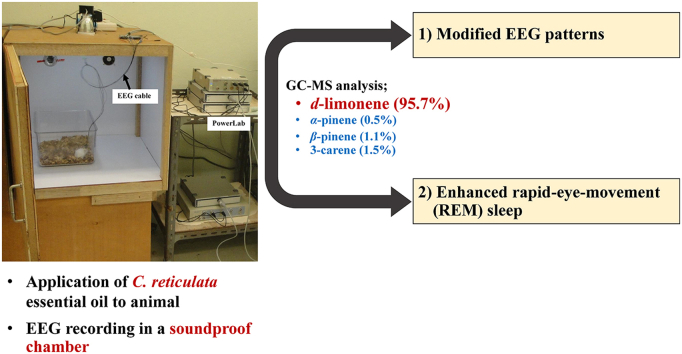
Abstract
Background
Citrus essential oil (EO) has been used for mood elevation and sedative hypnotic purposes. However, scientific proofs of its central nervous system (CNS) action remained largely unexplored. This study investigated chemotypes, electrical brain waves and sleep-wake effects of the essential oil from Citrus reticulata in rat model.
Methods
Chemical contents of citrus EO were analyzed using gas chromatography-mass spectrometry (GC–MS). Male Wistar rats implanted with electrodes on the frontal and parietal skulls were used for electroencephalographic (EEG) recording while inhaling the citrus EO (200 μl on cotton wool). Diazepam (10 mg/kg, p.o.) was used as a standard anxiolytic drug. EEG frequency analyses were performed by using Fast Fourier transform. All data were statistical analyzed using One-way ANOVA followed by Tukey's test.
Results
GC–MS analysis revealed d-limonene (95.7%) as a major constituent of citrus EO. The EEG results showed that overall EEG patterns of citrus EO effects were relatively similar to that of diazepam. However, significant differences between treatments were seen from sleep-wake analyses. Diazepam significantly increased episode numbers of awake and non-rapid eye movement (REM) sleep and reduced averaged episode duration. On the other hand, the citrus EO significantly decreased REM sleep latency and increased total time and episode numbers of REM sleep.
Conclusion
These findings demonstrated unique CNS effects of C. reticulata EO with EEG fingerprints and sleep-wake profiles. The data might be useful for citrus essential oil sub-classification and clinical application.
Keywords: Citrus reticulata, Sai-Nam-Phueng, Essential oil, GC/MS, EEG, REM sleep
Graphical abstract
At a glance of commentary
Scientific background on the subject
Previously, essential oils (EOs) have been used for emotional and sleep/wake modifications. Mostly, they are known to enhance alertness or sleepiness. However, specific effects of each essential oil including citrus EO remained to be explored.
What this study adds to the field
Citrus EO reduced time to enter sleep stage and increased REM sleep. EEG patterns induced by citrus EO were distinguished from that of diazepam, a standard anxiolytic drug. These data are useful as EEG fingerprint for sub-classification of citrus EO.
Essential oils (EOs) from various plant species have long been used in traditional medicine for emotional improvements and some other purposes. Moreover, EOs are added in many health products for inducing relaxation. In aromatherapy, several EOs are helpful in reducing anxiety symptoms [1]. They are important components of aromatherapy that have been widely used for multiple health purposes such as stress reduction [2], night time sleep modification [3], modulation of mood [4] or management of work-related stress perception [5]. In general, beneficial effects of EOs have been claimed and studied mostly by using questionnaires or subjective methods. In order to develop drug discovery of EOs, more scientific proofs of their biological effects are needed.
Various citrus EOs have been used in alternative therapies for mental problems. The effects of bergamot (Citrus bergamia) EO inhalation on mental health and well-being were reported [6]. Neroli (Citrus aurantium L.) EO inhalation is also an easy and applicable method to reduce anxiety in patients with acute coronary syndrome [7]. Moreover, Citrus aurantium blossom oil is an effective intervention to reduce anxiety during labor and first stage labor pain [8]. These findings strongly confirm that citrus EOs possess CNS effects and reduce the severity of stress in particular. Apart from the CNS effects, EO from Citrus limetta Risso (Rutaceae) also exhibited its in vitro and in vivo profiles the treatment of inflammation linked skin diseases [9]. In addition, antioxidant activity of this EO was confirmed by cell-free and cell-based assays [10]. These findings suggest multiple bioactive properties of citrus EOs.
In Thailand, Citrus reticulata Blanco. plants have been mostly cultivated and popularly used in orange juice industries. Previously, EO from Citrus spp. has gained some interests for its central nervous system (CNS) action in the treatment of anxiety and sleep problems [11]. In behavioral studies, oral administration of C. reticulata EO was found to reduce anxiety in mice using light–dark box and marble-burying tests and prolong sleeping time induced by ether inhalation [12]. However, it was important to compare anxiolytic effects of citrus EO on electrical brain waves and sleep-wake patterns to that of standard anxiolytic drug in experimental animals.
Electroencephalography (EEG) is a technique used to detect local field potential as a result of electrical potential synchronization and desynchronization of neuronal population in the brain. EEG patterns in response to drug administration known as electropharmacograms have been used to characterize drug action in specific brain areas [12,13]. In particular, EEG technique has frequently been used for determining quantitative sleep-wakefulness [14] and EEG power activity [15]. Therefore, this technique is practical to examine the effects of EOs on brain function.
However, effects of EO from C. reticulata have been rarely studied in terms of electrical brain activity or local field potential. Investigation of its CNS effects on electrical brain wave would provide EEG fingerprints or biomarkers. These data are necessary for classification and possible application of EOs as alternative choices for some mental illnesses.
The aim of this study was to investigate the effects of C. reticulata EO on EEG power activity and sleep-wake cycle in rats. Male rats with electrodes implanted on the skull over the frontal and parietal cortices were given the citrus EO inhalation while recording EEG signals. Quantitative data of sleep-wakefulness were analyzed following inhalation the citrus EO.
Materials and methods
Essential oil extraction
The fruit of C. reticulata Blanco. cv. Sai-Nam-Phueng was obtained from Khlong Lan district, Kamphaeng Phet province, Thailand. The peels were washed and dried with hot air oven (40–50 °C) and milled to minimize the approximate particle size 0.5 cm. Three kilograms of prepared peels were loaded into a high pressure stainless steel extractor tank. The extract-laden carbon dioxide was sent to the two separator tanks. These prepared peels were extracted with supercritical carbon dioxide. The best extraction condition gave the oleoresin deposit in the first separator and the second separator contained two immiscible phase of EOs and water. The EOs were separated and filtered. Anhydrous sodium sulphate was added for elimination of trace water. Temperature and pressure were controlled for the extractor (40 °C and 13 MPa), the first separator (40 °C and 9 MPa) and of the second separator (20 °C and 6 MPa). Time for equilibrium condition and extraction was 1.0 and 2.0 h, respectively. The EO was used as the pilot scale SCO2 (Guangzhou Masson New Separation Technology, China) which belongs to Thai Traditional and Herbal Development Center (TTHD), located at module 1, Biotechnology Pilot Plant, Thailand Science Park, Klong 1, Klongluang, Pathumtani, Thailand.
Gas chromatography-mass spectrometry (GC–MS) method
Chemical identification and quantification of EOs was investigated by Gas Chromatography-Mass spectrometry (Thermo electron Corporation, Fortune Scientific Thailand) at Thai Traditional and Herbal Development Center (TTHD), located at module 1, Biotechnology Pilot Plant, Thailand Science Park, Klong 1, Klongluang, Phathumtani, Thailand. The column was ZB-5ms. Helium (TIG) was used as the carrier gas at a flow rate of 1 ml/min. The programmed column temperature was ramped from 60 °C to 250 °C at a rate of 3 °C/min, and hold at 60 °C for an additional 5 min. The temperature at injection port was 200 °C, and at detector port was 270 °C. Detection was based on electron ionization (EI) mass spectrometry. Peak identification was carried out by comparisons with those available in Wiley 7n.L Database.
Animals
Adult male Wistar rats weighing 300–350 g were purchased from the Southern Animal Facility, Faculty of Science, Prince of Songkla University. The environmental condition was controlled with room temperature (24 ± 2 °C), humidity (55 ± 10%), and a 12/12 h light/dark cycle (light on 6:00). The rats were housed individually in plexiglas home cage and given food pellets and water ad libitum. The experimental protocols for the care and use of the rats were guided and approved by the Animals Ethical Committee of Prince of Songkla University. The project license number is MOE 0521.11/064.
Surgery
Skull surgery process was performed as previously described [16,17]. Briefly, the rats were anesthetized with intramuscular injection of 45 mg/kg Zoletil® 100 (Virbac, Thailand). To study cortical EEG in animals, stainless steel screw electrodes were permanently implanted on the skull to pick up electrical signal from epidural layer over the frontal and parietal cortices. This is a standard method used for detection of drug effects on electrical brain activity. In details, stainless steel screw electrodes were stereotaxically implanted over the frontal skull (AP; +2 mm, ML; 3 mm) and the parietal cortex (AP; −3.7 mm, ML; 3 mm) into the left side of the skull by using bregma as the landmark. The electrode fixed at the midline over the cerebellum (AP; −11.8 mm, ML; 0 mm) was used as a reference and ground electrode. Basically, the cerebellum mainly contributes its function on motor control. Moreover, ground electrode is needed to reduce noise interference and stabilize signal recording. Mostly, the cerebellum is used for these purposes. Bipolar electromyogram (EMG) stainless steel wire electrodes (15 μm in diameter; A-M Systems, Inc., Washington, United States) were inserted into bilateral dorsal neck muscles. All electrodes were secured in place with acrylic resin (Unifast Trad, Tokyo, Japan). Following the surgery, the rats were used for the experiment after a 10-day recovery period.
Drug administration
The rats were divided into 3 groups to receive either distilled water (n = 9), 10 mg/kg diazepam (n = 7) or 200 μL C. reticulata EO (n = 7). The sample size of animals per group was calculated by using the Resource Equation Approach [18] and following other related studies in similar field of research. N number was calculated from the equation n = DF/k + 1, where DF is degree of freedom (acceptable range for ANOVA is between 10 and 20). DF was normally estimated from the equation DF = N - k = kn - k = k (n - 1), where N = total number of subjects, k = number of groups, and n = number of subjects per group. This study had 3 groups and estimated DF at 15. Therefore, n was equal to 7.5. This was also justified in accordance with 3R's to determine minimum number of animals but maintain appropriate number to promote scientific progress.
The anxiolytic Zopam or diazepam (Global Pharm Co, Ltd., Thailand) was dissolved in distilled water. Diazepam 10 mg/kg body weight as a positive control was orally administered using stainless steel feeding gavage. Both C. reticulata EO (200 μL, i.h.) and distilled water (found to produce equal response of EEG pattern to that of sunflower oil, a non-aromatic general plant oil, in a preliminary study) were inhaled. Either the citrus EO or distilled water was pipetted on a cotton wool and put on saw dust in their home cage inside a soundproof chamber. The baseline EEG was recorded for 30 min with ambient air inhalation and followed with 1-h recording for the effects of either distilled water, diazepam or the citrus EO administration. All experiments were tested between 12:00 and 17:00 h.
EEG recording and data processing
Before the start of experiment, rats were acclimatized with recording condition being connected with EEG recording cables inside the soundproof chamber (60 cm × 60 cm x 60 cm, length x width x height) between 12:00 and 16:00 for 3 consecutive days. The EEG and EMG signals were amplified and digitized at 400 Hz (sampling rate) and filtered (EEG, a low-pass 100 Hz, high-pass 0.1 Hz; EMG, a low-pass 100 Hz, high-pass 10 Hz) by using a Bio Amp system and PowerLab 4/20 system (AD Instrument Pty Ltd, Sydney, Australia) with 12-bit A/D converter and stored in PC through the program LabChart v7.3.2 (AD Instrument Pty Ltd, Sydney, Australia). For the data processing, The EEG signals were processed through 0.8–100 Hz band-pass filter. The digitized EEG data were segmented into 512-point (50% overlap). The signals were converted to power spectra by the fast Fourier Transform algorithm (Hanning window cosine transform) by using the program LabChart v7.3.2. Then the power spectra of 1.28-sec sweeps of selected period were averaged to give the power spectra of the period. The power spectra were divided into multiple discrete frequency bands according to previous studies: Delta (0.8–3.9 Hz), Theta (4.7–7.0 Hz), Alpha (7.8–12.5 Hz), Beta1 (13.3–18.0 Hz), Beta2 (18.8–35.2 Hz), Gamma (35.9–100 Hz) [15,[19], [20], [21]]. These spectra in each frequency band of each group were averaged and relatively calculated as percent baseline.
In general, it is high deviation of EEG powers among individual animals. Different animals produced different baseline levels of EEG power. It's necessary to normalize data in relation to baseline level. The data of EEG power recorded during a 30-min period of pretreatment were used as baseline levels for values of each animals. The data of post-treatment of each rat were normalized in relation with its baseline level and expressed in % baseline values.
Continuous signals from a period of 1 h following the start of treatment were collected for analysis of EEG power spectrums. This was in a purpose for screening if there are particular frequencies sensitive to the treatment. However, this did not show temporal information of response that emerges in time scale to the treatment. Therefore, the present study was also designed to analyze and show the oscillation of some frequencies of interest in time domain. The data were analyzed and expressed every 1 min.
Sleep-wake state analysis
Three brain states of sleep-wake cycles were classified. There are wakefulness, non-rapid eye movement (non-REM) sleep or rapid eye movement (REM) sleep according to the criteria previously described by Cheaha, D. et al., 2016 [16], as shown in Fig. 5, Fig. 6. Firstly, wakefulness was characterized by low-amplitude EEG theta wave (6–9 Hz) from the frontal and parietal cortices, high-voltage EMG activities and high density of theta power in the parietal cortex seen in a spectrogram. Secondly, non-REM sleep was identified by high-voltage of slow EEG frequencies in both cortices, low EMG activities, and increased broad frequency activity in both cortices with particular highlight in delta range of spectrograms. Finally, REM sleep was seen with low frontal EEG amplitude but high parietal EEG amplitude, low EMG activities and high theta power in parietal a spectrogram. Sleep parameters were analyzed in terms of sleep latency of non-REM and REM sleeps, total time spent in each brain state, episode numbers of each brain state and averaged episode duration of each brain state.
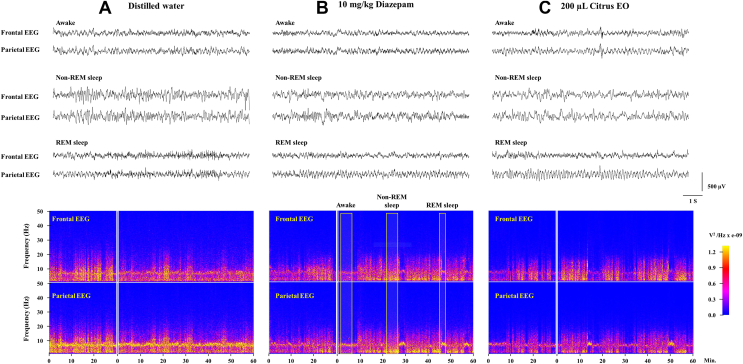
Fig. 5.
Raw EEG signals and spectrograms in the frontal and parietal cortices following oral administration of diazepam and inhalation of the citrus EO in comparison to that of control treatment (distilled water). Representative EEG tracings of each group were selected during awake (A), non-REM (B) and REM (C) sleep periods. Data were also converted into spectrograms to present electrical activity in frequency and time domains.
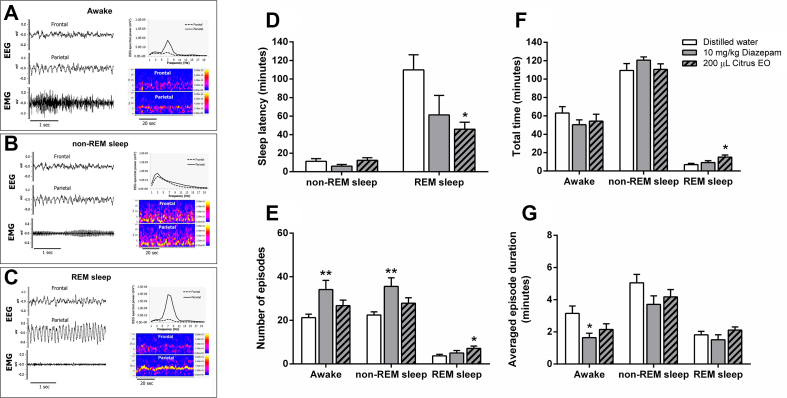
Fig. 6.
Identification of awake (A), non-REM (B) and REM (C) periods. Multiple brain states were identified by using EEG spectral powers and spectrograms. Sleep-wake effects of administration with diazepam and citrus EO were determined in terms of sleep latency (D), number of episode (E), total time spent (F) and averaged episode duration (G) (n = 7–9). ∗, ∗∗p < 0.05, 0.01, 0.001, respectively, compared with control levels (one-way ANOVA followed by Tukey's post hoc test).
Statistical analysis
EEG power (% baseline) was calculated from signals recorded during pre- and post-treatment periods. Baseline spectra values were calculated from pre-treatment period and set at 100%, while post-treatment spectra values were referenced with baseline to obtain percent baseline values. Percent baseline values were expressed as mean ± standard error of the mean (S.E.M.) of each group. Differences were determined using one-way analysis of variance (ANOVA), followed by multiple comparisons using Turkey's Method. Differences with p < 0.05 compared with control level were considered statistically significant.
Results
Phytochemical profile
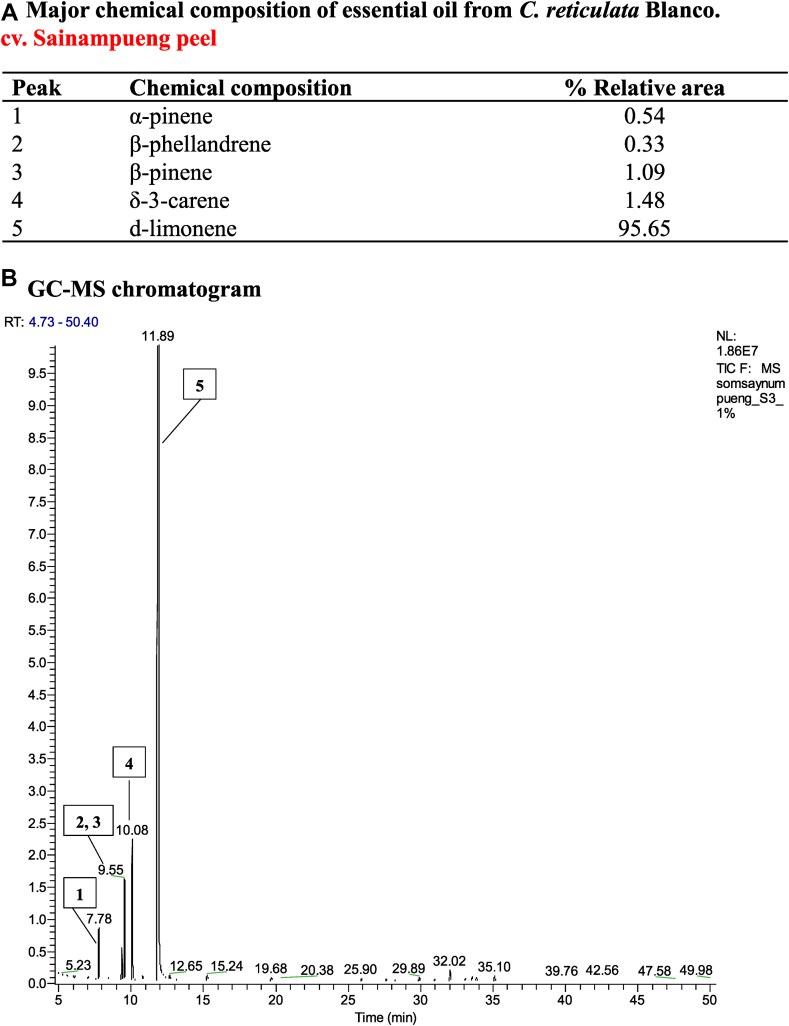
Chemical constituents were separated and percent yields of each component were shown [Fig. 1A]. The chemotypes of the EO from peels of Citrus reticulata Blanco. cv. Sai-Nam-Pueng were identified by GC–MS [Fig. 1B]. d-limonene (95.7%) was found to be a major constituent of this EO. α-pinene (0.5%), β-pinene (1.1%) and δ-3-carene (1.5%) were found as minor constituents.
Fig. 1.
Chemical composition of essential oil from C. reticulata Blanco. cv. Sai-Nam-Phueng peel (A). GC–MS chromatogram of C. reticulata cv. Sai-Nam-Pueung peel oil extracted by supercritical carbon dioxide method. (B) Essential oil from the peel of C. reticulata Blanco. cv. Sai-Nam-Pueung was analyzed by GC–MS. The major compositions were monoterpenes. d-Limonene was the main component of the essential oil.
EEG power spectrums induced by the citrus essential oil inhalation
Representative raw LFP signals recorded from the frontal and parietal cortices were shown [Fig. 5]. Frontal and parietal EEG signals following 3 different treatments were collected. Raw EEG data were processed through fast Fourier Transform for frequency analysis. Data were analyzed and expressed in spectrums of frequency power (% baseline) in the frequency range from 0.8 to 100 Hz.
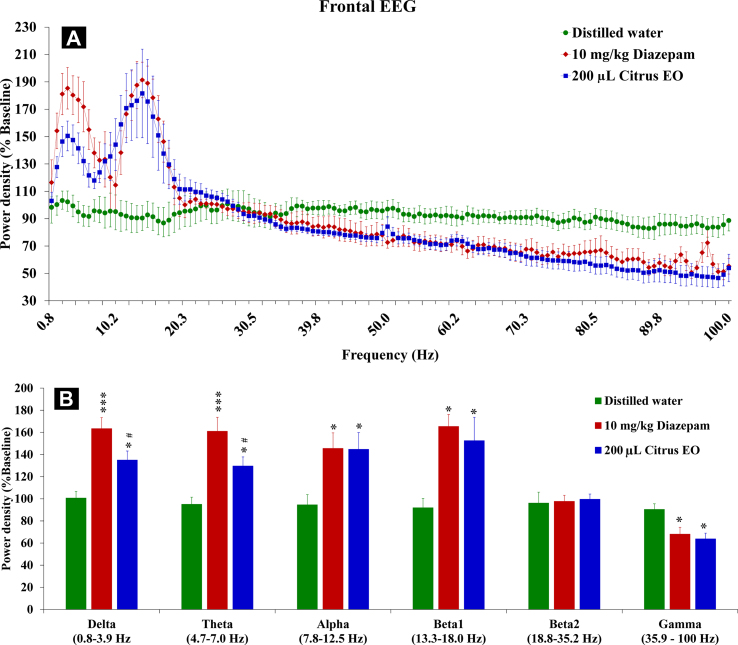
In the frontal cortex, C. reticulata EO appeared to increase powers of slow frequencies in comparison to control levels [Fig. 2A]. The citrus EO was found to produce similar effects to that of diazepam particularly for 10–20 Hz frequency range. Therefore, data were divided into powers of 6 frequency bands for statistical analysis [Fig. 2B]. One-way ANOVA revealed significant differences found in delta [F (2, 18) = 15.18, p < 0.001], theta [F (2, 18) = 13.98, p < 0.001], alpha [F (2, 18) = 5.10, p < 0.019], beta1 [F (2, 18) = 12.73, p < 0.01] and gamma [F (2, 18) = 5.67, p < 0.05] bands. Multiple comparison analyses also indicated significant increases in powers of these frequency bands. Altogether, the citrus EO produced similar effects to that of diazepam.
Fig. 2.
Effects of diazepam and citrus EO on frontal EEG powers of 6 discrete frequency ranges (n = 7–9). (A) Averaged percent baseline powers are expressed as means ± S.E.M. of spectral powers. (B) Powers of discrete frequency bands were divided for separately statistical analysis. ∗, ∗∗∗p < 0.05, 0.001, respectively, compared with control levels and #p < 0.05 compared with diazepam levels (one-way ANOVA followed by Tukey's post hoc test).
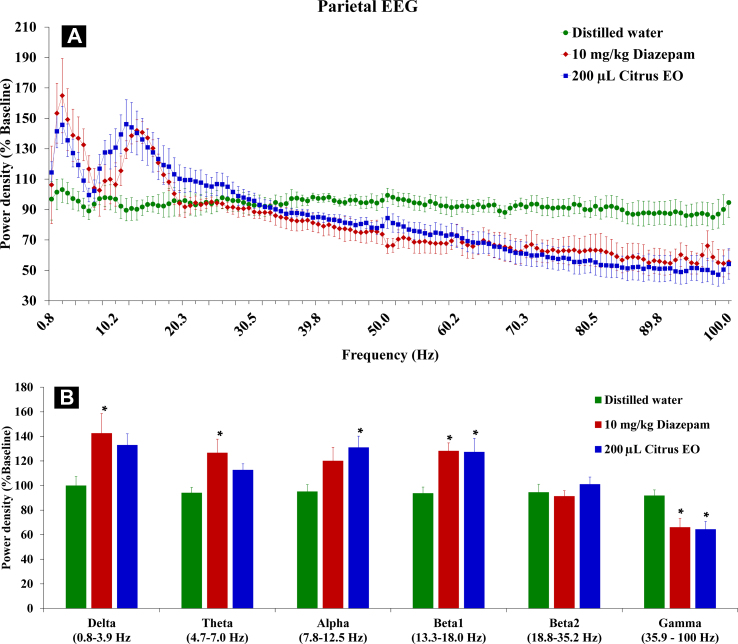
In the parietal cortex, effects of C. reticulata EO were also seen. The citrus EO produced similar patterns of power spectrum to that produced by diazepam [Fig. 3A]. When analyzed in each frequency band, significant changes were found in delta [F (2, 18) = 4.80, p < 0.05], theta [F (2, 18) = 6.25, p < 0.05], alpha [F (2, 18) = 10.16, p < 0.01], beta1 [F (2, 18) = 11.32, p < 0.01] and gamma [F (2, 18) = 4.87, p < 0.05] [Fig. 3B]. However, multiple comparisons indicated that the citrus EO significantly increased EEG powers only in alpha and beta1 and decreased EEG powers of gamma frequency ranges.
Fig. 3.
Effects of diazepam and citrus EO on parietal EEG powers of 6 discrete frequency ranges (n = 7–9). (A) Averaged percent baseline powers are expressed as means ± S.E.M. of spectral powers. (B) Powers of discrete frequency bands were divided for separately statistical analysis. ∗p < 0.05, compared with control levels (one-way ANOVA followed by Tukey's post hoc test).
Time-course effects of C. reticulata EO inhalation
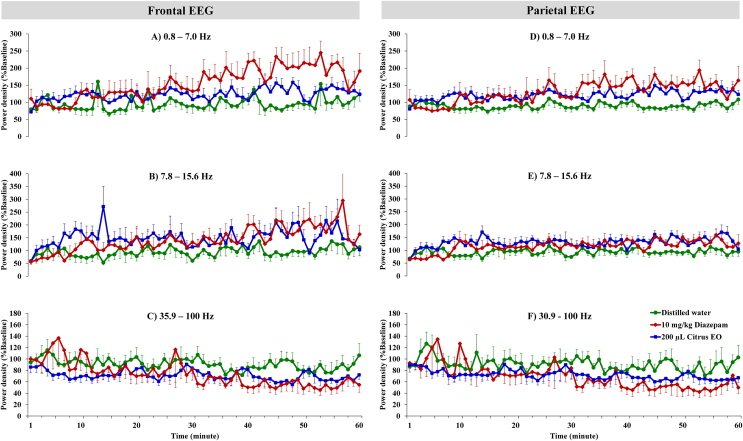
EEG powers of 3 frequency ranges (0.8–7.0, 7.8–15.6 and 35.9–100 Hz) following the inhalation were shown in time domain [Fig. 4]. In the frontal cortex, diazepam administration and the inhalation of C. reticulata EO appeared to increase power of 0.8–7.0, 7.8–15.6 Hz particularly at the end of 60-min period. Both treatments also decreased EEG powers of 35.9–100 Hz frequency range. Similar patterns of change in EEG power were seen in the parietal cortex. Diazepam administration and the inhalation of C. reticulata EO also increased powers of 0.8–7.0, 7.8–15.6 Hz and decreased EEG powers of 35.9–100 Hz frequency ranges.
Fig. 4.
Oscillations in the time domain of EEG powers in the frontal and parietal cortices. Percent baseline powers of 3 frequency ranges (0.8–7.0, 7.8–15.6 and 35.9–100 Hz) were analyzed every 1 min following administration with diazepam and citrus EO (n = 7–9).
Effects of C. reticulata EO inhalation on sleep-wake cycle
EEG signals from the frontal and parietal cortices and patterns of EMG signals were analyzed for classification of brain states of animals. Fast Fourier transform (FFT) and EEG spectrograms were used to reveal the overall EEG powers of broad frequency range. Awake periods were identified with fast frequency oscillation and high EMG activity [Fig. 6A]. Theta frequency from the parietal cortex appeared to dominate during wakefulness. Non-REM sleep was identified with slow wave activity from both cortices [Fig. 6B]. High power oscillations were seen especially for the slow frequencies. The most unique pattern of brain waves was observed during REM sleep [Fig. 6C]. During REM sleep period, extremely high theta activity was detected in the parietal but not the frontal cortex. Therefore, EEG signals recorded during a 1-hr period were analyzed for the identification of wakefulness, non-REM and REM sleep.
Sleep-wake patterns were evaluated in terms of sleep latency [Fig. 6D], episode number [Fig. 6E], total time spent [Fig. 6F] and averaged episode duration [Fig. 6G] of 3 brain states. Each parameter was separately analyzed for statistical determination. One-way ANOVA revealed significant difference in sleep latency [F (2, 20) = 4.54, p < 0.05]. Multiple comparisons also indicated a significant difference between REM sleep latencies of control and the citrus EO group. No significant difference was seen for non-REM sleep parameter. When analyzed in term of episode number of each parameter, significant changes were found for awake, non-REM and REM parameters [F (2, 20) = 5.48, p < 0.05, F (2, 20) = 6.37, p < 0.01 and F (2, 20) = 3.59, p < 0.05, respectively]. Multiple comparisons confirmed that diazepam significantly increased episode numbers of awake and non-REM sleep and the C. reticulata EO significantly increased episode numbers of REM sleep. Next, significant change was seen for total time of only REM sleep parameters [F (2, 20) = 4.65, p < 0.01]. The citrus EO was found to significantly increase time spent in REM sleep. Finally, averaged episode durations of each parameter were evaluated. Significant change was found only for the state of awake [F (2, 20) = 3.96, p < 0.05]. Multiple comparisons revealed that diazepam significantly decreased awake episode duration. Altogether, patterns of brain activity following different treatments can be observed from raw EEG tracings or spectrograms in time domain [Fig. 5A–C]. Particularly, the EEG spectrograms exhibited dynamics of sleep-wake rhythm.
Discussion
The present study identified chemotypes of the citrus EO from peels of Citrus reticulata Blanco. cv. Sai-Nam-Pueng. Its chemical profile was consistent with previous studies of the citrus EOs. Great proportion of limonene in various EOs from fruits that belong to the genus Citrus was consistently reported [22]. Basically, they comprise more than 90 percent volatile fractions [23,24]. The monoterpene hydrocarbons limonene is among other volatile constituents that appear to constitute the majority of the whole citrus oil [25]. In terms of bioactivity, limonene, a major component, or its related molecules have been demonstrated to produce anxiolytic effects [26]. Monoterpenes linalool and β-pinene, 2 minor constituents, also exhibited an antidepressant-like effect through interaction with the monoaminergic system [27]. EOs from various aromatic plants appear to contain different proportions of constituents. These might explain why the diversity is high among the effects of various EOs.
This study demonstrated the sensitive effects of C. reticulata EO on brain waves in animal model. It was demonstrated that gamma frequency reflected cortical hyperexcitability of stressed animals [28]. Reduction of EEG powers in this brain wave is correlated with stress attenuation. On the other hand, increased activities of lower frequency ranges were associated with anxiolytic or sedative effects as found in responses to diazepam and zolpidem [29]. Changes in these frequency ranges suggest partial common CNS actions between the effects of citrus EO and those standard anxiolytics. Previously, Citrus sp. EO inhalation was demonstrated to increase a wide frequency range which included theta, alpha1, alpha2, and beta1 activities in the frontal cortex and alpha2 and beta1 in the parietal cortex [30]. The present study also showed increases in powers of slow frequency ranges and decreases in powers of fast frequency ranges in the frontal cortex. Similar changes in EEG power activity were also found in the parietal cortex. These data appeared to be consistent with those previous findings. In addition, C. bergamia EO administration activated EEG power spectra in theta (4.25–8 Hz) band . Inhalation of (+)-limonene, a major component of citrus oil, was found to activate high beta (20–30 Hz) activities in the right temporal region of human [31]. These studies appeared to suggest the links between pharmacological modifications of neurotransmitter system and changes of discrete brain waves. Change in theta (4.75–6.75 Hz) activity was induced by a drug acting at norepinephrine α2 receptors [32]. An increase in alpha2 (9.75–12.5 Hz) oscillation in the frontal cortex was correlated with inactivation of the dopaminergic and opioid receptors [33]. Moreover, beta2 (18.75–35 Hz) activities were influenced by drugs acting at GABAA receptors as found in cases of diazepam treatment [34,35]. These reports suggest that C. reticulata EO mediates CNS effects via multiple neurotransmitter systems. An increase in alpha2 (9.75–12.5 Hz) oscillation in the frontal cortex was correlated with inactivation of the dopaminergic and opioid receptors [33]. Moreover, beta2 (18.75–35 Hz) activities were influenced by drugs acting at GABAA receptors as found in cases of diazepam treatment [34,35]. These reports suggest that C. reticulata EO mediates CNS effects via multiple neurotransmitter systems. Inhalation of (+)-limonene, a major component of citrus oil, was found to activate high beta (20–30 Hz) activities in the right temporal region of human [31]. These studies appeared to suggest the links between pharmacological modifications of neurotransmitter system and changes of discrete brain waves. Previously, an increase in the EEG theta-rhythm (4.3–7.2 Hz) in the cortex of freely moving rats was produced by a drug acting at norepinephrine α2 receptors [32]. An increase in alpha2 (9.75–12.5 Hz) oscillation in the frontal cortex was correlated with inactivation of the dopaminergic and opioid receptors [33]. Moreover, beta2 (18.75–35 Hz) activities were influenced by drugs acting at GABAA receptors as found in cases of diazepam treatment [34,35]. These reports suggest that C. reticulata EO mediates CNS effects via multiple neurotransmitter systems. An increase in alpha2 (9.75–12.5 Hz) oscillation in the frontal cortex was correlated with inactivation of the dopaminergic and opioid receptors [33]. Moreover, beta2 (18.75–35 Hz) activities were influenced by drugs acting at GABAA receptors as found in cases of diazepam treatment [34,35]. These reports suggest that C. reticulata EO mediates CNS effects via multiple neurotransmitter systems.
The data from time-course analyses also reflected the delay period for the citrus EO to be absorbed through various pathways such as respiratory or olfactory systems. The molecules of EO were believed to enter the bloodstream and cross the blood–brain barrier to act on the CNS. The molecules can be absorbed through the olfactory system and delivered to the CNS by dissolving in cerebrospinal fluid [36]. Alternatively, there were nerve impulses that transmitted olfactory sensation from mitral cells in the olfactory bulb to the piriform cortex and further to many brain areas involved in various emotional functions [37]. By the way, C. reticulata EO inhalation produced significant changes in EEG pattern mostly from 30 to 60 min. This might suggest slow actions of C. reticulata EO on the olfactory pathways in the brain.
In terms of mechanism, d-limonene exerted an anxiolytic-like effect not via benzodiazepine (GABAA) receptors [38]. Previously, d-limonene exerted sedative effect via adenosine (A2A) receptors [39]. Linalool and β-pinene, minor components of citrus EO, produced antidepressant via serotonin (5-HT1A) receptors, noradrenergic (α2 and β) receptors and dopamine (D1) receptors [27]. Therefore, C. reticulata EO is likely to produce its CNS action through multiple neurotransmitter systems that modulate some changes in EEG pattern.
The results from sleep-wake cycle analyses showed that C. reticulata EO enhanced REM sleep. These C. reticulata EO effects were totally different from that of diazepam. With the EO inhalation, animals spent less than half the time spent by control animals to enter REM sleep. C. reticulata group spent more time in REM sleep and entered REM state more frequently than control animals. These effects may rely on d-limonene that was found to stimulate adenosine A2A receptors [40]. Adenosine A2A agonist was demonstrated to increase pontine acetylcholine release [41]. Previously, REM sleep induction was associated with cholinergic activation in the pontine reticular formation [41,42]. It is well known that REM sleep disturbance was correlated with pathogenesis of aging [43] or Alzheimer's disease [44].
Diazepam effects on sleep parameters were confirmed with previous reports from related studies. Basically, diazepam was found to modify mean duration of non-REM sleep [45] and reduced non-REM sleep latency [46]. The present study demonstrated that diazepam produced a tendency of decrease in non-REM latency (non-significant) and significant increases in episode numbers of awake and non-REM sleep.
Diazepam reduces anxiety and promotes sleep via benzodiazepine site on GABAA receptors [47]. Anxiety is among chronic disorders that need prolonged treatment. Some undesirable effects of benzodiazepines such as sedation, muscle relaxant, dependence or tolerance were evidenced [48,49]. Therefore, searching for new alternative medicines with other modes of CNS action might be more preferable. Previously, citrus oils have been demonstrated to exhibit similar anxiolytic or sedative effects to that of diazepam. However, some different mechanisms were found to mediate different CNS actions. Anxiolytic-like effects induced by (+)-limonene was not blocked by flumazenil, a benzodiazepine receptor antagonist [38]. On the other hand, lemon EO exposure led to lower corticosterone release [50]. Later, limonene appeared to antagonize the effects of corticotropin-releasing factor in social interaction test in mice [51]. Previously, increases in exploratory activity and power of fast frequency hippocampal EEG were produced by the inhalation of bergamot EO (Citrus bergamia Risso et Poiteau) in rats [52]. Apart from the effects on EEG pattern, bergamot EO inhalation was also demonstrated to exhibit anxiolytic-like/relaxant effects in animal behavioural tasks even though these effects were not superimposable to those of the diazepam [53]. Consistently, antinociceptive effect of bergamot EO inhalation was seen in mice [54]. Recently, changes in cerebral activity produced by Origanum majorana essential oil inhalation were seen as improvements of stress treatment in bruxistic patients [55]. These findings support that electrical brain wave is sensitive for investigation of essential oil effects.
Conclusions
Particularly, the present EEG data clearly indicated the differences between frequency brain waves stimulated by C. reticulata EO and diazepam. These findings suggested that some CNS actions that mediate the effects of C. reticulata EO are distinct to that of benzodiazepines. Taken together, C. reticulata EO inhalation produced unique EEG patterns and modified sleep-wake parameters. In particular, it might be beneficial for sleep improvement in some neurodegenerative conditions.
Conflicts of interest
All authors declared that they have no conflict of interest.
Acknowledgments
This work was financially supported by grants from graduated school, the Natural Product Research Center of Excellence, Research Unit for EEG Biomarkers of Neuronal diseases, and Department of Physiology, Faculty of Science, Prince of Songkla University, Hatyai, Songkhla 90112, Thailand.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Malcolm B.J., Tallian K. Essential oil of lavender in anxiety disorders: ready for prime time? Ment Heal Clin. 2018;7:147–155. doi: 10.9740/mhc.2017.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fazlollahpour-Rokni F., Shorofi S.A., Mousavinasab N., Ghafari R., Esmaeili R. The effect of inhalation aromatherapy with rose essential oil on the anxiety of patients undergoing coronary artery bypass graft surgery. Complement Ther Clin Pract. 2019;34:201–207. doi: 10.1016/j.ctcp.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Goel N., Kim H., Lao R.P. An olfactory stimulus modifies nighttime sleep in young men and women. Chronobiol Int. 2005;22:889–904. doi: 10.1080/07420520500263276. [DOI] [PubMed] [Google Scholar]
- 4.Moss M., Hewitt S., Moss L., Wesnes K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int J Neurosci. 2008;118:59–77. doi: 10.1080/00207450601042094. [DOI] [PubMed] [Google Scholar]
- 5.Pemberton E., Turpin P.G. The effect of essential oils on work-related stress in intensive care unit nurses. Holist Nurs Pract. 2008;22:97–102. doi: 10.1097/01.HNP.0000312658.13890.28. [DOI] [PubMed] [Google Scholar]
- 6.Han X., Gibson J., Eggett D.L., Parker T.L. Bergamot (citrus bergamia) essential oil inhalation improves positive feelings in the waiting room of a mental health treatment center: a pilot study. Phytother Res. 2017;31:812–816. doi: 10.1002/ptr.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moslemi F., Alijaniha F., Naseri M., Kazemnejad A., Charkhkar M., Heidari M.R. Citrus aurantium aroma for anxiety in patients with acute coronary syndrome: a double-blind placebo-controlled trial. J Altern Complement Med. 2019;25:833–839. doi: 10.1089/acm.2019.0061. [DOI] [PubMed] [Google Scholar]
- 8.Namazi M., Ali Akbari S.A., Mojab F., Talebi A., Majd H.A., Jannesari S. Effects of citrus Aurantium (bitter orange) on the severity of first-stage labor pain. Iran J Pharm Res. 2014;13:1011–1018. [PMC free article] [PubMed] [Google Scholar]
- 9.Maurya A.K., Mohanty S., Pal A., Chanotiya C.S., Bawankule D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: in-vitro and in-vivo study. J Ethnopharmacol. 2018;212:86–94. doi: 10.1016/j.jep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Smeriglio A., Alloisio S., Raimondo F.M., Denaro M., Xiao J., Cornara L., et al. Essential oil of Citrus lumia Risso: phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem Toxicol. 2018;119:407–416. doi: 10.1016/j.fct.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho-Freitas M.I., Costa M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol Pharm Bull. 2002;25:1629–1633. doi: 10.1248/bpb.25.1629. [DOI] [PubMed] [Google Scholar]
- 12.Gargano A.C., Costa A., Costa M. Essential oils from Citrus latifolia and Citrus reticulata reduce anxiety and prolong ether sleeping time in mice. Tree For Sci Biotechnol. 2008;2:121–124. [Google Scholar]
- 13.Dimpfel W., Spüler M., Nickel B. Radioelectroencephalography (Tele-Stereo-EEG) in the rat as a pharmacological model to differentiate the central action of flupirtine from that of opiates, diazepam and phenobarbital. Neuropsychobiology. 1986;16:163–168. doi: 10.1159/000118319. [DOI] [PubMed] [Google Scholar]
- 14.Shinomiya K., Shigemoto Y., Okuma C., Mio M., Kamei C. Effects of short-acting hypnotics on sleep latency in rats placed on grid suspended over water. Eur J Pharmacol. 2003;460:139–144. doi: 10.1016/s0014-2999(02)02915-1. [DOI] [PubMed] [Google Scholar]
- 15.Dimpfel W., Schombert L., Gericke N. Electropharmacogram of Sceletium tortuosum extract based on spectral local field power in conscious freely moving rats. J Ethnopharmacol. 2016;177:140–147. doi: 10.1016/j.jep.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Cheaha D., Issuriya A., Manor R., Kwangjai J., Rujiralai T., Kumarnsit E. Modification of sleep-waking and electroencephalogram induced by vetiver essential oil inhalation. J Intercult Ethnopharmacol. 2016;5:72–78. doi: 10.5455/jice.20160208050736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheaha D., Keawpradub N., Sawangjaroen K., Phukpattaranont P., Kumarnsit E. Effects of an alkaloid-rich extract from Mitragyna speciosa leaves and fluoxetine on sleep profiles, EEG spectral frequency and ethanol withdrawal symptoms in rats. Phytomedicine. 2015;22:1000–1008. doi: 10.1016/j.phymed.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Arifin W.N., Zahiruddin W.M. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24:101–105. doi: 10.21315/mjms2017.24.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimpfel W. Preclinical data base of pharmaco-specific rat EEG fingerprints (tele-stereo-EEG) Eur J Med Res. 2003;8:199–207. [PubMed] [Google Scholar]
- 20.Dimpfel W. Pharmacological classification of herbal extracts by means of comparison to spectral EEG signatures induced by synthetic drugs in the freely moving rat. J Ethnopharmacol. 2013;149:583–589. doi: 10.1016/j.jep.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Dimpfel W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine. 2009;16:287–294. doi: 10.1016/j.phymed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Leite M.P., Fassin J., Jr., Baziloni E.M.F., Almeida R.N., Mattei R., Leite J.R. Behavioral effects of essential oil of Citrus aurantium L. inhalation in rats. Rev Bras Farmacogn. 2008;18:661–666. [Google Scholar]
- 23.Dugo P., Mondello L., Dugo L., Stancanelli R., Dugo G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J Pharmaceut Biomed Anal. 2000;24:147–154. doi: 10.1016/s0731-7085(00)00400-3. [DOI] [PubMed] [Google Scholar]
- 24.Mondello L., Stagno d'Alcontres I., Del Duce R., Crispo F. On the genuineness of citrus essential oils. Part XL. The composition of the coumarins and psoralens of Calabrian bergamot essential oil (Citrus bergamia Risso) Flavour Fragrance J. 1993;8:17–24. [Google Scholar]
- 25.Mondello L., Dugo P., Bartle K.D., Dugo G., Cotroneo A. Automated HPLC-HRGC: a powerful method for essential oils analysis. Part V. identification of terpene hydrocarbons of bergamot, lemon, Mandarin, sweet orange, bitter orange, grapefruit, clementine and mexican lime oils by coupled HPLC-HRGC-MS(ITD) Flavour Fragrance J. 1995;10:33–42. [Google Scholar]
- 26.de Almeida A.A., de Carvalho R.B., Silva O.A., de Sousa D.P., de Freitas R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol Biochem Behav. 2014;118:69–78. doi: 10.1016/j.pbb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Guzmán-Gutiérrez S.L., Bonilla-Jaime H., Gómez-Cansino R., Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Cheaha D., Sawangjaroen K., Kumarnsit E. Characterization of fluoxetine effects on ethanol withdrawal-induced cortical hyperexcitability by EEG spectral power in rats. Neuropharmacology. 2014;77:49–56. doi: 10.1016/j.neuropharm.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 29.van Lier H., Drinkenburg W.H., van Eeten Y.J., Coenen A.M. Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology. 2004;47:163–174. doi: 10.1016/j.neuropharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Kwangjai J., Hiranyachattada S., Wattanapiromsakul C., Kumarnsit E. Modification of electrical brain wave by Citrus sp. essential oil inhalation. J Physiol Biomed Sci. 2013;26:5–8. [Google Scholar]
- 31.Sowndhararajan K., Cho H., Yu B., Kim S. Effect of olfactory stimulation of isomeric aroma compounds, (+)-limonene and terpinolene on human electroencephalographic activity. Eur J Integr Med. 2015;7:561–566. [Google Scholar]
- 32.Akhmetova E.R., Vorob’ev V.V., Groznyi A.V., Kovalev G.I. [Participation of D1 dopamine and alpha2 adrenoreceptors in formation of the frequency spectrum of the cortical activity in rats] Eksp Klin Farmakol. 2000;63:7–10. Russian. [PubMed] [Google Scholar]
- 33.Dimpfel W. Pharmacological modulation of dopaminergic brain activity and its reflection in spectral frequencies of the rat electropharmacogram. Neuropsychobiology. 2008;58:178–186. doi: 10.1159/000191124. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Guasti A., del Río Portilla I.Y., Ugalde E., Corsi-Cabrera M. Diazepam and progesterone produce sexually dimorphic actions on the rat EEG: role of the neonatal sexual differentiation process. Psychoneuroendocrinology. 2003;28:85–100. doi: 10.1016/s0306-4530(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 35.Nickel B., Szelenyi I. Comparison of changes in the EEG of freely moving rats induced by enciprazine, buspirone and diazepam. Neuropharmacology. 1989;28:799–803. doi: 10.1016/0028-3908(89)90170-6. [DOI] [PubMed] [Google Scholar]
- 36.Lochhead J.J., Thorne R.G. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Brand G. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev. 2006;30:908–917. doi: 10.1016/j.neubiorev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Lima N.G., de Sousa D.P., Pimenta F.C., Alves M.F., de Souza F.S., Macedo R.O., et al. Anxiolytic-like activity and GC–MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol Biochem Behav. 2013;103:450–454. doi: 10.1016/j.pbb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Park H.M., Lee J.H., Yaoyao J., Jun H.J., Lee S.J. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A 2A receptors. Biochem Biophys Res Commun. 2011;404:345–348. doi: 10.1016/j.bbrc.2010.11.121. [DOI] [PubMed] [Google Scholar]
- 40.Satoh S., Matsumura H., Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol. 1998;351:155–162. doi: 10.1016/s0014-2999(98)00302-1. [DOI] [PubMed] [Google Scholar]
- 41.Coleman C.G., Baghdoyan H.A., Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem. 2006;96:1750–1759. doi: 10.1111/j.1471-4159.2006.03700.x. [DOI] [PubMed] [Google Scholar]
- 42.Bourgin P., Ahnaou A., Laporte A.M., Hamon M., Adrien J. Rapid eye movement sleep induction by vasoactive intestinal peptide infused into the oral pontine tegmentum of the rat may involve muscarinic receptors. Neuroscience. 1999;89:291–302. doi: 10.1016/s0306-4522(98)00290-5. [DOI] [PubMed] [Google Scholar]
- 43.Darchia N., Campbell I.G., Feinberg I. Rapid eye movement density is reduced in the normal elderly. Sleep. 2003;26:973–977. doi: 10.1093/sleep/26.8.973. [DOI] [PubMed] [Google Scholar]
- 44.Vitiello M.V., Prinz P.N., Williams D.E., Frommlet M.S., Ries R.K. Sleep disturbances in patients with mild-stage Alzheimer's disease. J Gerontol. 1990;45:M131–M138. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 45.Mailliet F., Galloux P., Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology (Berl) 2001;156:417–426. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 46.Radulovacki M., Sreckovic G., Zak R., Zahrebelski G. Diazepam and midazolam increase light slow-wave sleep (SWS1) and decrease wakefulness in rats. Brain Res. 1984;303:194–196. doi: 10.1016/0006-8993(84)90229-4. [DOI] [PubMed] [Google Scholar]
- 47.Xiong M., Li J., Wang D., Delphin E., Ye J.H. Intra-ventrolateral preoptic nucleus injection of γ-aminobutyric acid induces sedation in rats. Int J Physiol Pathophysiol Pharmacol. 2012;4:94–98. [PMC free article] [PubMed] [Google Scholar]
- 48.Rickels K., Schweizer E. The clinical presentation of generalized anxiety in primary-care settings: practical concepts of classification and management. J Clin Psychiatry. 1997;58:4–10. [PubMed] [Google Scholar]
- 49.Reynolds D.S. The value of genetic and pharmacological approaches to understanding the complexities of GABAA receptor subtype functions: the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 2008;90:37–42. doi: 10.1016/j.pbb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Ceccarelli I., Lariviere W.R., Fiorenzani P., Sacerdote P., Aloisi A.M. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats. Brain Res. 2004;1001:78–86. doi: 10.1016/j.brainres.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 51.Miguel T.T., Nunes-de-Souza R.L. Anxiogenic and antinociceptive effects induced by corticotropin-releasing factor (CRF) injections into the periaqueductal gray are modulated by CRF1 receptor in mice. Horm Behav. 2011;60:292–300. doi: 10.1016/j.yhbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Rombolà L., Corasaniti M.T., Rotiroti D., Tassorelli C., Sakurada S., Bagetta G., et al. Effects of systemic administration of the essential oil of bergamot (BEO) on gross behaviour and EEG power spectra recorded from the rat hippocampus and cerebral cortex. Funct Neurol. 2009;24:107–112. [PubMed] [Google Scholar]
- 53.Rombolà L., Tridico L., Scuteri D., Sakurada T., Sakurada S., Mizoguchi H., et al. Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules. 2017;22:614. doi: 10.3390/molecules22040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scuteri D., Crudo M., Rombolà L., Watanabe C., Mizoguchi H., Sakurada S., et al. Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia. 2018;129:20–24. doi: 10.1016/j.fitote.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Merino J., Parmigiani-Izquierdo J., López-Oliva M., Cabaña-Muñoz M. Origanum majorana essential oil inhalation during neurofeedback training reduces saliva myeloperoxidase activity at session-1 in bruxistic patients. J Clin Med. 2019;8:158. doi: 10.3390/jcm8020158. [DOI] [PMC free article] [PubMed] [Google Scholar]