Abstract
Toxicity induced by heavy metals deteriorates soil fertility status. It also adversely affects the growth and yield of crops. These heavy metals become part of the food chain when crops are cultivated in areas where heavy metals are beyond threshold limits. Cadmium (Cd) and nickel (Ni) are considered the most notorious ones among different heavy metals. The high water solubility of Cd made it a potential toxin for plants and their consumers. Accumulation of Ni in plants, leaves, and fruits also deteriorates their quality and causes cancer in humans when such a Ni-contaminated diet is used regularly. Both Cd and Ni also compete with essential nutrients of plants, making the fertility status of soil poor. To overcome this problem, the use of activated carbon biochar can play a milestone role. In the recent past application of activated carbon biochar is gaining more and more attention. Biochar sorb the Cd and Ni and releases essential micronutrients that are part of its structure. Many micropores and high cation exchange capacity make it the most acceptable organic amendment to improve soil fertility and immobilize Cd and Ni. In addition to improving water and nutrients, soil better microbial proliferation enhances the soil rhizosphere ecosystem and nutrient cycling. This review has covered Cd and Ni harmful effects on crop yield and their immobilization by activated carbon biochar. The focus was made to elaborate on the positive effects of biochar on crop yield and soil health.
Keyword: Biochar, Crops yield, Heavy metals, Toxicity, Soil fertility
1. Introduction
Heavy metals disturb the natural ecosystem due to their toxic effects (Abid et al., 2017, Danish et al., 2019a, Fiaz et al., 2014, Younis et al., 2015, Zafar-ul-Hye et al., 2020a, Zafar-ul-Hye et al., 2020c). These heavy metals are continuously becoming a part of the ecosystem through anthropogenic activities such as industrialization mining, overuse of pesticides in agriculture, and sewage water irrigation. It is a well-documented fact that any compound's accumulation beyond the soil's threshold limit becomes a soil pollutant (Zafar-ul-Hye et al., 2020b, Zafar-ul-Hye et al., 2020). The soil pollutants caused toxic effects on the plants and animals and soil microorganisms (Adriano, 2001). This also can affect the diversity, viability, and physiology of microbes in rhizosphere area which play very important role for healthy plant growth (Basu et al., 2021).
Such problematic soil conditions adversely affect the growth of plants and cause deterioration of crop productivity and quality. Among different environmental pollution, heavy metals are notorious that induce abiotic stresses in the plants. These heavy metals disturb the plant metabolism and restrict growth due to their high accumulation in different plant parts. In addition to plants, heavy metals are also dangerous for human and animal health that consumes metal contaminated food (Shah and Nongkynrih, 2007).
2. Cadmium and nickel as a pollutant
Cd (Zafar-ul-Hye et al., 2020a) and Ni (Gill and Tuteja, 2011) are the most notorious among different heavy metals. Both heavy metals have accumulated in Pakistan's soils with time in significant quantities (Bhutto et al., 2009). Indifferent biogeochemical and environmental cycles Cd enter through anthropogenic sources such as electroplating, industrial waste, pigments, plastic accessories, paints and metal alloys (Nriagu, 1996). In addition to the above sources, Cd also becomes a part of our environment through wastewater irrigation, mining of zinc, overuse of phosphorus fertilizer, uses automobile smoke, burning of fossil fuels, higher application of pesticides and cement industries (Dixit et al., 2011, Rao et al., 2011).
On the other hand, Ni is also a heavy metal, which also induced toxic effects in the plants beyond the required amount. It shares 3% composition in the earth and the 24th essential nutrient in the earth's crust. Emission of smoke from vehicles, mining of metals, burning fossil fuels, organic manure, industrial and municipal waste is also a major contributor of Ni in our environment. The role of anthropogenic activities is also crucial in that regard (Alloway, 1995).
3. Cd, Ni, agricultural soil and living organism health
In agricultural systems, Cd accumulation due to human activities has become one of the major issues globally, protecting crop productivity and making food poor quality (Chen et al., 2007). Most diseases caused by the higher accumulation of Cd in humans and mammals are not detectable because they show no symptoms. Search characteristics of Cd make it a potential toxin (López-Millán et al., 2009). Untreated sewage water is a major source of Cd contamination in both plants, especially vegetable crops, and soils (Hossny et al., 2001, Satarug et al., 2003). It has been observed that 80% of Cd becomes part of the human body by consuming Cd-contaminated cereal crops and vegetables (Satarug et al., 2010). Cancer, renal tubular dysfunction, low bone density, heart failure, nephritis, and nephrosis are essential diseases caused by Cd toxicity (Nishijo et al., 2006, Nordberg et al., 2002). As Cd can persist in our environment for more than 20 years, it makes it a potential life-toxic element for humans' survival (Ruiz et al., 2009).
Similarly, Ni becomes part of biota by involving precipitation, adsorption, and complexions with clay. It has been observed that a decrease in the soil pH significantly increases the bioavailability of Ni, especially in rural areas where crops are cultivated (Bencko, 1983). The distribution of Ni in the soil is mostly uniform. However, most Ni toxic effects are observed in the soil's upper layer with 3–100 ppm Ni concentrations (Bencko, 1983). The existence of Ni in the soil can be in several forms such as crystalline minerals (inorganic), on cations exchange surfaces which are inorganic, cations surfaces that are organic, as a free ion, water-soluble and chelated compounds (Scott-Fordsmand, 1997) which cause harmful impacts on plants (Chen et al., 2009). It is necessary to dispose of Cd contaminated waste materials with proper treatment to avoid its contamination in the environment. In 2010 Cd generated pollution was 21,000 tons; however, in 2011, it was up to 21,500 tons. Such conditions create alarming situations for crops cultivation in soils where Cd toxicity presents in dangerous concentrations (Pinto et al., 2004).
4. Cadmium as an essential nutrient vs pollutant
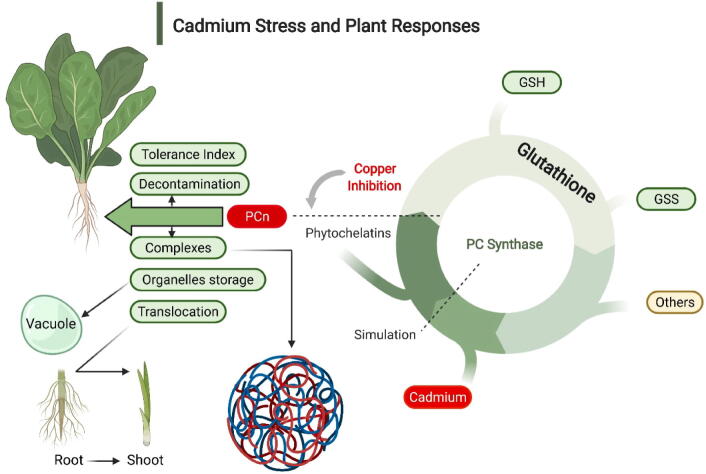
Cadmium is also required in small quantities to develop plants properly; however, its higher uptake in plants causes injuries (Reeves and Baker, 2000). So far, all the mechanism of poisoning caused by Cd is not well understood. Scientists are exploring the major mechanisms that Cd adopted two induced adverse effects in the plants (Fig. 1; Table 1). It also restricted physiological and metabolic activities, which decreased the growth attributes of crops. The higher Cd level in plants reduces transpiration (Inouhe, 2005) and photosynthesis rate (Bazzaz et al., 1974).
Fig. 1.
Cadmium stress and plant responses to mitigate the adverse effects of Cd.
Table 1.
Decrease in yield of different crops due to the toxicity of cadmium and nickel.
| Crop | Decrease in yield (%) | Heavy metal toxicity | References |
|---|---|---|---|
| Wheat | 72.0 | Ni | (Ouzounidou et al., 2006) |
| Barley | 27.2 | Ni | (Kumar et al., 2018) |
| Maize | 30.0 | Cd | (Dresler et al., 2015) |
| Bean | 36.5 | Ni | (Al-Qurainy, 2009) |
| Chickpea | 28.9 | Cd | (Hasan et al., 2008) |
| Sunflower | 50.0 | Ni | (Ahmad et al., 2011) |
| Radish | 52.0 | Ni | (Yadav et al., 2009) |
| Mustard | 43.8 | Cd | (Irfan et al., 2013) |
| Tomato | 80.0 | Ni | (Palacios et al., 1998) |
| Alfalfa | 33.2 | Cd | (Dražić et al., 2006) |
Less uptake of carbon dioxide due to Cd higher composition played an imperative role in disturbing the rate of photosynthesis in the plants (Larbi et al., 2002). It also decreases the germination of seeds when present in threshold limits in the soil. Low plant population due to poor germination causes a significant decrease in the yield (Larbi et al., 2002, Lozano-Rodríguez et al., 1997). Cd toxicity tolerance is different from different crops according to their stages; however, sitting stages are more susceptible to Cd toxicity (Sharma et al., 2010).
4.1. Antagonistic effects of cadmium
Cadmium also showed antagonistic relationships with the different essential nutrient elements that are required for the optimum growth of plants (Fig. 2). Higher intake and mobility of cadmium in the plants significantly decreased the iron uptake resulting in chlorosis (Genchi et al., 2020, Larbi et al., 2002). It also disturbs the optimum uptake of magnesium, potassium, and calcium; thus, plants suffer from nutritional deficiency stress (Dong et al., 2006, Greger et al., 1991, Larbi et al., 2002). In plants, Cd uptake beyond the threshold limit induces oxidative stress and restricts the electron transport chain activity, directly affecting the plant’s nucleic acid-associated mechanisms (Cuypers et al., 2010). Low uptake of zinc, iron, and manganese also disturb the plant cell's proper functioning (Lasat, 2002), which played an essential role in decreasing the yield (Dong et al., 2006).
Fig. 2.
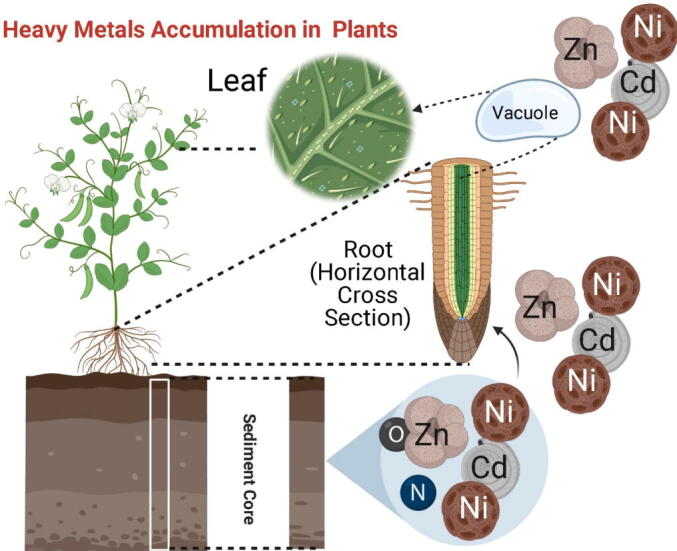
Heavy metals uptake and accumulation in the plants leaves vacuole.
5. Antagonistic effects of Ni on plant
In plants, the edible part of vegetables is a significant Ni accumulator that humans consume and other living organisms (Gupta et al., 2010, Olowoyo et al., 2012). Plants that suffer from Ni toxicity mostly show chlorosis and necrosis symptoms (Ahmad and Rasool, 2014). Likewise, Cd also decreases the uptake of iron, which adversely affects crop productivity (Kabata-Pendias, 2011). Ni also shows the antagonistic relationship between magnesium and calcium. Less uptake of magnesium deteriorates the structure of chlorophyll in the leaves. Low chlorophyll content in leaves ultimately resulted in the poor rate of photosynthesis in the plants (Piccini and Malavolta, 1992).
Seregin and Ivanov (2001) noted that mainly Ni is accumulated in the plant's laminar region and adversely affected photosystem II, which played a significant role in low photosynthesis (Maksymiec, 1998, Veeranjaneyulu and Das, 1982). It causes plastoquinone QA and Fe to plastoquinone QB and change the structure of the electron carrier (Krupa and Baszynski, 1995, Mohanty et al., 1989). Sheoran et al. (1990) observed restriction of Calvin cycle in the lives of Cajanus cajan. They argued that in 1 mM NiCl2, the Ni inhibits the activities of Rubisco, 3-phosphoglycerate kinase, fructose-1, 6-bisphosphatase, aldolase, and NAD. Search conditions decrease the rate of photosynthesis and result in the development of toxicity of Ni. Molas (1998) observed a significant reduction in the photosynthesis of Brassica oleracea in the presence of 10–20 g/m3 NiSO4⋅7H2O. They suggested that the cell's moisture contents were decreased when plants were cultivated in Ni toxicity and induced a condition of stress, which results in low photosynthetic activity of leaves.
Barsukova and Gamzikova (1999) noted that the reduction in the intake of Mg, Fe and Zn due to a higher intake of Ni (Calzado et al., 2005) resulted in the chlorosis (Khalid and Tinsley, 1980, Piccini and Malavolta, 1992). Pandolfini et al. (1992) noted a significant decrease in wheat's calcium and magnesium concentration when cultivated under 0.1–1 mM Ni concentration. Also, Ni shows an iron antagonistic relationship with potassium in the soil (Pulford and Watson, 2003).
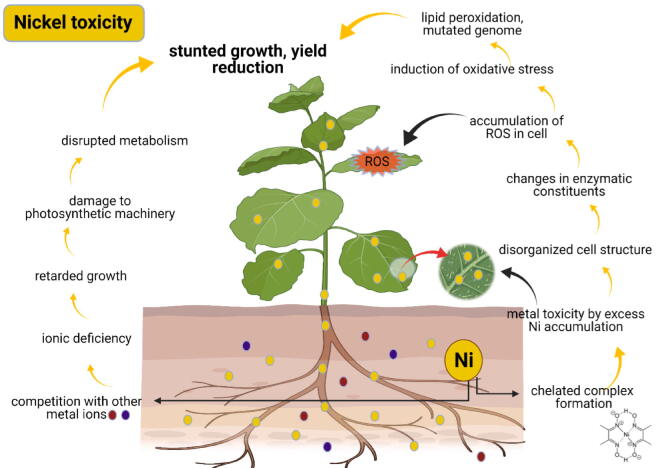
Furthermore, pigeon pea's mitotic activity is significantly decreased due to Ni higher concentration (Madhava Rao and Sresty, 2000). The toxicity of Ni also reduces the germination of plants (Madhava Rao and Sresty, 2000). In the case of cereals, mostly the wheat plants (Fig. 3), it retards the growth of shoot (Gajewska et al., 2006) and also decreased productivity due to low pods in seed formation (Tripathy et al., 1981). Therefore, the necessity of time is to introduce such an organic amendment that can detoxify these toxins from the soil on a long-term basis.
Fig. 3.
Adverse effects of nickel on plant (Chen et al., 2009).
6. Biochar as remediation
Activated black carbon fiber is one of such organic amendments that immobilizes heavy metals in the soil and decreases their bioavailability to the plants (Danish and Zafar-ul-Hye, 2020, Major, 2011, Radziemska et al., 2021, Sultan et al., 2020, Verheijen et al., 2010, Zafar-ul-Hye et al., 2020c). It is a fine black powder, a highly porous carbon structure that can be used as a fertilizer and soil conditioner. It can modify the physical, chemical, and biological attributes of the soil. Most physical properties of soil such as texture, structure, pore size distribution, and density with implications for soil aeration, water holding capacity (Danish et al., 2020, Danish et al., 2015a, Danish et al., 2015b, Danish and Zafar-ul-Hye, 2020, Danish and Zafar-ul-Hye, 2019, Fiaz et al., 2014, Zafar-ul-Hye et al., 2020c) and soil workability are positively and directly affected biochar's application in the soil as an amendment (Danish et al., 2019b, Danish et al., 2015b, Downie et al., 2012, Hashmi et al., 2019, Zafar-ul-Hye et al., 2019).
The history of biochar starts from the “Terra Preta de Indio” oxisols in Brazil which are suggested as Amazonian Dark Earth (ADE) developed about 7000 years ago. Most anthropogenic activities were involved in the establishment of these soils (Glaser, 2007). It has been observed that the fertility status of the soils was excellent due to the presence of significant amounts of char and organic debris, which act as natural soil fertilizers in the soil (Woods and Denevan, 2009). A significant increase in the yield of crops (Table 2) was observed in these soils compared to other soils where char was not applied as an amendment (Renner, 2007).
Table 2.
Increase in yield of different crops by variable application rate of biochar.
| Crop | Biochar (t/ha) | Yield increase (%) | References |
|---|---|---|---|
| Wheat | 25 | 21.5 | (Ali et al., 2019) |
| Barley | 10 | 39.5 | (Agegnehu et al., 2016) |
| Maize | 25 | 20.0 | (Arif et al., 2016) |
| Rice | 10.5 | 10.0 | (Liu et al., 2016) |
| Sorghum | 22 | 22.0 | (Laghari et al., 2015) |
| Winter rye | 20 | 14.5 | (Kraska et al., 2016) |
| Cotton | 20 | 21.9 | (Tian et al., 2018) |
| Soybean | 10 | 45.4 | (Van Zwieten et al., 2010) |
| Bean | 30 | 30.0 | (Rondon et al., 2004) |
| Radish | 10 | 33.5 | (Van Zwieten et al., 2010) |
| Carrot | 30 | 100 | (Rondon et al., 2004) |
| peanut | 8.5 | 45.6 | (Tando et al., 2017) |
| Tomato | 10 | 70.0 | (Hossain et al., 2010) |
Current assessments elaborated that biotech application in soil significantly increased the carbon pool of soil through pyrolysis and unlimited oxygen availability. Biochar is produced using the organic waste materials collected from agricultural fields (Danish and Zafar-ul-Hye, 2020, Lehmann et al., 2006, Woolf et al., 2010). When carbon-containing biomass is heated in the absence of oxygen at 450–6500C, a significant amount of volatile matter is admitted in gases. These gases can be collected in condensed to get bio-oils, which help decrease environmental pollution and provide an alternative energy source (Sohi et al., 2010). The biochar is mainly prepared by pyrolysis, which is divided into three major stages. In the first one, biomass having carbon is converted into unreacted water and residue (Fig. 4). In the second step, most of the volatile gases are emitted and left the biochar behind. In the last step, the structural and chemical modifications occur in this biochar (Demirbas, 2004).
Fig. 4.
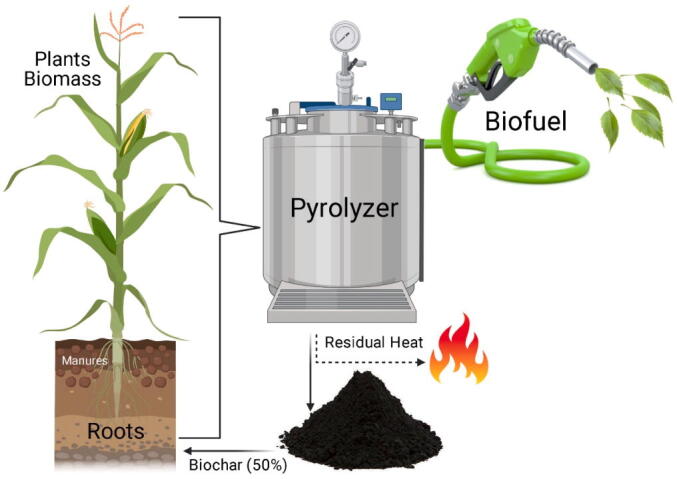
Preparation of Biochar.
Scientists nowadays are developing different biochar using different organic waste Biomass through pyrolysis (Park et al., 2011, Sohi et al., 2010). The application of biochar saved our environment from polluted gases and played an essential role in decreasing fertilizers' volatilization losses (Woolf et al., 2010), also use of biostimulant and foliar application of fertilizer reduses the direct application of fertilizer to the soil (Abbas et al., 2020, Izhar Shafi et al., 2020, Rafiullah et al., 2020, Ullah et al., 2020). Micropores of biochar significantly increased the soil's water holding capacity and decreased the soil infiltration rate. It also plays a crucial role in increasing the soil's surface area (Downie et al., 2012). It has been observed that chemical properties of soil such as pH, electrical conductivity, cation exchange capacity, nutrients holding capacity, and water holding capacity of soil become improved when biochar is applied as an amendment. A significant improvement in the microbial growth and soil population through biochar application validated its effectiveness as a soil amendment (Amonette and Joseph, 2009, Verheijen et al., 2010, Warnock, 2009). Furthermore, micro aggregates' stability is also enhanced due to activated carbon biochar's high binding ability (Lu et al., 2014).
Small pore spaces in biochar provide shelter to the microorganism present in the rhizosphere. Such conditions provide a chance for microbes to better floor acceleration and growth (Quilliam et al., 2013). As compared to organic matter, the shelf life of activated carbon is high. It remains in the soil for an extended period compared to the organic matter due to its high resistance against the composition process (Downie et al., 2012, Pathan et al., 2018, Thies and Rillig, 2009, Woods and Denevan, 2009). Activated carbon biochar has many functional groups that act as binding sites for heavy metals (Table 3).
Table 3.
Different functional group in different waste material produced biochar which can immobilize Ni and Cd.
| Waste material for biochar | Functional groups | Heavy metal which is absorbed | References |
|---|---|---|---|
| Rice straw | Carboxyl | Ni | (Ali et al., 2020) |
| Wood and bark chars | Hydroxyl | Cd | (Mohan et al., 2007) |
| Cotton seed hull char | Carbonyl, Carboxyl | Ni, Cd | (Uchimiya et al., 2011) |
| Green waste | Aromatic | Cd | (Park et al., 2011) |
| Wheat straw | Carbonyl | Cd | (Cui et al., 2012) |
| Rice straw | Carboxyl, Hydroxyl | Cd | (Jiang et al., 2012) |
| Rice straw | Carboxyl, Hydroxyl | Ni, Cd | (Deng et al., 2019) |
When biochar is applied in the soil, the heavy metals become bound on the biochar's active sites, significantly decreasing their mobility in soil and bioavailability to the plants (Machida et al., 2005). The above mineral nutrition, an integral part of the biochar structure, is also released in the soil and on the exchange sites that become readily available to the plants. Such conditions improve soil fertility and decrease the chances of heavy metals uptake, potentially toxins for plants, humans, and animals (Quilliam et al., 2013).
7. Conclusion and future perspective
Biochar is an effective organic amendment that can improve soil fertility status. Besides improving soil health by ameliorating the physio-chemical and biological properties of soil, it can mitigate Cd and Ni toxicity in different crops. The different scientist has done much work for manufacturing of thermo-pyrolyzed biochar. However, the need for time is to convert the production technology to chemically pyrolyzed biochar manufacturing. It decreases the potential hazards and can be easy for the industry to produce activated carbon on a large scale.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors would like to thank RMC-UTM and the financial support through industrial grant No. RJ130000.7609-4C187 and RJ130000.7609-4C240. This project was supported by Researchers Supporting Project Number (RSP-2021/301) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Uzma Younis, Email: uzmabotany@hotmail.com.
Subhan Danish, Email: sd96850@gmail.com.
Rahul Datta, Email: rahulmedcure@gmail.com.
References
- Abbas M., Anwar J., Zafar-ul-Hye M., Khan R.I., Saleem M., Rahi A.A., Danish S., Datta R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae. 2020;6:28. [Google Scholar]
- Abid M., Danish S., Zafar-ul-Hye M., Shaaban M., Iqbal M.M., Rehim A., Qayyum M.F., Naqqash M.N. Biochar increased photosynthetic and accessory pigments in tomato (Solanum lycopersicum L.) plants by reducing cadmium concentration under various irrigation waters. Environ. Sci. Pollut. Res. 2017;24:22111–22118. doi: 10.1007/s11356-017-9866-8. [DOI] [PubMed] [Google Scholar]
- Adriano, 2001. Trace elements in terrestrial environments. In: Biogeochemistry, bioavailability and risks of metals. Springer-Verlag, New York, p. 374. 10.2134/jeq2002.3740.
- Agegnehu G., Nelson P.N., Bird M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016;160:1–13. doi: 10.1016/j.still.2016.02.003. [DOI] [Google Scholar]
- Ahmad M.S.A., Ashraf M., Hussain M. Phytotoxic effects of nickel on yield and concentration of macro- and micro-nutrients in sunflower (Helianthus annuus L.) achenes. J. Hazard. Mater. 2011;185:1295–1303. doi: 10.1016/j.jhazmat.2010.10.045. [DOI] [PubMed] [Google Scholar]
- Ahmad, P., Rasool, S., 2014. Emerging technologies and management of crop stress tolerance, Emerging Technologies and Management of Crop Stress Tolerance: Biological Techniques. 10.1016/C2013-0-19047-2
- Al-Qurainy F. Toxicity of heavy metals and their molecular detection on Phaseolus vulgaris (L.) Aust. J. Basic Appl. Sci. 2009;3:3025–3035. [Google Scholar]
- Ali K., Wang X., Riaz M., Islam B., Khan Z.H., Shah F., Munsif F., Ijaz Ul Haq S. Biochar: an eco-friendly approach to improve wheat yield and associated soil properties on sustainable basis. Pakistan J. Bot. 2019;51:1255–1261. doi: 10.30848/PJB2019-4(7). [DOI] [Google Scholar]
- Ali U., Shaaban M., Bashir S., Gao R., Fu Q., Zhu J., Hu H. Rice straw, biochar and calcite incorporation enhance nickel (Ni) immobilization in contaminated soil and Ni removal capacity. Chemosphere. 2020;244 doi: 10.1016/j.chemosphere.2019.125418. [DOI] [PubMed] [Google Scholar]
- Alloway B.J. Heavy metals in soils. Heavy Metals in Soils. 1995:411–428. doi: 10.1007/978-94-011-1344-1. [DOI] [Google Scholar]
- Amonette J., Joseph S. In: Biochar for Environmental Management: Science and Technology. Amonette J., Joseph S., editors. Earthscan; London, UK: 2009. Characteristics of biochar - micro-chemical properties; pp. 33–52. [DOI] [Google Scholar]
- Arif M., Ali K., Jan M.T., Shah Z., Jones D.L., Quilliam R.S. Integration of biochar with animal manure and nitrogen for improving maize yields and soil properties in calcareous semi-arid agroecosystems. F. Crop. Res. 2016;195:28–35. doi: 10.1016/j.fcr.2016.05.011. [DOI] [Google Scholar]
- Barsukova V.S., Gamzikova O.I. Effects of nickel surplus on the element content in wheat varieties contrasting in Ni resistance. Agrokhimiya. 1999;1:80–85. [Google Scholar]
- Basu A., Prasad P., Das S.N., Kalam S., Sayyed R.Z., Reddy M.S., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent development, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- Bazzaz F.A., Carlson R.W., Rolfe G.L. The effect of heavy metals on plants: Part I. Inhibition of gas exchange in sunflower by Pb, Cd, Ni and Tl. Environ. Pollut. 1974;7:241–246. doi: 10.1016/0013-9327(74)90032-9. [DOI] [Google Scholar]
- Bencko V. Nickel: a review of its occupational and environmental toxicology. J. Hyg. Epidemiol. Microbiol. Immunol. 1983;27:237. [PubMed] [Google Scholar]
- Bhutto M.A., Zahida P., Sajid I., Mubarik A., Sahar N. Monitoring of heavy and essential trace metals contents in wheat procured from various countries by the Government of Pakistan in the year 2008–09. Int. J. Biol. Biotechnol. 2009;6:247–250. [Google Scholar]
- Calzado L.E., Gomez C.O., Finch J.A. Nickel recovered from solution by oxidation using ozone: some physical properties. Miner. Eng. 2005;18:537–543. doi: 10.1016/j.mineng.2004.09.003. [DOI] [Google Scholar]
- Chen C., Huang D., Liu J. Functions and toxicity of nickel in plants: recent advances and future prospects. Clean - Soil, Air, Water. 2009 doi: 10.1002/clen.200800199. [DOI] [Google Scholar]
- Chen F., Wu F., Dong J., Vincze E., Zhang G., Wang F., Huang Y., Wei K. Cadmium translocation and accumulation in developing barley grains. Planta. 2007;227:223–232. doi: 10.1007/s00425-007-0610-3. [DOI] [PubMed] [Google Scholar]
- Cui L., Pan G., Li L., Yan J., Zhang A., Bian R., Chang A. The reduction of wheat Cd uptake in contaminated soil via biochar amendment: a two-year field experiment. Bio Resour. 2012;7:5666–5676. [Google Scholar]
- Cuypers A., Plusquin M., Remans T., Jozefczak M., Keunen E., Gielen H., Opdenakker K., Nair A.R., Munters E., Artois T.J., Nawrot T., Vangronsveld J., Smeets K. Cadmium stress: an oxidative challenge. BioMetals. 2010;23(5):927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Danish S., Kiran S., Fahad S., Ahmad N., Ali M.A., Tahir F.A., Rasheed M.K., Shahzad K., Li X., Wang D., Mubeen M., Abbas S., Munir T.M., Hashmi M.Z., Adnan M., Saeed B., Saud S., Khan M.N., Ullah A., Nasim W. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019;185 doi: 10.1016/j.ecoenv.2019.109706. [DOI] [PubMed] [Google Scholar]
- Danish S., Tahir F.A., Rasheed M.K., Ahmad N., Ali M.A., Kiran S., Younis U., Irshad I., Butt B. Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric. 2019;4:381–390. doi: 10.1515/opag-2019-0034. [DOI] [Google Scholar]
- Danish S., Younis U., Akhtar N., Ameer A., Ijaz M., Nasreen S., Huma F., Sharif S., Ehsanullah M. Phosphorus solubilizing bacteria and rice straw biochar consequence on maize pigments synthesis. Int. J. Biosci. 2015;5:31–39. doi: 10.12692/ijb/5.12.31-39. [DOI] [Google Scholar]
- Danish S., Younis U., Nasreen S., Akhtar N., Iqbal M.T. Biochar consequences on cations and anions of sandy soil. J. Biodivers. Environ. Sci. 2015;6:121–131. [Google Scholar]
- Danish S., Zafar-ul-Hye M. Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton-Int. J. Exp. Bot. 2020;89:217–227. doi: 10.32604/phyton.2020.08523. [DOI] [Google Scholar]
- Danish S., Zafar-ul-Hye M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-42374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish S., Zafar-ul-Hye M., Mohsin F., Hussain M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230615. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Demirbas A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis. 2004;72:243–248. doi: 10.1016/j.jaap.2004.07.003. [DOI] [Google Scholar]
- Deng Y., Huang S., Laird D.A., Wang X., Meng Z. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere. 2019;218:308–318. doi: 10.1016/j.chemosphere.2018.11.081. [DOI] [PubMed] [Google Scholar]
- Dixit P., Mukherjee P.K., Ramachandran V., Eapen S. Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS One. 2011;6:16360. doi: 10.1371/journal.pone.0016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Wu F., Zhang G. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum) Chemosphere. 2006;64:1659–1666. doi: 10.1016/j.chemosphere.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Downie A., Crosky A., Munroe P., Crosky A., Munroe P. In: Biochar for Environmental Management: Science and Technology. Lehmann J., Joseph S., editors. Routledge; Earthscan, London: 2012. Physical properties of biochar; pp. 13–32. [DOI] [Google Scholar]
- Dražić G., Mihailović N., Lojić M. Cadmium accumulation in Medicago sativa seedlings treated with salicylic acid. Biol. Plant. 2006;50:239–244. doi: 10.1007/s10535-006-0013-5. [DOI] [Google Scholar]
- Dresler S., Wójcik M., Bednarek W., Hanaka A., Tukiendorf A. The effect of silicon on maize growth under cadmium stress. Russ. J. Plant Physiol. 2015;62:86–92. doi: 10.1134/S1021443715010057. [DOI] [Google Scholar]
- Fiaz K., Danish S., Younis U., Malik S.A., Raza Shah M.H., Niaz S. Drought impact on Pb/Cd toxicity remediated by biochar in Brassica campestris. J. Soil Sci. Plant Nutr. 2014;14:845–854. doi: 10.4067/S0718-95162014005000067. [DOI] [Google Scholar]
- Gajewska E., Skłodowska M., Słaba M., Mazur J. Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol. Plant. 2006;50:653–659. doi: 10.1007/s10535-006-0102-5. [DOI] [Google Scholar]
- Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal. Behav. 2011 doi: 10.4161/psb.6.2.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B. Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2007 doi: 10.1098/rstb.2006.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger M., Brammer E., Lindberg S., Larsson G., Idestam-almquist J. Uptake and physiological effects of cadmium in sugar beet (Beta vulgaris) related to mineral provision. J. Exp. Bot. 1991;42:729–737. doi: 10.1093/jxb/42.6.729. [DOI] [Google Scholar]
- Gupta N., Khan D.K., Santra S.C. Determination of public health hazard potential of wastewater reuse in crop production. World Rev. Sci. Technol. Sustain. Dev. 2010;7:328–340. doi: 10.1504/WRSTSD.2010.032741. [DOI] [Google Scholar]
- Hasan S.A., Hayat S., Ali B., Ahmad A. 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ. Pollut. 2008;151:60–66. doi: 10.1016/j.envpol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hashmi S., Younis U., Danish S., Munir T.M. Pongamia pinnata L. leaves biochar increased growth and pigments syntheses in Pisum sativum L. exposed to nutritional stress. Agric. 2019;9:153. doi: 10.3390/agriculture9070153. [DOI] [Google Scholar]
- Hossain M.K., Strezov V., Chan K.Y., Nelson P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum) Chemosphere. 2010;78:1167–1171. doi: 10.1016/j.chemosphere.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Hossny E., Mokhtar G., El-Awady M., Ali I., Morsy M., Dawood A. Environmental exposure of the pediatric age groups in Cairo City and its suburbs to cadmium pollution. Sci. Total Environ. 2001;273:135–146. doi: 10.1016/S0048-9697(00)00848-2. [DOI] [PubMed] [Google Scholar]
- Inouhe M. Phytochelatin. Brazilian J. Plant Physiol. 2005;17:65–78. [Google Scholar]
- Irfan M., Hayat S., Ahmad A., Alyemeni M.N. Soil cadmium enrichment: allocation and plant physiological manifestations. Saudi J. Biol. Sci. 2013;20(1):1–10. doi: 10.1016/j.sjbs.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar Shafi M., Adnan M., Fahad S., Wahid F., Khan A., Yue Z., Danish S., Zafar-ul-Hye M., Brtnicky M., Datta R. Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy. 2020;10:1224. [Google Scholar]
- Jiang J., Xu R., Jiang T., Li Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012;229:145–150. doi: 10.1016/j.jhazmat.2012.05.086. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A. CRC Press; 2011. Trace Elements in Soils and Plants; pp. 1–534. 10.1201/b10158-25. [Google Scholar]
- Khalid B.Y., Tinsley J. Some effects of nickel toxicity on rye grass. Plant Soil. 1980;55:139–144. doi: 10.1007/BF02149717. [DOI] [Google Scholar]
- Kraska P., Oleszczuk P., Andruszczak S., Kwiecińska-Poppe E., Różyło K., Pałys E., Gierasimiuk P., Michałojć Z. Effect of various biochar rates on winter rye yield and the concentration of available nutrients in the soil. Plant, Soil Environ. 2016;62:483–489. doi: 10.17221/94/2016-PSE. [DOI] [Google Scholar]
- Krupa Z., Baszynski T. Some aspects of heavy metals toxicity towards photosynthetic apparatus-direct and indirect effects on light and dark reactions. Acta Physiol. Plant. 1995;17:177–190. [Google Scholar]
- Kumar O., Singh S.K., Singh A.P., Yadav S.N., Latare A.M. Effect of soil application of nickel on growth, micronutrient concentration and uptake in barley (Hordeum vulgare L.) grown in Inceptisols of Varanasi. J. Plant Nutr. 2018;41:50–66. doi: 10.1080/01904167.2017.1381724. [DOI] [Google Scholar]
- Laghari M., Mirjat M.S., Hu Z., Fazal S., Xiao B., Hu M., Chen Z., Guo D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena. 2015;135:313–320. doi: 10.1016/j.catena.2015.08.013. [DOI] [Google Scholar]
- Larbi A., Morales F., Abadia A., Gogorcena Y., Lucena J.J., Abadia J. Effects of Cd and Pb in sugar beet plants grown in nutrient solution: induced Fe deficiency and growth inhibition. Funct. Plant Biol. 2002;29:1453–1464. doi: 10.1071/FP02090. [DOI] [PubMed] [Google Scholar]
- Lasat M.M. Phytoextraction of toxic metals: a review of biological mechanisms. J. Environ. Qual. 2002;31:109–120. [PubMed] [Google Scholar]
- Lehmann J., Gaunt J., Rondon M. Bio-char sequestration in terrestrial ecosystems – a review. Mitig. Adapt. Strateg. Glob. Chang. 2006;11:395–419. [Google Scholar]
- Liu Y., Lu H., Yang S., Wang Y. Impacts of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. F. Crop. Res. 2016;191:161–167. doi: 10.1016/j.fcr.2016.03.003. [DOI] [Google Scholar]
- López-Millán A.F., Sagardoy R., Solanas M., Abadía A., Abadía J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ. Exp. Bot. 2009;65:376–385. doi: 10.1016/j.envexpbot.2008.11.010. [DOI] [Google Scholar]
- Lozano-Rodríguez E., Hernandez L.E., Bonay P., Carpena-Ruiz R.O. Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J. Exp. Bota. 1997;48:123–128. [Google Scholar]
- Lu S.G., Sun F.F., Zong Y.T. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (Vertisol) Catena. 2014;114:37–44. doi: 10.1016/j.catena.2013.10.014. [DOI] [Google Scholar]
- Machida Y.J., Teer J.K., Dutta A. Acute reduction of an origin recognition complex (ORC) subunit in human cells reveals a requirement of ORC for Cdk2 activation. J. Biol. Chem. 2005;280:27624–27630. doi: 10.1074/jbc.M502615200. [DOI] [PubMed] [Google Scholar]
- Madhava Rao K.V., Sresty T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/S0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Major J. Biochar for soil remediation and land reclamation. IBI Res. Summ. 2011:1–6. [Google Scholar]
- Maksymiec W. Effect of copper on cellular processes in higher plants. Photosynthetica. 1998;34(3):321–342. doi: 10.1023/A:1006818815528. [DOI] [Google Scholar]
- Mohan D., Pittman C.U., Bricka M., Smith F., Yancey B., Mohammad J., Steele P.H., Alexandre-Franco M.F., Gómez-Serrano V., Gong H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007;310:57–73. doi: 10.1016/j.jcis.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Mohanty N., Vass I., Demeter S. Copper toxicity affects photosystem II electron transport at the secondary quinone acceptor. Q B. Plant Physiol. 1989;90:175–179. doi: 10.1104/pp.90.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas J. Changes in morphological and anatomical structure of cabbage (Brassica oleracea L.) outer leaves and in ultrastructure of their chloroplasts caused by an in vitro excess of nickel. Photosynthetica. 1998;34:513–522. doi: 10.1023/A:1006805327340. [DOI] [Google Scholar]
- Nishijo M., Morikawa Y., Nakagawa H., Tawara K., Miura K., Kido T., Ikawa A., Kobayashi E., Nogawa K. Causes of death and renal tubular dysfunction in residents exposed to cadmium in the environment. Occup. Environ. Med. 2006;63:545–550. doi: 10.1136/oem.2006.026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg G., Jin T., Bernard A., Fierens S., Buchet J.P., Ye T., Kong Q., Wang H. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio. 2002;31:478–481. doi: 10.1579/0044-7447-31.6.478. [DOI] [PubMed] [Google Scholar]
- Nriagu J.O. A history of global metal pollution. Science. 1996;272(5259):223. doi: 10.1126/science.272.5259.223. [DOI] [Google Scholar]
- Olowoyo J.O., Okedeyi O.O., Mkolo N.M., Lion G.N., Mdakane S.T.R. Uptake and translocation of heavy metals by medicinal plants growing around a waste dump site in Pretoria, South Africa. South African J. Bot. 2012;78:116–121. doi: 10.1016/j.sajb.2011.05.010. [DOI] [Google Scholar]
- Ouzounidou G., Moustakas M., Symeonidis L., Karataglis S. Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch. Environ. Contam. Toxicol. 2006;50:346–352. doi: 10.1007/s00244-005-5076-3. [DOI] [PubMed] [Google Scholar]
- Palacios G., Gómez I., Carbonell-Barrachina A., Navarro Pedreño J., Mataix J. Effect of nickel concentration on tomato plant nutrition and dry matter yield. J. Plant Nutr. 1998;21:2179–2191. doi: 10.1080/01904169809365553. [DOI] [Google Scholar]
- Pandolfini T., Gabbrielli R., Comparini C. Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant. Cell Environ. 1992;15:719–725. doi: 10.1111/j.1365-3040.1992.tb01014.x. [DOI] [Google Scholar]
- Park J.H., Choppala G.K., Bolan N.S., Chung J.W., Chuasavathi T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011;348:439. [Google Scholar]
- Pathan S.I., Větrovský T., Giagnoni L., Datta R., Baldrian P., Nannipieri P., Renella G. Microbial expression profiles in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil. 2018;433(1):401–413. [Google Scholar]
- Piccini D.F., Malavolta E. Effect of nickel on two common bean cultivars. J. Plant Nutr. 1992;15:2343–2350. [Google Scholar]
- Pinto A.P., Mota A.M., De Varennes A., Pinto F.C. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 2004;326:239–247. doi: 10.1016/j.scitotenv.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pulford I.D., Watson C. Phytoremediation of heavy metal-contaminated land by trees - A review. Environ. Int. 2003;29(4):529–540. doi: 10.1016/S0160-4120(02)00152-6. [DOI] [PubMed] [Google Scholar]
- Quilliam R.S., Glanville H.C., Wade S.C., Jones D.L. Life in the “charosphere” - Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013;65:287–293. doi: 10.1016/j.soilbio.2013.06.004. [DOI] [Google Scholar]
- Radziemska M., Gusiatin Z.M., Cydzik-Kwiatkowska A., Cerdà A., Pecina V., Bęś A., Datta R., Majewski G., Mazur Z., Dzięcioł J., Danish S., Brtnicky M. Insight into metal immobilization and microbial community structure in soil from a steel disposal dump that was phytostabilized with composted, pyrolyzed or gasified wastes. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2021.129576. [DOI] [PubMed] [Google Scholar]
- Rafiullah, Tariq, M., Khan, F., Shah, A.H., Fahad, S., Wahid, F., Ali, J., Adnan, M., Ahmad, M., Irfan, M., Zafar-ul-Hye, M., Battaglia, M.L., Zarei, T., Datta, R., Saleem, I.A., Hafeez-u-Rehman, Danish, S., 2020. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods 12, 32–40. 10.15586/qas.v12iSP1.793.
- Rao K., Mohapatra M., Anand S., Venkateswarlu P. Review on cadmium removal from aqueous solutions. Int. J. Eng. Sci. Technol. 2011;2:81–103. doi: 10.4314/ijest.v2i7.63747. [DOI] [Google Scholar]
- Reeves R.D., Baker A.J.M. John Wiley and Sons Ltd.; New York: 2000. Metal-accumulating plants. Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment. [Google Scholar]
- Renner R. Rethinking biochar. Environ. Sci. Technol. 2007:5932–5933. doi: 10.1021/es0726097. [DOI] [PubMed] [Google Scholar]
- Rondon, M., Ramirez, A., Hurtado, M., 2004. Charcoal additions to high fertility ditches enhance yields and quality of cash crops in Andean hillsides of Colombia, CIAT Annual Report. Cali, Colombia.
- Ruiz J.M., Blasco B., Ríos J.J., Cervilla L.M., Rosales M.A., Rubio-wilhelmi M.M., Sánchez-rodríguez E., Castellano R., Romero L. Distribution and efficiency of the phytoextraction of cadmium by different organic chelates. Terra Latinoam. 2009;27:295–301. [Google Scholar]
- Satarug S., Baker J.R., Urbenjapol S., Haswell-Elkins M., Reilly P.E.B., Williams D.J., Moore M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003:65–83. doi: 10.1016/S0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Satarug S., Garrett S.H., Sens M.A., Sens D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Fordsmand J.J. In: Reviews of Environmental Contamination and Toxicology. Ware G.W., Nigg H.N., Bevenue A., editors. Springer; New York, NY: 1997. Toxicity of nickel to soil organisms in Denmark; pp. 1–34. [DOI] [Google Scholar]
- Seregin I.V., Ivanov V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 2001;48:523–544. doi: 10.1023/A:1016719901147. [DOI] [Google Scholar]
- Shah K., Nongkynrih J.M. Metal hyperaccumulation and bioremediation. Biol. Plant. 2007;51(4):618–634. doi: 10.1007/s10535-007-0134-5. [DOI] [Google Scholar]
- Sharma S., Sharma P., Melhotra P. Bioaccumulation of heavy metals in Pisum sativum L. growing in fly ash amended soil. J. Amer. Sci. 2010;6:43–50. [Google Scholar]
- Sheoran I.S., Singal H.R., Singh R. Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.) Photosynth. Res. 1990;23:345–351. doi: 10.1007/BF00034865. [DOI] [PubMed] [Google Scholar]
- Sohi S.P., Krull E., Bol R. A review of biochar and its use and function in soil. Adv. Agron. 2010;105:47–82. doi: 10.1016/S0065-2113(10)05002-9. [DOI] [Google Scholar]
- Sultan H., Ahmed N., Mubashir M., Danish S. Chemical production of acidified activated carbon and its influences on soil fertility comparative to thermo-pyrolyzed biochar. Sci. Rep. 2020;10:595. doi: 10.1038/s41598-020-57535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tando, E., Nugroho, A., Islami, T., 2017. Effect of sago waste, manure and straw biochar on peanut (Arachis hypogaea L.) growth and yield on an Ultisol of Southeast Sulawesi. J. Degrad. Min. Lands Manag. 4, 749–757. 10.15243/jdmlm.2017.042.749.
- Thies J., Rillig M.C. Biochar for environmental management: Science and technology. Earthscan; London: 2009. Characteristics of biochar: Biological properties. [Google Scholar]
- Tian X., Li C., Zhang M., Wan Y., Xie Z., Chen B., Li W. Biochar derived from corn straw affected availability and distribution of soil nutrients and cotton yield. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy B.C., Bhatia B., Mohanty P. Inactivation of chloroplast photosynthetic electron-transport activity by Ni2+ BBA - Bioenerg. 1981;638:217–224. doi: 10.1016/0005-2728(81)90230-9. [DOI] [Google Scholar]
- Uchimiya M., Chang S.C., Klasson K.T. Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J. Hazard. Mater. 2011;190:432–441. doi: 10.1016/j.jhazmat.2011.03.063. [DOI] [PubMed] [Google Scholar]
- Ullah A., Ali M., Shahzad K., Ahmad F., Iqbal S., Habib M., Rahman U., Ahmad S., Iqbal M.M. Impact of seed dressing and soil application of potassium humate on cotton plants productivity and fiber quality. Plants. 2020;9:1444. doi: 10.3390/plants9111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zwieten L., Kimber S., Morris S., Chan K.Y., Downie A., Rust J., Joseph S., Cowie A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil. 2010;327:235–246. doi: 10.1007/s11104-009-0050-x. [DOI] [Google Scholar]
- Veeranjaneyulu K., Das V.S.R. Intrachloroplast localization of 65Zn and 63Ni in a zn-tolerant plant, Ocimum basilicum benth. J. Exp. Bot. 1982;33:1161–1165. doi: 10.1093/jxb/33.6.1161. [DOI] [Google Scholar]
- Verheijen F., Jeffery S., Bastos C., Van Der Velde M., Diafas I. Biochar application to soils. A critical scientific review of effects on soil properties, processes and functions. Environment. 2010;8:149. doi: 10.2788/472. [DOI] [Google Scholar]
- Warnock, D.D., 2009. Arbuscular mycorrhizal responses to biochars in soils - potential mechanisms of interaction and observed responses in controlled environments. Division of Biological Sciences and the Microbial Ecology and Department of Crop and Soil Sciences. University of Montana and Cornell University.
- Woods, W.I., Denevan, W.M., 2009. Amazonian dark earths: The first century of reports. In: Teixeira, W.I., Lehmann, W.G., Steiner, J., WinklerPrins, C., Rebellato, L. (Eds.), Amazonian Dark Earths: Wim Sombroek’s Vision. Springer, Dordrecht, pp. 1–14. 10.1007/978-1-4020-9031-8_1
- Woolf D., Amonette J.E., Street-Perrott F.A., Lehmann J., Joseph S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010;1:56. doi: 10.1038/ncomms1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.S., Shukla R., Sharma Y.K. Nickel toxicity on seed germination and growth in radish (Raphanus sativus) and its recovery using copper and boron. J. Environ. Biol. 2009;30:461–466. [PubMed] [Google Scholar]
- Younis U., Qayyum M.F., Shah M.H.R., Danish S., Shahzad A.N., Malik S.A., Mahmood S. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J. Plant Nutr. Soil Sci. 2015;178:209–217. doi: 10.1002/jpln.201400325. [DOI] [Google Scholar]
- Zafar-ul-Hye M., Danish S., Abbas M., Ahmad M., Munir T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy. 2019;9:343. doi: 10.3390/agronomy9070343. [DOI] [Google Scholar]
- Zafar-ul-Hye M., Naeem M., Danish S., Fahad S., Datta R., Abbas M., Rahi A.A., Brtnicky M., Holátko J., Tarar Z.H., Nasir M. Alleviation of cadmium adverse effects by improving nutrients uptake in bitter gourd through cadmium tolerant rhizobacteria. Environments. 2020;7:54. doi: 10.3390/environments7080054. [DOI] [Google Scholar]
- Zafar-ul-Hye M., Naeem M., Danish S., Khan M.J., Fahad S., Datta R., Brtnicky M., Kintl A., Hussain G.S., El-Esawi M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020;9:1386. doi: 10.3390/plants9101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar-ul-Hye M., Naeem M., Danish S., Khan M.J., Fahad S., Datta R., Brtnicky M., Kintl A., Hussain M.S., El-esawi M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020;9 doi: 10.3390/plants9101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar-ul-Hye M., Tahzeeb-ul-Hassan M., Abid M., Fahad S., Brtnicky M., Dokulilova T., Datta R., Danish S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-69183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]