Abstract
Hydatidosis is a zoonotic disease caused by Echinococcus granulosus larvae, which affects sheep worldwide, especially in rural communities. This study aims to determine the prevalence and structure of hydatid cyst in sheep. A total number of 1,198 sheep in different age groups G1 (<1 year), G2 (1–2 years) and G3 (>2 years) were slaughtered at Taif abattoirs, then examined for the presence of hydatid cysts in lung, liver, and mesentery. Prevalence of hydatid cyst infection in imported sheep (13.0%) was higher than of local sheep (10.2%). Particularly, as per gender, prevalence of imported females (71.9%) was higher than those of local females (28.1%), while that of imported males (66.3%) was higher than those of local males (33.7%). Large sizes of hydatid cysts and fertility recorded in G3 were higher in both local and imported sheep than those of G1 and G2. Morphometric analysis of pathological lesions in liver of all infected sheep showed a significant increase compared to non-infected healthy sheep (have no lesions) (P < 0.001). In addition, for all infected sheep, histochemical investigation with Masson’s trichrome stain showed collagen fibers inside the hydatid cyst capsules and in pericystic region. The collagen fibers content and the cellular laminated membranes took the green color, while immunohistochemical evaluation detected a positive reaction for CD3.
Keywords: Hydatidosis, Hydatid cyst, Sheep, Histomorphic, Histochemical, Immunohistochemistry
1. Introduction
Hydatid disease (cystic echinococcosis) is a chronic granulomatous zoonotic disease that affects sheep and results from the development of the intermediate larval stage of Echinococcus granulosus in various sites at different organs principally the liver, lungs and rare in mesentery (Beigh et al., 2017). Dogs and several other carnivores act as definitive hosts. Sheep are the most known intermediate hosts (Eckert and Deplazes, 2004, Kern et al., 2004). The reasons that may cause increased exposure risk of sheep to hydatidosis include inappropriate disposal of dead animals, access of farm dogs to the viscera and trimmings of a butchered sheep, farmers are careless in treating farm dogs with antihelminthics as well as leaving flocks of sheep grazing in fields where homeless dogs easily access to (Possenti et al., 2016, Murtaza et al., 2017). This disease causes widespread morbidity and mortality worldwide (Al-Hayyali, 2000, Al-Sabawi, 2001). Several studies indicated that hydatid disease is an endemic zoonotic disease in KSA affecting both humans and their domestic animals according to (Ibrahim, 2010) in Taif (Hayajneh et al., 2014). Epidemiology of hydatidosis is determined using cyst fertility as an important factor, where it depends on both the species of the intermediate host and the area affected by the disease (Ibrahim, 2010). As the disease causes production and economic losses according to previous studies (Torgerson et al., 2000, Torgerson and Dowling, 2001), it is characterized by the formation of single or multiple cysts of various sizes (Surhio et al., 2011). The cyst may remain dormant for many years. However, it poses a hazard to the rupture at any time, resulting either in the formation of daughter cysts or, uncommonly, in death due to asphyxiation or to a hypersensitivity reaction to the cyst contents (Burlew et al., 1990). Many studies have been conducted on cystic hydatidosis prevalence in slaughtered animals in different areas of Saudi Arabia, but a few or rare studies have addressed the histological appearance (Alsulami, 2019). This study aims to determine the prevalence and structure of hydatid cyst in local and imported slaughtered sheep based on the gender. Our selection of both imported and local sheep is to assess which category carries the highest hydatidosis prevalence. In turn, the obtained results will be reported to the respective local authorities to help them keeping public health of the living population at the safest status. In addition, the present study aims to perform histomorphic, histochemical and immunohistochemical evaluations for pathological alternation that takes place in lung, liver and mesentery accompanied hydatid cyst in sheep of different ages from Taif abattoirs.
2. Materials and method
2.1. Ethics approval
We have got the samples from animals slaughtered at Taif abattoirs, KSA, which is a normal daily work at abattoirs. Labelling of samples either as local or imported was the responsibility of the abattoir’s veterinarian who was dedicated to provide us with the samples. The first author follows ethical guidelines, and she has an approved certificate from The National Committee of Bioethics (NCBE) at King Abdulaziz City for Science and Technology (KACST), the highest authority gives ethical approvals in KSA, with number: 10023117, valid till 01 October 2023.
2.2. Study area
The study was conducted in Taif governorate (21.2819° N, 40.3841° E) located in western area of Saudi Arabia. Active survey was performed by visiting the abattoirs biweekly on periodic basis started from January to October 2020 to detect hydatid cysts in sheep obtained from two sources; local (from different local farms surrounding the slaughterhouse) and imported (from Sudan, Somalia and Romania). Grazing was the mostly followed husbandry practice for these animals before being slaughtered.
2.3. Sample size
A Total number of 1,198 sheep (561 local sources and 637 imported sources) of both sexes were inspected for detection of any hydatid cysts. Sheep examined were classified according to the age into 3 groups. The first group (G1) includes sheep of<1 year old, the second group (G2) includes sheep of 1–2 years old, and the third group (G3) includes sheep more than 2 years old. Post-mortem examination procedures employed visual examination, palpation, and systematic incision of each carcass and visceral organs, particularly the lung, liver, and mesentery according to the procedure recommended by FAO/UNEP/WHO (Eckert et al., 1984). The study data such as the source of animals, sex and age were recorded. The cysts were carefully removed from targeted organs and kept in a sterilized container. The samples were transferred to the lab at College of Science at Taif University.
2.4. Determination of cyst size
Cyst size determination was done for hydatid cysts taken from both local and imported sheep of different ages (G1, G2 and G3) by measuring the diameter of the cyst in centimeters using a measuring tape (Thrusfield, 2005). Then, according to size of the cysts, they were categorized as follows: small cyst (≤1 cm), medium cyst (2–5 cm) and large cyst (greater than5 cm).
2.5. Examination of cyst fertility
Cyst fertility was performed as per (Daryani et al., 2007). In brief, individual cysts were grossly examined to detect degeneration, then hydatid cysts in sheep were selected for fertility study according to the size (excluding those of too small size). Cyst walls were penetrated using a needle to reduce intracystic pressure, then it was cut with scalpel and scissors. After that, cyst contents were transferred into a sterile container. Cysts were divided into four categories, sterile (cyst filled with fluid, but without any protoscolices), calcified/nonviable (cysts with dead protoscolices) and viable or fertile (cysts with live protoscolices). Viability of the protoscolices was determined by placing a drop of cyst fluid on a microscopic slide, applying a cover slip then observed the flame cells motile activity like peristaltic movement (Fig. 1a). Slides were stained by adding a drop of aqueous eosin solution (0.1%), then examination was performed under a light microscope to confirm doubtful motility. Dead protoscolices take the dye, but the viable/fertile ones did not (Muluneh and Hailu, 2019).
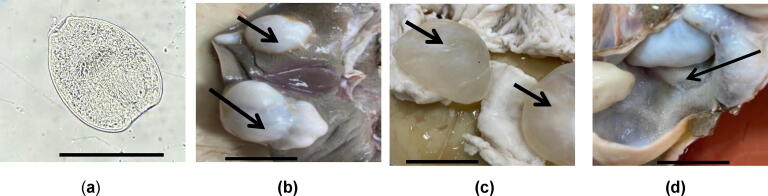
Fig. 1.
(a) Viable non-stained protoscolices. Scale-bar = 100 μm; (b) Hydatid cysts in liver of sheep (arrow); (c) Intact cyst wall adjacent mesentery, the cyst is popping out (arrow); (d) Cyst cavity. Scale-bar = 6 cm (for b, c and d).
2.6. Histopathological, histochemical and histomorphometric analysis
Specimens were grossly examined, and sections were taken from the wall of the cyst and from the neighboring lung, liver, and mesenteric tissues. Control sections were taken from 30 non infected sheep. All tissue sections, either infected or control, were fixed in 10.0% neutral buffered formalin solution for routine histopathological examination. The fixed specimens were trimmed, washed, dehydrated in ascending grades of alcohol, cleared in xylene, and embedded in paraffin. The embedded samples were sectioned at 3–5 μm thickness, stained with hematoxylin and eosin (H&E) for histomorphometric analysis. However, for histochemical analysis, samples were stained using Masson’s trichrome stain. All these procedures were applied as previously described by (Suvarna et al., 2013).
-
•
Histomorphometric analysis
Sections were prepared from lung, liver and mesenteric tissues taken from positive hydatidosis sheep (local (30), imported (30)) and from (30) non-infected ones, then stained with H&E for measurement of different alternation. Semi-automatic computer-assisted planimetry was used for direct morphometry on microscopic images by using a light microscope (BX50, Olympus, Tokyo, Japan) at a magnification 400x.
-
•
Immunohistochemical study.
Sections were stained using markers for CD3 and were counterstained with Harris hematoxylin, according to the protocol of Alborg pathology lab, Saudi Arabia. Our selection was based on the relation between CD3 expression and the nuclear immunopositive reaction (Ibrahim and Gameel, 2014).
2.7. Statistical analysis
The obtained data were collected and statistically analyzed to detect the infection rate of hydatidosis among local and imported sheep. Moreover, the analysis was conducted for correlation between age and both of sizes and types of hydrated cyst in infected sheep. In addition, analyses were conducted for hydated cyst distribution in lung, liver, and mesenteric tissues of infected local imported sheep along with and histopathological alternation of the same, which examined in lung from non-infected (control) and infected groups. For all tests, a P-value of (p < 0.001) was considered indicative of a statistically significant difference. The obtained results were statistically analyzed using Student t–test according to Steel and Torrie (1960).
3. Results
Prevalence of hydatid cyst was 10.2% and 13.0% for local and imported sheep, respectively (Table 1). The prevalence in imported sheep was significantly higher than of local sheep (at P < 0.05). In addition, the prevalence of hydatidosis was significantly different according to the sex of sheep. Regarding females, incidence of infection in 41 local female sheep was (71.9%) compared to (66.3%) in 55 imported female sheep. On the other hand, prevalence in 16 local male sheep was (28.1%) compared to (33.7%) in 28 imported ones (Table 1). Determination of cyst size revealed that the small size cysts for both groups; local and imported, in G1 (8.8%, 13.3%) were higher than the sizes in G2 (1.7%, 3.6%) and G3 (3.5%, 2.4%), respectively. However, the middle size cysts for both groups; local and imported, in G2 (10.5%, 8.4%) were higher than the sizes in G1 (5.3%, 6.0%) and G3 (1.7%, 3.6%), respectively. On the other hand, the large size cysts for both groups; local and imported, in G3 (33.7%, 38.6%) were higher than the sizes in G1 (1.7%, 1.2%) and G2 (28.1%, 27.2%), respectively (Table 2).
Table 1.
The incidence and prevalence of hydatidosis in both local and imported sheep based on their sex.
| Source of Sheep | Total | Non-infected (Incidence) |
Infected |
Percent of infection | |
|---|---|---|---|---|---|
| Female | Male | ||||
| (Incidence) n = 57 | |||||
| Local | 561 | 504 | Prevalence | 10.2% | |
| 41 (71.9%) | 16 (28.1%) | ||||
| Imported | 637 | 556 | (Incidence) n = 83* | 13.0% | |
| Prevalence | |||||
| 55 (66.3%) | 28 (33.7%) | ||||
Data expressed as mean ± SD: * significant (p < 0.05).
Table 2.
Correlation between hydatid cyst size and age of infected sheep.
| Cyst Diameter (cm) |
Local (n = 57) |
Imported (n = 83) |
||||
|---|---|---|---|---|---|---|
|
Age Group | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | |
| Small |
5 (8.8%) |
1 (1.7%) |
2 (3.5%) |
11 (13.3%) |
3 (3.6%) |
2 (2.4%) |
| Middle |
3 (5.3%) |
6 (10.5%) |
1 (1.7%) |
5 (6.0%) |
7 (8.4%) |
3 (3.6%) |
| Large |
1 (1.7%) |
16 (28.1%) |
22 (38.6%) |
1 (1.2%) |
23 (27.2%) |
28 (33.7%) |
Examination of the hydatid cyst types taken from different organs of local and imported sheep revealed that the fertile cyst type was the most common among both groups. Results show that the fertile cysts for both groups; local and imported, in G3 (38.6%, 39.8%) were higher than those of G2 (28.1%, 30.1%) and G1 (5.3%, 4.8%), respectively. While the number of sterile cysts for both groups; local and imported, in G1 (12.3%, 14.5%) was higher than that of G2 (10.5%, 6.0%) and G3 (1.7%, 1.2%), respectively. However, the calcified cyst showed the lowest incidence for both groups; local and imported, in G1 (0.0%, 0.0%), while it showed a higher incidence in G2 (1.7%, 2.4%), then G3 (1.7%, 1.2%) (Table 3).
Table 3.
Correlation between types of hydatid cyst and age of infected sheep.
| Types of Hydatid Cyst |
Local (n = 57) |
Imported (n = 83) |
||||
|---|---|---|---|---|---|---|
|
Age Group | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | |
| Sterile |
7 (12.3%) |
6 (10.5%) |
1 (1.7%) |
12 (14.5%) |
5 (6.0%) |
1 (1.2%) |
| Fertile |
3 (5.3%) |
16 (28.1%) |
22 (38.6%) |
4 (4.8%) |
25 (30.1%) |
33 (39.8%) |
| Calcified |
0 (0.0%) |
1 (1.7%) |
1 (1.7%) |
0 (0.0%) |
2 (2.4%) |
1 (1.2%) |
Distribution of hydatid cysts in organs showed that the most frequently affected organ was the liver, followed by the lung. However, the mesentery showed the least affected organ by infection, in both local and imported sheep groups from all ages (Table 4).
-
•
Histopathological and histochemical evaluation
Table 4.
Hydatid cysts distribution in lung, liver, and mesentery of infected local and imported sheep.
| Organ |
Local (n = 57) |
Imported (n = 83) |
||||
|---|---|---|---|---|---|---|
|
Age Group | ||||||
| G1 | G2 | G3 | G1 | G2 | G3 | |
| Lung |
2 (3.5%) |
8 (14.0%) |
6 (10.3%) |
4 (4.8%) |
9 (10.8%) |
11 (13.3%) |
| Liver |
6 (10.5%) |
15 (26.3%) |
16 (28.1%) |
10 (12.0%) |
19 (22.9%) |
23 (27.7%) |
| Mesentery |
0 (0.0%) |
1 (1.7%) |
3 (5.5%) |
2 (2.4%) |
4 (4.8%) |
1 (1.2%) |
3.1. Gross pathology
Hydatid cysts in lung, liver, and mesentery of infected sheep in different ages, sex and sources were grossly appeared in variable sizes, round shapes, and some of them were seen either bulging from the surface or deeply seated in the tissue (Fig. 1a-1c). Most cysts observed were soft and like dough when touching them. In addition, cysts were contained cavities with smooth membrane and filled with clear to somewhat turbid fluid (Fig. 1d). On the other hand, some cysts had firm appearance and with thickened contents. However, few cysts were found calcified, hard to cut and grainy. In addition, gross enlargement of affected organs was observed.
3.2. Lung
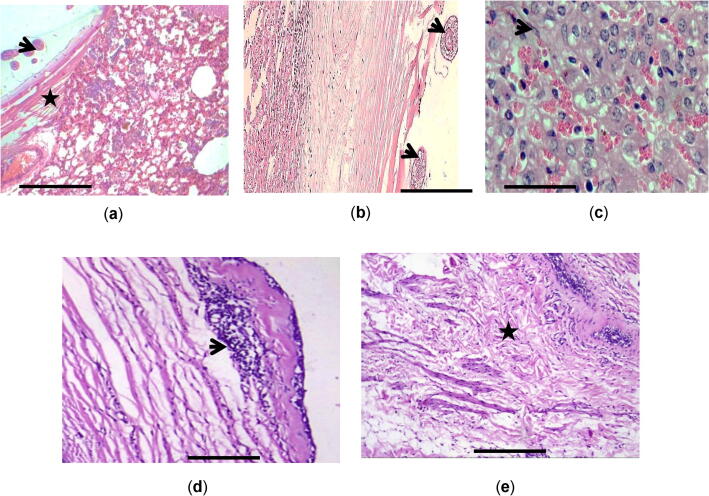
Histologically, lungs of non-infected sheep showed normal histological structures. However, lungs from infected local and imported sheep showed the cyst wall with its characteristic three layers structure; a thin inner germinal layer, a laminated layer, and an outer adventitial layer. Most examined sections had laminated membranes and germinal layers intact and containing brood capsules with protoscolices. Some cysts showed calcification in their walls, as well as inflammatory reactions due to eosinophils located directly nearby the cyst wall and reached to the surrounding alveoli and the adjacent bronchioles. Also, noticed hyperplasia of the bronchial and bronchiolar epithelium, thickening of the pulmonary interstitial structures by inflammatory cell infiltrate mainly lymphocytes and alveolar macrophages. Alveolar emphysema was seen adjacent to atelectatic alveoli nearby the cysts which lead to conventional bronchiectasis (BN). In addition, congestion of pulmonary vessels is observed along with hemorrhage in some sections (Fig. 2a). Morphometric analysis of pathological lesion in infected sheep included estimating the number of bronchiectasis, alveoli atelectasis (AA), areolar emphysema (AE), estimation of bronchiolar epithelial hyperplasia (EH), thickening of interalveolar septa (TIS), collagen fiber amount (CF), pulmonary vessels congestion (PC). Significant increase in these parameters was observed in infected sheep versus non-infected sheep (P < 0.05) and (P < 0.001) (Table 5).
Fig. 2.
(a) Hydatidosis affected lung showing parasitic membranes (star); Brood capsules containing protoscolices (arrowhead) and inflammatory reaction; thickening of pulmonary interstitial structures, alveolar emphysema adjacent to atelectatic H&E; (b) Liver showing laminated cyst wall containing protoscoilces (arrowhead); surrounded immediately by inflammatory cell infiltration (star) H&E. (c) Liver showing inflammatory cell infiltration mainly eosinophil’s (arrow), lymphocytes and macrophages. H&E. (d) Mesentery showing inflammatory reaction adjacent cyst wall (arrow) H&E. (e) Mesentery showing proliferation of collagen fiber H&E. Scale-bar = 50 μm.
Table 5.
Histopathological alteration in examined lung from non-infected (control) and infected group.
| Histopathological alteration |
Control (n = 30) |
Infected (n = 140) |
|---|---|---|
| (BN) | 0.00 | 13.00 ± 4.00* |
| (AA) | 0.00 | 9.00 ± 3.11* |
| (AE) | 0.00 | 14.30 ± 4.10* |
| (BH) | 24.01 ± 9.03 | 36.12 ± 13.20* |
| (TIS) | 70.03 ± 20.11 | 123.11 ± 23.09** |
| (CF) | 18.04 ± 5.13 | 86.00 ± 21.15** |
| (PC) | 0.00 | 28.00 ± 10.12** |
Data expressed as mean ± SD: * significant (p < 0.05), ** Highly significant (p < 0.001).
3.3. Liver
Liver from non-infected sheep showed normal architecture structure. While liver sections from infected local and imported sheep with hydatidosis revealed that the parenchyma adjacent to cysts was markedly congested with multiple small hemorrhagic areas. Widespread necrosis and degeneration were markedly obvious in the hepatic lobules. Central veins and sinusoidal spaces were severely congested, and oedemas were seen. In addition, inflammatory cell infiltration mainly eosinophils, macrophages, and lymphocytes were evident almost nearby the cyst and portal areas, (Fig. 2b and 2c). Observed changes in the biliary epithelium of some positive hydatidosis cases included hyperplasia and degeneration. Morphometric studies of pathological lesion in liver tissues of infected sheep revealed significant increase in hepatic lobules necrosis (HLN), hepatocytes degeneration (HD), cellular inflammation (CI), sinusoidal congestion (SC), Kupffer cell activation (KA), subcapsular edema (SE), and biliary hyperplasia (BH) compared with those of non-infected sheep (P < 0.001) (Table 6).
Table 6.
Histopathological alteration in examined liver for non-infected (control) and infected group.
| Histopathological alteration |
Control (n = 30) |
Infected (n = 140) |
|---|---|---|
| (HLN) | 0.00 | 13.00 ± 10.01** |
| (HD) | 0.00 | 22.00 ± 5.10** |
| (CI) | 0.00 | 23.00 ± 8.30** |
| (SC) | 0.00 | 21.00 ± 7.40** |
| (KA) | 0.00 | 19.00 ± 6.12** |
| (SE) | 0.00 | 19.00 ± 6.12** |
| (BH) | 0.00 | 19.00 ± 6.12** |
Data expressed as mean ± SD: ** Highly significant (p < 0.001).
3.4. Mesentery
Sections of mesenteric tissue showed the cyst wall with the characteristic layers as in lung and liver. In addition, infiltration of mononuclear inflammatory cells near the cyst wall and in mesenteric tissue (Fig. 2d) was observed, which was accompanied by proliferation of fibrous tissue (Fig. 2e). Marked decrease in cell population of the cortical follicles along with severe congestion was observed as well.
3.5. Histochemical results
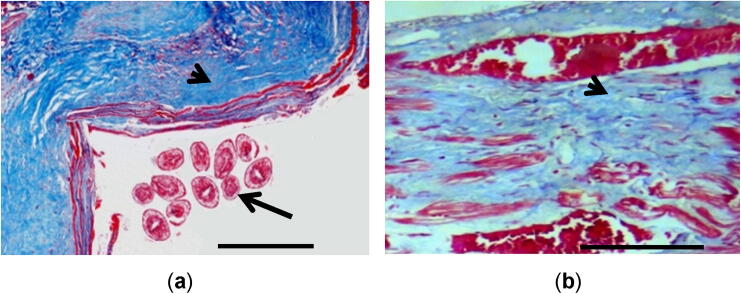
Masson’s trichrome stained sections from liver, lung, and mesentery of sheep showed collagen fibers inside the hydatid cyst capsules and in the pericystic region. The collagen fibers content and the cellular laminated membranes took the green color (Fig. 3a and 3b). In some cases, the laying down of collagen around the periphery of the cyst was not uniformly visible.
Fig. 3.
(a) Lung with hydatidosis showing collagen fibers found in the pericystic connective tissue (arrowhead), germinal layers, brood capsules, and protoscolices stained red in color (arrow). (Masson’s trichrome); (b) Liver revealing extensive fibrosis around cyst wall and surrounding parenchyma (arrowhead). Masson’s trichrome. Scale-bar = 50 μm.
3.6. Immunohistochemical findings
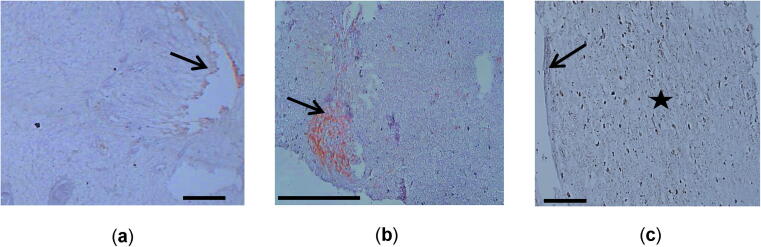
Slides stained with markers for CD3 showed positive reaction for CD3. High positivity reaction was observed, particularly in the cyst wall and cells in tissues neighboring the cyst wall. The reactions appeared as scattered brown stains in the examined tissue from lung, liver, and mesentery (Fig. 4a-c).
Fig. 4.
(a) Lung tissue with hydatosis showing positive reaction for CD3 marker and in the cyst wall (arrow); (b) Liver section showing positive brown stained cells reaction for CD3 marker and in the cyst wall (arrow) and parenchyma of liver tissue; (c) A section of mesentery showing positive brown stained cells reaction for CD3 marker in the cyst wall (arrow) and mesentery tissue (star). Scale-bar = 50 μm.
4. Discussion
Hydatid disease is one of the most widespread and serious helminth zoonotic infections in the world caused by Echinococcus granulosus. The disease poses an important public health problem in many areas. It is a common disease in sheep in Saudi Arabia, particularly among populations that practice sheep husbandry (Eckert et al., 2001, Almalki et al., 2017). The disease mostly remains asymptomatic in intermediate animal hosts and humans for a long period of infection (Alsulami, 2019).
In the present study, hydatidosis prevalence is high in imported versus local sheep, where infection incidence was (13.0%, 10.2%), respectively. Throughout the study, the difference in prevalence between the two animal sources could be related to differences in their environmental conditions such as, temperature, humidity, and the nature of the pasture along with social activities (Azlaf and Dakkak, 2006, Budke et al., 2006).
Furthermore, these variations could be relevant to the differences between E. granulosus strains in each source (Elmajdoub and Rahman, 2015). Gender of sheep is a possible determinant resulting in high hydatid cysts infection. In addition, females from local and imported sources in our studies recorded high infection rate as compared with males, where the prevalence in local and imported females was (71.9%, 66.3%), respectively. While prevalence in local and imported males was (28.1%, 33.7%), respectively. These findings agree with those of (Daryani et al., 2007; Murtaza et al., 2017). The reasons may be due to the longer age of females at the time of slaughtering as a result of keeping females for reproductive purposes (Anwar et al., 2000; Ibrahim, 2010; Ali et al., 2012). In addition, this may be due to hormonal differences, genetic composition, and the host-parasite relationship (Abdel-Baki et al., 2018). These results agree with previous studies carried out in Saudi Arabia (Almalki et al., 2017; Ibrahim, 2010), and in other countries (Hayajneh et al., 2014, Khadidja et al., 2014, Singh, 2018, Mehmood et al., 2020).
Very little information is available regarding the correlation between size of hydatid cyst with the age of sheep. We showed that the small size cysts in local and imported sheep of ages G1 (8.8%, 13.3%), respectively, were higher than those in G2 (1.7%, 3.6%) and G3 (3.5%, 2.4%), respectively. Accordingly, local and imported sheep with middle size cyst in G2 (10.5%, 8.4%), respectively, were higher than those in G1 (5.2%, 6.0%) and G3 (1.7%, 3.6%), respectively. While the large size cyst in local and imported sheep in G3 (33.7%, 38.6%), respectively, were higher than those in G1 (1.7%, 1.2%) and G2 (28.1%, 27.2%), respectively. As per (Anwar et al., 2000; Ibrahim, 2010) studies, the cyst requires 10–14 days for a very quick initial development. On the other hand, the cyst grows slowly in a variable time, depending on the cyst location and age of the animal. Fertile cyst requires more than 12 months, in most species, to achieve its complete structure. However, large hydatid cyst was reported in aged sheep and could be detected easily during postmortem examination (Ali et al., 2012, Mehmood et al., 2020).
Our findings showed that the sterile cyst prevalence in local and imported sheep in G1 (12.3%, 14.5%), respectively was higher than those in G2 (10.5%, 6.0%) and G3 (1.7%, 1.2%), respectively. Sterile hydatid cysts were noticed before by (Budke et al., 2006), who stated that the sterility of the acephalocyst might be due to the location of the parasite and the condition of the adventitious coat and due to infection by unspecific strain as well. The fertile cyst in our results for both local and imported sheep in G3 (38.6%, 39.8%), respectively, were higher than those in G1 (5.3%, 4.8%) and G2 (28.1%, 30.1%), respectively. This finding agrees with (Yildiz and Gurcan, 2003, Torgerson et al., 2009). Barnes et al. (2007) observed that the development of hydatid cysts was related to age and thus onset of fertility occurred in sheep. In addition, older livestock may have been exposed to more infective stages. Dueger and Gilman (2001) shows that age is a determinant potential factor and continuous source of infection in animals. On the other hand, (Ibrahim, 2010) reported that the age of the animal had no effect on the fertile cyst.
Data reported in our study showed that the most frequently affected organ is liver, followed by lungs, but mesentery reported the lowest infected rate. These findings also were supported by (Khan et al., 2001, Singh, 2018). Generally, the liver was more affected, in a proportional manner, by hydatidosis infection in all host species. E. granulosus clearly prefers liver because blood, after leaving the digestive tract, moves firstly to liver, taking oncospheres along in portal vein encountering hepatic and pulmonary filtration systems before entering any other organ (Singh et al., 2016).
Histologically, the cyst wall was observed with the characteristic three layers; a thin inner germinal layer, a laminated layer, and an outer adventitial layer. The results in the present study meet many previous studies (Ahmedullah et al., 2007, Verma and Swamy, 2009). According to Barnes et al. (2011), the adventitial layer in the multilocular cysts was thick in sheep which could be restrictive to growth, which accordingly protects the hydatid cyst from the host immune response. Saad (1985) reported that the fibrous capsule response was dense and diffuse in fertile cysts. In addition, different studies had described these features previously (Yildiz and Gurcan, 2003, Ahmedullah et al., 2007, Verma and Swamy, 2009, Kul and Yildiz, 2010, Barnes et al., 2011).
In this study, the pathological alternation in sections from lung, liver and mesentery of hydatidosis infected sheep showed inflammatory reaction comprising lymphocytes, macrophages, and eosinophils nearby the cyst wall and extended to the surrounding tissues. In addition, congestion of blood vessels is observed along with hemorrhage, as well as oedematous areas in some sections (Singh et al., 2016). These results are also attributed to host tissue immunological reactions against parasite (Ibrahim et al., 2008, Khadidja et al., 2014, Singh, 2018).
Observed also is hyperplasia of the bronchial epithelium in lungs, BH in liver, as well as a decrease in cell population in the cortical follicles and deformation of lymphatic follicles in mesentery (Singh, 2018). In addition, and according to Solcan et al. (2010), degenerative changes and BH were observed in biliary epithelium.
Alveolar emphysema was seen adjacent to atelectatic alveoli in the vicinity of the cysts which lead to conventional bronchiectasis. In addition, necrosis and degeneration in the hepatic lobules tissue was widespread. Such changes have been previously noted in hydatidosis affected lungs and livers were mainly attributed to the pressure effect exerted by the hydatid cyst (Beigh et al., 2017).
Morphometric analysis in our study of these pathological lesion in lung, liver and mesentery of infected sheep, revealed significant increase in the number of BN, AA, AE, EH, TIS in lung, HLN, HD, CI, KA, SE, and BH in liver compared to non-infected sheep. The morphometric analyses in our results were recorded for the first time accompanied with hydatidosis in lung, liver and mesentery of sheep.
Masson’s trichrome staining demonstrated collagen fibers found in the cyst wall of infected lung, liver, and mesentery sections. Also, demonstrated the fibrous tissue associated with hydatidosis in sheep was demonstrated. Presence of collagen indicates an inflammatory response to persistent irritation. This is essentially the basic protective response to contain the parasite, which in turn causes formation of the cyst wall. The noticeable fibrosis and cirrhosis in certain cases may be attributed to host tissue immunological reactions (Alsulami, 2019).
Immunohistochemical studies were performed to further characterize the lymphocyte infiltrate within liver, lungs and mesenteric tissues. The results showed that the inflammatory cells in all sections indicate to positive reaction for CD3 markers. CD3 represents positive T-lymphocytes and CD3 surface marker which is associated with the T cell receptors in both CD4 and CD8 cells. The number of CD3 positive T cells is an approximation of the sum of CD4 and CD8 T cells. This indicates that the cells infiltrating the hydatid cyst wall in the sections examined belonged to the CD4, CD8 T-lymphocytes which may play a role in the immune response of animal hydatidosis as suggested in case of human infection (Keir et al., 2002, Naji et al., 2007).
In conclusion, diagnosing hydatidosis, particularly postmortem diagnosis, will be efficiently conducted using gross pathological/histopathological examinations. Developing specially designed diagnostics will be achieved by highlighting epidemiology of economic animals and performing much more monitoring to reach to this target. In addition, age of cysts is considered as the key basis controlling the variable levels of pathogenicity to the individual cysts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We extend our appreciation to Taif University Researchers supporting Project number (TURSP-2020/299), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Baki A.S., Almalki E., Al-Quarishy S. Prevalence and characterization of hydatidosis in Najdi sheep slaughtered in Riyadh city, Saudi Arabia. Saudi Journal of Biological Sciences. 2018;25(7):1375–1379. doi: 10.1016/j.sjbs.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmedullah, F., Akbor, M., Haider, M., Hossain, M., Khan, M., Hossain, M., Shanta, I.S., 2007. Pathological investigation of liver of the slaughtered buffaloes in Barisal district. Bangl J Vet Med. (5), 81-5. https://doi:10.108010.3329/bjvm.v5i1.1321
- Al-Hayyali, F.Q., 2000. Histological and histochemical studies on hydatid cyst wall of Echinococcus granulosus in human and some intermediates hosts. M.Sc. Thesis (in Arabic), Coll. Sci. Univ. of Mosul, Iraq. https://www.iasj.net/iasj/download/2d02ed1ff06e1914
- Al-Sabawi, B.H., 2001. Immunomodulating effect of the black nightshade, Solanum nigrum L. on growth and development of Echinococcus grandulosus Batsch, 1786. Secondary hydatid cysts of human and sheep origin. Ph.D. Thesis (in Arabic), Coll. Of Sci. Univ. of Mosul, Iraq. https://www.iasj.net/iasj/download/2d02ed1ff06e1914
- Ali, E., Vahedi, N., Bokaei, S., 2012. Correlation between age of sheep and structural changes of sheep hydatid cyst. Iran J Vet Med. (6), 171-175. https://doi:10.22059/ijvm.2012.30004
- Almalki E., Al-Quarishy S., Abdel-Baki A.S. Assessment of prevalence of hydatidosis in slaughtered Sawakny sheep in Riyadh city, Saudi Arabia. Saudi Journal of Biological Sciences. 2017;24(7):1534–1537. doi: 10.1016/j.sjbs.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsulami, M., 2019. Prevalence and histopathological study on cystic hydatidosis in heart and spleen of goat slaughtered at Makkah, Saudi Arabi. Ann Parasitol. 65 (3), 225-36. https://doi:10.17420/ap6503.204 [PubMed]
- Anwar, A.H., Rana, S.H., Khan, M.N., Qudoos A., 2000. Hydatidosis: prevalence and biometrical studies in cattle (Bob Indicub). Pak J Agri Sci. 37 (1–2), 29–32. http://pakjas.com.pk/papers%5C690.pdf
- Azlaf R., Dakkak A. Epidemiological study of the cystic echinococcosis in Morocco. Veterinary Parasitology. 2006;137(1–2):83–93. doi: 10.1016/j.vetpar.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes T., Hinds L., Jenkins D., Bielefeldt-Ohmann H., Lightowlers M., Coleman G. Comparative Pathology of Pulmonary Hydatid Cysts in Macropods and Sheep. Journal of Comparative Pathology. 2011;144(2–3):113–122. doi: 10.1016/j.jcpa.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Beigh, A.B., Darzi, M.M., Bashir, S., Kashani, B., Shah, A., Shah S.A., 2017. Gross and histopathological alterations associated with cystic echinococcosis in small ruminants. J Parasit Dis. 41 (4), 1028-33. https://doi:10.1007/s12639-017-0929-z [DOI] [PMC free article] [PubMed]
- Budke C.M., Deplazes P., Torgerson P.R. Global Socioeconomic Impact of Cystic Echinococcosis. Emerg Infect Dis. 2006;12(2):296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew B.P., Cook E.W., Thiele J.S. Asymptomatic Pulmonary Cyst in a College Student. Chest. 1990;98(2):455–457. doi: 10.1378/chest.98.2.455. [DOI] [PubMed] [Google Scholar]
- Daryani A., Alaei R., Arab R., Sharif M., Dehghan M.H., Ziaei H. The prevalence, intensity and viability of hydatid cysts in slaughtered animals in the Ardabil province of Northwest Iran. J Helminthol. 2007;81(1):13–17. doi: 10.1017/S0022149X0720731X. [DOI] [PubMed] [Google Scholar]
- Dueger E.L., Gilman R.H. Prevalence, intensity, and fertility of ovine cystic echinococcosis in the central Peruvian Andes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95(4):379–383. doi: 10.1016/s0035-9203(01)90188-9. [DOI] [PubMed] [Google Scholar]
- Eckert, J., Deplazes, P., 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev.17 (1), 107-35. https://doi:10.1128/cmr.17.1.107-135.2004 [DOI] [PMC free article] [PubMed]
- Eckert, J., Gemmell, M.A., Meslin, F.-X., 2001. Pawlowski, Z.S.; World Health Organization. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; World Health Organization and the World Organisation for Animal Health (OIE—Office International des Epizooties): Paris, France, pp. 31–34. https://apps.who.int/iris/handle/10665/42427
- Eckert J., Gemmell M.A., Matyas Z., Soulsby E.J.L. 2nd ed.; World Health Organization:; Geneva, Switzerland: 1984. World Health Organization, Veterinary Public Health Unit. Guidelines for surveillance, prevention and control of Echinococcosis/Hydatidosis; pp. 19–22. [Google Scholar]
- Elmajdoub, L.O., Rahman, W.A., 2015. Prevalence of Hydatid Cysts in Slaughtered Animals from Different Areas of Libya. OJVM. 05 (01), 1-10. https://doi:10.4236/ojvm.2015.51001
- Hayajneh, F.M.F., Althomali, A.M.H., Nasr A.T.M., 2014. Prevalence and characterization of hydatidosis in animals slaughtered at Al Taif abattoir, Kingdom of Saudi Arabia. OJAS. 04 (01), 38-41. https://doi:10.4236/ojas.2014.41006
- Ibrahim M.M. Study of cystic echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: interaction between some biotic and abiotic factors. Acta Trop. 2010;113(1):26–33. doi: 10.1016/j.actatropica.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Ibrahim, M.M., Ghamdi, M., Gahmdi, M., 2008. Helminths community of veterinary importance of livestock in relation to some ecological and biological factors. Turkiye Parazitol Derg. 32 (1), 42-7. https://pubmed.ncbi.nlm.nih.gov/18351550/ [PubMed]
- Ibrahim S.E.A., Gameel A.A. In Proceedings of 5th annual conference-agricultural and veterinary research. 2014. Pathological, histochemical and Immunohistochemical studies of lungs and livers of cattle and sheep infected with hydatid disease; pp. 1–17. [Google Scholar]
- Keir, M.E., Rosenberg, M.G., Sandberg, J.K., Jordan, K.A., Wiznia, A., Nixon, D.F., 2002. Stoddart CA, McCune JM.. Generation of CD3+CD8lowThymocytes in the HIV Type 1-Infected Thymus. J Immunol. 169 (5), 2788-96. 10.4049/jimmunol.169.5.2788 [DOI] [PubMed]
- Kern P., Ammon A., Kron M., Sinn G., Sander S., Petersen L.R., Gaus W., Kern P. Risk factors for alveolar echinococcosis in humans. Emerg Infect Dis. 2004;10(12):2088–2093. doi: 10.3201/eid1012.030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadidja, H., Achour, Y., Houcin, B., Vasile, C., 2014. Histological Appearance of Echinococcus Granulosus in the Camel Species in Algeria. Bulletin UASVM Vet Med. 71 (1), 79–84. https://agris.fao.org/agris-search/search.do?recordID=RO2014100440
- Khan A.H., El-Buni A.A., Ali M.Y. Fertility of the cysts of Echinococcus granulosus in domestic herbivores from Benghazi, Libya, and the reactivity of antigens produced from them. Ann Trop Med Parasitol. 2001;95(4):337–342. doi: 10.1080/00034980120053258. [DOI] [PubMed] [Google Scholar]
- Kul O., Yildiz K. Multivesicular cysts in cattle: characterisation of unusual hydatid cyst morphology caused by Echinococcus granulosus. Vet Parasitol. 2010;170(1–2):162–166. doi: 10.1016/j.vetpar.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Mehmood N., Arshad M., Ahmed H., Simsek S., Muqaddas H. Comprehensive Account on Prevalence and Characteristics of Hydatid Cysts in Livestock from Pakistan. Korean J Parasitol. 2020;58(2):121–127. doi: 10.3347/kjp.2020.58.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muluneh, H.; Hailu, M., 2019. Prevalence Major Metacestodes of Ruminant Slaughtered at Elfora Export Abattoir and Public Health Importance. JDVS. 21, 10 (5), 555797. https://doi: 10.19080/JDVS.2019.10.555797
- Murtaza, M., Suzan A. Al-Azizz, Abdulhameed, F.M., Kadhim, L., 2017. Active survey of hydatid cysts in slaughtered sheep at Basrah abattoirs, Basrah province. Iraq. J Entomol Zool Stud. 5 (5), 951-954. https://www.entomoljournal.com/archives/?year=2017&vol=5&issue=5&ArticleId=2425
- Naji A., Le Rond S., Durrbach A., Krawice-Radanne I., Creput C., Daouya M., Caumartin J., LeMaoult J., Carosella E.D., Rouas-Freiss N. CD3+CD4low and CD3+CD8low are induced by HLA-G: novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood. 2007;110(12):3936–3948. doi: 10.1182/blood-2007-04-083139. [DOI] [PubMed] [Google Scholar]
- Possenti A., Manzano-Román R., Sánchez-Ovejero C., Boufana B., La Torre G., Siles-Lucas M., Casulli A. Potential Risk Factors Associated with Human Cystic Echinococcosis: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(11) doi: 10.1371/journal.pntd.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M.B. University of Khartoum; Sudan: 1985. Hydatidosis/ Echinococcosis in the Sudan with emphasis on epidemiology, experimental transmission, and histopathology. Ph.D. Thesis. [Google Scholar]
- Singh B.B., Sharma R., Sharma J.K., Mahajan V., Gill J.P.S. Histopathological changes associated with E. granulosus echinococcosis in food producing animals in Punjab (India) J Parasit Dis. 2016;40(3):997–1000. doi: 10.1007/s12639-014-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.K. Mesenteric Hydatid Cyst. Nep J. Radiology. 2018;8(2):43–46. doi: 10.3126/njr.v8i2.22988. [DOI] [Google Scholar]
- Steel, R.G.D., Torrie, J.H., 1960. Principles and procedures of statistics with special reference to the biological sciences, 1st ed.; McGraw-Hill Book Company: New York. 10.1002/bimj.19620040313
- Surhio, A.S., Bhutto B., Gadahi J.A., Akhtar N., Arijo A., 2011. Studies on the prevalence of caprine and ovine hydatidosis at slaughter houses of Larkana, Pakistan. Res Opin Anim Vet Sci. 1 (1), 40-43. https://agris.fao.org/agris-search/search.do?recordID=DJ2012071826
- Suvarna K.S., Layton C., Bancroft J.D. 7th ed. Churchill Livingstone; United Kingdom: 2013. Bancroft's Theory and Practice of Histological Techniques. https://www.elsevier.com/books/bancrofts-theory-and-practice-of-histological-techniques/suvarna/978-0-7020-4226-3. [Google Scholar]
- Thrusfield, M., 2005. Veterinary Epidemiology, 3rd ed.; Wiley-Blackwell: Singapore, pp. 233. https://www.wiley.com/en-au/Veterinary+Epidemiology%2C+3rd+Edition-p-9781118713419
- Torgerson P., Ziadinov I., Aknazarov D., Nurgaziev R., Deplazes P. Modelling the age variation of larval protoscoleces of Echinococcus granulosus in sheep. International Journal for Parasitology. 2009;39(9):1031–1035. doi: 10.1016/j.ijpara.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Carmona C., Bonifacino R. Estimating the economic effects of cystic echinococcosis: Uruguay, a developing country with upper-middle income. Ann Trop Med Parasitol. 2000;94(7):703–713. doi: 10.1080/00034983.2000.11813594. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Dowling P.M. Estimating the economic effects of cystic echinococcosis. Part 2: an endemic region in the United Kingdom, a wealthy, industrialized economy. Ann Trop Med Parasitol. 2001;95(2):177–185. doi: 10.1080/00034980020030948. [DOI] [PubMed] [Google Scholar]
- Verma, Y., Swamy, M., 2009. Prevalence and pathology of hydatidosis in buffalo liver. Buffalo Bull. (28), 207–211. https://ibic.lib.ku.ac.th/e-bulletin/2009-207.pdf
- Yildiz K., Gurcan S. Prevalence of hydatidosis and fertility of hydatid cysts in sheep in Kirikkale. Turkey. Acta Veterinaria Hungarica. 2003;51(2):181–187. doi: 10.1556/AVet.51.2003.2.6. [DOI] [PubMed] [Google Scholar]