Abstract
The knowledge about surfactant biology is now deeper and recent research has allowed to clarify its role in several human lung disorders. The balance between surfactant production and consumption is better known and the same applies to their regulatory mechanisms. This has allowed to hypothesize and investigate several new and original strategies to protect surfactant and enhance its activity. These interventions are potentially useful for several disorders and particularly for acute respiratory distress syndrome. We here highlight the mechanisms regulating surfactant consumption, encompassing surfactant catabolism but also surfactant injury due to other mechanisms, in a physiopathology-driven fashion. We then analyze each corresponding strategy to protect surfactant and enhance its activity. Some of these strategies are more advanced in terms of research & development pathway, some others are still investigational, but all are promising and deserve a joint effort from clinical-academic researchers and the industry.
Keywords: Surfactant, Protection, Phospholipase A2
After 50 years from the first studies on surfactant replacement in preterm neonates [1], surfactant biology has eventually seen relevant advancements. There is a newly growing interest for the development of new approaches optimizing surfactant therapy for the treatment of respiratory distress syndrome (RDS) due to primary surfactant deficiency of preterm babies [2]. Moreover, there are promising data regarding the possibility to expand surfactant use beyond RDS and treat other critical respiratory conditions [3].
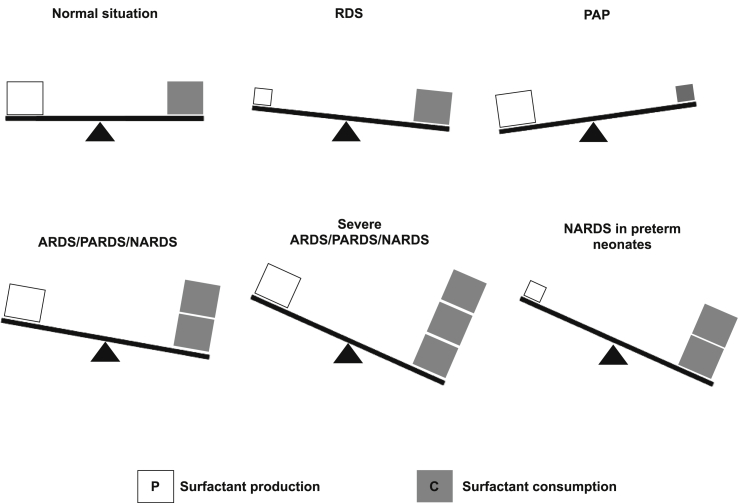
In fact, surfactant system can be affected by various types of disorders [Fig. 1] characterized by imbalance between surfactant production and consumption. Surfactant production has been well characterized and its mechanisms are quite well known [4]. Surfactant consumption refers to a more general process encompassing various mechanisms, from the surfactant recycling, which may be increased and lead to surfactant catabolism, to molecular injuries caused by mechanisms different from the recycling/catabolism. All these can cause quantitative and qualitative surfactant dysfunction. In normal conditions, surfactant production and consumption are well balanced and ensure a low alveolar surface tension (<5 mN/m) and the absence of relevant lung tissue inflammation. RDS and pulmonary alveolar proteinosis (PAP) are characterized by decreased production and consumption, respectively [2,5]. Thus, In RDS decreased production leads to low surfactant levels, whereas in PAP decreased consumption leads to high surfactant levels. Acute respiratory distress syndrome in adult (ARDS) [6], pediatric (PARDS) [7] and neonatal (NARDS) [8] patients are more complex disorders where surfactant is variously damaged by an increased catabolism and/or various molecular injuries and this can also be associated with a relatively insufficient production in preterm neonates [3,9]. The biological and biochemical agents responsible for surfactant catabolism and molecular injury are numerous and can interact between them. We are now aware of these mechanisms and there are promising molecules and techniques holding potential to protect surfactant and preserve its functions during increased catabolism or in presence of surfactant-injuring agents. However, it is crucial to increase our knowledge about the interplay between surfactant catabolism and surfactant-injuring agents to provide more efficient surfactant protection strategies.
Fig. 1.
Physiopathology of surfactant system in various human diseases. In normal situations surfactant production and consumption are balanced, and this allows to achieve low alveolar surface tension and lack of significant lung tissue inflammation. In RDS, which is usually affecting preterm neonates, surfactant production is reduced. In patients with PAP, surfactant consumption is relatively reduced compared to its production. ARDS in adults, in pediatric (PARDS) or neonatal (NARDS) patients are disorders of various severity characterized by increased surfactant catabolism and normal primary surfactant production. NARDS may also occur in preterm neonate: in this case a relative primary surfactant deficiency can co-exist.
Surfactant recyclying and catabolism
Here we intend to briefly summarize surfactant recycling and catabolism. The former is the physiological mechanism of re-uptake and substitution of surfactant phospholipids and proteins when needed [10], while the latter may result in a pathological process when increased surfactant phospholipid or protein destruction in absence of physiological needs occur and it is elicited by some pathological triggers. Type-II pneumocytes (i.e.: the same cells responsible for surfactant production) perform both surfactant recycling and catabolism and they are responsible for most of the recycling [10]. Conversely, alveolar macrophages (the cell type involved in the inflammatory cascade) are involved in surfactant catabolism and particularly they have a predominant role during pathological situations characterized by high lung tissue inflammation [10].
Catabolism is up-regulated when increasing doses of exogenous surfactant are administered in animal models [11], whereas it is insufficient in PAP which is caused by autoimmune or hereditary disfunction in GM-CSF signaling, leading to fewer and less active alveolar macrophages [5]. This leads to the abnormal accumulation of less functional and oxidized surfactant components in the alveolar space with a reduction in the pulmonary diffusion capacity [12].
Alveolar macrophages are the main cells producing the isotype-IIA of secretory phospholipase A2 (sPLA2-IIA) [13]. The activity of this enzyme is increased by GM-CSF treatment [14] and there is a significant correlation between GM-CSF and sPLA2-IIA expression and activity [15].
From a molecular point of view, surfactant lipid recycling and catabolism are due to several distinct mechanisms that may involve various surfactant components and may differentially be involved in either recycling or catabolism. For instance, by promoting membrane fragmentation [12], surfactant protein-D and -C (SP-D and SP-C), may affect the structure and conversion of surfactant large aggregates - surface-active large aggregates of heavy density - into small aggregates. The latter are small vesicles with light density and low interfacial activity that probably may also result from breathing mechanics. Interestingly, Surfactant Protein A (SP-A) may interact with these small aggregates, favoring their uptake by type-II pneumocytes [12]. It has been suggested that SP-D and SP-C may also play a role in macrophages clearance of surfactant phospholipid and cholesterol, under both physiological conditions and aging [12]. A further mechanism that also regulates both surfactant phospholipid recycling and catabolism involves sPLA2 enzymes, which hydrolyze phospholipid by detaching a free fatty acid from their sn-2 position. This hydrolysis has several consequences on the biophysical and biological function of surfactant. In fact, sPLA2 hydrolysis is directly proportional to the alveolar surface tension [16] and, as consequence, inversely proportional to lung compliance and gas exchange function [17,18].
PLA2 hydrolysis is a complex process as various enzyme subtypes are expressed in human lung tissue and they have different phospholipid substrate specificity although they can all variously contribute to the impairment of surfactant biophysical activity [19,20]. Beyond these biophysical consequences, sPLA2 hydrolysis also leads to the production of free fatty acids, especially arachidonic acid (C20H32O2, 20:4), making it available for the further steps of the inflammatory cascade [8]. Therefore, sPLA2 enzymes represent a crucial dual target since, from one side, they can impair surfactant function, and, from the other side, they may increase lung tissue inflammation which, in its turn, injures surfactant. This represents the so-called “ARDS vicious cycle” where surfactant catabolism and inflammation perpetuate each other, since pro-inflammatory cytokines (notably tumor necrosis factor-α and GM-CSF [21,22]) increase the production of sPLA2-IIA which again catabolizes surfactant phospholipids [13]. As a consequence, sPLA2 activity in broncho-alveolar lavage samples from ARDS patients also shows correlation with relevant clinical outcomes [18,[23], [24], [25]].
The role of sPLA2 enzymes is not only crucial in surfactant recycling and catabolism but there are also important interplays with several proteins. These include the sPLA2 receptor which would allow several effects independent from hydrolysis [26], and other enzymes such as cytosolic phospholipases [27] depicting a complex interactome, whose description is out of our scopes. We will however detail the main mechanisms modulating sPLA2 actions as these are the basis for possible surfactant protection strategies.
Surfactant protein-A (SP-A) is a hydrophilic surfactant protein with important anti-infectious and anti-inflammatory properties. In details, the SP-A2 isoform is involved in anti-inflammatory and immunomodulatory properties, whereas SP-A1 contributes more to lipid aggregation and helps in both adsorption and spreading of massive amounts of surfactant material at the air-liquid interface [12]. Notably, SP-A is able to decrease sPLA2-IIA expression but also to inhibit its activity by a protein-to-protein calcium dependent interaction [21,28]. SP-A represents the most potent agent to this end, but it is not the only one, since a negatively charged phospholipid, the dioleoylphosphatidylglycerol (DOPG; C42H79O10P, PG 36:2) is also able to downregulate sPLA2-IIA expression [21,22]. Compared to SP-A, that also plays an effect on surfactant biophysical function (by facilitating surfactant adsorption and spreading), DOPG is a minor surfactant constituent with no effect on surface tension. However both these molecules are important as negative feedback agents modulating the role of sPLA2 [13]. Other sPLA2-modulating agents are surfactant protein-B (SP-B) which seems to reduce surfactant hydrolysis caused by both sPLA2 subtype-IB and -IIA: this seems to be related to a lower availability of lipid substrate for the catalytic site of sPLA2 enzymes, due to SP-B creating packed phospholipid layers [29]. Interestingly, SP-B seems also to be increased as acute-phase protein during pulmonary infections [30], thus its anti-sPLA2 effect may have a protective role when the inflammation is too much extended. Club cell secretory protein (CCSP) is produced in the airway epithelium and has multiform potent anti-inflammatory properties [31]. Particularly, CCSP interacts with sPLA2 enzymes by blocking neutrophil influx and fibroblast migration mediated by sPLA2 [32,33] and seems to be involved in the mitigation of ventilator-induced lung injury [34,35] and the mechanisms of ARDS in patients of any age [25,[36], [37], [38], [39], [40]]. As sPLA2 activity is extremely important to guarantee a balance between surfactant production and consumption, it is not surprising that several molecules may modulate sPLA2 pathway, guaranteeing a certain redundancy.
Surfactant injury mechanisms not linked to catabolism
There are several agents able to injure surfactant through molecular mechanisms unrelated to surfactant catabolism, but still able to cause relevant quantitative and qualitative surfactant dysfunction. Table 1 lists the main surfactant-injuring agents playing a role in human diseases: the biochemical mechanisms of these injuries are numerous [9,[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]]. Blood proteins may compete with surfactant aggregates, reducing surfactant adsorption (e.g. albumin) or change surfactant fluidity/structure (e.g. C-Reactive Protein), significantly impacting on its biophysical function. Moreover, upon pathological mechanisms, the presence of proteins which are not normally found in the alveolar space may also affect other biological functions by interacting with surfactant-proteins. On top of this effect, hemoglobin may contribute to the inflammation processes, causing protein and lipid oxidation. The latter is also a transversal mechanism, triggered by the rise in reactive species of oxygen that may happen under several circumstances and negatively influence surfactant function. Cholesterol is an important surfactant component but, when it is increased, it worsen surfactant function, since it is a small amphiphilic molecule and makes surfactant complexes more fluid [12]. Bile acids seem to enhance the cholesterol distribution in surfactant membranes and act as co-enzyme of sPLA2, facilitating surfactant phospholipid hydrolysis and production of inflammatory mediators, including the release of free fatty acids, which may in turn change surfactant fluidity and structure. More coarse injuring agents, such as smoke, sea water, milk, food, or gastric secretion, may be aspirated and create various damages: they can modify surfactant structure or fluidity, dilute surfactant and create an osmotic injury. They can also have a direct cytopathic effect on the alveolar and airway epithelium and obstruct small airways with debris preventing the physiological diffusion of surfactant toward the upper airways via the mucociliary movement [10]. This process may contribute to surfactant recycling and help keeping small airways open. Moreover, the resulting injury may also facilitate local infections which, in turn, may contribute to the epithelium damages and increase local inflammation and oxidation, affecting both surfactant structure and activity.
Table 1.
The main agents capable to injure surfactant with mechanisms different from direct catabolism and associated human diseases.
| Surfactant-injuring agent | Mechanisms of action | Relevant disorder |
|---|---|---|
| Albumin [9,41] | Change surfactant fluidity and structure | Indirect (secondary) ARDS |
| Hemoglobin [42] | Increased inflammation, capillary occlusion, protein/lipid oxidation. | Indirect (secondary) ARDS, Maternal blood aspirationa, Pulmonary hemorrhagea |
| Other plasma proteins [9,41] | Change surfactant fluidity and structure, interaction with surfactant proteins | Indirect (secondary) ARDS |
| Cholesterol [43] | Change surfactant fluidity | Indirect (secondary) ARDS, Meconium aspirationa |
| Bile acids [44] | Change surfactant film structure and facilitate sPLA2-substrate interaction, increase inflammation | Neonatal bile acid pneumoniaa Bile aspirationa |
| Milk/Gastric secretions [45,46] | Change surfactant fluidity and structure, direct cytopathic damage | Milk/gastric content aspirationa |
| Inflammatory cytokines [47,48] | Epithelial injury; change surfactant fluidity and structure | Indirect (secondary) or direct (primary) ARDS |
| Free fatty acids [49] | Change surfactant fluidity and structure | Indirect (secondary) ARDS |
| Water [50] | Osmotic damage Dilution of surfactant | Near drowninga |
| Viral infection [51,52] | Cytolysis, direct cytopathic effect | Viral pneumoniaa |
| Oxidative stress [53,54] | Protein oxidation | Indirect (secondary) or direct (primary) ARDS |
| Antigen-Antibody response [55] | Cellular injury induced by alloantibodies | TRALI |
| Smoke [53,56] | Epithelial injury, susceptibility to infections, change surfactant fluidity and structure and other afore-mentioned mechanisms | Direct (primary) ARDS |
Abbreviations: ARDS: acute respiratory distress syndrome; TRALI: transfusion-related acute lung injury.
These disorders can eventually qualify as or evolve in ARDS, if they are enough clinically severe and fulfill relevant ARDS definitions.
These mechanisms are involved in the pathogenesis of various types of ARDS in patients of diverse ages and can also be simultaneously present in each patient. This explains the pathobiological complexity of ARDS: this syndrome, although appears with a common clinical trait, may have different underlying mechanisms of injury and the prevalent one may vary. Therefore, it is important to recognize different ARDS subtypes according to its pathobiology, as this may help to choose one strategy to protect surfactant over the others: several promising therapies have failed because they were trialed in mixed and inhomogeneous populations of patients who had different physiopathology context [3]. Unfortunately, recognizing the physiopathology behind the different subtypes of ARDS is not yet totally and easily possible, as these mechanisms of injury can be overlapped and they are not always measurable at the bedside. However, some distinctions have been recently possible, and, for instance, we know that primary (direct) ARDS has a more evident epithelial injury and alveolar inflammation pattern, while the secondary (indirect) form of the syndrome is preferentially characterized by endothelial injury and systemic inflammation [57,58].
A particular case is represented by neonatal bile acid pneumonia, typical of neonates born to mothers with intrahepatic cholestasis of pregnancy [59,60]. This is relatively rare disorder that can appear as mild or severe respiratory failure caused by bile acids reaching the lung through the systemic circulation [61]. The physiopathology of bile acid pneumonia is complex and represents an example of overlap of surfactant catabolism and other types of surfactant injury. In fact, bile acids act as sPLA2 co-enzyme facilitating the enzyme–substrate interaction [44,62], but they can also alter surfactant biophysical properties, changing surfactant fluidity directly or act as mediators to mobilize and transfer cholesterol into surfactant complexes [12]. Finally, bile acids also increase pro-inflammatory cytokines and seem to reduce hydrophilic surfactant protein which have anti-inflammatory and immune defense roles [63,64]. The understanding of the pathobiological pattern of surfactant injury will be crucial in the future to develop and eventually choose surfactant protection strategies.
Possible strategies to protect surfactant
There are several possible strategies to protect surfactant and enhance its activity or at least preserve it. The various strategies correspond to the different injury mechanisms described above and are represented [Table 2] by several drug candidates but also by some techniques, such as whole-body hypothermia, repeated surfactant bolus or lavage with diluted surfactant solutions.
Table 2.
Main strategies to protect surfactant with the aim to enhance its activity or at least preserve it.
| Strategy | Agent or technique | Ref |
|---|---|---|
| Increased surfactant function | Surfactants with different profiles (for ex.: enhanced with DOPG, SP-D) Whole-body hypothermia | [[65], [66], [67]] |
| sPLA2 activity inhibition | Varespladib and indolic inhibitors | [[68], [69], [70], [71]] |
| sPLA2 expression inhibition | Budesonide and other steroids, Whole-body hypothermia | [[72], [73], [74], [75], [76], [77]] |
| Inflammation reduction | Steroids, CCSP, SP-D, interleukin inhibitors, whole body hypothermia | [31,[78], [79], [80], [81], [82]] |
| Increased active surfactant pool | Higher or repeated surfactant doses | [12,83,84] |
| Restored surfactant phospholipid profile | Higher or repeated surfactant doses | [12,83,84] |
| Oxidative stress reduction | Surfactants enhanced with antioxidant agents | [[85], [86], [87], [88], [89]] |
| Injuring agent removal | Lavage with surfactant solutions | [3,[90], [91], [92]] |
The strategies may be represented by drugs or techniques which are at different stages of clinical development, despite having a promising rationale. More details are given in the text and examples of relevant references in the field are given for each strategy. Abbreviations: CCSP: Club Cell Secretory Protein; DOPG: dioleoylphosphatidylglycerol; SP-D: surfactant protein-D; sPLA2: secretory phospholipase A2.
These strategies act through the following mechanisms:
-
-
availability of more surface-active surfactants or catabolism-resistant surfactants (for instance, by changing the phospholipid profile or adding hydrophilic proteins to surfactant composition or using controlled whole-body hypothermia);
-
-
surfactant protection using direct sPLA2-inhibitors competing for the phospholipid substrate (for instance, indolic inhibitors such as varespladib);
-
-
reduction of inflammation using steroids, CCSP, hydrophilic surfactant proteins or interleukin inhibitors that, in some cases, might also reduce sPLA2 expression (some of these agents may be vehicled by surfactant itself and reach the alveoli in high concentration). Similar results might be obtained using controlled whole-body hypothermia;
-
-
reduction of oxidation using antioxidant agents (some of these agents may be vehicled by surfactant itself and reach the alveoli in high concentration);
-
-
administration of higher or repeated surfactant doses to overcome the inactivation and/or restore a functional phospholipid profile;
-
-
removal of injuring agents performing lung lavages with diluted surfactant solutions.
These strategies vary in terms of their research and development stage. Some drugs have been clinically tested in large populations such as surfactant-vehicled budesonide in preterm neonates with RDS [93] and/or NARDS [94] or systemic steroids in adults with ARDS [95] and they only need explanatory clinical investigations to be refined. Other drugs have a very promising safety and efficacy profiles but, unfortunately, have not been tested yet for a lack of industrial interest, despite the clinical unmet need and their potential. For some others, such as surfactant protein-D [65], the clinical research is underway, while, for anti-interleukin agents, their use for ARDS during the pandemics might allow to quickly accumulate an unexpected clinical experience [78].
There are also strategies based on techniques rather than drugs, such as the use of whole-body hypothermia, repeated surfactant dose or surfactant lavages. Controlled whole-body hypothermia can reduce lung tissue inflammation, sPLA2 expression and activity, and improve surfactant function by modifying its composition and structure [[96], [97], [98], [99], [100]]. Based on these effects, hypothermia has been efficaciously used for NARDS due to meconium aspiration [101] or for primary ARDS induced by various triggers [102,103].
It is interesting to note that despite surfactant being used since decades a clear dose-finding study has not been performed. Clinical and pharmacodynamic data show that 200 mg/kg is the dose to be preferred in preterm neonates with RDS [104] but not much is known about the ideal dose for different types of ARDS in patients of various age. This area represents a field to be urgently explored [105] to provide an efficacious treatment for human diseases involving surfactant system. Finally, lavage with diluted surfactant has been extensively studied in neonates with primary NARDS due to meconium aspiration and seems to be efficacious in removing meconial agents able to injure surfactant [90], but many details are still to be clarified, such as the volume, concentration, total dose, technique and timing of lavage. The same technique could theoretically be beneficial in other types of primary ARDS, but the experience is more limited. The literature regarding each of these strategies is listed in Table 2 and could be useful to guide future research steps of basic and clinical researchers.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
Prof. De Luca has received research and educational grants from Chiesi Pharmaceuticals spa and ABBVIE inc. He served as lecturer for Airway Therapeutics, Chiesi Pharmaceuticals spa and ABBVIE inc. He is a scientific consultant and equity owner for OPHIREX inc. Finally, he has been member of advisory boards for Chiesi Pharmaceuticals spa, ABBVIE inc and Airway Therapeutics. These companies produce surfactants, surfactant proteins or related products, but had no role in design, preparation, review, approval of the manuscript or decision to submit it for publication. The declared conflicts are all unrelated to the present manuscript.
The other author has no conflicts of interest to disclose.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Fujiwara T., Maeta H., Chida S., Morita T., Watabe Y., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 2.De Luca D., Autilio C., Pezza L., Shankar-Aguilera S., Tingay D.G., Carnielli V.P. Personalized medicine for the management of RDS in preterm neonates. Neonatology. 2021;118:127–138. doi: 10.1159/000513783. [DOI] [PubMed] [Google Scholar]
- 3.De Luca D., Cogo P., Kneyber M.C., Biban P., Semple M.G., Perez-Gil J., et al. Surfactant therapies for pediatric and neonatal ARDS: ESPNIC expert consensus opinion for future research steps. Crit Care. 2021;25:75. doi: 10.1186/s13054-021-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnielli V.P., Giorgetti C., Simonato M., Vedovelli L., Cogo P. Neonatal respiratory diseases in the newborn infant: novel insights from stable isotope tracer studies. Neonatology. 2016;109:325–333. doi: 10.1159/000444891. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell B.C., Nakata K., Bonella F., Campo I., Griese M., Hamilton J., et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5:16. doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Khemani R.G., Smith L., Lopez-Fernandez Y.M., Kwok J., Morzov R., Klein M.J., et al. Pediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE) investigators; pediatric acute lung injury and sepsis investigators (PALISI) network. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7:115–128. doi: 10.1016/S2213-2600(18)30344-8. Epub 2018 Oct 22. Erratum in: Lancet Respir Med. 2018 Nov 13; Erratum in: Lancet Respir Med. 2019;7:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Luca D., van Kaam A.H., Tingay D.G., Courtney S.E., Danhaive O., Carnielli V.P., et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5:657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]
- 9.Günther A., Ruppert C., Schmidt R., Markart P., Grimminger F., Walmrath D., et al. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2:353–364. doi: 10.1186/rr86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olmeda B., Martínez-Calle M., Pérez-Gil J. Pulmonary surfactant metabolism in the alveolar airspace: biogenesis, extracellular conversions, recycling. Ann Anat. 2017;209:78–92. doi: 10.1016/j.aanat.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Kramer B.W., Ikegami M., Jobe A.H. Surfactant phospholipid catabolic rate is pool size dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2000;279:L842–L848. doi: 10.1152/ajplung.2000.279.5.L842. [DOI] [PubMed] [Google Scholar]
- 12.Autilio C., Pérez-Gil J. Understanding the principle biophysics concepts of pulmonary surfactant in health and disease. Arch Dis Child Fetal Neonatal Ed. 2019;104:F443–F451. doi: 10.1136/archdischild-2018-315413. [DOI] [PubMed] [Google Scholar]
- 13.Touqui L., Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol Med Today. 1999;5:244–249. doi: 10.1016/s1357-4310(99)01470-7. [DOI] [PubMed] [Google Scholar]
- 14.Reed J.A., Ikegami M., Cianciolo E.R., Lu W., Cho P.S., Hull W., et al. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol. 1999;276:L556–L563. doi: 10.1152/ajplung.1999.276.4.L556. [DOI] [PubMed] [Google Scholar]
- 15.De Luca D., Shankar-Aguilera S., Autilio C., Raschetti R., Vedovelli L., Fitting C., et al. Surfactant-secreted phospholipase A 2 interplay and respiratory outcome in preterm neonates. Am J Physiol Lung Cell Mol Physiol. 2020;319:L95–L104. doi: 10.1152/ajplung.00462.2019. [DOI] [PubMed] [Google Scholar]
- 16.Hite R.D., Seeds M.C., Jacinto R.B., Balasubramanian R., Waite M., Bass D. Hydrolysis of surfactant-associated phosphatidylcholine by mammalian secretory phospholipases A2. Am J Physiol. 1998;275:L740–L747. doi: 10.1152/ajplung.1998.275.4.L740. [DOI] [PubMed] [Google Scholar]
- 17.De Luca D., Baroni S., Vento G., Piastra M., Pietrini D., Romitelli F., et al. Secretory phospholipase A2 and neonatal respiratory distress: pilot study on broncho-alveolar lavage. Intensive Care Med. 2008;34:1858–1864. doi: 10.1007/s00134-008-1224-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakos G., Kitsiouli E., Hatzidaki E., Koulouras V., Touqui L., Lekka M.E. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:772–779. doi: 10.1097/01.ccm.0000158519.80090.74. [DOI] [PubMed] [Google Scholar]
- 19.Seeds M.C., Jones K.A., Duncan Hite R., Willingham M.C., Borgerink H.M., Woodruff R.D., et al. Cell-specific expression of group X and group V secretory phospholipases A 2 in human lung airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:37–44. doi: 10.1165/ajrcmb.23.1.4034. [DOI] [PubMed] [Google Scholar]
- 20.Hite R.D., Seeds M.C., Safta A.M., Jacinto R.B., Gyves J.I., Bass D.A., et al. Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A(2)-mediated surfactant dysfunction. Am J Physiol Lung Cell Mol Physiol. 2005;288:L618–L624. doi: 10.1152/ajplung.00274.2004. [DOI] [PubMed] [Google Scholar]
- 21.Berger A., Havet N., Vial D., Arbibe L., Dumarey C., Watson M.L., et al. Dioleylphosphatidylglycerol inhibits the expression of type II phospholipase A2 in macrophages. Am J Respir Crit Care Med. 1999;159:613–618. doi: 10.1164/ajrccm.159.2.9805053. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y.Z., Medjane S., Chabot S., Kubrusly F.S., Raw I., Chignard M., et al. Surfactant protein-A and phosphatidylglycerol suppress type IIA phospholipase A2 synthesis via nuclear factor-κb. Am J Respir Crit Care Med. 2003;168:692–699. doi: 10.1164/rccm.200304-467OC. [DOI] [PubMed] [Google Scholar]
- 23.Kim D.K., Fukuda T., Thompson B.T., Cockrill B., Hales C., Bonventre J.V. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol. 1995;269:L109–L118. doi: 10.1152/ajplung.1995.269.1.L109. [DOI] [PubMed] [Google Scholar]
- 24.De Luca D., Lopez-Rodriguez E., Minucci A., Vendittelli F., Gentile L., Stival E., et al. Clinical and biological role of secretory phospholipase A2 in acute respiratory distress syndrome infants. Crit Care. 2013;17:R163. doi: 10.1186/cc12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Luca D., Minucci A., Tripodi D., Piastra M., Pietrini D., Zuppi C., et al. Role of distinct phospholipases A2 and their modulators in meconium aspiration syndrome in human neonates. Intensive Care Med. 2011;37:1158–1165. doi: 10.1007/s00134-011-2243-z. [DOI] [PubMed] [Google Scholar]
- 26.String Consortium. https://string-db.org/network/9606.ENSP00000283243/; 2021 [accessed 28 October 2021].
- 27.Kitsiouli E., Nakos G., Lekka M.E. Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta. 2009;1792:941–953. doi: 10.1016/j.bbadis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Arbibe L., Koumanov K., Vial D., Rougeot C., Faure G., Havet N., et al. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest. 1998;102:1152–1160. doi: 10.1172/JCI3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hite R.D., Grier B.L., Waite B.M., Veldhuizen R.A., Possmayer F., Yao L.-J., et al. Surfactant protein B inhibits secretory phospholipase A 2 hydrolysis of surfactant phospholipids. Am J Physiol Lung Cell Mol Physiol. 2012;302:L257–L265. doi: 10.1152/ajplung.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Aronco S., Simonato M., Vedovelli L., Baritussio A., Verlato G., Nobile S., et al. Surfactant protein B and A concentrations are increased in neonatal pneumonia. Pediatr Res. 2015;78:401–406. doi: 10.1038/pr.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilon A.L. Rationale for the development of recombinant human CC10 as a therapeutic for inflammatory and fibrotic disease. Ann N Y Acad Sci. 2000;923:280–299. doi: 10.1111/j.1749-6632.2000.tb05536.x. [DOI] [PubMed] [Google Scholar]
- 32.Schrama A.J., Elferink J.G., Hack C.E., de Beaufort A.J., Berger H.M., Walther F.J. Pulmonary secretory phospholipase A2 in infants with respiratory distress syndrome stimulates in vitro neutrophil migration. Neonatology. 2010;97:1–9. doi: 10.1159/000220766. [DOI] [PubMed] [Google Scholar]
- 33.Lesur O., Bernard A., Arsalane K., Lauwerys R., Bégin R., Cantin A., et al. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am J Respir Crit Care Med. 1995;152:290–297. doi: 10.1164/ajrccm.152.1.7541278. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa S., Miyahara T., Reynolds S.D., Stripp B.R., Anghelescu M., Eyal F.G., et al. Clara cell secretory protein and phospholipase A 2 activity modulate acute ventilator-induced lung injury in mice. J Appl Physiol. 2005;98:1264–1271. doi: 10.1152/japplphysiol.01150.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa S., King J.A., Reynolds S.D., Stripp B.R., Parker J.C. Time and pressure dependence of transvascular Clara cell protein, albumin, and IgG transport during ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L604–L612. doi: 10.1152/ajplung.00283.2003. [DOI] [PubMed] [Google Scholar]
- 36.Wang S.Z., Rosenberger C.L., Bao Y.X., Stark J.M., Harrod K.S. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 37.De Luca D., Minucci A., Cogo P., Capoluongo E.D., Conti G., Pietrini D., et al. Secretory phospholipase A2 pathway during pediatric acute respiratory distress syndrome: a preliminary study. Pediatr Crit Care Med. 2011;12 doi: 10.1097/PCC.0b013e3181dbe95e. e20–4. [DOI] [PubMed] [Google Scholar]
- 38.Jorens P.G., Sibille Y., Goulding N.J., van Overveld F.J., Herman A.G., Bossaert L., et al. Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur Respir J. 1995;8:1647–1653. doi: 10.1183/09031936.95.08101647. [DOI] [PubMed] [Google Scholar]
- 39.Lesur O., Langevin S., Berthiaume Y., Légaré M., Skrobik Y., Bellemare J.F., et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med. 2006;32:1167–1174. doi: 10.1007/s00134-006-0235-1. [DOI] [PubMed] [Google Scholar]
- 40.Hermans C., Knoops B., Wiedig M., Arsalane K., Toubeau G., Falmagne P., et al. Clara cell protein as a marker of Clara cell damage and bronchoalveolar blood barrier permeability. Eur Respir J. 1999;13:1014–1021. doi: 10.1034/j.1399-3003.1999.13e14.x. [DOI] [PubMed] [Google Scholar]
- 41.Aman J., van der Heijden M., van Lingen A., Girbes A.R., van Nieuw Amerongen G.P., van Hinsbergh V.W., et al. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:89–97. doi: 10.1097/CCM.0b013e3181feb46a. [DOI] [PubMed] [Google Scholar]
- 42.Janz D.R., Ware L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J Intensive Care. 2015;3:20. doi: 10.1186/s40560-015-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keating E., Rahman L., Francis J., Petersen A., Possmayer F., Veldhuizen R., et al. Effect of cholesterol on the biophysical and physiological properties of a clinical pulmonary surfactant. Biophys J. 2007;93:1391–1401. doi: 10.1529/biophysj.106.099762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Luca D., Minucci A., Zecca E., Piastra M., Pietrini D., Carnielli V.P., et al. Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration. Intensive Care Med. 2009;35:321–326. doi: 10.1007/s00134-008-1321-3. [DOI] [PubMed] [Google Scholar]
- 45.Chin C., O'Hare B., Lerman J., Endo J. Comparison of exogenous surfactant and positive end-expiratory pressure therapies in a model of human breast milk-induced acute lung injury in rabbits. Br J Anaesth. 2000;84:600–607. doi: 10.1093/bja/84.5.600. [DOI] [PubMed] [Google Scholar]
- 46.Davidson B.A., Knight P.R., Wang Z., Chess P.R., Holm B.A., Russo T.A., et al. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol. 2005;288:L699–L708. doi: 10.1152/ajplung.00229.2004. [DOI] [PubMed] [Google Scholar]
- 47.Calfee C.S., Janz D.R., Bernard G.R., May A.K., Kangelaris K.N., Matthay M.A., et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calfee C.S., Delucchi K., Parsons P.E., Thompson B.T., Ware L.B., Matthay M.A., et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall S.B., Hyde R.W., Notter R.H. Changes in subphase aggregates in rabbits injured by free fatty acid. Am J Respir Crit Care Med. 1994;149:1099–1106. doi: 10.1164/ajrccm.149.5.8173747. [DOI] [PubMed] [Google Scholar]
- 50.Jin F., Li C. Seawater-drowning-induced acute lung injury: from molecular mechanisms to potential treatments. Exp Ther Med. 2017;13:2591–2598. doi: 10.3892/etm.2017.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habashi N.M., Camporota L., Gatto L.A., Nieman G. Functional pathophysiology of SARS-CoV-2-induced acute lung injury and clinical implications. J Appl Physiol. 2021;130:877–891. doi: 10.1152/japplphysiol.00742.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herold S., Becker C., Ridge K.M., Budinger G.R.S. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J. 2015;45:1463–1478. doi: 10.1183/09031936.00186214. [DOI] [PubMed] [Google Scholar]
- 53.Leikauf G.D., McDowell S.A., Bachurski C.J., Aronow B.J., Gammon K., Wesselkamper S.C., et al. Functional genomics of oxidant-induced lung injury. Adv Exp Med Biol. 2001;500:479–487. doi: 10.1007/978-1-4615-0667-6_73. [DOI] [PubMed] [Google Scholar]
- 54.Machado L.U., Fiori H.H., Baldisserotto M., Ramos Garcia P.C., Vieira A.C.G., Fiori R.M. Surfactant deficiency in transient tachypnea of the newborn. J Pediatr. 2011;159:750–754. doi: 10.1016/j.jpeds.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Wu T.J., Teng R.J., Tsou Yau K.I. Transfusion-related acute lung injury treated with surfactant in a neonate. Eur J Pediatr. 1996;155:589–591. doi: 10.1007/BF01957910. [DOI] [PubMed] [Google Scholar]
- 56.Oulton M., Moores H.K., Scott J.E., Janigan D.T., Hajela R. Effects of smoke inhalation on surfactant phospholipids and phospholipase A2 activity in the mouse lung. Am J Pathol. 1991;138:195–202. [PMC free article] [PubMed] [Google Scholar]
- 57.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinha P., Calfee C.S. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawakita T., Parikh L.I., Ramsey P.S., Huang C.C., Zeymo A., Fernandez M., et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2015;213:570.e1–570.e8. doi: 10.1016/j.ajog.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zecca E., De Luca D., Marras M., Caruso A., Bernardini T., Romagnoli C. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome. Pediatrics. 2006;117:1669–1672. doi: 10.1542/peds.2005-1801. [DOI] [PubMed] [Google Scholar]
- 61.Zecca E., De Luca D., Baroni S., Vento G., Tiberi E., Romagnoli C. Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics. 2008;121:e146–e149. doi: 10.1542/peds.2007-1220. [DOI] [PubMed] [Google Scholar]
- 62.Herraez E., Lozano E., Poli E., Keitel V., De Luca D., Williamson C., et al. Role of macrophages in bile acid-induced inflammatory response of fetal lung during maternal cholestasis. J Mol Med. 2014;92:359–372. doi: 10.1007/s00109-013-1106-1. [DOI] [PubMed] [Google Scholar]
- 63.D'Ovidio F., Mura M., Ridsdale R., Takahashi H., Waddell T.K., Hutcheon M., et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 64.D'Ovidio F., Mura M., Tsang M., Waddell T.K., Hutcheon M.A., Singer L.G., et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 65.Arroyo R., Kingma P.S. Surfactant protein D and bronchopulmonary dysplasia: a new way to approach an old problem. Respir Res. 2021;22:141. doi: 10.1186/s12931-021-01738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preuß S., Scheiermann J., Stadelmann S., Omam F.D., Winoto-Morbach S., Lex D., et al. 18:1/18:1-Dioleoyl-phosphatidylglycerol prevents alveolar epithelial apoptosis and profibrotic stimulus in a neonatal piglet model of acute respiratory distress syndrome. Pulm Pharmacol Therapeut. 2014;28:25–34. doi: 10.1016/j.pupt.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Arroyo R., Grant S.N., Gouwens K.R., Miller D.M., Kingma P.S. Evaluation of recombinant human SP-D in the rat premature lung model. Ann Anat. 2021;235:151670. doi: 10.1016/j.aanat.2020.151670. [DOI] [PubMed] [Google Scholar]
- 68.De Luca D., Minucci A., Piastra M., Cogo P.E., Vendittelli F., Marzano L., et al. Ex vivo effect of varespladib on secretory phospholipase A2 alveolar activity in infants with ARDS. PloS One. 2012;7 doi: 10.1371/journal.pone.0047066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Luca D., Minucci A., Trias J., Tripodi D., Conti G., Zuppi C., et al. Varespladib inhibits secretory phospholipase A2 in bronchoalveolar lavage of different types of neonatal lung injury. J Clin Pharmacol. 2012;52:729–737. doi: 10.1177/0091270011405498. [DOI] [PubMed] [Google Scholar]
- 70.De Luca D., Vendittelli F., Trias J., Fraser H., Minucci A., Gentile L., et al. Surfactant and varespladib Co-administration in stimulated rat alveolar macrophages culture. Curr Pharmaceut Biotechnol. 2013;14:445–448. doi: 10.2174/1389201011314040010. [DOI] [PubMed] [Google Scholar]
- 71.Kokotou M.G., Limnios D., Nikolaou A., Psarra A., Kokotos G. Inhibitors of phospholipase A 2 and their therapeutic potential: an update on patents (2012-2016) Expert Opin Ther Pat. 2017;27:217–225. doi: 10.1080/13543776.2017.1246540. [DOI] [PubMed] [Google Scholar]
- 72.Triggiani M., Granata F., Petraroli A., Loffredo S., Frattini A., Staiano R.I., et al. Inhibition of secretory phospholipase A2-induced cytokine production in human lung macrophages by budesonide. Int Arch All Immunol. 2009;150:144–155. doi: 10.1159/000218117. [DOI] [PubMed] [Google Scholar]
- 73.Yeh T.F., Chen C.M., Wu S.Y., Husan Z., Li T.C., Hsieh W.S., et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193:86–95. doi: 10.1164/rccm.201505-0861OC. [DOI] [PubMed] [Google Scholar]
- 74.Yeh T.F., Lin H.C., Chang C.H., Wu T.S., Su B.H., Li T.C., et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121:e1310–e1318. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]
- 75.Kothe T.B., Kemp M.W., Schmidt A., Royse E., Salomone F., Clarke M.W., et al. Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2019;316:L888–L893. doi: 10.1152/ajplung.00477.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kothe T.B., Royse E., Kemp M.W., Schmidt A., Salomone F., Saito M., et al. Effects of budesonide and surfactant in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2018;315:L193–L201. doi: 10.1152/ajplung.00528.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 78.Nissen C.B., Sciascia S., de Andrade D., Atsumi T., Bruce I.N., Cron R.Q., et al. The role of antirheumatics in patients with COVID-19. Lancet Rheumatol. 2021;3:e447–e459. doi: 10.1016/S2665-9913(21)00062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calfee C.S., Matthay M.A. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Luca D., Piastra M., Tosi F., Pulitano S., Mancino A., Genovese O., et al. Pharmacological therapies for pediatric and neonatal ALI/ARDS: an evidence-based review. Curr Drug Targets. 2012;13:906–916. doi: 10.2174/138945012800675687. [DOI] [PubMed] [Google Scholar]
- 81.Park W.Y., Goodman R.B., Steinberg K.P., Ruzinski J.T., Radella F 2nd, Park D.R., et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 82.Dani C., Corsini I., Burchielli S., Cangiamila V., Romagnoli R., Jayonta B., et al. Natural surfactant combined with beclomethasone decreases lung inflammation in the preterm lamb. Respiration. 2011;82:369–376. doi: 10.1159/000328928. [DOI] [PubMed] [Google Scholar]
- 83.Hite R.D., Morris P.E. Acute respiratory distress syndrome: pharmacological treatment options in development. Drugs. 2001;61:897–907. doi: 10.2165/00003495-200161070-00001. [DOI] [PubMed] [Google Scholar]
- 84.Boet A., Brat R., Aguilera S.S., Tissieres P., De Luca D. Surfactant from neonatal to pediatric ICU: bench and bedside evidence. Minerva Anestesiol. 2014;80:1345–1356. [PubMed] [Google Scholar]
- 85.Poggi C., Dani C. Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxid Med Cell Longev. 2014;2014:721043. doi: 10.1155/2014/721043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dani C., Corsini I., Longini M., Burchielli S., Dichiara G., Cantile C., et al. Natural surfactant combined with superoxide dismutase and catalase decreases oxidative lung injury in the preterm lamb: surfactant Plus SOD and CAT for RDS. Pediatr Pulmonol. 2014;49:898–904. doi: 10.1002/ppul.22955. [DOI] [PubMed] [Google Scholar]
- 87.Dani C., Corsini I., Burchielli S., Cangiamila V., Longini M., Paternostro F., et al. Natural surfactant combined with beclomethasone decreases oxidative lung injury in the preterm lamb: oxidative Lung Injury in the Preterm Lamb. Pediatr Pulmonol. 2009;44:1159–1167. doi: 10.1002/ppul.21145. [DOI] [PubMed] [Google Scholar]
- 88.Dani C., Buonocore G., Longini M., Felici C., Rodriguez A., Corsini I., et al. Superoxide dismutase and catalase activity in naturally derived commercial surfactants. Pediatr Pulmonol. 2009;44:1125–1131. doi: 10.1002/ppul.21116. [DOI] [PubMed] [Google Scholar]
- 89.De Luca D., Cogo P., Zecca E., Piastra M., Pietrini D., Tridente A., et al. Intrapulmonary drug administration in neonatal and paediatric critical care: a comprehensive review. Eur Respir J. 2011;37:678–689. doi: 10.1183/09031936.00024910. [DOI] [PubMed] [Google Scholar]
- 90.Choi H.J., Hahn S., Lee J., Park B.J., Lee S.M., Kim H.S., et al. Surfactant lavage therapy for meconium aspiration syndrome: a systematic review and meta-analysis. Neonatology. 2012;101:183–191. doi: 10.1159/000329822. [DOI] [PubMed] [Google Scholar]
- 91.Wiswell T.E., Knight G.R., Finer N.N., Donn S.M., Desai H., Walsh W.F., et al. A multicenter, randomized, controlled trial comparing surfaxin (lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics. 2002;109:1081–1087. doi: 10.1542/peds.109.6.1081. [DOI] [PubMed] [Google Scholar]
- 92.Gommers D., Eijking E.P., So K.L., van’t Veen A., Lachmann B. Bronchoalveolar lavage with a diluted surfactant suspension prior to surfactant instillation improves the effectiveness of surfactant therapy in experimental acute respiratory distress syndrome (ARDS) Intensive Care Med. 1998;24:494–500. doi: 10.1007/s001340050602. [DOI] [PubMed] [Google Scholar]
- 93.Rüegger C.M., Bassler D. Alternatives to systemic postnatal corticosteroids: inhaled, nebulized and intratracheal. Semin Fetal Neonatal Med. 2019;24:207–212. doi: 10.1016/j.siny.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Deliloglu B., Tuzun F., Cengiz M.M., Ozkan H., Duman N. Endotracheal surfactant combined with budesonide for neonatal ARDS. Front Pediatr. 2020;8:210. doi: 10.3389/fped.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meduri G.U., Annane D., Confalonieri M., Chrousos G.P., Rochwerg B., Busby A., et al. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. 2020;46:2284–2296. doi: 10.1007/s00134-020-06289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Autilio C., Echaide M., De Luca D., Pérez-Gil J. Controlled hypothermia may improve surfactant function in asphyxiated neonates with or without meconium aspiration syndrome. PloS One. 2018;13:e0192295. doi: 10.1371/journal.pone.0192295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Luca D., Vázquez-Sánchez S., Minucci A., Echaide M., Piastra M., Conti G., et al. Effect of whole body hypothermia on inflammation and surfactant function in asphyxiated neonates. Eur Respir J. 2014;44:1708–1710. doi: 10.1183/09031936.00117714. [DOI] [PubMed] [Google Scholar]
- 98.Autilio C., Shankar-Aguilera S., Minucci A., Touqui L., De Luca D. Effect of cooling on lung secretory phospholipase A2 activity in vitro, ex vivo, and in vivo. Am J Physiol Lung Cell Mol Physiol. 2019;316:L498–L505. doi: 10.1152/ajplung.00201.2018. [DOI] [PubMed] [Google Scholar]
- 99.Autilio C., Echaide M., Cruz A., García-Mouton C., Hidalgo A., Da Silva E., et al. Molecular and biophysical mechanisms behind the enhancement of lung surfactant function during controlled therapeutic hypothermia. Sci Rep. 2021;11:728. doi: 10.1038/s41598-020-79025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Autilio C., Echaide M., Dell'Orto V., Perez-Gil J., De Luca D. Effect of whole body hypothermia on surfactant function when amniotic fluid is meconium stained. Ther Hypothermia Temp Manag. 2020;10:186–189. doi: 10.1089/ther.2017.0012. [DOI] [PubMed] [Google Scholar]
- 101.De Luca D., Tingay D.G., van Kaam A., Brunow de Carvalho W., Valverde E., Christoph Roehr C., et al. Hypothermia and meconium aspiration syndrome: international multicenter retrospective cohort study. Am J Respir Crit Care Med. 2016;194:381–384. doi: 10.1164/rccm.201602-0422LE. [DOI] [PubMed] [Google Scholar]
- 102.Duan M., Berra L., Kumar A., Wilcox S., Safford S., Goulet R., et al. Use of hypothermia to allow low-tidal-volume ventilation in a patient with ARDS. Respir Care. 2011;56:1956–1958. doi: 10.4187/respcare.01211. [DOI] [PubMed] [Google Scholar]
- 103.Cruces P., Cores C., Casanova D., Pizarro F., Díaz F. Successful use of mild therapeutic hypothermia as compassionate treatment for severe refractory hypoxemia in COVID-19. J Crit Care. 2021;63:260–263. doi: 10.1016/j.jcrc.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cogo P.E., Facco M., Simonato M., Verlato G., Rondina C., Baritussio A., et al. Dosing of porcine surfactant: effect on kinetics and gas exchange in respiratory distress syndrome. Pediatrics. 2009;124:e950–e957. doi: 10.1542/peds.2009-0126. [DOI] [PubMed] [Google Scholar]
- 105.Foligno S., De Luca D. Carelessness about surfactant dose—a cultural problem, a legal issue, or an open research question? JAMA Pediatr. 2019;173:211–212. doi: 10.1001/jamapediatrics.2018.4296. [DOI] [PubMed] [Google Scholar]