Abstract
It has been over 40 years since Lasagna's Law was first introduced and its main postulates are still permeating the clinical trial arena.
This article attempts to identify the most pressing roadblocks in patient recruitment, categorizes them based on the stakeholders involved and provides suggestions on how to identify and mitigate the risks involved.
1. Introduction
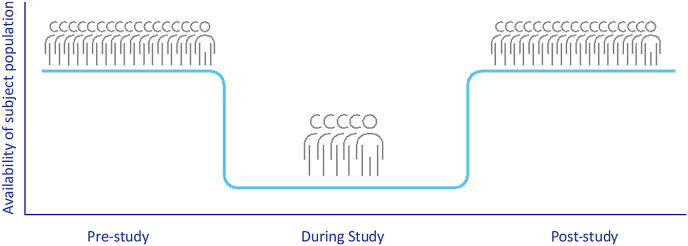
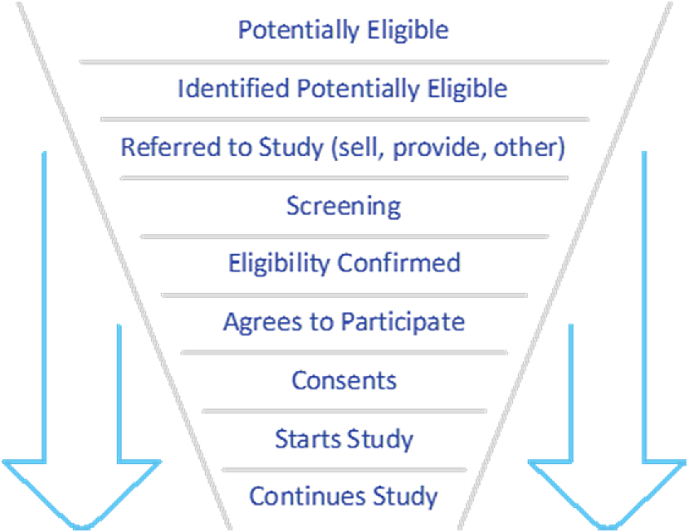
Louis Lasagna, known as the “father of modern pharmacology”, is also credited with highlighting a problematic phenomenon that has long perplexed clinical researchers suggesting that “the number of patients available to join a trial drop by 90% the day the trial begins. They re-appear as soon as the study is over” i.e., the number of participants actually available for recruitment in a study, usually turns out to be much lower than estimated in advance (graph 1). His observation in 1979 has since become known as “Lasagna's Law”. In 2001 Alvan R. Feinstein, founding editor of the Journal of Clinical Epidemiology, described it as follows: “the number of patients who are actually available for a trial is about 1/10 to 1/3 of what was originally estimated” [1]. Patient numbers continue to diminish from the time of study inception until it is completed. Researchers from the University of Rochester have devised a graphic representation of the “recruitment funnel” (Graph 2) and jokingly noted that “the best time to plan a controlled trial is after the trial has finished’’.
Graph 1.
Patient engagement pre-, during and post-study.
Graph 2.
Recruitment funnel.
Over 40 years since Lasagna's Law was first observed, not much has changed. Today, 11% of clinical research sites fail to enroll even a single participant, and nearly 90% of clinical trials experience significant delays due to issues with patient recruitment.
A 2015 analysis of registered trials revealed that 19% were terminated early because they could not recruit enough subjects [2]. A 2013 study found that over 80% of conducted clinical trials missed their recruitment targets [3]. According to Briel et al., 76% of discontinued clinical trials were terminated due to poor recruitment [4].
Given these data, the consensus on patient recruitment, being the most important and the most challenging part of any clinical program, is unequivocal. Different seven-digit figures have been proposed to describe the costs to a sponsor when marketing authorization is delayed merely by one day. Therefore, getting a realistic picture of what trial recruitment will look like and preemptively addressing the identifiable risks, is critically important for all stakeholders in drug development. It seems counterintuitive, but despite the obviousness of the problem, a large proportion of the studies do not proactively implement any tactics to improve patient recruitment [5].
So, why does Lasagna's Law continue to permeate the field of drug development? While there is no single solution that would meet the needs of an individual clinical program, there are several common challenges that remain true for any trial.
It helps to separate patient recruitment challenges into categories based on the stakeholders involved.
2. Sponsors
2.1. Accurate assessment of disease prevalence and evaluation of recruitment potential
It is important to differentiate between the overall disease incidence and prevalence and real-life patient eligibility. The available epidemiologic studies are often dated or limited in their scope. Thus, a thorough feasibility analysis is critical in asserting true prevalence and patient availability. Wherever possible, it is therefore necessary to include electronic health record queries, ICD-10 deidentified records, and geo-targeting disease data. Every feasibility should include direct communication with the prospective investigators and the KOLs. This task is often best suited for the CROs that have vast databases and longstanding relationships with the research teams.
Investigators should be asked about their historical patient encounters with the sought-after patient population. Better yet, if investigators had participated in similar trials in the recent past, inquiring about the number of patients that they were able to recruit and the difficulties they had faced would be invaluable.
2.2. Clinical trial protocol development
Quite often the sponsors utilize more of a scientific approach to what they would like to study and are less concerned with how they are going to study it. This applies both to the selection of the endpoints and to the inclusion/exclusion criteria. A clear scientific question should always be the driver in clinical trial design.
The sponsors aren't always cognizant of the challenges associated with selecting too many endpoints for their study. This applies to selecting more than one primary and too many secondary or exploratory endpoints. When designing a protocol, it is important to put yourself in the position of both the investigators and the patients. The investigators not only look at the rationality and ethics of the study design, but also at its logistical complexity. A considerable burden is added when the trial protocol demands too many unjustifiable patient visits, laboratory and radiographic evaluations or invasive procedures. Not only is it time consuming, but it can also have a negative impact on patient recruitment. As an example, if the protocol dictates that the investigator must administer 10 different scales at each patient visit, they know a priori that many patients may have a problem with lengthy visits. If the protocol includes excessive invasive tests or procedures, the investigators, who operate under the doctrine of “primum non nocere” (“first, do no harm”), may be hesitant to participate in the study or will put little effort into patient recruitment. This will also apply to the patients who may fear or dislike having multiple IV contrast injections or tissue biopsies. Even if the patients initially agree to participate, there is a higher likelihood that they may drop out of the trial, should they be unable or unwilling to follow the protocol.
When it comes to inclusion and exclusion criteria, similar logic needs to be followed. Excessive restrictions and tedious inclusion/exclusion criteria have a negative impact on investigators’ fervor and may, in turn hamper patient recruitment.
Clearly, the balance between appropriate inclusion of vital metrics of trial efficacy and safety needs to be weighed against real-life circumstances. The best way, to streamline the protocol is to start discussing its particulars with prospective investigators and opinion leaders. This requires a well written trial synopsis that includes the endpoints, the inclusion/exclusion criteria, and the schedule of events to start such a dialogue. This dialogue can be led by the sponsor or be outsourced to a CRO. It can start with electronic questionnaires and continue with targeted interviews as well as with ad hoc advisory board meetings.
2.3. Trial funding
While prudent allocation of study resources is central to staying within the study budget, it is critical to make sure that the investigators and their study teams are well compensated. In some instances, assuring that the research staff is available during the off-hours, at nights and on weekends, can make a dramatic difference in the rates of recruitment.
Patient reimbursement should also be seriously considered. Many patients experience financial difficulties because of their illness and may not have the funds to travel and take the time off for trial-related activities. Covering travel expenses, room and board, and in some situations, providing a stipend for the time spent on participation in the trial, can significantly improve both recruitment and retention.
3. Geographic coverage
The decision about where to conduct a trial can have a tremendous impact on the speed of recruitment. This is especially relevant to later-stage trials where large patient cohorts are required and in rare/orphan diseases where low disease prevalence requires global reach. It is best to make the decision to expand internationally early in the study planning phase. Learning about local regulatory requirements and standards of care may have an impact on inclusion/exclusion criteria, diagnostic preferences, and other aspects of protocol development.
4. Patients
A UK survey discovered that of the patients who were aware of a clinical trial, 71% opted not to participate. Of these patients, 37% did not participate because they felt that the standard treatment was better, 31% were concerned about receiving a placebo, 22% cited fear of being treated like guinea pigs, and 21% cited both travel time and distance as major barriers to participation [6].
As stated earlier, when devising a protocol, special attention needs to be paid to patient-related logistics. Questions include:
How much time will the patients have to spend at the clinical site?
Could the trial-related procedures be viewed as excessive or dangerous?
What are the chances of being included in the placebo group?
What would be the standard of care should this happen?
These and many other questions should be answered ahead of study start.
In some clinical trials the use of direct-to-patient awareness campaigns may be useful and should be devised before the trial is launched. A metanalysis of 45 trials with over 43 000 participants found the following interventions to be effective in increasing recruitment:
Telephone reminders to non-respondents.
Use of opt-out rather than opt-in procedures for contacting potential participants.
Open designs where participants know what treatment they are receiving [3].
Patient advocacy groups, foundations and consortia can add to trial popularization and patient engagement. This is especially true when the trial is in rare and orphan disease indications.
5. Investigators
Investing into investigator engagement is imperative to ensuring clinical trial success. Whenever possible, the investigators should be selected based on their track record. This is where utilization of an experienced CRO with a dynamic and comprehensive investigator database can help. This doesn't mean that less experienced or new investigators shouldn't be considered but balancing the complexity of the trial and the enthusiasm of a less experienced investigator can be challenging.
This also applies to selection of key opinion leaders (KOLs), who can be tremendous assets as their influence among peers can drive the awareness of the drug candidate pre- and post-launch. Having a KOL as a principal investigator increases trial credibility and investigator engagement. However, most KOLs have demanding academic and clinical roles and often aren't the most prolific recruiters. When considering KOLs for a trial, it is important to assess their clinical research team's capabilities and workload. If their teams are engaged in multiple competing trials, chances are high that they won't be focused on yours.
Approaching KOLs and investigators early and getting their opinion on trial design could be very helpful. Their clinical insight gives them a different perspective not only on how the trial will be received by the patients, but also on the validity of the scientific questions that are being asked.
6. CROs
The role of CROs in mitigating the impacts of Lasagna's Law cannot be overestimated. Many CROs can perform a more thorough feasibility assessment and suggest better trial sites than even an experienced in-house team can. If there is a chance to bring in a CRO early in the study development cycle, its input on study design can be very valuable.
Clearly the goal of any CRO is to attract business, and sponsors rightfully view some CRO's optimistic recruitment projections as a marketing strategy. Therefore, devising a risk-sharing model with selected CROs can have a significant impact on their projections. Agreeing upon and instituting financial penalties for under recruitment and conversely devising a bonus structure for high enrollers, can be a very powerful tool.
7. Utilization of novel trial designs
One of the significant barriers to recruitment is a patients’ reluctance to participate in trials where the chance of receiving a placebo is high. Thus, any trial design changes that reduce the placebo cohort should be considered, wherever possible. This may involve employing 1:2 or 1:3 instead of 1:1 randomization, and using crossover designs. In some instances, introduction of a master protocol (basket, umbrella and platform trials) [7] could be advantageous. Clearly, the selection of the most appropriate design will depend on the scientific question that is being asked and on the sort of interventions that are being planned in the trial.
The least disruptive the trial-related procedures and interventions, the least time-consuming they are, the higher the likelihood of patient participation in the trial. For example, decentralized clinical trials, which are increasingly popular, can significantly reduce the need for patient visits via the use of telemedicine, wearable devices, remote drug dispensation and at-home diagnostic testing. The costs of such interventions should be individually weighed against the potential advantages for patient recruitment and retention.
8. Conclusions
Recruitment and retention of patients are integral to the success of any clinical study. Over 40 years after Dr. Lasagna came up with his law of progressively diminishing returns in patient recruitment, Lasagna Law still looms large over clinical trials and drug development. Overcoming patient recruitment challenges requires proactively identifying and mitigating risks. It also needs active engagement with diverse stakeholders including investigators, KOLs, CROs and patient groups. Furthermore, these strategies must be consistently reviewed and revised as needed when approaching each new study in order for them to remain effective.
Biography

Louis Cesare Lasagna (February 22, 1923–August 6, 2003)
Dr. Lasagna was a renowned expert in clinical pharmacology. He graduated from Rutgers University, earned his medical degree from Columbia University, and completed an anesthesia fellowship at Harvard Medical School. In 1954 Dr. Lasagna established the first ever clinical pharmacology department at Johns Hopkins University. He subsequently chaired the Department of Pharmacology and Toxicology at the University of Rochester and then became dean of the Sackler School of Graduate Biomedical Sciences at Tufts University.
References
- 1.Feinstein A.R. Chapman and Hall/CRC; 2001. Principles of Medical Statistics; p. 508. [Google Scholar]
- 2.Carlisle B., Kimmelman J., Ramsay T., MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin. Trials. 2015 Feb;12(1):77–83. doi: 10.1177/1740774514558307. Epub 2014 Dec 4. PMID: 25475878; PMCID: PMC4516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treweek S., Lockhart P., Pitkethly M., et al. Methods to improve recruitment to randomized controlled trials: Cochrane systematic review and metaanalysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briel M., Olu K.K., von Elm E., Kasenda B., Alturki R., Agarwal A., Bhatnagar N., Schandelmaier S. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J. Clin. Epidemiol. 2016 Dec;80:8–15. doi: 10.1016/j.jclinepi.2016.07.016. Epub 2016 Aug 3. PMID: 27498376. [DOI] [PubMed] [Google Scholar]
- 5.Strategies to improve recruitment to randomised trials. Cochrane Database Syst. Rev. 2018 Feb 22;2(2) doi: 10.1002/14651858.MR000013.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn R. Surveys identify barriers to participation in clinical trials. J. Natl. Cancer Inst. 2000 Oct 4;92(19):1556–1558. doi: 10.1093/jnci/92.19.1556. PMID: 11018085. [DOI] [PubMed] [Google Scholar]
- 7.Bogin V. Master protocols: new directions in drug discovery. Contemp Clin Trials Commun. 2020 Apr 25;18 doi: 10.1016/j.conctc.2020.100568. PMID: 32395664; PMCID: PMC7205752. [DOI] [PMC free article] [PubMed] [Google Scholar]