Abstract
Biochemical, antioxidant, serum, and urine profiles together with physical examination can deliver important information regarding animal health status, and are vital in the diagnosis and treatment of patients. CCl4, a potent nephrotoxin, was used for causing toxicity in rat kidneys. The present study aimed at exploring the nephroprotective potential of P. jacquemontiana leaves methanol extract (PJM) and P. hydaspidis whole-plant methanol extract (PHM) on kidney cells of male rats after oxidative stress and DNA damage was instigated by CCl4. Various parameters including enzymatic levels, serum profiles, urine profiles, genotoxicity, and histological studies were conducted. In renal samples of rats treated with CCl4, the antioxidant enzymes (POD, SOD, CAT), PH level, protein level, and glutathione contents were significantly (p < 0.05) declined whereas renal biochemicals (H2O2, TBARS, and nitrite), specific gravity, level of urea, urobilinogen, serum BUN and creatinine were markedly (p < 0.05) increased relative to control group. Co-administration of PJM and PHM with CCl4 displayed protective ability against CCl4 intoxication by restoring activities of antioxidant enzymes, urine profile, biochemical parameters, and serum profile in rats. CCl4 also induced prominent DNA damages and glomerular atrophy with abnormal appearance of glomerulus and Bowman’s capsule. These damages results in impaired corticular sections, edema in Bowman’s capsule, accumulation of necrotic cells, dilation of convoluted tubules, and narrowing of space between Bowman’s capsule, which were successfully ameliorated after co-administration of PJM and PHM fractions in a dose-dependent manner (200 and 400 mg/kg b.w.). The results obtained suggest the therapeutic role of PJM and PHM in oxidative-stress related disorders of kidney and may be helpful in kidney trauma.
Keyword: P. jacquemontiana, Periploca hydaspidis, Nephrotoxic, CCl4, DNA, Comet, Bowman’s capsule
1. Introduction
CCl4-induced tissue injury is documented as the most commonly studied and validated model deliberated in rats so far. The toxicant; CCl4 causes tissue damage via generating and elevating ROS (reactive oxygen species) levels under oxidative stress (Alsheblak et al., 2016). This oxidative stress underlies severe pathological conditions e.g. hypertension, diabetes mellitus, cancer, hepatic ailments, renal disorders, and death (Al-Olayan et al., 2014, Usman et al., 2020). CCl4 is not only declared hepatotoxic to humans but also nephrotoxic. It mainly damages lungs, heart, brain, liver, testes, and kidney with maximum effect on liver cells followed by kidney cells. CCl4-induced oxidative stress contributes to nephrotoxicity after inducing acute and chronic renal deteriorations leading to a variety of pathological conditions (Sajid et al., 2016, Rahmouni et al., 2017). The mechanism in either case includes the initiation of free-radicals leading to an increase in LPO (lipid peroxidation) products i.e. TBARS (thio-barbituric acid substances), H2O2 (hydrogen peroxide), and nitrite (Ali et al 2021a). Biochemical parameters including AST (aspartate transaminase), ALT (alanine transaminase), and ALP (alkaline phosphatase) are altered whereas endogenous levels of antioxidant enzymes i.e. CAT (catalase), SOD (superoxide dismutase), GSH (glutathione), and POD (peroxidase) are decreased. These changes lead to degradation of cellular molecules (DNA, lipids, proteins) resulting in failure of that respective organ.
These CCl4-induced cell damages are evaluated by its possible effects on genotoxicity, histology, and activity/expression of antioxidant enzymes machinery (Aayadi et al., 2017). It not only prompts the lipid peroxidation (LPO) and altered redox status of tissues but also cause serious DNA damage and histopathological appearance (Ali et al., 2021a, Ali et al., 2021b). A single injection of CCl4 instigates DNA oxidative damage via generating DNA-strand breaks in blood lymphocytes detected by comet assay (Chu et al., 2016) whereas interstitial mononuclear cell-infiltration, renal-tubule dilation, glomerular atrophy, and fibrosis in tissue samples (Hozzein et al., 2019). Wide-spread DNA-strand breaks caused via CCl4 toxicity may cause cell transformation and ultimate death of that cell (Sajid et al., 2016). Comet assay has emerged as a broadly-used tool for in vitro genotoxic testing including the assessment of mutagenicity and genotoxic potential of several chemicals and herbal products in vivo (Abou Gabal et al., 2015).
All living organisms have naturally-embedded defense system which fights against oxidative stress generated via CCl4 (Karakuş et al., 2017). It acts by increasing the level of endogenous antioxidant enzymes and decreasing LPO (Bellassoued et al., 2018). Allah Almighty has blessed Pakistan with great diversity of medicinal plants which are rich in natural bioactive compounds such as alkaloids, flavonoids, terpenoids, tannins etc (Abbasi et al., 2019, Abbasi et al., 2020, Abbasi et al., 2021). Medicinal plants are highly rich in different antioxidants to combat various types of reactive oxygen species (Iqbal et al., 2019, Iqbal et al., 2020). Antioxidants such as vitamin-E, n-acetyl cysteine, naringenin, silymarin, rhein, and quercetin display health-promoting effects against oxidative damage via declining LPO and partially improving tissue injury (Sahreen et al., 2015, Iqbal et al., 2017). Black-tea extract, Gingko-biloba, vitamin-C & E, and melatonin are well-known antioxidants reported for reducing CCl4-induced renal toxicity (Ogeturk et al., 2005). Plant materials and products have abundant amounts of these antioxidant compounds (Iqbal et al., 2018a, Iqbal et al., 2018b, Iqbal et al., 2018c). Many plant-containing phytochemicals have been used in the treatment of hepatic and renal diseases, either given independently or collectively (Karakuş et al., 2017, Usman et al., 2020, Hameed et al., 2021, Uddin et al., 2021). Two such phytochemical-rich and antioxidant-rich medicinal plants reported for their significant antioxidant, anti-inflammatory, hepatoprotective (Ali et al., 2017, Ali et al., 2018b), antimicrobial (Ali et al., 2018a, Ullah et al., 2015), anticancer and wound healing (Ali et al., 2021c, Ali et al., 2021d) potential is Parrotiopsis jacquemontiana (Decne) Rehder and Periploca hydaspidis Falc. The current study evaluates the plants of interest for its nephroprotective activities after CCl4-induced oxidative stress was generated in male experimental rats.
2. Materials and methods
2.1. Collection and identification of plant sample
The leaves of P. jacquemontiana were sampled from Upper Dir district, Pakistan from May to June 2019. The global positing system (GPS) coordinates calculated for the respective plant was 35.34°N and 71.96°E, whereas whole plant of P. hydaspidis was collected from Charbagh (coordinates: 34°49″33′N 72°27″02′E) district Swat from April to June 2019. The identification and collection of plants were done by Dr. Irfan Ullah Farhad and Dr. Syed Afzal Shah from biological sciences department of Quaid-i-Azam University (QAU), Islamabad and voucher # 063214 and 23651 were allotted at Herbarium of Pakistan, Quaid-i-Azam University, Islamabad, Pakistan.
2.2. Processing of plant samples
The leaves of P. jacquemontiana and whole plant of P. hydaspidis were properly washed using distilled water to remove dust and other debris. The plants were air dried at room temperature and stored under shade for two to four weeks. Electric grinder was used to get fine powder of plant material. Powdered plant material was soaked in pure methanol for one week. Filtration was done through Whatman number.1 filter paper to get refined methanolic plant extract. Rotary evaporator was used to evaporate the methanol solvent from respective fractions to obtain P. jacquemontiana methanol extract (PJM) and P. hydaspidis methanol extract (PHM). For in vivo evaluation assays, the extract was further dried and stored at 4 °C.
2.3. CCl4-induced toxicity studies
Crude methanol extracts of P. jacquemontiana and P. hydaspidis were used for its protective potential on CCl4-induced renal damages in rats. Toxicity was induced by CCl4 which was monitored by quantifying the levels of enzymes (antioxidant and serum) and other parameters (histological and comet).
2.3.1. Experimental rats used
Male Sprague-dawley rats (180–250 g) obtained from primate facility of QAU, Islamabad were used in the current study and the protocols regarding different assays were approved (BCH-275) by the ethical committee of QAU. The guidelines of NIH (national institute of health) were strictly followed and the experimental rats were gently treated, grown in ventilated cages, and fed with clean water and standard laboratory feed for rats daily.
2.3.2. Acute-toxicity testing
For acute-toxicity screening, the methanol fraction of P. jacquemontiana (PJM) and P. hydaspidis (PHM) was administered intra-gastrically (50 mg/kg b.wt) to each of three male experimental rats and observed for two weeks to check the behavioral changes and mortality risks. Although no toxicity signs and mortality were observed yet, the augmented order of fraction doses ranging from 100 to 4000 mg/kg b.wt was administered intra-gastrically to experimental rats. Toxicity signs, altered behavior and mortality was not even recorded against the highest dose administered of the fraction. Therefore 1/10th of 4000 mg/kg served as high dose (400 mg/kg b.wt) and 1/20th of 4000 mg/kg served as low dose (200 mg/kg b.wt) for the evaluation of nephroprotective potential of PJM and PHM in rats.
2.3.3. Experimental design of rat groups
A total of eight groups (having 7 rats each = 8) comprising Sprague-dawley rats (180–250 g) was designed for each nephro-toxicity study. The first group (Group 1) was not administered with any treatment and characterized the control group. Group II was treated with olive oil and DMSO in equal ratio (1 mL/kg body wt.; 1:1 v/v) whereas Group III was intra-peritoneally injected with CCl4 in olive oil (1 mL/kg body wt.; 2:8 v/v). Group IV was treated with reference control; silymarin (50 mg/kg body wt.) after 24 h following CCl4 treatment. In Group V and VI, PJM/PHM (200 mg/kg and 400 mg/kg body wt.) doses were administered orally following CCl4 treatment after 24 h’ whereas in Group VII and VIII, the rats were only administered with PJM/PHM (200 mg/kg and 400 mg/kg body wt.) doses. The respective treatments were continued for 4 weeks given on alternative days (three times a week) in morning time. After experiment completion, the rats were treated with ether anesthesia, euthanized, and blood was withdrawn from their heart carefully via injections. Kidney was removed from each of the rats and placed in saline solution. The kidneys were divided into two equal portions; one of which was preserved in liquid nitrogen (for enzymatic and biochemical assays) while the other part in 10% formalin (for histopathological studies).
2.3.4. Anti-oxidant enzyme studies of kidney
Tissue homogenate (10%) was prepared in KH2PO4 buffer (100 mM) containing EDTA (1 mM; PH 7.4) followed by centrifugation (12,000g) at 4 °C for half an hour. The collected supernatant was used for the determination of following parameters.
2.3.4.1. Catalase assay
Catalase (CAT) is commonly located in cellular organelles and peroxisomes. CAT undergoes the conversion of H2O2 into O2 and H2O. Different tumors have the reduced potential to detoxify hydrogen peroxide due to significant decrease in catalase levels. To determine CAT activity, the protocol of (Chance and Maehly, 1955) was followed. Briefly, 100 μL of supernatant was taken and 400 μL of 5.9 mM H2O2 and 2500 μL of 50 mM of phosphate buffer were added to it and mixed together. The alteration in absorbance of mixture was observed at 520 nm after 1 min, where one unit CAT activity meant change in absorbance of 0.01units/min.
2.3.4.2. Peroxidase assay
Protocol of Chance and Maehly, (1955) was utilized to investigate the activity of POD with slight modifications. The reaction mixture consisted of 40 mM of 300 μL H2O2, 20 mM of 100 μL of guaiacol, 25 mM of 2500 μL of phosphate buffer (pH 5) and 1000 μL of supernatant. Change in absorbance was recorded at 470 nm after 1 min interval. Change in absorbance of 0.01 units/min was equivalent to one unit POD activity.
2.3.4.3. Superoxide dismutase assay
Activity of SOD was determined by following the protocol of Kakkar et al., (1984). Reaction mixture of the assay consisted of 186 mM of 0.1 mL of phenazine methosulphate, 0.052 mM of 1.2 mL of sodium pyrophosphate buffer and 0.3 mL of supernatant. Centrifugation of the mixture was done at 1500g for 10 min and then once more centrifuged at 10,000g for 15 min. Enzymatic reaction in the mixture was started the addition of of 0.2 mL of 780 μM NADH. The optical density was determined at 560 nm and results were assessed in units/mg protein.
2.3.5. Biochemical parameter studies of kidney
The kidney tissue homogenates were analyzed for the assessment of total protein, GSH content, TBARS, H2O2, and nitrite quantification assays.
2.3.5.1. Total protein assessment
Estimation of total soluble protein within tissues was examined by following the protocol of Lowry et al. (1951). 80 mg of kidney portion was weighed and homogenized in potassium phosphate buffer. The reaction mixture was centrifuged at 10,000g for 15–20 min at 4 °C to obtain supernatant. The supernatant (0.1 mL) was incubated for 10 min and dissolved in 1 mL of alkaline copper solution. Similar proportion (1:1) Folin Ciocalteu Phenol reagent was added to the dissolved supernatant and mixed carefully by vortex machine. Incubation was done at room temperature for half an hour and absorbance was taken at 595 nm via spectrophotometer. The total solubilized protein was deliberated by standard curve of bovine serum albumin.
2.3.5.2. Reduced glutathione assay
Reduced glutathione (GSH) activity was evaluated using the methodology of Jollow et al., (1974) with some modifications. First 1000 μL of homogenate mixture was taken and dissolved in 1000 μL 0f 4% sulfosalicylic acid, then this solution was incubated at 4 °C for one hour followed by centrifugation at 4 °C for 20 min at 1200g. 100 μL of supernatant was poured in 2.7 mL of phosphate buffer (pH 7.4) and 200 μL of 100 mM DTNB was added into it. The reaction between GSH and DTNB produced yellow color indicating reduced glutathione. The optical density was measured via spectrophotometer at 412 nm.
2.3.5.3. Lipid peroxidation (TBARS) assay
Assessment of TBARS was done by adapting the protocol of Iqbal and Wright, (1996) with slight adjustments. The reaction cocktail comprised of 0.1 M of 580 μL of phosphate buffer (pH 7.4), 100 mM of 20 μL of ferric chloride, 100 mM of 200 μL ascorbate and 200 μL of homogenized sample. This mixture was incubated for 60 min in shaking water bath at 37 °C. Finally 10% of 1000 μL trichloroacetic acid (TCA) and 0.67% of 500 μL of thiobarbituric acid (TBA) were added. The reaction tubes were put in boiling water bath for 20 min and then stored in crushed ice bath for 5 min. Centrifugation was done at 1200g for 10 min to obtain supernatant and the amount of TBARS was quantified by taking absorbance of supernatant at 535 nm in comparison with blank reagent. Lipid peroxidation activity was determined as nM TBARS/min/mg at 37 °C by using molar extinction coefficient (1.56 × 105 M−1 cm-1).
2.3.5.4. Hydrogen peroxide (H2O2) assay
The activity of H2O2 was determined by following the protocol of Pick and Keisari, (1981). The oxidation of phenol red is stimulated by H2O2 mediated horseradish peroxidase enzyme. The reaction mixture contained 2 mL of tissue homogenate dissolved in 0.28 nM of 1 mL of phenol red, 5.5 nM dextrose, 8.5 units of horse radish peroxidase, and 0.05 mM phosphate buffer. The whole mixture was incubated for 60 min at optimum temperature (37 °C). After incubation 10 N of 10 μL NaOH was added to impede the reaction and centrifugation was done for 5 min at 800g. The supernatant was collected and optical density was measured at 610 nm against blank reagent. The production of H2O2 was measured in nM H2O2/min/mg tissue using oxidized phenol red as standard curve.
2.3.5.5. Nitrite quantification assay
Griess reagent was utilized for evaluation of nitrite assay by following the protocol of Grisham et al., (1996). The master mix comprised of 1 mL of tissue homogenate which was dissolved in 3 M of 100 μL NaOH and 5% ZnSO4 solution. The samples were centrifuged at 6400g for 15–20 min. The obtained supernatant (20 μL) was dissolved in 1 mL Griess reagent. Change in color was observed and absorbance was measured at 540 nm. For estimation of nitrite concentration in tissue samples, standard curve of sodium nitrite was used.
2.3.6. Serum and urine analyses studies of kidney
The level of serum markers (total protein, albumin, BUN, and creatinine) and urine analysis profile (PH, specific gravity, urea, creatinine, albumin, and urobilinogen) was determined following manufacturer’ guidelines mentioned on AMP diagnostic kits purchased from AMP Company.
2.3.6.1. Quantification of urea
Reagent-1 consisted of urease (140U/mL), biocides (1 2 0), 2-oxogluterate (10 mM/L), Tris buffer (125 mM/L) and GIDH (U/mL) whereas Reagent-2 consisted of NADH (1.5 mM/L). Briefly, 800 μL of Reagent-1 and 200 μL of Reagent-2 were dissolved in 10 μL of serum sample. After 1 to 2 min, the optical density was recorded at 340 nm. Distilled water was used as blank whereas 50 mg/dL urea served as standard. Urea was estimated by the following formula:
2.3.6.2. Quantification of creatinine
According to the AMP diagnostic kit procedure, 1 mL of working reagent solution was mixed with 0.1 mL of sample/standard and the absorbance was recorded immediately after 25 s at 500 nm as an initial reading. The second absorbance reading was noted after 2 min and creatinine was estimated using the following equation:
2.3.6.3. Quantification of albumin
Colorimetric method was performed to estimate serum albumin by utilizing bromocresol green (BCG) at pH (4.20). According to AMP company kit procedure, the reaction mixture consisted of 10 μL sample added to 1 mL of reagent having Brij 35, succinate buffer reagent and bromocresol green. The absorbance was measured at 625 nm after 5 min of incubation. Distilled H2O was used as blank and concentration of serum albumin was evaluated by using the formula:
2.4. Comet assay
The extent of DNA damages were assessed using the procedure of Dhawan et al., (2009) with some amendments. The slides were sterilized and dipped in hot normal melting-point agar (NMPA; 1%) followed by solidification at room temperature. A small section of renal tissue was put in cold lysing solution (1 mL), minced into small pieces, and mixed with low melting-point agarose (LMPA; 75 µL). The mixture formed was coated onto the already coated slides and a cover-slip was carefully placed over it. This slide was positioned on ice-packs for 8–12 min after which the cover-slip was removed and LMPA was again added followed by solidification on ice-packs. After successful coating with LMPA, the slide was put in lysing solution for 10 min and eventually placed in refrigerator for 02 h. Electrophoresis was performed before staining the slide with ethidium bromide (1%) for visualization under fluorescent microscope. An image analysis software; CASP-1.2.3.b was used to determine the amount of DNA damage. 50–100 cells in each test sample were analyzed for calculating DNA content in head and tail of renal cells nuclei, tail moment, tail length, head length, and comet length.
2.5. Histopathological analysis
For assessing the histopathology of kidney, paraffin-fixed stained slices were used. The multi-step methodology was used to fix testing samples, avert tissue decay, and conserve the morphology of tissues. For this purpose, the kidney sections preserved in 10% formalin were used. Variable concentrations of alcohol in ascending order i.e. 50%, 70%, 90% and 100% were applied to wash the fixed tissues. The main objective of this step was to fix the tissue on rigid solid matrix which makes it easy to cut thin slices of tissues. The final step was the formation of slides by making 3–4 thin layers of tissue samples followed by hematoxylin and eosin staining. Light microscope was used for slide examination at 40X and HDCE-50B was used for photography.
2.6. Statistical analysis
The results of all assays were communicated as average ± standard deviation. Graphpad-prism-5 software calculated inhibition percentages. Statistix-8.1software derived sample differences between different treatment groups at p ≤ 0.05 by applying one-way ANOVA and Tukey’s-HSD multi-comparison tests. CASP-1.2.3.b software was used to determine the amount of DNA damage in comet assay.
3. Results
3.1. Protective effects of PJM and PHM on nephro-toxicity
3.1.1. Effect on anti-oxidant enzymes of kidney
CCl4 treatment generated ROS which considerably declined the activity of antioxidant enzymes of kidney. Table 1, Table 6 displays a significant decrease (p < 0.05) in enzyme levels of SOD, CAT, and POD after CCl4 treatment relative to control group, while the treatment with PJM, PHM and silymarin markedly reduced (p < 0.05) the toxicity caused by CCl4 by restoring the renal level of SOD (4.49 ± 0.30, 4.43 ± 0.33 and 4.68 ± 0.41 U/milligram), CAT (4.53 ± 0.29, 4.61 ± 0.61 and 4.64 ± 0.53 U/minute), and POD (8.11 ± 0.57, 8.05 ± 0.58 and 8.56 ± 0.30 U/minute) towards normal, similar to control rat group (5.23 ± 0.31 U/milligram, 6.06 ± 0.48 U/minute, and 10.61 ± 0.60 U/minute). After conducting multiple comparison of various treatment groups under study, it was observed that PJM and PHM (200 mg/kg) was least effective compared to control group whereas it’s higher dose; PJM/PHM (400 mg/kg) and reference drug; silymarin successfully restored all enzymatic levels and hence the antioxidant enzyme activity. However, when PJM and PHM was treated (200 and 400 mg/kg) alone, no alteration was caused in the above parameters, similar to control group (Table 1, Table 6).
Table 1.
Effect of PJM on kidney antioxidant enzymes:
| Treatment | CAT (U/min) | POD (U/min) | SOD (U/mg protein) |
|---|---|---|---|
| Control | 6.06 ± 0.48ab | 10.6 ± 0.60a | 5.23 ± 0.31ab |
| Control (vehicle) | 5.79 ± 0.28b | 10.2 ± 0.54a | 5.05 ± 0.45abc |
| CCl4 (1 mL/kg) | 1.53 ± 0.42e | 2.37 ± 0.49d | 1.36 ± 0.24e |
| CCl4 + Silymarin | 4.64 ± 0.53c | 8.56 ± 0.30b | 4.68 ± 0.41bc |
| CCl4 + PJM (200 mg/kg) | 3.43 ± 0.40d | 6.39 ± 0.25c | 3.60 ± 0.32d |
| CCl4 + PJM (400 mg/kg) | 4.53 ± 0.29c | 8.11 ± 0.57b | 4.49 ± 0.30c |
| PJM (200 mg/kg) | 5.64 ± 0.65b | 9.97 ± 0.74a | 5.01 ± 0.31abc |
| PJM (400 mg/kg) | 6.62 ± 0.26a | 10.4 ± 0.38a | 5.45 ± 0.35a |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PJM: Parrotiopsis jacquemontiana methanol fraction, CCl4: Carbon tetra-chloride.
Table 6.
Effect of PHM on kidney antioxidant enzymes:
| Treatment | CAT (U/min) | POD (U/min) | SOD (U/mg protein) |
|---|---|---|---|
| Control | 6.06 ± 0.48a | 10.6 ± 0.60a | 5.23 ± 0.31ab |
| Control (vehicle) | 5.79 ± 0.28a | 10.2 ± 0.54a | 5.05 ± 0.45abc |
| CCl4 (1 mL/kg) | 1.53 ± 0.42d | 2.37 ± 0.49d | 1.36 ± 0.24e |
| CCl4 + Silymarin | 4.64 ± 0.53b | 8.56 ± 0.30b | 4.68 ± 0.41bc |
| CCl4 + PHM (200 mg/kg) | 3.57 ± 0.13c | 6.12 ± 0.52c | 3.48 ± 0.18d |
| CCl4 + PHM (400 mg/kg) | 4.61 ± 0.61b | 8.05 ± 0.58b | 4.43 ± 0.33c |
| PHM (200 mg/kg) | 6.09 ± 0.54a | 10.0 ± 0.87a | 5.31 ± 0.57ab |
| PHM (400 mg/kg) | 6.15 ± 0.43a | 10.4 ± 0.53a | 5.42 ± 0.40a |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PHM: Periploca hydaspidis methanol fraction, CCl4: Carbon tetra-chloride.
3.1.2. Effect on biochemical markers of kidney
The ameliorative effect of PJM and PHM on biochemical markers of kidney after CCl4-induced injury is represented in Table 2, Table 7. CCl4 injected rat group showed a significant increase (p < 0.05) in level of TBARS, H2O2, and nitrite whereas a decrease (p < 0.05) in total protein and GSH level relative to control rat group. Co-treatment of PJM (200 and 400 mg/kg) markedly reduced the levels of TBARS (41.1 ± 2.11 and 33.8 ± 1.84 nM/minute/mg proteins), H2O2 (12.7 ± 0.50 and 10.8 ± 0.48 nM/minute/mg tissue), and nitrite (91.9 ± 2.17 and 75.3 ± 2.16 μM/mL) compared to CCl4-treated group (49.8 ± 1.73 nM/minute/mg proteins, 16.6 ± 0.99 nM/minute/mg tissue, and 110.5 ± 2.57 μM/mL). Similar results were shown by the co-administration of PHM (200 and 400 mg/kg) which evidently decreased the quantity of TBARS (38.1 ± 0.93 and 32.6 ± 1.22 nM/minute/mg proteins), H2O2 (11.7 ± 0.62 and 10.3 ± 0.59 nM/minute/mg tissue), and nitrite (89.9 ± 2.16 and 72.1 ± 1.44 μM/mL) relative to CCl4 group, respectively. The content of total protein (6.61 ± 0.41/6.91 ± 0.53 and 7.28 ± 0.52 μg/mg tissue) and GSH (22.7 ± 1.12/23.4 ± 0.79 and 27.3 ± 1.46 nM/minute/mg tissue) in kidney tissue was also elevated substantially (p < 0.05) upon co-administration of PJM/PHM and silymarin with CCl4 relative to CCl4 only treated group. After conducting multiple comparisons of various treatment groups under study, it was observed that PJM and PHM (400 mg/kg) had a restorative potential on the content of GSH, TBARS, H2O2, nitrite, and total protein which was found to be statistically similar to silymarin (standard drug). However, when PJM/PHM was treated (200 and 400 mg/kg) alone, then no alteration (p < 0.05) was caused in the aforementioned renal parameters, similar to control group (Table 2, Table 7).
Table 2.
Effect of PJM on kidney biochemical parameters:
| Treatment | Protein (µg/mg tissue) | GSH (nM/min/mg protein) | TBARS (nM/min/mg protein) | H2O2 (nM/min /mg tissue) | Nitrite (µM/ml) |
|---|---|---|---|---|---|
| Control | 8.52 ± 0.36a | 28.4 ± 0.93c | 25.5 ± 1.04d | 6.11 ± 0.33fg | 58.8 ± 1.13e |
| Control (vehicle) | 8.21 ± 0.42a | 30.7 ± 0.98b | 26.6 ± 0.94d | 6.04 ± 0.17g | 59.0 ± 2.18e |
| CCl4 (1 mL/kg) | 2.38 ± 0.38e | 11.6 ± 1.18f | 49.8 ± 1.73a | 16.6 ± 0.99a | 110.5 ± 2.57a |
| CCl4 + Silymarin | 7.28 ± 0.52b | 27.3 ± 1.46c | 34.5 ± 1.16c | 8.63 ± 0.32d | 71.3 ± 1.71d |
| CCl4 + PJM (200 mg/kg) | 5.34 ± 0.33d | 16.5 ± 1.11e | 41.1 ± 2.11b | 12.7 ± 0.50b | 91.9 ± 2.17b |
| CCl4 + PJM (400 mg/kg) | 6.61 ± 0.41c | 22.7 ± 1.12d | 33.8 ± 1.84c | 10.8 ± 0.48c | 75.3 ± 2.16c |
| PJM (200 mg/kg) | 8.14 ± 0.17a | 31.6 ± 1.04ab | 25.0 ± 2.05d | 7.17 ± 0.34e | 59.7 ± 1.30e |
| PJM (400 mg/kg) | 8.49 ± 0.40a | 32.6 ± 0.72a | 24.5 ± 2.01d | 6.87 ± 0.29ef | 58.3 ± 1.06e |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PJM: Parrotiopsis jacquemontiana methanol fraction, CCl4: Carbon tetra-chloride.
Table 7.
Effect of PHM on kidney biochemical parameters:
| Treatment | Protein (µg/mg tissue) | GSH (nM/min/mg protein) | TBARS (nM/min/mg protein) | H2O2 (nM/min /mg tissue) | Nitrite (µM/ml) |
|---|---|---|---|---|---|
| Control | 8.52 ± 0.36a | 28.4 ± 0.93b | 25.5 ± 1.04e | 6.11 ± 0.33e | 58.8 ± 1.13d |
| Control (vehicle) | 8.21 ± 0.42a | 30.7 ± 0.98a | 26.6 ± 0.94de | 6.04 ± 0.17e | 59.0 ± 2.1d |
| CCl4 (1 mL/kg) | 2.38 ± 0.38d | 11.6 ± 1.18e | 49.8 ± 1.73a | 16.6 ± 0.99a | 110.5 ± 2.57a |
| CCl4 + Silymarin | 7.28 ± 0.52b | 27.3 ± 1.46b | 34.5 ± 1.16c | 8.63 ± 0.32d | 71.3 ± 1.71c |
| CCl4 + PHM (200 mg/kg) | 6.13 ± 0.50c | 19.3 ± 0.74d | 38.1 ± 0.93b | 11.7 ± 0.62b | 89.9 ± 2.16b |
| CCl4 + PHM (400 mg/kg) | 6.91 ± 0.53bc | 23.4 ± 0.79c | 32.6 ± 1.22c | 10.3 ± 0.59c | 72.1 ± 1.44c |
| PHM (200 mg/kg) | 8.63 ± 0.61a | 30.8 ± 1.27a | 27.9 ± 1.63d | 6.77 ± 0.49e | 60.6 ± 2.24d |
| PHM (400 mg/kg) | 8.95 ± 0.56a | 31.1 ± 2.02a | 26.2 ± 1.44de | 6.22 ± 0.34e | 59.7 ± 3.04d |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PHM: Periploca hydaspidis methanol fraction, CCl4: Carbon tetra-chloride
3.1.3. Effect on serum markers of kidney
The ameliorative effect of PJM and PHM on renal serum profile is summarized in Table 3, Table 8. Following CCl4 treatment, the rats demonstrated elevated levels of BUN (68.4 ± 2.04 mg/dl) and serum creatinine (8.19 ± 0.70 mg/dl) while reduced levels of total protein (47.2 ± 2.09 mg/dl) and serum albumin (9.93 ± 0.60 mg/dl) compared to control group (43.5 ± 1.77, 3.70 ± 0.64, 98.3 ± 2.18, and 22.0 ± 0.67 mg/dl), respectively. Co-administration of PJM and PHM (400 mg/kg) reduced CCl4-induced nephrotoxicity after reverting the aforementioned parameters back to normal and exhibited close values to control group, conforming to BUN (52.0 ± 2.14, and 54.5 ± 2.06 mg/dl), serum creatinine (5.24 ± 0.19, and 5.19 ± 0.14 mg/dl), total protein (88.3 ± 1.87, and 17.7 ± 0.65 mg/dl), and serum albumin (16.5 ± 0.78, and 86.8 ± 3.19 mg/dl). However, when PJM/PHM was treated (200 and 400 mg/kg) alone, then no alteration (p < 0.05) was caused in above serum parameters, similar to control group (Table 3, Table 8).
Table 3.
Effect of PJM on serum parameters of kidney:
| Treatment | Protein mg/dl | Albumin mg/dl | BUN mg/dl | Creatinine mg/dl |
|---|---|---|---|---|
| Control | 98.3 ± 2.18a | 22.0 ± 0.67ab | 43.5 ± 1.77d | 3.70 ± 0.64cd |
| Control (vehicle) | 96.4 ± 3.00a | 21.8 ± 0.93b | 44.1 ± 2.37d | 4.35 ± 0.27cd |
| CCl4 (1 mL/kg) | 47.2 ± 2.09d | 9.93 ± 0.60f | 68.4 ± 2.04a | 8.19 ± 0.70a |
| CCl4 + Silymarin | 85.0 ± 1.82b | 18.7 ± 0.72c | 49.9 ± 2.79c | 4.96 ± 0.67bc |
| CCl4 + PJM (200 mg/kg) | 71.7 ± 2.17c | 13.5 ± 0.65e | 57.5 ± 3.17b | 5.55 ± 0.35b |
| CCl4 + PJM (400 mg/kg) | 88.3 ± 1.87b | 16.5 ± 0.78d | 52.0 ± 2.14c | 5.24 ± 0.19b |
| PJM (200 mg/kg) | 98.4 ± 2.42a | 22.6 ± 0.80ab | 43.9 ± 2.10d | 4.34 ± 0.22 cd |
| PJM (400 mg/kg) | 99.9 ± 2.30a | 23.2 ± 0.76a | 42.8 ± 1.60d | 4.21 ± 0.30 cd |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PJM: Parrotiopsis jacquemontiana methanol fraction, CCl4: Carbon tetra-chloride.
Table 8.
Effect of PHM on serum parameters of kidney:
| Treatment | Protein mg/dl | Albumin mg/dl | BUN mg/dl | Creatinine mg/dl |
|---|---|---|---|---|
| Control | 98.3 ± 2.18a | 22.0 ± 0.67a | 43.5 ± 1.77e | 3.70 ± 0.64d |
| Control (vehicle) | 96.4 ± 3.00a | 21.8 ± 0.93a | 44.1 ± 2.37e | 4.35 ± 0.27 cd |
| CCl4 (1 mL/kg) | 47.2 ± 2.09d | 9.93 ± 0.60d | 68.4 ± 2.04a | 8.19 ± 0.70a |
| CCl4 + Silymarin | 85.0 ± 1.82b | 18.7 ± 0.72b | 49.9 ± 2.79d | 4.96 ± 0.67bc |
| CCl4 + PHM (200 mg/kg) | 69.7 ± 2.33c | 15.5 ± 0.69c | 60.1 ± 1.89b | 5.36 ± 0.19b |
| CCl4 + PHM (400 mg/kg) | 86.8 ± 3.19b | 17.7 ± 0.65b | 54.6 ± 2.06c | 5.19 ± 0.14b |
| PHM (200 mg/kg) | 99.7 ± 2.76a | 21.6 ± 0.79a | 45.9 ± 1.36e | 4.41 ± 0.15 cd |
| PHM (400 mg/kg) | 100.5 ± 2.15a | 22.7 ± 0.82a | 44.6 ± 2.15e | 4.09 ± 0.18d |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PHM: Periploca hydaspidis methanol fraction, CCl4: Carbon tetra-chloride.
3.1.4. Effect on urine profile of kidney
PJM and PHM presented a protective effect on urine profile of kidney as presented in Table 4, Table 9. CCl4 injected group showed reduced level of urinary albumin (4.01 ± 0.76 mg/dl) and PH (6.41 ± 0.38) whereas an increased level of urea (92.9 ± 1.54 mg/dl), specific gravity (1.38 ± 0.09), urobilinogen (8.18 ± 0.74 mg/dl) and urinary creatinine (5.76 ± 0.25 mg/dl) relative to control rat group, respectively. Co-treatment with PJM and PHM (400 mg/kg) reversed CCl4-induced nephro-toxic effects by significantly (p < 0.05) elevating urinary albumin (6.95 ± 0.52, and 7.28 ± 0.65 mg/dl) and PH (7.05 ± 0.02, and 6.91 ± 0.31) and decreasing urea (74.7 ± 1.99, and 72.3 ± 2.10 mg/dl), specific gravity (1.16 ± 0.04, and 1.17 ± 0.07), urobilinogen (5.17 ± 0.24, and 5.28 ± 0.40 mg/dl), and urinary creatinine (3.29 ± 0.16, and 3.15 ± 0.36 mg/dl), similar to control rat group. Treatment with PJM/PHM (200 and 400 mg/kg) alone caused no significant change (p > 0.05) in aforementioned urine parameters, unlike CCl4 treated group (Table 4, Table 9).
Table 4.
Effect of PJM on urine profile of kidney:
| Treatment | PH | Specific gravity | Urea mg/mL | Creatinine mg/mL | Albumin mg/mL | Urobilinogen mg/mL |
|---|---|---|---|---|---|---|
| Control | 7.25 ± 0.15ab | 1.04 ± 0.05d | 65.6 ± 1.34e | 2.81 ± 0.11d | 8.48 ± 0.33a | 3.85 ± 0.58d |
| Control (vehicle) | 7.34 ± 0.27a | 1.01 ± 0.06d | 66.2 ± 1.71e | 2.83 ± 0.10d | 8.22 ± 0.59a | 4.17 ± 0.19cd |
| CCl4 (1 mL/kg) | 6.41 ± 0.38d | 1.38 ± 0.09a | 92.9 ± 1.54a | 5.76 ± 0.25a | 4.01 ± 0.76d | 8.18 ± 0.74a |
| CCl4 + Silymarin | 6.98 ± 0.04bc | 1.10 ± 0.05cd | 69.6 ± 2.24d | 3.01 ± 0.40cd | 7.55 ± 0.55ab | 4.87 ± 0.72bc |
| CCl4 + PJM (200 mg/kg) | 6.70 ± 0.06cd | 1.27 ± 0.06b | 79.0 ± 1.20b | 3.76 ± 0.28b | 6.43 ± 0.56c | 5.60 ± 0.45b |
| CCl4 + PJM (400 mg/kg) | 7.05 ± 0.02ab | 1.16 ± 0.04c | 74.7 ± 1.99c | 3.29 ± 0.16c | 6.95 ± 0.52bc | 5.17 ± 0.24b |
| PJM (200 mg/kg) | 7.11 ± 0.05ab | 1.07 ± 0.03cd | 65.2 ± 1.64e | 2.82 ± 0.11d | 8.31 ± 0.70a | 4.02 ± 0.29d |
| PJM (400 mg/kg) | 7.29 ± 0.18ab | 1.06 ± 0.01d | 64.4 ± 1.84e | 2.81 ± 0.18d | 8.36 ± 0.11a | 3.93 ± 0.19d |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PJM: Parrotiopsis jacquemontiana methanol fraction, CCl4: Carbon tetra-chloride.
Table 9.
Effect of PHM on urine profile of kidney:
| Treatment | pH | Specific gravity | Urea mg/dl | Creatinine mg/dl | Albumin mg/dl | Urobilinogen mg/dl |
|---|---|---|---|---|---|---|
| Control | 7.25 ± 0.15ab | 1.04 ± 0.05d | 65.6 ± 1.34e | 2.81 ± 0.11c | 8.48 ± 0.33abc | 3.85 ± 0.58e |
| Control (vehicle) | 7.34 ± 0.27a | 1.01 ± 0.06d | 66.2 ± 1.71e | 2.83 ± 0.10c | 8.22 ± 0.59bcd | 4.17 ± 0.19de |
| CCl4 (1 mL/kg) | 6.41 ± 0.38d | 1.38 ± 0.09a | 92.9 ± 1.54a | 5.76 ± 0.25a | 4.01 ± 0.76f | 8.18 ± 0.74a |
| CCl4 + Silymarin | 6.98 ± 0.04abc | 1.10 ± 0.05 cd | 69.6 ± 2.24 cd | 3.01 ± 0.40bc | 7.55 ± 0.55cde | 4.87 ± 0.72 cd |
| CCl4 + PHM (200 mg/kg) | 6.68 ± 0.08 cd | 1.24 ± 0.05b | 75.8 ± 2.31b | 3.46 ± 0.37b | 6.76 ± 0.77e | 5.76 ± 0.45b |
| CCl4 + PHM (400 mg/kg) | 6.91 ± 0.31bc | 1.17 ± 0.07bc | 72.3 ± 2.10c | 3.15 ± 0.36bc | 7.28 ± 0.65de | 5.28 ± 0.40bc |
| PHM (200 mg/kg) | 7.14 ± 0.32ab | 1.09 ± 0.02 cd | 66.4 ± 1.77e | 2.90 ± 0.40c | 8.73 ± 0.27ab | 4.35 ± 0.31de |
| PHM (400 mg/kg) | 7.19 ± 0.11ab | 1.08 ± 0.02 cd | 67.1 ± 1.32de | 2.88 ± 0.31c | 9.19 ± 0.15a | 4.11 ± 0.15de |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PHM: Periploca hydaspidis methanol fraction, CCl4: Carbon tetra-chloride.
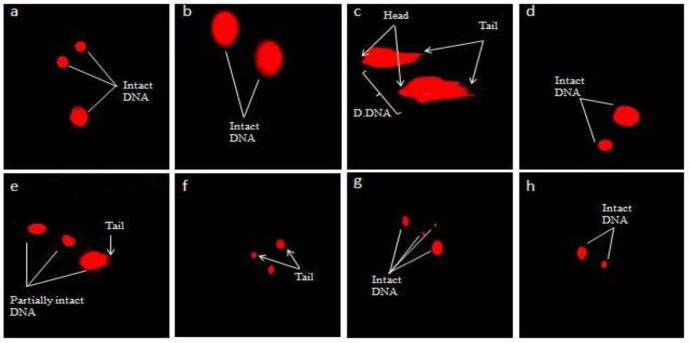
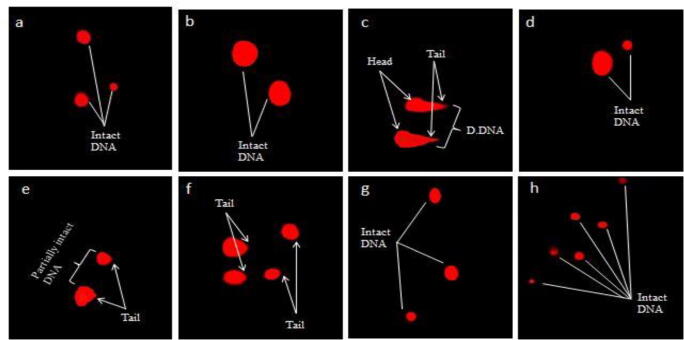
3.1.5. Effect on comet parameters
Table 5, Table 10 and Figs. (1 and 3) display the ameliorative effect of PJM and PHM on comet parameters post CCl4-induced toxicity in renal cells of rats. CCl4-treated group revealed a marked increase (p < 0.05) in tail moment, tail length, comet length, and percent DNA in tail relative to control group whereas a considerable decrease (p < 0.05) in head length and percent DNA of head compared to control group. The extent of DNA damage was evident in renal cells of CCl4-administered group rats (Figs. 1c and 3c). The total amount of DNA migrated from head of comet towards the tail in CCl4-treated group was 30.6% whereas PJM and PHM administration (400 mg/kg) reduced the DNA migration to 2.35 and 5.45%, respectively. Although the least quantity of migrated DNA was recorded for silymarin treated rat group (0.98%) compared to control group yet, PJM and PHM administration (200 and 400 mg/kg) lessened the toxic effects induced by CCl4 and retreated all altered parameters back to normal, resembling control group. After conducting multiple comparisons between various treatment groups, the higher dose of PJM/PHM (400 mg/kg) was directed to significantly (p < 0.05) restore all the comet parameters (Table 5, Table 10) and display protective effect (Figs. 1f and 3f), similar to silymarin administered group (Figs. 1d and 3d). Treatment with PJM/PHM (200 and 400 mg/kg) alone caused no significant alteration (p > 0.05) in comet parameters, similar to control group.
Table 5.
Effect of PJM on DNA damages of kidney cells employing comet assay:
| Treatment | Comet length (µm) | Head length (µm) | Tail length (µm) | % DNA in head | % DNA in tail | Tail moment |
|---|---|---|---|---|---|---|
| Control | 45.2 ± 2.21cd | 36.4 ± 1.36a | 8.72 ± 1.47d | 91.1 ± 1.96a | 9.95 ± 1.25c | 0.86 ± 0.09d |
| Control (vehicle) | 44.5 ± 2.56d | 35.5 ± 2.20ab | 8.97 ± 2.28d | 90.3 ± 2.42a | 10.6 ± 1.30c | 0.95 ± 0.11d |
| CCl4 (1 mL/kg) | 64.2 ± 2.25a | 22.4 ± 1.98d | 41.8 ± 2.94a | 75.3 ± 1.82c | 24.9 ± 2.17a | 10.4 ± 0.47a |
| CCl4 + Silymarin | 46.2 ± 2.44cd | 36.3 ± 1.93a | 9.85 ± 1.95d | 90.13 ± 2.10a | 9.70 ± 1.69c | 0.95 ± 0.15d |
| CCl4 + PJM (2 0 0) | 59.1 ± 2.57b | 27.6 ± 1.69c | 31.5 ± 1.72b | 85.8 ± 1.56b | 14.6 ± 1.44b | 4.61 ± 0.43b |
| CCl4 + PJM (4 0 0) | 49.0 ± 3.04c | 32.9 ± 1.62b | 16.1 ± 1.77c | 88.2 ± 1.49ab | 12.3 ± 1.63bc | 1.98 ± 0.16c |
| PJM (2 0 0) | 45.6 ± 2.36cd | 36.3 ± 2.37a | 9.26 ± 1.26d | 90.6 ± 1.50a | 10.7 ± 1.81c | 0.99 ± 0.14d |
| PJM (4 0 0) | 45.2 ± 2.43cd | 36.8 ± 1.34a | 8.43 ± 1.20d | 91.2 ± 1.68a | 9.67 ± 1.79c | 0.81 ± 0.28d |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PJM: Parrotiopsis jacquemontiana methanol fraction, CCl4: Carbon tetra-chloride.
Table 10.
Effect of PHM on DNA damages of kidney cells employing comet assay:
| Treatment | Comet length (µm) | Head length (µm) | Tail length (µm) | % DNA in head | % DNA in tail | Tail moment |
|---|---|---|---|---|---|---|
| Control | 46.4 ± 1.39d | 42.7 ± 1.69a | 3.74 ± 0.58d | 95.7 ± 1.33a | 4.67 ± 0.49d | 0.17 ± 0.09d |
| Control (vehicle) | 45.9 ± 2.17d | 42.0 ± 2.41a | 3.95 ± 0.79d | 95.5 ± 1.73a | 5.61 ± 0.71d | 0.22 ± 0.11d |
| CCl4 (1 mL/kg) | 73.4 ± 2.37a | 23.6 ± 1.67c | 49.8 ± 1.50a | 64.6 ± 1.02d | 35.3 ± 2.25a | 17.5 ± 0.50a |
| CCl4 + Silymarin | 45.4 ± 1.65d | 40.5 ± 1.62a | 4.91 ± 0.63d | 94.7 ± 2.05a | 5.65 ± 1.00d | 0.27 ± 0.12d |
| CCl4 + PHM (2 0 0) | 61.2 ± 2.69b | 33.9 ± 2.14b | 27.2 ± 1.53b | 81.2 ± 2.10c | 19.7 ± 1.70b | 5.38 ± 0.53b |
| CCl4 + PHM (4 0 0) | 51.4 ± 2.11c | 39.6 ± 1.93a | 11.8 ± 0.82c | 89.8 ± 2.24b | 10.12 ± 0.84c | 1.20 ± 0.30c |
| PHM (2 0 0) | 47.0 ± 1.27d | 42.3 ± 1.33a | 4.68 ± 0.67d | 94.5 ± 1.55a | 6.04 ± 0.47d | 0.28 ± 0.10d |
| PHM (4 0 0) | 46.2 ± 1.47d | 42.3 ± 1.84a | 3.87 ± 0.38d | 95.6 ± 2.01a | 4.83 ± 0.43d | 0.18 ± 0.07d |
Mean ± SD (n = 7) having different superscript letters within a column denote significance at
p < 0.05. PHM: Periploca hydaspidis methanol fraction, CCl4: Carbon tetra-chloride.
Fig. 1.
Fluorescence micrograph of kidney cells and protective potential of PJM on genotoxicity (a) Control group (b); Vehicle control (c) CCl4 (1 mL/kg bw) (d) CCl4 + Silymarin (e) CCl4 + PJM (200 mg/kg) (f) CCl4 + PJM (400 mg/kg) (g) PJM (200 mg/kg) (h) PJM (400 mg/kg). PJM: P.jacquemontiana methanol extract, D.DNA: Damaged DNA.
Fig. 3.
Fluorescence micrograph of kidney cells and protective effect of PHM on genotoxicity (a) Control group (b) Vehicle control (c) CCl4 (1 mL/kg bw) (d) CCl4 + Silymarin (e) CCl4 + PHM (200 mg/kg) (f) CCl4 + PHM (400 mg/kg) (g) PHM (200 mg/kg) (h) PHM (400 mg/kg). PHM: P. hydaspidis methanol extract, D.DNA: Damaged DNA.
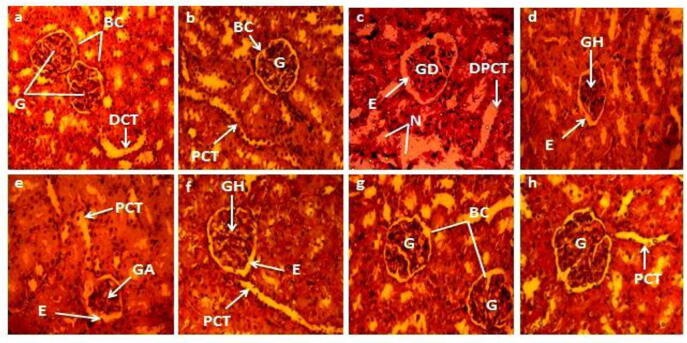
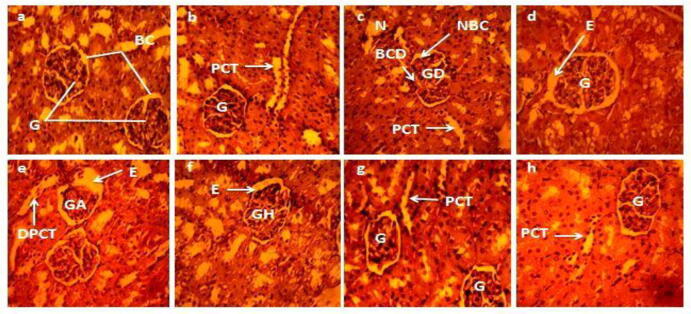
3.1.6. Effect on histo-architecture of kidney
Histopathological alterations in the architecture of renal tissues are presented in Fig. 2, Fig. 4. The control and vehicle group of rats displayed normal morphology and histo-architecture of renal tissues (Figs. 2a,b and 4a,b) with prominent glomeruli, Bowman’s capsule, distal and proximal convoluted tubules etc. whereas severe pathological abnormalities were observed in CCl4 administered group i.e. glomeruli disruption with impaired corticular sections, accumulation of necrotic cells, distortion of Bowman’s capsule and the narrowing of space between it (Figs. 2c and 4c). However, these renal damages were successfully ameliorated by the co-administration of PJM (400 mg/kg) (Fig. 2f), PHM (400 mg/kg) (Fig. 4f), and silymarin (Figs. 2d and 4d) with CCl4 in such a way that its histo-architecture resembled the control group. Though all co-treated groups showed slight edema but glomerular hypertrophy was obvious in PJM and PHM high-dose administered groups only (Figs. 2f and 4f). On the other hand, both PJM and PHM treated low doses (200 mg/kg) groups exhibited edema and glomerular atrophy as presented in Figs. 2e and 4e. PJM/PHM treatment alone (200 and 400 mg/kg) did not instigate any histopathological changes in renal tissues (Figs. 2g,h and 4g,h) and resembled the control group.
Fig. 2.
Protective potential of PJM on renal histopathology of rats (40X magnification using hematoxylin-eosin stain). (a) Control group (b) Vehicle control (c) CCl4 (1 mL/kg bw) (d) CCl4 + Silymarin (e) CCl4 + PJM (200 mg/kg) (f) CCl4 + PJM (400 mg/kg) (g) PJM (200 mg/kg) (h) PJM (400 mg/kg). BC: Bowman’s capsule, G: Glomerulus, PCT: Proximal convoluted tubule, DCT: Distal convoluted tubule, N: Necrosis, GD: Glomerular disruption, E: Edema, DPCT: Dilated proximal convoluted tubule, GH: Glomerular hypertrophy, GA: Glomerular atrophy, PJM: P.jacquemontiana methanol extract.
Fig. 4.
Protective potential of PHM on renal histopathology of rats (40X magnification using hematoxylin-eosin stain). (a) Control group (b) Vehicle control (c) CCl4 (1 mL/kg bw) (d) CCl4 + Silymarin (e) CCl4 + PHM (200 mg/kg) (f) CCl4 + PHM (400 mg/kg) (g) PHM (200 mg/kg) (h) PHM (400 mg/kg). BC: Bowman’s capsule, G: Glomerulus, PCT: Proximal convoluted tubule, N: Necrosis, GD: Glomerular disruption, BCD: Bowman’s capsule disruption, NBC: Narrowing of Bowman’s capsule, E: Edema, DPCT: Dilated proximal convoluted tubule, GH: Glomerular hypertrophy, GA: Glomerular atrophy, PJM: P.hydaspidis methanol extract.
4. Discussion
As herbal plants have been used since ancient times for the management of human ailments without having adverse effects on the body/living organs and keeping in mind the current treatments available in developed and underdeveloped countries which are highly costly, time-consuming, and with serious side-effects (Batool et al., 2019, Batool et al., 2020, Zahra et al., 2021a, Zahra et al., 2021b). The present study demonstrates the protective potential of P. jacquemontiana and P. hydaspidis on CCl4-intoxicated kidney cells of male experimental rats for the first time.
The toxicity of CCl4 to kidneys depends upon inflammatory processes, free-radical generation, and oxidative stress. Single-dose administration of CCl4 can cause oxidative stress and LPO resulting in cellular damage and leakage of enzymes to blood-stream (El-Haskoury et al., 2018). Keeping in view the high sensitivity of kidney to CCl4, the formation of less urine by kidneys may cause H2O and waste-products to build-up in the blood and body. CCl4 administration increases ROS levels and leads to oxidative stress which in turn decreases antioxidant enzyme levels (Alm-Eldeen et al., 2016, Zangeneh et al., 2018). Our study showed declined levels of POD, SOD, GSH, and CAT in CCl4-injected group while the co-treatment of PJM/PHM, particularly high dose (400 mg/kg) with CCl4 markedly (p < 0.05) elevated the levels of antioxidant enzymes, similar to silymarin- treated and control rat group. This coincides with the research conducted by Safhi, 2018, who demonstrated a significant (p > 0.001) increase in antioxidant (POD, SOD, GSH, GPX, GR, and CAT) enzymes after co-treatment of Zingerone (100 mg/kg) with CCl4 relative to CCl4-treated group only. Under reduced levels of CAT, SOD, and GSH oxidative stress may take place. Diminished GSH content causes impairment in H2O2 clearance and promotes formation of hydroxyl radicals. Reduced SOD levels are unable to catalyze dismutation of superoxide radicals while reduced levels of CAT are unable to reduce H2O2 → H2O and O2, hence being incapable of preventing cell damage induced via oxidative stress (Safhi, 2018).
Xenobiotic consumption elevates lipid peroxidation (TBARS) and leads to overproduction of superoxide that causes dismutation of H2O2 and singlet O2 which can be readily converted into highly reactive hydroxyl (OH˙) radicals. This singlet O2 and OH˙ radicals have a high potential in initiating long-chain reactions of free-radicals linked to LPO (Suzek et al., 2017). In current study, an increased level of TBARS, nitrite, and H2O2 was witnessed in CCl4-injected rats. Earlier studies have reported a marked elevation in LPO causing membranous damage and many pathological alterations comprising acute and chronic renal damages (Alm-Eldeen et al., 2016). The pathologies were reversed by treatment with PJM and PHM (400 mg/kg) fractions which is in accordance with the findings of Karakuş et al., 2017, who stated the extracts of Taraxacum officinale and Silybum marianum to overcome oxidative stress via increasing antioxidant enzyme levels. Likewise, the nephroprotective potential of PJM and PHM may be accredited to an increase in antioxidant enzymatic status which in turn overpowers the oxidative stress post CCl4 damage (Khan et al., 2010, Zangeneh et al., 2018).
The current study presented increased concentrations of BUN and creatinine while decreased concentrations of albumin and proteins in serum of CCl4-intoxicated rats. Normally, creatinine and urea are measured for assessing kidney function and protein metabolism whereby its increased level in blood is deliberated as a marker for kidney impairment (Usman et al., 2020). CCl4-administered group revealed high creatinine levels, which is considered an investigative marker for cell-membrane injury and cellular leakage in kidney tissues (Mika and Guruvayoorappan, 2013). A recent study reported similar results demonstrating elevated level of uric acid and creatinine as indices of nephro-toxicity from the fact that they are final products of nitrogenous base (purine) and can modify glomerular filtration-rate. Any alteration occurring in glomerular filtration-rate elevates uric acid and serum creatinine levels which are associated with kidney damage (Ullah et al., 2013).
The physiological integrity and functional condition of kidney is also shown by urine analysis as reported in earlier studies, where malfunctioned kidneys demonstrated distressed urine profiles. The detection of urobilinogen, RBC’s, and acidic pH in urine profile with increased level of urinary creatinine, WBC’s, and urea whereas declined levels of albumin and urinary protein indicated serious renal damage (Sahreen et al., 2015). The obtained data exhibited the manifestation of aforementioned parameters in urine analysis of CCl4-treated rat group, henceforth verifying that damage had taken place. Also, the increased specific gravity of urine found in CCl4-intoxicated rat group correlates with osmolality and is a well-documented symptom of renal diseases including renal artery steatosis, dehydration, proteinuria, necrosis, and reduced blood flow towards kidney (Sahreen et al., 2015). The kidney injuries were effectively reverted towards control rat group after co-treatment with PJM/PHM (400 mg/kg). Treated rat groups displayed normal urine profiles without toxic parameters. These measurement levels of urine highlighted the pharmacological significance of PJM and PHM fraction. The current study strengthens the research findings of Shah et al., 2017, who reported the nephro-protective effect of Sida cordata Ethyl-acetate fraction (SCEE) against CCl4-induced nephrotoxicity using 150 and 300 mg/kg doses. A significant increase (p < 0.05) in urinary albumin, urinary protein levels, and pH while considerable reduction in RBCs, WBCs, urobilinogen, specific gravity, and urea were observed after SCEE administration relative to CCl4-treated group, thereby indicating improved kidney function.
DNA strand breakages detected at single-cell level are interpreted by a responsive and adaptable technique known as comet assay. The assay is used to assess the DNA damages induced via ROS in kidney tissues (Sajid et al., 2016). Our study demonstrated an increase in comet length, tail length, and tail moment whereas a decrease in percentage DNA of head in CCl4-administered rats. The considerable increase in tail parameters i.e. comet length, percentage DNA in tail, tail length and tail moment result from the amount of DNA transferred from comet head → comet tail and specify CCl4-induced kidney damage. Co-administration of PJM/PHM (200 and 400 mg/kg) markedly reduced (p < 0.05) the DNA-migration parameters and uplifted the head length and percentage DNA in comet head, thus representing the nephro-protective effects against CCl4 induced DNA damages. Furthermore, the DNA-strand breaks were calculated as 09.41 in CCl4-treated group which were noticeably reduced (p < 0.05) to 0.98 and 0.20 after treating with PJM and PHM high dose (400 mg/kg), respectively. The present study directs the ameliorative potential of PJM and PHM fractions against DNA fragmentations, DNA disruptions, and oxidative stress ensuring cell viability. The current research data appears similar to the results of Younis et al., 2018, who documented high dose (400 mg/kg) of Fraxinus xanthoxyloides to markedly (p < 0.01) re-establish the CCl4-induced altered parameters of comet towards control, resembling silymarin-treated group.
Renal injury caused via CCl4 administration was validated further by performing histopathological analysis of kidney tissues. Analysis through histology not only indicated toxicity caused by CCl4 in CCl4-injected rat group but also revealed the effectiveness of PJM and PHM on renal cells post treatment. CCl4-treated group displayed abnormal appearance of glomeruli and Bowman’s capsule with impaired corticular segments, aggregation of necrotic cells, dilation of convoluted tubules, edema in Bowman’s capsule, and space narrowing between Bowman’s capsule. Earlier study accomplished by Kalantari et al., 2018 revealed the histopathology of CCl4 group to be characterized by epithelial cell detachment, widening of tubular lumen, vacuolization, atrophy, and tubular necrosis which modifies the nephron functioning by altering the capability of tubular absorption leading to kidney dysfunction and injury. This kidney injury was successfully reversed by co-treatment of PJM/PHM (particularly 400 mg/kg) fraction, thereby exhibiting normal architecture of renal cells, resembling control group. Hypertrophy of glomerulus was observed in PJM and PHM high doses which may prove favorable, as reported elsewhere that hyperplasia and hypertrophy when present after tissue injury considerably contributes to the process of regeneration (Marongiu et al., 2017). Our study is in match with the findings of AL-Ghamdi et al., 2019, who reported the co-administration (200 mg/kg) of methanol (ZS-1) and aqueous (ZS-4) fractions of Zizyphus spina-christi (L) to display characteristic glomerular and tubular structures of kidney resembling control group.
5. Conclusion
The present findings provide additional scientific evidence unravelling P. jacquemontiana and P. hydaspidis as strong antioxidants capable of defending the kidney from CCl4-induced damages. These medicinal plants are declared safe after no toxicity was observed in experimental rats, even when provided at higher doses. The in vitro high free-radical scavenging capability assessed earlier was hereby implicated endogenously on rat model in vivo. P. jacquemontiana and P. hydaspidis manifest the compounds which efficiently work on kidney to keep it functioning normally. It appears to effectively reserve the structural integrity of glomerulus, Bowman’s capsule, proximal and distal convoluted tubules when injury takes place via inhibiting CCl4-instigated oxidative stress chain reactions, preventing DNA damage, and restoring cellular antioxidant status. The current studies confirmed that it may be promising candidates for phytomedicine development against various renal ailments. However, additional in vivo and clinical trials are needed to further justify and evaluate the effectiveness and biocompatibility of these plants as nephroprotective agents in humans.
CRediT authorship contribution statement
Saima Ali: Conceptualization, Formal analysis, Investigation, Methodology, Writing -original draft. Muhammad Rashid Khan: Project administration, Resources. Javed Iqbal: Conceptualization, Investigation, Methodology, Validation. Banzeer Ahsan Abbasi: Validation. Tabassum Yaseen: Data curation. Riffat Batool: Formal analysis. Muhammad Delwar Hussain: Funding acquisition. Mohsin Kazi: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP 2021/301), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aayadi H., Mittal S.P., Deshpande A., Gore M., Ghaskadbi S.S. Protective effect of geraniin against carbon tetrachloride induced acute hepatotoxicity in Swiss albino mice. Biochem. Biophys. Res. Commun. 2017;487:62–67. doi: 10.1016/j.bbrc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Abou Gabal A., Aboul-Ela H.M., Ali E., Ahemd E., Shalaby O.K. Hepatoprotective, DNA damage prevention and antioxidant potential of Spirulina platensis on CCl4-induced hepatotoxicity in mice. Am. J. Biomed. Res. 2015;3:29–34. [Google Scholar]
- Ali S., Khan M.R., Iqbal J., Batool R., Naz I., Yaseen T., El-Serehy H.A. Chemical composition and pharmacological bio-efficacy of Parrotiopsis jacquemontiana (Decne) Rehder for anticancer activity. Saudi J. Biol. Sci. 2021;28(9):4969–4986. doi: 10.1016/j.sjbs.2021.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Batool R., Shah S.A., Iqbal J., Abbasi B.A., Althobaiti F. Characterization and phytochemical constituents of Periploca hydaspidis Falc crude extract and its anticancer activities. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi M., Ahmed A., El-Zohri M., Shahat A.A. Hepatoprotective, nephroprotective, anti-amylase, and antiglucosidase effects of Ziziphus spina-christi (L.) against carbon tetrachloride-induced toxicity in rats. Trop. J. Pharm. Res. 2019;18 [Google Scholar]
- Abbasi B.A., Iqbal J., Ahmad R., Bibi S., Mahmood T., Kanwal S., Hameed S. Potential phytochemicals in the prevention and treatment of esophagus cancer: A green therapeutic approach. Pharmacol. Rep. 2019;71:644–652. doi: 10.1016/j.pharep.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Abbasi B.A., Iqbal J., Khan Z., Ahmad R., Uddin S., Shahbaz A., Mahmood T. Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc. Res. Tech. 2021;84(2):192–201. doi: 10.1002/jemt.23577. [DOI] [PubMed] [Google Scholar]
- Abbasi B.A., Iqbal J., Kiran F., Ahmad R., Kanwal S., Munir A., Mahmood T. Green formulation and chemical characterizations of Rhamnella gilgitica aqueous leaves extract conjugated NiONPs and their multiple therapeutic properties. J. Mol. Struct. 2020;1218 [Google Scholar]
- Ali S., Khan M.R., Sajid M. Protective potential of Parrotiopsis jacquemontiana (Decne) Rehder on carbon tetrachloride induced hepatotoxicity in experimental rats. Biomed. Pharmacother. 2017;95:1853–1867. doi: 10.1016/j.biopha.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Sajid M., Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement. Altern. Med. 2018;18:43. doi: 10.1186/s12906-018-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Shah S.A., Batool R., Maryam S., Majid M., Zahra Z. Protective aptitude of Periploca hydaspidis Falc against CCl 4 induced hepatotoxicity in experimental rats. Biomed. Pharmacother. 2018;105:1117–1132. doi: 10.1016/j.biopha.2018.06.039. [DOI] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Khan R. Green synthesized AgNPs from Periploca hydaspidis Falc. and its biological activities. Microsc. Res. Tech.. 2021;2021:1–18. doi: 10.1002/jemt.23780. [DOI] [PubMed] [Google Scholar]
- Ali S., Sulaiman S., Khan A., Khan M.R., Khan R. Green synthesized silver nanoparticles (AgNPs) from Parrotiopsis jacquemontiana (Decne) Rehder leaf extract and its biological activities. Microsc. Res. Tech.. 2021;2021:1–16. doi: 10.1002/jemt.23882. [DOI] [PubMed] [Google Scholar]
- Alm-Eldeen A.A., El-Naggar S.A., El-Boray K.F., Elgebaly H.A., Osman I.H. Protective role of Commiphora molmol extract against liver and kidney toxicity induced by carbon tetrachloride in mice. Trop. J. Pharm. Res. 2016;15:65–72. [Google Scholar]
- Al-Olayan E.M., El-Khadragy M.F., Aref A.M., Othman M.S., Kassab R.B., Abdel Moneim A.E. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid. Med. Cell. Longev. 2014;2014:01–12. doi: 10.1155/2014/381413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsheblak M.M., Elsherbiny N.M., El-Karef A., El-Shishtawy M.M. Protective effects of L-carnosine on CCl4-induced hepatic injury in rats. Eur. Cytokine Netw. 2016;27:6–15. doi: 10.1684/ecn.2016.0372. [DOI] [PubMed] [Google Scholar]
- Bellassoued K., Hsouna A.B., Athmouni K., van Pelt J., Ayadi F.M., Rebai T., Elfeki A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018;17:1–14. doi: 10.1186/s12944-017-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batool R., Aziz E., Salahuddin H., Iqbal J., Tabassum S., Mahmood T. Rumex dentatus could be a potent alternative to treatment of micro-bial infections and of breast cancer. J. Tradit. Chin. Med. 2019;39:772–779. [PubMed] [Google Scholar]
- Batool R., Aziz E., Iqbal J., Salahuddin H., Tan B.K.H., Tabassum S., Mahmood T. In vitro antioxidant and anti-cancer activities and phytochemical analysis of Commelina benghalensis L. root extracts. Asian Pac. J Trop. Biomed. 2020;10:417. [Google Scholar]
- Chance B., Maehly A. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Chu C.C., Chen S.Y., Chyau C.C., Fu Z.H., Liu C.C., Duh P.D. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury, in vivo. J. Funct. Foods. 2016;26:585–597. [Google Scholar]
- Dhawan A., Bajpayee M.M., Pandey A.K., Parmar D. Protocol for the single cell gel electrophoresis/comet assay for rapid genotoxicity assessment. Sigma. 2009;1077:1. [Google Scholar]
- El-haskoury R., Al-Waili N., Kamoun Z., Makni M., Al-Waili H., Lyoussi B. Antioxidant Activity and Protective Effect of Carob Honey in CCl4-induced Kidney and Liver Injury. Arch. Med. Res. 2018;49:306–313. doi: 10.1016/j.arcmed.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Grisham M.B., Johnson G.G., Lancaster J.R. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- Hozzein W.N., Al-Khalaf A.A., Mohany M., Ahmed O.M., Amin A.A., Alharbi H.M. Efficacy of two actinomycete extracts in the amelioration of carbon tetrachloride–induced oxidative stress and nephrotoxicity in experimental rats. Environ. Sci. Pollut. Res. 2019:1–10. doi: 10.1007/s11356-019-05730-w. [DOI] [PubMed] [Google Scholar]
- Hameed S., Khalil A.T., Ali M., Iqbal J., Rahman L., Numan M., Shinwari Z.K. Precursor effects on the physical, biological, and catalytic properties of Fagonia indica Burm. f. mediated zinc oxide nanoparticles. Microsc. Res. Tech. 2021 doi: 10.1002/jemt.23867. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Wright D.J. Host resistance to insecticides can confer protection to endo-larval parasitoids. Bull. Entomol. Res. 1996;86:721–723. [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Mahmood T., Kanwal S., Ali B., Badshah H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018;108:752–756. doi: 10.1016/j.biopha.2018.09.096. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Batool R., Mahmood T., Ali B., Khalil A.T., Ahmad R. Potential phytocompounds for developing breast cancer therapeutics: nature’s healing touch. Eur. J. Pharmacol. 2018;827:125–148. doi: 10.1016/j.ejphar.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Khalil A.T., Ali B., Mahmood T., Kanwal S., Ali W. Dietary isoflavones, the modulator of breast carcinogenesis: Current landscape and future perspectives. Asian Pac. J. Trop. Med. 2018;11:186–193. [Google Scholar]
- Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7:1129–1150. [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Batool R., Mahmood T., Ali B., Munir A. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019;109:1381–1393. doi: 10.1016/j.biopha.2018.10.107. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Mahmoodi M., Munir A., Zahra S.A., Capasso R. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines. 2020;8(5):117. doi: 10.3390/biomedicines8050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow D., Mitchell J., Zampaglione N.A., Gillette J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kalantari H., Pajou M.D., Kheradmand P., Goodarzian M., Zeidooni L. Nephroprotective Effect of Hydroalcoholic Extract Allium jesdianum Boiss against Carbon Tetrachloride Induced Nephrotoxicity via Stress Oxidative in Mice. Pharm. Sci. 2018;24:89–96. [Google Scholar]
- Karakuş A., Değer Y., Yıldırım S. Protective effect of Silybum marianum and Taraxacum officinale extracts against oxidative kidney injuries induced by carbon tetrachloride in rats. Ren. Fail. 2017;39:1–6. doi: 10.1080/0886022X.2016.1244070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.A., Khan M.R., Sahreen S., Bokhari J. Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol. 2010;48:2469–2476. doi: 10.1016/j.fct.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marongiu F., Marongiu M., Contini A., Serra M., Cadoni E., Murgia R., Laconi E. Hyperplasia vs hypertrophy in tissue regeneration after extensive liver resection. World J. Gastroenterol. 2017;23:1764. doi: 10.3748/wjg.v23.i10.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika D., Guruvayoorappan C. The effect of Thespesia populnea on cisplatin induced nephrotoxicity. J. Cancer Res. Ther. 2013;9:50. doi: 10.4103/0973-1482.110362. [DOI] [PubMed] [Google Scholar]
- Ogeturk M., Kus I., Colakoglu N., Zararsiz I., Ilhan N., Sarsilmaz M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J. Ethnopharmacol. 2005;97:273–280. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell. Immunol. 1981;59:301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Rahmouni F., Hamdaoui L., Badraoui R., Rebai T. Protective effects of Teucrium polium aqueous extract and ascorbic acid on hematological and some biochemical parameters against carbon tetrachloride (CCl4) induced toxicity in rats. Biomed. Pharmacother. 2017;91:43–48. doi: 10.1016/j.biopha.2017.04.071. [DOI] [PubMed] [Google Scholar]
- Safhi M.M. Nephroprotective effect of Zingerone against CCl4-induced renal toxicity in Swiss albino mice: molecular mechanism. Oxid. Med. Cell. Longev. 2018:01–07. doi: 10.1155/2018/2474831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahreen S., Khan M.R., Khan R.A., Alkreathy H.M. Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. J. Food Nutr. Research. 2015;59:28438. doi: 10.3402/fnr.v59.28438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajid M., Khan M.R., Shah N.A., Ullah S., Younis T., Majid M., Ahmad B., Nigussie D. Proficiencies of Artemisia scoparia against CCl4 induced DNA damages and renal toxicity in rat. BMC Complement. Altern. Med. 2016;16:1–10. doi: 10.1186/s12906-016-1137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.A., Khan M.R., Nigussie D. Phytochemical investigation and nephroprotective potential of Sida cordata in rat. BMC Complement. Altern. Med. 2017;17:388. doi: 10.1186/s12906-017-1896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek H., Celik I., Dogan A. Nephroprotective hepatoprotective potential and antioxidant role of carob pods (Cerotonia siliqua L.) against carbon tetrachloride-induced toxicity in rats. Indian J. Pharm. Educ. Res. 2017;51:312–320. [Google Scholar]
- Ullah N., Khan M., Khan T., Ahmad W. Cymbopogon citratus protects against the renal injury induced by toxic doses of aminoglycosides in rabbits. Indian J. Pharm. Sci. 2013;75:241. [PMC free article] [PubMed] [Google Scholar]
- Uddin S., Safdar L.B., Anwar S., Iqbal J., Laila S., Abbasi B.A., Quraishi U.M. Green Synthesis of Nickel Oxide Nanoparticles from Berberis balochistanica Stem for Investigating Bioactivities. Molecules. 2021;26:1548. doi: 10.3390/molecules26061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah R., Bakht J., Shafi M. Antimicrobial and anti-oxidant potential of Periploca hydaspidis. Bangladesh J. Pharmacol. 2015;10:645–651. [Google Scholar]
- Usman J.G., Madziga H.A., Sandabe U.K., Sodipo O.A. Nephroprotective effects of methanolic extract of Cucumis metuliferus fruit in cockerels. Int. J. Herb. Med. 2020;8(3):01–07. [Google Scholar]
- Younis T., Rasul A., Jabeen F., Hussain G., Altaf J., Jafri L., Rani R., Khan M.R., Sarfraz I., Ali M. Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl4-challanged nephrotoxicity in rats. Pak. J. Pharm. Sci. 2018:31. [PubMed] [Google Scholar]
- Zangeneh M.M., Zangeneh A., Tahvilian R., Moradi R. Evaluation of the nephroprotective effect of Glycyrrhiza glabra L aqueous extract on CCl4-induced nephrotoxicity in mice. J. Comp. Pathol. 2018;27:1119–1126. [Google Scholar]
- Zahra S.A., Iqbal J., Abbasi B.A., Shahbaz A., Kanwal S., Shah S.L., Mahmood T. Antimicrobial, cytotoxic, antioxidants, enzyme inhibition activities, and scanning electron microscopy of Lactuca orientalis (Boiss.) Boiss. Seeds. Microsc Res Tech. 2021;84:1284–1295. doi: 10.1002/jemt.23687. [DOI] [PubMed] [Google Scholar]
- Zahra S.A., Iqbal J., Abbasi B.A., Yaseen T., Hameed A., Shahbaz A., Ahmad P. Scanning electron microscopy of Sophora alopecuroides L. seeds and their cytotoxic, antimicrobial, antioxidant, and enzyme inhibition potentials. Microsc. Res. Tech. 2021;84:1809–1820. doi: 10.1002/jemt.23740. [DOI] [PubMed] [Google Scholar]