Abstract
The purpose of this study was to evaluate the genetic variation of the PIK3CA gene and the histopathological changes in liver tissue of patients with chronic Schistosomiasis to predict hepatocellular carcinoma. In this retrospective, the study samples were taken from 20 patients, divided into chronic schistosomiasis infected group of people (S) and chronic schistosomiasis uninfected group of people (C). The liver tissue biopsy samples for histological examinations were obtained only from chronic Schistosomiasis patients (n = 9). The blood samples were obtained from groups S and C for the mutational analysis of the PIK3CA and TP53 genes. The results suggest that the patients diagnosed with chronic Schistosomiasis were 9 (55%), and healthy patients without Schistosomiasis were 11 (45%). Histological results found that proliferation of fibrosis was observed in the hepatocytes of schistosomiasis patients. A total of 8 mutations (5 male, 3 female) were detected in PIK3CA and TP53 genes. Including 1634 A > G substitution mutations in PIK3CA, which was the only mutation found in males and females among the 8 mutations, accounting 22.22%. PIK3CA gene mutations were found more predominant in male groups as compared to other TP53 gene mutations. In conclusion, this study found that patients with chronic Schistosomiasis are at risk of PIK3CA gene mutations, eventually leading to hepatocytes fibrosis and liver cancer.
Keywords: Hepatocellular-carcinoma, Mutations, PIK3CA, Schistosomiasis, TP53
1. Introduction
The liver is a vital organ responsible for various physiological functions, such as metabolism, immune response, digestion, and detoxification (Hwang and Yang, 2021). However, chronic Schistosomiasis is a severe disease that causes more than 500,000 deaths each year in the world (Wilson et al., 2007). The main form of this disease is chronic granulomatous, which response to parasite eggs trapped in host tissues. In some cases, granulomas can develop into periportal fibrosis and portal hypertension, leading to fatal complications. The immunopathological damage of Schistosomiasis is mainly due to the granulomatous inflammation around the parasite eggs in the host's liver during acute phase. It can lead to liver fibrosis and ultimately cause death (Burke et al., 2009, Wilson et al., 2007). When liver cirrhosis is caused by chronic schistosomiasis, leading to liver cell fibrosis, the proliferation of liver cells decreases and the destruction of liver cells increases. About 15% of patients with cirrhosis have dysplastic nodules (Thorgeirsson and Grisham, 2002). In this process, the molecular interaction between the granulomatous response to the parasite eggs trapped in the host tissue and the host cell mechanism. The changes begin with the increase of pre-neoplastic and dysplastic hepatocytes and primary hepatocellular carcinoma (Nakashima et al., 1975, Thorgeirsson et al., 2002). When the frequency of genetic damage increases, the malignant phenotype of hepatocytes progression may also increase, leading to the development of phenotype and genetic heterogeneity of HCCs (Lu et al., 2016).
The phosphoinositide-3-kinase-catalysis-α (PIK3CA) gene mutation in HCC cases in Italy was 28% (Colombino et al., 2012) and 35.6% in China (Lee et al., 2005). Cell proliferation, angiogenesis and apoptosis are affected by PIK3CA when it regulates the phosphatase and tensin homolog (PTEN)-AKT pathway. In addition, several anti-cancer drugs that can regulate the PI3K/Akt pathway have also achieved good results in combating malignant tumors, and it is particularly important to understand the mutation status of the PIK3CA gene (Carnero, 2009). Researchers have evaluated the PIK3CA gene on HCC patients with different causes in different world regions to combat liver cancer. However, the role of genetic variations of the PIK3CA gene in patients infected with chronic Schistosomiasis following hepatocellular carcinoma is not clear. Therefore, this study was planned to find the variation of the PIK3CA gene in humans infected with Schistosomiasis to predict hepatocellular carcinoma.
2. Materials and methods
2.1. Informed consent and ethical approval
All patients in this study signed written informed consent. A questionnaire was also provided to obtain their medical history. The retrospective research plan was approved by the National Research Council of King Abdulaziz City of Science and Technology (KACST), King Fahd Medical City (KFMC), Kingdom of Saudi Arabia (KSA) (IRB log number: 20 −545E). The data used in this study was secondary. However, the privacy statements of the participants were also collected.
2.2. Research design
This retrospective study was conducted over 20 patients at KFMC, Riyadh, KSA, between November 2019 to May 2020. Initially, the patients were divided into chronic schistosomiasis infected group of people (S) and chronic schistosomiasis uninfected group of people (C). There were 9 patients in group S (7 males and 2 females), age 28–47 years old. Total 11 healthy patients were in group C (4 males and 7 females), age 27–44 years old. The profile of each participant contained entailed information is summarized in Table 1.
Table 1.
Benchmark demographic profiles showing clinical characteristics of patients.
| Cases (Numbers) | Age (Years) | Gender Male/Female |
Diagnosis Schistosomiasis Yes/No |
Cancer in family (Medical history) Yes/No |
|---|---|---|---|---|
| 1 | 34 | M | No | No |
| 2 | 38 | M | Yes | No |
| 3 | 42 | M | No | No |
| 4 | 28 | M | Yes | No |
| 5 | 39 | F | Yes | No |
| 6 | 36 | M | No | No |
| 7 | 29 | M | No | No |
| 8 | 41 | M | Yes | No |
| 9 | 44 | F | No | No |
| 10 | 25 | F | No | No |
| 11 | 27 | F | No | No |
| 12 | 33 | M | Yes | No |
| 13 | 43 | M | Yes | No |
| 14 | 37 | M | Yes | No |
| 15 | 47 | M | Yes | No |
| 16 | 31 | F | No | No |
| 17 | 35 | F | No | No |
| 18 | 23 | F | No | No |
| 19 | 37 | F | No | N |
| 20 | 40 | F | Yes | No |
2.3. Tissue samples
The liver tissue biopsy samples of approximately 7–8 mm were obtained from chronic Schistosomiasis patients only (n = 9) with informed consent for histological examinations. Furthermore, the obtained biopsy samples were divided into two parts. The first part was stored in RNA Later at − 80 °C (Ambion, Austin, TX), and the second part was stained with Hematoxylin and eosin (H&E) and Masson’s trichrome (MT) (Kononen et al., 1998, Li et al., 2021). All 9 liver biopsies included in this study were histologically confirmed as hepatocellular carcinoma (HCC). The samples were fixed with 4% paraformaldehyde, dehydrated, embedded in paraffin, and stained with H&E and MT. The sample was then sectioned and inspected under an Olympus BX51 microscope.
2.4. DNA extraction
The blood samples were obtained from the 20 infected (S) and uninfected chronic Schistosomiasis (C) patients to analysis the genetic variation. Following the manufacturer's instructions, commercial blood and tissue kit (Qiagen, Hilden, Germany) was used to extract DNA from the collected blood samples. Furthermore, UV spectrophotometer (NanoDrop™ 1000, Thermo Fisher Scientific, Waltham, MA, USA) was used to confirm the purity of the extracted DNA (Piskata et al., 2019).
2.5. Mutational analysis of PIK3CA and TP53 gene
PCR amplification was carried out targeting exons 9 and 20 of the PIK3CA gene. The forward primer used for exon 9 was 5′-CATCTGTGAATCCAGAGGGGA-3′ and 5′- AGCACTTACCTGTGACTCCA-3′ as reverse primer. Whereas, for exon 20, 5′-CTCTGGAATGCCAGAACTAC-3′ was used as forward primer and 5′-ATGCTGTTTAATTGTGTGGAAG-3′ was used as reverse primer. The research trial and experimental procedures in this study was followed as per guidelines of International Agency for Research on Cancer (IARC) (http://www-p53.iarc.fr/Download/TP53_DirectSequencing_IARC.pdf), including the analyses process of TP53 gene and PCR reactions. All PCR reactions were performed using 10 to 100 ng genomic DNA in a 50 μl reaction mixture. Primm Srl Laboratories (Milan, Italy) performs two-way direct sequencing analysis on all samples containing sufficient DNA. The PCR mixure contained 50 ng of genomic DNA, and the reaction volume was 20 µl, which contained 5 µM specific oligonucleotide primers and HotStarTaq Master Mix (Qiagen, Valencia, CA). The temperature cycle was followed, initially denaturation at 95 °C for 10 min, then 32 cycles of reaction at 95 °C for 30 s. Furthermore, annealing for 30 s at 56 °C and 45 s at 72 °C. Then performed a single extension step at 72 °C for 10 min. Nucleic Fast 96 PCR Clean-up Kit was used to purify the PCR products, and Big Dye v1.1 Cycle Sequencing Kit was used for sequencing. Then, PCR products were processed in ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA). Sequencher software (Gene Codes Corporation, Ann Arbor, MI) was used for analyses and sequencing comparisons with the normal DNA. All samples were subjected to bidirectional direct sequencing analysis (Tornesello et al., 2013).
2.6. Statistical analysis
The quantitative results of this experiment were expressed in standard deviations and mean (P-value < 0.05 were considered statically significant). Sigmastat programming adaptation 3.5 (Systat Software, San Jose, CA, USA) was used for statistical analysis.
3. Results
3.1. Characterization of patients
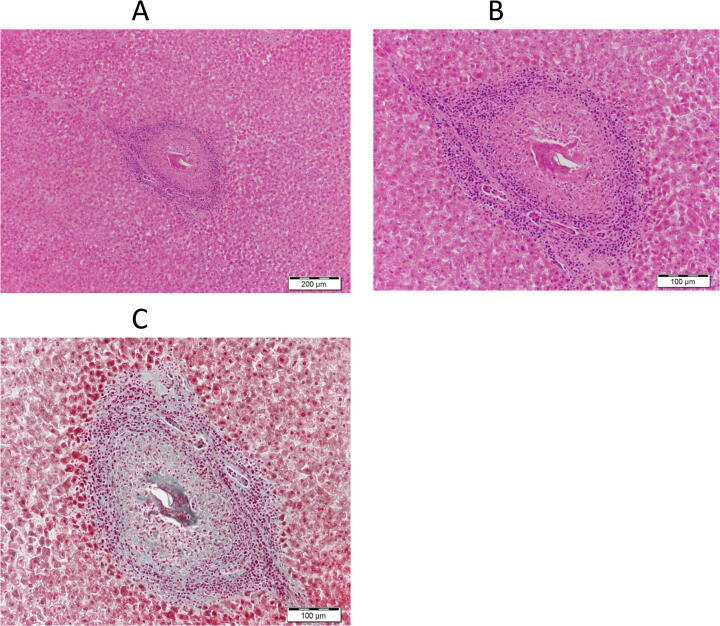
The clinicopathological features of the 20 patients with and without chronic Schistosomiasis are presented in Table 1. In total 11 patients with 55% were male, and 9 patients with 45% were female. The range of age was 23 to 47 years, with an average of 35.5 years. The patients diagnosed with chronic Schistosomiasis were 9 (55%) and found healthy without Schistosomiasis were 11 (45%). The gender percentage of patients diagnosed with chronic Schistosomiasis was 77.77% male and 22.22% female. The biopsy samples were only taken from 9 patients with chronic Schistosomiasis for histological features representation, shown in Fig. 1.
Fig. 1.
Histological features of liver sections stained with hematoxylin and eosin. A and B showing the fibrosis proliferation between the hepatocytes. C showing the distribution of collagen in blue. The liver fibrosis was observed higher magnification photomicrograph of a liver section stained with Masson's trichrome (MT).
3.2. Histological assessment
Fig. 2 shows the hepatic fibrosis in microscopic images obtained from the patients with chronic schistosomiasis liver condition. The morphological changes after hematoxylin and eosin staining were observed in the liver of chronic schistosomiasis patients, and later diagnosed as hepatocellular carcinoma. Pathologically it has been indicated that the dysregulation of proliferation and proliferation of fibrosis was observed in the hepatocytes of schistosomiasis patients (Fig. 2 A, B). In addition, a fibrotic procession in hepatocytes due to chronic liver schistosomiasis was due to the PIK3CA and TP53 gene mutation.
Fig. 2.
Somatic mutations in TP53 and PIK3CA genes’ frequencies for 9 patients infected with chronic Schistosomiasis.
Predominantly progression was detected on MT staining, which contained inflammatory cells in the most fibrous septa with loose collagen fibers. The activity of the cirrhotic progression is being reflected with these results, which implies cirrhosis due to chronic Schistosomiasis (Fig. 2C).
3.3. PIK3CA gene mutation
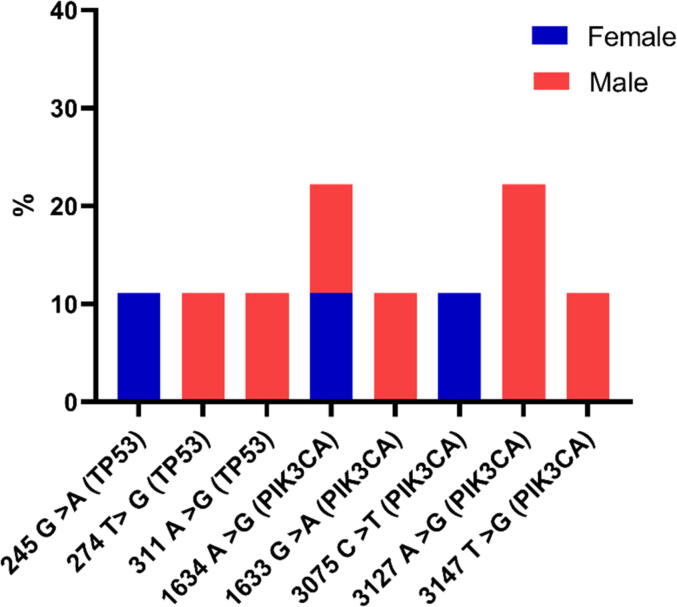
The study included 9 samples, 7 male and 2 female with chronic Schistosomiasis and hepatocellular carcinoma. The mutations were detected within exons 9 and 20 of the PIK3CA gene. The PIK3CA gene mutation carriers were diagnosed with chronic Schistosomiasis and hepatocellular carcinoma within the age of 47 for males and 40 for females. Total five distinct mutations of PIK3CA gene were identified as 1634 A > G, 1633 G > A, 3075C > T, 3127 A > G and 3147 T > G with substitution mutation. The mutation frequency ranged from 22.2 to 11.1%, and the highest substitution mutation in 1634 A > G (male and female) was 22.22%. However, 3127 A > G (male) was 22.22%. The substitution frequencies of the other three mutations (two males and one female) were 1633 G > A, 3147 T > G, and 3075C > T, respectively, which was 11.1%. All of these mutations were found in patients diagnosed with hepatocellular carcinoma and chronic Schistosomiasis. In addition, there was no medical history of any family members of the patients who have been previously diagnosed with cancer (Table 2). Total frequency was counted 77.7% for PIK3CA gene mutation in 9 chronic schistosomiasis and hepatocellular carcinoma patients.
Table 2.
The frequencies of PIK3CA gene mutations in patients with chronic Schistosomiasis and hepatocellular carcinoma.
| Patients | Sex | Mutations | Mutation type | Mutation frequency (%) |
|---|---|---|---|---|
| 2 | M & F | 1634 A > G | Substitution | 2/9 (22.2%) |
| 1 | M | 1633 G > A | Substitution | 1/9 (11.1%) |
| 1 | F | 3075C > T | Substitution | 1/9 (11.1%) |
| 2 | M | 3127 A > G | Substitution | 2/9 (22.2%) |
| 1 | M | 3147 T > G | Substitution | 1/9 (11.1%) |
| Total | 5 | 7/9 (77.7%) |
3.4. TP53 gene mutation
A total of 3 mutations in the TP53 gene were detected for exons 4–9, including 1 female and 2 males out of 9 patients. The substitution mutation frequency of 11.1% was found in each 245 G > A (female), 274 T > G, and 311 A > G (male). Total frequency was 33.3% for TP53 gene mutation in 9 chronic schistosomiasis and hepatocellular carcinoma patients (Table 3).
Table 3.
The frequencies of TP53 gene mutations in patients with chronic Schistosomiasis and hepatocellular carcinoma.
| Patients | Sex | Mutations | Mutation type | Mutation frequency (%) |
|---|---|---|---|---|
| 1 | F | 245 G > A | Substitution | 1/9 (11.1%) |
| 1 | M | 274 T > G | Substitution | 1/9 (11.1%) |
| 1 | M | 311 A > G | Substitution | 1/9 (11.1%) |
| Total | 3 | 3/9 (33.3%) |
3.5. PIK3CA and TP53 gene mutation
Fig. 2 shows the distribution of PIK3CA and TP53 gene mutations in 9 patients with chronic Schistosomiasis and hepatocellular carcinoma. A total of 8 mutations (5 male, 3 female) were detected in PIK3CA and TP53 genes. Including 1634 A > G substitution mutations in PIK3CA, the only mutation found in males and females among the 8 mutations, accounting for 22.22%. However, 3127 A > G mutation was found 22.22% in male-only. PIK3CA gene mutations were found more predominant in male groups than other TP53 gene mutations (Fig. 2).
4. Discussion
Cancer is a genetic disease. Researchers are paying more attention to genetics and genomics to find treatments based on mutation analysis. However, carcinogenesis is the process by which normal cells evolve until they become cancer cells. The cause of carcinogenesis is complex and multifactorial and may involve cellular, molecular, genetic, epigenetic, and environmental changes (Pitot, 1993). According to Global Cancer Watch (GLOBOCAN 2008) estimates, liver cancer is divided into primary and secondary, and it is one of the second common malignant tumors that causes deaths in the world (Ding et al., 2016). It is believed that the onset of primary liver cancer is a complex process involving many factors (Gan et al., 2018). Mutations of mtDNA and reduction of mtDNA copy number are often found in cancer cells and are considered the driving factors of carcinogenesis (Reznik et al., 2016).
Schistosomiasis is a tropical disease which has a high incidence in the Middle East, South America, Southeast Asia, and Africa. Infectious larvae invade the human host's skin and cause the disease, which initially grows as an intermediate host. Mature Schistosoma mansoni and japonicum lives in the mesentery or the veins of the pelvic cavity, where they lay eggs and secrete in feces/urine. Furthermore, those eggs trapped in the surrounding tissues of liver to cause granluomas, which can cause disease (McManus et al., 2018).
Our histological results found fibrotic procession in hepatocytes of Schistosomiasis and hepatocellular cancer due to the PIK3CA and TP53 gene mutation. The hepatocytes can be seen in Fig. 2 as irregular proliferation and occurrence of fibrosis. Previously, it was reported that Schistosomiasis could evade the immune system and survive for several years in the host body, causing severe damage to tissues. Its repair leads to the accumulation and deposition of extracellular matrix proteins, leading to liver fibrosis. The manifestations of this disease are largely achieved by the connective tissue growth factor (CTGF) gene, which encodes pro-fibrotic molecules produced by endothelial cells and hepatic stellate cells, myofibroblasts, and hepatocytes (Dessein et al., 2009, Isnard et al., 2010). Subsequent studies also showed that miRNAs activate hepatic stellate cells (HSC) by down-regulating miR-203, thereby increasing the expression of interleukin 33 (IL-33), stimulating the production of interleukin 13 (IL-13) and subsequent hepatic lymphocytes (Hong et al., 2017). In addition, IL-13 activates HSC differentiation, which is another effective source of collagen and is also involved in fibrosis (Angeles et al., 2020, Kamdem et al., 2018). In schistosomiasis japonicum infection, the proliferation of HSC caused by soluble egg antigen (SEA) induces collagen I and III secretions, causing damage to living tissue (Kong et al., 2019). The cytokine profile in other studies also showed the progression of interleukin 5 (IL-5) and IL-13 expression in untreated patients with long-term severe liver fibrosis (Magalhães et al., 2004).
Our data indicated that the total frequency of PIK3CA gene mutation in chronic schistosomiasis patients following HCC was 77.7%, and 33.3% for tumor suppressor gene TP53. It could be speculated that after PIK3CA gene mutation, hepatocytes grow uncontrollably and cause fibrosis in the liver tissues due to chronic schistosomiasis. In addition, it causes HCCs, and the mutation of the tumor suppressor gene TP53 as 33.3%. Previously, studies conducted have not found the role of PIK3CA gene mutation in patients with hepatocellular carcinoma after chronic Schistosomiasis, but only cancer. Therefore, it may be challenging to explain this in detail due to the lack of available literature. However, our results indicated that the PIK3CA gene mutation in patients with Schistosomiasis indicates HCC. It was previously reported that hepatitis B (HBV) and hepatitis C (HBV) are responsible for 54% and 31% of HCC, respectively. In Africa and Asia more than 60% of liver cancer cases are caused by HBV infection, and HCV causes 20%, rest are caused by alcohol and aflatoxin. Around 60% of HCC cases are related to HCV in the United States, Europe, Egypt, and Japan, wherase 20% are caused by HBV (El-Serag, 2012, Tornesello et al., 2013). In Southern Italy, 61% of HCC cases can be attributed to HCV infection (Franceschi et al., 2006, Fusco et al., 2008). It has been reported that mutations cause 35% of HCC cases in China in the PIK3CA gene (Lee et al., 2005), 28% in Italy (Colombino et al., 2012). When PIK3CA gene regulates the phosphatase and tensin homolog (PTEN)-AKT pathway, it affects cell proliferation, angiogenesis, and apoptosis. In addition, several anti-cancer drugs that can regulate the PI3K/Akt pathway have also achieved good results in combating malignant tumors. However, few studies have evaluated the PIK3CA gene mutation in HCC related cases. Therefore, it is particularly important to understand the mutation status of the PIK3CA gene (Carnero, 2009). It is indicated in a study that HCV-positive cases may develop liver cancer (El-Serag, 2002, Giannini et al., 2013). TP53 is a tumor suppressor gene, which is most important related to cancer research. Mutation and genetic changes in the TP53 gene cause human malignancies (Munro et al., 2005, Nigro et al., 1989, Petitjean et al., 2007a), 90% mutations were found non-synonymous and occurred due to the changes in single amino acid (Petitjean et al., 2007b). Inaddition, DNA binding activity reduced when p53 protein affectedly change the tertiary structure (Martin et al., 2002). After mutation, p53 protein inactivates wild-type p53 protein by forming mutant and wild-type dimer structures (Chan et al., 2004, De Vries et al., 2002). Inddition, mutant p53 gene increased the cell proliferation, drug resistance, cell migration and promote the angiogenesis (Blandino et al., 1999, Bossi et al., 2006). Several studies have shown that exposure to specific carcinogens, inducing certain mutations in the TP53 gene develope specific cancer (Hussain et al., 2000, Vogelstein and Kinzler, 1992). Exposure to ultraviolet light (UV) cause skin cancer, which alter the CC to TT (Giglia‐Mari and Sarasin, 2003), Smoking cause liver cancer with changing G to T in the base pairs of TP53 gene (Hainaut et al., 2001, Pfeifer et al., 2002). Aristolochic acid in the diet change A to T and cause endemic nephropathy (Janković et al., 2011), and aflatoxin B1 (AFB1) cause liver cancer after transversion of AGG to AGT (Hsu et al., 1991, Hussain et al., 2007).
5. Conclusion
It is conluded that the liver fibrosis in patients with chronic Schistosomiasis leads to develop cancer, which may be related to the mutation of the PIK3CA gene. Hepatocellular carcinoma could be predicted based on PIK3CA gene mutations in chronic schistosomiasis patients. The risk to get cancer in chronic schistomosiasis patients is very high. Based on our study, it could be speculated that if the patients with chronic schistosomiasis not treated early may become cancerous in future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Angeles J.M.M., Mercado V.J.P., Rivera P.T.J.F., i. I Behind enemy lines: immunomodulatory armamentarium of the Schistosome parasite. 2020;11:1018. doi: 10.3389/fimmu.2020.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino G., Levine A.J., Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. 1999;18(2):477–485. doi: 10.1038/sj.onc.1202314. [DOI] [PubMed] [Google Scholar]
- Bossi G., Lapi E., Strano S., Rinaldo C., Blandino G., Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. 2006;25(2):304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- Burke M., Jones M., Gobert G., Li Y., Ellis M., McManus D.J.P., i Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- Carnero A.J.E., o. o. i. d Novel inhibitors of the PI3K family. 2009;18:1265–1277. doi: 10.1517/13543780903066798. [DOI] [PubMed] [Google Scholar]
- Chan W.M., Siu W.Y., Lau A., Poon R.Y.J.M., biology, c How many mutant p53 molecules are needed to inactivate a tetramer? 2004;24:3536–3551. doi: 10.1128/MCB.24.8.3536-3551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombino, M., Sperlongano, P., Izzo, F., Tatangelo, F., Botti, G., Lombardi, A., Accardo, M., Tarantino, L., Sordelli, I., Agresti, M. J. C. d., and disease (2012). BRAF and PIK3CA genes are somatically mutated in hepatocellular carcinoma among patients from South Italy. 3, e259-e259. [DOI] [PMC free article] [PubMed]
- De Vries A., Flores E.R., Miranda B., Hsieh H.-M., Van Oostrom C.T.M., Sage J., Jacks T.J.P., o. t. N. A. o. S Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. 2002;99:2948–2953. doi: 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Chevillard C., Arnaud V., Hou X., Hamdoun A.A., Dessein H., He H., Abdelmaboud S.A., Luo X., Li J.J.T.J., o. e. m Variants of CTGF are associated with hepatic fibrosis in Chinese. Sudanese, and Brazilians infected with SchistosomesCTGF and severe hepatic fibrosis. 2009;206:2321–2328. doi: 10.1084/jem.20090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.-J., Li Y., Wang S.-D., Wang X.-S., Fang F., Wang W.-Y., Lv P., Zhao D.-H., Wei F., Qi L.J.I., j. o. m. s Long noncoding RNA lncCAMTA1 promotes proliferation and cancer stem cell-like properties of liver cancer by inhibiting CAMTA1. 2016;17:1617. doi: 10.3390/ijms17101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. 2012;142(6):1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H.B.J.H. Hepatocellular carcinoma and hepatitis C in the United States. 2002;36:s74–s83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- Franceschi S., Montella M., Polesel J., La Vecchia C., Crispo A., Dal Maso L., Casarin P., Izzo F., Tommasi L.G., Chemin I., Trépo C., Crovatto M., Talamini R. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. 2006;15(4):683–689. doi: 10.1158/1055-9965.EPI-05-0702. [DOI] [PubMed] [Google Scholar]
- Fusco M., Girardi E., Piselli P., Palombino R., Polesel J., Maione C., Scognamiglio P., Pisanti F.A., Solmone M., Cicco P.D., Ippolito G., Franceschi S., Serraino D. Epidemiology of viral hepatitis infections in an area of southern Italy with high incidence rates of liver cancer. 2008;44(6):847–853. doi: 10.1016/j.ejca.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Gan H., Li N., Zhang Q., Feng Z-Z. Silencing FOXA1 gene regulates liver cancer cell apoptosis and cell proliferation. 2018;22:397–404. doi: 10.26355/eurrev_201801_14187. [DOI] [PubMed] [Google Scholar]
- Giannini E.G., Marenco S., Bruzzone L., Savarino V., Farinati F., Del Poggio P., Rapaccini G.L., Di Nolfo M.A., Benvegnù L., Zoli M., Borzio F., Caturelli E., Chiaramonte M., Trevisani F. Hepatocellular carcinoma in patients without cirrhosis in Italy. 2013;45(2):164–169. doi: 10.1016/j.dld.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Giglia-Mari G., Sarasin A. TP53 mutations in human skin cancers. 2003;21(3):217–228. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- Hainaut P., Olivier M., Pfeifer G.P.J.M. TP53 mutation spectrum in lung cancers and mutagenic signature of components of tobacco smoke: lessons from the IARC TP53 mutation database. 2001;16:551–553. doi: 10.1093/mutage/16.6.551. [DOI] [PubMed] [Google Scholar]
- Hong Y., Fu Z., Cao X., Lin J.J.P., i Changes in microRNA expression in response to Schistosoma japonicum infection. 2017;39 doi: 10.1111/pim.12416. [DOI] [PubMed] [Google Scholar]
- Hsu, I., Metcalf, R., Sun, T., Welsh, J., Wang, N., and Harris, C. J. N. (1991). Mutational hot spot in the p53 gene in human hepatocellular carcinomas. 350, 427-428. [DOI] [PubMed]
- Hussain S.P., Schwank J., Staib F., Wang X.W., Harris C.C. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. 2007;26(15):2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- Hussain S.P., Harris C.C. Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens. 2000;462:311–322. doi: 10.1016/s1383-5742(00)00015-6. [DOI] [PubMed] [Google Scholar]
- Hwang, S., and Yang, Y. M. J. A. o. P. R. (2021). Exosomal microRNAs as diagnostic and therapeutic biomarkers in non-malignant liver diseases. 1-14. [DOI] [PMC free article] [PubMed]
- Isnard, A., He, H., Kouriba, B., and Chevillard, C. J. e. (2010). Genetic Factors Involved in Human Susceptibility to Infection by Schistosomiasis. [DOI] [PMC free article] [PubMed]
- Janković, S., Bukvić, D., Marinković, J., Janković, J., Marić, I., and Djukanović, L. J. N. D. T. (2011). Time trends in Balkan endemic nephropathy incidence in the most affected region in Serbia, 1977–2009: the disease has not yet disappeared. 26, 3171-3176. [DOI] [PubMed]
- Kamdem S.D., Moyou-Somo R., Brombacher F., Nono J.K.J.F., i. i Host regulators of liver fibrosis during human schistosomiasis. 2018;9:2781. doi: 10.3389/fimmu.2018.02781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D.-L., Kong F.-Y., Liu X.-Y., Yan C., Cui J., Tang R.-X., Zheng K.-Y.-J.-P., vectors Soluble egg antigen of Schistosoma japonicum induces pyroptosis in hepatic stellate cells by modulating ROS production. 2019;12:1–12. doi: 10.1186/s13071-019-3729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononen J., Bubendorf L., Kallionimeni A., Bärlund M., Schraml P., Leighton S., Torhorst J., Mihatsch M.J., Sauter G., Kallionimeni O.-P.-J.-N., m Tissue microarrays for high-throughput molecular profiling of tumor specimens. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Lee, J. W., Soung, Y. H., Kim, S. Y., Lee, H. W., Park, W. S., Nam, S. W., Kim, S. H., Lee, J. Y., Yoo, N. J., and Lee, S. H. J. O. (2005). PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. 24, 1477-1480. [DOI] [PubMed]
- Li Y., Zhang P., Zhang X., Bi X., Wu M., Zou J., Wang Z., Lu F., Dong Z., Gao J.J.A. Adipose matrix complex: a high-rigidity collagen-rich adipose-derived material for fat grafting. 2021;13:14910. doi: 10.18632/aging.203120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.-C., Hsu C.-H., Hsu C., Cheng A.-L. Tumor heterogeneity in hepatocellular carcinoma: facing the challenges. Liver cancer. 2016;5(2):128–138. doi: 10.1159/000367754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães A., Miranda D.G., Miranda R.G., Araújo M.I., Jesus A.A.de., Silva A., Santana L.B., Pearce E., Carvalho E.M., Jesus Amélia.R.de. Cytokine profile associated with human chronic schistosomiasis mansoni. 2004;99(suppl 1):21–26. doi: 10.1590/s0074-02762004000900004. [DOI] [PubMed] [Google Scholar]
- Martin A.C.R., Facchiano A.M., Cuff A.L., Hernandez-Boussard T., Olivier M., Hainaut P., Thornton J.M. Integrating mutation data and structural analysis of the TP53 tumor-suppressor protein. 2002;19(2):149–164. doi: 10.1002/humu.10032. [DOI] [PubMed] [Google Scholar]
- McManus D.P., Dunne D.W., Sacko M., Utzinger Jürg, Vennervald B.J., Zhou X.-N. Schistosomiasis. Nature Reviews Disease Primers. 2018;4(1) doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- Munro A., Lain S., Lane D.J.B., j. o. c P53 abnormalities and outcomes in colorectal cancer: a systematic review. 2005;92:434–444. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Okuda K., Kojiro M., Sakamoto K., Kubo Y., Shimokawa Y.J.C. Primary liver cancer coincident with schistosomiasis japonica. A study of 24 necropsies. Cancer. 1975;36:1483–1489. doi: 10.1002/1097-0142(197510)36:4<1483::aid-cncr2820360441>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Nigro J.M., Baker S.J., Preisinger A.C., Jessup J.M., Hosteller R., Cleary K., Signer S.H., Davidson N., Baylin S., Devilee P., Glover T., Collins F.S., Weslon A., Modali R., Harris C.C., Vogelstein B. Mutations in the p53 gene occur in diverse human tumour types. 1989;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Petitjean A., Achatz M.I.W., Borresen-Dale A.L., Hainaut P., Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. 2007;26(15):2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- Petitjean A., Mathe E., Kato S., Ishioka C., Tavtigian S.V., Hainaut P., Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53. 2007;28(6):622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- Pfeifer G.P., Denissenko M.F., Olivier M., Tretyakova N., Hecht S.S., Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. 2002;21(48):7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- Piskata Z., Servusova E., Babak V., Nesvadbova M., Borilova G. The quality of DNA isolated from processed food and feed via different extraction procedures. 2019;24(6):1188. doi: 10.3390/molecules24061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H.C. The molecular biology of carcinogenesis. 1993;72(S3):962–970. doi: 10.1002/1097-0142(19930801)72:3+<962::aid-cncr2820721303>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Reznik, E., Miller, M. L., Şenbabaoğlu, Y., Riaz, N., Sarungbam, J., Tickoo, S. K., Al-Ahmadie, H. A., Lee, W., Seshan, V. E., and Hakimi, A. A. J. e. (2016). Mitochondrial DNA copy number variation across human cancers. 5, e10769. [DOI] [PMC free article] [PubMed]
- Thorgeirsson S.S., Grisham J.W.J.N., g Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Tornesello M.L., Buonaguro L., Tatangelo F., Botti G., Izzo F., Buonaguro F.M. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. 2013;102(2):74–83. doi: 10.1016/j.ygeno.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K.W. Carcinogens leave fingerprints. 1992;355(6357):209–210. doi: 10.1038/355209a0. [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Mentink-Kane M.M., Pesce J.T., Ramalingam T.R., Thompson R., Wynn T.A.J.I., biology, c Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]