Abstract
PURPOSE
To investigate microperimetry testing of retinitis pigmentosa GTPase regulator gene (RPGR)-associated retinopathy in a cohort of children and adults.
DESIGN
Prospective observational case series.
METHODS
The coefficient of repeatability and intraclass correlation coefficient (ICC) of mean sensitivity (MS) were calculated for mesopic microperimetry. Best-corrected visual acuity (BCVA), contrast sensitivity (CS), MS, total volume (VTOT), and central 3-degree field volume (V3) from volumetric and topographic analyses were acquired.
RESULTS
The study recruited 76 individuals with RPGR (53 adults, 23 children). The mean follow-up period was 2.8 years. The ICC values for MS, VTOT, and V3 were 0.982 dB (95% CI, 0.969-0.989 dB), 0.970 dB-steradian (sr) (95% CI, −0.02658 to 0.03691 dB-sr), and 0.986 dB-sr (95% CI, 0.978-0.991), respectively. The r values for interocular MS, VTOT, and V3 were 0.97 (P < .01), 0.97 (P < .01), and 0.98 (P < .01), respectively, indicating strong interocular correlation. The interocular correlation of progression for MS, VTOT, and V3 was 0.81 (P < .01), 0.64 (P < .01), and 0.81 (P < .01), respectively. There was no statistically significant difference in the interocular progression rates for MS or VTOT. V3 did show a statistically significant difference. Most patients lost retinal sensitivity rapidly during their second and third decades of life.
CONCLUSIONS
The high degree of reproducibility of results and the good interocular correlation lends this method to accurately monitoring disease progression, as well as supporting validation of the use of MP in assessing the outcomes of gene therapy clinical treatment trials.
HIGHLIGHTS
-
•
Retinitis pigmentosa GTPase regulator gene (RPGR)-associated retinopathy is a predominantly symmetric test.
-
•
Microperimetry has a good test-retest reliability for the disease and can track progression of retinal functional loss.
-
•
The high degree of reproducibility of results, good interocular correlation, and accurate tracking of change over time lends this modality well to assessing the outcomes of gene therapy trials.
X-linked retinitis pigmentosa (XLRP) is a subset of genetically heterogenous conditions that fall under the broad phenotypic group of retinitis pigmentosa (RP). Affected individuals typically present with nyctalopia and progressive peripheral visual loss. In the later stages of the condition, central vision becomes affected, resulting in severe visual impairment.1
XLRP accounts for 5% to 15% of all RP cases.2,3 Pathogenic sequence variants in the RP GTPase regulator gene (RPGR) have been shown to be responsible for 75% of cases of XLRP. RPGR variants have also been associated with cone dystrophy (COD), cone-rod dystrophy (CORD), and sector RP.4, 5, 6 RPGR-associated RP (XLRP-RPGR) has a particularly severe phenotype, characterized by early onset of symptoms, usually in early childhood and particularly rapid progression of visual loss. It is currently the target of several gene therapy trials (NCT04671433, NCT03252847, NCT03116113, NCT03316560, and NCT04517149) that aim to arrest progression and improve retinal function. A recent publication of the initial results of a gene therapy trial for XLRP-RPGR included microperimetry (MP) testing as part of the secondary outcomes.7 This trial used the mesopic Macular Integrity Assessment (MAIA) MP assessment (CenterVue MP Systems, Padova, Italy), which was central to achieving an objective assessment of retinal sensitivity changes after treatment and for the monitoring of inflammatory complications of gene therapy and documenting their resolution.
Retinal sensitivity measures are widely used as part of retinal functional assessment and often constitute key metrics for monitoring disease progression. Common methods for measuring retinal sensitivity include full-field dynamic and static perimetry or MP (fundus-guided perimetry). Test-retest repeatability of MP in an RPGR patient cohort using the MAIA system has been reported.8 Previously published data from our group explored repeatability, interocular symmetry, and rate of progression using full-field static perimetry in XLRP-RPGR.9 We have developed a customized testing protocol for RPGR-associated retinopathy using the Nidek MP1 (Nidek Technologies, Padova, Italy), aiming for a standardized and reproducible assessment of point-by-point retinal sensitivity, as surrogate measures of retinal function and disease progression. Further analysis of retinal sensitivity was undertaken using the Visual Field Monitoring and Analysis software (VFMA: Office of Technology Transfer & Business Development, Portland, OR).10, 11, 12 This uses all of the point-by-point retinal sensitivity measures obtained from the MP testing grid to generate a comprehensive volume plot of retinal sensitivity, which is also a more sensitive measure of change over time. A total hill of vision (VTOT) or any other volumetric measure of sensitivity can be generated within defined retinal areas; for example, within the central 3° (V3).
We aimed to characterize retinal function in detail, including disease symmetry and natural history, in a large, molecularly confirmed cohort of RPGR-associated retinopathy, by using a range of MP-derived metrics. The correlation of MP metrics with contrast sensitivity (CS) and best corrected visual acuity (BCVA) was investigated. This study also provides data to support the validation of MP-derived metrics as clinically meaningful end points for patient stratification and monitoring of treatment effect in both on-going and future gene therapy trials.
METHODS
Ethical approval was received from the Moorfields Eye Hospital Ethics Committee at (London, United Kingdom) for this study. The study adhered to the Declaration of Helsinki.
STUDY PARTICIPANTS
All participants were affected males with molecular genetic confirmation of disease-causing variants in RPGR. Participants aged younger than 18 years were classified as children, and those older than 18 were classified as adults.
MOLECULAR GENETICS
All patients were recruited from the Moorfields Eye Hospital retinal genetics service. Patients were genetically screened by variable protocols, as previously described.13
ASSESSMENT OF VISUAL FUNCTION
Patients attended research appointments at 6-month intervals for 2 years and consequently for annual visits. Visual function assessments included BCVA at 4 m, using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, followed by CS testing at a distance of 1 m with the Pelli-Robson chart. BCVA was recorded in logMAR units and CS as logCS units.
ASSESSMENT OF RETINAL FUNCTION
Mesopic fundus-guided perimetry was performed using the Nidek MP-1. Pupils were dilated using phenylephrine hydrochloride 2.5% solution (Bausch & Lomb, Inc) and tropicamide 1% ophthalmic solution (Akorn, Inc). Fixation was monitored by a dedicated ophthalmic technician throughout each assessment. A single horizontal transfoveal optical coherence tomography (OCT) scan performed on a Spectralis OCT (Heidelberg Engineering) was imported into the perimeter to facilitate the accurate centration of the testing grid over the anatomical fovea. The radial pattern 44-point testing grid developed for these assessments is shown in the Supplemental Figure. Background illumination was set to 4 apostilbs (1.27 cd/m2) and a Goldmann size III stimulus was presented for 200 milliseconds. The testing protocol used a 4- to 2-dB full-threshold bracketing test strategy. A mean sensitivity (MS) value was automatically computed by the manufacturer's software. The intrinsic reliability reports from the device were collected; however, in the absence of robust evidence of their value, they were not used as an exclusion criterion. Fixation stability within 4° was used as a primary reliability assessment, and testing was repeated if test results fell below 90%. Those with fixation stability of less than 90% were included in the analysis only if repeat testing remained consistent in MS and the volumetric sensitivity indices. Participants with sensitivities below the testing threshold (recorded sensitivity of 0) were also excluded from further testing or analysis.
VOLUMETRIC INDICES OF RETINAL FUNCTION
Test data were exported from the software as comma-separated value (csv) files for analysis in the VFMA software. This software generates a 3-dimensional hill-of-vision (HOV) model of the visual field and allows the calculation of a volume of sensitivity beneath the surface of the model, based upon the total sensitivity across the solid angle of the base of the test grid, in decibel-steradian (dB-sr) units.14 This is an objective numerical measure of the total sensitivity of the examined retinal area (termed Vtotal/VTOT) and can be subanalyzed by area. In our study, we also analyzed the visual field contained within a central circle of 3° radius (termed V3), based on the total sensitivity across the solid angle of a central 6°, to reflect the function of the central visual field.
PROGRESSION ANALYSIS
Progression rates for each individual eye were obtained from gradients of linear trend lines fitted to data points using the least-squares method. Photoreceptor degeneration and the decline of visual function in RP, both in animal models and humans, follows an exponential pattern over an extended time period.15, 16, 17, 18, 19, 20 Nevertheless, shorter follow-up periods can be successfully modeled using a linear best-fit line to capture a short-range progression snapshot.9 Only eyes with a minimum of 3 data points and a minimum follow-up of 1 year were included.
DISEASE SYMMETRY
The Bland-Altman analysis was used to assess interocular differences in MS, VTOT, and V3 at baseline and for the interocular progression rates. The Spearman correlation coefficient was used to investigate interocular correlation between baseline MS, VTOT, and V3, and progression rates for VTOT and V3.
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS Statistics software (IBM Corp). Significance for all statistical tests was set at P < .05. The Shapiro-Wilk test was used to test for normality for all variables. Test-retest reliability was investigated with the intraclass correlation coefficient (ICC) based on absolute agreement and a 2-way mixed-effects model using results from the right eye to minimize the clustering effect.
RESULTS
DEMOGRAPHICS AND GENETICS
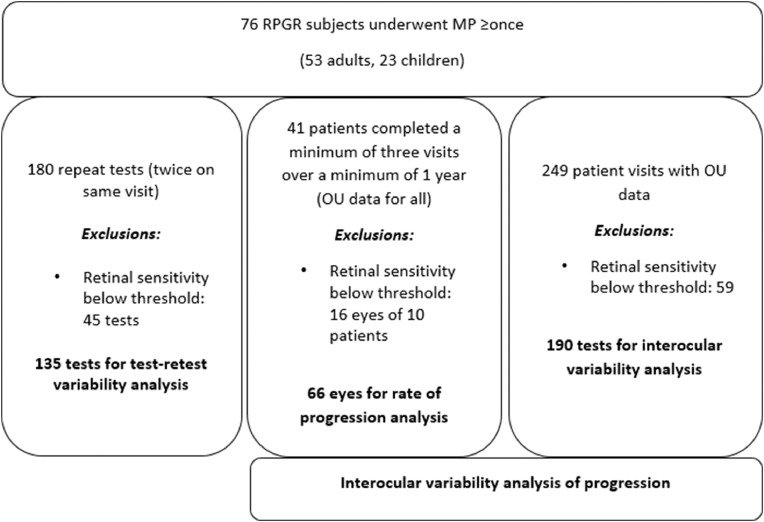
MP testing was performed in 76 individuals (53 adults, 23 children) with RPGR retinopathy. The age range of patients was 6.9 to 55.8 years (mean, 25; median, 24) at baseline testing. The mean follow-up period was 2.8 years (range, 1.2-5.3). The flow chart of patient participation is given in Figure 1.
FIGURE 1.
Flow chart of subject recruitment and participation in testing. MP = microperimetry; RPGR = retinitis pigmentosa GTPase regulator.
Disease-causing variants in RPGR were present in the open reading frame 15 (ORF15) in 49 participants (64%) or in exons 1 to 14 in 27 (36%). Of those with variants in the ORF15 region, there were 2 patients expressing a COD phenotype, 1 patient with CORD, and 1 patient with sectoral RP. All other patients had XLRP.
FUNCTIONAL ASSESSMENT
The baseline BCVA, CS, MS, VTOT, and V3 metrics are presented in Table 1. Annual progression rates for these parameters are also reported.
Table 1.
Functional Assessment of Progression
| OD Progression Rate |
OS Progression Rate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Metric | OD Baseline Mean (SD) | OD Baseline Median (IQR) | Mean (SD) | Median (IQR) | OS Baseline Mean (SD) | OS Baseline Median (IQR) | Mean (SD) | Median (IQR) |
| BCVA, LogMAR | 0.41 (0.34) | 0.34 (0.34) | 0.00 (0.05) | 0.00 (0.04) | 0.44 (0.40) | 0.39 (0.28) | 0.03 (0.07) | 0.01 (0.07) |
| CS, logCS | 1.19 (0.45) | 1.30 (0.63) | 0.02 (0.09) | 0.02 (0.08) | 1.15 (0.42) | 1.23 (0.56) | 0.00 (0.08) | 0.00 (0.07) |
| MS, dB | 6.75 (6.47) | 3.9 (10.00) | 0.82 (0.87) | 0.74 (1.10) | 6.75 (6.84) | 3.30 (10.96) | 0.67 (0.86) | 0.54 (1.06) |
| VTOT, dB-sr | 0.30 (0.36) | 0.12 (0.54) | 0.04 (0.06) | 0.0289 (0.069) | 0.31 (0.39) | 0.10 (0.53) | 0.04 (0.06) |
0.03 (0.06) |
| V3, dB-sr | 0.06 (0.06) | 0.04 (0.11) | 0.01 (0.01) |
0.01 (0.01) |
0.05 (0.06) | 0.04 (0.08) | 0.01 (0.01) |
0.01 (0.01) |
Mean and median best-corrected visual acuity (BCVA) in LogMAR units, mean sensitivity (MS) in decibels (dB) and the volumetric indices VTOT and V3 in decibel-steradians (dB-sr) for the right eye (OD) and left eye (OS). Progression rates calculated per year. SD = standard deviation, IQR = interquartile range
TEST-RETEST RELIABILITY
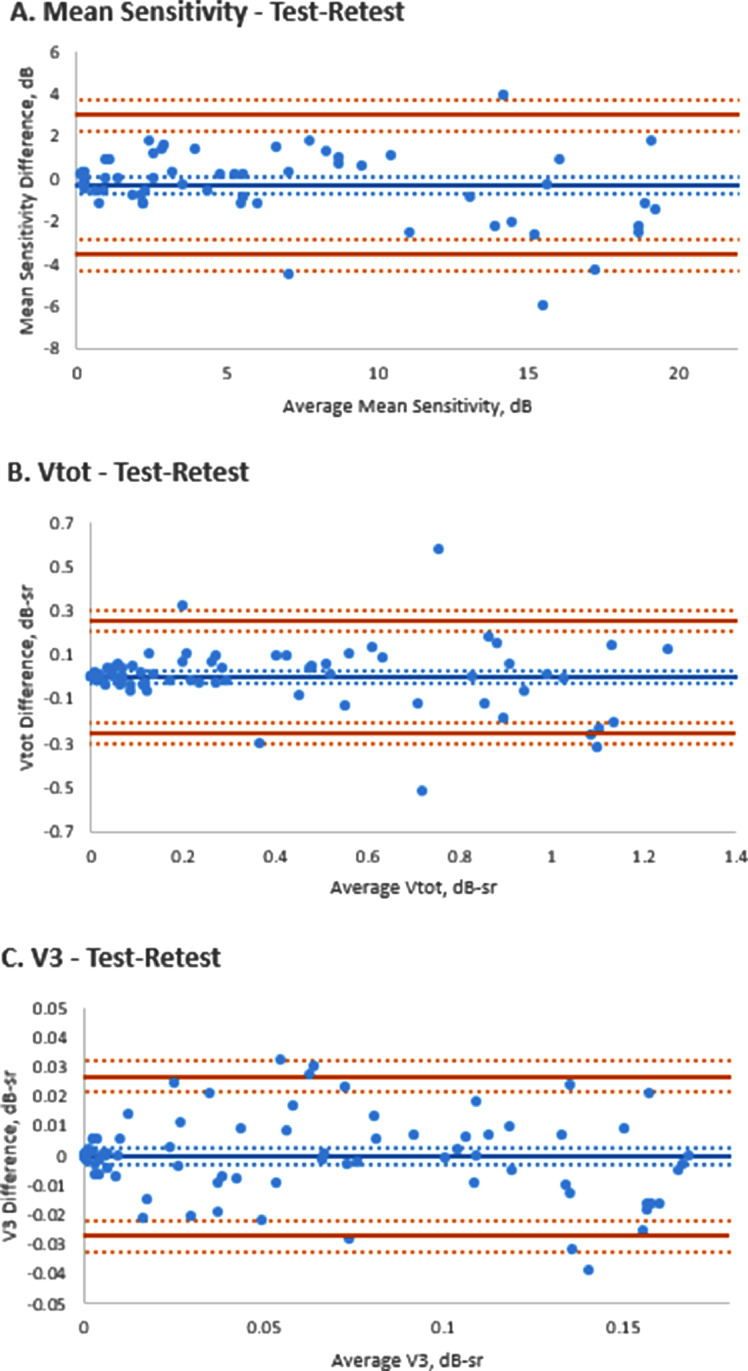
After exclusions for subthreshold sensitivity (MS value of 0 dB), 135 pairs of tests were analyzed. All paired same-day tests passing the exclusion criteria were included, not only those at baseline. The ICC values in this subgroup for MS, VTOT, and V3 were 0.982 (95% CI, 0.969-0.989), 0.970 (95% CI, −0.02658 to 0.03691), and 0.986 (95% CI, 0.978-0.991), respectively. This indicates a strong correlation between separate consecutive tests and a high test-retest reliability for this method. Bland-Altman plots for the test-retest reliability of right eye MS, VTOT, and V3 are presented in Figure 2.
FIGURE 2.
Test-retest reliability assessment. Bland-Altmann plots of the test-retest reliability of the (A) mean sensitivity measurements in dB, and the volumetric measurement in dB-steradian (dB-sr) of (B) total retinal sensitivity (VTOT) and (C) of the fovea-centered area of radius 3° (V3).
INTEROCULAR SYMMETRY
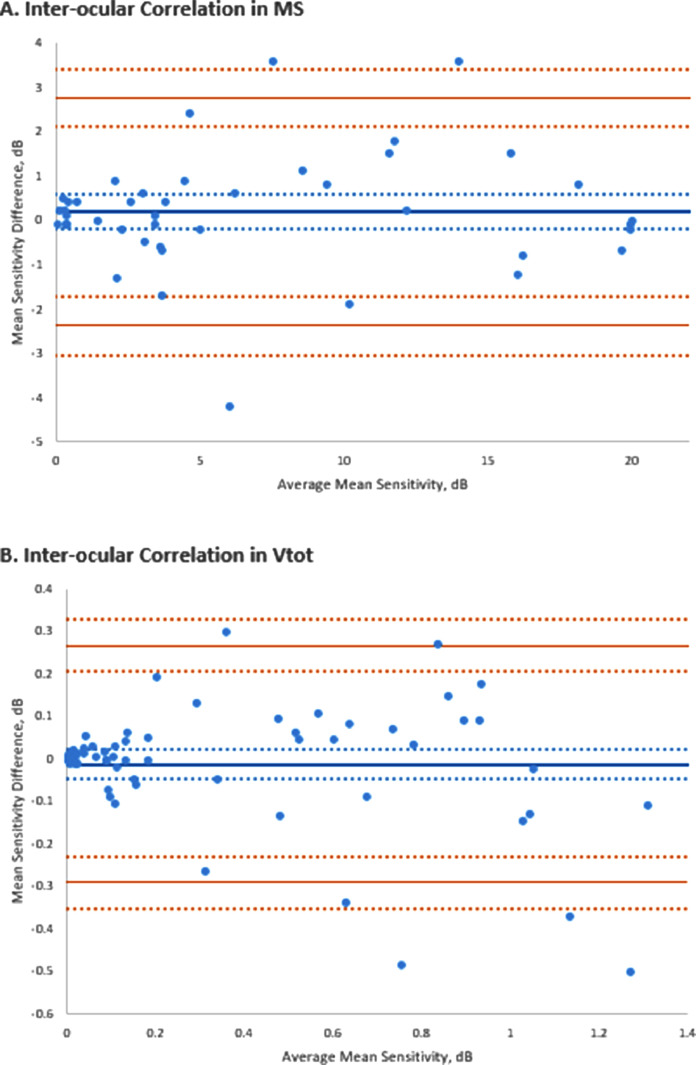
Data from 190 test pairs for right and left eyes at baseline were analyzed after the application of the aforementioned exclusion criteria. The r values (Pearson correlation coefficient) for interocular MS, VTOT, and V3, were 0.97 (P < .01), 0.97 (P < .01), and 0.98 (P < .01), respectively, indicating strong interocular correlation of all metrics. The interocular correlation (Spearman correlation coefficient) of progression for MS, VTOT, and V3 were 0.81 (P < .01), 0.64 (P < .01), and 0.81 (P < .01), respectively. The Bland-Altman plots for the interocular MS and VTOT are presented in Figure 3. There was no statistically significant difference in the interocular progression rates for MS (P = .26) or VTOT (P = .70) by paired t test. V3 did show a statistically significant difference at P = .01.
FIGURE 3.
Interocular symmetry assessment. Bland-Altmann plots of the (A) interocular symmetry of the mean sensitivity (MS) and (B) the volumetric measurement of total retinal sensitivity (VTOT). dB-sr = in dB-steradian.
RATE OF PROGRESSION
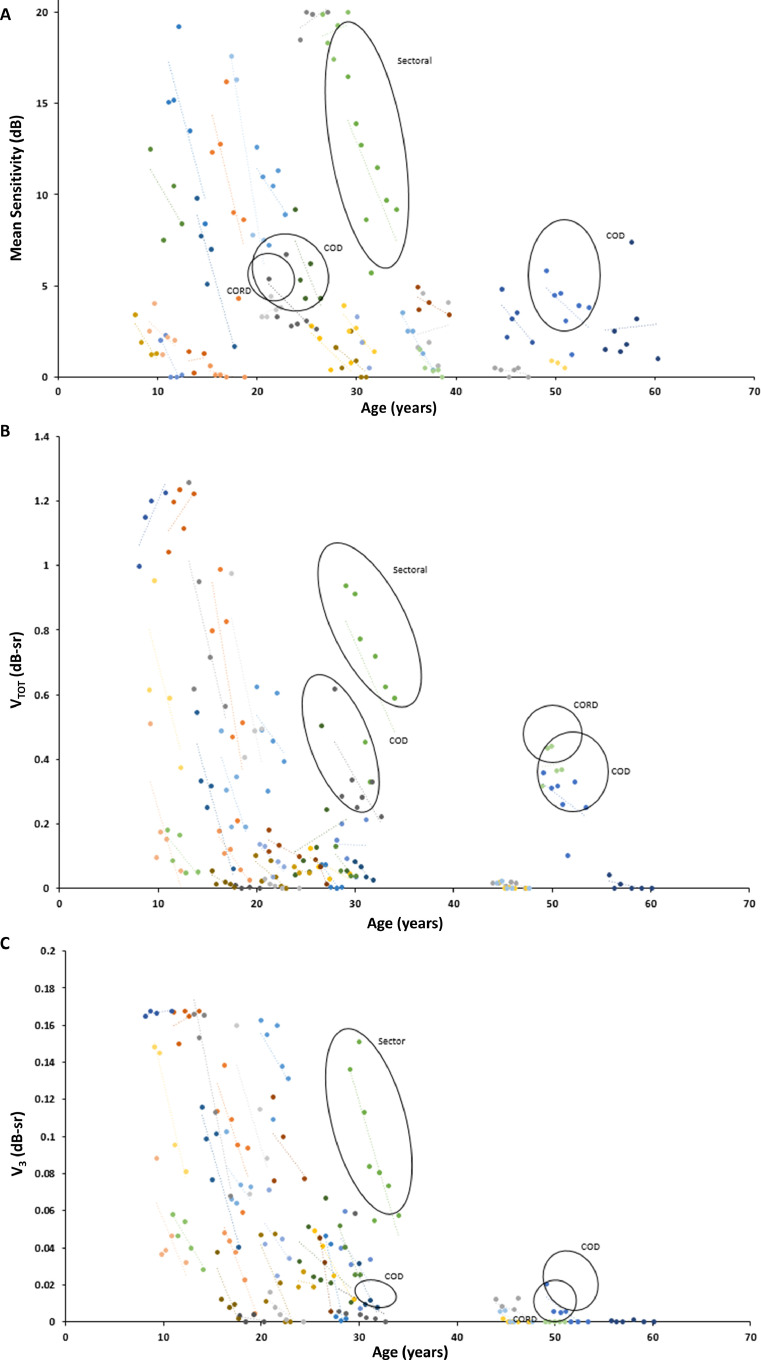
Because there was a strong interocular correlation at baseline, only OD data were selected for analysis of progression as being representative of the cohort. The mean rates of annual progression of MS, VTOT, and V3 were 0.82 dB/y, 0.04 dB-sr/y, and 0.01 dB-sr/y respectively (Table 1). Figure 4 shows the progression plots for MS, VTOT, and V3.
FIGURE 4.
Progression of retinal functional loss. Plots with best-fit lines of all individuals (X-linked retinitis pigmentosa [XLRP], 27; cone dystrophy [COD], 2; cone-rod dystrophy [CORD], 1; and sector retinitis pigmentosa [RP], 1) in the cohort with ≥3 consecutive retinal sensitivity measurements. A. The mean sensitivity. The volumetric measurement of (B) total retinal sensitivity (VTOT) and (C) fovea-centered area of radius 3° (V3) are presented for the analyzed right eyes. dB-sr = dB-steradian.
The rate of progression in the ORF15 genotype subgroup was comparable to that of the subgroup with disease-causing variants in exons 1 to 14 (MS, 0.81 dB/y in both subgroups; VTOT, 0.042 and 0.039 dB-sr/y, respectively; and V3, 0.009 and 0.013 dB-sr/y, respectively). None of the differences reached statistical significance (P= .667 for MS, P = .535 for VTOT, and P = .395 for V3)
Most patients lost retinal sensitivity rapidly during their second and third decades of life. Patients with COD, CORD, and sectoral RP phenotypes were outliers, as would be anticipated. For example, the patient with sectoral RP demonstrated a similar characteristic rapid decline in MS, VTOT, and V3; however, this was noted later in life. One of the 2 COD patients and the CORD patient demonstrated unique relative preservation of VTOT into later life, however, with characteristic reductions in MS and V3.
CORRELATION WITH BCVA AND CS
The correlation between baseline BCVA and CS, and the MS, VTOT and V3 metrics was tested using the Spearman correlation coefficient. There was a positive statistically significant correlation between baseline BCVA and CS, BCVA and MS, BCVA and VTOT, and BCVA and V3 (0.80, 0.62, 0.58, and 0.77, respectively). There was also a positive statistically significant correlation between baseline CS and MS, CS and VTOT, and CS and V3 (0.70, 0.65, and 0.85, respectively).
The correlation between progression rates of BCVA and CS, and the MS, VTOT, and V3 metrics was also investigated. There was a weak positive correlation between the annual progression rates of BCVA and CS (0.14) and weak positive correlations between the progression rates of BCVA and the MS, VTOT, and V3 metrics (0.06, 0.14, and 0.03, respectively). There was also a weak positive correlation between the annual progression rates of CS and MS, VTOT, and V3 (0.21, 0.29, and 0.26, respectively). These were not statistically significant.
DISCUSSION
To our knowledge, this is the largest prospective report on retinal sensitivity using MP in a molecularly confirmed group of RPGR individuals including adults and children. Traditional assessments of visual function, such as visual acuity (VA) and CS, are not ideal for monitoring disease progression in RPGR, because most changes occur outside of the fovea until late in the disease course. This means that relative preservation of the VA and CS parameters does not reflect the rapid progressive changes in visual function that occurs. Furthermore, clinical treatment trials need to review the response to treatment over relatively short time periods, over which VA and CS have been observed to remain largely stable. Hence surrogate markers of retinal function need to be used for accurate monitoring.
INTEROCULAR SYMMETRY
There was good overall symmetry in the MS and the volumetric indices of retinal function, VTOT, and V3 at baseline, as demonstrated by the r values of the interocular correlation coefficient.
Interocular symmetry of visual function has previously been demonstrated in individuals with RPGR.9,21 The interocular correlation of progression of the MS, VTOT, and V3 metrics was more variable. However, the variability did not reach statistical significance for MS (P = .264) and VTOT (P = .705) by paired t test. Interocular variability in the progression of V3 was statistically significant at P = .01. What this signifies clinically is not clear; however, we postulate that the absolute values of retinal sensitivity are so small for the V3 metric that the effect of small differences and any outlying values is magnified and can skew the analysis.
Clinical trials of treatment frequently use fellow eyes as controls, and hence, good interocular correlation is useful in monitoring treatment response vs control progression. We believe adequate interocular symmetry is demonstrated in our participants to facilitate this; however, we would recommend acquiring sufficient baseline testing to establish a reliable and robust starting point and focusing assessments on the MS and VTOT metrics. Furthermore, with high levels of interocular disease symmetry demonstrated, the advent of approved gene therapy in the future promises potentially similar benefit to both eyes from receiving treatment.
TEST-RETEST RELIABILITY
The ICC results for MS, VTOT, and V3 indicate strong test-retest reliability. The MS is a traditional measure of visual function; however, because it is a mathematical average, it lacks the precision necessary to distinguish point-by-point sensitivity and does not possess the comprehensive reflection of total retinal sensitivity such as that achieved by the volumetric indices VTOT and V3. Good test-retest reliability of these metrics lends support to their use in monitoring visual function progression in this cohort of patients.
MP is a faster test to perform compared with full-field perimetry such as the Octopus900 (Haag-Streit AG) and, hence, may be easier to include in testing regimens for both monitoring of progression and recording the response to treatment trials. It was also reasonably well tolerated by the children in our study, with participants as young as 8 years being able to reliably perform testing on the Nidek MP-1 in this cohort. Given the early onset of visual loss in XLRP, this lends it well to detect changes in childhood. A recent report,8 using the MAIA perimeter reported good test-retest reliability for testing in an XLRP-RPGR cohort (with no data provided on the age of these individuals, but likely to have been adults only).They found greater variability between the first 2 tests participant performs than between tests 2 and 3, indicating a learning phenomenon, resulting in the recommendation to perform 3 baseline assessments in cases of clinical trials and use the final macular sensitivity result as baseline. The ICC results for MS and the volumetric indices in our study confirm high levels of test-retest reliability for MP measurements on the Nidek MP-1 perimeter. This further supports the potential use of this device to monitor progression and treatment effect in XLRP-RPGR.
RATE OF PROGRESSION
The mean rates of annual progression of MS, VTOT, and V3 were 0.82 dB/y, 0.04 dB-sr/y, and 0.01 dB-sr/y, respectively, in our cohort. This is comparable to the progression rates previously reported by Tee and associates,8 based on full-field static perimetry using the Octopus 900 perimeter, with the V30 and V5 mean annual rates of progression of right eyes being reported as 0.6819 and 0.0056 dB-sr, respectively. Together, these findings support the anticipated progression of the natural history of XLRP-RPGR and lend weight to prognostic information that can be imparted to patients.
The correlation of the baseline BCVA and CS with the MS and the volumetric indices is robust and statistically significant. The greatest correlation was observed between baseline BCVA and CS, with the second strongest correlation being noted between both BCVA and V3, and CS and V3. This implies that baseline BCVA, CS, and V3 may be useful as surrogate markers for disease severity at baseline. However, there was only a weak, not statistically significant correlation, between the progression rates of BCVA and CS and the MS and VTOT. This supports the observation that progression rates of central visual loss can differ significantly from full visual field loss in this primarily rod-centric phenotype, with central macular function remaining relatively preserved until the latest stages of disease.
It is notable that the progression rates of loss of retinal sensitivity are comparable between the molecularly heterogenous cohort of XLRP patients, with similar progression rates being recorded between the ORF15 and the exons 1 to 14 subgroups. A previous study investigating progression rates using full-field Octopus perimetry found an increased rate of progression (although not statistically significant) in the exon 1 to 14 subgroup, although their findings may have been confounded by an age disparity between the 2 molecularly distinct cohorts.8 Another study, carried out in the Asian population, also supported the finding that patients with variants in exons 1 to 14 retained less visual acuity than patients with ORF15 variants and deteriorated faster.22 Similar findings were identified in a cohort of 14 Japanese patients.23
LIMITATIONS
The study has several limitations. For evaluating progression rates, a longer time period would be desirable, which would allow modeling of progression according to established exponential models. In our study with shorter follow-up, a linear trend line fit was more appropriate for estimating progression rates. The Nidek-MP-1 has a relatively narrow dynamic range of 0 to 20 dB, and this meant that younger participants in our cohort were sometimes able to reach the ceiling of sensitivity, and maximum sensitivity values were recorded for sequential tests. Similarly, older patients frequently fell below the sensitivity threshold, and values of 0 were generated for their retinal sensitivity results, despite some preserved level of retinal function (BCVA and CS). These floor and ceiling effects could be addressed with wider bracketing strategies for the testing protocol, which is not possible to achieve on the Nidek MP-1. Newer MP devices have a wider dynamic range of stimuli intensities. It is nevertheless useful to analyze the data acquired on the Nidek MP-1, because most of our cohort of patients fell well within its sensitivity brackets, and so meaningful progression data were collected.
Furthermore, the testing protocol used only a mesopic testing strategy. The latest MP devices can perform across a range of illuminations and record sensitivities under photopic, mesopic, and dark-adapted scotopic conditions, as well as using rod- and cone-specific color stimuli (cyan and red, respectively). XLRP-RPGR is a condition affecting first the rod and subsequently cone system. It is therefore of interest to specifically probe the rod system in XLRP-RPGR to more fully assess disease progression, particularly in the early stages, and directly probe the cone system for later disease stages.
The Nidek-MP-1 tests a mixture of rod and cone function under standard mesopic protocol conditions. An alternative to the latest MP devices may be the dark-adapted chromatic Medmont M700 perimeter (Medmont International Pty Ltd), which has been designed to probe different photoreceptor mechanisms24,25 but requires further investigation. Further exploration of retinal function in affected females with RPGR retinopathy will be of value26 as well as correlation of MP with advanced cellular imaging techniques.27,28
In conclusion, we report detailed findings on interocular symmetry, test-retest reliability, and progression of retinal functional loss, using mesopic MP in the largest molecularly confirmed RPGR cohort to date, including both adults and children. The high degree of reproducibility of results, good interocular correlation, and accurate tracking of change over time lends this method well to monitoring disease progression as well as supporting the validation of the use of MP in assessing the outcomes of gene therapy trials.
Acknowledgments
Funding/Support: Supported by grants from the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital National Health Service Foundation Trust and University College London (UCL) Institute of Ophthalmology, The Wellcome Trust (099173/Z/12/Z), Moorfields Eye Charity, Retina UK, and the Foundation Fighting Blindness (USA).
Financial Disclosures: Michel Michaelides consults for MeiraGTx. The other authors indicate no conflicts of interest. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Supplemental Material available at AJO.com.
Appendix. Supplementary materials
References
- 1.Tee JJ, Smith AJ, Hardcastle AJ, Michaelides M. RPGR-associated retinopathy: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2016;100(8):1022–1027. doi: 10.1136/bjophthalmol-2015-307698. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Guan L, Shen T, et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum Genet. 2014;133(10):1255–1271. doi: 10.1007/s00439-014-1460-2. [DOI] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 4.Georgiou M, Grewal PS, Narayan A, et al. Sector retinitis pigmentosa: extending the molecular genetics basis and elucidating the natural history. Am J Ophthalmol. 2021;221:299–310. doi: 10.1016/j.ajo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman N, Georgiou M, Khan KN, Michaelides M. Macular dystrophies: clinical and imaging features, molecular genetics and therapeutic options. Br J Ophthalmol. 2020;104(4):451–460. doi: 10.1136/bjophthalmol-2019-315086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill JS, Georgiou M, Kalitzeos A, Moore AT, Michaelides M. Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy. Br J Ophthalmol. 2019;103(5):711–720. doi: 10.1136/bjophthalmol-2018-313278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cehajic-Kapetanovic J, Xue K, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26(3):354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley TMW, Jolly JK, Menghini M, Wood LJ, Nanda A, MacLaren RE. Test-retest repeatability of microperimetry in patients with retinitis pigmentosa caused by mutations in RPGR. Clin Exp Ophthalmol. 2020;48(5):714–715. doi: 10.1111/ceo.13753. [DOI] [PubMed] [Google Scholar]
- 9.Tee JJL, Yang Y, Kalitzeos A, et al. Characterization of visual function, interocular variability and progression using static perimetry-derived metrics in RPGR-associated retinopathy. Invest Ophthalmol Vis Sci. 2018;59(6):2422–2436. doi: 10.1167/iovs.17-23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiou M, Singh N, Kane T, et al. Long-term investigation of retinal function in patients with achromatopsia. Invest Ophthalmol Vis Sci. 2020;61(11):38. doi: 10.1167/iovs.61.11.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanna P, Georgiou M, Aboshiha J, et al. Cross-sectional and longitudinal assessment of retinal sensitivity in patients with childhood-onset Stargardt disease. Transl Vis Sci Technol. 2018;7(6):10. doi: 10.1167/tvst.7.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumaran N, Rubin GS, Kalitzeos A, et al. A cross-sectional and longitudinal study of retinal sensitivity in RPE65-associated Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2018;59(8):3330–3339. doi: 10.1167/iovs.18-23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontikos N, Arno G, Jurkute N, et al. Genetic basis of inherited retinal disease in a molecularly characterized cohort of more than 3000 families from the United Kingdom. Ophthalmology. 2020;127(10):1384–1394. doi: 10.1016/j.ophtha.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weleber RG, Smith TB, Peters D, et al. VFMA: topographic analysis of sensitivity data from full-field static perimetry. Transl Vis Sci Technol. 2015;4(2):14. doi: 10.1167/tvst.4.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48(3):1298–1304. doi: 10.1167/iovs.06-0971. [DOI] [PubMed] [Google Scholar]
- 16.Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85(1):7–14. doi: 10.1016/j.exer.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke G, Collins RA, Leavitt BR, et al. A one-hit model of cell death in inherited neuronal degenerations. Nature. 2000;406(6792):195–199. doi: 10.1038/35018098. [DOI] [PubMed] [Google Scholar]
- 18.Grover S, Fishman GA, Anderson RJ, Alexander KR, Derlacki DJ. Rate of visual field loss in retinitis pigmentosa. Ophthalmology. 1997;104(3):460–465. doi: 10.1016/s0161-6420(97)30291-7. [DOI] [PubMed] [Google Scholar]
- 19.Holopigian K, Greenstein V, Seiple W, Carr RE. Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology. 1996;103(3):398–405. doi: 10.1016/s0161-6420(96)30679-9. [DOI] [PubMed] [Google Scholar]
- 20.Massof R, Dagnelie G, Benzschawel T, Palmer R, Stein D. First order dynamics of visual field loss in retinitis pigmentosa. Clin Vision Sci. 1990;5:1–26. [Google Scholar]
- 21.Bellingrath JS, Ochakovski GA, Seitz IP, et al. High symmetry of visual acuity and visual fields in RPGR-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2017;58(11):4457–4466. doi: 10.1167/iovs.17-22077. [DOI] [PubMed] [Google Scholar]
- 22.Zou X, Fang S, Wu S, et al. Detailed comparison of phenotype between male patients carrying variants in exons 1-14 and ORF15 of RPGR. Exp Eye Res. 2020;198 doi: 10.1016/j.exer.2020.108147. [DOI] [PubMed] [Google Scholar]
- 23.Mawatari G, Fujinami K, Liu X, et al. Clinical and genetic characteristics of 14 patients from 13 Japanese families with RPGR-associated retinal disorder: report of eight novel variants. Hum Genome Var. 2019;6:34. doi: 10.1038/s41439-019-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser RG, Tan R, Ayton LN, Caruso E, Guymer RH, Luu CD. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(13):5436–5442. doi: 10.1167/iovs.16-19295. [DOI] [PubMed] [Google Scholar]
- 25.Bennett LD, Klein M, Locke KG, Kiser K, Birch DG. Dark-adapted chromatic perimetry for measuring rod visual fields in patients with retinitis pigmentosa. Transl Vis Sci Technol. 2017;6(4):15. doi: 10.1167/tvst.6.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgiou M, Ali N, Yang E, et al. Extending the phenotypic spectrum of PRPF8, PRPH2, RP1 and RPGR, and the genotypic spectrum of early-onset severe retinal dystrophy. Orphanet J Rare Dis. 2021;16(1):128. doi: 10.1186/s13023-021-01759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiou M, Kalitzeos A, Patterson EJ, Dubra A, Carroll J, Michaelides M. Adaptive optics imaging of inherited retinal diseases. Br J Ophthalmol. 2018;102(8):1028–1035. doi: 10.1136/bjophthalmol-2017-311328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daich Varela M, Esener B, Hashem SA, Cabral de Guimaraes TA, Georgiou M, Michaelides M. Structural evaluation in inherited retinal diseases. Br J Ophthalmol. 2021;12 doi: 10.1136/bjophthalmol-2021-319228. Published online May. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.