Abstract
In the current study the potential use of aqueous and methanolic extracts of Ephedra alata aerial parts as biological control agent against pathogenic bacteria and especially Staphylococcus aureus methicillin resistant isolated from auricular infections was evaluated. Chemical tests and spectrophotometric methods were used for screening and quantification of phytochemicals. The assessment of the antioxidant activity was accomplished by DPPH and ABTS radicals scavenging assays. Extracts were evaluated for their antibacterial efficacy by diffusion and microdilution methods. Biofilm inhibition was tested using XTT assay and the cytotoxicity of extracts was carried out on Vero cell line. The GC-FID analysis revealed that E. alata was rich in unsatured fatty acids. In addition, the aqueous extract had the highest flavonoid and protein contents (30.82 mg QE /g dry extract and 98.92 mg BSAE/g dry extract respectively). However, the methanolic extract had the highest phenolic, sugars and tannins. The antioxidant activity demonstrated that the aqueous extract exhibited the strong potency (IC50 ranged between 0.001 and 0.002 mg/mL).
Both extracts displayed antimicrobial activity on Gram negative and positive strains. They were effective against S. aureus isolated from auricular infections. The tested extracts were able to inhibit biofilm formation with concentration-dependent manner.
Moreover, no cytotoxic effect on Vero cells line was demonstrated for the extracts. Overall, our findings highlight the potential use of E. alata extract as a novel source of bioactive molecules with antioxidant, antibacterial and antiobiofilm effects for the control of infectious disease especially those associated to S. aureus methicillin resistant.
Keywords: Ephedra alata, Mineral composition, Fatty acids, Antioxidant effect, Antibacterial potency, Antibiofilm activity, Cytotoxicity
1. Introduction
Since the emergence of bacterial resistant strains phenomenon, infectious diseases become a serious and widespread public health problem due to the World Health Organization (WHO) (Organization, 2006). Among the antibiotic resistant bacteria, Methicillin-Resistant Stapylococcus aureus (MRSA) is the principal nosocomial and skin colonizer for humans and primordial causes of the hospital and community-acquired infections (Hassoun et al., 2017). Recently, several convential antibiotics such as vancomycin, clindamycin, levofloxacin, betalactamics, quinolones and glycopeptides used to combat these bacteria have been failed (Lee et al., 2018). Thus, phytotherapy is required, that can be effective therapeutic option, for the treatment of infectious diseases caused by MRSA.
Indigenous medicinal plants are used by around three quarters of the world’s population (Dey and De, 2012). Plants natural products (pure compounds or extracts) are chosen by the rural areas people as a primary method to treat diseases because of the unavailability of modern drugs, high cost of synthetic drugs, reduced side effects of medicinal plants and knowledge of target and effective plant against a particular disease (Bhat et al., 2014, Shahidi and Ambigaipalan, 2015). Ephedra is a genus of gymnosperm belonging to the family of Ephedraceae (D'Auria et al., 2012). This genus includes approximately 67 species distributed in arid environments and the desert from the north hemisphere, Asia, Americas, the southern part of Europe and North Africa (Xie et al., 2013). In Tunisia, 4 species are found including E. altissima, E. nebrodensis, E. fragilis and E. alata (Cuénod, 1954, Le Floch et al., 2010). Ephedra alata Decne (Arabic common names: “Alenda”, “Alanda Mujanaa”, “Theel maiz”, “Anab bahar”, “Ather,” “Jashia”) is a perennial stiff shrub, light green dioecious species, up to 40–100 cm tall, which extend on the steppes and the deserts of the southern regions (Rjim Maâtoug and El Borma). It is commonly used by Tunisian people for pasture and fuel (Nabli, 1991, Chaieb and Boukhris, 1998). The decoction of E. alata aerial parts has been reported in folk medicine to relieve nasal decongestion, in the treatment of asthma, digestive system disorders and as a general respiratory decongestant. The hot broth of the dried green stems is used in traditional medicine, as hot tea, after abortion and to treat bacterial and fungal infections (Gupta et al., 2008, Nawwar et al., 1984). Previous in vitro and in vivo studies showed that E. alata extracts have several biological properties including anti-inflammatory, anticancer, antibacterial, antioxidant, antidiabitic, antiobesity and antiviral effects (Hyuga et al., 2016). The hydro-alcoholic extract from E. alata present antioxidant, antimicrobial and anticancer effect against the MCF-7, mammalien cell line (Danciu et al., 2019). The methanolic extract of E. alata enhanced the anticancer activity of cisplatin in Breast Cancer cells (4T1) in Vitro and in Vivo (Sioud et al., 2020a), and reduced oxidative stress, genotoxicity against cisplatin-nephrotoxicity and hepatotoxicity damage (Sioud et al., 2020b). Moreover, the decoction of the aerial parts of E. alata has an anti-diabetogenic activity in vitro and in vivo (Lamine et al., 2019). Health beneficial effects of E. alata plant extracts are by dint of the presence of pharmacologically active substances, like alkaloids, phenolic acids, flavonoids, proanthocyanidins, tannins, saponins reducing sugars and cardiac glycosides (Ibragic and Sofić, 2015, Sioud et al., 2020b).
The current work aims to characterize the phytochemical contents of E. alata extracts as well as its mineral and fatty acids compositions. To evaluate the possible use of the aqueous and methanolic extracts as bioactive molecules to control pathogens associated to infectious diseases, antimicrobial assay against S. aureus isolated from auricular infections was carried out. Other biological activities such as antioxidant, antibiofilm and cytotoxic were investigated.
2. Material and methods
2.1. Plant material samples and extracts preparation
Ephedra alata was freshly collected from Kef Derbi area (latitude: 34°41′, longitude: 9°29′), Governorate of Gafsa, TUNISIA on March 2018 (Fig. 1). The plant identification was conducted by Prof. Diego Rivera Núñez, (Faculty of Biology of Murcia, Spain). The whole aerial parts of the plant was rinsed with deionized water, dried for 3 weeks in a well ventilated dark room and milled to powder Fig. 1.
Fig. 1.
Map of the different location sites of Ephedra alata in Tunisia.
Powder samples of E. alata were extracted in dark flasks using the shaking method at a sample to solvent ratio of (10:1 (v/w)) with distilled water and methanol (Merck; 97%). The mixtures were extracted using an incubator shaker at 150 rpm and 25 °C for 24 h and 48 h for aqueous and methanolic extracts, respectively. The extracts were filtered twice through Whatman No. 1 filter paper (Whatman, Fisher Scientific, Schwerte, Germany). The water extracts were lyophilized using freeze dryer (Bioblock Scientific, Illkirch, France) while methanol solvents were removed at 40 °C using a rotary vaccum evaporator. All crude extracts were stored in dark bottles at 4 °C until analysis. The yield percentage of the dried extracts was determined as the following equation (1):
| (1) |
where W1 is the weight of the extract after evaporation of solvent, and W2 is the weight of the E. alata.
2.2. Proximate and minerals composition analysis
Moisture and ash were estimated using AOAC methods (AOAC, 2000). Minerals composition (calcium, potassium, phosphore, sodium, and fer) was determined using the AOAC procedure (930.05) and performed by atomic absorption spectrometry (PerkinElmer, Waltham, MA, USA) (Mosbah et al., 2019). The results were presented as mg per 100 g DW.
2.3. Fatty acid analysis
Total lipids were extracts from powdred E. alata aerial parts according to Beligh and Dayer (Bligh and Dyer, 1959). For total fatty acids analysis, the lipid extract was transesterified with 0.1 mL BF3-MeOH (14%). After incubation for 30 min at 70 °C, the reaction was stopped by adding 0.5 mL ultrapure water. The fatty acid methyl esters (FAMS) were analyzed by GC-FID according to the European Union Commission modified regulation EEC.2568/91. FAMS were identified by comparing their relative and absolute retention times to those of TFA standards and results were expressed as percent of total area.
2.4. Determination of the qualitative phytochemical content
The occurrence of major secondary metabolite in the E. alata extracts was evaluated using standard procedures. The screening was performed as follow: tannins (Banso and Adeyemo, 2006), flavonoids, saponins, terpenoids and alkaloids (Raaman, 2006), sterols, anthraquinones and cardiac glycosides (Khandelwal, 2008), proteins (Krishnaiah et al., 2009).
2.5. Quantitative phytochemical content
Total phenolic contents in E. alata extracts was determined using Folin-Ciocalteau method (Singleton and Rossi, 1965). A calibration curve of gallic acid was prepared and the results were expressed as mg gallic acid equivalents (GAE)/ g dry extract.
Total flavonoid content in the different extracts was determined by a colorimetric method of Kim et al. (2002). A calibration curve of quercetin was used and the results were reported as mg quercetin equivalent (QE) /g dry extract. The total sugar contents were measured as described by Dubois et al. (1956). The results were expressed as mg glucose equivalent (GE) /g dry extract. The concentration of condensed tannins was calculated by the procedure of Julkunen-Tiitto. (1985). Tannic Acid was used for the calibration curve and the results were presented as mg acid tannic equivalent (ATE) /g dry extract.
The total protein content was accomplished according to Marks et al. (1985) and the results were reported as mg Bovine Serum Albumin equivalent (BSA) /g dry extract.
2.6. Antioxidant activities
The antioxidant potential of E. alata extracts have been tested through 2 methods as follow:
2.6.1. DDPH scavenging activity
The power of the E. alata extracts to inhibit the formation of free radicals was assessed as described previously by Hwang et al. (2001) with slight modifications. 2 mL of E. alata extracts at various concentrations (1–1000 μg/mL) were added to 2 mL of ethanolic DPPH solution (0.2 mM) shacked and left in the dark for 30 min at ambiant temperature. The decrease of absorbance was recorded at 517 nm. Ascorbic acid was employed as standard.
The DPPH scavenging ability of extracts was calculated based on the following equation (2):
| (2) |
2.6.2. ABTS radical scavenging activity
The antiradical power of extracts was determined using the 2,2-azino-bis-3-ethylbenzothiazoline- 6-sulfonic acid (ABTS+•) as reported by Montedoro et al. (1992) with some modifications. In Brief, a stock solution was prepared by mixing ABTS solution (7 mM) and sodium persulfate solution (2 mM) and incubated for 15 h in a dark room at 4 °C. A working solution was prepared by adding the water to the incubated solution until reaching an absorbance of 0.7 ± 0.02 at 734 nm. Afterwards, 900 µL of diluted ABTS were added to 100 µL of each plant extracts at various concentrations (1–1000 μg/mL). Readings of absorbance of samples were performed at 734 nm after incubation for 30 min at ambient temperature. The percentage of ABTS+scavenging activity (ABTS+-SA) was determined as the following equation (3):
| (3) |
2.7. Antibacterial assay
The antibacterial activity of E. alata plant extracts using agar disk diffusion method was tested against five clinical methicillin resistant Staphylococcus aureus strains: Sa12, Sa16, Sa26, Sa27 and Sa32 previously isolated from auricular infections in hospital (Kairouan, Tunisia) (Zmantar et al., 2008). To evaluate their activity spectra, the extracts were tested against different Gram positive and negative references strains (Salmonella typhimirum DT104 and Enterococcus faecalis ATCC 29212). The extracts were filtered using sterile filters (Thermo Fisher Scientific, Germany) of 0.22 µm diameter. The strains were grown in Mueller–Hinton (MH) broth (Oxoid) at 37 °C for 24 h and suspensions were adjusted to 0.5 McFarland standard turbidity. Then, 100 μL of each precultured suspension was spreaded onto plates containing MH agar. Sterile filter paper discs (6 mm in diameters) were impregnated with 20 μL of the different extracts and placed on agar. The treated plates were putted at 4◦C for 1 h and then incubated at 37 °C overnight. After incubation, the halo of the inhibition zone around the discs was determined. Each sample was tested in triplicate.
The minimum inhibitory concentration (MIC) was evaluated as recommended by Taheur et al. (2016). Briefly, serial dilutions of the extracts (0.05–25 mg/mL) were filled in 96 U bottomed-wells plates (Nunc, Roskilde, Denmark) with MH broth and the target bacteria. The treated plates were incubated at 37 ◦C overnight. The MIC was reported as the lowest concentration of the sample that did not allow the growth of microorganisms and do not show visible turbidity of the broth medium. The minimum bactericidal concentration (MBC) was evaluated by transferring 10 µL from the well showing no bacteria growth after MIC assay, on MH agar. After 24 h period of incubation at 37 °C, the bacterial growth was examined and the MBC was determined as the lowest concentration of the sample having bactericidal activity.
2.8. Effect of E. alata extracts on biofilm formation
The ability of E. alata extracts to inhibit biofilm formation was evaluated using colorimetric assay XTT (2,3-bis(2-methyloxy-4-nitro-5-sulfophenyl)–2H-tetrazolium-5-carboxanilide) (Sigma-Aldrich) as described previously by Taheur et al. (2016). The E. alata extracts (0.05–25 mg/mL) were mixed with pathogenic bacteria suspension (grown in TSB, for 24 h at 37 °C; 105 CFU/mL) in 96 U bottomed-wells plates (Nunc, Roskilde, Denmark) containing Tryptic Soy Broth (TSB) (Oxoid) with 2% glucose (w/v). Wells containing only TSB with 2% glucose and TSB with 2% glucose, inoculated with pathogenic strain were served as negative and positive control, respectively. After an overnight of incubation at 37 °C, the plates were rinsed 3 times with PBS. XTT solution (1 mg /mL PBS) and Menadione (Sigma-Aldrich) solution (0.4 mM in acetone) were prepared and filtered-sterilized before use. Next, 180 μL of of PBS and 20 µL of XTT-Menadione solution (12.5: 1 v/v) were added to each well. After incubation (3 h, 37 °C, in darkness), the optical density at 492 nm was been recorded and the reduction of biofilm biomass was determined.
2.9. Cytotoxicity assay for E. alata extracts on VERO cells
The cytotoxic effect of the E. alata extracts was evaluated on VERO cell line as described by Taheur et al. (2020). The concentrated extracts were diluted in RPMI 1640 medium to obtain concentration ranging from 100 to 1000 µg/mL. Vero cells (5 × 105/well) were seeded in 96-well plates and incubated for 24 h at 37 °C under 5% CO2. Then, the medium was removed and the cells were treated with samples and grown in CO2 (5%) incubator at 37 °C. After 24 h, the growth medium was discarted and 200 µL MTT solution (3 mg/mL) was added in each well and incubated for 4 h at 37 °C. The resultant formazan crystal was dissolved in DMSO and the Optical density (OD) was measured at 570 nm. The experiment was performed in triplicate.
2.10. Statistical analyses
All tests were performed in triplicate. The averages and standard of the results obtained were calculated using Microsoft Excel. The differences were analyzed by SPSS statistical package version 16.0 using Duncan-test. Values with (p < 0.05) were considered as significant.
3. Results
3.1. Proximate and mineral composition analysis
The results showed that Ephedra alata aerial parts have a content of 7.76% and 8.28% for moisture and ash, respectively.
The content of micro- and macroelement in E. alata aerial parts plant, given as mg per 100 g DW, is presented in Table 1. Calcium content was the highest, with about 1540 followed by potassium 1380 and phosphorus 160 mg/100 g.
Table 1.
Proximate and mineral composition of Ephedra alata aerial parts.
| Proximate composition | Mineral composition | ||||||
|---|---|---|---|---|---|---|---|
| Plant | Moisture (%) | Ash (%) | Phosphorus (P) (mg/100 g DW) | Calcium (Ca) (mg/100 g DW) | Iron (Fe) (mg/100 g DW) | Sodium (Na) (mg/100 g DW) | Potassium (k) (mg/100 g DW) |
| Ephedra alata | 7.76 ± 0.05 | 8.28 ± 0.00 | 160 ± 0.00 | 1540 ± 0.03 | 6 ± 0.00 | 20 ± 0.30 | 1380 ± 0.00 |
Values presented are means ± SD of triplicate measurements. DW: dry weight.
3.2. Fatty acids analysis
The fatty acids profile of E. alata aerial parts are shown in Table 2 and allowed to the elucidation of 27 compounds. The predominant fatty acids were γ-linolenic acid (23.69%) , followed by octadecadienoic acid (23.08%), palmitic acid (18.91%), oleic acid (17.43%), α-linolenic acid (3.56%), stearic acid (2.28%), eicosatrienoic acid (1.55%) and vaccenic acid (1.33%).
Table 2.
Fatty acids analysis of Ephedra alata aerial parts.
| Fatty acid | Abbreviation | (%) composition |
|---|---|---|
| Lauric acid | C12:0 | 0.54 |
| Myristic acid | C14:0 | 0.63 |
| Myristoleic acid | C14:1 | 0.78 |
| Palmitic acid | C16:0 | 18.91 |
| Palmitoleic acid | C16:1 | 0.77 |
| Margaric acid | C17:0 | 0.54 |
| Heptadecenoic acid | C17:1 | 0.26 |
| Stearic acid | C18:0 | 2.28 |
| Oleic acid | C18:1 cis 9 | 17.43 |
| Vaccenic acid | C18:1 cis 11 | 1.33 |
| Trans-9, cis-12-Octadecadienoic acid | C18:2 (cis 9, cis12) | 23.08 |
| α-Linolenic acid | C18:3 w3 | 3.56 |
| γ-Linolenic acid | C18:3 w6 | 23.69 |
| Arachidic acid | C20:0 | 0.48 |
| Eicosenoic acid | C20:1 | 0.86 |
| Eicosadienoic acid | C20:2 | 0.17 |
| Eicosatrienoic acid | C20:3 w3 | 1.55 |
| Dihomo-γ-linolenic acid | C20:3 w6 | 0.75 |
| Heneicosylic acid | C21:0 | 0.57 |
| Behenic acid | C22:0 | 0.20 |
| Erucic acid | C22:1 w9 | 0.47 |
| Docosadienoic acid | C22:2 | 0.35 |
| Docosatrienoic acid | C22:3 | 0.05 |
| Docosatetraenoic acid | C22:4 | 0.09 |
| Docosapentaenoic acid | C22:5 | 0.24 |
| Lignoceric acid | C24:0 | 0.33 |
| Nervonic acid | C24:1 | 0.07 |
| Total satured fatty acids | 24.48 | |
| Total polyunsatured fatty acids | 21.97 | |
| Total monounsatured fatty acids | 53.53 |
3.3. Qualitative and quantitative phytochemical content
The results of phytochemical screening of E. alata aerial parts extracts are summarized in Table 3. The aqueous crude extract exhibited high abundance of saponins and flavonoids and the absence of terpenoids, steroids and anthraquinones. On the other hand, the methanolic extract exhibited high amounts of alkaloids and low amounts of tannins, flavonoids terpenoids and proteins. Steroids, saponins, and anthraquinones were not detected.
Table 3.
Phytochemical screening of E. alata aerial parts extracts.
| Aqueous extract | Methanolic extract | |
|---|---|---|
| Tannins | + | + |
| Flavonoids | +++ | + |
| Steroids | – | – |
| Cardiac glycosides | + | ++ |
| Saponins | +++ | – |
| Proteins | ++ | + |
| Terpenoids | – | + |
| Anthraquinones | – | – |
| Alkaloids | ++ | +++ |
+++: relatively high in abundance; ++: relatively moderate in abundance; +: relatively low presence; -: not detected.
The extract yields and the phytochemical characterization of E. alata extracts were determined and results are depicted in Table 4. The extract yield increased with the increasing polarity of the solvent. In fact, aqueous extract recorded the highest yield 38.29% while the lowest yield (5%) was registered for the methanolic extract. Flavonoids and proteins contents were high in the aqueous extract with values of 30.82 mg QE/g dry extract and 98.62 mg BSAE/g dry extract, respectively. However, the highest contents of phenols, sugars and tannins were recorded for methanolic extract with values of 125.73 mg GAE/g dry extract, 2.02 mg GE /g dry extract and 0.55 mg ATE/g dry extract, respectively.
Table 4.
Total bioactive components of E. alata aerial parts extracts.
| Extracts | Extracts Yields (%) | Total flavonoids (mg QE /g dry extract) | Total phenols (mg GAE/g dry extract) | Total sugars (mg GE /g dry extract) | Total tannins (mg TAE/g dry extract) | Total proteins (mg BSAE/g dry extract) |
|---|---|---|---|---|---|---|
| Aqueous | 38.2 ± 0.2 | 30.82 ± 0.49 | 124.11 ± 1.18 | 0.08 ± 0.02 | 5.54 ± 0.29 | 98.62 ± 2.29 |
| Methanolic | 5.23 ± 0.0 | 27.89 ± 0.44 | 125.73 ± 1.68 | 2.02 ± 0.07 | 5.55 ± 0.09 | 97.12 ± 0.19 |
Values presented are means ± SD of triplicate measurements. GAE: Gallic Acid Equivalent; QE: Quercetin Equivalent; GE: Glucose Equivalent; TAE: Tannic Acid Equivalent; BSA: Bovine Serum Albumin Equivalent.
3.4. Antioxidant activity
The tested extracts displayed important antioxidant power as presented in Table 5. In the developed tests, aqueous extract was the most potent with IC50 values (0.001 and 0.002 mg/mL) considerably lower than the methanolic extract.This was confirmed based on the
Table 5.
Antioxidant activities of E. alata extracts.
| IC50 (mg/mL) |
||
|---|---|---|
| Extracts | DPPH | ABTS |
| Methanolic | 0.557 ± 0.028c | 0.143 ± 0.014c |
| Aqueous | 0.001 ± 0.0001a | 0.002 ± 0.0001a |
| Ascorbic acid | 0.014 ± 0.0001b | 0.015 ± 0.002b |
Values presented are means ± SD of triplicate measurements.
The letters (a–c) indicate a significant difference in the same line between the different antioxidant methods according to the Duncan test (p < 0.05).
IC50: The concentration of extracts at which 50% of antioxidant activity were shown.
DPPH scavenging ability and ABTS assays, whereas the aqueous extract showed the significantly highest antioxidant power (p < 0.05) compared to ascorbic acid.
3.5. Antibacterial activity
The antibacterial activity of E. alata aerial parts extracts was tested in vitro using the disc diffusion and the microdilution assays. Results are given as diameters of the inhibition zone (DIZ), MICs and MBCs (Table 6). Both extracts showed a dose-dependent antibacterial activity against all bacteria strains. Based on the DIZ values, the aqueous and methanolic extracts were effective on Gram positive and negative bacterial strains. The MIC and MBC were 1.56 mg/mL and 6.25 mg/mL, respectively, for Enterococcus faecalis. On the other hand, the both extracts are able to inhibit the growth of S. aureus. In fact, results demonstrated that methanolic extract was effective with a low MIC starting from 0.02 mg/mL and MBC of 12.5 mg/mL.
Table 6.
Antibacterial activities of the Ephedra alata extracts.
| Bacteria organisms | Aqueous extract |
Methanolic extract |
||||
|---|---|---|---|---|---|---|
| DIZ ± SD | MIC (mg/mL) | MBC (mg/mL) | DIZ ± SD | MIC (mg/mL) | MBC (mg/mL) | |
| References strains | ||||||
| Salmonella typhimirum DT104 | 12 ± 0 | 12.5 | >25 | 16 ± 0.33 | 6.25 | >25 |
| Enterococcus faecalis ATCC 29212 | 11 ± 0 | 12.5 | 12.5 | 12 ± 0.33 | 1.56 | 6.25 |
| Auricular infections strains | ||||||
| Sa12 | 8 ± 0 | 6.25 | >25 | 13 ± 0.33 | 3.125 | 12.5 |
| Sa16 | 11 ± 0 | 3.12 | 25 | 11 ± 0 | 0.09 | 12.5 |
| Sa26 | 10 ± 1.4 | 12.5 | >25 | 11.5 ± 0.7 | 3.12 | >25 |
| Sa27 | 9 ± 0 | 25 | >25 | 11 ± 0 | 3.12 | 12.5 |
| Sa32 | 10 ± 0 | 12.5 | >25 | 13.5 ± 0.7 | 0.02 | >25 |
DIZ: diameter inhibition zone; SD: standard deviation; MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration.
3.6. Antibiofilm activity
The effect of E. alata extracts on the antibiofilm formation was quantified by XTT method and the results were summarized in Table 7. The tested extracts inhibited bacterial biofilm formation. Aqueous and methanolic extracts were the most potent against S.thyphimirium with BIC50 values of 4.62 and 3.18 mg/mL, respectively. Regarding the clinical strains, the 2 extracts were able to inhibit the biofilm formation by almost the MRSA strains. Sa12 and Sa26 seemed to be the most sensitive towards both extracts with BIC50 values ranging from 4.55 to 13.96 mg/mL.
Table 7.
Antibiofilm activity of Ephedra alata extracts against references and oral strains.
| BI50 (mg/mL) | ||
|---|---|---|
| Aqueous extract | Methanolic extract | |
| References strains | ||
|
Enterococcus faecalis ATCC 29212 Salmonella typhimirumDT104 |
6.78 4.62 |
6.74 3.18 |
| Auricular infections strains | ||
| Sa12 | 5.96 | 4.55 |
| Sa16 | >25 | >25 |
| Sa26 | 12.25 | 13.96 |
| Sa27 | 17.13 | 17.67 |
| Sa32 | >25 | >25 |
BI50: minimum biofilm inhibition concentration of E. alata extracts able to inhibit 50% on the biofilm formation.
3.7. Cytotoxicity assay for E. alata extracts on VERO cells
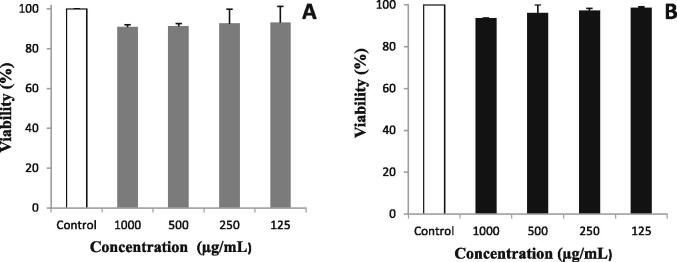
The cytotoxic activity of E. alata aerial parts extracts was evaluated in vitro by the determination of cell growth effects on Vero cell lines using MTT assay (Fig. 2). No effect on the viability of the VERO cell line was observed by the incubation with aqueous and methanolic extracts ranging from 125 to 1000 µg /mL.The high concentration of the aqueous and methanolic extracts (1000 µg/mL) did not revealed cytotoxic activity with percentage of viability ranging from 90.92 to 93.60, respectively. Thus, our data indicated that both extracts were not toxic with an IC50 values (>1000 μg/mL).
Fig. 2.
Cytotoxic activity (expressed as % of viability) of VERO cell line after 24 h of incubation with aqueous (A) or methanolic (B) extracts obtained from aerial parts of E. alata. White bars represent control. Values are presented as mean ± SD of triplicate measurements.
4. Discussion
Medicinal plants have been extensive consumed all over the world due its nutritional values and beneficial health effects. Many researchers have demonstrated the correlation between the mineral plant contents and its therapeutic proprieties (Adnan et al., 2010, Tomescu et al., 2015). Minerals deficiency is among the main causes of several chronic diseases. The data revealed that our potent plant is very abundant on mineral elements. The higher amount on calcium gives significant value of the Ephedra, because this element is necessary for bone formation and renewal, muscle and heart contraction, blood clotting, cellular exchanges, release of hormones and the transmission of nerve impulses (Humenczuk, 1988, Payne and Wilson, 1999). Potassium is the second major element found in Ephedra. Provides its usefulness in regulating heart rate and blood pressure, it participates in the transmission of nerve impulses and muscle contraction (Arinathan et al., 2003). The considerable content of phosphorus in the sample contributes to the maintenance of pH, participates in numerous chemical reactions of the organism, and enters into the composition of DNA and RNA of cells (Payne and Wilson, 1999). Iron deficiency has been estimated as the 16th leading risk factors underlying the global burden of disease (Collaborators, 2018). Hence, it caused anemia for nearly 2 billion people worldwide (Graham et al., 2012). The consumption of 100 g of E. alata aerial parts could cover around 37.5% and 75%, dietary Fer intake for man and women, respectively. Calcium, potassium and iron contents were excessive higher, whereas sodium level was lower than those reported by Soua et al. (2020) for polysaccharides extracted from E. alata grown in Mahdia (delegation in the center-east of Tunisia).
Additionally, fatty acids were known for their beneficial health effects which can be preventive and therapeutic for various diseases. To the best of the authors’ knowledge, this is the first study on the fatty-acid composition of E. alata growing in Gafsa (delegation in the southwestern of Tunisia). Our data underline the richness of this plant in fatty acids and especially unsatured fatty acids (α-Linolenic acid, γ-linolenic acid octadecadienoic acid) which has anti-inflammatory effect and prevent cancer cell proliferation (Booyens et al., 1984, Chang et al., 2010). Oleic acid was found to prevent cardiovascular diseases (Ranalli and Angerosa, 1996).
The qualitative phytochemical assays of our potent plant confirm the presence of various metabolite compounds with different rates in both extracts. Our findings are in agreement with those reported previously by Jaradat et al., 2015, Kittana et al., 2017.
When comparing with other Ephedra species collected from the southern Lebanon, results indicated that the methanolic and ethanolic solvents extract are richer than aqueous extract in plant bioactive components (Kallassy et al., 2017). Furthermore, the group of Al-Awaida et al (2018) have studied the Ephedra species collected from south Jordan and demonstrated that the chloroformic and methanolic extracts are rich in phenolic compounds. Whereas, ethanolic extract of aerial parts of Ephedra species collected from Balochistan was higher in phytochemical content than methanolic extract (Gul et al., 2017).
The flavonoids and phenols are documented as the most dynamic ingredients to which biological activities of many plant species can be attributed. Therefore, quantitative analyses were carried out. The screening of phytochemical contents of the tested extracts showed that the presence of flavonoids and phenols in high concentration was detected in both aqueous and methanolic extracts of E. alata aerial parts. In our work, the highest flavonoids content was registered in the aqueous extract (30.82 mg quercetin /g extract). Whereas, the methanolic extract displayed the highest phenols content (125.73 mg gallic acid/g extract). Contrariwise, the use of polar solvents like water helps to co-extract the biomolecules attached to phenolic compounds (glycosides organic acids tannins salts proteins and sugars) which interferes with the phenolic quantification (Zieliński and Kozłowska, 2000). Likewise, the study of Sioud et al. (2020b) reports that the methanolic extract of the same species harvested from the sahara of Tataouine (delegation in the southeast of Tunisia) was rich in phenolic and flavonoid compounds with values of 205 mg GAE/g extract and 40.5 mg rutin /g extract, respectively. The hydro-alcoholic extract obtained from aerial parts of E. alata from Djerba (island in the south of Tunisia) had a high polyphenol amount in the order of 156.226 mg gallic acid/g extract (Danciu et al., 2019). However, Benabderrahim et al. (2019) reported lower polyphenol content (28.43 mg gallic acid/g) in the hydro-alcoholic extract (50:50 V/V) obtained from aerial parts of E. alata from Djerba. In fact, environmental conditions, the region position, plant organs used and the extraction procedure can influence phenolic contents of the plant.
To deepen the phytochemical characterization of E. alata aerial parts extracts, the tannin, sugar and protein contents were also quantified. The methanolic extract had the highest content of tannin and sugar, while; the aqueous extract is rich on protein. Benabderrahim et al. (2019) reported that E. alata, collected from Medenine (delegation in the South-East of Tunisia) had 7.81 mg TAE/ g DW of condensed tannin. To the best of our knowledge, protein and sugar amounts have not been studied earlier for Ephedra species extracts.
Currently, the high interest on studying and searching bio-antioxidants derived from medicinal plants and its biomolecules is due to their safe use in pharmaceutical, cosmetic or food industries (Tlili et al., 2019). The results of the current study showed that the two extracts obtained from E. alata, especially aqueous extract displayed excellent antioxidant capacity, confirmed by DPPH antiradical activity and ABTS radicals scavenging assay. Sioud et al. (2020b) found that the methanolic extract of E. alata exhibited a potent DPPH radical scavenger with IC50 value of 0.0147 mg/mL. Furthermore, Danciu and coworkers reported that the hydroethanolic extract of E. alata possessed strong antioxidant activity in order of 7453 µmolTrolox/g (Danciu et al., 2019). In fact, Benabderrahim et al. (2019) reported high rates of DPPH% and ABTS+% radical scavenging abilities, with values of 33.51 and 37.86 mg TEAC/100 g extract, respectively. Jerbi et al. (2016) showed that the methanolic extract of E. alata collected from Kbeli (delegation in the south of Tunisia) exhibited the strongest free radical-scavenging activity with an IC50 value of 0.027 mg/mL, followed by the ethyl acetate extract (IC50 = 0.107 mg/mL), as compared with BHT (IC50 = 0.042 mg/mL) and vitamin E (IC50 = 0.046 mg/mL). Also, the DPPH antiradical ability of the methanolic extract of E. alata was evaluated and found that IC50 was 16.03 μg/mL. Variations among the antioxidant activity of plant extracts could be explained by the difference of the bioactive constituents present in the different plant extracts and their contents (Mokrani and Madani, 2016).
Nowadays, biological control of multidrug resistant pathogens by medicinal plants and its phenolic compounds has become an effective alternative with especially low toxic effects.
In our study, the extracts had the potency to inhibit the growth of references pathogenic strains, and especially Staphylococcus aureus methicillin resistant bacteria. The antibacterial activity of the hydroethanolic extract from E. alata was evaluated and the data demonstrated that this extract inhibit the growth of different pathogenic bacteria like Klebsiella pneumonia, Shigella flexneri, Salmonella enterica, Staphylococcus aureus with low MIC and MBC concentrations (Danciu et al., 2019). Moreover, Jerbi et al. (2016) reported the antibacterial activity of E. alata extracts against bacteria and demonstrated that methanolic extract, ethyl acetate extract, hexanic extract were the most active. Also, Ziani et al. (2019) indicated that the hydroethanolic extract of aerial parts of E. alata collected from Algeria revealed strong activity against Methicillin resistant and susceptible S. aureus strains. Recently, Manilal et al. (2020) reported the significant inhibitory effect of M. stenopetala and R. officinalis extracts on the growth of MRSA.
Also, the extracts tested in this study were able to decrease biofilm formation of the pathogenic references bacteria and the MRSA clinical isolates cultured on polystyrene surface. By now, there is no investigation on the antibiofilm activity of E. alata extracts. Contrariwise, many scientific reports have studied the antibiofilm activity of other species of medicinal plants. Chemsa et al. (2018) tested the antibiofilm activity of the methanolic extract and the essential oil of Anthemissti parum against different pathogenic. They reported that at the presence of concentrations between 25 µL/mL to 100 µL/mL of the essential oil showed antibiofilm potency (8.25–45.41%) while at the presence of concentrations between 1.56 mg/mL to 25 mg/mL of the methanolic extract showed higher antibiofilm percentages (5.09–80.02%). The crude extract of Vetiveria zizanioides, Moringa stenopetal has been reported to decrease the biofilm formation of Staphylococcus aureus methicillin-resistant (Kannappan et al., 2017, Manilal et al., 2020).
Evaluating the toxicological proprieties of crude plant extracts or active compounds is a crucial assay to ensure their safety consumption by animals or humans. In the current study, toxicity of E. alata extracts were assessed in vitro using Vero cell lines. Our data showed that both tested extracts were non toxic at IC50 values (>1000 μg/mL) because a crude extract with cytotoxic activity was when IC50 < 30 μg/mL, according to the American National Cancer Institute (Suffness and Pezzuto, 1990). Our findings were in accordance with those obtained by (Al-Awaida et al., 2018), who reported the less cytotoxicity activity of various solvent extracts of Ephedra aphylla on Vero cells. Furthermore, Sioud et al. (2020a) evaluated the cytotoxicity of E. alata methanolic extract on, 4T1, carcinoma cell line and showed inhibition of cells and induction of apoptosis. By the same way, the hydro-alcoholic extract of E. alata inhibited the proliferation, presented a pro-apoptotic and cytotoxic potential against the MCF-7, human mammary cell line (Danciu et al., 2019).
5. Conclusion
In the current study, qualitative and quantitative phytochemical analysis revealed that aqueous and methanolic extracts are sources of various metabolite compounds with interesting biological activities. In fact, the different extracts showed an antioxidant power without any cytotoxic effect. Also they displayed antibacterial and antibiofilm activities against the human selective pathogenic organism, MRSA, in a dose dependant- manner. These properties may allow these extracts to be considered as an effective source of bioactive molecules to control pathogenic bacteria associated to infectious disease like S. aureus methicillin resistant isolated from auricular infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adnan M., Hussain J., Tahir Shah M., Shinwari Z.K., Ullah F., Bahader A., Khan N., Latif Khan A., Watanabe T. Proximate and nutrient composition of medicinal plants of humid and sub-humid regions in North-west Pakistan. J. Med. Plants Res. 2010;4:339–345. [Google Scholar]
- Al-Awaida W., Al-Hourani B.J., Akash M., Talib W.H., Zein S., Falah R.R., Aburubaiha Z. In vitro anticancer, anti-inflammatory, and antioxidant potentials of Ephedra aphylla. J. Cancer Res. Ther. 2018;14:1350. doi: 10.4103/0973-1482.196760. [DOI] [PubMed] [Google Scholar]
- AOAC, I . AOAC international Gaithersburg; MD: 2000. AOAC official methods of analysis. [Google Scholar]
- Arinathan V., Mohan V., John De Britto A. Chemical composition of certain tribal pulses in South India. Int. J. Food Sci. Nutr. 2003;54:209–217. doi: 10.1080/09637480120092026. [DOI] [PubMed] [Google Scholar]
- Banso A., Adeyemo S. Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri. 2006;18 [Google Scholar]
- Benabderrahim M.A., Yahia Y., Bettaieb I., Elfalleh W., Nagaz K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind. Crops Prod. 2019;138 [Google Scholar]
- Bhat P., Hegde G.R., Hegde G., Mulgund G.S. Ethnomedicinal plants to cure skin diseases—an account of the traditional knowledge in the coastal parts of Central Western Ghats, Karnataka, India. J. Ethnopharmacol. 2014;151:493–502. doi: 10.1016/j.jep.2013.10.062. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Booyens J., Engelbrecht P., Le Roux S., Louwrens C., Van der Merwe C., Katzeff I. Some effects of the essential fatty acids linoleic acid and alpha-linolenic acid and of their metabolites gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and of prostaglandins A1 and E1 on the proliferation of human osteogenic sarcoma cells in culture. Prostaglandins, Leukotrienes Med. 1984;15:15–33. doi: 10.1016/0262-1746(84)90053-2. [DOI] [PubMed] [Google Scholar]
- Chaieb, M., Boukhris, M., 1998. Flore succincte et illustrée des zones arides et sahariennes de Tunisie.(Eds.) Association pour la protection de la nature et de l’environnement. Sfax 67.
- Chang C.-S., Sun H.-L., Lii C.-K., Chen H.-W., Chen P.-Y., Liu K.-L. Gamma-linolenic acid inhibits inflammatory responses by regulating nf-κB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation. 2010;33:46–57. doi: 10.1007/s10753-009-9157-8. [DOI] [PubMed] [Google Scholar]
- Chemsa A.E., Zellagui A., Öztürk M., Erol E., Ceylan O., Duru M.E., Lahouel M. Chemical composition, antioxidant, anticholinesterase, antimicrobial and antibiofilm activities of essential oil and methanolic extract of Anthemis stiparum subsp. sabulicola (Pomel) Oberpr. Microb. Pathog. 2018;119:233–240. doi: 10.1016/j.micpath.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Collaborators, G.L.R.o.S., 2018. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. New England Journal of Medicine 379, 2429-2437. [DOI] [PMC free article] [PubMed]
- Cuénod A. Impr; SEFAN: 1954. Flore analytique et synoptique de la Tunisie. [Google Scholar]
- D'Auria M., Emanuele L., Racioppi R. FT-ICR-MS analysis of lignin. Nat. Prod. Res. 2012;26:1368–1374. doi: 10.1080/14786419.2011.586947. [DOI] [PubMed] [Google Scholar]
- Danciu C., Muntean D., Alexa E., Farcas C., Oprean C., Zupko I., Bor A., Minda D., Proks M., Buda V. Phytochemical characterization and evaluation of the antimicrobial, antiproliferative and pro-apoptotic potential of Ephedra alata Decne. Hydroalcoholic extract against the MCF-7 breast cancer cell line. Molecules. 2019;24:13. doi: 10.3390/molecules24010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., De J.N. Ethnobotanical survey of Purulia district, West Bengal, India for medicinal plants used against gastrointestinal disorders. J. Ethnopharmacol. 2012;143:68–80. doi: 10.1016/j.jep.2012.05.064. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers, P.t., Smith, F Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Graham R.D., Knez M., Welch R.M. How much nutritional iron deficiency in humans globally is due to an underlying zinc deficiency? Adv. Agronomy. Elsevier. 2012:1–40. [Google Scholar]
- Gul, R., Jan, S.U., Faridullah, S., Sherani, S., Jahan, N., 2017. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. The Scientific World Journal 2017. [DOI] [PMC free article] [PubMed]
- Gupta M., Mandowara D., Jain S. Medicinal plants utilized by rural women of Rajasthan. Asian Agri-history. 2008;12:321–326. [Google Scholar]
- Hassoun A., Linden P.K., Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—a review of recent developments in MRSA management and treatment. Crit. Care. 2017;21:1–10. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humenczuk, M., 1988. V. Hegarty, Decisions in Nutrition, From CV Mosby, Box 28430, St. Louis, MO 63146 (1988), 590 pp., softcover, $33.95. Elsevier.
- Hwang B.Y., Kim H.S., Lee J.H., Hong Y.S., Ro J.S., Lee K.S., Lee J.J. Antioxidant benzoylated flavan-3-ol glycoside from Celastrus orbiculatus. J. Nat. Prod. 2001;64:82–84. doi: 10.1021/np000251l. [DOI] [PubMed] [Google Scholar]
- Hyuga S., Hyuga M., Oshima N., Maruyama T., Kamakura H., Yamashita T., Yoshimura M., Amakura Y., Hakamatsuka T., Odaguchi H. Ephedrine alkaloids-free Ephedra Herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J. Nat. Med. 2016;70:571–583. doi: 10.1007/s11418-016-0979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragic S., Sofić E. Chemical composition of various Ephedra species. Bosnian J. Basic Med. Sci. 2015;15:21. doi: 10.17305/bjbms.2015.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaradat N., Hussen F., Al Ali A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J. Mater. Environ. Sci. 2015;6:1771–1778. [Google Scholar]
- Jerbi A., Zehri S., Abdnnabi R., Gharsallah N., Kammoun M. Essential oil composition, free-radical-scavenging and antibacterial effect from stems of Ephedra alata alenda in Tunisia. J. Essential Oil Bearing Plants. 2016;19:1503–1509. [Google Scholar]
- Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food. Chem. 1985;33:213–217. [Google Scholar]
- Kallassy H., Fayyad-Kazan M., Makki R., El-Makhour Y., Rammal H., Leger D.Y., Sol V., Fayyad-Kazan H., Liagre B., Badran B. Chemical composition and antioxidant, anti-inflammatory, and antiproliferative activities of Lebanese Ephedra Campylopoda plant. Med. Sci. Monit. Basic Res. 2017;23:313. doi: 10.12659/MSMBR.905056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannappan A., Gowrishankar S., Srinivasan R., Pandian S.K., Ravi A.V. Antibiofilm activity of Vetiveria zizanioides root extract against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2017;110:313–324. doi: 10.1016/j.micpath.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Khandelwal K.R. Pragati Books Pvt; Ltd: 2008. Practical pharmacognosy. [Google Scholar]
- Kim D.O., Lee K.W., Lee H.J., Lee C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food. Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Kittana N., Abu-Rass H., Sabra R., Manasra L., Hanany H., Jaradat N., Hussein F., Zaid A.N. Topical aqueous extract of Ephedra alata can improve wound healing in an animal model. Chin. J. Traumatol. 2017;20:108–113. doi: 10.1016/j.cjtee.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah D., Devi T., Bono A., Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009;3:067–072. [Google Scholar]
- Lamine J.B., Boujbiha M.A., Dahane S., Cherifa A.B., Khlifi A., Chahdoura H., Yakoubi M.T., Ferchichi S., El Ayeb N., Achour L. Α-amylase and α-glucosidase inhibitor effects and pancreatic response to diabetes mellitus on wistar rats of ephedra alata areal part decoction with immunohistochemical analyses. Environ. Sci. Pollut. Res. 2019;26:9739–9754. doi: 10.1007/s11356-019-04339-3. [DOI] [PubMed] [Google Scholar]
- Le Floch, É., Boulos, L., Vela, E., 2010. Catalogue synonymique commenté de la flore de Tunisie. Simpact.
- Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:1–23. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- Manilal A., Sabu K.R., Shewangizaw M., Aklilu A., Seid M., Merdekios B., Tsegaye B. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D.L., Buchsbaum R., Swain T. Measurement of total protein in plant samples in the presence of tannins. Anal. Biochem. 1985;147:136–143. doi: 10.1016/0003-2697(85)90019-3. [DOI] [PubMed] [Google Scholar]
- Mokrani A., Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016;162:68–76. [Google Scholar]
- Montedoro G., Servili M., Baldioli M., Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food. Chem. 1992;40:1571–1576. [Google Scholar]
- Mosbah H., Chahdoura H., Adouni K., Kamoun J., Boujbiha M.A., Gonzalez-Paramas A.M., Santos-Buelga C., Ciudad-Mulero M., Morales P., Fernández-Ruiz V. Nutritional properties, identification of phenolic compounds, and enzyme inhibitory activities of Feijoa sellowiana leaves. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.13012. [DOI] [PubMed] [Google Scholar]
- Nabli, M., 1991. Diversité floristique en Tunisie. conservation des resources végétales,(Eds.), Rejdali M. et Heywood VH Actes Editions Rabat, Maroc.
- Nawwar M.A., El-Sissi H.I., Barakat H.H. Flavonoid constituents of Ephedra alata. Phytochemistry. 1984;23:2937–2939. [Google Scholar]
- Organization W.H. The world health report 2006: working together for health. World Health. 2006 Organization. [Google Scholar]
- Payne W.J.A., Wilson R.T. Blackwell Science; 1999. An introduction to animal husbandry in the tropics. [Google Scholar]
- Raaman N. New India Publishing; 2006. Phytochemical techniques. [Google Scholar]
- Ranalli A., Angerosa F. Integral centrifuges for olive oil extraction. The qualitative characteristics of products. J. Am. Oil. Chem. Soc. 1996;73:417–422. [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- Sioud, F., Amor, S., Toumia, I.b., Lahmar, A., Aires, V., Chekir-Ghedira, L., Delmas, D., 2020a. A new highlight of ephedra alata decne properties as potential adjuvant in combination with cisplatin to induce cell death of 4T1 breast cancer cells in vitro and in vivo. Cells 9, 362. [DOI] [PMC free article] [PubMed]
- Sioud F., Toumia I.B., Lahmer A., Khlifi R., Dhaouefi Z., Maatouk M., Ghedira K., Chekir-Ghedira L. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ. Sci. Pollut. Res. 2020:1–10. doi: 10.1007/s11356-020-07904-3. [DOI] [PubMed] [Google Scholar]
- Soua L., Koubaa M., Barba F.J., Fakhfakh J., Ghamgui H.K., Chaabouni S.E. Water-Soluble Polysaccharides from Ephedra alata Stems: Structural Characterization, Functional Properties, and Antioxidant Activity. Molecules. 2020;25:2210. doi: 10.3390/molecules25092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffness M., Pezzuto J.M. Academic Press; London: 1990. Methods in plant biochemistry: assays for bioactivity; pp. 71–133. [Google Scholar]
- Taheur F.B., Kouidhi B., Fdhila K., Elabed H., Slama R.B., Mahdouani K., Bakhrouf A., Chaieb K. Anti-bacterial and anti-biofilm activity of probiotic bacteria against oral pathogens. Microb. Pathog. 2016;97:213–220. doi: 10.1016/j.micpath.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Taheur F.B., Mansour C., Jeddou K.B., Machreki Y., Kouidhi B., Abdulhakim J.A., Chaieb K. Aflatoxin B1 degradation by microorganisms isolated from Kombucha culture. Toxicon. 2020;179:76–83. doi: 10.1016/j.toxicon.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Tlili N., Kirkan B., Sarikurkcu C. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crops Prod. 2019;128:147–152. [Google Scholar]
- Tomescu, A., Rus, C., Pop, G., Alexa, E., Radulov, I., Imbrea, I.M., Negrea, M., 2015. Researches regarding proximate and selected elements composition of some medicinal plants belonging to the lamiaceae family. Agronomy Series of Scientific Research/Lucrari Stiintifice Seria Agronomie 58.
- Xie M., Yang Y., Wang B., Wang C. Interdisciplinary investigation on ancient Ephedra twigs from Gumugou Cemetery (3800 B.P.) in Xinjiang region, northwest China. Microsc. Res. Tech. 2013;76:663–672. doi: 10.1002/jemt.22216. [DOI] [PubMed] [Google Scholar]
- Ziani B.E., Heleno S.A., Bachari K., Dias M.I., Alves M.J., Barros L., Ferreira I.C. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019;116:312–319. doi: 10.1016/j.foodres.2018.08.041. [DOI] [PubMed] [Google Scholar]
- Zieliński H., Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food. Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]
- Zmantar T., Chaieb K., Abdallah F.B., Kahla-Nakbi A.B., Hassen A.B., Mahdouani K., Bakhrouf A. Multiplex PCR detection of the antibiotic resistance genes in Staphylococcus aureus strains isolated from auricular infections. Folia Microbiol. 2008;53:357–362. doi: 10.1007/s12223-008-0055-5. [DOI] [PubMed] [Google Scholar]