Abstract
Genes for flagellin A (FlaA) proteins from European borrelial strains of Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii were cloned and sequenced. An identity of 92 to 93% was observed in the flaA sequences of the different species. Polyhistidine-tagged recombinant FlaA (rFlaA) proteins were produced in Escherichia coli and used as antigens in Western blotting (WB) and enzyme-linked immunosorbent assay (ELISA). In immunoglobulin G (IgG) WB, 71% (10 of 14) of the sera from neuroborreliosis and 86% (12 of 14) of those from Lyme arthritis patients reacted with one to three rFlaAs. In IgG ELISA, 74% (14 of 19) and 79% (15 of 19) of patients with neuroborreliosis and arthritis, respectively, were positive. The immunoreactivity in local European patient sera was stronger against rFlaA from B. garinii and B. afzelii than against rFlaA from B. burgdorferi sensu stricto. Neither IgG nor IgM ELISA was sensitive in the serodiagnosis of erythema migrans. Serum samples from patients with syphilis and systemic lupus erythematosus showed mild cross-reactivity in IgG tests. Sera from Yersinia enterocolitica or beta-hemolytic Streptococcus infections showed only occasional responses. With IgM ELISA, 58% (11 of 19) and 37% (7 of 19) of patients with neuroborreliosis and arthritis, respectively, were positive. Cross-reactive antibodies to FlaA, especially in serum samples from patients with rheumatoid factor positivity and Epstein-Barr virus infection, reduced the specificity of IgM serodiagnosis. Therefore, rFlaA seems to have a limited role for IgM serodiagnosis, yet rFlaA might be useful in the IgG serodiagnosis of disseminated Lyme borreliosis.
Laboratory diagnosis of Lyme borreliosis (LB) is mainly based on serology, although the present serologic tests have unsatisfactory sensitivity and specificity (34). Routine laboratory testing uses enzyme-linked immunosorbent assays (ELISA) with borrelial whole-cell lysate (WCL) or flagella (consisting mainly of polymerized FlaB protein) as the most commonly used antigens. A two-step approach with ELISA followed by a confirmatory Western blot (WB) has been recommended for positive or borderline results (19, 36). Especially in Europe, the applicability of this procedure has, however, remained doubtful (3, 14) because three or more borrelial species cause LB (38). Several difficulties complicate LB serology. Firstly, immunoglobulin G (IgG) antibody responses are often delayed during the early stages of LB. Even at late-stage LB, 5 to 10% of patients do not have elevated antibody levels (29), perhaps due to diversion of the host immune response towards Th1 immunity by borrelial factors (17). Secondly, viral infections cause false-positive IgM results in several LB tests (4). Thirdly, in a subgroup of patients antibody levels may stay high after successful treatment of LB even for prolonged periods (6, 15).
Several recombinant borrelial antigens (OspA, OspB, OspC, OspE, OspF, p22, BmpA, BBK32, BBK50, VlsE, p100, 14-kDa internal flagellin fragment) (5, 7, 16, 21, 22, 24, 25, 31, 35) and chimeric borrelial proteins OspA, OspB, OspC, flagellin (p41), and p93 (13) have been studied to improve serologic diagnosis. Of these proteins, BmpA (32) and OspC (8, 26, 27, 30, 31) have been suggested as antigens which induce early IgM responses. However, IgG antibodies to recombinant BmpA have been detected mainly in long-standing disease (29, 32). A limiting factor in the use of OspC as a diagnostic antigen is the extensive structural variation of the molecule between borrelial species (18, 23). Use of recombinant antigens has increased the specificity of serologic assays, but the sensitivity of tests using single antigens has thus far remained disappointing. In Europe, where the sequence heterogeneity of antigenic proteins in various borrelial species and strains complicates LB serology (33), supplementary information is needed on the differences between the antigenic properties of the borrelial species.
Flagellin A (FlaA) is a 37-kDa outer sheath protein of the periplasmic Borrelia burgdorferi flagella. It has been suggested that FlaA could potentially be a useful antigen for detecting antibodies in early LB. Gilmore et al. (12) obtained promising results for erythema migrans (EM) patients with IgM WB using recombinant FlaA (rFlaA) as an antigen. In contrast, Ge et al. (9) failed to show any useful serologic role in LB for another rFlaA construct. The purpose of the present study was to expand our knowledge of FlaA proteins in B. burgdorferi sensu lato spirochetes. We present the cloning and expression of FlaA proteins from three European borrelial strains of B. burgdorferi sensu stricto, B. afzelii, and B. garinii and the results of WB assays and ELISAs using FlaA recombinants as antigens.
MATERIALS AND METHODS
Borrelial strains.
Domestic borrelial strains of B. burgdorferi sensu stricto (ia) isolated from cerebrospinal fluid and B. afzelii (A91) and B. garinii (46) isolated from skin biopsies of Finnish patients with LB were used. The genotyping of these strains was performed by PCR of flaB and subsequent sequencing of the PCR products, as described previously (20).
Borrelia culture and DNA isolation.
Borrelial strains were cultured in Barbour-Stoenner-Kelly-H medium (Sigma) at 33°C and in a 5% CO2 atmosphere until the growth was approximately 2 × 108 cells/ml. The genomic DNA was isolated with a DNeasy Tissue kit (Qiagen, Hilden, Germany).
PCR and cloning of the genes.
For each borrelial strain the flaA sequence was studied by PCR amplification of the genomic DNA (Table 1). Approximately 1 ng of template DNA was used and the parameters in the PCR amplification reaction were 30 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min 30 s with AmpliTaq Gold DNA polymerase (Perkin Elmer). DNA products were visualized by gel electrophoresis on a 1% Agarose NA gel (Amersham Pharmacia, Uppsala, Sweden) containing ethidium bromide. The PCR products were cloned into the pCR 2.1-TOPO vector (Invitrogen, Groningen, The Netherlands). Escherichia coli INFαF (Invitrogen) host cells were used for cloning.
TABLE 1.
Primers used in PCR reactions for flaA sequencing and expression of the respective protein
| Target DNA | Primer (5′-3′)a | Location (bp) |
|---|---|---|
| Sequencing | ||
| 1 | ATA TAA GGA GTT GGT TTA CAT | −19–2 |
| 2 | ATG AAA AGG AAA GCT AAA AGT | 1–21 |
| 3 | GCT ATT CTC AAT CAT CTG C | 386–404 |
| 4 | TTT ATG AGA CTA GCG GAA CT | 860–879 |
| 5 | TTA AAT AAA CCT TGC CAT CAA | 1,438–1,418 |
| 6 | TTT TCT AAA TCT AAT ATT TCC AT | 1,117–1,094 |
| 7 | CAT TTT ACT TGA AGC AAG AG | 717–698 |
| Expression | ||
| FlaABbia | CCG GAT CCG ATG GAT TAG CAG AGG GTT | 67–85 |
| CCG GTA CCC TAA TTT TTC GGA GAT GAT TC | 1,026–1,006 | |
| FlaABaA91 | CCG GAT CCG ATG GAT TAA CAG AGG GC | 67–84 |
| CCG GTA CCC TAA TTT TCT GAA GAT GAT TC | 1,026–1,006 | |
| FlaABg46 | CCG GAT CCG ATG GAT TGC CAG AAG GC | 67–84 |
| CCG GTA CCC TAC TTT TTT AAG GAT GAT TC | 1,029–1,009 |
Underlining indicates BamHI and KpnI cleavage sites.
DNA sequencing.
Plasmid DNA containing flaA inserts was isolated from E. coli by using a QIAprep-Spin plasmid kit (Qiagen). DNA sequencing was performed with the DyePrimer (T7, M13Rev) cycle sequencing kit (Applied Biosystems Inc.) in accordance with the manufacturer‘s instructions by the Core Facility of the Haartman Institute, University of Helsinki. Sequencing reactions were run and analyzed by an automated sequencing apparatus model 373A (Applied Biosystems, Inc.). DNA and protein sequences were analyzed with Lasergene software (DNASTAR, Inc.). To eliminate possible sequencing errors, the whole gene was sequenced twice, and in some cases three times, following discordant results.
Construction of the expression plasmid and expression of rFlaA.
The starting codon for the flaA construct was chosen according to previous successful experiences of other investigators to express FlaA (12). The flaA constructs included the protein coding sequence without the sequence for the leader and three subsequent codons encoding the first three amino acids after a predicted signal peptidase I cleavage site (28) (Fig. 1). Expression primers for flaA of each strain were designed individually (Table 1), and the gene area to be expressed was multiplied by PCR and inserted into the pCR 2.1-TOPO vector as above. The plasmid containing the insert was purified and digested with BamHI and KpnI. The cleaved flaA was ligated into similarly cut pQE-30 expression plasmid (Qiagen) with T4 ligase. The ligation mixture was used to transform E. coli M15 host cells as described in the manufacturer‘s instructions (Qiagen). The transformation mixture was plated onto Luria-Bertani agar containing 100 μg of ampicillin and 25 μg of kanamycin per ml. A primary culture for expression of rFlaA was started by inoculating a single colony from a fresh transformant plate into 50 ml of Luria-Bertani broth containing antibiotics as described above. The culture was incubated at 37°C with shaking overnight. This starter culture was diluted 1:100 to 1,500 ml of Luria-Bertani broth with antibiotics as described above and incubated at 37°C for 3 h (the growth reached the mid-log phase; the optical density at 600 nm was ca. 0.6). Isopropyl-β-d-thiogalactoside (Calbiochem) was added to a final concentration of 0.6 mM, and an additional incubation of 3 h was performed. The cells were harvested, washed, and sonicated. The expressed rFlaA was recovered as insoluble protein in the centrifugation pellet. This was dissolved in 4 M guanidine hydrochloride and attached by its 6xHistidine tag to a Chelating Sepharose Fast Flow column (Amersham Pharmacia) containing Ni2+. rFlaA was subsequently eluted from the column by 160 mM imidazole–4 M guanidine hydrochloride eluting buffer. Protein expression and purity were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
FIG. 1.
FlaA amino acid sequence comparison of the translated full-length gene in B. burgdorferi sensu stricto strains B31, 212, and ia (Bbia), B. afzelii A91 (BaA91), and B. garinii 46 (Bg46). Boxes indicate amino acid heterogeneity. The starting point of our recombinant constructs is indicated by an arrow.
Purification of rFlaA.
rFlaAs originating from B. burgdorferi sensu stricto ia, B. afzelii A91, and B. garinii 46 (referred to as rFlaABbia, rFlaABaA91, and rFlaABg46, respectively) were dialyzed in 10 mM potassium phosphate (pH 7.2). After centrifugation the resulting insoluble protein pellet was resolubilized in 4 M guanidine hydrochloride when used for WB or in 5 M urea when used for ELISA.
The insolubility of the rFlaA protein complicated reliable measurement of the protein concentration by either spectrophotometry or chemical assay methods. For WB and ELISA, protein concentrations of rFlaA preparations were estimated by comparing the intensity of FlaA bands with known concentrations of bovine serum albumin (BSA) in SDS-PAGE after Coomassie blue staining or Ponceau S staining after transfer to nitrocellulose.
WB.
rFlaA was applied to SDS-PAGE gels (12.5% gels) and transferred to a nitrocellulose membrane (Bio-Rad) by semidry transfer with 40 mM glycine–50 mM Tris (pH 9.0)–0.375% (wt/vol) SDS–20% (vol/vol) methanol buffer. For IgM WB, serum samples were incubated at a dilution of 1:500 in BSA (5 mg/ml) in 155 mM NaCl–0.04% Tween 20 buffer (BSA-NaCl-Tween) for 2 h. For IgG WB, a serum dilution of 1:75 in 0.1% Tween 20–0.9% NaCl was used. After washing, the blots were incubated with alkaline phosphatase-conjugated rabbit anti-human IgM or IgG (Jackson Immuno Research Laboratories Inc.) for 2 h. In control experiments the anti-IgM conjugate showed a faint FlaA band in the absence of serum. Therefore, the conjugate was absorbed with rFlaA from B. afzelii. FlaA in coupling buffer (0.6 M NaCl–125 mM NaHCO3) was added to tresyl-activated Sepharose 4B (Amersham Pharmacia) beads. After incubation for 16 h and centrifugation the beads were washed with the coupling buffer, and the conditions were alternated three times between 0.1 M Tris buffer (pH 8.0)–0.5 M NaCl and 0.1 M acetate buffer (pH 4.0)–0.5 M NaCl. The IgM conjugate was added to the beads and centrifuged after 2 h of incubation. The supernatant was used at a 1:8,000 final dilution for the IgM WB. The IgG conjugate was used without FlaA immunoabsorption at a 1:5,000 dilution. After washing, the visualization was done with 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium (Sigma). The intensity of WB bands was evaluated visually and with MacBAS 2.5 software (Koshin Graphic Systems, Inc.). In the MacBAS program the mean of the intensity values of blood donor samples was calculated. Values above the mean plus 2 standard deviations (SD) were defined as positive.
ELISA.
For rFlaA ELISA, microtiter plate wells were coated with 200 ng of rFlaA in 5 M urea overnight at +4°C. With intervening washes the sera and reagents were sequentially incubated in the wells as follows: serum samples at a dilution of 1:100 in BSA-NaCl-Tween overnight at +4°C, alkaline phosphatase-conjugated rabbit anti-human IgM or IgG (Jackson Immuno Research Laboratories Inc.) at a dilution of 1:5,000 in BSA-NaCl-Tween for 2 h, and 4-nitro-phenylphosphate (Boehringer Mannheim, Mannheim, Germany) substrate in diethanolamine buffer (pH 10.0) for 15 min. The absorbance at 405 nm was measured by using a Multiscan photometer (Thermo Labsystems, Helsinki, Finland).
Patients.
Human serum samples were collected from patients with typical LB (primary EM, neuroborreliosis [NB], or Lyme arthritis [LA]). For all patients, the diagnosis of LB was based on clinical guidelines for diagnosis set forth by the Centers for Disease Control and Prevention, Atlanta, Ga. (39). The clinical diagnosis of NB and LA was confirmed by demonstrating antibodies in serum by ELISA against WCL from B. afzelii strain SK1 and flagella from B. afzelii DK1 (Dako, Ely, United Kingdom), and for patients with NB, the diagnosis was confirmed by demonstrating antibodies to flagella in cerebrospinal fluid. For all patients with EM, the diagnosis was confirmed by culturing B. burgdorferi from a skin biopsy (11 B. afzelii and 4 B. garinii). Serum samples were obtained from patients with syphilis, rheumatoid factor (RF) positivity, antistreptolysin (ASO) positivity, Epstein-Barr virus (EBV) infection, high Yersinia enterocolitica antibody titers with positive stool culture, and clinically and serologically verified systemic lupus erythematosus (SLE), and samples from healthy blood donors were used as controls.
Nucleotide sequence accession numbers.
flaA sequences from B. burgdorferi strain ia, B. afzelii A91, and B. garinii 46 have been assigned to the GenBank database with accession numbers AY057222, AY057223, and AY057224, respectively. Published flaA sequences from B. burgdorferi sensu stricto strains B31 and 212 were obtained from GenBank (accession no. AE001168 and U62900, respectively).
RESULTS
Cloning and nucleotide sequence analysis of flaA.
flaA nucleotide sequences of the borrelial strains were obtained by PCR and sequencing. The primers were designed according to the published flanking region sequences to ensure correct sequences at the gene ends. The analyzed sequences were compared with the published flaA sequences of B. burgdorferi sensu stricto strains B31 and 212. The flaA sequence of the B. burgdorferi sensu stricto strain ia was identical with that of the American strain B31 whereas it differed by nine nucleotides (identity of 99% at the nucleotide level) from the flaA sequence in strain 212. The identity of flaA sequences between the Finnish strains B. burgdorferi sensu stricto ia and B. afzelii A91, between B. burgdorferi sensu stricto ia and B. garinii 46, and between B. afzelii A91 and B. garinii 46 was 93, 92, and 93%, respectively. The identity of flaA in B. afzelii A91 and in B. garinii 46 compared to flaA in strain 212 was 92 and 91%, respectively.
Protein sequence analysis.
The deduced amino acid sequences of FlaABbia, FlaABaA91, and FlaABg46 were 95% identical (Fig. 1). The identity of both FlaABaA91 and FlaABg46 with FlaA of B. burgdorferi sensu stricto 212 was 94%. The flaA gene of B. garinii 46 has an additional triplet of nucleotides, AAA, at positions 1000 to 1002, leading to a lysine insertion into an otherwise hydrophilic sequence area. None of the sequence differences change the local hydrophilicity of the sequence area, indicating conservation of the overall structure (Fig. 1). The deduced amino acid sequence of FlaA protein (without the leader peptide) was rich in phenylalanine and tyrosine (15 and 16 amino acids, respectively) but contained no cysteine. The count of these amino acids was identical in all three FlaA proteins examined in this study. Thirty-three percent of the deduced amino acids were hydrophobic. The calculated (Lasergene Software; DNASTAR, Inc.) isoelectric points of FlaABbia, FlaABaA91, and FlaABg46 for the mature protein without leader sequence were 5.70, 5.33, and 7.27, respectively. Three additional lysines and fewer aspartic acids in FlaABg46 than in FlaABbia and FlaABaA91 may account for the higher isoelectric point of FlaABg46.
IgG WB.
For the WB analysis, serum samples from 14 patients with NB, 14 with LA, 10 with syphilis, and 13 healthy blood donors were tested. Ten samples from patients with NB (71%) and 12 from patients with LA (86%) reacted with one or more of the rFlaAs. Two of 10 patients with syphilis and 1 out of 13 healthy blood donors were positive. The combined results of the WB analysis are presented in Table 2. rFlaABg46 and rFlaABaA91 were superior to rFlaABbia in detecting antibodies to FlaA.
TABLE 2.
IgG WB results with rFlaA antigen from B. garinii, B. afzelii, and B. burgdorferi sensu stricto
| Antigen | No. of patients with a positive result for the indicated antigen
|
|||
|---|---|---|---|---|
| NB patients (n = 14) | LA patients (n = 14) | Syphilis patients (n = 10) | Healthy blood donors (n = 13) | |
| rFlaABg46 | 9 | 12 | 0 | 1 |
| rFlaABaA91 | 9 | 6 | 0 | 0 |
| rFlaABbia | 6 | 5 | 2 | 0 |
| Totala | 10 | 12 | 2 | 1 |
Total refers to the total number of patients in each group having antibodies to one or more of the rFlaA proteins.
IgM WB.
In repeated experiments using serum samples from patients with EM or NB, we consistently observed moderate to strong IgM immunoreactivity to rFlaAs, yet samples from patients with EBV infection or RF positivity were similarly immunoreactive. However, the evaluation of the immunoblot bands visually and with the MacBAS program showed differences in the intensity of the bands (data not shown). Based on the intensities of healthy blood donor serum reactions, one of five samples from patients with EM, four of five from patients with NB, two of five from patients with EBV, and two of five with RF positivity gave positive IgM reactions for one or more of the rFlaAs. Alterations in the dilution of the serum (1:25 to 1:500), the buffer (varying the protein and using different salt concentrations), or the applied conjugate did not lead to any better discrimination of the IgM bands between the patients and the controls (data not shown).
IgG ELISA.
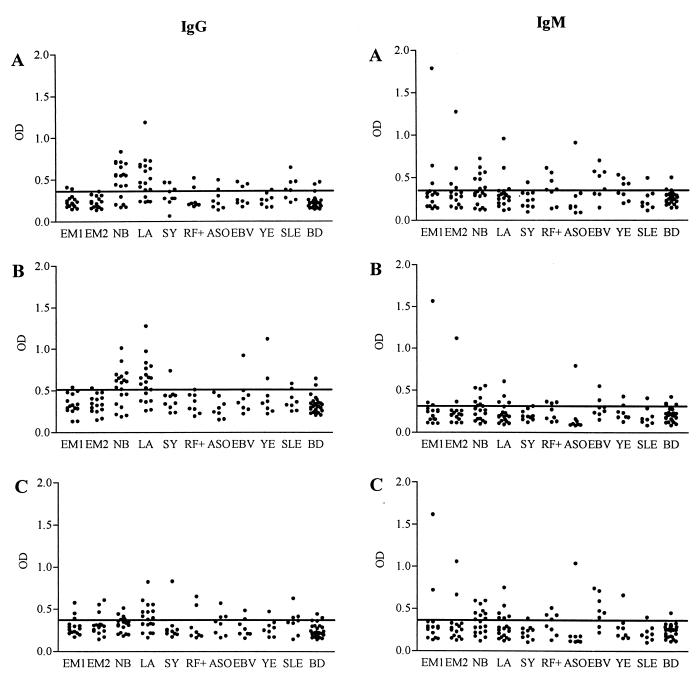
For IgG ELISA optical density values above the means plus 2 SD of values of healthy blood donors were defined as positive. Results for IgG ELISA of LB patients were comparable with the results obtained from IgG WB analysis: positive reactions were found mainly against rFlaABg46 and rFlaABaA91 while reactions to rFlaABbia were less frequent (Fig. 2). Of the samples from 15 patients with EM, two (13%) of the acute-phase samples and three (20%) of the convalescent-phase samples reacted with one or more of the rFlaAs. Samples from 14 of the 19 (74%) patients with NB and from 15 of 19 (79%) patients with LA reacted with either rFlaABg46 or rFlaABaA91. The 19 patient samples of NB and LA used here include those examined by IgG WB. None of the samples from patients with NB or LA reacted with rFlaABbia only. Twelve of the 14 positive samples from NB patients recognized both rFlaABaA91 and rFlaABg46, and the remaining 2 recognized rFlaABg46 only. Of the 15 positive samples from LA patients, 11 recognized both antigens, 2 recognized rFlaABaA91 only, and 2 recognized rFlaABg46 only. Depending on the rFlaA antigen used, 1 to 3 of 10 patients with syphilis, 1 to 2 of 8 patients with RF positivity, 0 to 3 of 8 patients with ASO positivity, 1 to 3 of 8 patients with EBV infection, 0 to 2 of 8 patients with Yersinia infection, 2 to 3 of 8 patients with SLE, and 2 of 27 healthy blood donors were positive.
FIG. 2.
IgG and IgM ELISA results with rFlaA proteins from B. garinii (Bg46) (A), B. afzelii (BaA91) (B), and B. burgdorferi sensu stricto (Bbia) (C) as antigens. Optical density (OD) values for each patient group and control samples are shown separately. Serum samples were from patients with EM at the acute phase (EM1), EM at the convalescent phase (EM2), NB, LA, syphilis (SY), RF positivity (RF+), ASO positivity, EBV infection, Yersinia infection (YE), and SLE and from healthy blood donors (BD). The cutoff level (mean + 2 SD of blood donor samples) for each antigen is indicated by a line.
IgM ELISA.
IgM ELISA was performed with the same panel of patient and control samples as the IgG ELISA. Of the 15 samples from patients with EM, 3 (20%) of the acute-phase samples and 4 (27%) of the convalescent-phase samples reacted with one or more of the rFlaAs. Samples from 11 of the 19 (58%) patients with NB and from 7 of 19 (37%) patients with LA reacted with one or more of the rFlaAs. Depending on the rFlaA antigen used, 0 to 1 of 10 patients with syphilis, 2 to 4 of 8 patients with RF positivity, 1 of 8 patients with ASO positivity, 2 to 6 of 8 patients with EBV infection, 1 to 4 of 8 patients with Yersinia infection, 1 of 8 patients with SLE, and 1 of 27 healthy blood donors were positive. Results with three variant rFlaAs as antigen in the IgM ELISA are shown in Fig. 2.
DISCUSSION
In the present study, flaA sequences of B. afzelii and B. garinii are reported for the first time. The identity of the flaA sequences of B. afzelii, B. garinii, and B. burgdorferi sensu stricto was over 90%. Although the exact function of the FlaA protein is not known, it has been shown that periplasmic flagellae of B. burgdorferi comprise a core protein FlaB and an outer sheath protein FlaA (11). Given the essential role of flagella in motility of the spirochete it is not surprising that the flaA was highly conserved among B. burgdorferi sensu lato strains.
FlaA has been evaluated as a WB antigen in LB serodiagnosis in only a few studies, and the results have been contradictory (9, 12). No previous studies using rFlaA in ELISA have been published. Our aim was to study the antigenic potential of FlaA in the European context. Therefore, we cloned flaA from all three borrelial species and produced the respective recombinant proteins. For IgG serodiagnosis of NB and LA, FlaA appeared to be a sensitive antigen. Seventy-one or seventy-four percent of patients with NB were positive either by WB or ELISA, respectively. The corresponding positivities for LA patient samples were 86 and 79%. The specificity in our series was slightly lowered due to low positive values occurring more frequently in samples from other diseases than in samples from healthy controls. Although the sequences of the three FlaA proteins were highly homologous, rFlaA antigens from B. garinii and B. afzelii seemed to perform better regarding sensitivity and specificity than rFlaA from B. burgdorferi sensu stricto. Obviously, various FlaA proteins have both common and divergent antigenic epitopes. In Europe, B. garinii and B. afzelii are the most prevalent genospecies (38). This concurs with the predominance of immunoreactivity to rFlaA from these two genospecies.
In IgM serology our patient selection differed from the series by Gilmore et al. (12). They studied EM patient sera with antibodies to the B. burgdorferi 37-kDa protein (putative FlaA antigen) in WCL immunoblot which also reacted with the rFlaA. However, only 38 and 57% of all patients with EM at the acute and convalescent phase, respectively, had antibodies to rFlaA (12). Serum samples from EM patients with no observed immunoreactivity against the B. burgdorferi 37-kDa protein did not recognize rFlaA (12). It is not clear how the group of EM patients without immunoreactivity to rFlaA differed from those reacting positively with rFlaA. In our study with unselected culture-confirmed EM patients, sensitivity of the IgM serology in WB and ELISA assays was only 20 to 27%. Another study failed to show FlaA as an immunodominant antigen in LB (9). The authors did not specify whether an IgM or IgG WB or early- or convalescent-phase serum samples of LB patients were used. In our assays for EM patient samples, IgG ELISA performed almost equally with IgM ELISA. For NB patients, IgG FlaA ELISA had better sensitivity than IgM ELISA. It is possible that our patient selection, including NB patients at early and late stages, may account for this finding. Furthermore, cross-reactive antibodies to FlaA reduced the specificity of IgM serodiagnosis both in WB and ELISA experiments. Possibly, antibodies to FlaA of ubiquitous bacteria account for the cross-reactivity in IgM serology. Therefore, we do not see rFlaA as a useful antigen in IgM serodiagnosis for early LB.
As is typical for signal sequences, the leader of the FlaA protein includes a central hydrophobic segment (10). Trials to express a construct with the N-terminal leader have failed (12), perhaps due to toxicity to the E. coli host cell. However, as shown by Gilmore et al. (12) and in our study, FlaA without the leader peptide was well expressed, yet our recombinant protein tended to form inclusion bodies. The Protein Analysis program (Lasergene) predicted a β-sheet-rich region between amino acids 50 and 180 of the full-length protein (data not shown). We anticipate that this region of FlaA is involved in binding to the FlaB protein in the flagellar structure and may contribute to its property to form inclusions. A more hydrophilic construct formed by exclusion of the N-terminal part of FlaA might lead to a soluble recombinant protein. However, in another laboratory a construct where the first 79 amino acids were excluded failed to lead to successful expression (12).
Unlike in other spirochetes, B. burgdorferi FlaA is expressed at a considerably lower level than FlaB (11). The surface of borrelial flagella may have only a small amount of FlaA protein attached to the FlaB backbone, leaving a large part of the FlaB surface uncovered. This is in accordance with the observed difficulty of demonstrating flagellar sheaths in Borrelia (2). The smaller amount of FlaA protein expressed by Borrelia spp. during infection than of FlaB may account for the difficulty in finding specific IgM or IgG to FlaA and to the lower proportion of patients with positive immunoreactivity to FlaA than to FlaB. Alternatively, differences in the time of collection of sera with respect to clinical course might cause discrepancies in the immunoreactivity.
Besides Borrelia immunity, FlaA in other bacteria may be associated with interesting phenomena. In another spirochete disease, syphilis, the endoflagellar sheath protein of Treponema pallidum, TpN37, which is the product of flaA, has been shown to elicit a strong T-cell response (1). A recent study reported an amino acid sequence in FlaA of Campylobacter jejuni for which an association with Guillain-Barré syndrome was proposed (37).
In conclusion, FlaA seemed to be a sensitive antigen in the IgG serodiagnosis of disseminated LB. In IgM serology, low sensitivity in early LB and poor specificity may constrain its use to routine serodiagnosis. Regardless of the high homology of FlaA proteins within Borrelia genospecies, the present results suggest that recombinant proteins from B. garinii and/or B. afzelii should be preferred when rFlaA is used in the IgG serodiagnosis of LB in Europe.
ACKNOWLEDGMENTS
We thank Klaus Hedman for providing the EBV serum samples and Matti Viljanen for donating the B. burgdorferi strains used in this study. The critical comments of Malcolm Richardson are gratefully acknowledged.
This study was supported by grants from National Technology Agency (Tekes), Helsinki, Finland; Helsinki Central Hospital Research Funds; Clinical Research Institute of Helsinki University Central Hospital; the Paulo Foundation; and Finska Läkaresällskapet, Helsinki, Finland.
REFERENCES
- 1.Arroll T W, Centurion-Lara A, Lukehart S A, van Voorhis W C. T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect Immun. 1999;67:4757–4763. doi: 10.1128/iai.67.9.4757-4763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaauw A A M, van Loon A M, Schellekens J F P, Bijlsma J W J. Clinical evaluation of guidelines and two-test approach for Lyme disease. Rheumatology. 1999;38:1121–1126. doi: 10.1093/rheumatology/38.11.1121. [DOI] [PubMed] [Google Scholar]
- 4.Brown S L, Hansen S L, Langone J J. Role of serology in the diagnosis of Lyme disease. JAMA. 1999;282:62–66. doi: 10.1001/jama.282.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Buckert S, Rössler D, Munchhoff P, Wilske B. Development of enzyme-linked immunosorbent assays using recombinant borrelial antigens for serodiagnosis of Borrelia burgdorferi infection. Med Microbiol Immunol. 1996;185:49–57. doi: 10.1007/s004300050014. [DOI] [PubMed] [Google Scholar]
- 6.Craft J E, Grodzicki R L, Steere A C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149:789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- 7.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 8.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Y, Charon N W. FlaA, a putative flagellar outer sheath protein, is not an immunodominant antigen associated with Lyme disease. Infect Immun. 1997;65:2992–2995. doi: 10.1128/iai.65.7.2992-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge Y, Charon N W. An unexpected flaA homolog is present and expressed in Borrelia burgdorferi. J Bacteriol. 1997;179:552–556. doi: 10.1128/jb.179.2.552-556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Li C, Corum L, Slaughter C A, Charon N W. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J Bacteriol. 1998;180:2418–2425. doi: 10.1128/jb.180.9.2418-2425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore R D, Jr, Murphree R L, James A M, Sullivan S A, Johnson B J B. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J Clin Microbiol. 1999;37:548–552. doi: 10.1128/jcm.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes-Solecki M J C, Dunn J J, Luft B J, Castillo J, Dykhuizen D E, Yang X, Glass J D, Dattwyler R J. Recombinant chimeric Borrelia proteins for diagnosis of Lyme disease. J Clin Microbiol. 2000;38:2530–2535. doi: 10.1128/jcm.38.7.2530-2535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goossens H A T, van den Bogaard A E, Nohlmans M K E. Evaluation of fifteen commercially available serological tests for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1999;18:551–560. doi: 10.1007/s100960050347. [DOI] [PubMed] [Google Scholar]
- 15.Hammers-Berggren S, Lebech A-M, Karlsson M, Andersson U, Hansen K, Stiernstedt G. Serological follow-up after treatment of Borrelia arthritis and acrodermatitis chronica atrophicans. Scand J Infect Dis. 1994;26:339–347. doi: 10.3109/00365549409011804. [DOI] [PubMed] [Google Scholar]
- 16.Hauser U, Lehnert G, Wilske B. Diagnostic value of proteins of three Borrelia species (Borrelia burgdorferi sensu lato) and implications for development and use of recombinant antigens for serodiagnosis of Lyme borreliosis in Europe. Clin Diagn Lab Immunol. 1998;5:456–462. doi: 10.1128/cdli.5.4.456-462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante-Duarte C, Horton H F, Byrne M C, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 18.Jauris-Heipke S, Liegl G, Preac-Mursic V, Rössler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsson B J B, Robbins K E, Bailey R E, Cao B-L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 20.Junttila J, Peltomaa M, Soini H, Marjamäki M, Viljanen M K. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J Clin Microbiol. 1999;37:1361–1365. doi: 10.1128/jcm.37.5.1361-1365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrenz M B, Hardham J M, Owens R T, Nowakowski J, Steere A C, Wormser G P, Norris S J. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol. 1999;37:3997–4004. doi: 10.1128/jcm.37.12.3997-4004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang F T, Steere A C, Marques A R, Johnson B J B, Miller J N, Philipp M T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livey I, Gibbs C P, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 24.Magnarelli L A, Fikrig E, Padula S J, Anderson J F, Flavell R A. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Ijdo J W, Padula S J, Flavell R A, Fikrig E. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J Clin Microbiol. 2000;38:1735–1739. doi: 10.1128/jcm.38.5.1735-1739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathiesen M J, Holm A, Christiansen M, Blom J, Hansen K, Ostergaard S, Theisen M. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun. 1998;66:4073–4079. doi: 10.1128/iai.66.9.4073-4079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathiesen M J, Christiansen M, Hansen K, Holm A, Åsbrink E, Theisen M. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1998;36:3474–3479. doi: 10.1128/jcm.36.12.3474-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Oksi J, Uksila J, Marjamäki M, Nikoskelainen J, Viljanen M K. Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J Clin Microbiol. 1995;33:2260–2264. doi: 10.1128/jcm.33.9.2260-2264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padula S J, Dias F, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauer S, Spohn N, Rasiah C, Neubert U, Vogt A. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J Clin Microbiol. 1998;36:857–861. doi: 10.1128/jcm.36.4.857-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roessler D, Hauser U, Wilske B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J Clin Microbiol. 1997;35:2752–2758. doi: 10.1128/jcm.35.11.2752-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryffel K, Peter O, Rutti B, Suard A, Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J Clin Microbiol. 1999;37:4086–4092. doi: 10.1128/jcm.37.12.4086-4092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigal L H. Pitfalls in the diagnosis and management of Lyme disease. Arthritis Rheum. 1998;41:195–204. doi: 10.1002/1529-0131(199802)41:2<195::AID-ART3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Theisen M, Frederiksen B, Lebech A-M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevejo R T, Krause P J, Sikand V K, Schriefer M E, Ryan R, Lepore T, Porter W, Dennis D T. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J Infect Dis. 1999;179:931–938. doi: 10.1086/314663. [DOI] [PubMed] [Google Scholar]
- 37.Tsang R S W, Figueroa G, Bryden L, Ng L-K. Flagella as a potential marker for Campylobacter jejuni strains associated with Guillain-Barré syndrome. J Clin Microbiol. 2001;39:762–764. doi: 10.1128/JCM.39.2.762-764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G, van Dam A P, Schwartz I, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev. 1999;12:633–653. doi: 10.1128/cmr.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wharton M, Chorba T L, Vogt R L, Morse D L, Buehler J W. Case definitions for public health surveillance. Morb Mortal Wkly Rep. 1990;39:19–21. [PubMed] [Google Scholar]