Abstract
The stinging catfish, Heteropneustes fossilis (Bloch, 1974) is a commercially important fish species in Asia. This is an important food fish as is enriched with high amounts of protein, iron and calcium. The current research demonstrates the population structure, size at sexual maturity, spawning- and peak-spawning season and fecundity of H. fossilis in an important wetland ecosystem - Gajner Beel in northwestern Bangladesh with an aim of its sustainable conservation through induced breeding and aquaculture practices. A total of 426 stinging catfish captured from the Gajner Beel through monthly sampling from January to December 2019 used in the study. Total length (TL), standard length (SL) and body weight (BW) of individual fishes were measured. The size ranges were with 6.70–24.10 cm TL, 1.37–83.94 g BW. Gonads were removed carefully through ventral dissection and weighted. Lm was 14.02, 13.5, 13.0 and 15.0 cm based on maximum length (Lmax), TL vs. GSI (%), TL vs. SL and logistic model, respectively. Monthly variations of GSI and maturation stages were confirmed in April to August as spawning season and June as peak spawning month. Fulton’s condition factor (KF) was found to be with significant relations with GSI values. Fecundity was 1,730 to 23,870 and significantly correlated with both TL and BW. Temperature has been increasing 0.029 °C/year with the falling of rainfall at 2.96 mm/year in the study area. Environmental factors -Temperature, rainfall, dissolved oxygen and pH were found to be significantly correlated with GSI. We found the optimal range of temperature (29–31 °C), rainfall (350–380 mm), dissolved oxygen (5.0–6.0 mg/l) and pH (7.1–7.5) for spawning of H. fossilis. The paper recommended the policy guidelines to pave the ways of the aquaculture, conservation and management of H. fossilis in the changing eco-climatic events through specific management measures.
Keywords: Fecundity, Gajner Beel, Heteropneustes fossilis, Optimum eco-climatic factors, Size at sexual maturity, Spawning season
1. Introduction
The Order Siluriformes (catfish) comprises 4,100 species, which constitute 6.3% of all vertebrate species and 12% of all fish species. Catfish have long been playing key role in biological research, as they are similar to a common fishery parent than most bony fish (infraclass Teleostei) because of their phylogenetic position. Nutritionally, catfish are well-known as a valuable source of dietary protein worldwide (Liu et al., 2016). The stinging catfish Heteropneutes fossilis (Bloch, 1794), under the Family Heteropneustidae is generally known as the ‘Shingi or Singhee’ among Bengali speaking people in Bangladesh and India and diaspora living in other parts of the world (Rahman et al., 2019). The benthopelagic catfish is widely distributed in Bangladesh, India, Laos, Myanmar, Nepal, Sri Lanka, and Thailand (Talwar and Jhingran, 1991). It inhabits mainly in slow and shallow water bodies - ponds, ditches, swamps, marshlands, and sometimes in muddy rivers. As it provides large concentrations of protein, iron (226 mg/100 g) and calcium the fish is dubbed as an essential food fish (Saha and Guha, 1939) and occupy high price in the markets (Alok et al., 1993). IUCN Bangladesh, 2015, IUCN, 2000 declared the fish as least concern (LC) in Bangladesh and the world, respectively.
Fruitful fisheries management largely depends on exact assessment of biological parameters, such as reproduction, growth and assessment of stock (Tracey et al., 2007). Understanding of reproductive biology of fish species is vital and a prerequisite for conservation and relevant management approaches (Brewer et al., 2008, Grandcourt et al., 2009, Muchlisin et al., 2010). Besides, conscientious evaluations of reproductive behavior unearth dynamic causes that may influence survival in addition to recruitment of different fish species (Khatun et al., 2019). Fish population replenishment as well as individual potency is mostly dependent on fruitful reproduction (Hossain et al., 2017a). Subsequently, the knowledge of gonadosomatic index, maturation size and spawning period are considered as a key element for the biological researches in fishes (Hossain et al., 2012).
Lm of fish is important in exploring the causes of variations in the length of maturation (Templeman, 1987). In addition, maturation size of fishes is broadly used as a sign of minimum-allowable capture size (Lucifora et al., 1999, Hasan et al., 2021). Estimation of spawning/breeding season in fish is essential mainly for the conservation of adult or mature individuals from heavy fishing pressure (Templeman, 1987, Mawa et al., 2021). The condition factor indicates the level of wellness of the fish in their natural environment. It is an essential measure of different ecological and biological variables such as the degree of fitness, the production of gonads and the suitability of the atmosphere with respect to the feeding state (Mac Gregoer, 1959).
Fecundity is a vital element of fish biology that must be recognized to understand variations in population levels and to make attempts to raise the high amount of harvest capability. (Akter et al., 2007). The fecundity referred the total number of eggs that is produced by a female during the usual life-span. The number of ripening eggs in female ovaries before spawning can also be recognized (Shrivastava, 1999, Hossain et al., 2021). It must be known to assess the reproductive potentialities of a fish stock (Lagler, 1956) and to understand of reproductive strategy (Nazari et al., 2003).
The reproductive cycle is an equitable mechanism related to the environmentally regulated routine of organisms living in different aquatic environments. Therefore, the normal breeding mechanism of the fish community may be influenced by some unfavorable environmental factors and may eventually inhibit the process of recruiting into the stock by spawning and hampering the migration process. Environmental factors (e.g. rainfall, temperature) and changes in water hydrological parameters (e.g. Do, pH, and alkalinity) have effects on fishes especially in their ecology and growth (Lappalainen et al., 2008, Britton et al., 2013, Hossain et al., 2021). In addition, temperature is a fundamental physical regulator in fish life, and its influence is strongly embodied in the management of all reproductive processes, from gamete growth and maturation, ovulation and permeation, breeding, embryonic development and laying eggs, to larval and juvenile growth and survival (Pankhurst and Munday, 2011). Water temperature is also known as a major element of fish growth i.e. the growth rate of aquatic organism is reduced with the decrease of water temperature (Blanck and Lamouroux, 2007, Lappalainen et al., 2008).
Fisheries management is sometimes regarded as a reaction to 'common failure,' in which the lack of exclusive property rights means that fish are overfished and resources and labor are wasted (Wilson and McCay, 2001). Aquaculture, or water farming, is the rearing, breeding, and cultivation of fish, shellfish, and aquatic plants. It has been considered as an effective and environmental friendly form of production of food and commercial goods, ensures ecosystem sustainability and restores threatened or endangered organisms (NOAA, 2020).
H. fossilis has immense aquaculture potential and may easily be farmed in ponds and shallow ditches. The culture of this fish is yet to flourish in Bangladesh owing to a lack of effective culture technologies (Roy et al., 2019). Most of the Beels are dried out during half of pre-monsoon and post monsoon period due to the increasing temperature and decreasing rainfall. In addition, lacks of good quality seed is also an obstacle in the way of developing aquaculture of H. fossilis. For the profitable and sustainable fish farming, seed is very important. Fish seeds (fertilized eggs, spawns, fry and fingerlings) collected from open water bodies are preferred by the fish farmers over the seed produced in the hatchery.
There are a few studies conducted on H. fossilis reproduction (Table 1) including the study on reproductive biology (Talwar and Jhingran, 1991, Joy and Tharakan, 1999) and on fecundity (Bhatt et al., 1977). However to date no study explored the reproductive biology and its relation with eco-climatic factors for this important fish species. The present study was the first attempt that covered the key aspects of reproduction, including length at first sexual maturity and spawning seasons, and fecundity using various models considering the changes in climatic factors as well as the aquaculture, conservation and management aspects of H. fossilis in the wetland ecosystem of Bangladesh.
Table 1.
Available works on different aspects of Heteropneustes fossilis world wide.
| Aspects | Water body/Country | References |
|---|---|---|
| Biometrics relationships | ||
| Length-weight relationships | Ganga River | Khan et al. (2012) |
| length-weight relationship and conditions | Ramsar site, Assam, India | Das et al. (2015) |
| length-length, length-weight relationship and conditions | Nageshwari, Banglaesh | Ferdaushy and Alam (2015) |
| Length-weight relationships | Gajner beel floodplain | Hossain et al. (2017b) |
| length-weight relationship and conditions | Indus River, Pakistan | Muhammad et al. (2017) |
| length-weight relationship and conditions | Atrai and Brahmaputra Rivers | Islam et al. (2017) |
| Morphometrics and meristics | Gajner Beel | Rahman et al. (2019) |
| Reproduction | ||
| Spawning season | Bangladesh | Shafi and Quddus (1982) |
| Induced spawning of the Indian catfish | India | Joy and Tharakan (1999) |
| Fisheries of Bangladesh | Saud et al. (2015) | |
| Fecundity of the freshwater catfishes | India | Bhatt et al. (1977) |
| Freshwater fishes of Bangladesh | Bangladesh | Rahman (1989) |
| Fisheries of Bangladesh | Bangladesh | Shafi and Quddus (2001) |
| Gonado-somatic index and fecundity | Brahmaputra River, Assam, India | Saud et al. (2015) |
| Growth | ||
| Impact of eco-hydrological factor on growth | Gajner beel | Hasan et al. (2020) |
| Age and Growth | Tangail, River Brahmaputra | De Graaf (2003) |
| Population parameters | Medi beel, Netrokona District | Mustafa and De Graaf (2008) |
| Aquaculture and Hatchery | ||
| Water quality, growth and production performance | Bangladesh | Roy et al. (2019) |
| Effect of stocking density on growth, survival and production | Bangladesh | Monir and Rahman (2015) |
| Induced Breeding | Bangladesh | Rahman et al. (2013) |
| Induced spawning of catfish | India | Alok et al. (1993) |
| Biochemical impacts of salinity | Bangladesh | Ahmmed et al. (2017) |
| Culture potentials | Bangladesh | Rahman et al. (2014) |
| Effect of feeding frequency on surfacing activity | India | Marian et al. (1982) |
| Effects of dietary protein levels | India | Siddiqui and Khan (2009) |
| Comparative growth performance assessment | Bangladesh | Nushy et al. (2020) |
| Impacts of different diets on growth | Bangladesh | Ahmmed et al. (2016) |
| Optimum ration level for better growth | India | Khan and Abidi (2010) |
| Seed Production an Urgent Need | India | Haniffa et al. (2017) |
2. Materials and methods
2.1. Sampling site and fish measurement
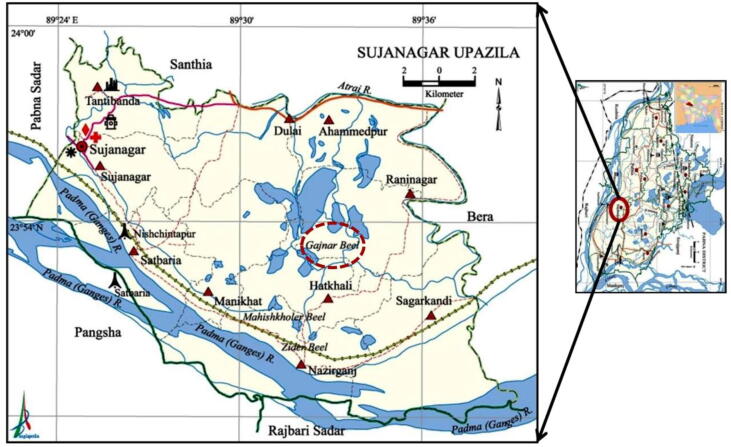
Monthly samplings were done from the Gajner Beel wetland ecosystems, northwestern Bangladesh during the January to December 2019 (Fig. 1). Cast net (1.5–2.5 cm, day time: 8.30 to 9.30 am) was used to collect the samples with the help of local fishers. Specimens were immediately preserved in ice and transported to the laboratory and preserved in a deep freezer until further analysis. Before taking measurements, each specimen was dipped with water, washed, and taken on a tray. Then lengths were measured (TL, and SL, to closest 0.1 cm) by a measuring board and total weight was taken (BW, to nearest 0.01 g) by an electronic balance. Sex identification was done through observing gonads under a microscope and by evaluating the meristic and morphometric characters of fishes. Length frequency distribution for H. fossilis was calculated individually with 1 cm intermissions of TL.
Fig. 1.
Sampling sites in a wetland ecosystem (Gajner Beel) (indicated by red circle), northwestern Bangladesh.
2.2. Size at sexual maturity (Lm)
The Lm was estimated using several functions (i) Based on maximum length, log (Lm) = -0.1189 + 0.9157* log (Lmax), (Binohlan and Froese, 2009). (ii) The relationship of TL vs. GSI, GSI (%) = (GW/BW) × 100. (Nikolsky, 1963) (iii) The alteration point of TL value from TL-SL statistics helped to estimate Lm using the equation of Huxley (1932): y = a + bx. A discrete linear regression was calculated for each subset of the data and arranged at 0.5 cm intervals of the break point above the total size frequency of females. The calculations were repeated several times. From the minimum residual sum of squares (RSS), the change was identified in the subsequent two subsets of data (Pankhurst and Munday, 2011, Ahamed and Ohtomi, 2014). Furthermore, the growth type was identified by regression analysis from untransformed TL-SL data. For this length-length relationship, significant discrepancy of b values from the hypothetical isometric value (b = 1) of growth was either positively (b > 1) or negatively allometric (b < 1) (Hartnoll, 1982). ANCOVA (analysis of covariance) was used to check for significant deviation in slopes and intercepts between the two subsets of data (Zar, 1984). (iv) A logistic curve was fitted to the maturity information by distributing the percentage of mature individuals (PMI) against TL class. PMI = 100/ [1 + exp{-f (TLm - TL50)}], where f refers to growth coefficient and TLm is the midpoint of every TL class (King, 2007). PMI never exceeds 100%, even though the biggest TL classes of all mature individuals in a population are simultaneously in a reproductive cycle. Thus, the data were adjusted to overcome an unnecessarily high estimation of TL50 according to the method of King (2007).
2.3. Spawning season
Spawning season was estimated using two methods (i) monthly changes in gonadosomatic Index (GSI) (Khatun et al., 2019) and (ii) seasonal progression of gonads (maturation stages of gonad) (Zhang et al., 2009). Gross physical (morphological) analysis of the ovary reported macroscopic determination of gonad maturity and seasonal progression of the gonad. Depending on the macroscopic features, the maturation stage was defined by the opacity of the gonads, the durability and vascularization and the overall coloring of the gonads (Shinkafi et al., 2011, Nath, 2013).
2.4. Condition and prey-predatory status
Fulton’s condition factor (Fulton, 1904) was estimated as a percentage, i.e., (KF) = 100*(W/L3). Relative weight (WR) was considered through the formula of Froese (2006): WR = (W/WS) × 100, WS = a*Lb
2.5. Fecundity
According to Murua et al. (2003) total fecundity (FT) was estimated by gravimetric method. The relationships within FT, TL and BW were done by, FT = m × (TL)n (non-linear) and FT = m + n × (BW) (linear regression).
2.6. Eco-climatic factors
Hydrological data were collected in every month from sampling station by HACH (HQ40d) digital multi-meter parameter. Monthly dissolved oxygen (mg/l), temperature (°C), pH, total dissolved solids (TDS) mg/l and total alkalinity (mg/l) were assessed. A specific sampling time (9:00–11:00 am) was maintained. Climatological data (rainfall and air temperature) were obtained from the meteorological station of Dhaka, Bangladesh.
2.7. Statistical analyses
All statistical studies were performed by Microsoft® Excel, past 4.03 and GraphPad Prism 6.5 software. Normality of data was confirmed by Shapiro-wilk test. The non-parametric Spearman-rank test examined the relationships between (a) eco-climatic information and GSI and (b) condition factors with GSI at a 5% (p < 0.05) significance level. The correlation analysis helped to discern the essential environmental features that induced egg maturity and spawning period.
3. Results
3.1. Population structure
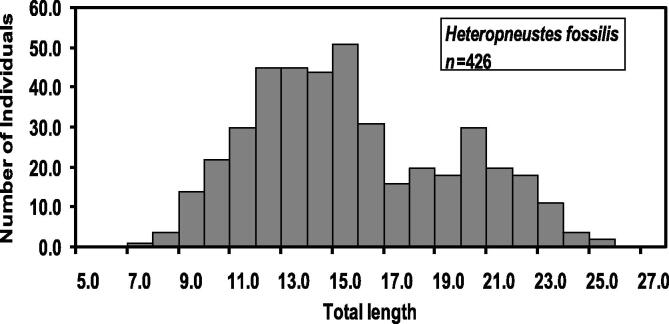
From a total of 845H. fossilis individuals, 426 female specimens were analyzed. The total length (TL) of the analyzed female fishes ranged between 6.70 and 24.10 cm TL and body weight (BW) ranged between 1.37 and 83.94 g. Weight of gonad was 0.01–25.90 g. Table 2 represented minimum, maximum, mean and 95% CL of TL, BW, and GW. Length-frequency distribution of H. fossilis is shown in Fig. 2.
Table 2.
Descriptive statistics on the lengths (cm), body weight (g) and gonad weight (g) measurements of Heteropneustes fossilis (Bloch, 1794) in the Gajner Beel of northwestern Bangladesh during January -December 2019.
| Characters | n | Min | Max | Mean ± SD | 95 %CI |
|---|---|---|---|---|---|
| Total length TL (cm) | 6.70 | 24.10 | 14.68 ± 3.88 | 14.31 – 15.05 | |
| Standard length SL (cm) | 426 | 6.00 | 21.80 | 13.18 ± 3.57 | 12.84 – 13.52 |
| Body weight BW (g) | 1.37 | 83.94 | 20.87 ± 17.50 | 19.20 – 22.54 | |
| Gonad weight GW (g) | 0.01 | 25.90 | 1.16 ± 3.09 | 0.87 – 1.46 |
n, sample size; Min, minimum; Max, maximum, SD, standard deviation; CI, confidence intervals.
Fig. 2.
Length-frequency distributions of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
3.2. Sexual maturity
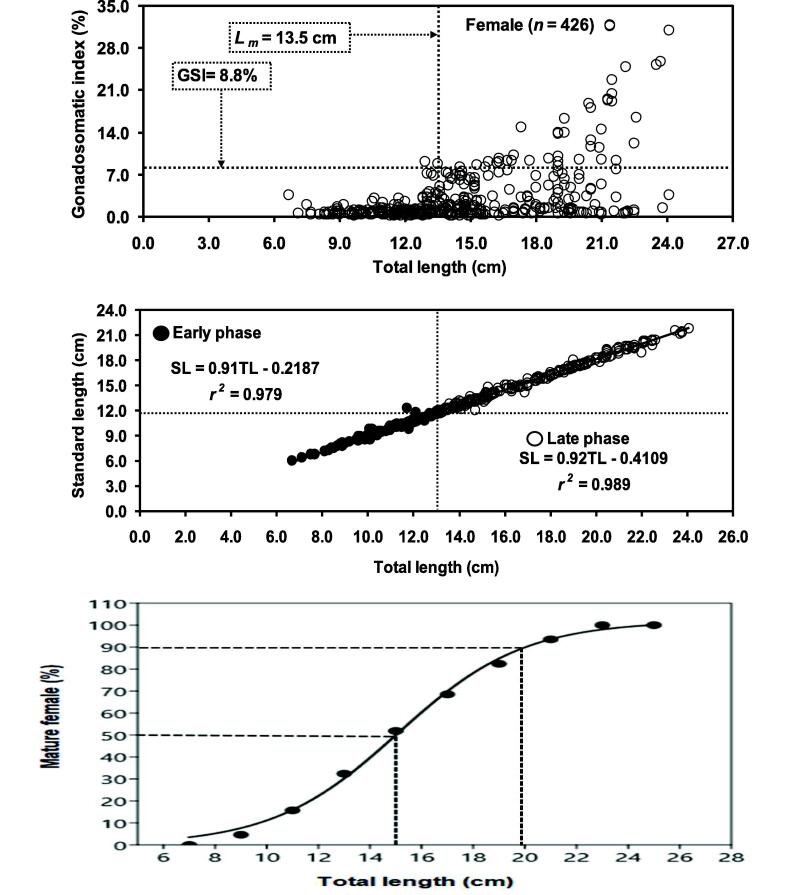
The calculated Lm of H. fossilis was 14.02 cm (95% CL = 10.99–17.84 cm) in TL based on maximum length. We also calculated the size at first sexual maturity (Lm) of H. fossilis from different worldwide water-bodies based on maximum length which was shown in Table 3. On TL vs. GSI relationship, we found the lowest and highest values of gonadal weight studied of the H. fossilis were 0.01 g and 25.90 g. The GSI was smaller than 13.50 cm TL was low (<8.80%). The GSI (>8.80%) around 13.50 cm in TL rose sharply for maximum of the female of H. fossilis. Therefore, Lm may consider being started from 13.50 cm in TL for H. fossilis in the Gajner Beel, northwestern Bangladesh. The relationship between TL vs. GSI of H. fossilis are given in Fig. 3. Spearman rank correlation test revealed significant correlation between TL vs. GSI (rs = 0.1172; p = 0.0155) indicating that GSI was dependent on body size. Through regression analysis at 1.0 cm intervals of total size range of females, a break point was found with an individual TL value, depending on the lowest total RSS value and comparative growth of TL-SL of H. fossilis as distributed into two phases (Table 4). The comparative growth rate of SL was estimated as 13.0 cm TL (Fig. 3). SL rose more promptly than TL after this size and indicated a negative allometric growth pattern (Table 4). ANCOVA showed significant difference in slopes (p = 0.01) above by below the alteration point for the observed regression (Table 5). In addition, the logistic equation of mature females for every length class (TL) group was expressed the relations between TL and the mature individuals’ percentage values (Fig. 3). We found that the smallest size of mature female was 12.0 cm and a TL of 15.0 cm corresponded to 50% of sexually mature H. fossilis females in Gajner Beel. Based on above four models, range of Lm was 13.50 to 15.00 cm of H. fossilis.
Table 3.
The calculated size at first sexual maturity (Lm) of Heteropneustes fossilis (Bloch, 1794) from worldwide different water-bodies based on maximum length.
| Water body | Max TL (cm) | Lm | 95% CI of Lm | References |
|---|---|---|---|---|
| Ganges River, India | 31.00 | 17.65 | 13.70 –22.64 | Khan et al. (2012) |
| Deepar Beel, Assam, India | 19.10 | 11.33 | 8.96 –14.31 | Das et al. (2015) |
| Nageshwari, Bangladesh | 15.50 | 9.36 | 7.47 –11.75 | Ferdaushy and Alam (2015) |
| Gajner Beel, Bangladesh | 16.50 | 9.91 | 7.89–12.46 | Hossain et al. (2017b) |
| Indus River, Pakistan | 13.00 | 7.96 | 6.40 –9.94 | Muhammad et al. (2017) |
| Atrai and Brahmaputra Rivers | 13.71 | 8.36 | 6.71–10.46 | Islam et al. (2017) |
| Gajner Beel, Bangladesh | 26.80 | 15.45 | 12.06–19.72 | Rahman et al. (2019) |
| Gajner Beel, Bangladesh | 24.10 | 14.02 | 10.99–17.84 | Present Study |
TL, Total length; Lm, Size at first sexual maturity; CI, confidence intervals.
Fig. 3.
(i) Relationship between gonadosomatic index (GSI) vs. total length (TL) (ii) total length vs.standard length and (iii) total length (TL) vs. adjusted percentage of Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
Table 4.
Estimated slope and intercept of linear equation representing the relationship between total length and standard length of female Heteropneustes fossilis in the Gajner Beel, Bangladesh and RSS in different sets of two phases.
| Break pointTL (cm) |
Early phase |
Late phase |
Total RSS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | n | RSS | Slope | Intercept | n | RSS | ||
| 7.0 | 0.69 | 1.4245 | 5 | 0.020 | 0.92 | −0.3184 | 421 | 24.379 | 24.399 |
| 8.0 | 0.89 | −0.0089 | 19 | 0.191 | 0.92 | −0.3278 | 407 | 24.226 | 24.417 |
| 9.0 | 0.89 | −0.0087 | 41 | 0.767 | 0.92 | −0.3420 | 385 | 23.613 | 24.380 |
| 10.0 | 0.88 | 0.0157 | 71 | 0.898 | 0.92 | −0.3403 | 355 | 23.469 | 24.367 |
| 11.0 | 0.90 | 0.1621 | 116 | 5.394 | 0.92 | −0.3724 | 310 | 18.976 | 24.370 |
| 12.0 | 0.89 | −0.0600 | 160 | 7.170 | 0.91 | −0.3151 | 265 | 17.103 | 24.273 |
| 13.0 | 0.91 | −0.2373 | 204 | 19.240 | 0.92 | −0.3876 | 221 | 4.645 | 23.885 |
| 14.0 | 0.91 | −0.2190 | 256 | 11.900 | 0.92 | −0.3548 | 170 | 12.495 | 24.395 |
| 15.0 | 0.92 | −0.2678 | 287 | 14.597 | 0.93 | −0.4620 | 139 | 9.818 | 24.415 |
| 16.0 | 0.91 | −0.2343 | 303 | 16.999 | 0.92 | −0.4006 | 123 | 7.383 | 24.382 |
| 17.0 | 0.91 | −0.2216 | 323 | 17.708 | 0.91 | −0.1385 | 103 | 6.578 | 24.286 |
| 18.0 | 0.91 | −0.2318 | 341 | 18.110 | 0.89 | 0.2007 | 85 | 6.060 | 24.170 |
| 19.0 | 0.91 | −0.2622 | 371 | 8.655 | 0.84 | 1.3603 | 55 | 15.754 | 24.409 |
| 20.0 | 0.92 | −0.3204 | 390 | 21.632 | 0.81 | 2.0120 | 35 | 2.489 | 24.121 |
| 21.0 | 0.92 | −0.3492 | 409 | 22.699 | 0.91 | −0.3122 | 17 | 1.514 | 24.213 |
| 22.0 | 0.92 | −0.3252 | 420 | 24.129 | 0.83 | 1.7024 | 6 | 0.245 | 24.374 |
n, Number of individuals; TL, Total length; RSS, Residual sum of square; SL, Standard length.
Table 5.
Allometric coefficients measured as slope for linear regressions (by least square estimate, with log-transformed data) between TL and SL of Heteropneustes fossilis.
| Relationship | n |
Allometric coefficient |
r2 | Significance level | ||
|---|---|---|---|---|---|---|
| b | 95 %CL of b | |||||
| TL vs. SL | Early phase | 179 | 0.91** | 0.88–0.92 | 0.979 | p = 0.01 |
| Late phase | 247 | 0.92** | 0.91–0.93 | 0.989 | ||
Allometric coefficient significantly (p = 0.05) different from 1.0, TL, Total length, SL, Standard length; n, Sample size; b, Slopes; 95% CI, Confidence interval, r2, Coefficient of determination.
3.3. Spawning season
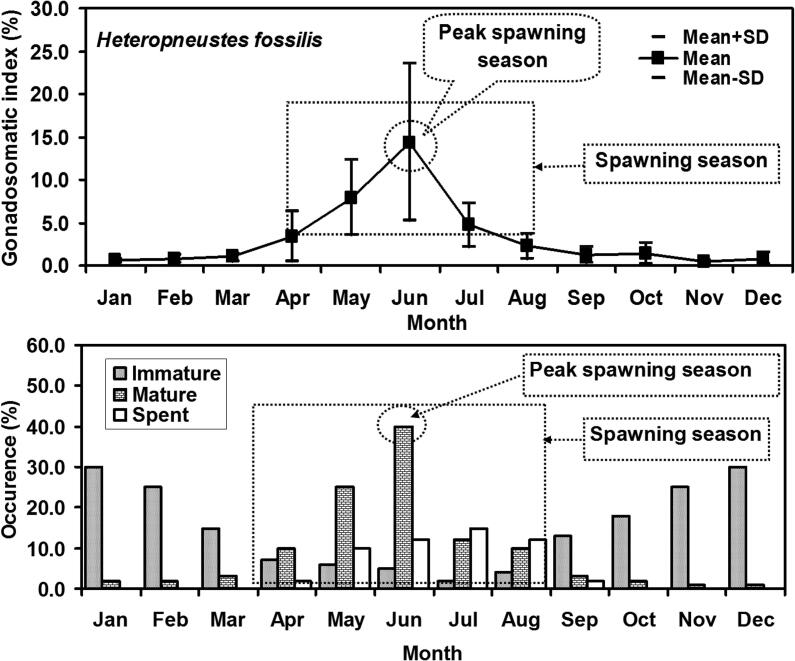
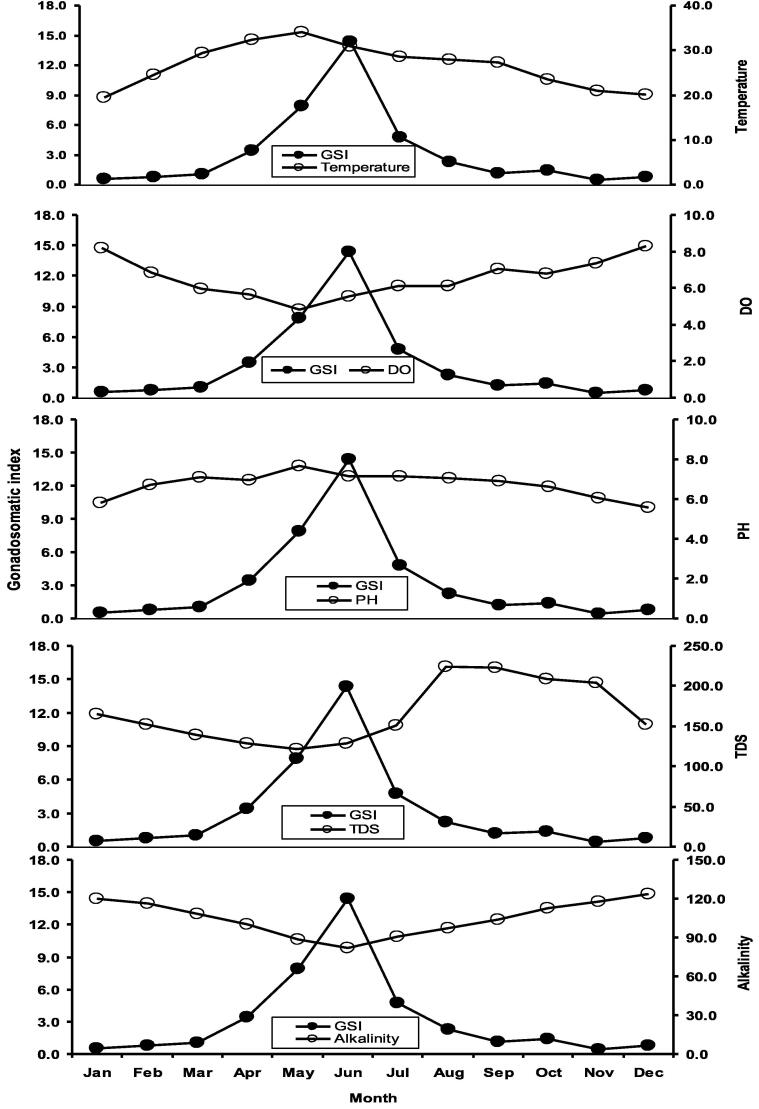
Monthly variations in GSI values are represented in Fig. 4. The ovary started to mature in March and continued until August. The highest GSI values were observed in April to August to define the full reproductive period of H. fossilis. In addition the highest GSI values were observed in the June that defines the peak season for spawning of H. fossilis.
Fig. 4.
Monthly variations of gonadosomatic index (GSI) and maturation stage based on colour with maximum and minimum values of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
Five maturity phases were identified from macroscopic investigation (Table 6). Temporal variation in gameto genic patterns of females has showed that the developed condition of gonads (Mature) was found in every month. The highest percentage of mature gonads was observed in June and the lowest was in November. Based on macroscopic features, fishes with gonads with mature stages were considered as spawning and peak spawning season of fishes (Fig. 4).
Table 6.
Macroscopic and description of gonad maturation stages of Heteropneustes fossilis in the Gajner Beel, northwestern Bangladesh.
| Stages | Brief description of gonad |
|---|---|
| Stage 1: Immature | Ovary cord like, ova invisible to the necked eye, thick membrane. |
| Stage 2: Maturing | Greenish, more than two and half quarter of body cavity, opaque ova, granular appearance, lobular. |
| Stage 3: Mature | Green, large occupies all the body cavity, delicate ovary membrane. Ova flow out with gentle pressure. |
| Stage 4: Ripe | Deep green to blackish, large occupies all the body cavity, delicate ovary membrane. Ova flow out with gentle pressure. |
| Stage 5: Spent | Flaccid empty sac, reddish bloody with many tiny opaque green residual ova. |
3.4. Condition factors
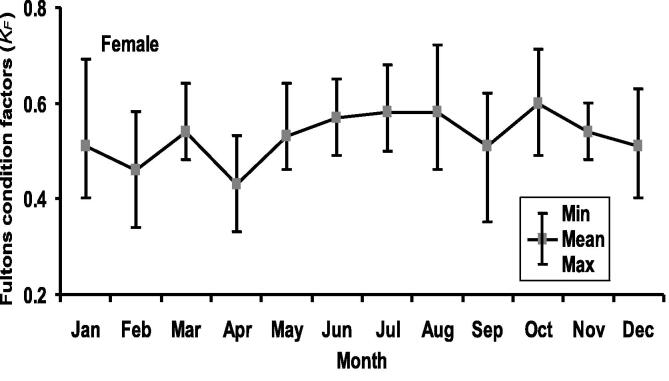
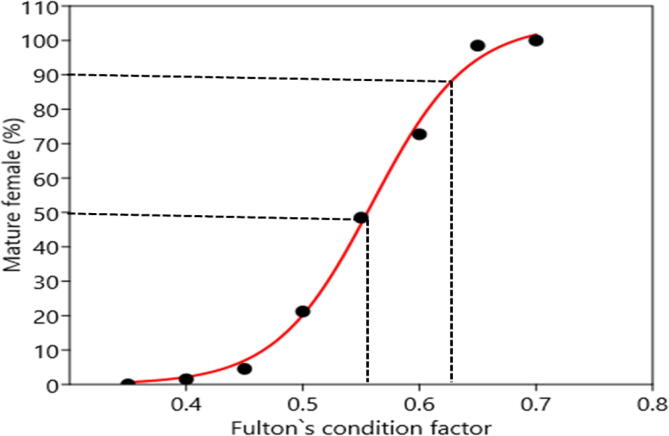
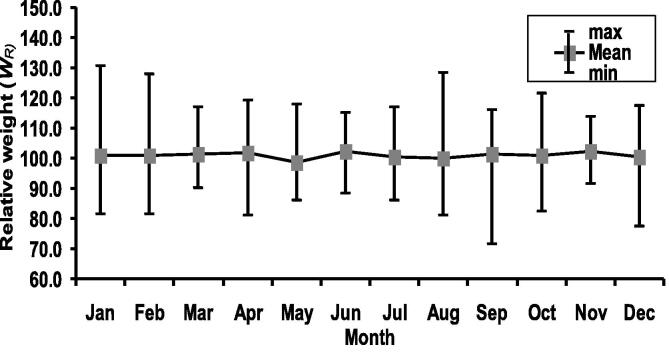
The lowermost and uppermost KF values for females were recorded in April (0.33) and August (0.72), respectively, with an average 0.53 ± 0.07 (Fig. 5). Conferring to Spearman rank test, KF and TL were significantly correlated for female H. fossilis (rs = 0.2116; p < 0.0001). Further, KF revealed significant correlation with GSI values (rs = 0.2495; < 0.0001). Half of the female H. fossilis spawned when KF was 0.55 (Fig. 6). The calculated lowest value of WR for females was 71.44 (Mean ± SD, 101.35 ± 11.05) in September and the highest was 130.51 (101.11 ± 8.6) in January (Fig. 7). Wilkoxon sign ranked test showed significant differences from 100 for females (p = 0.0122).
Fig. 5.
Monthly variations of Fulton’s condition factor (KF) of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
Fig. 6.
Adjusted percentage of mature females of Heteropneustes fossilis versus condition factor showing the logistic curve fitted to the data.
Fig. 7.
Monthly variations of relative weight (WR) of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
3.5. Fecundity
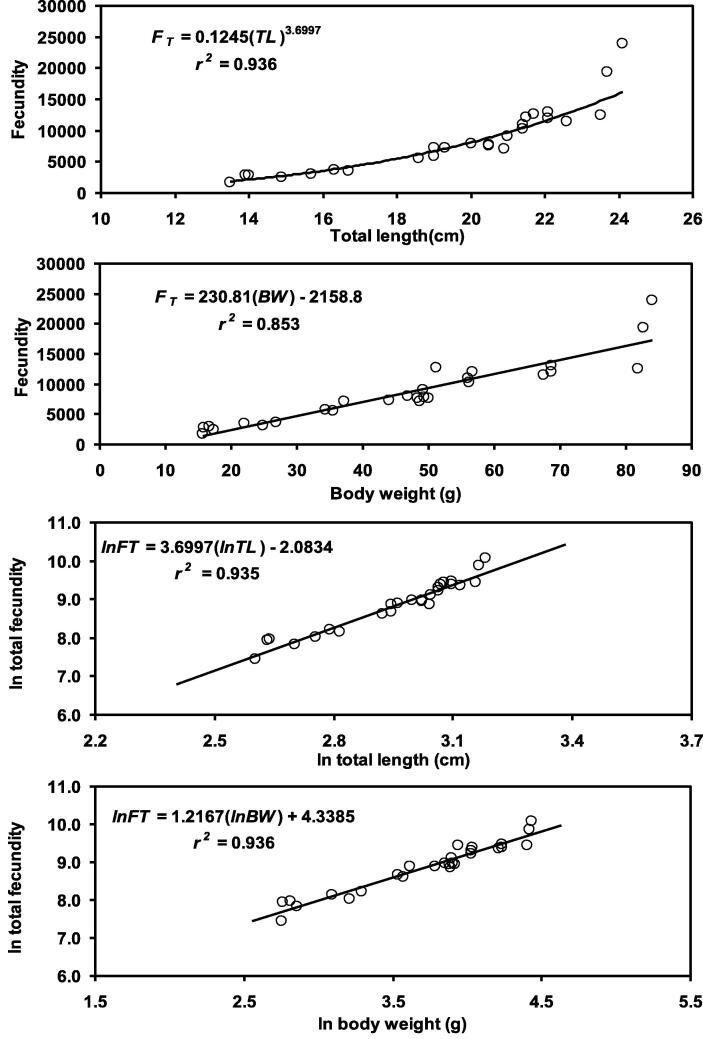
The mean total fecundity was 11897.78 ± 5027.28 and ranged between 5942 and 49852. Significant correlation was observed between TL and FT (rs = 0.967 and p < 0.0001) and between BW and FT (rs = 0.923 and p < 0.0001). Significant linear relationships were also found for natural log (ln) transferred TL vs. FT (rs = 0.967 and p < 0.0001) and BW vs. FT (rs = 0.965 and p < 0.0001). The relationships were shown in Fig. 8.
Fig. 8.
Relationships between TL vs. FT and BW vs. FT and ln (TL) vs. ln (FT) and ln (BW) vs. ln (FT) of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
3.6. Eco-climatic factors
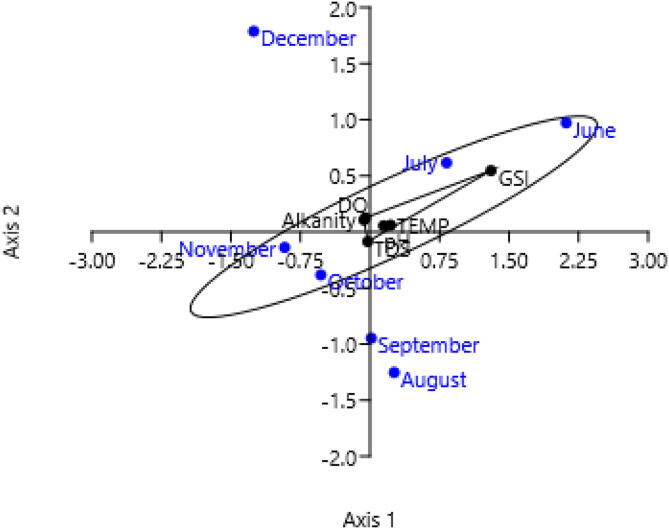
In our study, several environmental parameters were inspected for their influences on gonadal development and spawning of H. fossilis. Temperature, total alkalinity, pH and dissolved oxygen were found to be correlated with GSI (Table 7). The relationships between GSI and water quality factors are shown in Fig. 9, Fig. 10.
Table 7.
Relationship between hydrological parameters with GSI of Heteropneustes fossilis (Bloch, 1794) in the Gajner Beel of northwestern Bangladesh during January to December 2019.
| Relationship | rs value | 95% CL of rs | p values | Significance |
|---|---|---|---|---|
| Temperature vs. GSI | 0.8182 | 0.4450 to 0.9492 | 0.0019 | * |
| DO vs. GSI | −0.8392 | −0.9555 to −0.4972 | 0.0011 | * |
| pH vs. GSI | 0.8266 | 0.4657 to 0.9518 | 0.0015 | * |
| TDS vs. GSI | −0.5068 | −0.8351 to 0.1399 | 0.1075 | ns |
| Total alkalinity vs. GSI | −0.9231 | −0.9794 to −0.7337 | <0.0001 | * |
GSI, Gonadosomatic index; DO, Dissolved Oxygen; TDS, Total Dissolved Solids; r, Spearman rank correlation values; CL, confidence limit; p, shows the level of significance; ns, not significant;
significant (p ≤ 0.05).
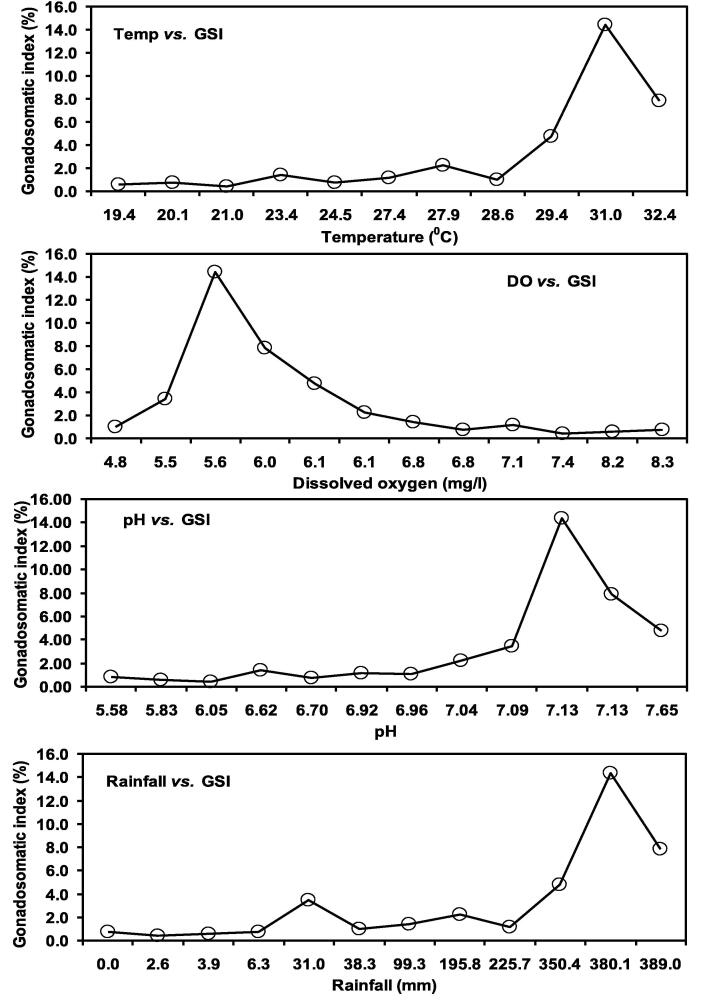
Fig. 9.
Relationship between gonadosomatic index (GSI) and different hydrological parameters of female Heteropneustes fossilis in the Gajner Beel, NW Bangladesh.
Fig. 10.
Correlation of environmental parameters and gonadosomatic index (GSI) of Heteropneustes fossilis in Gajner Beel, northwestern Bangladesh.
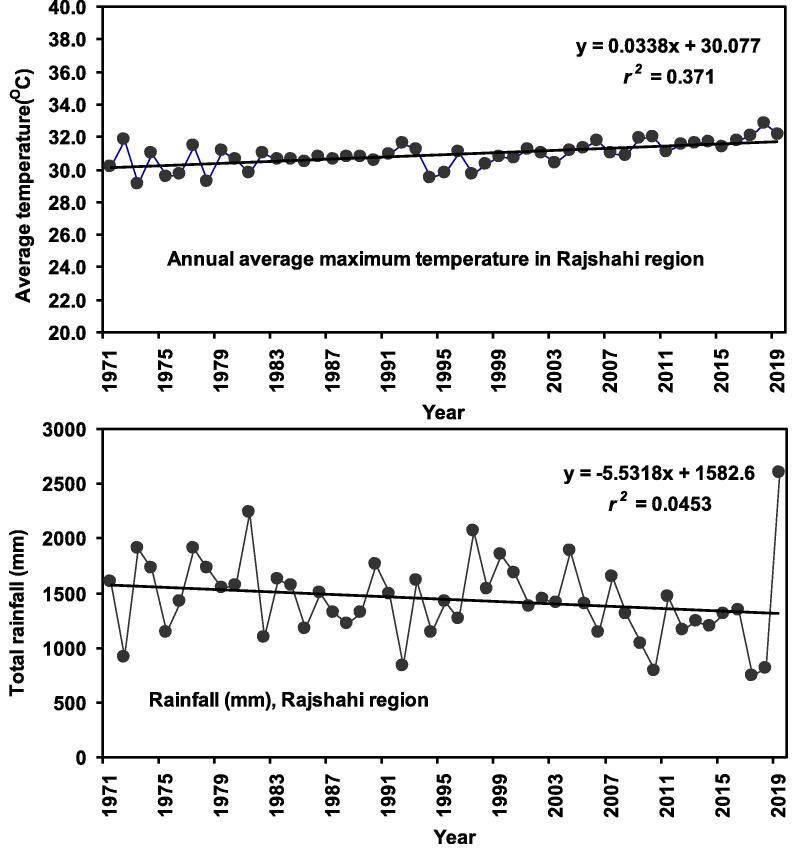
The optimal hydrological and physicochemical factors for the spawning of H. fossilis as observed in the current study are – temperature − 29 to 31 °C, rainfall − 350 to 380 mm, dissolve oxygen − 5 to 6 mg/l and pH − 7.1 to 7.5 (Fig. 11). A lengthy time series data over five decades (1971 to 2019) on air temperature and rainfall were evaluated to assess its changing temporal pattern. In the study area, annual air temperature revealed a rising trend of 0.029 °C/year, with the coefficient of determination value (r2 = 0.371) whereas the annual average rainfall indicated a dropping trend of 2.98 mm/year (r2 = 0.045) Fig. 12).
Fig. 11.
Determination of suitable eco-hydrological parameters by the relationship between temperature, DO, pH and rainfall vs. GSI of Heteropneustes fossilis in Gajner Beel, northwestern Bangladesh.
Fig. 12.
Annual average maximum temperature (oC) and rainfall (mm) in the Rajshahi region (including Gajner Beel) northwestern Bangladesh during 1971 to 2019.
3.7. Seed production and farming for H. Fossilis were reviewed from the existing literatures
The seeds of H. fossilis should be collected after two to three months of peak spawning season. Based on SGR (%), survival (%) and production, the maximum stocking density of H. fossilis in the culture systems should not exceed 125,000/ha for monoculture and 24,000/ha for polyculture with Indian major carp and other farmed fishes (Table 8). The feeding rates and feeding frequency should be 12–5% (from nursery phase to grow-out, rate gradually decreases as the individual weight increases) and twice/day, respectively, to optimize the production and cost of production for the species. Dietary protein should be 40% and optimum ration level should remain 5% BW day−1 (Table 8). The suitable range of temperature was 27.4–29.8 °C, pH 6.8–7.8, dissolved oxygen 6.8 to 8.6 mg/l, ammonia (NH3) < 0.1 mg/l for good aquaculture.
Table 8.
Accumulated the optimum parameters from different available works of Heteropneustes fossilis on aquaculture aspect.
| Aspects |
Comparison parameter |
Reference | |||
|---|---|---|---|---|---|
| Stocking density | Quantity | SGR% | Survival% | Production (Mean) | |
| 1,85,000/ha | 1.23 ± 0.021 | 71.61 ± 3.17 | 9708.16 ± 421.40 | Rahman et al. (2014) | |
| 1, 25,000/ha* | 2.36 ± 0.16 | 87 | 4537.12 ± 227.4 | Roy et al. (2019) | |
| 24000/ha (Poly culture) | 1.29 ± 0.02 | 81.78 ± 0.65 | 693.42 ± 3.08 | Hossain et al. (2018) | |
| Feeding | Feeding rate | Feeding frequency | SGR (%) | FCR | |
| 12–4% | Twice/day | 1.23 ± 0.021 | 2.51 ± 0.04 | Rahman et al. (2014) | |
| 12–5%* | Twice/day* | 1.23 ± 0.04 | 1.60 ± 0.01 | Nushy et al. (2020) | |
| Protein level | DP (%) | SGR (%) | PER | PRE (%) | |
| 40* | 1.76 | 1.75 | 31.7% | Siddiqui and Khan (2009) | |
| Optimum ration level | Ration level (BW day−1) | (FCR) | PRE (%) | ERE (%) | |
| 5* | 1.59 ± 0.03 | 30.8 ± 0.03 | 59.73 ± 0.24 | Khan and Abidi (2010) | |
| Induced breeding | Ovaprim | Time | Fertilization % | Hatching rate % | |
| 0.5 ml/kg | 10 | – | 82.48 | Vijaykumar et al. (1998) | |
| 0.4 ml/kg | 10- | ||||
| 12 | – | 96 | Nayak et al. (2001) | ||
| 0.5 ml/kg | 18–24 | 75.0 | 60 | Haniffa and Sridhar (2002) | |
| 0.5 ml/kg | – | 92.33 | 94.87 | Karl Marx and Chakrabarty (2007) | |
| 0.6 ml/kg | 10 | 86.67 | 76.92 | Rahman et al. (2013) | |
| 0.7 ml/kg* | – | 93.6 | 95.5 | Arcockiaraj et al. (2001) | |
Indicated the best. DP, Dietary protein; SGR, Specific growth rate; PER, Protein efficiency ratio; PRE, Protein retention efficiency; FCR, feed conversion ratio; PRE, Protein retention efficiency; ERE, Energy retention efficiency.
A healthy broods produced a healthy offspring. In the hatchery good quality of broods are insufficient so we can easily collect the broods from the spawning season. It’s also help to avoid the cross breeding in hatchery. According to survey results all researchers are agreed that ovaprim are the best hormone for induced breeding of H. fossilis than other hormones. But different researcher used different dose of ovaprim among them we recommend 0.7 ml/kg body weight is best dose for induced breeding of H. fossilis fish based on highest fertilizing and hatching rate (Table 8). Reproductive performance, development, feed efficiency and feed intake of fish, normally regulated by a few environmental variables. Here we detected optimum range of some environmental factors (temp was 29 to 31 °C, DO was 5.0 to 6.0 mg/l, and pH was 7.1 to 7.5) which can be applying in hatchery for artificial breeding and also can be used in nursery pond for better growth of fingerlings. Mixed diet (commercial feed and snail meat) is the best for highest growth of H. fossilis larvae.
4. Discussion
The study exposes the reproductive feature (sexual maturity, spawning season and fecundity) condition, prey predator status and described the relation within reproductive features and eco-climatic factors. It also deals with the optimum range of eco-climatic factors in relation to reproduction of H. fossilis using multiple indices. As a widely used tool for fisheries biologists and managers, careful estimation of the maturity status of fish, helps to ensure proper assessment and management of exploited stocks (Rahman et al., 2018). Nevertheless, the size at sexual maturity and spawning season of fish were estimated by several models including brooding of eggs over time, appearance of ovary and maturation stages over time (King, 2007), relative weight of gonad (TL vs. gonadosomatic index, modified gonadosomatic index and dobriyal index) over time (Hossain et al., 2017a, Ahamed et al., 2018, Khatun et al., 2019) and histological analyses (Chelemal et al., 2009, Lucano-Ramírez et al., 2019, Jan and Ahmed, 2019). Though histological analyses of gonads is the best way of assessing maturity, where the facilities are lacking, other biotic parameters can easily be measured as indicators of maturity status, (West, 1990, Lowerre-Barbieri et al., 2011, Khatun et al., 2019). GSI was used effectively by a good number of researchers to assess the maturity status of fish (Fontoura et al., 2009, Khatun et al., 2019). A significant population parameter in the fisheries management of exploited stocks is the size at maturity (Jennings et al., 2001). It is vital for fisheries biologists to manage and conserve a particular fish population in any aquatic ecosystem (Lucifora et al., 1999). In our study, the size at first sexual maturity for H. fossilis was estimated by four models. On the basis of observed maximum length Lm, which was 14.02 cm. TL vs. GSI relationship also provides an efficient maturity assessment (Ahamed et al., 2018, Hossen et al., 2019). Results of our study estimated Lm of female H. fossilis as 13.5 cm TL based on GSI in the Gajner Beel. Several studies have described the comparative growth of TL and SL, wherever an unexpected intensification in one of these length sections is indicative of sexual development (Yamada et al., 2007, Khatun et al., 2019). The comparative growth frequency of SL changed at 13.0 cm TL in our study which may suggest gonadal maturity. Based on the comparative growth of different body parts, the variation point could generally represent sexual maturity (Gab-Alla et al., 1990, Cha et al., 2004). Finally, we used a logistic model to assess Lm of H. fossilis. GSI information for a logistic function is more appropriate for fish species with an unrestricted sampling program (Fontoura et al., 2009). The estimated Lm in our study was 15.0 cm for female H. fossilis through the logistic equation. Talwar and Jhingran (1991) reported that Lm of H. fossilis was 12 cm, however, the results of our study revealed the Lm for the species ranged between 13 and 15 cm. This dissimilarity can be attributed to sampling (variation in sample sizes or shrinking body size with formalin preservation) or ecologic differences in population densities, food availability or water temperature (Khatun et al., 2019). This study provides comprehensive information about the length at first sexual maturity of H. fossilis by four reliable models. The finding can be useful in selecting mesh size of nets and avoid catching smaller fish thus allowing them to spawn (Khatun et al., 2019).
Spawning season is crucial to assess spawning time and migration of a fish population for breeding purpose (Wilding et al., 2000, Khatun et al., 2019). The maximum values of GSI as an indication of spawning season were determined during April to August in our study. Besides, highest GSI values were observed in June that suggests peak spawning for H. fossilis in the Gajner Beel. We found same results, based on monthly changes of maturation stages. A number of other studies observed the spawning season of H. fossilis in different months - May and August (Shafi and Quddus, 1982), July to August (Joy and Tharakan, 1999) and April to May and peak in April (Saud et al., 2015). Variation among studies may be ascribed to geographic area, fish population densities, environmental parameters (especially water temperature and rainfall), and/or food availability.
Condition factor as an indicator of success of development, reproduction and survival is a quantitative parameter of the well-being of fish that assesses the performance of the present and future populations (Richter, 2007). Fulton's condition factor (KF) is an index illustrating correlations between biotic and abiotic factors in fish physiological conditions. It indicates the well-being of the population at different points of the development cycle (Angelescu et al., 1958). In the current study, KF ranged from 0.33 to 0.72 for female H. fossilis. Ferdaushy and Alam, 2015, Muhammad et al., 2017 reported that the KF of this species was 0.96 and 0.48, respectively. In our study, KF was found to be significantly related with monthly GSI values. The study revealed that the spawning period of H. fossilis began in April with a peak in June and ended in August when the upper values of KF represented their recovery in the Gajner Beel, NW Bangladesh.
Relative weight (WR) is the most widely used index for determining the status of fish in the ecosystem (Froese, 2006). WR values below 100 for individuals or populations suggest problems such as low prey accessibility or high predator density, whereas values above 100 indicate a prey surplus or low predator density (Rypel and Richter, 2008). In our study, monthly deviation of WR from 100 in female H. fossilis populations revealed the disparity in habitat regarding the abundance of food items and the presence of prey and predator organisms (Anderson and Neumann, 1996) in Gajner Beel.
The estimation of the fecundity and explanation of reproductive strategies is essential in fish biology, physiology and population dynamics (Hunter et al., 1992). Fecundity in fishes is a crucial factor to comprehend differences in population size and is very pertinent to fishery management (Rahman et al., 2018, Hossain et al., 2021). In the present study, fecundity of H. fossilis was 1730 to 23,870 in the Gajner Beel, NW Bangladesh. In the past, varying numbers of fecundity for this fish were observed by different authors − 1375 to 46,737 (Bhatt et al., 1977), 4200 to 15,750 (Rahman, 1989), 2,843 to 44,724 (Shafi and Quddus, 2001) and 2431 to 23,002 (Saud et al., 2015). The variations in fecundity occurred due to difference in habitat conditions and presence of nutrient and physicochemical factors within, stock variations (Ruzzante et al., 1998) and differences in methodological approaches used for fecundity assessment (Alonso-Fernández (2009)).
Global climate change is impacting the fish and fisheries and will continue to do so in the future (Roessig et al., 2004). Most of the studies into the impact of climate change on fish populations have been carried out on pelagic fishes (Loukos et al., 2003, Lloret et al., 2004). Environmental conditions like temperature, DO, pH and total alkalinity affect reproductive migrations of H. fossilis in the Gajner Beel wetland ecosystem. The maximum GSI values of females were from March to August, when water temperatures were highest, so GSI may be reacting to water conditions as seen for tropical weather fishes in general (Khatun et al., 2019). The impact of temperature in regulating gonadal development and fish reproduction is corporate by various studies (Ridha et al., 1998, Rideout et al., 2005, Khatun et al., 2019). Hence, alterations in peak spawning behavior may result from increasing global temperatures (Peer and Miller, 2014, Khatun et al., 2019). According to our findings, the lowest temperatures were found in January, and the highest was in June suggesting a substantial connection between water temperature and spawning season. The GSI values were high in April to July when temperatures were 29 to 31 °C. So, 29–31 °C may be best for reproduction. We also found DO, pH and total alkalinity ranges of 5.0–6.0 mg/l, 7.1–7.5 and 90–100 mg/l respectively, from April to July when GSI high. So, these ranges should be optimum for reproduction of H. fossilis. No other studies are available for H. fossilis to relate to our findings. In contrast, the lowest GSI was found at a water temperature of 25 °C. Primary sex cell and segregation of female gonads stimulated by lower temperature (<20 °C) though Chmilevskiy and Lavrova (1990) is dissimilar to our results. Based on climate data (1971–2019) a thermal increase of 0.029 °C yr−1 temperature is apparent in northwestern Bangladesh. The start of sexual maturity is controlled by environmental and climate driven conditions. Spawning events are normally associated with climatic variability, particularly for rainfall and temperature (Parkinson et al., 1999, Wilding et al., 2000, Khatun et al., 2019, Sabbir et al., 2021). Usually, the annual rainfall sequence of the seasonal tropics is linked to the timing of spawning for Southeast Asian cyprinids (Rainboth, 1991). The maximum rainfall was perceived in April to July in our study, when GSI value was at apex suggesting a connection between GSI and rainfall. The maximum rainfall was reported in June (380 mm) when GSI was highest. GSI was also lowest when rainfall was lowest in January, when no reproduction was occurring. Fluctuation in the spawning season for H. fossilis is predicted by exploration of climate data (1971–2019) which showed that rainfall is decreasing by 2.96 mm/year in northwestern Bangladesh. Absence of information prevents comparisons of our findings. But for a temperate freshwater teleost, Parkinson et al. (1999) reported that the high rainfall and temperature influenced final maturation of gonads and spawning season which is similar to our findings. Also increase of air temperature has impact on ecosystem and catfish fishery such as changes in ecosystem, blockage of migratory route and high turbidity of water (Khatun et al., 2019, Sarkar et al., 2020).
We suggest that stocking density 1, 25,000/ha is the best for H. fossilis whereas Rahman et al. (2014) suggested 1, 85,000/ha. We found 0.7 ml/kg ovaprim is the optimum for induced breeding of H. fossilis. According to Nayak et al. (2001) the best dose is 0.4 ml/kg. Vijaykumar et al., 1998, Haniffa and Sridhar, 2002, Karl Marx and Chakrabarty, 2007 revealed 0.5 ml/kg is the best dose for H. fossilis induced breeding.
5. Management policies
The management of fisheries is aimed at optimizing the advantages of the unit of output (fish stock) that is being managed. Based on our study outcomes, some management policies are suggested here for the sustainable management of H. fossilis in the wetlands of Bangladesh and in the region. For instance, the Lm was 13.0 to 15.0 cm of H. fossilis in the Gajner Beel, NW Bangladesh that’s mean 50% fishes spawn at this length, so smaller sized fishes than the Lm are strongly prohibited to catch and only bigger than 15.0 cm fishes are recommended for exploitation. Banned period should be established based on peak spawning season. In that time fishing would be strictly prohibited. The suitable range of KF (0.33–0.72) should be maintained in hatchery for artificial breeding of this species. Warming temperatures may be causing earlier river flow pulses which may be responsible for the changes in breeding pattern of fishes (Sarkar et al., 2019). We found that temperature was increasing and rainfall was decreasing day by day with an obvious impact on the shifting of the spawning season of H. fossilis so, short term management policies should be established for the management and conservation of the wild stock of this species.
6. Conservation strategies
To protect the diversity of catfishes, numerous measures have been implemented, including in situ conservation, habitat fingerprinting, ex-situ conservation, and the development of live gene banks (Sarkar et al., 2012). We should also restore wetlands because they are an important aspect of the riverine ecosystem, and fish survival is largely dependent on the quality of water bodies. Due to extensive siltation, several wetlands have already lost their link to the main waterway. Fishes use wetlands as breeding and rearing grounds so, research efforts should be translated into action in order to restore those crucial habitats.
7. Conclusion
The air-breathing stinging catfish (H. fossilis) is considered to be highly nourishing, palatable and tasty and well preferred because of its less spine, less fat and high digestibility in many parts of Indian subcontinent. Attempt has been taken to describe the changes in reproductive biology, relation to eco-climatic factors and suggesting a policy for aquaculture, conservation and management of H. fossilis. In fine, to conserve the wild stock of H. fossilis and to ensure the effective and sustainable management of this important fish, it is essential to set the mesh size of nets and establishment of ban period during peak spawning season through the Lm and spawning season, correspondingly. This is vital for the protection of wild stock of H. fossilis in the Gajner Beel and other open water bodies. H. fossilis seeds can be collected from the natural water bodies, after 2–3 months followed by the peak spawning in June and should be farmed following suggested stocking density, feed, feeding rate, feeding frequency and protein levels ensuring good aquaculture practices. H. fossilis broods can be collected during the spawning season and artificially breed in the hatchery using recommended doses of hormone. Analyses of time series data on temperature and rainfall revealed a rising trend of temperature and declining drift in rainfall. These eco-climatic factors play key role in the onset of spawning and entire lifecycle thereafter and are responsible for the shift in the breeding season including spawning peak. Any management policy and conservation guideline such as ban period, gear selectivity, amending or proposing new mesh size of nets – must take into account the impact of the eco-climatic factors to pave the way of successful management of H. fossilis in the wetland ecosystem.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was carried out with the financial assistance from the Bangladesh Agriculture Research Council (ID: 484) and National Science and Technology, Bangladesh.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahamed F., Ohtomi J. Relative growth and sexual maturity of the pandalid shrimp Plesionika izumiae (Decapoda, Caridea) in Kagoshima Bay, Southern Japan. Crustaceana. 2014;87:1567–1577. [Google Scholar]
- Ahamed, F., Saha, N., Ahmed, Z.F., Hossain, M.Y., Ohtomi, J., 2018. Reproductive biology of Apocryptes bato (Gobiidae) in the Payra River, southern Bangladesh. J. Appl. Ichthyol. 3, 1169–1175.
- Ahmmed M.K., Ahmmed F., Kabir K.A., Faisal M., Ahmed S.I., Ahsan M.N. Biochemical impacts of salinity on the catfish, Heteropneustes fossilis (Bloch, 1794), and possibility of their farming at low saline water. Aquac. Res. 2017;48:4251–4261. [Google Scholar]
- Ahmmed M.K., Ghosh S.K., Sarker J., Islam M.M., Ahsan M.N. Impacts of different diets on growth and survival of stinging catfish, Heteropneustes fossilis Bangladesh. J. Vet. Anim. Sci. 2016;4:22–26. [Google Scholar]
- Akter M.A., Hossain M.D., Hossain M.K., Afza R., Bhuyian A.S. The fecundity of Hilsa ilisha from the River Padma near Godagari of Rajshahi district. Rajshahi Univ. J. Zool. 2007;26:41–44. [Google Scholar]
- Alok D., Krishnan T., Talwar G.P., Garg L.C. Induced spawning of catfish, Heteropneustes fossilis (Bloch), using D-Lys6 salmon gonadotropin-releasing hormone analog. Aquacult. 1993;115:159–167. [Google Scholar]
- Alonso-Fernández, A., Vallejo, A.C., Saborido-Rey, F., Murua, H., Trippel, E.A., 2009. Fecundity estimation of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) of Georges bank: Application of the autodiametric method. Fish. Res. 99, 47–54.
- Anderson R.O., Neumann R.M. In: In fisheries techniques. 2nd ed. Murphy B.R., Willis W.D., editors. American fisheries society Bethesda; Maryland: 1996. Length, weight and associated structure indices; pp. 447–482. [Google Scholar]
- Angelescu V., Gneri F.S., Nani A. La merluza del mar argentine (biologia e taxonomia) Secretaria de marina servicio de hidrografia naval. 1958:1–224. [Google Scholar]
- Arcockiaraj, A.J., Haniffa, M.A., Perumalsamy, P.R.R., Marimuthu, K., Muruganandam, M., 2001. Induced spawning of the freshwater catfish singhi Heteropneustes fossilis using non-piscine pituitary extracts. Aquacult. 2, 5–8.
- Bhatt V.S., Dalal S.G., Abidi S.A.H. Fecundity of the freshwater catfishes Mystus seenghala (Sykes), Mystus cavasius (Ham), Wallagonia attu (Bloch) and Hetereopneustes fossilis (Bloch) from the plains of northern India. Hydrobiol. 1977;54:219–224. [Google Scholar]
- Binohlan C., Froese R. Empirical equations for estimating maximum length from length at first maturity. J. Appl. Ichthyol. 2009;25:611–613. [Google Scholar]
- Blanck A., Lamouroux N. Large-scale intraspecific variation in life-history traits of European freshwater fish. J. Biogeogr. 2007;34:862–875. [Google Scholar]
- Brewer S.K., Rabeni C.F., Papoulias D.M. Comparing histology and gonadosomatic index for determining spawning condition of small-bodied riverine fishes. Ecol. Freshw. Fish. 2008;17:54–58. [Google Scholar]
- Britton J.R., Davies G.D., Pegg J. Spatial variation in the somatic growth rates of European barbel Barbus barbus: a UK perspective. Ecol. Freshw. Fish. 2013;22:21–29. [Google Scholar]
- Cha H.K., Oh C.W., Choi J.H. Biology of the cocktail shrimp, Trachysalambria curvirostris (Decapoda: Penaeidae) in the Yellow Sea of Korea. J. Mar. Biol. Associ. U.K. 2004;84:351–357. [Google Scholar]
- Chmilevskiy D.A., Lavrova T.V. The influence of temperature on oogenesis in Tila-pia, Oreochromis mossambicus. J. Ichthyol. 1990;30:14–24. [Google Scholar]
- Chelemal M., Jamili S., Sharifpour I. Reproductive biology and histological studies in Abu Mullet, Liza abu in the water of the Khuzestan province. J. Fish. Aquat. Sci. 2009;4:1–11. [Google Scholar]
- Das P., Rahman W., Talukdar K., Deka P. Length-weight relationship and relative condition factor of Heteropneustes fossilis (Bloch) of Deepar Beel, a Ramsar site of Assam. India. Int. J. Appl. Res. 2015;12:1024–1027. [Google Scholar]
- De Graaf G. The flood pulse and growth of floodplain fish in Bangladesh. Fish. Manag. Ecol. 2003;10:241–247. [Google Scholar]
- Ferdaushy M.H., Alam M.M. Length-length and length-weight relationships and condition factor of nine freshwater fish species of Nageshwari. Bangladesh. Int. J. Aquat. Biol. 2015;3:149–154. [Google Scholar]
- Fontoura N.F., Braun A.S., Milani P.C.C. Estimating size at first maturity (L50) from gonadosomatic index (GSI) data. Neotrop. Ichthyol. 2009;7:217–222. [Google Scholar]
- Froese R. Cube law, condition factor and weight length relationship. History meta-analysis and recommendations. J. Appl. Ichthyol. 2006;22:241–253. [Google Scholar]
- Fulton, T.W., 1904. The rate of growth of fishes, 22nd annual report, part III. Edinburg: fisheries board of Scotland.
- Gab-Alla A.F., Hartnoll R.G., Ghobashy A.F., Mohammed S.Z. Biology of Penaeid prawns in the Suez Canal lakes. Mar. Biol. 1990;107:417–426. [Google Scholar]
- Grandcourt E.M., Al-Abdessalaam T.Z., Francis F., Al-Shamsi A.T., Hartmann S.A. Reproductive biology and implications for management of the orange-spotted grouper Epinephelus coioides in the southern Arabian Gulf. J. Fish. Biol. 2009;74:820–841. doi: 10.1111/j.1095-8649.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- Haniffa M.A., Jafar S.S., Bhat A.A. Seed production an urgent need for singhi (Heteropneustes fossilis) farming – A review. Ann. Aquac. Res. 2017;4:1038. [Google Scholar]
- Haniffa, M.A., Sridhar, S., 2002. Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis). Vet. Archiv. 72, 51–56.
- Hartnoll R.G. In: The biology of Crustacea. Bliss D.E., editor. Academic Press; New York: 1982. Growth; pp. 111–197. [Google Scholar]
- Hasan M.R., Hossain M.Y., Mawa Z., Tanjin S., Rahman M.A., Sarkar U.K., Ohtomi J. Evaluating the size at sexual maturity for 20 fish species (Actinopterygii) in wetland (Gajner Beel) ecosystem, north-western Bangladesh through multi-model approach: A key for sound management. Acta. Ichthyol. Piscat. 2021;51(1):29–36. [Google Scholar]
- Hasan M.R., Mawa Z., Ul-Hassan H., Rahman M.A., Tanjin S., Abro A.N., Gabol K., Bashar M.A., Jasmine S., Ohtomi J., Hossain M.Y. Impact of eco-hydrological factors on growth of the Asian stinging catfish Heteropneustus fossilis (Bloch, 1794) in a wetland ecosystem. Egypt. J. Aquat. Biol. Fish. 2020;24:77–94. [Google Scholar]
- Hossain F., Halim K.M.A., Siddique A.B., Shanta S.M., Rabbi M.F., Islam R., Azam R.M., Rahman M.R. Polyculture of stinging catfish (Heteropneustes fossilis) with Indian major carps in ponds. Int. J. Fish. Aquat. Stud. 2018;6:37–42. [Google Scholar]
- Hossain M.Y., Mawa Z., Hasan M.R., Rahman M.A., Tanjin S., Khatun M.M., Jasmine S. Assessing reproductive Biology of Macrobrachium lamarrei in the Ganges River (NW Bangladesh) in relation to environmental parameters. Saudi. J Biol Sci. 2021 doi: 10.1016/j.sjbs.2021.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.Y., Hossen M.A., Islam M.S., Jasmine S., Nawer F., Rahman M.M. Reproductive biology of Pethia ticto (Cyprinidae) from the Gorai River (SW Bangladesh) J. Appl. Ichthyol. 2017;33:1007–1014. [Google Scholar]
- Hossain M.Y., Hossen M.A., Ahmed Z.F., Hossain M.A., Pramanik M.N.U., Nawer F., Paul A.K., Khatun D., Haque N., Islam M.A. Length–weight relationships of 12 indigenous fish species in the Gajner Beel floodplain (NW Bangladesh) J. Appl. Ichthyol. 2017;33:842–845. [Google Scholar]
- Hossain M.Y., Jewel M.A.S., Nahar L., Rahman M.M., Naif A., Ohtomi J. Gonadosomatic index-based size at first sexual maturity of the catfish Eutropiichthys vacha (Hamilton 1822) in the Ganges River (NW Bangladesh) J. Appl. Ichthyol. 2012;28:601–605. [Google Scholar]
- Hossen M.A., Hossain M.Y., Pramanik M.N.U., Rahman M.A., Islam M.A., Nawer F., Parvin M.F. Biometry, sexual maturity, natural mortality and fecundity of endangered halfbeak Dermogenys pusilla (Zenarchopteridae) from the Ganges River in northwestern Bangladesh. Indian J. Geo- Mar. Sci. 2019;48:1548–1555. [Google Scholar]
- Hunter J.R., Macewicz B.J., Lo N.C.H., Kimbrell C.A. Fecundity, spawning and maturity of female dover sole, Microstomus pacifius, with an evaluation of assumptions and precision. Fish. Bull. 1992;90:101–128. [Google Scholar]
- Huxley J.S. Methuen and Co.; Ltd., London: 1932. Problems of relative growth; p. 276. [Google Scholar]
- Islam M.R., Azom M.G., Faridullah M., Mamun M. Length-weight relationship and condition factor of 13 fish species collected from the Atrai and Brahmaputra Rivers. Bangladesh. J. Biodiv. Env. Sci. 2017;10:123–133. [Google Scholar]
- IUCN Bangladesh, 2015. Red list of Bangladesh. volume 5: Freshwater fishes. IUCN, international union for conservation of nature, Bangladesh country office, Dhaka, Bangladesh. p. 360.
- IUCN, 2000. IUCN red list of threatened species. International union for conservation of nature and natural resources, Gland, Switzerland. Available:http://www.redlist.org/.
- Jan M., Ahmed I. Reproductive biology and histological studies of ovarian development of Schizothorax plagiostomus in River Lidder from Kashmir Himalaya. J. Appl. Ichthyol. 2019;35:512–519. [Google Scholar]
- Jennings S., Kaiser M.J., Reynolds J.D. Blackwell; Oxford: 2001. Marine fisheries ecology. [Google Scholar]
- Joy K.P., Tharakan B. Induced spawning of the Indian catfish, Heteropneustes fossilis, by GnRH analogue alone or in combination with dopamine-affecting drugs. J. Appl. Aquacult. 1999;9:23–32. [Google Scholar]
- Karl Marx, K., Chakrabarty, R.A., 2007. Comparative study on the induced breeding and in vitro fertilization performance by various inducing agents in catfish, Heteropneustes fossilis (Bloch). Bangladesh J. Fish. Res. 11, 1–5.
- Khan, M.A., Abidi, S.F., 2010. Optimum ration level for better growth, conversion efficiencies and body composition of fingerling Heteropneustes fossilis (Bloch) Aquacult. Int. 18, 175–188.
- Khan M.A., Khan S., Miyan K. Length–weight relationship of giant snakehead, Channa marulius and stinging catfish, Heteropneustes fossilis from the River Ganga. J. Appl. Ichthyol. 2012;28:154–155. [Google Scholar]
- Khatun D., Hossain M.Y., Nawer F., Mostafa A.A., Al-Askar A.A. Reproduction of Eutropiichthys vacha (Schilbeidae) in the Ganges River (NW Bangladesh) with special reference to potential influence of climate variability. Environ. Sci. Pollut. Res. 2019;26:10800–10815. doi: 10.1007/s11356-019-04523-5. [DOI] [PubMed] [Google Scholar]
- King M. 2nd edition. Oxford press; London: 2007. Fisheries biology, assessment and management; p. 382. [Google Scholar]
- Lagler K.F. In freshwater fishery biology. 2nd Edn. W.M. Brown company publishers; Dubuque: 1956. Enumeration of fish eggs; pp. 106–110. [Google Scholar]
- Lappalainen J., Tarkan A.S., Harrod C. A meta-analysis of latitudinal variations in life-history traits of roach, Rutilus rutilus, over its geographical range: linear or non-linear relationships? Freshw. Biol. 2008;53:1491–1501. [Google Scholar]
- Liu Z., Liu S., Yao J., Bao L., Zhang J., Li Y., Jiang C., Sun L., Wang R., Zhang Y., Zhou T., Zeng Q., Fu Q., Gao S., Li N., Koren S., Jiang Y., Zimin A., Xu P., Phillippy A.M., Geng W.X., Song L., Sun F., Li C., Wang X., Chen A., Jin Y., Yuan Z., Yang Y., Tan S., Peatman E., Lu J., Qin Z., Dunham R., Li Z., Sonstegard T., Feng W.J., Danzmann R.G., Schroeder S., Scheffler B., Duke M.V., Ballard L., Kucuktas H., Kaltenboeck L., Liu H., Armbruster J., Xie Y., Kirby M.L., Tian Y., Flanagan M.E., Mu W., Waldbieser G.C. The channel catfish genome sequence provides insights into the evolution of scale formation in teleosts. Nat. commun. 2016;11:757. doi: 10.1038/ncomms11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret J., Palomera I., Salat J., Sole I. Impact of freshwater input and wind on landings of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in shelf waters surrounding the Ebre (Ebro) River delta (north-western Mediterranean) Fish. Oceanogr. 2004;13:102–110. [Google Scholar]
- Loukos H., Monfray P., Bopp L., Lehodey P. Potential changes in skipjack tuna (Katsuwonus pelamis) habitat from a global warming scenario: Modeling approach and preliminary results. Fish. Oceanogr. 2003;12:474–482. [Google Scholar]
- Lowerre-Barbieri S.K., Brown-Peterson N.J., Murua H., Tomkiewicz J., Wyanski D.M., Saborido-Rey F. Emerging issues and methodological advances in fisheries reproductive biology. Mar. Coast. Fish. 2011;3:32–51. [Google Scholar]
- Lucano-Ramírez G., Gómez-García M.D.J., Ruiz-Ramírez S., González-Sansón G., Aguilar Betancourt C., Flores-Ortega J.R. Reproductive characteristics of the sole Achirus mazatlanus (Pleuronectiformes: Achiridae) in the Barra de Navidad coastal lagoon. Jalisco, México, Ciencias Marinas. 2019;45:47–58. [Google Scholar]
- Lucifora L.O., Valero J.L., García V.B. Length at maturity of the Greeneye Spurdog Shark, Squalus mitsukurii (Elasmobranchii: Squalidae), from the SW Atlantic, with comparisons with other regions. Mar. Freshw. Res. 1999;50:629–632. [Google Scholar]
- Mac Gregoer J.S. Relation between fish condition and population size in the sardine (Sardinops cacrulea). U.S. Fishery wild service. Fish. Bull. 1959;60:215–230. [Google Scholar]
- Marian M.P., Ponniah A.G., Pitchairaj R., Narayanan M. Effect of feeding frequency on surfacing activity and growth in the air-breathing fish, Heteropneustes fossilis. Aquacul. 1982;26:237–244. [Google Scholar]
- Mawa Z., Hossain M.Y., Hasan M.R., Tanjin S., Rahman M.A., Sarmin M.S., Habib K.A. First record on size at sexual maturity and optimum catchable length of 10 marine fishes from the Bay of Bengal (Bangladesh) through multi-models approach: a key for sound fisheries management. Environ. Sci. Pollut. Res. 2021;28:38117–38127. doi: 10.1007/s11356-021-13491-8. [DOI] [PubMed] [Google Scholar]
- Monir M.S., Rahman S. Effect of stocking density on growth, survival and production of shing (Heteropneustes fossilis) fingerlings under nursery ponds in Northern region of Bangladesh. Int. J. Fish. Aquat. Stud. 2015;2:81–86. [Google Scholar]
- Muchlisin Z.A., Musman M., Siti-Azizah M.N. Spawning seasons of Rasbora tawarensis in Lake Laut Tawar, Aceh Province. Indonesia. Reprod. Biol. Endocrin. 2010;8:49. doi: 10.1186/1477-7827-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad H., Iqbal Z., Bashir Q., Hanif M.A. Length-weight relationship and condition factor of catfish species from Indus River. Pakistan. Punjab Univ. J. Zool. 2017;32:35–38. [Google Scholar]
- Murua H.G., Kraus F., Saborido-Rey P.R., Witthames A., Thorsen A., Junquera S. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J. Northwest Atl. Fish. Sci. 2003;33:33–54. [Google Scholar]
- Mustafa M.G., De Graaf G. Population parameters of important species in inland fisheries of Bangladesh. Asian Fish. Sci. 2008;21:147–158. [Google Scholar]
- Nath A.K. UGC major research project. p; India: 2013. Studies on hilsa fisheries in Hooghly-estuarine system of west Bengal; p. 198. [Google Scholar]
- Nayak, P.K., Mishra, T.K., Singh, B.N., Pandey, A.K., Das, R.C., 2001. Induced maturation and ovulation in Heteropneustes fossilis by using LHRHa, pimozide and ovaprim for production of quality eggs and larvae. Indian J. Fish. 48, 269–275.
- Nazari E.M., Simoes-Costa M.S., Muller Y.M.R., Ammar D., Dias M. Comparisons of fecundity, egg size, and egg mass volume of the freshwater prawns Macrobrachium potiuna and Macrobrachium olfersi (Decapoda, Palaemonidae) J. Crust. Biol. 2003;23:862–868. [Google Scholar]
- Nikolsky G.V. Academic Press Inc.; London: 1963. The ecology of fishes. [Google Scholar]
- NOAA., 2020. Historical Maps and Charts audio podcast. National Ocean Service website, https://oceanservice.noaa.gov/podcast/November20/nop08-historical-maps charts.html, accessed on 12/12/20.
- Nushy N.H., Zafar A., Khatun M., Rohani F., Rana M. Comparative growth performance assessment of Shing (Heteropneustes fossilis) feeding with prepared and commercial diet. J. Aquac. Mar. Biol. 2020;9:10–13. [Google Scholar]
- Pankhurst N.W., Munday P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011;62:1015–1026. [Google Scholar]
- Parkinson D., Philipport J.C., Barras E. A preliminary investigation of spawning migration of grayling in a small stream determined by radio-tracking. J. Fish. Biol. 1999;55:172–182. [Google Scholar]
- Peer A.C., Miller T.J. Climate change, migration phenology, and fisheries management interact with unanticipated consequences. N. Am. J. Fish. Manag. 2014;34:94–110. [Google Scholar]
- Rahman, A.K.A., 1989. Freshwater fishes of Bangladesh, 1st edition, zoological society of Bangladesh, Department of zoology, University of Dhaka, Dhaka-1000, pp. 170–171.
- Rahman M.A., Hasan M.R., Hossain M.Y., Islam M.A., Khatun D., Rahman O., Mawa Z., Islam M.S., Chowdhury A.A., Parvin M.F., Khatun H. Morphometric and meristic characteristics of the Asian stinging catfish Heteropneustes fossilis (Bloch, 1794): A key for identification. Jordan J. Biol. Sci. 2019;12:467–470. [Google Scholar]
- Rahman M.M., Hossain M.Y., Hossain M.I., Provhat S.J., Islam M.S., Hossain M.B. Induced breeding of the stinging catfish, Heteropneustes fossilis: comparison among different inducing agents. Turkish J. Fish. Aquat. Sci. 2013;13:523–527. [Google Scholar]
- Rahman M.M., Hossain M.Y., Tumpa A.S., Hossain M.I., Billah M.M., Ohtomi J. Size at sexual maturity and fecundity of the mola carplet Amblypharyngodon mola (Hamilton 1822) (Cyprinidae) in the Ganges River. Bangladesh. Zool. Ecol. 2018;28:429–436. [Google Scholar]
- Rahman S., Monir M.S., Hossain M., Mou M.H. Culture potentials of stinging catfish shing (Heteropneustes fossilis) under different stocking densities in northern region of Bangladesh. Tren. Fish. Res. 2014;3:2319–4758. [Google Scholar]
- Rainboth W.J. In: Cyprinids fishes, systematics, biology and exploitation. Winfiled I.J., Nelson J.S., editors. Chapman and Hall; London: 1991. Cyprinids of Southeast Asia. [Google Scholar]
- Richter T.J. Development and evaluation of standard weight equations for bridge-lip suckers and large-scale suckers. N. Am. J. Fish Manag. 2007;27:936–939. [Google Scholar]
- Rideout R.M., Rose G.A., Burton M.P.M. Skipped spawning in female iteroparous fishes. Fish. Fish. 2005;6:50–72. [Google Scholar]
- Ridha M.T., Cruz E.M., Al-Ameeri A.A., Al-Ahmed A.A. Effects of controlling temperature and light duration on seed production in tilapia, Oreochromis spilurus (Gunther) Aquacult. Res. 1998;29:403–410. [Google Scholar]
- Roessig, J., Woodley, Cech, C.J., Hansen, L., 2004. Effects of global climate change on marine and estuarine fishes and fisheries. Rev. Fish Biol. Fish. 14, 251–275.
- Roy D., Al Masud A., Saha P.K., Kutubuddin M.M., Islam M.M. Water quality, growth and production performance of stinging catfish, Heteropneustes fossilis (Bloch) in cemented tanks with two different stocking densities. Bangladesh J. Zool. 2019;47:107–119. [Google Scholar]
- Ruzzante D.E., Taggar T.C.T., Cook D. A nuclear DNA basis for shelf and bank-scale population structure in northwest Atlantic cod (Gadus morhua): Labrador to Georges bank. Mol. Ecol. 1998;7:1663–1680. [Google Scholar]
- Rypel A.L., Richter T.J. Empirical percentile standard weight equation for the blacktail redhorse. N. Am. J. Fish. Manag. 2008;28:1843–1846. [Google Scholar]
- Sabbir W., Hossain M.Y., Rahman M.A., Hasan M.R., Mawa Z., Tanjin S., Ohtomi J. First report on reproductive features of the Hooghly croaker Panna heterolepis Trewavas, 1977 from the Bay of Bengal in relation to environmental factors. Environ. Sci. Pollut. Res. 2021;28(18):23152–23159. doi: 10.1007/s11356-020-12310-w. [DOI] [PubMed] [Google Scholar]
- Saha K.C., Guha B.C. Nutritional investigation of Bengal fish. Indian J. Med. Res. 1939;26:921–927. [Google Scholar]
- Sarkar U.K., Bakshi S., Lianthuamluaia L., Mishal P., Ghosh B.D., Saha S., Karnatak G. Understanding enviro-climatological impact on fish biodiversity of the tropical floodplain wetlands for their sustainable management. Sustain. Water Resour. Manag. 2020;6(5):1–12. [Google Scholar]
- Sarkar U.K., Naskar M., Srivastava P.K., Roy K., Sarkar S.D., Gupta S., Bose A.K., Nandy S.K., Verma V.K., Sudheesan D., Karnatak G. Climato-environmental influence on breeding phenology of native catfishes in river Ganga and modeling species response to climatic variability for their conservation. Int. J. Biometeorol. 2019;63:991–1004. doi: 10.1007/s00484-019-01703-3. [DOI] [PubMed] [Google Scholar]
- Sarkar U.K., Pathak A.K., Sinha R.K., Sivakumar K., Pandian A.K., Pandey A., Dubey V.K., Lakra W.S. Freshwater fish biodiversity in the River Ganga (India): Changing pattern, threats and conservation perspectives. Rev. Fish. Biol. Fish. 2012;22:251–272. [Google Scholar]
- Saud B.J., Landge A.T., Borah S. Gonado-somatic index and fecundity of Heteropneustes fossilis (Bloch) from the lower reaches of Brahmaputra River, Assam. India. J. Exp. Zool. B. 2015;18:657–660. [Google Scholar]
- Shafi M., Quddus M.A.A. Bangla Academy; Dhaka: 1982. Bangladesher Matshya Sampad (in Bengeli) pp. 314–319. [Google Scholar]
- Shafi M., Quddus M.M.A. Kabir publication. Dhaka; Bangladesh: 2001. Bangladesher Matsho Shampad (Fisheries of Bangladesh) (in Bengali) pp. 231–239. [Google Scholar]
- Shinkafi, B.A., Ipinjolu, J.K., Hassan, W.A., 2011. Gonad maturation stages of Auchenoglanis occidentalis (Valenciennes 1840) in River Rima, north-western Nigeria. J. Fish. Aquat. Sci. 6, 236–246.
- Shrivastava C.B.L. A text book of fishery Science and Indian fisheries. Kitab Mahal. Soc. (Sci.) 1999;20(1):1–5. [Google Scholar]
- Siddiqui T.Q., Khan M.A. Effects of dietary protein levels on growth, feed utilization, protein retention efficiency and body composition of young Heteropneustes fossilis (Bloch) Fish Physiol. Biochem. 2009;35(3):479–488. doi: 10.1007/s10695-008-9273-7. [DOI] [PubMed] [Google Scholar]
- Talwar P.K., Jhingran A.G. Inland fishes of India and adjacent countries. 1991;Vol. 2:1027–1028. [Google Scholar]
- Templeman W. Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiate) from the northwest Atlantic. J. Northwest. Atl. Fish. Sci. 1987;7:155–167. [Google Scholar]
- Tracey S.R., Lyle J., Haddon M. Reproductive biology and per-recruit analyses of striped trumpeter (Latris lineata) from Tasmania, Australia: Implications for management. Fish. Res. 2007;84:358–368. [Google Scholar]
- Vijaykumar, C., Sridhar, S., Haniffa, M.A., 1998. Low cost breeding and hatching techniques of the catfish (Heteropneustes fossilis) for small scale farmers. Naga. 21, 15–17.
- West G. Methods of assessing ovarian development in fishes: a review. Mar. Freshw. Res. 1990;41:199–222. [Google Scholar]
- Wilding, T., Yong, K., Pitkethley, R., 2000. Bay of plenty freshwater fish calendar, In: Environmental report 00/26. Environment bay of plenty, Whakatane.
- Wilson D.C., McCay B.J. 2nd ed. CA Academic Press; San Diego: 2001. Encyclopedia of Ocean Sciences. [Google Scholar]
- Yamada, R., Kodama, K., Yamakawa, T., Horiguchi, T., Aoki, I., 2007. Growth and reproductive biology of the small penaeid shrimp Trachysalambria curvirostris in Tokyo Bay. Mar. Biol. 151, 961–971.
- Zar J.H. 2nd Edn. Prentice-Hall Inc.; Englewood Cliffs, New Jersey, USA: 1984. Biostatistical Analysis. [Google Scholar]
- Zhang, J., Takita, T., Zhang, C., 2009. Reproductive biology of Ilisha elongate (Teleostei: Pristigasteridae) in Ariake Sound, Japan: Implications for estuarine fish conservation in Asia. Estuar. Coast. Shelf Sci. 81, 105–113.