thank Even Heir, National Institute of Public Health, Oslo, Norway, and Henning Sørum, The Norwegian School of Veterinary Science, for helpful discussions and constructive comments on this paper. We are also grateful to the laboratory and scientific staff at the Cattle Health Section, National Veterinary Institute, Oslo, Norway.
This study was partially funded by The Research Council of Norway (grant 140723/110).
REFERENCES
- 1.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray R A. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramley A J, Dodd F H. Reviews of the progress of dairy science: mastitis control—progress and prospects. J Dairy Res. 1984;51:481–512. doi: 10.1017/s0022029900023797. [DOI] [PubMed] [Google Scholar]
- 3.Evans J, Dyke K G H. Characterization of the conjugation system associated with the Staphylococcus aureus plasmid PJE1. J Gen Microbiol. 1988;134:1–8. doi: 10.1099/00221287-134-1-1. [DOI] [PubMed] [Google Scholar]
- 4.Grinius L, Dreguniene G, Goldberg E B, Liao C H, Projan S H. A staphylococcal multidrug resistance gene product is a member of a new protein family. Plasmid. 1992;27:119–129. doi: 10.1016/0147-619x(92)90012-y. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 6.Heir E, Sundheim G, Holck A L. Resistance to quaternary ammonium compounds in Staphylococcus spp. isolated from the food industry and nucleotide sequence of the resistance plasmid pST827. J Appl Bacteriol. 1995;79:149–156. doi: 10.1111/j.1365-2672.1995.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 7.Heir E, Sundheim G, Holck A L. Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. Int J Food Microbiol. 1999;48:211–219. doi: 10.1016/s0168-1605(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 8.Heir E, Sundheim G, Holck A L. The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J Appl Microbiol. 1999;86:378–388. doi: 10.1046/j.1365-2672.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- 9.Heir E, Sundheim G, Holck A L. The Staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol Lett. 1998;163:49–56. doi: 10.1111/j.1574-6968.1998.tb13025.x. [DOI] [PubMed] [Google Scholar]
- 10.Hugo W B, Russell A D. Types of antimicrobial agents. In: Russell A D, Hugo W B, Ayliffe G A J, editors. Principles and practice of disinfection, preservation and sterilisation. 2nd ed. Oxford, United Kingdom: Blackwell Scientific Publications; 1992. pp. 37–38. [Google Scholar]
- 11.Im S H, Yoon S J, Kim W K, Shin C K, Lee D W, Moon K H. Characterization of cryptic plasmid of multidrug-resistant Staphylococcus aureus SA2. J Microbiol Biotechnol. 1996;6:145–146. [Google Scholar]
- 12.International Dairy Federation. Laboratory methods for use in mastitis work. Doc. 132. Brussels, Belgium: IDF; 1981. [Google Scholar]
- 13.Leelaporn A, Paulsen I T, Tennent J M, Littlejohn T G, Skurray R A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J Med Microbiol. 1994;40:214–220. doi: 10.1099/00222615-40-3-214. [DOI] [PubMed] [Google Scholar]
- 14.Leelaporn A, Firth N, Paulsen I T, Hettiaratchi A, Skurray R A. Multidrug resistance plasmid pSK108 from coagulase-negative staphylococci; relationships to Staphylococcus aureus qacC plasmids. Plasmid. 1995;34:62–67. doi: 10.1006/plas.1995.1034. [DOI] [PubMed] [Google Scholar]
- 15.Littlejohn T G, DiBerardino D, Messerotti L J, Spiers S J, Skurray R A. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene. 1991;101:59–66. doi: 10.1016/0378-1119(91)90224-y. [DOI] [PubMed] [Google Scholar]
- 16.Littlejohn T G, Paulsen I T, Gillespie M T, Tennent J M, Midgley M, Jones I G, Purewal A S, Skurray R A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1992;95:259–266. doi: 10.1016/0378-1097(92)90439-u. [DOI] [PubMed] [Google Scholar]
- 17.Macrina F I, Kopecko D J, Jones K R, Ayers D J, McCowen S M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- 18.McDonnel G, Russell A D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novick R P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- 20.Pankey J W, Eberhart R J, Cuming A L, Daggett R D, Farnsworth R F, McDuff C K. Uptake on postmilking teat antisepsis. J Dairy Sci. 1984;67:1336–1353. doi: 10.3168/jds.S0022-0302(84)81443-5. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci USA. 1996;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen I T, Skurray R A, Tam R, Saier M H, Jr, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 23.Rouch D A, Cram D S, DiBerardino D, Littlejohn T G, Skurray R A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus—common ancestry with tetracycline-transport and sugar-transport proteins. Mol Microbiol. 1990;4:2051–2062. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 24.Russell A D, Hugo W B. Chemical disinfectants. In: Linton A H, Hugo W B, Russell A D, editors. Disinfection in veterinary and farm animal practice. Oxford: Blackwell Scientific Publications; 1987. pp. 20–23. [Google Scholar]
- 25.Russell A D. Plasmids and bacterial resistance to biocides. J Appl Microbiol. 1997;83:155–165. doi: 10.1046/j.1365-2672.1997.00198.x. [DOI] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson R. DNA-sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasatsu M, Shima K, Shibata Y, Kono M. Nucleotide sequence of a gene that encodes resistance to ethidium bromide from a transferable plasmid in Staphylococcus aureus. Nucleic Acids Res. 1989;17:10103. doi: 10.1093/nar/17.23.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasatsu M, Shibata Y, Noguchi N, Kono M. High-level resistance to ethidium bromide and antiseptics in Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:109–113. doi: 10.1016/0378-1097(92)90514-o. [DOI] [PubMed] [Google Scholar]
- 29.Seery L T, Nolan N C, Sharp P M, Devine K M. Comparative-analysis of the PC194 group of rolling circle plasmids. Plasmid. 1993;30:185–196. doi: 10.1006/plas.1993.1051. [DOI] [PubMed] [Google Scholar]
- 30.Smith T H, Fox L K, Middleton J R. Outbreak of mastitis caused by one strain of Staphylococcus aureus in a closed dairy herd. J Am Vet Med Assoc. 1998;212:553–556. [PubMed] [Google Scholar]
- 31.State Veterinary Laboratories of Norway. Routines for mastitis diagnostics. Oslo, Norway: State Veterinary Laboratories; 1993. [Google Scholar]
- 32.Sundheim G, Hagtvedt T, Dainty R. Resistance of meat associated staphylococci to a quaternary ammonium compound. Food Microbiol. 1992;9:161–167. [Google Scholar]
- 33.Tennent J M, Lyon B R, Midgley M, Jones I G, Purewal A S, Skurray R A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989;135:1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- 34.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]