Abstract
Background
Osteoporotic intertrochanteric fractures often have postoperative complications despite a perfect reduction and an optimal fixation. We describe a simple technique using bone substitute augmentation and hypothesize that this method would prevent excessive sliding of the lag screw and potential subsequent complications.
Methods
Between January 2009 and July 2017, patients with osteoporotic intertrochanteric fractures who were treated with a dynamic hip screw (DHS) were enrolled in this retrospective cohort study. DHS group patients received conventional DHS treatment and BSA-DHS group patients received bone-substitute augmented DHS treatment. Factors such as demographics, Parker and Palmer mobility scores, health-related quality of life (HRQoL) scores (short-form Health Survey-12 Physical Component Summary [SF-12-PCS], and SF-12 Mental Component Summary [SF-12-MCS]), morbidities, mortality, and radiographic outcomes were compared.
Results
We enrolled 85 patients: DHS group = 37 and BSA-DHS group = 48. There was significant lag-screw sliding (mean: 9 mm and 3 mm, p < 0.001), varus collapse (mean: 7° and 3°, p < 0.001), and femoral shortening (mean: 10 mm and 3 mm, p < 0.001) in the DHS group compared to the BSA-DHS group. The ability to get around the house was significantly different between the DHS and BSA-DHS groups (p = 0.031) at 3 months. Postoperative scores were not significantly different after 6, 9 or 12 months, however. Scores for the ability to get out of the house and to go shopping and the SF-12-PCS were significantly worse in the DHS group at 3 and 6 months. Malunion and lag-screw cutout were also significantly worse in the DHS group (p = 0.037 and p = 0.033, respectively).
Conclusions
Bone-substitute augmentation appears effective to prevent typical postoperative complications experienced by the DHS group patients, and to improve functional outcomes. Additional prospective randomized large-scale cohort studies are necessary to confirm this conclusion.
Level of evidence
Therapeutic Level III.
Keywords: Intertrochanteric fracture, Osteoporosis, Bone substitute, Health-related quality of life (HRQoL)
At a glance of commentary.
Scientific background on the subject
Osteoporotic intertrochanteric fractures are common among elderly patients. This injury often leads to complications despite a perfect reduction and an optimal fixation. Poor bone stock is the major problem. Several techniques have been reported to improve stability and prevent fixation failure. However the complication rate is still high.
What this study adds to the field
We describe a simple technique using bone substitute augmentation. This simple technique is to compress the bone substitutes into cancellous bone interstices of the proximal fragment. Bone-substitute augmentation appears effective to increase fixation stability, to prevent typical postoperative complications experienced by DHS group patients, and to improve functional outcomes.
Osteoporotic intertrochanteric fractures are relatively common among elderly patients. This injury has potentially devastating effects on patients and health-care systems [[1], [2], [3], [4]]. Except under conditions of extremely poor health, osteosynthesis is the standard treatment: it provides pain relief, allows early weight-bearing, allows early return to daily activities, and prevents complications related to long-term confinement to bed [[1], [2], [3]].
Despite the numerous implants that have been developed to replace the dynamic hip screw (DHS), the DHS is widely used [[5], [6], [7], [8]]. However, osteoporotic intertrochanteric fractures often lead to complications despite a perfect reduction and an optimal tip-apex distance (TAD) index [9,10]. The poor bone stock of elderly patients is the major problem, and the complication might be as high as 19% [11]. Poor bone stock leads to poor pull-out strength of implants and excessive sliding of the lag screw, and contributes to femoral shortening, rotation of the femoral head, varus collapse of the proximal fragment, cutout of the lag screw, and various functional impairments [1,2,12,13].
Several techniques that use autografts, cement, or bone substitutes have been reported [[11], [12], [13], [14]] to augment the bone stock or to improve the bone-implant interface to provide adequate primary stability and prevent fixation failure. Because bone autografts have poor mechanical properties, and because harvesting the autograft may lead to donor-site morbidity, there are legitimate concerns about using them [12]. Cement-augmentation has been widely used to facilitate fixation stability and provide early postoperative weight-bearing, but there is no consensus about the area of augmentation or method of delivery [1,[11], [12], [13], [14]]. In addition, conventional DHS and cement-augmented DHS fail in different ways, which can make the subsequent surgery difficult [13].
In this study we describe a simple technique to compress the bone substitutes into cancellous bone interstices of the proximal fragment. We compared complications and outcomes between a bone substitute-augmented DHS (BSA-DHS) and a conventional DHS. We hypothesized that the BSA-DHS would be ideal for preventing excessive sliding of the lag screw in patients with osteoporotic intertrochanteric fractures and for providing satisfactory radiographic and clinical outcomes after a minimum follow-up of 12 months.
Materials and methods
Ethics
This retrospective study was approved by the Ethics Committee and Institutional Review Board of our institution, and all patients provided signed informed consent forms.
Demographics
Between January 2009 and July 2017, all patients who underwent surgery for osteoporotic intertrochanteric fracture at the authors’ institution were routinely entered into our departmental computer databases. According to Arbeitsgemeinschaft für Osteosynthesefragen/Orthopedic Trauma Association (AO/OTA) classification [15], the fractures were classified as either stable (31-A1 and 31-A2.1) or unstable (31-A2.2, 31-A2.3, and 31-A3) [16]. We routinely collected the clinical data of the patients: age, gender, body weight, body height, body mass index (BMI), fracture pattern, American Society of Anesthesiologists (ASA) classification, length of hospital stay, surgical methods used, surgical duration, complications, and the preoperative, postoperative radiographic, and clinical functional assessments.
The three required inclusion criteria were: (1) ≥ 65 years old, (2) had undergone a stable intertrochanteric fracture (AO/OTA 31-A1 and 31-A2.1), and (3) had been followed-up for a minimum of 12 months. The exclusion criteria were: (1) unacceptable reduction of fractures; (2) multiple fractures, pathologic fractures, fracture of the opposite hip; (3) previous ipsilateral hip or femur surgery; (4) developmental abnormality; (5) inability to ambulate preoperatively; and (6) incomplete medical records, radiographic analyses, or functional assessments.
To minimize surgeon-related confounding factors all surgical operations were performed by a single surgeon. To minimize implant-related confounding factors all enrolled patients were treated with a DHS. To minimize drug-related variables, patients who had taken osteoporosis medications before and after surgery were excluded.
Patients were divided into two groups. The DHS group included patients with an osteoporotic intertrochanteric fracture who had conventional DHS treatment. The BSA-DHS group included patients who had bone-substitute augmented DHS treatment. To determine an adequate sample size, an a priori power analysis using the two-sided hypothesis test with a power of 90% and a significance of 0.05 was done. The independent t-test was used to obtain the sample size. The sliding distance of the lag screw was the primary variable. Base on Lee et al. [1], we assumed that the mean change in the sliding distance of the lag screw was 5.8 mm ± 5.6 mm, which yielded a sample size calculation of 34 patients required in each group.
Surgical technique
All surgical operations were performed by a single surgeon with extensive experience using DHS. The quality of the fracture reduction was considered acceptable when all of the following criteria were met: (1) anatomic or slight valgus alignment on the anteroposterior (AP) view, (2) alignment with parallel or slight cervical anteversion on the lateral view, and (3) no more than 1 cm of displacement between two major fracture fragments [1].
Conventional DHS
In the conventional DHS group, a DHS was inserted using a standard surgical procedure and C-arm fluoroscope assistance. After the reduction was deemed acceptable, a guide wire was inserted into the femoral neck and aimed beneath the center of the femoral head without penetrating the hip joint. A 135-degree side plate, with at least four holes available below the fracture, was used. Before the side plate was secured to the lateral aspect of the femur, the traction was released to ensure cortical contact between fragments.
Bone substitute-augmented DHS
We used bone substitutes (Foramic® Bone Graft Substitute; Maxigen Biotech Inc., Taipei, Taiwan) to infiltrate the bony defects and surrounding cancellous bone interstices. A 3-cc syringe was used to deliver the bone substitutes. The end with a nozzle that connected to a needle was removed with a knife; the end of a hollow barrel with a piston was kept to carry the bone substitutes [Fig. 1]. After the fracture fragment had been adequately reduced, the guide wire was inserted [Fig. 2A]. The lag screw channel was reamed using a DHS reamer, but the DHS tap was not used. A Kirschner wire (3.0-mm in diameter) was inserted superiorly parallel to the guide wire and the lag screw hole, and then the guide wire was removed. The resected hollow barrel with bone substitutes was placed into the screw tract. The bone substitutes were pushed using the plunger and then using gentle hammer taps against the DHS impactor [Fig. 2B]. The guide wire was reinserted, the assembled lag screw was inserted into the drilled hole, and then the DHS side plate was positioned against the femoral shaft [Fig. 2C].
Fig. 1.

A 3-cc syringe was used to deliver the bone substitutes. The end with a nozzle that connects to a needle was removed with a knife; the end of a hollow barrel with a piston was preserved to carry the bone substitutes.
Fig. 2.
Intraoperative fluoroscopy. (A) Adequately reducing fracture fragments, a guide wire was inserted. (B) The lag screw channel was reamed using a DHS reamer. A Kirschner wire (3.0 mm in diameter) was inserted above and parallel to the guide wire and lag screw hole; the guide wire was then removed. The bone substitutes were pushed using the plunger and then gentle hammer taps against the DHS impactor. (C) The guide wire reinserted, and then an assembled lag screw was inserted into the drilled hole.
Postoperative rehabilitation protocol
The postoperative rehabilitation protocol was identical for both groups. Quadriceps strengthening and hip and knee range of motion exercises were begun immediately after surgery. Patients were encouraged to get out of bed and walk on the second postoperative day. Partial weight bearing with the aid of a walker frame and at the limit of pain was authorized for all patients and continued for at least 8 weeks. Subsequently, patients could be advanced to full weight bearing based on the appearance of recanalization or bridging callus on follow-up radiographs.
Assessment
Radiographic assessment
The bone mineral density (BMD) of the opposite hip was measured using dual energy X-ray absorptiometry (DXA) (DXA QDR 4500; Hologic Inc., Waltham, MA) and was recorded and analyzed by a research associate who was blinded to the surgical technique.
All patients enrolled in the study were preoperatively and postoperatively assessed using a radiographic examination of an AP view of pelvis, and AP and lateral views of the affected hip. Any change in the position of the implants present on the AP view at the last follow-up was recorded. The radiographic evaluation was reviewed by two independent surgeons. The intra-observer and inter-observer reliability—good to very good in this study—was assessed using the intra-class correlation coefficient (ICC) [17].
The TAD was measured using AP and lateral radiographs of the affected hip [18]. The initial postoperative and the last follow-up radiographs were compared. A decrease in the neck-shaft angle was measured as varus collapse [19]. The telescoping of the lag screw was measured as lag screw sliding, the magnitude of bone shortening was measured using the method described elsewhere [20]. Fracture union was defined as recanalization of the trabeculae or bridging callus visible on both radiograph views; delayed union was defined as no sign of fracture healing after 24 weeks; nonunion was defined as the absence of bone union after 36 weeks postoperatively; and malunion was defined as femoral shortening of more than 20 mm or varus collapse of more than 15° compared with the opposite side [1].
Failure of the treatment was defined when the following events occurred: (1) the screw penetrated the hip joint; (2) the barrel-plate or its screws broke; or (3) the patient underwent a second operation because of other implant failures.
Clinical assessment
Postoperative functional scores were calculated using the Parker and Palmer new mobility score [21]. Hip pain was graded on a four-point scale: (1) no pain; (2) mild pain not affecting walking or requiring regular analgesic medication; (3) moderate pain affecting walking, or requiring regular medication, or both; (4) severe pain [1]. General health-related quality of life scores were obtained using the 12-item Short Form Health Survey Physical Component Summary and the Mental Component Summary (SF-12 MCS) [22]. Each subscale is scored from 0 to 100, with higher scores representing better functions. Mobility scores, pain scores, and HRQoL scores were assessed at 3, 6, 9, and 12 months postoperatively. Clinical assessments were reviewed and analyzed by a research associate.
Statistical analysis
All data were entered into an Excel spreadsheet (Microsoft Corp, Redmond, WA) and subsequently copied into SPSS 13.0 for Windows (SPSS Inc., Chicago, IL). The data were analyzed by an independent statistician blinded to the group allocation. The independent t-test was used for continuous variables. A χ2 test or the Fisher's exact test was used for binary variables. We used repeated measure ANOVA to detect the differences between different time points (post-operation 3 months, 6 months, 9 months and 12 months) in each group. Significance was set at p < 0.05.
Results
We reviewed the records of 180 patients (180 hips) who met our inclusion criteria. However, 69 patients who were taking antiosteoporotic agents, 15 with incomplete data, and 11 lost to follow-up were excluded. Finally, 22 men and 63 women (mean age: 78 years; age range: 65–91 years) at the time of surgery were included. Their mean body height was 154 cm (range: 140–177 cm), mean body weight was 54 kg (range: 37–84 kg), and mean BMI was 24 kg/m2 (range: 19–33 kg/m2).
The DHS group contained 37 patients and the BSA-DHS group contained 48 patients. At the time of the operation, there were no significant differences in age, gender, BMI, BMD of the contralateral hip, ASA classification, or duration of hospital stay [Table 1]. The mean of duration of surgery in each group was 75 min (DHS) and 89 min (BSA-DHS) (p = 0.014) [Table 1].
Table 1.
Demographic Data of Patients.
| Demographic Data | DHS |
BSA-DHS |
p |
|---|---|---|---|
| (n = 37) | (n = 48) | ||
| Age at the time of operation (years) | 79 ± 6 | 78 ± 8 | 0.780 |
| Gender | 0.618 | ||
| Male | 11 (30%) | 11 (23%) | |
| Female | 26 (70%) | 37 (77%) | |
| Body height (cm) | 155 ± 9 | 154 ± 9 | 0.812 |
| Body weight (kg) | 55 ± 11 | 54 ± 9 | 0.754 |
| Body mass index (kg/m2) | 23 ± 4 | 24 ± 4 | 0.935 |
| BMD of contralateral hip (T-score) | −2.8 ± 1.2 | −2.9 ± 1.1 | 0.880 |
| ASA classification | 0.812 | ||
| ASA I | – | – | |
| ASA II | 27 (73%) | 34 (71%) | |
| ASA III | 10 (27%) | 14 (29%) | |
| Duration of surgery (min) | 75 ± 14 | 89 ± 12 | 0.014∗ |
| Duration of hospital stay (days) | 8 ± 1 | 8 ± 1 | 0.990 |
Values shown are mean ± standard deviation (SD) or n (%).
DHS: patients treated with conventional DHS.
BSA-DHS: patients treated with bone substitute-augmented DHS.
p-values for between-group comparisons were determined using a χ2 test and Fisher's exact test for categorical variables, and independent t-test for continuous variables.
∗p < 0.05.
Radiographic analyses showed no significant differences in TAD (p = 0.771) or mean union time (p = 0.760). However, there were significant differences in sliding of the lag screw (mean: DHS = 9 mm and BSA-DHS = 3 mm; p < 0.001). The varus collapse and femoral shortening were also significantly different (all: p < 0.001) between the two groups [Table 2].
Table 2.
Radiographic Parameters of Patients.
| Radiographic Parameters | DHS |
BSA-DHS |
p |
|---|---|---|---|
| (n = 37) | (n = 48) | ||
| Tip Apex Distance (mm) | 19 ± 2 | 19 ± 2 | 0.771 |
| Union time (weeks) | 13.6 ± 2.5 | 13.9 ± 2.3 | 0.760 |
| Sliding of lag screw (mm) | 9 ± 4 | 3 ± 2 | <0.001∗ |
| Varus collapse (degrees) | 7 ± 3 | 3 ± 2 | <0.001∗ |
| Femoral shortening (mm) | 10 ± 6 | 3 ± 2 | <0.001∗ |
Values are shown as mean ± standard deviation.
DHS: patients treated with conventional DHS.
BSA-DHS: patients treated with bone substitute-augmented DHS.
p-values for between-group comparisons were determined using independent t-test.
∗p < 0.05.
The pain score and mobility score at different time intervals were demonstrated in Table 3. Pain scores were significantly different at 3 months (mean: DHS = 2.5 and BSA-DHS = 1.6; p = 0.003) and at 6 months (mean = 1.8 and 1.3, respectively [ p = 0.013]). These differences were no longer significant at 9 months and 12 months [Fig. 3]. The ability to get around the house at 3 months postoperatively was significantly different between the DHS and BSA-DHS groups (p = 0.031). These differences were no longer significant at 6 months, 9 months and 12 months [Fig. 4]. With regard to the ability to get out of the house, there were significant differences at 3 months (mean: DHS = 0.9 and BSA-DHS = 1.5; p < 0.001) and at 6 months (mean = 1.5 and 1.8, respectively [p = 0.044]) [Fig. 5]. There were also significant differences in the ability to go shopping at 3 months (mean = 0 and 1.1, respectively [p < 0.001]) and at 6 months (mean = 0.7 and 1.6, respectively [p = 0.011]) [Fig. 6].
Table 3.
Mobility score (Parker and Palmer) and Pain score at Different Time Intervals.
| Mobility and Pain Scores | Postoperative Period |
|||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| Pain score (1–4) | ||||
| DHS Group | 2.5 ± 0.6∗ | 1.8 ± 0.6∗ | 1.4 ± 0.5 | 1.2 ± 0.5 |
| BSA-DHS Group | 1.6 ± 0.5 | 1.3 ± 0.5 | 1.2 ± 0.4 | 1.1 ± 0.3 |
| Ability to get around the house (0–3) | ||||
| DHS Group | 1.4 ± 0.6∗ | 2.3 ± 0.6 | 2.8 ± 0.4 | 2.9 ± 0.3 |
| BSA-DHS Group | 2.0 ± 0.3 | 2.4 ± 0.4 | 2.9 ± 0.3 | 2.9 ± 0.3 |
| Ability to get out of the house (0–3) | ||||
| DHS Group | 0.9 ± 0.3∗ | 1.5 ± 0.6∗ | 2.1 ± 0.3 | 2.2 ± 0.3 |
| BSA-DHS Group | 1.5 ± 0.3 | 1.8 ± 0.5 | 2.2 ± 0.3 | 2.3 ± 0.2 |
| Ability to go shopping (0–3) | ||||
| DHS Group | 0∗ | 0.7 ± 0.4∗ | 1.5 ± 0.5 | 1.6 ± 0.5 |
| BSA-DHS Group | 1.1 ± 0.2 | 1.6 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.2 |
Values are shown as mean ± standard deviation.
DHS: patients treated with conventional DHS.
BSA-DHS: patients treated with bone substitute-augmented DHS.
p-values for between-group comparisons were determined using independent t-test.
∗p < 0.05.
Fig. 3.
Mean hip pain scores. Differences of mean hip pain scores were found between the DHS and BSA-DHS groups at the postoperation periods of 3 and 6 months. (The error bars are for standard deviation).
Fig. 4.
The score of the ability to get around the house (indoor walking) was significantly worse in DHS group at postoperation 3 months. (The error bars are for standard deviation).
Fig. 5.
For the ability to get out of the house (outdoor walking), significant differences were noted at postoperation 3 and 6 months between the DHS and BSA-DHS groups. (The error bars are for standard deviation).
Fig. 6.
Evaluation of the ability to walking during shopping. For patients without bone-substitute augmentation, the mean score of the ability to go shopping was significantly lower than the scores in BSA-DHS group at postoperation 3 and 6 months. (The error bars are for standard deviation).
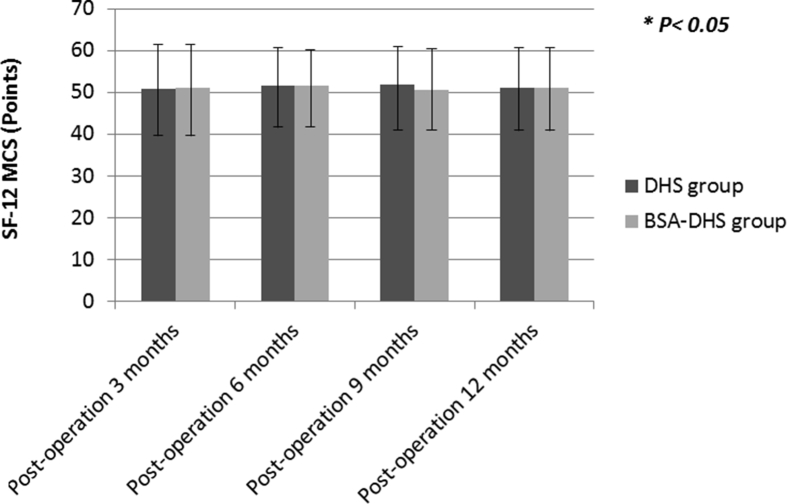
HRQoL at different time intervals was demonstrated in Table 4. HRQoL scores were significantly different at 3 months (mean: DHS = 18.2 and BSA-DHS = 26.9; p < 0.001) and at 6 months (mean = 27.2 and 35.8, respectively [p = 0.013]). The differences in the HRQoL scores between the 2 groups were no longer significant at 9 months and 12 months [Fig. 7]. SF-12 MCS scores were not significantly different between the groups [Fig. 8].
Table 4.
Health-Related Quality of Life (HRQoL) at Different Time Intervals.
| Postoperative Period |
||||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| SF-12 PCS | ||||
| DHS Group | 18.2 ± 8.3∗ | 27.2 ± 10.0∗ | 37.1 ± 10.1 | 38.1 ± 10.6 |
| BSA-DHS Group | 26.9 ± 8.6 | 35.8 ± 10.7 | 39.1 ± 10.7 | 39.3 ± 11.3 |
| SF-12 MCS | ||||
| DHS Group | 50.8 ± 11.1 | 51.7 ± 9.3 | 51.9 ± 10.6 | 51.2 ± 10.5 |
| BSA-DHS Group | 51.0 ± 10.9 | 51.5 ± 9.0 | 50.6 ± 10.1 | 51.2 ± 10.4 |
Values are shown as mean ± standard deviation.
DHS: patients treated with conventional DHS.
BSA-DHS: patients treated with bone substitute-augmented DHS.
p-values for between-group comparisons were determined using independent t-test.
∗p < 0.05.
Fig. 7.
The scores of the SF-12 PCS were significantly better in BSA-DHS group at postoperation 3 months and 6 months. (The error bars are for standard deviation).
Fig. 8.
SF-12 MCS scores were not significantly different between the groups during the study period. (The error bars are for standard deviation).
We used repeated measure ANOVA to detect the differences between different time points (post-operation 3 months, 6 months, 9 months and 12 months) in each group. Pain scores were significantly different between 3 and 6 months (p = 0.023), 3 and 9 months (p = 0.004), 3 and 12 months (p < 0.001), 6 and 9 months (p = 0.041), and 6 months and 12 months (p = 0.026) in DHS group. In BAS-DHS group, however, significant differences were detected between 3 and 6 months (p = 0.044), 3 and 9 months (p = 0.035), 3 and 12 months (p = 0.034).
Regarding the ability to get around of the house, there were significant differences between 3 and 6 months (p = 0.002), 3 and 9 months (p < 0.001), 3 and 12 months (p < 0.001) in DHS group. Similar results were noted in BAS-DHS group (p = 0.047, p = 0.028, and p = 0.028, respectively). In DHS group, significant differences were detected between 3 and 6 months (p = 0.008), 3 and 9 months (p < 0.001), 3 and 12 months (p < 0.001), 6 and 9 months (p = 0.034), and 6 months and 12 months (p = 0.033) in the ability to get out of the house. In BAS-DHS group, significant differences were detected between 3 and 6 months (p = 0.046), 3 and 9 months (p = 0.030), 3 and 12 months (p = 0.028). There were similar intragroup differences in the ability to go shopping between 3 and 6 months (p < 0.001), 3 and 9 months (p < 0.001), 3 and 12 months (p < 0.001), 6 and 9 months (p = 0.003), and 6 months and 12 months (p = 0.003) in DHS group. In BAS-DHS group, significant differences were detected between 3 and 6 months (p = 0.018), 3 and 9 months (p = 0.012), 3 and 12 months (p = 0.010).
HRQoL scores were significantly different between 3 and 6 months (p = 0.022), 3 and 9 months (p = 0.004), 3 and 12 months (p = 0.002), 6 and 9 months (p = 0.031), and 6 months and 12 months (p = 0.030) in DHS group. In BAS-DHS group, significant differences were detected between 3 and 6 months (p = 0.037), 3 and 9 months (p = 0.013), 3 and 12 months (p = 0.012). SF-12 MCS scores were not significantly different among different time points in both groups.
A summary of complications during the 12-month follow-up showed significant differences in malunion between the patients in the DHS and BSA-DHS groups (p = 0.037). Similar intergroup differences were seen when comparing the lag screw cutout (p = 0.033). Cutout only occurred in 4 hips in the DHS group. Three of these patients had been treated with bipolar hemiarthroplasty and the fourth with total hip arthroplasty (THA). There were no significant differences in the rate of superficial wound infection, deep wound infection, pneumonia, urinary tract infection, delayed union, nonunion, or implant failure. Mortality between the groups—three patients in the DHS group and one in the BSA-DHS group — deceased during the follow-up period for reasons unrelated to the surgery (p = 0.313) [Table 5].
Table 5.
Postoperative Complications of Patients.
| Postoperative Complications | DHS |
BSA-DHS |
p |
|---|---|---|---|
| (n = 37) | (n = 48) | ||
| Superficial wound infection | 3 (8.1%) | 2 (4.2%) | 0.649 |
| Deep wound infection | 0 | 0 | – |
| Pneumonia | 3 (8.1%) | 1 (2.1%) | 0.313 |
| Urinary tract infection | 4 (10.8%) | 2 (4.2%) | 0.396 |
| Delayed union | 0 | 0 | – |
| Malunion | 7 (19.9%) | 2 (4.2%) | 0.037∗ |
| Nonunion | 0 | 0 | – |
| Cutout of the lag screw | 4 (10.8%) | 0 | 0.033∗ |
| Implant failure | 0 | 0 | – |
| Mortality | 3 (8.1%) | 1 (2.1%) | 0.313 |
Values are shown as n (%).
DHS: patients treated with conventional DHS.
BSA-DHS: patients treated with bone substitute-augmented DHS.
p-values for between-group comparisons were determined using χ2 and Fisher's exact tests.
∗p < 0.05.
Discussion
The most important finding of this study is that, compared with the conventional DHS treatment, the BSA-DHS treatment lessens lag screw sliding, varus collapse, and femoral shortening, and reduces complications in elderly patients. This benefit also translates into better functional outcomes.
The major advantage of the DHS is that the sliding of the lag screw allows impaction of the fracture fragments, which promotes bone healing. However, osteoporosis, which is characterized by reduced BMD and reduced bone strength, leads to increased lag screw sliding and, in turn, fixation failure [12]. Even when the screw is inserted at the optimal site, or the implant design and various surgical fixation techniques are improved, the lack of bony support and insufficient contact between the fracture fragments might contribute to an excessive sliding of the lag screw, followed by femoral shortening, varus collapse of the proximal fragment, femoral head cutout, and, finally, various functional impairments when treating intertrochanteric fractures [1,2,9,10]. The decrease in bone regenerative capacity in elderly patients is another critical factor: it might delay the repair of a femoral metaphyseal defect. Insufficient bony support between fracture fragments contributes to an excessive sliding of the lag screw. In addition, before fracture healing, the implant sustains most of the mechanical loads. Delayed union is caused by decreased regenerative capacity, and it might increase the risk of failure of the side-plate construct.
Autografts provide osteoconduction, osteoinduction, and osteogenesis. It is desirable to augment the bone stock or to improve the quality of the bone-implant interface to provide adequate stability and to prevent fixation failures, but the poor mechanical properties and donor-site morbidity caused by autografts limit their clinical use [12]. Polymethyl methacrylate (PMMA) cements can be used as gap-filling internal fixation devices and for supporting struts in the bony defect to facilitate reconstruction and load transfer, and to improve anchorage of the implant-bone construct [12]. Moreover, augmentation might increase the anchorage of the lag screw, insertional torque, and cutout strength, and it might foster early postoperative weight bearing [12,14,23]. Lee et al. [1] used PMMA cement in the proximal fragment, which converted a conventional DHS into a one-piece rigid device and significantly reduced lag screw sliding, femoral shortening, and fixation failures. Cement augmentation provides a better texture stability. However, Cement augmentation-DHS has its own set of failure modes — delayed union, nonunion, osteonecrosis of the femoral head, side-plate screw pullout, screw breakage, and plate breakage — all of which cause subsequent revision surgery to be more complex and technically demanding [13,14,23,24]. Another potential complication of improper surgical technique is the extravasation of PMMA cement into the hip joint. PMMA cement is not biodegradable and leads to impingement or further destruction of the hip joint. This is a considerable disadvantage, particularly for PMMA [13,14,25,26].
Biomechanical as well as clinical studies have reported that the implantation of bisphenol A dimethacrylate (Bis-DMA) cement or injectable calcium-phosphate cement into the bony defect may “buy time” for preventing side-plate construct failure before fracture healing. In contrast to PMMA, the Bis-DMA cement is hypothesized to lessen the potential risk of thermal-related bone necrosis, but it is quite expensive, and limited information about it is available in the current literature [27]. Injectable calcium-phosphate cement has valuable osteoconductive properties, and it causes less thermal damage than do PMMA cements [[28], [29], [30], [31]]. In addition, calcium-phosphate cements can be remodeled and replaced by bone in vivo [30,31]. Mattsson et al. [32], in a randomized and controlled study, concluded that intertrochanteric fractures were successfully stabilized using calcium-phosphate augmented DHS. Despite these potential advantages, less stiffness and accelerated resorption might lead to early cement failure before bone healing [19,26,27]. In addition, while calcium-phosphate cement is implanted, it is in liquid phase, and there is still a risk of cement leakage into the fracture site or hip joint.
In this study, we used a bone graft substitute that is a biphasic mixture with a composition of 60% hydroxyapatite and 40% β-tricalcium phosphate (Foramic® Bone Graft Substitute, Maxigen Biotech Inc., Guishan District, Taoyuan City, Taiwan) for augmentation. It was intended to prevent the excessive lag screw sliding commonly encountered in osteoporotic fractures with a lack of bony support. It is safe, has excellent biocompatibility, and can be replaced by natural bone. In this study, BSA-DHS fixations effectively prevented excessive lag screw sliding and inadequate bone anchoring of the lag screw [Fig. 9]. Bone substitutes reinforced bone stock and prevented lag screw migration in the femoral head. Additionally, bone substitutes introduced around the shaft of the lag screw limited its excessive sliding. All these allowed adequate impaction of the fracture fragments, which promoted bone healing.
Fig. 9.
(A) A 84-year-old man with an osteoporotic intertrochanteric fracture (AO/OTA type 31-A2.1). (B) The fracture was fixed with a bone substitute-augmented DHS (BSA-DHS). (C) Lag screw sliding (2 mm) and femoral shortening (2 mm) occurred 2 months postoperatively. (D) Uneventful union of the fracture was noted at 4 months after surgery.
This study has some limitations. First, this is a retrospective study with all the inherent weakness and biases of such study designs. Second, the number of patients was small. Although we determined an adequate sample size (34 hips per group), this study might still be too underpowered to provide significant differences. A randomized controlled trial involving large sample sizes and long-term follow-up is important for evidence-based recommendations. Third, the current study was limited to AO/OTA 31-A1 and 31-A2.1 pertrochanteric fractures. We had no instances of AO/OTA 31-A2.2, 31-A2.3 or AO/OTA 31-A3 pertrochanteric fractures; thus we are unable to comment on whether the BSA-DHS treatment is advantageous for treating them. Finally, all hips reported in this study were treated by DHS, and any hips treated by cephalomedullary device were excluded. The 31-A2.2, 31-A2.3 and 31-A3 show comminution around the intertrochanteric area and therefore always belong to the unstable patterns. Cephalomedullary device is increasingly becoming the golden standard for unstable pertrochanteric fractures [16]. However, in treating AO/OTA 31-A2.1 pertrochanteric fractures, the orthopedic community is divided between those using a cephalomedullary device for these fractures and those who choose a DHS. Some surgeons abandoned the DHS because of the inferior biomechanical properties. However, many AO/OTA 31-A2.1 pertrochanteric fractures can be successfully managed by DHS by strictly adhering to surgical principles involving a TAD index and quality of reduction [2,16,33]. In our institution, we prefer to treat AO/OTA 31-A2.1 pertrochanteric fractures using a DHS. It is currently unclear to us whether the augmentation of bone substitute could also benefit those hips treated with cephalomedullary device.
Conclusion
Our data suggest that BSA-DHS fixation can be an effective alternative for reducing lag screw sliding, varus collapse, and femoral shortening, and for complications when treating osteoporotic intertrochanteric fractures. This benefit translated into better functional outcomes and HRQoL. Prospective randomized large-scale cohort studies are necessary for evidence-based recommendations.
Conflicts of interest
All authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Lee P.C., Hsieh P.H., Chou Y.C., Wu C.C., Chen W.J. Dynamic hip screws for unstable intertrochanteric fractures in elderly patients–encouraging results with a cement augmentation technique. J Trauma. 2010;68:954–964. doi: 10.1097/TA.0b013e3181c995ec. [DOI] [PubMed] [Google Scholar]
- 2.Huang T.W., Yang T.Y., Huang K.C., Peng K.T., Lee M.S., Hsu R.W. Effect of teriparatide on unstable pertrochanteric fractures. BioMed Res Int. 2015;2015:568390. doi: 10.1155/2015/568390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen S.H., Huang K.C., Tsai Y.H., Yang T.Y., Lee M.S., Ueng S.W., et al. Risk analysis for second hip fracture in patients after hip fracture surgery: a nationwide population-based study. J Am Med Dir Assoc. 2014;15:725–731. doi: 10.1016/j.jamda.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Chen I.J., Chiang C.Y., Li Y.H., Chang C.H., Hu C.C., Chen D.W., et al. Nationwide cohort study of hip fractures: time trends in the incidence rates and projections up to 2035. Osteoporos Int. 2015;26:681–688. doi: 10.1007/s00198-014-2930-z. [DOI] [PubMed] [Google Scholar]
- 5.Strauss E., Frank J., Lee J., Kummer F.J., Tejwani N. Helical blade versus sliding hip screw for treatment of unstable intertrochanteric hip fractures: a biomechanical evaluation. Injury. 2006;37:984–989. doi: 10.1016/j.injury.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Windolf M., Braunstein V., Dutoit C., Schwieger K. Is a helical shaped implant a superior alternative to the Dynamic Hip Screw for unstable femoral neck fractures? A biomechanical investigation. Clin Biomech (Bristol, Avon) 2009;24:59–64. doi: 10.1016/j.clinbiomech.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Windolf M., Muths R., Braunstein V., Gueorguiev B., Hänni M., Schwieger K. Quantification of cancellous bone-compaction due to DHS Blade insertion and influence upon cut-out resistance. Clin Biomech (Bristol, Avon) 2009;24:53–58. doi: 10.1016/j.clinbiomech.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Socci A.R., Casemyr N.E., Leslie M.P., Baumgaertner M.R. Implant options for the treatment of intertrochanteric fractures of the hip: rationale, evidence, and recommendations. Bone Joint J. 2017;99:128–133. doi: 10.1302/0301-620X.99B1.BJJ-2016-0134.R1. [DOI] [PubMed] [Google Scholar]
- 9.Gardner M.J., Lorich D.G., Lane J.M. Osteoporotic femoral neck fractures: management and current controversies. Instr Course Lect. 2004;53:427–439. [PubMed] [Google Scholar]
- 10.Lorich D.G., Geller D.S., Nielson J.H. Osteoporotic pertrochanteric hip fractures: management and current controversies. Instr Course Lect. 2004;53:441–454. [PubMed] [Google Scholar]
- 11.Bramlet D.G. Use of the talon hip screw in intertrochanteric fractures of the hip. Clin Orthop Relat Res. 2004 Aug:93–100. doi: 10.1097/01.blo.0000132628.90667.e6. [DOI] [PubMed] [Google Scholar]
- 12.Lindner T., Kanakaris N.K., Marx B., Cockbain A., Kontakis G., Giannoudis P.V. Fractures of the hip and osteoporosis: the role of bone substitutes. J Bone Joint Surg Br. 2009;91:294–303. doi: 10.1302/0301-620X.91B3.21273. [DOI] [PubMed] [Google Scholar]
- 13.Wu M.H., Lee P.C., Peng K.T., Wu C.C., Huang T.J., Hsu R.W. Complications of cement-augmented dynamic hip screws in unstable type intertrochanteric fractures--a case series study. Chang Gung Med J. 2012;35:345–353. doi: 10.4103/2319-4170.106135. [DOI] [PubMed] [Google Scholar]
- 14.Stoffel K.K., Leys T., Damen N., Nicholls R.L., Kuster M.S. A new technique for cement augmentation of the sliding hip screw in proximal femur fractures. Clin Biomech (Bristol, Avon) 2008;23:45–51. doi: 10.1016/j.clinbiomech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Fracture and dislocation compendium. Orthopaedic trauma association committee for coding and classification. J Orthop Trauma. 1996;10:1–154. [PubMed] [Google Scholar]
- 16.Schipper I.B., Marti R.K., van der Werken C. Unstable trochanteric femoral fractures: extramedullary or intramedullary fixation Review of literature. Injury. 2004;35:142–151. doi: 10.1016/s0020-1383(03)00287-0. [DOI] [PubMed] [Google Scholar]
- 17.Park J., Shin J.M., Lee D.K., Lee S.S., Paik S.H., Lee B.H. The effect of synthetic osteoconductive bone graft material for augmentation of internally fixed unstable trochanteric fractures. BioMed Res Int. 2019:5879089. doi: 10.1155/2020/5014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgaertner M.R., Curtin S.L., Lindskog D.M., Keggi J.M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg Am. 1995;77:1058–1064. doi: 10.2106/00004623-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Tsukada S., Okumura G., Matsueda M. Postoperative stability on lateral radiographs in the surgical treatment of pertrochanteric hip fractures. Arch Orthop Trauma Surg. 2012;132:839–846. doi: 10.1007/s00402-012-1484-9. [DOI] [PubMed] [Google Scholar]
- 20.Leung F., Gudushauri P., Yuen G., Lau T.W., Fang C., Chow S.P. Dynamic hip screw blade fixation for intertrochanteric hip fractures. J Orthop Surg (Hong Kong) 2012;20:302–306. doi: 10.1177/230949901202000307. [DOI] [PubMed] [Google Scholar]
- 21.Parker M.J., Palmer C.R. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br. 1993;75:797–798. doi: 10.1302/0301-620X.75B5.8376443. [DOI] [PubMed] [Google Scholar]
- 22.Ware J. Jr, Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Szpalski M., Descamps P.Y., Hayez J.P., Raad E., Gunzburg R., Keller T.S. Prevention of hip lag screw cut-out by cement augmentation: description of a new technique and preliminary clinical results. J Orthop Trauma. 2004;18:34–40. doi: 10.1097/00005131-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Cheng C.L., Chow S.P., Pun W.K., Leong J.C. Long-term results and complications of cement augmentation in the treatment of unstable trochanteric fractures. Injury. 1989;20:134–138. doi: 10.1016/0020-1383(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 25.von der Linden P., Gisep A., Boner V., Windolf M., Appelt A., Suhm N. Biomechanical evaluation of a new augmentation method for enhanced screw fixation in osteoporotic proximal femoral fractures. J Orthop Res. 2006;24:2230–2237. doi: 10.1002/jor.20299. [DOI] [PubMed] [Google Scholar]
- 26.Augat P., Rapp S., Claes L. A modified hip screw incorporating injected cement for the fixation of osteoporotic trochanteric fractures. J Orthop Trauma. 2002;16:311–316. doi: 10.1097/00005131-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 27.DiMaio F.R. The science of bone cement: a historical review. Orthopedics. 2002;25:1399–1407. doi: 10.3928/0147-7447-20021201-21. [DOI] [PubMed] [Google Scholar]
- 28.Larsson S., Bauer T.W. Use of injectable calcium phosphate cement for fracture fixation: a review. Clin Orthop Relat Res. 2002 Feb:23–32. doi: 10.1097/00003086-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Oda H., Nakamura K., Matsushita T., Yamamoto S., Ishibashi H., Yamazaki T., et al. Clinical use of a newly developed calcium phosphate cement (XSB-671D) J Orthop Sci. 2006;11:167–174. doi: 10.1007/s00776-005-0993-6. [DOI] [PubMed] [Google Scholar]
- 30.Bauer T.W. An overview of the histology of skeletal substitute materials. Arch Pathol Lab Med. 2007;131:217–224. doi: 10.5858/2007-131-217-AOOTHO. [DOI] [PubMed] [Google Scholar]
- 31.De Long W.G. Jr, Einhorn T.A., Koval K., McKee M., Smith W., Sanders R., et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: a critical analysis. J Bone Joint Surg Am. 2007;89:649–658. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 32.Mattsson P., Alberts A., Dahlberg G., Sohlman M., Hyldahl H.C., Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures: a prospective, randomized multicentre study. J Bone Joint Surg Br. 2005;87:1203–1209. doi: 10.1302/0301-620X.87B9.15792. [DOI] [PubMed] [Google Scholar]
- 33.Huang T.W., Chuang P.Y., Lin S.J., Lee C.Y., Huang K.C., Shih H.N., et al. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine (Baltim) 2016;95:e3626. doi: 10.1097/MD.0000000000003626. [DOI] [PMC free article] [PubMed] [Google Scholar]