Abstract
Diabetes mellitus (DM) is one of the most serious threats in the 21th century throughout the human population that needs to be addressed cautiously. Nowadays, stem cell injection is considered among the most promising protocols for DM therapy; owing to its marked tissues and organs repair capability. Therefore, our 4 weeks study was undertaken to elucidate the probable beneficial effects of two types of adult mesenchymal stem cells (MSCs) on metabolism disturbance and some tissue function defects in diabetic rats. Animals were classified into 4 groups; the control group, the diabetic group, the diabetic group received a single dose of adipose tissue-derived MSCs and the diabetic group received a single dose of bone marrow-derived MSCs. Herein, both MSCs treated groups markedly reduced hyperglycemia resulting from diabetes induction via lowering serum glucose and rising insulin and C-peptide levels, compared to the diabetic group. Moreover, the increased lipid fractions levels were reverted back to near normal values as a consequence to MSCs injection compared to the diabetic untreated rats. Furthermore, both MSCs types were found to have hepato-renal protective effects indicated through the decreased serum levels of both liver and kidney functions markers in the treated diabetic rats. Taken together, our results highlighted the therapeutic benefits of both MSCs types in alleviating metabolic anomalies and hepato-renal diabetic complications.
Keyword: Diabetes, Diabetic nephropathy, Hyperlipidemia, MSCs
Abbreviations: AD-MSCs, Adipose-derived mesenchymal stem cells; AGEs, Advanced glycation end products; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BM-MSCs, Bone marrow-derived mesenchymal stem cells; BUN, Blood urea nitrogen; CD, Cluster of differentiation; D, Diabetic; DM, Diabetes mellitus; DMEM, Dulbecco's modified Eagle's medium; DN, Diabetic nephropathy; FBG, Fasting blood glucose; FBS, Fetal bovine serum; γ-GT, gamma glutamyl transferase; HbA1c, Glycosylated hemoglobin; HDL-C, High-density lipoprotein cholesterol; HO-1, Heme-oxygenase 1; IPCs, Insulin producing cells; ISCT, International Society for Cellular Therapy; LDL-C, Low-density lipoprotein cholesterol; LPO, Lipid peroxidation; MSCs, Mesenchymal stem cells; PBS, Phosphate-buffered saline; ROS, Reactive oxygen species; SEM, Standard error of mean; SPSS, Statistical Package for Social Scientists; STZ, Streptozotocin; T1DM, Type 1 diabetes mellitus; TC, Total cholesterol; TG, Triglycerides; TL, Total lipids
1. Introduction
The substantial increase in diabetes mellitus (DM) has been a major public health problem affecting 12% of the global population. It is a multiple etiology metabolic disorder that is considered the 7th leading cause of death worldwide (Kodidela et al., 2020, Lima Júnior et al., 2021). In 2017, 425 million patients were diagnosed with diabetes, which is expected to rise by 2045 to 629 million (Buchade et al., 2021). Of note, severe hypoinsulinemia due to pancreatic β-cells autoimmune targeting is considered a characteristic feature of type 1 diabetes mellitus (T1DM). Destruction of β-cells located in the pancreas could lead to blood insulin regulation defect with elevated blood glucose levels; initiating deleterious carbohydrate, protein and lipid metabolic disturbance resulting in marked hyperglycemia; which could in turn lead to various complications progression in different organs such as neuropathy, nephropathy and retinopathy (Williams et al., 2020). In such patients, continuous exogenous insulin therapy is the most common therapeutic strategy used that cannot be avoided; however, it cannot compensate for the normal β-cells’ sensitive adjustment (Takahashi et al., 2019).
Since, in some cases, insulin therapy could not properly control the progression of diabetes and its complications, other alternative therapies might be desirable. For this reason, new approaches of treatment are being searched. Earlier traditional treatments had focused only on the insulin level regulation without curing the diabetic complications, while, modern therapeutic approaches not only mitigate the symptoms of the disease but also improve organs’ function. Cell-based therapy, such as mesenchymal stem cells (MSCs), has been proposed as a promising therapeutic strategy for a number of degenerative disorders and their complications including T1DM; regarding to their regeneration potential and multilineage differentiation (Peng et al., 2018, Aminzadeh et al., 2020). Among various cell types that have been used, mesenchymal stem cells (MSCs) are considered to be one of the most promising types of stem cells for translational application because of their rich tissue sources, multilineage differentiation capacity, easy amplification in vitro, low immunogenicity, regeneration potential, and unique immune biological properties (Ma et al., 2021). MSCs are multipotent, non-hematopoietic stem cells, which can be collected from various sources (bone marrow, liver, kidney, adipose tissue, urine, umbilical cord blood, umbilical tissue Wharton’s jelly, placenta). MSCs are identified by their expression of surface markers, CD73, CD90, and CD105, and lack of expression of hematopoietic markers (CD11b, CD19, CD34, CD45, CD79, HLA-DR). Owing to their multipotency, MSCs can replicate and differentiate into specialized cells to repopulate injured tissues (Birtwistle et al., 2021).
MSCs are multipotent, non-hematopoietic stem cells, which can be collected from various sources (bone marrow, liver, kidney, adipose tissue, urine, umbilical cord blood, umbilical tissue Wharton’s jelly, placenta). MSCs are identified by their expression of surface markers, CD73, CD90, and CD105, and lack of expression of hematopoietic markers (CD11b, CD19, CD34, CD45, CD79, HLA-DR). Owing to their multipotency, MSCs can replicate and differentiate into specialized cells to repopulate injured tissues (Birtwistle et al., 2021). Because bone marrow-mesenchymal stem cells (BM-MSCs) are easily obtained by clinical procedures, it has become one of the researches focuses of cell therapy for diabetes. Several in vitro studies have revealed the reprogramming potency of BM-MSCs to become functional insulin producing cells (IPCs), in addition to, regenerating pancreatic tissues, which could overcome most of the diabetic complications as a consequence to the marked hyperglycemia (Wu et al., 2017, Zang et al., 2017). However, last years have witnessed a significantly increasing interest in adult adipose mesenchymal stem cells (AD-MSCs) as well, as a promising candidate for translational medicine applications. The abundant and renewable source of AD-MSCs, in addition to simple isolation procedure, are only some of the reasons for this success (Argentati et al., 2018, Si et al., 2019).
Since stem cells suitable source selection is critical to ensure their proper differentiation into IPCs to repair diabetic complications, here we performed a head-to-head study comparing the possible therapeutic benefits of both AD-MSCs and BM-MSCs for the treatment of T1DM and its related complications in diabetic rat model while uniformizing all study conditions regarding the rat’s gender, age, weight, housing conditions, and blood glucose level, in addition to, the cell count, phenotype, passage of cells, and the route of injection.
2. Materials and methods
2.1. Chemicals
Streptozotocin (STZ) and culture media constituents of both BM-MSCs and AD-MSCs were purchased from Sigma Aldrich Co. (St. Louis, Mo 6, USA); and were of pure chemical gradient.
2.2. Biochemical assays
Serum glucose concentration was estimated by Trinder (1984) method using SPINREACT Co. diagnostics kit, Spain. Insulin measurement occurred by ELISA kit purchased from Boehringer Mannheim Co., Germany, according to the method of Flier et al. (1976) using Boehringer Analyzer ES 300. While, C-peptide measurement occurred by enzyme immunoassay (EIA) kit purchased from Bio Vision Co., USA, according to the method of Flier et al. (1976). Glycosylated hemoglobin (HbA1c), advanced glycation end products (AGEs) and heme-oxygenase 1 (HO-1) were estimated according to the methods of Gonen and Rubenstein (1978) by using kits obtained from Teco Diagnostics Co., USA. However, Biodiagnostic Co., Egypt kits have been used for the measurement of serum lipid profile markers as total cholesterol (TC), triglycerides (TG), total lipids (TL), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C); according to methods of Young, 2001, Fossati and Prencipe, 1982, Zollner and Kirsch, 1962, Friedewald, 1972, Grove, 1979, respectively. Using kits from Diamond Co., Egypt, serum total proteins were measured according to Henry, (1964), while albumin and globulins were estimated according to Young (2001). Serum liver function markers like gamma glutamyl transferase (γ-GT), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymatic activities were determined according to Scherwin et al. (2003), while alkaline phosphatase (ALP) and total bilirubin was analyzed quantitatively according to Young (2001). Serum kidney function as creatinine, urea and uric acid levels were determined using Biodiagnostic Co., Egypt kits, according to Young (2001) methods. All assessments were carried out according to the instructions of the supplier.
2.3. BM-MSCs and AD-MSCs preparation
Fresh bone marrow and subcutaneous adipose tissues were obtained from male 6–8-week-old rats and used to isolate BM-MSCs and AD-MSCs respectively. To prepare BM-MSCs, each end of the femur and tibia was cut to expose the marrow cavity, then washed three times with phosphate-buffered saline (PBS). Fresh bone marrow was collected and centrifuged at 2000 rpm for 10 min. Pelleted cells were suspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin with 100 μg /ml streptomycin as an antibiotic at 37 °C in a 5% CO2 atmospheric state with 95% humidity, then centrifuged at 2000 rpm for 10 min (Hamza et al., 2017). On the other hand, 1–2 mg fresh rats’ subcutaneous adipose tissues (epididymal fat) were harvested by lipoaspiration, minced, washed extensively three times in PBS and incubated into a digestion solution containing 0.075 % collagenase type I (prepared in PBS) at 37° for 3 h, then centrifuged for 10 min at 2000 rpm. After discarding the supernatant, cells were collected as a pellet and suspended in DMEM (10% FBS, 2 mM L-glutamine, and 100 U/ml penicillin with 100 μg /ml streptomycin as an antibiotic) at 37 °C in 5% CO2 with 95% humidity (Chen et al., 2020). The growth mediums of both MSCs types were changed every 3 days, and non-adherent cells were removed. All MSCs used in this study, were from passage 3–4, and transferred chilled for transplantation within 2 h.
2.4. BM-MSCs and AD-MSCs characterization
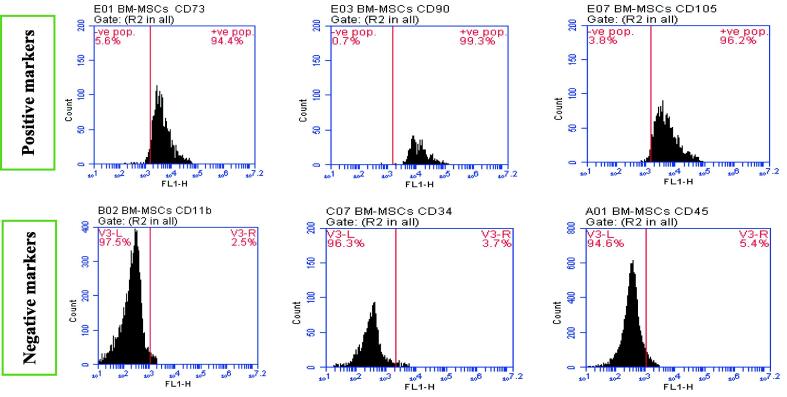
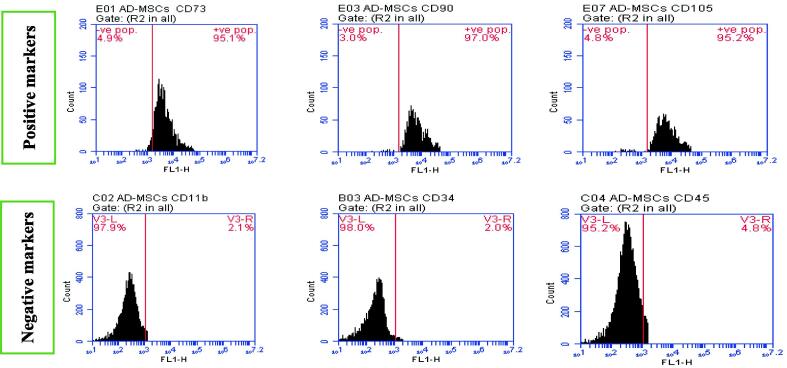
The inverted microscope has been used to perform both BM-MSCs and AD-MSCs morphological characterization for confirming their identity. Moreover, the stemness of cultured cells was confirmed by positive and negative surface markers (CD11b, CD34, CD45, CD73, CD90 and CD105) % for characterization of the isolated BM-MSCs and AD-MSCs phenotyping, Fig. 1A, Fig. 1B. This was determined via Sigma Aldrich Company (USA) kits through flow cytometric analysis to confirm retaining of their phenotype before performing the animal study; according to the minimal criteria defined by ISCT. A fresh tissue specimen was washed with isotonic tris EDTA buffer, dissolved in 250 ml of distilled water at 7.5 pH (using 1N HCl), and centrifuged for 10 min at 1800 rpm., then aspire supernatant. Cells are fixed in ice-cold 96-100% ethanol (1 ml for each sample), then stored in the refrigerator. Regarding cells staining, the fluorochrome is directly linked to the primary antibody (Phycoerythrin (PE) conjugate). Cell suspension adjusted (10 cell/mL) with PBS/BSA buffer pH 7.4. The cell suspension was Aliquoted into test tubes (100 µL each), then a 7µL antibody was added for each tube, mixed well, and incubated for 30 min at room temperature. Then, cells were washed with 2 mL of PBS/BSA and centrifuged for 5 min at 4000 rpm. However, the resulting supernatant was discarded and cells were resuspended in 0.2 mL of PBS/BSA (Tribukait et al., 1975). Finally, data were acquired by flow cytometry using FACS Caliber flow cytometer (Becton Dickinson, Sunnyvale, CA, USA), equipped with a compact air cooked low power 15 m watt argon ion laser beam (488 nm). The average evaluated cells per specimen was 10.000. Dean and Jett’s computer program for mathematical analysis is used to obtain the DNA histograms (Dean and Jett, 1974).
Fig. 1A.
BM-MSCs positive surface markers (CD 73: 94.4 %, CD 90: 99.3 %, CD 105: 96.2 %)andBM-MSCs negative surface markers (CD 11b: 2.5 %, CD 34: 3.7 %, CD 45: 5.4 %).
Fig. 1B.
AD-MSCs positive surface markers (CD 73: 95.1 %, CD 90: 97.0 %, CD 105: 95.2 %)andAD-MSCs negative surface markers (CD 11b: 2.1 %, CD 34: 2.0 %, CD 45: 4.8 %).
2.5. Experimental animals and maintenance
Twenty-four, 6–8 weeks, male Wistar rats weighed 100–120 g were maintained at controlled temperature (22 ± 2 °C) with a 12:12 h light: dark cycle and humidity of 50 ± 5%, with free access to water and chow. Following two weeks of acclimatization, rats were randomly allocated into 4 groups each of 6 animals. The experiential protocol was continued for four consecutive weeks. All experimental procedures were approved and supervised by the Institutional Ethical Committee for the care and use of laboratory animals in the Faculty of Science, Arish University, North Sinai, Egypt. All efforts were made to minimize animal suffering.
2.6. Induction of diabetes
Overnight fasting rats for 12 h were injected intraperitoneally with a single dose of freshly prepared STZ solution (45 mg/kg b.w.) dissolved in 0.05 M cold sodium citrate buffer, pH 4.5; while control rats received the vehicle alone. The STZ-induced diabetic animals were allowed to drink 5% glucose solution overnight to overcome drug-induced hypoglycemia. Three days after induction, diabetes was confirmed by examining blood glucose level from tail vein using Glukotest of diagnosis glucose level by ACCU–CHEKGo apparatus (Roche Company, Germany). Rats with fasting blood glucose level over 200 mg/dl were selected for randomized grouping and considered as diabetic (Kodidela et al., 2020).
2.7. Experimental design
-
1.
Control group: Injected intraperitoneally with a single dose of sodium citrate buffer (pH 4.5).
-
2.
Diabetic (D) untreated group: Injected intraperitoneally with a single dose of STZ (45 mg/kg bw) dissolved in sodium citrate buffer (pH 4.5).
-
3.
Diabetic AD-MSCs treated group: Injected intravenously with a single dose of AD-MSCs (1x106 cell/rat), following diabetes induction confirmation.
-
4.
Diabetic BM-MSCs treated group: Injected intravenously with single dose of BM-MSCs (1x106 cell/rat), following diabetes induction confirmation.
2.8. Sample’s collection
After 4 weeks experimentation period, overnight fasted rats were anesthetized using diethyl ether before being dissected and blood samples were immediately withdrawn directly from the heart. For HbA1c assessment, a few droplets of blood samples were placed in clean heparinized tubes. On the other hand, the remaining of blood samples were collected in clean non-heparinized centrifuge tubes, then let to stand for 15 min, and centrifuged for 15 min at 3000 rpm. Finally, blood sera were carefully separated, labeled and kept at −20 °C for subsequent biochemical analysis.
2.9. Statistical analysis
Obtained data were statistically evaluated with ANOVA followed by Post-Hoc Tukey multiple range tests using Statistical Package for the Social Sciences (SPSS/17.5 software version) for Windows. All the results were expressed as the mean ± SEM for 6 animals in each group. Statistical significance was set at P ≤ 0.05.
3. Results
Fig. 1A, Fig. 1B showed characterization of BM‑MSCs and AD‑MSCs through analyzing a panel of surface markers by flow cytometry. BM‑MSCs and AD‑MSCs were prepared from freshly harvested bone marrow and adipose tissues of a male Wistar rat. The expressions of CD11b, CD34, CD45, CD73, CD90, and CD105 were measured according to fluorescence intensity over respective isotype and unstained negative control Ig in (A) BM‑MSCs and (B) AD‑MSCs. Both MSCs types were positive for CD73, CD90, and CD105, while were negative for CD11b, CD34 and CD45; with variable degrees. Such results confirm both BM-MSCs and AD-MSCs identity after their extraction. Table 1 showed a marked serum glucose, HbA1c and AGEs elevation coupled with a significant insulin, C-peptide and HO-1 levels decline in diabetic group; compared to control. Regarding diabetic rats, all measured parameters in both MSCs-treated groups revealed an obvious enhancement. However, data of diabetic rats injected with AD-MSCs detected a marked hypoglycemic superiority when compared to those of BM-MSCs.
Table 1.
Serum glucose, insulin, C-peptide, HbA1c, AGEs and HO-1 levels.
| Control | Diabetic (D) | D + AD-MSCs | D + BM-MSCs | |
|---|---|---|---|---|
| Glucose(mg/ 100 ml) | 93.91 ± 4.42 | 405.40 ± 16.46 a | 96.75 ± 5.70b | 150.50 ± 5.22abc |
| Insulin (µ I U/ml) | 17.15 ± 0.66 | 8.02 ± 0.33a | 16.90 ± 0.34b | 13.32 ± 0.57abc |
| C-peptide (ng/ml) | 0.84 ± 0.03 | 0.30 ± 0.03a | 0.81 ± 0.01b | 0.75 ± 0.02abc |
| HbA1c (%) | 2.95 ± 0.11 | 5.90 ± 0.20a | 2.93 ± 0.14b | 3.49 ± 0.10abc |
| AGEs (AU/mg protein) | 2.80 ± 0.11 | 8.46 ± 0.35a | 3.00 ± 0.16b | 4.12 ± 0.12abc |
| HO-1 (P mol/mg) | 260.00 ± 13.53 | 87.00 ± 3.60 a | 264.66 ± 8.12b | 218.00 ± 9.04abc |
Values expressed as mean ± SEM (n = 6). a, b and c are Significant differences (P ≤ 0.05) comparing to control, diabetic and diabetic AD-MSCs treated groups respectively.
Illustrated data in Table 2 highlighted a marked rise in all lipid fractions; except for a significant HDL-C decline in diabetic rats; comparable to control rats. However, both treated diabetic groups showed a marked improvement in all lipid parameters; regarding the diabetic group. Notably, diabetic rats injected with AD-MSCs recorded a great amelioration in all tested parameters; comparing to BM-MSCs treated rats.
Table 2.
Serum TL, TG, TC, LDL-C and HDL-C levels.
| Control | Diabetic (D) | D + AD-MSCs | D + BM-MSCs | |
|---|---|---|---|---|
| TL (mg/dl) | 290.80 ± 10.33 | 533.00 ± 29.04a | 295.00 ± 16.07b | 325.60 ± 15.58abc |
| TG (mg/dl) | 56.10 ± 3.30 | 122.00 ± 8.00 a | 58.25 ± 3.27b | 65.00 ± 4.22abc |
| TC (mg/dl) | 76.00 ± 3.16 | 160.20 ± 7.44a | 84.00 ± 4.22b | 89.30 ± 4.46abc |
| LDL-C (mg/dl) | 105.34 ± 0.23 | 166.65 ± 0.54a | 109.68 ± 072b | 120.44 ± 1.07abc |
| HDL-C (mg/dl) | 41.50 ± 2.07 | 18.66 ± 0.96 a | 40.33 ± 1.78b | 36.40 ± 1.82 abc |
Values expressed as mean ± SEM (n = 6). a, b and c are Significant differences (P ≤ 0.05) comparing to control, diabetic and diabetic AD-MSCs treated groups respectively.
Table 3 results demonstrated a significant elevation in kidney function markers of diabetic rats when compared to normal rats. Meanwhile, marked decreases were detected in all tested parameters in both MSCs-diabetic treated rats; in comparison with the diabetic untreated rats. However, values of tested parameters were still significantly higher; in case of BM-MSCs treated group; in regard to control. Interestingly, all kidney function markers exhibited a marked amelioration in AD-MSCs-treated diabetic rats comparing to the treatment with BM-MSCs.
Table 3.
Serum creatinine, urea and uric acid levels.
| Control | Diabetic (D) | D + AD-MSCs | D + BM-MSCs | |
|---|---|---|---|---|
| Creatinine (mg/dl) | 0.44 ± 0.03 | 1.62 ± 0.06a | 0.45 ± 0.02b | 0.69 ± 0.03abc |
| Urea (mg/dl) | 20.43 ± 1.34 | 78.23 ± 4.56a | 22.00 ± 2.87b | 35.22 ± 2.98abc |
| Uricacid (mg/dl) | 1.43 ± 0.05 | 3.22 ± 0.34a | 1.46 ± 0.02b | 1.88 ± 0.04abc |
Values expressed as mean ± SEM (n = 6). a, b and c are Significant differences (P ≤ 0.05) comparing to control, diabetic and diabetic AD-MSCs treated groups respectively.
Data revealed in Table 4 showed significant increases in all tested liver function markers, accompanied by a marked decline in globulins, albumin and total proteins levels in diabetic untreated rats; in regard to control group. All tested parameters were significantly improved in both MSCs treated groups, when compared to the diabetic group. AD-MSCs group data were reverted back to near normal levels when compared to control group. However, BM-MSCs group data still significantly different, comparing to control group. Surprisingly, treatment of diabetic rats with AD-MSCs exhibited an obvious enhancement over the BM-MSCs treatment.
Table 4.
Serum AST, ALT, ALP, γ-GT, total bilirubin, total proteins, albumin and globulins levels.
| Control | Diabetic (D) | D + AD-MSCs | D + BM-MSCs | |
|---|---|---|---|---|
| AST (u/l) | 56.60 ± 3.25 | 115.50 ± 5.38a | 61.20 ± 3.57b | 77.23 ± 4.22abc |
| ALT (u/l) | 30.00 ± 2.75 | 69.83 ± 4.72a | 31.75 ± 2.53b | 45.70 ± 3.21abc |
| ALP (u/l) | 230.50 ± 12.45 | 423.00 ± 34.76a | 233.20 ± 11.3b | 298.46 ± 12.73abc |
| γ-GT (u/l) | 30.00 ± 1.40 | 82.47 ± 4.65a | 32.00 ± 2.08b | 47.82 ± 3.02abc |
| Bilirubin (mg/dl) | 0.58 ± 0.02 | 2.12 ± 0.15a | 0.60 ± 0.03b | 0.77 ± 0.03 abc |
| Total proteins (g/dl) | 7.30 ± 0.32 | 3.50 ± 0.08a | 7.27 ± 0.34b | 5.67 ± 0.26 abc |
| Albumin (g/dl) | 4.31 ± 0.19 | 2.11 ± 0.07a | 4.24 ± 0.15b | 3.31 ± 0.24abc |
| Globulins (g/dl) | 2.90 ± 0.16 | 1.34 ± 0.07a | 2.82 ± 0.22b | 2.20 ± 0.16 abc |
Values expressed as mean ± SEM (n = 6). a, b and c are Significant differences (P ≤ 0.05) comparing to control, diabetic and diabetic AD-MSCs treated groups respectively.
4. Discussion
Mounting evidence suggests that innovative MSCs therapies appear to show promise in regenerative medicine research. Although emerging data from animal studies have demonstrated that injection of MSCs reduces blood glucose levels and restores lipid metabolic balance, the features of MSCs derived from different sources vary greatly. The therapeutic efficacy of MSCs is highly linked to their physiological state, dose, and tissues from where they are derived from. Compared to BM-MSCs, AD-MSCs have become popular for therapy because of the ease of acquisition, high proliferative capacity, low immunogenicity, and high levels of multipotency (Ma et al., 2021). However, the molecular mechanism by which MSCs participate in the treatment of diabetes remains unclear. The possible mechanisms include promoting islet cell regeneration, reducing insulin resistance in peripheral tissues, increasing insulin sensitivity, regulating the immune system, protecting islet β-cells, and improving diabetic complications (Ma et al., 2021, Xue et al., 2021). Stem cells were suggested to exert a therapeutic effect mainly by replacing damaged tissues and paracrine pathways. The effects were initially attributed to MSCs implanting damaged tissue, differentiating, and replacing damaged cells. The subsequent evidence suggests that MSCs also act through the secretion of a broad repertoire of bioactive paracrine growth factors, chemokines, cytokines that could transmit chemical signals between cells, and/or release of microvesicles with paracrine action which could deliver biomolecules with immunomodulatory and pro-regenerative properties to neighboring cell (Birtwistle et al., 2021, Lin et al., 2021, Xue et al., 2021).
4.1. Carbohydrate and glycemic control
Herein, the increase in fasting blood glucose (FBG) in diabetic rats is well expected and agrees with earlier reports cleared that diabetic subjects often exhibited huge serum glucose, AGEs and HbA1c levels, coupled with a marked HO-1, C-peptide, and insulin levels decline compared to the control subjects (Amer et al., 2018, Samaha et al., 2020). Regarding diabetes treatment with MSCs, our results suggested the huge elevation in the level of serum C-peptide in both MSCs injected groups markedly resulted in an obvious rise in the endogenous insulin secretion that could greatly minimize the need for exogenous insulin injection; which clearly points out their efficacy as a DM therapeutic adjunct. Consistent with our data, (Burt et al., 2002) reported that AD-MSCs were found to be not only an IPCs donor source but also greatly supported the resident pancreatic islets as well as local BM-MSCs do. In (Thakkar et al., 2015) study, after two years of injecting IPCs derived from AD-MSCs into 20 patients with T1DM, patients exhibited a significant exogenous insulin requirement decrease, elevated serum C-peptide levels, and decreased HbA1c levels. In this line, (Kono et al., 2014) injected human AD-MSCs in STZ-diabetic mice and reported a marked hypoglycemia coupled with a significant elevation in serum insulin and enhanced glucose tolerance which have been attributed to the capability of AD-MSCs to release numerous cytokines contribute to both β-cell death prevention and β-cell proliferation. (Bassi et al., 2012) also noted improved hyperglycemia in diabetic mice administrated allogeneic AD-MSCs via triggering the expansion of the T-regs cells and attenuating the Th1-related immune response. Moreover, (Amer et al., 2018) found that STZ-diabetic rats received IPCs differentiated from AD-MSCs; showed an apparent islet cells proliferation and regeneration that leads to a marked C-peptide increase accompanied with insulin secretion elevation; in glucose-dependent manner. On the other hand, owing to their ability to stimulate the damaged pancreatic β-cells proliferation and regeneration. BM-MSCs therapeutic effects on diabetes have been also confirmed by many reports. (El-Kholy et al., 2018) revealed a marked serum HO-1, C-peptide and insulin levels elevation in contrast to significant glucose, HbA1c and AGEs serum levels decline; in STZ-diabetic rats. According to (Thakkar et al., 2017), the BM-MSCs hypoglycemic therapeutic potential could be attributed to their (i) direct effect of IPCs differentiation and their intrinsic β-cell regenerative capacity, insulin secretion, and maintenance of the β-cell mass remnant; with (ii) the immunomodulators secretion indirect effect; resulting in a significant autoimmune β-cells damage arrest that induced by T-cells. (Zang et al., 2017) also reported that BM-MSCs showed a powerful glycemic control indicated by the 50% decline in insulin requirements; through restoring islet function, endogenous islet cells protection, induction of islet cell regeneration and differentiation into IPCs; followed by marked restoration of β-cell mass and significant hypoglycemia; in STZ-diabetic mice. Similar results were recently reported by many other studies (Lin et al., 2021, Ma et al., 2021, Xue et al., 2021).
4.2. Lipid profile
A proportional link between impaired glycemic regulation and dyslipidemia progression was greatly confirmed by many scientists (Kubota et al., 2020, Yang et al., 2020). Consistent with our data, (Antony et al., 2017) and (Lin et al., 2017) found dramatically lipoprotein disturbance in STZ diabetic rats; as a result of the significant serum TL, TG, TC, LDL-C and VLDL-C levels increase with HDL-C decrease. Once accumulated in tissues, lipids can generate species that will lead to increased inflammation and oxidative stress inducing the progression of many diabetic complications (Carlier et al., 2020). The hyperlipidemic effect of STZ was indicated by the increase of serum lipid levels in addition to the histology of liver tissue which displayed the presence of lipid accumulation in hepatocytes of diabetic rats compared with the control group (Kilari et al., 2021). However, in harmony with our results, (El-Kholy et al., 2018) reported marked amelioration in the lipid profile markers in BM-MSCs-treated diabetic rats; compared to the diabetic untreated rats. Meanwhile, excessive lipid accumulation prevention was noticed in STZ-diabetic mice; following 8 weeks of BM-MSCs injection (Nagaishi et al., 2014). According to (Gao et al., 2014), MSCs could alleviate the abnormal function of diabetic adipocytes, through both PI3K/AKT insulin signaling pathway and GLUT4 expression up-regulation; accompanied by an elevated release of MSCs insulin-like growth factor-1 (IGF-1). Also, in accordance with our results, AD-MSCs administration to diabetic animals significantly improved the lipid profile as shown by the significant decrease in TGs, TC, LDL-C, while a significant increase in HDL-C, compared to the diabetic untreated mice (Xue et al., 2021) as well as the diabetic untreated rats (Liao et al., 2017). Recently, (Ma et al., 2021) found that AD-MSCs could reduce lipid deposition in the liver of type II diabetic mice, as evidenced by markedly decreased hepatic levels of TL, TC, TG, and LDL-C compared to diabetic untreated mice. Taken together, the hypolipidemic activity of MSCs in DM was clearly confirmed; suggested they usage in atherosclerosis diseases treatment.
4.3. Kidney functions
Since many renal cell types are insulin-sensitive, too high blood glucose levels can cause diabetic nephropathy (DN), a major long-term complication, resulting in severe kidney dysfunction (Rogal et al., 2019). Similar to our findings, (El-Kholy et al., 2018) and (Kodidela et al., 2020) found dramatic glucose and ROS elevation, resulting in serious kidney damage; confirmed via the marked increase in serum kidney function markers (creatinine, uric acid, and urea) levels, in diabetic rats with respect to the control. On the other hand, based on their confirmed therapeutic role in regenerative medicine, MSCs have been suggested as a prominent promising tool to enhance and arrest DN development and progression. Many previous reports highlighted the positive BM-MSCs therapy role in initiating a significant renal function recovery in diabetic rats with DN; as indicated by the marked decline in serum uric acid, urea, and creatinine levels compared to the untreated rats (El-Kholy et al., 2018, Hamza et al., 2017). However, the exact mechanisms of nephroprotection of MSCs remain unclear at present. To date, several potential mechanisms have been proposed. MSCs therapeutic potential involves mainly a paracrine mechanism based on modulation of the immune response, extracellular vesicles and trophic factors release, exertion of anti-fibrotic effects, and renal tissue repair (Li et al., 2018a, Li et al., 2018b). Interestingly, MSCs infusion at the early stages of DN was found to induce marked renal cytokine and macrophage infiltration suppression, nephrocyte apoptosis inhibition, and renal impairment attenuation in diabetic rats; preventing glomerular defects and kidney dysfunction (Sun et al., 2018). Additionally, immunoregulation is one important aspect, encompassing anti-inflammatory, antiapoptotic, and antioxidant action (Lin et al., 2021). Overall, the role of MSCs in the treatment of DN is highly prospective.
4.4. Liver functions
This study results strongly supported the confident relationships between diabetes and liver damage which has previously been established by many studies, in addition to confirming the ameliorating effect of both AD-MSCs and BM-MSCs on restoring the normal hepatic functions. Likely, BM-MSCs therapy could enhance the endogenous hepatocyte regenerative processes; owing to their characteristic self-renewal and differentiation potential capabilities; that could ameliorate liver function in advanced chronic liver disease (Kumar et al., 2011). In accordance with our results, (El-Kholy et al., 2018) highlighted BM-MSCs protective ability on both hepatocytes structure and function in STZ-diabetic rats; indicated via the marked amelioration in the serum total bilirubin, γ-GT, ALP, ALT and levels. In this regard, MSCs injection was found to improve liver function in a mouse model of hepatic failure (Lee et al., 2018). Moreover, (Liao et al., 2017) found that AD-MSCs transplantation was able to alleviate hyperglycemia and insulin resistance of type II diabetic rats, besides protecting against the pathogenic changes of liver fibrosis and recovering the injured liver function; as evidenced through reduced serum levels of AST, ALT, ALP, and TB compared with those in the diabetic untreated group; suggesting that AD-MSCs transplantation may be an effective therapeutic approach for T2DM patients with liver fibrosis. Also, (Ma et al., 2021) reported that AD-MSCs treatment significantly ameliorated hepatic functions in type II diabetic mice, as evidenced by markedly decreased serum levels of ALT and AST and dramatic reduced histological lesion of liver tissue compared to diabetic untreated mice. Indeed, MSCs could release paracrine factors needed for modulation of the immune response, cell proliferation stimulation, functional liver restoration, and attenuation of cell death mechanisms; which are collectively induced and support the MSCs hepatocyte differentiation potential (Wang et al., 2017a, Wang et al., 2017b). Thus, we can assume that the MSCs administration has a great hepato-protective role in DM which could enhance liver function and restoration of appropriate protein metabolism balance.
5. Conclusion
Recent breakthroughs in deriving glucose-responsive β-like cells from MSCs have provided encouragement for β cell replacement therapy. This study explored the potential of both AD-MSCs and BM-MSCs for the treatment of T1DM. Under the same experimental criteria, we found that AD-MSCs had a stronger ability over BM-MSCs to alleviate hyperglycemia, insulin deficiency, and dyslipidemia besides restoring normal renal and hepatic functions. Hence, we could suggest AD-MSCs, in particular, offer an ideal alternative for β-cells transplantation that might aid in the reversal of T1DM hazards.
6. Authors’ contributions
SGE and FA conceived and designed the study. Experiments and lab work were done by SGE, HMR, EIH, RMA, and ES; while data tabulating and acquisition, searching for literature and preparing the first draft of the manuscript were performed by SGE, AA, and EF All authors have read and agreed to the published version of the manuscript.
7. Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
8. Ethics approval
All experimental procedures were done according to research protocols approved by the Animal Care and Bioethics Committee of the Faculty of Science, Arish University, North Sinai, Egypt.
9. Funding
This study was supported by Taif University Researchers Supporting Project (TURSP-2020/222), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amer M.G., Embaby A.S., Karam R.A., Amer M.G. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018;654:87–94. doi: 10.1016/j.gene.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Aminzadeh A., Maroof N.T., Mehrabani M., Juybari K.P., Sharifi A.M. Investigating the Alterations of Oxidative Stress Status, Antioxidant Defense Mechanisms, MAP Kinase and Mitochondrial Apoptotic Pathway in Adipose-Derived Mesenchymal Stem Cells from STZ Diabetic Rats. Cell J. 2020;22(1):38–48. doi: 10.22074/cellj.2020.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony P.J., Gandhi G.R., Stalin A., Balakrishna K., Toppo E., Sivasankaran K., Ignacimuthu S., Al-Dhabi N.A. Myoinositol ameliorates high-fat diet and streptozotocin-induced diabetes in rats through promoting insulin receptor signaling. Biomed. Pharmacother. 2017;88:1098–1113. doi: 10.1016/j.biopha.2017.01.170. [DOI] [PubMed] [Google Scholar]
- Argentati C., Morena F., Bazzucchi M., Armentano I., Emiliani C., Martino S. Adipose Stem Cell Translational Applications: From Bench-to-Bedside. Int. J. Mol. Sci. 2018;19(11):3475. doi: 10.3390/ijms19113475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi E.J., Moraes-Vieira P.M.M., Moreira-Sa C.S.R., Almeida D.C., Vieira L.M., Cunha C.S., Hiyane M.I., Basso A.S., Pacheco-Silva A., Camara N.O.S. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61(10):2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtwistle L, Chen X, Pollock C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchade S., Desai S., Bhonde R., Kazi H., Sainani S., Rode K. Stem Cells: A Golden Therapy for Diabetic Wounds. Curr Diabetes Rev. 2021;17(2):156–160. doi: 10.2174/1573399816666200716200450. [DOI] [PubMed] [Google Scholar]
- Burt R.K., Oyama Y., Traynor A., Kenyon N.S. Hematopoietic stem cell therapy for type 1 diabetes: Induction of tolerance and islet cell neogenesis. Autoimmun Rev. 2002;1:133–138. doi: 10.1016/s1568-9972(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Carlier A., Phan F., Szpigel A., Hajduch E., Salem J.-E., Gautheron J., Le Goff W., Guérin M., Lachkar F., Ratziu V., Hartemann A., Ferré P., Foufelle F., Bourron O. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Reports Med. 2020;1(9):100154. doi: 10.1016/j.xcrm.2020.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-S., Lai P.-F., Kuo C.-H., Day CeciliaHsuan, Chen R.-J., Ho T.-J., Yeh Y.-L., Mahalakshmi B., Padmaviswanadha V., Kuo W.-W., Huang C.-Y. Resveratrol enhances therapeutic effect on pancreatic regeneration in diabetes mellitus rats receiving autologous transplantation of adipose-derived stem cells. Chin J. Physiol. 2020;63(3):122. doi: 10.4103/CJP.CJP_3_20. [DOI] [PubMed] [Google Scholar]
- Dean P.N., Jett J.H. Mathematical analysis of DNA distributions derived from flow microfluorometry. Cell Biol. J. 1974;60:523–524. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kholy W.M., Hussein R.H., Khalil D.Y. Assessment of the potential ameliorating effects of BM-MSCs or insulin on the altered metabolic status of pancreas, liver and kidney in STZ-diabetic rats. Int. J. Adv. Res. 2018;6(8):18–34. [Google Scholar]
- Flier J.S., Kahn C.R., Jarrett D.B., Roth J. Characterization of antibodies to the insulin receptor: A cause of insulin-resistant diabetes in man. J. Clin. Investigation. 1976;58(6):1442–1449. doi: 10.1172/JCI108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined calorimetrically with an enzyme that procedures hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Friedewald Determination of serum low density lipoprotein cholesterol (LDL-C) Clin. Chem. 1972;25:560–562. [Google Scholar]
- Gao D., Xie J., Zhang J., Feng C., Yao B., Ma K., Li J., Wu X., Huang S., Fu X. MSC attenuate diabetes-induced functional impairment in adipocytes via secretion of insulin-like growth factor-1. Biochem. Biophys. Res. Commun. 2014;452(1):99–105. doi: 10.1016/j.bbrc.2014.08.060. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A.H. Estimation of glycosylated hemoglobin. Diabetologia. 1978;15:1–3. [Google Scholar]
- Grove T. Effect of reagent pH on determination of HDL Cholesterol by precipitation with sodium phosphotungstate-magnesium. Clin Chem. 1979;25:560–562. [PubMed] [Google Scholar]
- Hamza A.H., Al-Bishri W.M., Damiati L.A., Ahmed H.H. Mesenchymal stem cells: a future experimental exploration for recession of diabetic nephropathy. Ren. Fail. 2017;39(1):67–76. doi: 10.1080/0886022X.2016.1244080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.J. Determination of total protein by colorimetric method. Clin chem. 1964;3:181–182. [Google Scholar]
- Júnior J.P., Franco R.R., Saraiva A.L., Moraes I.B., Espindola F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J. Ethnopharmacol. 2021;268:113667. doi: 10.1016/j.jep.2020.113667. [DOI] [PubMed] [Google Scholar]
- Kilari B.P., Mudgil P., Azimullah S., Bansal N., Ojha S., Maqsood S. Maqsood S. Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats. J. Dairy Sci. 2021;104(2):1304–1317. doi: 10.3168/jds.2020-19412. [DOI] [PubMed] [Google Scholar]
- Kodidela S., BegumShaik F., Chinta V., AliMohammad S., Pasala C., Mittameedi S. Possible ameliorative role of green tea on chronic alcohol mediated renal toxicity of STZ -induced diabetic rats. Clin Nutrition Experimental J. 2020;34:1–25. [Google Scholar]
- Kono T.M., Sims E.K., Moss D.R., Yamamoto W., Ahn G., Diamond J. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32(7):1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Watanabe R., Hosojima M., Saito A., Sasou A., Masumura T., Harada Y., Hashimoto H., Fujimura S., Kadowaki M. Rice bran protein ameliorates diabetes, reduces fatty liver, and has renoprotective effects in Zucker Diabetic Fatty rats. J. Funct. Foods. 2020;70:103981. doi: 10.1016/j.jff.2020.103981. [DOI] [Google Scholar]
- Kumar A., Pati N., Sarin S. Use of Stem Cells for Liver Diseases: Current Scenario. J. Clin. Experimental Hepatol. 2011;1(1):17–26. doi: 10.1016/S0973-6883(11)60114-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Chen Y.-F., Wu H.-H., Lee O.K. Historical Perspectives and Advances in Mesenchymal Stem Cell Research for the Treatment of Liver Diseases. Gastroenterology. 2018;154(1):46–56. doi: 10.1053/j.gastro.2017.09.049. [DOI] [PubMed] [Google Scholar]
- Li H., Rong P., Ma X., Nie W., Chen C., Yang C. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018;215:113–118. doi: 10.1016/j.lfs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu J., Liao G., Zhang J., Chen Y., Li L. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int. J. Mol. Med. 2018 doi: 10.3892/ijmm10.3892/ijmm.2018.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Li H, Yang Q, et al. Administration of mesenchymal stem cells in diabetic kidney disease: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12(34) doi: 10.1186/s13287-020-02108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Qi Y., Huang C., Wu M., Wang C., Li F. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin. Nutrition. 2017;37(4):1423–1429. doi: 10.1016/j.clnu.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Liao N, Zheng Y, Xie H, et al. Adipose tissue-derived stem cells ameliorate hyperglycemia, insulin resistance and liver fibrosis in the type 2 diabetic rats. Stem Cell Res Ther. 2017;8 doi: 10.1186/s13287-017-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang L, Yang s, et al. The tissue origin of human mesenchymal stem cells dictates their therapeutic efficacy on glucose and lipid metabolic disorders in type II diabetic mice. Stem Cell Res Ther. 2021;12(358) doi: 10.1186/s13287-021-02463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi K., Ataka K., Echizen E., Arimura Y., Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic hepatocyte damage in mice by inhibiting infiltration of bone marrow-derived cells. Hepatology. 2014;59(5):1816–1829. doi: 10.1002/hep.26975. [DOI] [PubMed] [Google Scholar]
- Peng B., Dubey N.K., Mishra V.K., Tsai F.C., Dubey R., Deng W. Addressing Stem Cell Therapeutic Approaches in Pathobiology of Diabetes and Its Complications. J. Diabetes Res. 2018;2018:1–16. doi: 10.1155/2018/7806435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogal J., Zbinden A., Schenke-Layland K., Loskill P. Stem-cell based organ-on-a-chip models for diabetes research. Adv. Drug Deliv. Rev. 2019;140:101–128. doi: 10.1016/j.addr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Samaha M.M., Said E., Salem H.A. Modulatory role of imatinib mesylate on pancreatic β-cells' secretory functions in an STZ rat model of diabetes mellitus. Chemico-Biological Interaction. 2020;328:109197. doi: 10.1016/j.cbi.2020.109197. [DOI] [PubMed] [Google Scholar]
- Scherwin J.E., Kaplan L.A., Pesce A.J., Kazmierczak S.C. Liver function. Clin. Chem. 2003;4:492–496. [Google Scholar]
- Si Z., Wang X., Sun C., Kang Y., Xu J., Wang X., Hui Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. & Pharmacother. 2019;10 doi: 10.1016/j.biopha.108765. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhao F., Zhang W., Lv J., Lv J., Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signaling pathway. J. Cell Mol. Med. 2018;22:4840–4855. doi: 10.1111/jcmm.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Sakata N., Yoshimatsu G., Hasegawa S., Kodama S. Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. J. Clin. Med. 2019;8(2):16–25. doi: 10.3390/jcm8020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar U.G., Trivedi H.L., Vanikar A.V., Dave S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015;17(7):940–947. doi: 10.1016/j.jcyt.2015.03.608. [DOI] [PubMed] [Google Scholar]
- Thakkar U.G., Vanikar A., Trivedi H. Should we practice stem cell therapy for type 1 diabetes mellitus as precision medicine? Cytotherapy. 2017;19(5):574–576. doi: 10.1016/j.jcyt.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Tribukait B, Moberger G, Zetterberg A. Methodological aspects of rapid-flow cytofluorometry for DNA analysis of human urinary bladder cells. European Press. 1975;1:50–60. [Google Scholar]
- Trinder Acolorimetric method for determination of glucose. Ann. Clin. Biochem. 1984;6:24–26. [Google Scholar]
- Wang I., Tao T., Su W., Yu H., Yu Y., Qin J. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevive. Lab Chip. 2017;17:1749–1760. doi: 10.1039/c7lc00134g. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yan J., Zou X., Guo K., Zhao Y., Meng C. Bone marrow mesenchymal stem cells repair cadmium-induced rat testis injury by inhibiting mitochondrial apoptosis. Chem. Biol. Interact. 2017;271:39–47. doi: 10.1016/j.cbi.2017.04.024. [DOI] [PubMed] [Google Scholar]
- Williams D.M., Nawaz A., Evans M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020;11(6):1199–1216. doi: 10.1007/s13300-020-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.H., Liu C.P., Xu K.F., Mao X.D., Zhu J., Jiang J.J. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J. Gastroenterol. 2017;13(24):3342–3349. doi: 10.3748/wjg.v13.i24.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Xiao X, Tu T, et al. Mesenchymal stem cells modified by FGF21 and GLP1 ameliorate lipid metabolism while reducing blood glucose in type 2 diabetic mice. Stem Cell Res Ther. 2021;12(133) doi: 10.1186/s13287-021-02205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhou L., Wang X., Wang W., Wang J. Inhibition of HMGB1 involved in the protective of salidroside on liver injury in diabetes mice. Int. Immunopharmacol. 2020;89(Part:A). doi: 10.1016/j.intimp.106987. [DOI] [PubMed] [Google Scholar]
- Young D.S. Effects of disease on clinical laboratory tests. Clin Chem. 2001;4:17–18. [Google Scholar]
- Zang L., Hao H., Liu J., Han W., Mu Y. Mesenchymal stem cell therapy in type II diabetes mellitus. Diabetology Metabolic Syndrome. 2017;9(36):1–11. doi: 10.1186/s13098-017-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner N., Kirsch K. Micro-determination of lipids by the sulphophosphovanillin reaction. Reaction Exp. Med. 1962;135:545–548. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.