Abstract
An assessment of 27 mutant tomato lines from four countries (Germany, USA, Russia, Bulgaria) was carried out for resistance to five Alternaria alternata strains under conditions of the South of Russia. Five strains of the A. alternata fungus were isolated from naturally infected plants selected in five agroclimatic zones of Krasnodar Krai: Central - strain 1, Western - strain 6, North - strain 11, South Foothill - strain 12, Chernomorskaya - strain 13. The assessment was carried out in the field during 2018–2020, in a greenhouse and under the laboratory conditions three times for each studied strain. In the field, the plants were treated every year with a spore suspension of A. alternata strain 1. Mutant lines obtained from the United States: 868, 663, 533, 544 and 898 showed the greatest resistance to Alternaria in 2018–2020, the lesion of which averaged 4.5–8.0% over three years. 13 mutant lines: 17, 40, 688, 722 (Germany), 311, 394, 418, 542, 728, 743, 917 (USA), 322 (Russia), 159 (Bulgaria) showed average resistance with the development of the disease 10.2–24.9% over three years of the research. Mutant lines 743, 663, 868, 544 obtained from the USA possessed relatively high resistance to all the studied strains under greenhouse conditions; moreover, no signs of damage with strains 1 and 11 were observed on Mo 868, signs of damage by strain 11 of A. alternata were not observed on Mo 743. Under laboratory conditions, mutant lines 663, 743, 868, obtained from the United States, were most resistant. Mo 663 showed resistance to strains 1, 13; line 743 - to strains 11, 12; line 868 - to strains 1, 11. There was a predominantly positive correlation between the results of field, greenhouse and laboratory assessments, which indicates a strong connection between them and the possibility of using these methods to assess the resistance of tomato samples to Alternaria independently of each other.
Keywords: Tomato, Genetic collection, Alternaria, Alternaria alternata, Resistance, Breeding
1. Introduction
Tomato (Solanum lycopersium Mill.) is among the most cultivated and consumed vegetable crops around the world, including Russia (Al-Amri, 2013, Nekoval et al., 2020a, Salman et al., 2020). Krasnodar Krai is one of the leading regions of Russia in terms of area and production of open ground tomato. According to the Russian Statictics Agency, in 2020, 893.4 thousand tons of tomato were produced in the industrial sector of vegetable growing in Russia on an area of 17.9 thousand hectares. In Krasnodar Krai, 9.08 thousand tons of tomato were produced on an area of 0.47 thousand hectares (AB-Center, 2020).
Tomato is extremely susceptible to the influence of abiotic and biotic factors that affect the yield and product quality. Tomato is infected with up to 200 diseases caused by viruses, bacteria, nematodes and fungi. The most common and economically significant disease affecting tomato at all stages of the growing season is considered to be alternaria caused by the Alternaria fungi (Gannibal, 2011, Gannibal et al., 2011, Tewari and Vishunavat, 2012, Shoaib et al., 2019). In Russia, Alternaria species are extremely widespread and very diverse (Gannibal, 2011, Gannibal et al., 2011, Gannibal et al., 2011, Gannibal, 2011, Orina, 2011). 5 species are economically important: Alternaria solani Sorauer (syn. Macrosporium solani Ellis et G. Martin), Alternaria linariae (Neerg.) E.G. Simmons, Alternaria tenuissima (Nees et T. Nees: Fr.) Wiltshire, Alternaria arborescens E.G. Simmons, Alternaria alternata (Fr.) Keissl. (Simmons, 2007, Gannibal, 2011, Gannibal et al., 2011, Gannibal et al., 2011, Gannibal, 2011).
Symptoms of alternaria are characterized by brown to dark brown necrotic spots with concentric rings on foliage, stem and fruit. This disease, which in severe cases can lead to complete defoliation, and, accordingly, rotting of fruits and loss of yield, is most dangerous for tomatoes in regions with heavy rains, high humidity and rather high temperatures − 24–29.0° C. It is known that the infestation of plants of the family Solanaceae species of the genus Alternaria can reach 80% in the field, and 30% in a greenhouse (Van der Waals et al., 2001, Soleimani and Kirk, 2012).
Currently, the main practice for controlling alternaria is the use of fungicides, but environmental problems require a significant reduction in the use of chemical pesticides. The use of fungicides during wet and rainy periods is often ineffective (Shoaib et al., 2020). Also, pathogenic microorganisms show resistance to fungicides (Pasche et al., 2005, Luo et al., 2007, Rosenzweig et al., 2008). It is necessary to study alternative methods of alternaria control. Resistant varieties are one of the best strategies for long-term control of this disease (Odilbekov et al., 2014, Medic-Pap et al., 2016, Moghaddam et al., 2019, Akhtar et al., 2019).
It should be noted that among the known cultivated tomato varieties there are no registered varieties completely resistant to alternaria (Pawar et al., 2016, Adhikari et al., 2017, Vloutoglou and Kalogerakis, 2000, Chaerani and Voorrips, 2006, Chaerani et al., 2007). The cultivated tomato has a narrow genetic diversity, which is the result of its intense selection and inbreeding during evolution. As a result, cultivated tomato species are more susceptible to disease attack than wild and mutant varieties. Thus, crop losses due to pathogenic infections can be eliminated by developing resistant varieties using plant breeding methods with resistance genes from wild species and mutant forms of tomato (Delgado and Gomez-Cordoves, 1998, Da Motta and Soares, 2001, Logrieco et al., 2003, Gannibal, 2011, Gannibal et al., 2011, Nekoval et al., 2020b).
To develop resistant varieties, breeders need tomato genetic collections with identified genes. One of such collections is maintained at the Federal State Budgetary Scientific Institution Federal Research Center of Biological Plant Protection, Krasnodar, and the work on a comprehensive study of the collection and selection of parental material for practical use in breeding is being carried out (Nekoval et al., 2016a, Nekoval et al., 2016b, Nekoval et al., 2018).
Screening of tomato plants for resistance to alternaria is carried out by various methods widely studied by many authors (Locke, 1948, Bussey and Stevenson, 1991, Foolad et al., 2000, Vloutoglou and Kalogerakis, 2000, Gannibal, 2011, Gannibal et al., 2011, Nekoval et al., 2020b). The most widespread field screening, which makes it possible to assess the resistance of plants to a pathogen in the natural environment. However, this screening method is time-consuming, labor-intensive and highly dependent on environmental conditions. Also, methods are used to study the resistance of tomato to alternaria in a greenhouse and in a laboratory conditions. These methods facilitate faster screening of plants for resistance. However, there are conflicting opinions regarding the correlation between field, greenhouse and laboratory resistance of tomato to alternaria (Locke, 1948, Bussey and Stevenson, 1991, Foolad et al., 2000, Vloutoglou and Kalogerakis, 2000, Gannibal, 2011, Gannibal et al., 2011, Nekoval et al., 2020b). Therefore, in our research, we set two tasks: 1) Select promising mutant tomato lines from different countries of the world for inclusion in the breeding process to develop tomato varieties resistant to various strains of A. alternata isolated in the South of Russia; 2) Compare different methods of screening tomato for resistance to alternaria and determine the most reliable one.
2. Materials and methods
2.1. Plant material
As an experimental material, 100 mutant tomato lines from different countries of the world were used (Bocharnikova and Kozlova, 1992), gathered into a collection of mutant tomato forms by Academician A.A. Zhuchenko, which is stored, replenished and studied in the laboratory of the tomato genetic collection of the Federal State Budgetary Scientific Institution “Federal Research Center of Biological Plant Protection” (FSBSI FRCBPP), Krasnodar. Based on the results of long-term field observations against a provocative infectious background, 27 mutant lines were selected from four countries (Russia, Germany, Bulgaria, the United States) that showed the greatest resistance to various pests in the South of Russia (Nekoval et al., 2016a, Nekoval et al., 2016b, Nekoval et al., 2018) (Table 1).
Table 1.
Mutant forms of tomato from the collection of the FSBSI FRCBPP.
| Mo* | Mutant code according to international classification | Gene | Locus name | Origin | Received |
|---|---|---|---|---|---|
| 17 | 341/1/66 | cm | curly motted | SPON | Germany |
| 40 | 3–105 | apn | albo-punctata | CHEM | Germany |
| 43 | 382/66 | alb | albescent | SPON | Germany |
| 130 | – | ep, obl | easy peeling,oblate fruit | RAD | Bulgaria |
| 159 | La 0330 | bk | beaked | SPON | Bulgaria |
| 163 | – | – | – | SPON | Russia |
| 220 | – | – | – | SPON | Russia |
| 311 | La 0618 | op | opaca | RAD | USA |
| 322 | – | – | – | SPON | Russia |
| 374 | – | – | – | SPON | Russia |
| 394 | La 3563 | sp, u | self-pruning, uniform ripening | SPON | USA |
| 409 | La 0786 | nv | netted virescent | SPON | USA |
| 418 | La 3722 | oli | olivacea | RAD | USA |
| 421 | La 0643 | l-2 | lutescent-2 | SPON | USA |
| 533 | La 0588 | bc | bicolor | RAD | USA |
| 542 | 68–1242 | de | declinata | RAD | USA |
| 544 | La 3767 | ds | dwarf sterile | SPON | USA |
| 663 | 78–3075-1 | rvt, vo, d, gf, sp | red vascular tissue, virescent orange, dwarf, green flesh, self-pruning | SPON | USA |
| 688 | La 0539 | car | carinata | RAD | Germany |
| 722 | La 0846 | mup | multiplicata | RAD | Germany |
| 728 | MLP148/78 | pma | praemortua | SPON | USA |
| 743 | 78–3095-3 | per | perviridis | RAD | USA |
| 858 | La 1997 | bn | blunt | SPON | USA |
| 868 | La 2–33 | ms-4 | male-sterile-4 | SPON | USA |
| 898 | 2–137 | pi | pistillate | SPON | USA |
| 916 | La 0705 | ste | sterilis | RAD | USA |
| 917 | La 0708 | ta | tarda | RAD | USA |
Mutant code in the collection of the FSBSI FRCBPP
2.2. Selection and cultivation of Alternaria alternata isolates.
Accurate identification and description of isolates or races of a pathogen is critical for breeding programs on disease resistance (Akhtar et al., 2019).
Isolation of isolates into a pure culture was carried out by stimulating the sporulation of affected plant parts: samples with injuries were placed in a wet chamber, before that they were washed with distilled water and surface disinfected with 1% sodium hypochlorite. The samples were incubated at a temperature of 20–25° C for 2–5 days, then, by transferring individual conidia or several conidia from one chain with a sterile needle (Gannibal et al., 2011, Gannibal, 2011) into tubes with Czapek's medium, a pure culture was obtained (Nikitina and Reshetnik, 2006).
We obtained five strains of p. Alternaria, from naturally infected plants selected in five agroclimatic zones of Krasnodar Krai (Central - strain 1, Western - strain 6, Northern - strain 11, South Foothill - strain 12, Chernomorskaya - strain 13). These strains were identified on the basis of the known morphological characteristics of the genus Alternaria (macroscopic and microscopic) (Nekoval et al., 2020b) and molecular methods.
During the morphological identification of these strains, their belonging to the A. alternata species complex was established, which was further confirmed as a result of genetic analysis - based on sequencing of the ITS1 region when checking these sequences for similarity with those already available in the GeneBank NCBI database, all strains were identified as species A. alternata: (Alternaria_alternata_strain_13 MW659929; Alternaria_alternata_strain_6 MW659930; Alternaria_alternata_strain_12 MW659931; Alternaria_alternata_strain_1 MW659932; Alternaria_alternata_strain_11 MW659933) (described in the previous study) (Nekoval et al., 2020b).
2.3. Field assessment
Screening of 27 mutant tomato lines for resistance to alternaria was carried out under the field conditions of the Federal State Budgetary Scientific Institution Federal Research Center of Biological Plant Protection against an artificial infectious background in 2018–2020.
Plants were placed in randomized blocks in triplicate. One replication included 10 plants of each genotype. Planting pattern: 1.5 m × 0.75 m (row spacing × plant spacing). During the seasons, fungicide treatments were not carried out.
To evenly distribute the inoculum over the experimental plots, each year the plants were treated with a spore suspension of strain 1 (isolated in the experimental plot in the second part of July 2017) at a concentration of 5 × 104 conidia / ml using a spray bottle. The counts were carried out from the middle of June (interphase period of plant development - budding – flowering: code of growth stages 51 … 69 on the BBCH scale) with an interval of 9–12 days, starting from the appearance of the first signs of the disease, only four to five counts per season (Foolad et al., 2000, Gannibal et al., 2011, Gannibal, 2011). At each count, the percentage of leaf necrotic area was visually assessed using a modified Horsfall-Barratt rating scheme, where 0 indicates no visible symptoms of alternaria disease and 100 indicates plant death (Horsfall and Barratt, 1945). To assess the resistance of tomato lines to alternaria, a modified 5-point scale was used (Table 2) (Vakalounakis, 1983, Gannibal et al., 2011, Gannibal, 2011).
Table 2.
Scale for assessing the tomato damage with alternaria.
| Damage score | Damage degree | Resistance degrree |
|---|---|---|
| 0 | There are no visible symptoms of damage | High resistance |
| 1 | Up to 10% of the leaf area is damaged | Relatively high resistance |
| 2 | From 10 to 25 % of the leaf area is damaged | Moderate resistance |
| 3 | From 25 to 50 % of the leaf area is damaged | Moderate susceptibility |
| 4 | From 50 to 75 % of the leaf area is damaged | Susceptibility |
| 5 | More than 75 % of the leaf area is damaged | High susceptibility |
2.4. Greenhouse experiments.
Four week old seedlings of 27 genotypes were individually transplanted into 5 L pots and grown in five separate greenhouse sections (GH). In each section, the plant resistance to one of the five A. alternata strains was assessed. The study was carried out in three replicates, in one replication − 5 plants of each genotype. Plants were grown at day / night temperatures of 22/17° C, with a 12 h photoperiod. At the late seedling stage (6 to 7 weeks), plants were individually inoculated with A. alternata strains. After inoculation, the plants were kept in the dark for 12 h. After the plants were maintained for a 12 h photoperiod. Six days after inoculation, the plants were individually assessed for disease symptoms. For each plant, the percentage of leaf area covered by A. alternata foci was visually assessed on three plant tiers (upper, middle, and lower). Of the intensity of alternaria development percentage for each plant was calculated as the average of the three sections (using a modified technique described in Foolad et al., 2000).
2.5. Laboratory assessment
To determine the resistance of mutant tomato lines against an artificial infectious background in laboratory conditions, we used tomato leaves of the middle tier. Inoculum was prepared in Petri dishes with cultures of 1–2 weeks of age, occupying the entire surface and having abundant sporulation; 3–5 ml of sterile water was added, the spores were carefully separated from the surface of the colony with a spatula, the number of spores in the suspension was counted using a Goryaev chamber (Gannibal et al., 2011, Gannibal, 2011).
The separated leaf lobes were placed in a humid chamber on filter paper, and 1 drop of inoculum (concentration 5 × 103 conidia / ml) was applied using an automatic dispenser on each side of the central vein. After 24 h, the drops were dried with filter paper and the leaves were moistened with sterile water from a spray bottle every day. The size of the lesion was measured daily for 5 days, starting on the 4th day of incubation. The radius of each necrosis was calculated as half the mean of the two perpendicular diameters. Five days later, the final degree of disease damage (percentage of necrotic tissue in leaf area) was visually assessed (Foolad et al., 2000, Gannibal et al., 2011, Gannibal, 2011). The experiment was repeated three times for all studied A. alternata strains.
2.6. Data analysis
The lesion area under the disease progression curve (AUDPC) was calculated for each genotype in each replication and in each year using the formula:
;
AUDPC – lesion area under the disease progression curve;
x – degree of disease development in ith count;
t – the number of days between counts.
Statistical data processing was performed by standard methods using MS Excel and STATISTICA for Windows. To compare the variability of the traits taken into account in the experiment within each genotype between the years of the study, a one-way analysis of variance was carried out, in which the year of the study appeared as a factor. To compare genotypes with each other, a one-way analysis of variance was carried out, in which the belonging of a tomato plant to a certain genotype appeared as a factor. Analysis of variance traditionally ended by comparing the mean values of the features and calculating the error of the mean: its results demonstrated which values of the features were statistically significantly different. In this case, the Duncan test was used, and the differences were considered statistically significant at the p ≤ 0.05 level. For a comprehensive analysis of the variability of the traits taken into account in the experiment, a two-factor analysis of variance was carried out, where the factors were: belonging of a plant to a certain genotype of tomato and the year of study. This analysis made it possible to reveal the specificity of the reaction of plants of various tomato genotypes to changes in the conditions of the year.
Pearson's correlation coefficients for various variables were calculated using the statistical analysis software StatSoft Statistica V. 10.0.
3. Results
3.1. Field assessment
The first symptoms of alternaria on tomato were recorded in the middle of June on the field station of the Federal State Budgetary Scientific Institution FRCBPP under the conditions of an artificial infectious background in 2018–2020. During the growing season of the culture, alternaria developed on all aerial parts of plants. In particular, dark brown or black spots appeared on stems, leaves, petioles, peduncles, sepals, and fruits, increasing in size as the infection progressed.
The intensity of the disease progression depended on the weather conditions of the year, since the hydrometeorological conditions of the growing seasons of 2018–2020 varied. So, the disease progression was the highest in 2019–31.2%. In 2018, the least development of alternaria was noted for the three years of the research − 13.4%. In 2020, the disease progression was 19.8%.
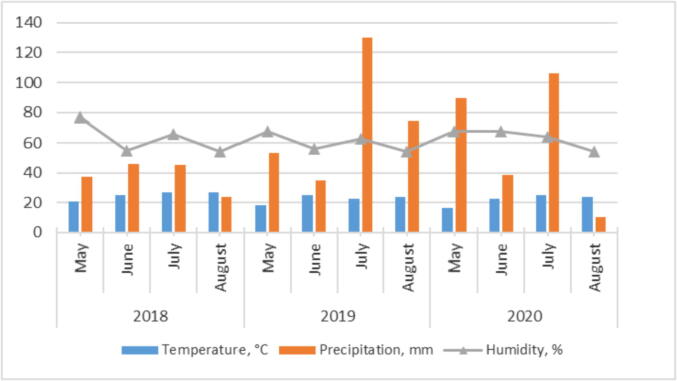
The period from the end of May to the end of June in 2018 was characterized by high temperatures (2.8 … 3.4° C higher than the average annual values) and a deficit of precipitation (20 … 30% of the average annual norm). June – July were dry, also with high temperatures (3.2 … 3.4° C higher than the average long-term value). On the contrary, the growing season of 2019 was characterized by excessive moisture, which is associated with a more intensive development of the disease than in the previous and subsequent periods. So, in July the amount of precipitation was 130 mm, which is 70 mm higher than the norm. The conditions of the growing season of 2020 were also favorable for the development of alternaria: June was characterized by fluctuations in low night temperatures with high daytime ones, with periodic precipitation at the beginning and in the middle of the month; at the beginning of July, hot and predominantly dry weather was noted; in the second part of July, the weather was moderately hot with heavy rains. However, in the growing season of 2020, precipitation fell by 47.2 mm less than in the previous one, which was reflected in a less intensive development of the disease (Fig. 1).
Fig. 1.
Meteorological conditions during the 2018–2020 research.
To analyze the variability of the value of the degree of disease development in ith count; of alternaria development and AUDPC of the studied tomato lines during 2018–2020 a two-way analysis of variance was carried out, in which the factors were: a certain mutant tomato line; year of research; the interaction of these two factors. To comply with the condition of the normal distribution of the characteristic, of the degree of disease development in ith count; of alternaria development was converted to 2 * Asin (root (p)) (Table 3, Table 4).
Table 3.
Analysis of variance (two-factor model) of the degree of disease development in ith count; of alternaria development variability of mutant tomato lines over three years of the study (2018–2020).
| Variability | df | mS | F | Dispersion | Share of total variance,% |
|---|---|---|---|---|---|
| General | 112030,2 | 103025,1* | 255,92 | 100,0 | |
| Among lines | 26 | 1552,9 | 1428,1* | 172,43 | 67,4 |
| Over years | 2 | 6593,4 | 6063,4* | 81,39 | 31,8 |
| Interaction | 52 | 83,7 | 76,9* | 1,02 | 0,4 |
| Remaining | 162 | 1,1 | 1,09 | 0,4 |
Table 4.
Analysis of variance (two-factor model) of the AUDPC variability of tomato mutant lines over three years of the study (2018–2020).
| Variability | df | mS | F | Dispersion | Share of total variance,% |
|---|---|---|---|---|---|
| General | 1 | 63,839,871 | 69785,5* | 142869,08 | 100,0 |
| Among lines | 26 | 937,610 | 1024,9* | 104077,22 | 72,8 |
| Over years | 2 | 3,031,473 | 3313,8* | 37414,30 | 26,2 |
| Interaction | 52 | 38,399 | 42,0* | 462,77 | 0,3 |
| Remaining | 162 | 915 | 914,80 | 0,6 |
Analysis of the data obtained showed that the greatest influence on the above traits was exerted by the belonging of a tomato plant to a certain mutant line, the contribution of this factor to the total variance was 67.4% for the degree of disease development in ith count; of alternaria development, and 72.8% for AUDPC.
The effect of the year factor was slightly lower: 31.8% and 26.2% of the total variability, respectively. A small but statistically significant contribution of the interaction of factors to the variance of the studied traits was shown; it was 0.4% for the degree of disease development in ith count; of alternaria development, and 0.3% for AUDPC. The interaction effect means a specific reaction of tomato lines to a change in the year of cultivation, therefore, it can be concluded that in most cases the reaction of tomato lines to a change in the year of cultivation had a general tendency. This conclusion is confirmed by the statistical analysis using the Duncan test, which characterizes the statistically significant differences in each line between the years of the study (Table 5). This table presents data on the degree of the disease development on each genotype during the 2018–2020 research.
Table 5.
Variability of the degree of alternaria development on mutant tomato lines, 2018–2020.
| Mo | Diseas Mean ± SE |
p-level | ||
|---|---|---|---|---|
| 2018 г. | 2019 г. | 2020 г. | ||
| 17 | 14,6 ± 0,35a | 24,8 ± 0,51c | 20,7 ± 0,30b | 0,000* |
| 40 | 16,4 ± 0,45a | 20,3 ± 0,54c | 18,2 ± 0,42b | 0,003* |
| 43 | 49,1 ± 0,49a | 67,2 ± 0,40c | 58,3 ± 0,58b | 0,000* |
| 130 | 20,4 ± 0,65a | 51,2 ± 0,73c | 26,2 ± 0,70b | 0,000* |
| 159 | 13,7 ± 0,72a | 15,8 ± 0,65a | 15,0 ± 0,81a | 0,194* |
| 163 | 19,4 ± 0,62a | 30,6 ± 0,60c | 25,1 ± 0,73b | 0,000* |
| 220 | 13,2 ± 0,71a | 50,7 ± 0,76c | 23,6 ± 0,84b | 0,000* |
| 311 | 6,5 ± 0,47a | 24,5 ± 0,50c | 18,4 ± 0,44b | 0,000* |
| 322 | 8,3 ± 0,45a | 27,9 ± 0,56c | 18,9 ± 0,51b | 0,000* |
| 374 | 25,2 ± 0,96a | 48,2 ± 0,92c | 30,8 ± 1,01b | 0,000* |
| 394 | 5,9 ± 0,27a | 15,6 ± 0,35c | 10,5 ± 0,38b | 0,000* |
| 409 | 17,2 ± 0,59a | 37,5 ± 0,67c | 24,7 ± 0,70b | 0,000* |
| 418 | 7,6 ± 0,69a | 24,5 ± 0,62c | 15,8 ± 0,76b | 0,000* |
| 421 | 33,1 ± 0,78a | 61,9 ± 0,93c | 42,4 ± 0,84b | 0,000* |
| 533 | 2,7 ± 0,25a | 12,2 ± 0,42c | 5,5 ± 0,31b | 0,000* |
| 542 | 5,8 ± 0,34a | 19,4 ± 0,72c | 7,2 ± 0,32b | 0,000* |
| 544 | 3,0 ± 0,25a | 14,7 ± 0,46c | 5,1 ± 0,42b | 0,000* |
| 663 | 0,0a | 14,7 ± 0,43c | 2,5 ± 0,21b | 0,000* |
| 688 | 9,5 ± 0,48a | 27,1 ± 0,67c | 19,6 ± 0,60b | 0,000* |
| 722 | 11,7 ± 0,60a | 31,0 ± 0,95c | 24,0 ± 0,92b | 0,000* |
| 728 | 6,6 ± 0,11a | 45,2 ± 0,92c | 22,8 ± 0,78b | 0,000* |
| 743 | 6,4 ± 0,16a | 15,1 ± 0,35c | 9,2 ± 0,33b | 0,000* |
| 858 | 24,3 ± 0,94a | 56,9 ± 1,05c | 31,7 ± 1,12b | 0,000* |
| 868 | 0,0a | 11,3 ± 0,30c | 2,3 ± 0,29b | 0,000* |
| 898 | 4,0 ± 0,23a | 15,7 ± 0,43b | 4,3 ± 0,26a | 0,000* |
| 916 | 22,6 ± 0,94a | 53,7 ± 1,32c | 36,2 ± 1,21b | 0,000* |
| 917 | 13,7 ± 0,45a | 24,5 ± 0,52c | 17,1 ± 0,50b | 0,000* |
| Cpeднee | 13,4 ± 0,48a | 31,2 ± 0,64c | 19,8 ± 0,60b | 0,000* |
* Differences were considered statistically significant at p ≤ 0.05; data represent the mean of disease development on 10 plants of each genotype, coupled with standard error of the mean.
Of the 27 mutant tomato lines, statistically significant differences in the degree of damage over the years of the study were not observed in one line − 159. In the 898 mutant line, one study year (2019) was significantly different from the other two (2018 and 2020). In 25 mutant lines, statistically significant differences in the degree of damage were found over all the years of the study.
Table 6 presents data on the AUDPC trait for each genotype in the 2018–2020 research period.
Table 6.
Variability of AUDPC on mutant tomato lines, 2018–2020.
| Mo | Diseas Mean ± SE |
p-level | ||
|---|---|---|---|---|
| 2018 г. | 2019 г. | 2020 г. | ||
| 17 | 14,6 ± 0,35a | 24,8 ± 0,51c | 20,7 ± 0,30b | 0,000* |
| 40 | 16,4 ± 0,45a | 20,3 ± 0,54c | 18,2 ± 0,42b | 0,003* |
| 43 | 49,1 ± 0,49a | 67,2 ± 0,40c | 58,3 ± 0,58b | 0,000* |
| 130 | 20,4 ± 0,65a | 51,2 ± 0,73c | 26,2 ± 0,70b | 0,000* |
| 159 | 13,7 ± 0,72a | 15,8 ± 0,65a | 15,0 ± 0,81a | 0,194* |
| 163 | 19,4 ± 0,62a | 30,6 ± 0,60c | 25,1 ± 0,73b | 0,000* |
| 220 | 13,2 ± 0,71a | 50,7 ± 0,76c | 23,6 ± 0,84b | 0,000* |
| 311 | 6,5 ± 0,47a | 24,5 ± 0,50c | 18,4 ± 0,44b | 0,000* |
| 322 | 8,3 ± 0,45a | 27,9 ± 0,56c | 18,9 ± 0,51b | 0,000* |
| 374 | 25,2 ± 0,96a | 48,2 ± 0,92c | 30,8 ± 1,01b | 0,000* |
| 394 | 5,9 ± 0,27a | 15,6 ± 0,35c | 10,5 ± 0,38b | 0,000* |
| 409 | 17,2 ± 0,59a | 37,5 ± 0,67c | 24,7 ± 0,70b | 0,000* |
| 418 | 7,6 ± 0,69a | 24,5 ± 0,62c | 15,8 ± 0,76b | 0,000* |
| 421 | 33,1 ± 0,78a | 61,9 ± 0,93c | 42,4 ± 0,84b | 0,000* |
| 533 | 2,7 ± 0,25a | 12,2 ± 0,42c | 5,5 ± 0,31b | 0,000* |
| 542 | 5,8 ± 0,34a | 19,4 ± 0,72c | 7,2 ± 0,32b | 0,000* |
| 544 | 3,0 ± 0,25a | 14,7 ± 0,46c | 5,1 ± 0,42b | 0,000* |
| 663 | 0,0a | 14,7 ± 0,43c | 2,5 ± 0,21b | 0,000* |
| 688 | 9,5 ± 0,48a | 27,1 ± 0,67c | 19,6 ± 0,60b | 0,000* |
| 722 | 11,7 ± 0,60a | 31,0 ± 0,95c | 24,0 ± 0,92b | 0,000* |
| 728 | 6,6 ± 0,11a | 45,2 ± 0,92c | 22,8 ± 0,78b | 0,000* |
| 743 | 6,4 ± 0,16a | 15,1 ± 0,35c | 9,2 ± 0,33b | 0,000* |
| 858 | 24,3 ± 0,94a | 56,9 ± 1,05c | 31,7 ± 1,12b | 0,000* |
| 868 | 0,0a | 11,3 ± 0,30c | 2,3 ± 0,29b | 0,000* |
| 898 | 4,0 ± 0,23a | 15,7 ± 0,43b | 4,3 ± 0,26a | 0,000* |
| 916 | 22,6 ± 0,94a | 53,7 ± 1,32c | 36,2 ± 1,21b | 0,000* |
| 917 | 13,7 ± 0,45a | 24,5 ± 0,52c | 17,1 ± 0,50b | 0,000* |
| Average | 13,4 ± 0,48a | 31,2 ± 0,64c | 19,8 ± 0,60b | 0,000* |
* Differences were considered statistically significant at the level, p ≤ 0.05; data represent the mean of disease progression on 10 plants and standard error.
Thus, all the studied mutant lines had statistically significant differences in the AUDPC trait over the years of the study. In mutant lines 40, 43, 159, 322, 663, 898, one year of the study was significantly different from the other two. In 21 mutant lines, statistically significant differences in resistance were found over all three years of the study.
To compare mutant lines of tomato according to the degree of disease development in ith count; of development of alternaria in different years of the study, analysis of variance was used, completed by comparing the mean values, the results of which are presented in Table 7.
Table 7.
Comparison of mutant tomato lines by the degree of disease development in ith count; of Alternaria alternata development in the field, 2018–2020.
| 2018 | 2019 |
2020 |
Mean |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mo | Diseas Mean± SD |
Comparing the mean values | Mo | Diseas Mean± SD |
Comparing the mean values | Mo | Diseas Mean ± SD | Comparing the mean values | Mo | Diseas Mean ± SD |
| 868 | 0,0a | A | 868 | 11,3 ± 0,30 | A | 868 | 2,3 ± 0,29 | A | 868 | 4,5 |
| 663 | 0,0a | A | 533 | 12,2 ± 0,42 | A | 663 | 2,5 ± 0,21 | A | 663 | 5,7 |
| 533 | 2,7 ± 0,25 | B | 663 | 14,7 ± 0,43 | B | 898 | 4,3 ± 0,26 | B | 533 | 6,8 |
| 544 | 3,0 ± 0,25 | B | 544 | 14,7 ± 0,46 | B | 544 | 5,1 ± 0,42 | B | 544 | 7,6 |
| 898 | 4,0 ± 0,23 | B | 743 | 15,1 ± 0,35 | B | 533 | 5,5 ± 0,31 | B | 898 | 8,0 |
| 542 | 5,8 ± 0,34 | C | 394 | 15,6 ± 0,35 | B | 542 | 7,2 ± 0,32 | C | 743 | 10,2 |
| 394 | 5,9 ± 0,27 | C | 898 | 15,7 ± 0,43 | B | 743 | 9,2 ± 0,33 | D | 394 | 10,7 |
| 743 | 6,4 ± 0,16 | CD | 159 | 15,8 ± 0,65 | B | 394 | 10,5 ± 0,38 | D | 542 | 10,8 |
| 311 | 6,5 ± 0,47 | CD | 542 | 19,4 ± 0,72 | C | 159 | 15,0 ± 0,81 | E | 159 | 14,8 |
| 728 | 6,6 ± 0,11 | CD | 40 | 20,3 ± 0,54 | C | 418 | 15,8 ± 0,76 | EF | 418 | 15,9 |
| 418 | 7,6 ± 0,69 | DE | 917 | 24,5 ± 0,52 | D | 917 | 17,1 ± 0,50 | FG | 311 | 16,5 |
| 322 | 8,3 ± 0,45 | EF | 311 | 24,5 ± 0,50 | D | 40 | 18,2 ± 0,42 | GH | 40 | 18,3 |
| 688 | 9,5 ± 0,48 | F | 418 | 24,5 ± 0,62 | D | 311 | 18,4 ± 0,44 | GH | 322 | 18,4 |
| 722 | 11,7 ± 0,60 | G | 17 | 24,8 ± 0,51 | D | 322 | 18,9 ± 0,51 | H | 917 | 18,4 |
| 220 | 13,2 ± 0,71 | H | 688 | 27,1 ± 0,67 | E | 688 | 19,6 ± 0,60 | HI | 688 | 18,7 |
| 159 | 13,7 ± 0,72 | H | 322 | 27,9 ± 0,56 | E | 17 | 20,7 ± 0,30 | I | 17 | 20,0 |
| 917 | 13,7 ± 0,45 | H | 163 | 30,6 ± 0,60 | F | 728 | 22,8 ± 0,78 | J | 722 | 22,2 |
| 17 | 14,6 ± 0,35 | H | 722 | 31,0 ± 0,95 | F | 220 | 23,6 ± 0,84 | JK | 728 | 24,9 |
| 40 | 16,4 ± 0,45 | I | 409 | 37,5 ± 0,67 | G | 722 | 24,0 ± 0,92 | JK | 409 | 26,5 |
| 409 | 17,2 ± 0,59 | I | 728 | 45,2 ± 0,92 | H | 409 | 24,7 ± 0,70 | KL | 220 | 29,2 |
| 163 | 19,4 ± 0,62 | J | 374 | 48,2 ± 0,92 | I | 163 | 25,1 ± 0,73 | KL | 163 | 30,9 |
| 130 | 20,4 ± 0,65 | J | 220 | 50,7 ± 0,76 | J | 130 | 26,2 ± 0,70 | L | 130 | 32,6 |
| 916 | 22,6 ± 0,94 | K | 130 | 51,2 ± 0,73 | J | 374 | 30,8 ± 1,01 | M | 374 | 34,7 |
| 858 | 24,3 ± 0,94 | L | 916 | 53,7 ± 1,32 | K | 858 | 31,7 ± 1,12 | M | 916 | 37,5 |
| 374 | 25,2 ± 0,96 | L | 858 | 56,9 ± 1,05 | L | 916 | 36,2 ± 1,21 | N | 858 | 37,6 |
| 421 | 33,1 ± 0,78 | M | 421 | 61,9 ± 0,93 | M | 421 | 42,4 ± 0,84 | O | 421 | 45,8 |
| 43 | 49,1 ± 0,49 | N | 43 | 67,2 ± 0,40 | N | 43 | 58,3 ± 0,58 | P | 43 | 58,2 |
| LSD | 1,3667 | 2,0256 | 1,652 | |||||||
In the table, the value of the degree of alternaria development is given as it increases. Analysis of variance made it possible to obtain a quantitative assessment of the influence of the factor “belonging of a tomato plant to a certain mutant line” on the degree of the development of the disease; a comparison of the mean values showed between which mutant lines there are statistically significant differences. Thus, the highest value of the degree of damage to tomato plants by alternaria over three years of the research was noted in the mutant line 43–49.1% in 2018, 67.2% in 2019, 58.3% in 2020, damage to plants by alternaria was noted in lines 868 and 663–0.0% in 2018 and, respectively, 2.3% and 2.5% in 2020; for lines 868 and 533–11.3% and 12.2% in 2019, respectively.
In 2018, mutant lines 868, 663; 533, 544, 898; 542, 394; 688, 722; 220, 159, 917, 17; 40, 409; 163, 130; 916; 858, 374; 421; 43 differed from each other statistically significantly in terms of the variability of the degree of the alternaria development; the rest of the lines had intermediate values of the studied trait. In 2019, the mutant lines 868, 533; 663, 544, 743, 394, 898, 159; 542, 40; 917, 311, 418, 17; 688, 322; 163, 722; 409; 728; 374; 220, 130; 916; 858; 421; 43 differed from each other statistically significantly in terms of the degree of the alternaria development. In 2019, mutant lines 868, 663; 898, 544, 533; 542; 743, 394; 159; 322; 17, 728; 130; 374, 858; 916, 421, 43 differed from each other statistically significantly in terms of the degree of the alternaria develoment; the rest of the lines had intermediate values of the studied trait.
Despite the fact that the temperature, precipitation amount, relative humidity and, as a consequence, the intensity of the lesion were different in different years of the study, there was a significant correlation between the degree of disease development and AUDPC over all years of the study (r = 0.83–0.99, at P < 0.01) (Table 8).
Table 8.
Pearson correlation coefficients for parameters of field, laboratory and greenhouse assessments of resistance to alternaria.
|
Field (Fld) |
Greenhouse (GH) | Laboratory | ||||||
|---|---|---|---|---|---|---|---|---|
| Dev-181 | Dev-19 | Dev-20 | AUDPC-182 | AUDPC-19 | AUDPC-20 | GH-Dev-13 | Rad-13 | |
| Fld-Dev-19 | 0,83** | |||||||
| Fld-Dev-20 | 0,94** | 0,91** | ||||||
| Fld-AUDPC-18 | 0,98** | 0,84** | 0,94** | |||||
| Fld-AUDPC-19 | 0,83** | 0,98** | 0,92** | 0,84** | ||||
| Fld-AUDPC-20 | 0,93** | 0,93** | 0,98** | 0,93** | 0,94** | |||
| GH-Dev-1 | 0,62** | 0,57** | 0,68** | 0,62** | 0,60** | 0,71** | ||
| Rad-14 | 0,65** | 0,62** | 0,74** | 0,66** | 0,65** | 0,76** | 0,90** | |
| Rate-15 | 0,61** | 0,60** | 0,69** | 0,61** | 0,64** | 0,71** | 0,83** | 0,95** |
| Sev-16 | 0,68** | 0,62** | 0,74** | 0,65** | 0,66** | 0,75** | 0,91** | 0,92** |
Dev-18 – final degree of development of alternaria in the field in year 2018 (A. alternata strain 1), 2 AUDPC-18 – area under disease progress curve in the field in year 2018 (A. alternata strain 1), 3GH-Dev-1 – final degree of development of alternaria in a greenhouse (A. alternata strain 1), 4 Rad-1 – average final lesion radius caused by the infection by A. alternata strain 1 in the laboratory, 5 Rate-1 – average rate (mm/day) of lesion expansion caused by the infection by A. alternata strain 1 in the laboratory, 6 Sev-1 – average final percentage disease severity at the detached-leaflet level caused by the infection by A. alternata strain 1 in the laboratory.
Analysis of three-year data showed that genotypes Mo 868, 663, 533, 544 and 898 showed relative resistance to alternaria, the damage of which in field conditions averaged 4.5–8.0%. Moreover, in 2018, under less favorable weather conditions for the development of the pathogen, no visible signs of alternaria were detected on lines 868 and 663. Average resistance over three years of the research was shown by 13 mutant lines: 743, 394, 542, 159, 418, 311, 40, 322, 917, 688, 17, 722 and 728. The degree of alternaria development varied within 10.2–24.9 %. Average susceptibility was demonstrated by eight mutant lines − 409, 220, 163, 130, 374, 916, 858, 421 with a degree of development of 26.5–45.8%. The most susceptible was mutant line 43, with the damage averaged 58.2% over three years.
3.2. Greenhouse experiments
The first signs of the tomato plants damage by alternaria appeared 3 days after inoculation. 7 days after inoculation, the degree of plant damage by the pathogen was assessed (Table 9).
Table 9.
Assessment of the damage of tomato mutant lines by A. alternata in a greenhouse.
| Mo | Strain 1, Diseas Mean ± SD | Strain 6, Diseas Mean ± SD |
Strain 11, Diseas Mean ± SD |
Strain 12, Diseas Mean ± SD |
Strain 13, Diseas Mean ± SD |
Average, |
|---|---|---|---|---|---|---|
| 17 | 63,8 ± 1,27 | 62,6 ± 1,23 | 44,2 ± 0,54 | 71,2 ± 1,38 | 63,2 ± 1,37 | 61,0 ± 1,22 |
| 40 | 32,7 ± 0,98 | 57,9 ± 1,12 | 49,1 ± 0,67 | 71,8 ± 1,49 | 74,6 ± 1,39 | 57,2 ± 1,28 |
| 43 | 64,2 ± 0,62 | 41,0 ± 0,98 | 52,6 ± 0,85 | 43,1 ± 0,84 | 24,4 ± 0,43 | 45,0 ± 0,97 |
| 130 | 25,7 ± 0,60 | 19,3 ± 0,43 | 9,6 ± 0,18 | 15,8 ± 0,31 | 22,1 ± 0,38 | 18,5 ± 0,34 |
| 159 | 8,0 ± 0,28 | 12,5 ± 0,25 | 37,4 ± 0,62 | 30,8 ± 0,73 | 10,6 ± 0,11 | 19,8 ± 0,31 |
| 163 | 15,3 ± 0,64 | 40,6 ± 1,19 | 11,7 ± 0,18 | 52,9 ± 1,25 | 72,5 ± 1,37 | 38,6 ± 0,75 |
| 220 | 20,3 ± 0,46 | 28,8 ± 0,63 | 60,4 ± 1,35 | 35,0 ± 0,79 | 32,8 ± 0,58 | 35,5 ± 0,62 |
| 311 | 28,6 ± 0,53 | 52,9 ± 1,21 | 61,0 ± 1,14 | 54,1 ± 1,19 | 73,5 ± 1,35 | 54,0 ± 1,13 |
| 322 | 20,1 ± 0,50 | 17,9 ± 0,43 | 15,4 ± 0,28 | 36,2 ± 0,64 | 32,3 ± 0,64 | 24,4 ± 0,23 |
| 374 | 62,3 ± 0,54 | 71,8 ± 1,15 | 53,7 ± 0,84 | 45,5 ± 0,92 | 48,6 ± 0,92 | 56,4 ± 0,45 |
| 394 | 25,7 ± 0,43 | 35,1 ± 0,64 | 42,7 ± 0,80 | 47,6 ± 0,97 | 40,0 ± 0,83 | 38,2 ± 0,42 |
| 409 | 57,7 ± 0,37 | 61,3 ± 1,24 | 72,5 ± 1,16 | 48,0 ± 0,86 | 30,9 ± 0,75 | 54,1 ± 0,74 |
| 418 | 31,0 ± 0,48 | 33,7 ± 0,76 | 30,6 ± 0,58 | 45,7 ± 0,77 | 47,2 ± 0,70 | 37,6 ± 0,43 |
| 421 | 28,4 ± 0,39 | 67,3 ± 1,08 | 77,3 ± 0,94 | 60,1 ± 1,17 | 56,9 ± 0,85 | 58,0 ± 1,06 |
| 533 | 5,0 ± 0,33 | 10,1 ± 0,21 | 12,3 ± 0,15 | 7,4 ± 0,16 | 29,7 ± 0,22 | 12,9 ± 0,12 |
| 542 | 15,5 ± 0,15 | 11,4 ± 0,35 | 17,6 ± 0,24 | 13,0 ± 0,20 | 18,2 ± 0,19 | 15,1 ± 0,17 |
| 544 | 9,5 ± 0,12 | 6,3 ± 0,12 | 6,9 ± 0,10 | 9,4 ± 0,09 | 11,8 ± 0,13 | 8,8 ± 0,11 |
| 663 | 3,2 ± 0,07 | 6,2 ± 0,16 | 5,8 ± 0,12 | 1,5 ± 0,02 | 0,8 ± 0,03 | 3,5 ± 0,10 |
| 688 | 17,6 ± 0,34 | 24,8 ± 0,31 | 10,2 ± 0,11 | 28,0 ± 0,36 | 33,7 ± 0,43 | 22,9 ± 0,26 |
| 722 | 62,7 ± 0,32 | 30,7 ± 0,59 | 35,4 ± 0,45 | 21,3 ± 0,29 | 11,5 ± 0,14 | 32,3 ± 0,47 |
| 728 | 19,5 ± 0,43 | 6,8 ± 0,27 | 18,3 ± 0,23 | 15,0 ± 0,16 | 22,8 ± 0,23 | 16,5 ± 0,38 |
| 743 | 2,7 ± 0,10 | 4,8 ± 0,20 | 0,0 | 3,5 ± 0,05 | 3,0 ± 0,02 | 2,8 ± 0,09 |
| 858 | 34,8 ± 0,37 | 31,0 ± 0,74 | 42,6 ± 0,82 | 26,9 ± 0,17 | 30,1 ± 0,76 | 33,1 ± 0,68 |
| 868 | 0,0 | 4,2 ± 0,10 | 0,0 | 7,4 ± 0,14 | 6,5 ± 0,10 | 3,6 ± 0,13 |
| 898 | 11,4 ± 0,16 | 8,5 ± 0,11 | 9,6 ± 0,15 | 10,0 ± 0,18 | 16,3 ± 0,24 | 11,2 ± 0,18 |
| 916 | 81,5 ± 0,80 | 78,2 ± 1,28 | 58,4 ± 1,07 | 33,9 ± 0,58 | 47,4 ± 0,94 | 59,9 ± 1,21 |
| 917 | 45,7 ± 0,55 | 39,0 ± 0,56 | 30,7 ± 1,00 | 54,1 ± 1,26 | 12,3 ± 0,12 | 36,4 ± 0,58 |
| Average | 29,4 ± 0,44 | 32,0 ± 0,64 | 32,0 ± 0,54 | 32,9 ± 0,62 | 32,3 ± 0,56 | 31,7 ± 0,53 |
Strain 1. On line 868, no signs of disease were detected. On 26 susceptible tomato lines, the disease development varied within 2.7–81.5%. Lines 743, 663, 533, 159 and 544 showed relatively high resistance with the lowest degree of plant damage (2.7–9.5%). Lines 898, 163, 542, 688, 728, 322 and 220 were allocated to the group with moderate resistance - the disease development was 11.4–20.3%. The moderate susceptibility (25.7–45.7) was shown by lines 130, 394, 421, 311, 418, 40, 858, 917. The susceptible group included lines 409, 374, 722, 17, 43 - the degree of disease development was 57, 7–64.2%. Line 916 was the most susceptible (81.5%).
According to the results of screening mutant tomato lines in a greenhouse and a field for resistance to A. alternata strain 1, a significant positive correlation (r = 0.56–0.70, p ≤ 0.01) was noted between the degree of damage to plants by alternaria in the greenhouse and in the field and AUDPC (Table 8).
Strain 6. No resistant genotypes have been identified for this strain. Lines 868, 743, 663, 544, 728, 898 had a relatively high resistance with a degree of damage of 4.2–8.5%; moderate resistance (10.1–24.8%) was shown by lines 533, 542, 159, 322, 130, 688. Lines 220, 722, 858, 418, 394, 917, 163, 43 were assigned to the group with moderate susceptibility to the disease (28.8–41.0%); lines 311, 40, 409, 17, 421, 374 were susceptible with a lesion rate of 52.9–67.3%. Line 916 (78.2%) also showed the greatest susceptibility.
Strain 11. On two lines (743, 868), there were no signs of alternaria infection. Lines 663, 544, 130, 898 showed relatively high resistance, the degree of damage varied within 5.8–9.6%. Moderate resistance (10.2–18.3%) was shown by lines 688, 163, 533, 322, 542, 728; moderate susceptibility with a degree of damage of 30.6–49.1% - lines 418, 917, 722, 159, 858, 394, 17, 40; susceptibility (52.6–72.5%) - lines 43, 374, 916, 220, 311, 409. Line 421 showed high susceptibility with a degree of alternaria development of 77.3%.
Strain 12. When infected with this strain, all lines showed a different percentage of the intensity of the disease development. Lines 663, 743, 533, 868, 544 showed relatively high resistance, the degree of damage to the leaf surface was 1.5–9.4%. Lines 898, 542, 728, 130, 722 showed moderate resistance (10.0–21.3%); the moderate susceptibility (26.9–48.0%) was shown by lines 858, 688, 159, 916, 220, 322, 374, 418, 394, 409. Lines 163 were allocated to the susceptible group (52.9–71.8%) , 311, 917, 421, 17, 40. Tomato lines highly susceptible to strain 12 were not identified.
Strain 13. No resistant genotypes were identified. Relatively high resistance was observed in lines 663, 743, 868, the degree of damage was 0.8–6.5%. Lines 159, 722, 544, 917, 898, 542, 130, 728, 43 were allocated to the group with moderate resistance with plant damage within 10.6–24.4%; with the moderae susceptibility (29.7–48.6%) lines 533, 858, 409, 322, 220, 688, 394, 418, 916, 374; susceptibility (56.9–74.6) - lines 421, 17, 163, 40. Genotypes highly susceptible to strain 13 were not identified.
The results of screening of mutant tomato lines in a greenhouse showed that none of the tested genotypes was resistant to all studied A. alternata strains. According to the results of the analysis of the average values of the degree of damage to plants, it was noted that 4 lines had relatively high resistance: 743, 663, 868, 544; moderate resistance − 8 lines: 898, 533, 542, 728, 130, 159, 688, 322; moderate susceptibility − 8 lines: 722, 858, 220, 917, 418, 394, 163, 43; the highest susceptibility − 7 lines: 311, 409, 374, 40, 421, 916, 17.
It was noted that the greatest aggressiveness was shown by strain 12 - on average, the degree of damage to all mutant lines was 32.9%; the least aggressiveness - strain 1 (degree of damage − 29.4%). Also we noted that genotypes that showed greater resistance to some strains were less resistant or susceptible to others. For example, line 163, moderately resistant to strains 1, 11, showed moderate susceptibility and susceptibility to strains 6, 12, 13.
3.3. Laboratory assessment
To analyze the resistance of mutant tomato lines to A. alternata strains against an artificial infectious background under the laboratory conditions, the radius of necrosis, the rate of increase in necrosis, and the final degree of disease damage (percentage of necrotic tissue from the leaf area) were assessed on separated leaf lobes (Table 10).
Table 10.
Assessment of the damage of mutant tomato lines by A. alternata strains under the laboratory conditions.
| Mo | Strain 1 |
Strain 6 |
Strain 11 |
Strain 12 |
Strain 13 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion radius (±SD) (mm)1 |
Rate (±SD) (mm/day)2 | Severity (±SD) (%)3 |
Lesion radius (±SD) (mm) |
Rate (±SD) (mm/day) |
Severity (±SD) (%) |
Lesion radius (±SD) (mm) |
Rate (±SD) (mm/day) |
Severity (±SD) (%) |
Lesion radius (±SD) (mm) |
Rate (±SD) (mm/day) |
Severity (±SD) (%) |
Lesion radius (±SD) (mm) |
Rate (±SD) (mm/day) |
Severity (±SD) (%) |
|
| 17 | 11,9 ± 0,60 | 1,31 ± 0,07 | 34,8 ± 1,51 | 10,7 ± 0,53 | 1,23 ± 0,06 | 29,3 ± 1,39 | 10,5 ± 0,57 | 1,20 ± 0,09 | 31,1 ± 1,46 | 13,6 ± 0,65 | 1,59 ± 0,07 | 45,3 ± 1,54 | 15,1 ± 0,65 | 1,52 ± 0,07 | 62,7 ± 1,84 |
| 40 | 8,7 ± 0,48 | 1,01 ± 0,07 | 25,6 ± 1,81 | 10,1 ± 0,37 | 1,32 ± 0,07 | 36,5 ± 1,55 | 9,2 ± 0,36 | 1,08 ± 0,05 | 30,1 ± 1,70 | 14,3 ± 0,61 | 1,65 ± 0,08 | 55,8 ± 1,88 | 14,7 ± 1,16 | 1,49 ± 0,08 | 62,4 ± 0,75 |
| 43 | 13,8 ± 0,60 | 1,46 ± 0,07 | 51,0 ± 1,78 | 8,6 ± 0,21 | 1,07 ± 0,04 | 24,7 ± 1,75 | 11,3 ± 0,46 | 1,38 ± 0,07 | 44,7 ± 1,15 | 12,2 ± 0,45 | 1,40 ± 0,07 | 40,2 ± 1,64 | 9,2 ± 0,53 | 1,10 ± 0,06 | 23,8 ± 1,58 |
| 130 | 7,5 ± 0,27 | 0,87 ± 0,05 | 12,6 ± 1,25 | 7,9 ± 0,16 | 0,85 ± 0,06 | 17,4 ± 1,45 | 9,0 ± 0,41 | 1,12 ± 0,07 | 21,5 ± 0,65 | 8,9 ± 0,41 | 1,02 ± 0,03 | 27,4 ± 1,70 | 9,7 ± 0,45 | 1,17 ± 0,06 | 26,5 ± 1,41 |
| 159 | 2,5 ± 0,24 | 0,24 ± 0,04 | 4,2 ± 0,73 | 8,0 ± 0,25 | 0,92 ± 0,05 | 14,5 ± 1,16 | 10,8 ± 0,41 | 1,19 ± 0,08 | 25,7 ± 0,55 | 8,5 ± 0,38 | 0,95 ± 0,05 | 26,2 ± 1,59 | 4,0 ± 0,16 | 0,53 ± 0,04 | 13,0 ± 1,03 |
| 163 | 7,6 ± 0,43 | 0,89 ± 0,06 | 15,5 ± 1,07 | 10,0 ± 0,32 | 1,25 ± 0,08 | 29,7 ± 1,68 | 4,6 ± 0,15 | 0,43 ± 0,07 | 12,6 ± 1,17 | 10,4 ± 0,52 | 1,30 ± 0,07 | 35,4 ± 1,61 | 13,8 ± 0,48 | 1,48 ± 0,07 | 52,2 ± 1,60 |
| 220 | 6,0 ± 0,49 | 0,73 ± 0,08 | 13,5 ± 1,19 | 9,8 ± 0,37 | 1,16 ± 0,07 | 29,0 ± 1,62 | 11,7 ± 0,70 | 1,26 ± 0,06 | 34,1 ± 1,52 | 11,1 ± 0,39 | 1,18 ± 0,06 | 29,8 ± 1,26 | 10,4 ± 0,61 | 1,22 ± 0,06 | 30,9 ± 1,47 |
| 311 | 8,2 ± 0,48 | 0,90 ± 0,07 | 15,3 ± 1,58 | 12,4 ± 0,39 | 1,46 ± 0,07 | 31,1 ± 1,70 | 13,5 ± 0,72 | 1,53 ± 0,08 | 36,2 ± 1,60 | 11,7 ± 0,33 | 1,35 ± 0,07 | 31,8 ± 1,63 | 14,1 ± 0,46 | 1,55 ± 0,06 | 52,4 ± 1,63 |
| 322 | 6,3 ± 0,32 | 0,67 ± 0,05 | 13,6 ± 1,06 | 7,5 ± 0,33 | 0,81 ± 0,02 | 11,7 ± 0,94 | 7,7 ± 0,29 | 0,83 ± 0,03 | 10,8 ± 0,58 | 9,9 ± 0,41 | 1,12 ± 0,06 | 25,9 ± 0,95 | 11,5 ± 0,69 | 1,38 ± 0,05 | 27,0 ± 1,67 |
| 374 | 12,1 ± 0,40 | 1,22 ± 0,07 | 41,6 ± 1,65 | 13,3 ± 0,28 | 1,52 ± 0,10 | 55,2 ± 1,84 | 10,8 ± 0,54 | 1,35 ± 0,07 | 33,5 ± 1,47 | 12,5 ± 0,47 | 1,32 ± 0,08 | 30,3 ± 1,42 | 9,6 ± 0,49 | 1,09 ± 0,04 | 24,1 ± 1,52 |
| 394 | 8,5 ± 0,57 | 0,89 ± 0,07 | 19,1 ± 1,49 | 9,1 ± 0,19 | 1,15 ± 0,05 | 22,4 ± 0,68 | 11,7 ± 0,69 | 1,34 ± 0,08 | 26,0 ± 1,73 | 11,0 ± 0,65 | 1,35 ± 0,07 | 29,7 ± 1,53 | 10,1 ± 0,52 | 1,19 ± 0,05 | 25,5 ± 1,56 |
| 409 | 11,6 ± 0,74 | 1,28 ± 0,08 | 30,1 ± 1,73 | 11,8 ± 0,15 | 1,25 ± 0,05 | 34,5 ± 1,57 | 14,8 ± 0,49 | 1,57 ± 0,07 | 51,7 ± 1,66 | 12,6 ± 0,50 | 1,34 ± 0,07 | 32,8 ± 1,38 | 9,5 ± 0,45 | 1,07 ± 0,05 | 22,4 ± 1,39 |
| 418 | 9,1 ± 0,30 | 1,15 ± 0,05 | 18,7 ± 1,46 | 10,7 ± 0,28 | 1,21 ± 0,06 | 26,3 ± 0,75 | 8,0 ± 0,42 | 1,05 ± 0,04 | 22,6 ± 0,57 | 12,1 ± 0,37 | 1,38 ± 0,08 | 30,9 ± 1,17 | 11,3 ± 0,60 | 1,34 ± 0,08 | 34,7 ± 1,56 |
| 421 | 9,8 ± 0,53 | 1,20 ± 0,06 | 35,8 ± 1,66 | 13,6 ± 0,53 | 1,57 ± 0,07 | 41,3 ± 1,61 | 16,1 ± 0,72 | 2,03 ± 0,15 | 72,5 ± 1,96 | 13,8 ± 0,44 | 1,53 ± 0,09 | 64,2 ± 1,85 | 13,7 ± 0,46 | 1,55 ± 0,09 | 49,4 ± 1,57 |
| 533 | 3,1 ± 0,42 | 0,43 ± 0,07 | 6,5 ± 1,18 | 5,9 ± 0,28 | 0,63 ± 0,03 | 12,0 ± 1,04 | 8,8 ± 0,43 | 1,17 ± 0,04 | 26,1 ± 1,37 | 4,1 ± 0,11 | 0,50 ± 0,04 | 13,8 ± 1,07 | 10,3 ± 0,54 | 1,38 ± 0,07 | 25,1 ± 1,52 |
| 542 | 7,3 ± 0,38 | 0,84 ± 0,01 | 23,1 ± 0,58 | 8,4 ± 0,46 | 0,94 ± 0,02 | 30,2 ± 1,42 | 6,5 ± 0,31 | 0,72 ± 0,05 | 20,4 ± 0,47 | 5,3 ± 0,12 | 0,55 ± 0,02 | 17,3 ± 1,19 | 5,1 ± 0,32 | 0,58 ± 0,03 | 13,9 ± 1,14 |
| 544 | 3,0 ± 0,09 | 0,54 ± 0,01 | 9,5 ± 0,23 | 5,6 ± 0,23 | 0,60 ± 0,06 | 16,2 ± 0,52 | 7,9 ± 0,41 | 0,86 ± 0,03 | 34,2 ± 1,72 | 4,5 ± 0,16 | 0,48 ± 0,02 | 13,2 ± 1,12 | 8,6 ± 0,44 | 0,94 ± 0,05 | 24,7 ± 1,37 |
| 663 | 0,0 | 0,0 | 0,0 | 1,8 ± 0,13 | 0,22 ± 0,02 | 2,5 ± 0,15 | 2,0 ± 0,11 | 0,25 ± 0,02 | 3,1 ± 0,14 | 2,2 ± 0,08 | 0,28 ± 0,01 | 5,2 ± 0,32 | 0,0 | 0,0 | 0,0 |
| 688 | 8,9 ± 0,49 | 1,21 ± 0,11 | 28,4 ± 1,10 | 8,3 ± 0,44 | 1,05 ± 0,09 | 19,0 ± 1,25 | 6,4 ± 0,24 | 0,71 ± 0,05 | 15,3 ± 1,12 | 10,5 ± 0,52 | 1,19 ± 0,06 | 29,3 ± 1,46 | 10,1 ± 0,38 | 1,15 ± 0,06 | 31,8 ± 1,59 |
| 722 | 12,8 ± 0,42 | 1,25 ± 0,32 | 44,7 ± 1,05 | 9,7 ± 0,52 | 1,30 ± 0,27 | 24,1 ± 1,58 | 10,7 ± 0,35 | 1,42 ± 0,32 | 36,4 ± 1,21 | 7,3 ± 0,36 | 0,82 ± 0,06 | 21,0 ± 0,64 | 4,0 ± 0,25 | 0,51 ± 0,02 | 9,5 ± 0,96 |
| 728 | 7,5 ± 0,38 | 0,83 ± 0,07 | 16,2 ± 1,14 | 4,7 ± 0,39 | 0,52 ± 0,02 | 13,4 ± 1,12 | 8,3 ± 0,44 | 1,05 ± 0,12 | 25,2 ± 1,13 | 7,6 ± 0,39 | 0,78 ± 0,05 | 20,1 ± 0,61 | 7,5 ± 0,27 | 0,96 ± 0,04 | 17,3 ± 1,20 |
| 743 | 3,4 ± 0,19 | 0,45 ± 0,03 | 5,8 ± 0,23 | 2,2 ± 0,32 | 0,36 ± 0,03 | 5,5 ± 0,17 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 | 0,0 | 2,0 ± 0,13 | 0,28 ± 0,02 | 3,6 ± 0,68 |
| 858 | 9,7 ± 0,50 | 1,27 ± 0,10 | 28,9 ± 1,70 | 10,2 ± 0,49 | 1,33 ± 0,11 | 24,0 ± 1,21 | 12,2 ± 0,31 | 1,40 ± 0,21 | 33,7 ± 1,76 | 8,2 ± 0,40 | 0,84 ± 0,07 | 19,5 ± 1,17 | 9,7 ± 0,51 | 1,22 ± 0,07 | 31,8 ± 1,44 |
| 868 | 0,0 | 0,0 |
0,0 |
3,1 ± 0,19 | 0,43 ± 0,03 | 6,3 ± 0,25 | 0,0 | 0,0 | 0,0 | 5,5 ± 0,25 | 0,57 ± 0,04 | 11,2 ± 1,05 | 2,6 ± 0,14 | 0,36 ± 0,03 | 4,1 ± 0,73 |
| 898 | 1,9 ± 0,07 | 0,28 ± 0,01 | 3,2 ± 0,14 | 3,6 ± 0,15 | 0,39 ± 0,02 | 7,4 ± 1,04 | 5,5 ± 0,27 | 0,58 ± 0,04 | 12,3 ± 1,02 | 3,2 ± 0,20 | 0,37 ± 0,03 | 10,2 ± 0,95 | 6,0 ± 0,28 | 0,72 ± 0,05 | 13,9 ± 1,07 |
| 916 | 15,2 ± 0,68 | 1,98 ± 0,14 | 68,6 ± 2,29 | 16,2 ± 0,60 | 2,13 ± 0,19 | 72,9 ± 2,37 | 13,0 ± 0,48 | 1,52 ± 0,09 | 49,7 ± 1,22 | 10,6 ± 0,59 | 1,09 ± 0,05 | 24,3 ± 0,67 | 13,6 ± 0,52 | 1,51 ± 0,09 | 52,8 ± 1,69 |
| 917 | 12,6 ± 0,45 | 1,55 ± 0,09 | 38,1 ± 1,61 | 10,7 ± 0,51 | 1,32 ± 0,12 | 23,5 ± 1,33 | 8,6 ± 0,51 | 1,14 ± 0,10 | 24,1 ± 1,53 | 13,7 ± 0,62 | 1,76 ± 0,11 | 52,2 ± 1,43 | 3,7 ± 0,14 | 0,44 ± 0,02 | 9,6 ± 0,86 |
| Average | 7,7 ± 0,39 | 0,90 ± 0,07 | 22,4 ± 1,16 | 8,7 ± 0,34 | 1,03 ± 0,07 | 24,5 ± 1,23 | 8,8 ± 0,40 | 1,04 ± 0,08 | 27,2 ± 1,13 | 9,1 ± 0,38 | 1,03 ± 0,06 | 27,5 ± 1,22 | 8,9 ± 0,43 | 1,03 ± 0,05 | 27,6 ± 1,29 |
Average final lesion radius caused by the infection by A. alternata strains, 2 average rate (mm/day) of lesion expansion caused by the infection by A. alternata strains, 3 average final percentage disease severity at the detached-leaflet level caused by the infection by A. alternata strains.
Strain 1. The study of the resistance of mutant tomato lines to A. alternata strain 1 showed that there were no symptoms of damage upon inoculation with the pathogen on lines 663 and 868. The radius of necrosis among susceptible lines varied within 1.9–15.2 mm, the degree of leaf damage within 3.2–68.6. The lowest degree of leaf damage (3.2–13.6%) was observed in lines 898, 159, 743, 533, 544, 220, 322. Lines 374, 917, 722, 43, 916 - the radius of necrosis showed high susceptibility to this strain ranged from 12.1 to 15.2 mm, the degree of damage - from 38.1 to 68.6%.
Strain 6. Lines resistant to A. alternata strain 6 were not observed. The radius of necrosis among susceptible lines varied in the range of 1.8–16.2 mm, the degree of leaf damage was in the range of 2.5–72.9%. The lowest degree of leaf damage (2.5–14.5%) was recorded in 8 lines (663, 743, 868, 898, 322, 533, 728, 159). Lines 421, 374, 916 were highly susceptible with a lesion rate of 41.3–72.9%.
Strain 11. No damage was noted on lines 743, 868. The radius of necrosis among susceptible lines varied in the range of 2.0–14.8 mm, the degree of leaf damage was in the range of 3.1–72.5%. The lowest degree of leaf damage (3.1–12.6%) was observed in lines 663, 322, 898, 163. Lines 43, 916, 409, 421 showed the greatest susceptibility: the radius of necrosis was 11.3–16.1 mm, the degree of leaf damage is 44.7–72.5%.
Strain 12. Mutant line 743 showed high resistance to this strain, no necrosis of leaf tissues was observed. Among susceptible lines, the radius of necrosis varied from 2.2 to 14.3 mm, the degree of leaf damage - from 5.2 to 64.2%. The lowest degree of leaf damage (5.2–13.8%) was recorded in lines 663, 898, 868, 544, 533. Lines 43, 17, 917, 40, 421 showed high susceptibility with a degree of damage 40.2–64.2 %.
Strain 13. Line 663 was not damaged by A. alternata strain 13. The radius of necrosis among susceptible lines varied from 2.0 to 15.1 mm, with the degree of leaf damage from 3.6 to 62.7%. Lines 743, 868, 722, 917, 159, 542, 898 were damaged to a lesser extent (3.6–13.9%). Lines 421, 163, 311, 916, 40, 17 showed a high susceptibility to this strain - the radius of necrosis varied from 13.6 to 15.1 mm, the degree of damage varied from 49.4 to 62.7%.
In general, the differences between strains and genotypes in terms of the radius of the lesion, the rate of increase in necrosis, and the damage degree of the disease were significant (at P < 0.01, P < 0.05) (table). The correlation was insignificant between the parameters of the radius of necrosis (r = 0.37, P < 0.05), the rate of increase in necrosis (r = 0.34, P < 0.05), the degree of damage (r = 0.38, P < 0 , 05) by strain 13 and the degree of damage by strain 1.
Assessment of the virulence of A. alternata strains on 27 mutant lines showed that completely immune samples to all the studied strains were not found. However, line 663 showed resistance to strains 1, 13; line 743 - to strains 11, 12; line 868 - to strains 1, 11. No lines resistant to A. alternata strain 6 were found. Lines 421, 916 were highly susceptible to four strains; to three - line 43; to two - lines 17, 40, 374, 917; to one - lines 163, 311, 409, 722.
3.4. Comparison of the methods for assessing the resistance of mutant tomato lines in the field, greenhouse and laboratory.
It has been determined that there is a predominantly high correlation between the three testing methods. Thus, there was a significant positive correlation (r = 0.57–0.91, p ≤ 0.01) between the degree of damage to plants by A. alternata strain in the greenhouse, the parameters of damage in the field and under the laboratory conditions (Table 8).
The correlation between the results of assessing the parameters of tomato plants damage by the five studied strains in the greenhouse and under the laboratory conditions was mainly positive (r = 0.57–0.91, p ≤ 0.01) (Table 11), and its enhancement was observed when comparing the parameters of damage by the identical strains. The correlation between the degree of damage to tomato lines by strain 1 in the greenhouse and the parameters of damage by strain 13 under the laboratory conditions was insignificant (r = 0.34–0.38, p ≤ 0.05). Also, the correlation between the degree of damage to tomato lines by strain 13 in the greenhouse, the degree of damage to tomato lines by strain 1 in the greenhouse (r = 0.34, p ≤ 0.05) and under the laboratory conditions (r = 0.32, p ≤ 0.05).
Table 11.
Pearson correlation coefficients for the parameters of the laboratory assessments of resistance to A. alternata.
| Rad1-1 | Rate2-1 | Sev3-1 | Rad-6 | Rate-6 | Sev-6 | Rad-11 | Rate-11 | Sev-11 | Rad-12 | Rate-12 | Sev-12 | Rad-13 | Rate-13 | Sev-13 | GH-Dev4-1 | GH-Dev-6 | GH-Dev-11 | GH-Dev-12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate-1 | 0,95** | ||||||||||||||||||
| Sev-1 | 0,92** | 0,89** | |||||||||||||||||
| Rad-6 | 0,80** | 0,79** | 0,75** | ||||||||||||||||
| Rate-6 | 0,80** | 0,81** | 0,78** | 0,96** | |||||||||||||||
| Sev-6 | 0,73** | 0,74** | 0,78** | 0,89** | 0,87** | ||||||||||||||
| Rad-11 | 0,64** | 0,60** | 0,57** | 0,78** | 0,71** | 0,62** | |||||||||||||
| Rate-11 | 0,66** | 0,61** | 0,60** | 0,77** | 0,71** | 0,61** | 0,96** | ||||||||||||
| Sev-11 | 0,63** | 0,63** | 0,65** | 0,74** | 0,68** | 0,67** | 0,89** | 0,89** | |||||||||||
| Rad-12 | 0,70** | 0,66** | 0,57** | 0,79** | 0,73** | 0,60** | 0,65** | 0,63** | 0,57** | ||||||||||
| Rate-12 | 0,67** | 0,62** | 0,52** | 0,74** | 0,67** | 0,54** | 0,60** | 0,59** | 0,51** | 0,97** | |||||||||
| Sev-12 | 0,59** | 0,57** | 0,49** | 0,67** | 0,63** | 0,49** | 0,59** | 0,59** | 0,59** | 0,88** | 0,89** | ||||||||
| Rad-13 | 0,48* | 0,50** | 0,37 ns | 0,66** | 0,61** | 0,57** | 0,57** | 0,53** | 0,51** | 0,66** | 0,62** | 0,59** | |||||||
| Rate-13 | 0,45* | 0,48* | 0,34 ns | 0,62** | 0,58** | 0,52** | 0,58** | 0,55** | 0,52** | 0,61** | 0,56** | 0,54** | 0,98** | ||||||
| Sev-13 | 0,46* | 0,49** | 0,38 ns | 0,67** | 0,66** | 0,61** | 0,50** | 0,47* | 0,48* | 0,65** | 0,64** | 0,64** | 0,94** | 0,89** | |||||
| GH-Dev-1 | 0,90** | 0,83** | 0,90** | 0,73** | 0,73** | 0,74** | 0,61** | 0,63** | 0,62** | 0,61** | 0,56** | 0,46* | 0,38 ns | 0,34 ns | 0,38 ns | ||||
| GH-Dev-6 | 0,78** | 0,74** | 0,75** | 0,88** | 0,86** | 0,87** | 0,68** | 0,66** | 0,69** | 0,78** | 0,74** | 0,71** | 0,66** | 0,59** | 0,70** | 0,79** | |||
| GH-Dev-11 | 0,64** | 0,59** | 0,61** | 0,79** | 0,75** | 0,71** | 0,87** | 0,83** | 0,85** | 0,72** | 0,66** | 0,65** | 0,52** | 0,49** | 0,51** | 0,67** | 0,82** | ||
| GH-Dev-12 | 0,63** | 0,58** | 0,48* | 0,73** | 0,69** | 0,57** | 0,58** | 0,55** | 0,50** | 0,89** | 0,90** | 0,89** | 0,69** | 0,62** | 0,74** | 0,54** | 0,81** | 0,71** | |
| GH-Dev-13 | 0,45* | 0,44* | 0,32 ns | 0,67** | 0,63** | 0,60** | 0,46* | 0,43* | 0,40* | 0,68** | 0,67** | 0,64** | 0,89** | 0,83** | 0,87** | 0,34 ns | 0,73** | 0,53** | 0,80** |
Rad– average final lesion radius caused by the infection by A. alternata strains, 2 Rate– average rate (mm/day) of lesion expansion caused by the infection by A. alternata strains, 3 Sev – average final percentage disease severity at the detached-leaflet level caused by the infection by A. alternata strains, 4 GH-Dev – final degree of development of alternaria in a greenhouse, *, ** – significant at P < 0.05 and P < 0.01, respectively, ns – not significant at P < 0.05.
4. Discussion
The development, maintenance and practical use of genetic collections of plants are the important factors in increasing the efficiency of breeding and genetic research.
The collection of mutant tomato forms of the Federal State Budgetary Scientific Institution “Federal Research Center of Biological Plant Protection” is part of the tomato gene pool. It collects and maintains mutant forms of tomato, numbering more than 500 samples. A collection of mutant tomato forms was collected by Academician A.A. Zhuchenko. Professors C. Rick (University of California) and H. Stubble, Chr. Lehmann (Germany) kindly provided mutant forms from their collections. The greatest number of mutations is obtained when S. lycopersium is exposed to physical and chemical mutagens. Mutations in the lines have undergone long-term selection and are not cleaved when the tomato is sown. These samples are adapted to the climatic conditions of the South of Russia, for more than 15 years they have been grown on the experimental plot of the Federal State Budgetary Scientific Institution FRCBPP (Krasnodar) in the field and a greenhouse.
In genetic studies, collections of mutant forms of tomato are used quite widely (Hao et al., 2017, Das et al., 2019). Plant resources in genetic banks contain identified donors of genetic resistance to abiotic and biotic stressors (Bocharnikova and Kozlova, 1992, Hammer, 2003, Halewood et al., 2018).
However, not all plant samples from genetic banks have identified marker loci. Only 1% of plants in the world collection have fetotypic and genotypic characteristics. This leads to the fact that about 10% of genetic collections are actively used in breeding. Although the genetic maps of chromosomes for the tomato culture are quite complete, the potential possibilities of using this crop for breeding programs for immunity are not thoroughly studied, and information on the economically valuable and adaptive genetic potential of tomato species is limited (Zhuchenko, 2001). Introgression of resistance genes from S. lycopersicum is a viable and promising strategy. This research should be based on knowledge of the source of the resistance prior to its introduction into a breeding program in the future.
The importance of cultivated plant species and varieties adapted to local conditions is extremely high, since it is their biological properties that make it possible to withstand external stress factors (Zhuchenko, 2001), which is of great importance in a changing climate.
According to our observations in Russia, the most dangerous pathogens on tomato plantings are alternaria (Alternaria sp.), late blight (Phytophtora infestans (Mont.) de Bary), fusarium wilt (Fusarium sp.), verticillium wilt (Verticillium sp.), black mold (Aspergillus niger Tieghem), mildew (Erysiphaceae), cladosporium (Cladosporium fulvum Cooke) and various viruses. In the south of Russia, meteorological conditions often go beyond the norm, which accordingly affects the development and distribution of many harmful objects.Thus, due to hot weather with frequent alternation of dry and rainy days, an increase in the harmfulness of Alternaria sp. fungi.
In the course of long-term field research against a provocative infectious background, 27 mutant lines were selected from four countries of the world (Russia, Germany, Bulgaria and the United States), which showed the greatest resistance to various pests in the conditions of the South of Russia.
In the search for the plants most resistant to the pathogen, the selection method is extremely important. The combination of methods - field, greenhouse and laboratory - allows the most complete assessment of the resistance of samples to disease.
The advantage of the field method is obvious: the complex action of environmental factors, both biotic and abiotic (temperature, precipitation, humidity), makes it possible to test samples in natural conditions and draw a conclusion about the resistance and adaptability of plants in a specific agroclimatic zone. Under greenhouse and laboratory conditions, parameters such as temperature, humidity, inoculum amount are regulated, which creates a stronger infectious load on plants and allows you to quickly assess the resistance of tomato plants to disease. Therefore, these methods have an undeniable advantage over the field method, since they allow an assessment of resistance in years when there is no strong development of the disease in the field (Popkova, 1979, Chaerani et al., 2007).
However, the data obtained by inoculation under isolation conditions of the plants may not coincide with the data obtained during field experiments. Also, when individual leaves are infected, the integrity of the organism is violated, the defense reactions actively occurring in it are slowed down (usually by 5–6 days), and resistance to infection is limited to isolated leaf tissues (Popkova, 1979). Thus, studies by Foolad et al., 2000 showed a relationship between the results of field and greenhouse studies on replicates, years and genotypes of tomato. However, the method of leaf inoculation was not recognized as reliable, since the data of laboratory experiments did not correlate with either the results of studies in the greenhouse or with field data. However, in the studies of Locke, 1948, Nekoval et al., 2020b, when inoculating the separated leaf lobes with mycelial suspension in laboratory analysis, the effectiveness of this method was noted, and a positive correlation was found with the results of field and greenhouse assessments. Also, Chaerani et al., 2007 showed in studies that the drip method of inoculation is easy to use and allows you to objectively assess the severity of alternaria. However, it should be noted that the authors used this method not on separated leaf lobes in laboratory conditions, but on individual leaves on whole plants in a greenhouse.
According to the results of our studies in the field, despite different weather conditions in the years of the experiment, and, as a consequence, different percentages of the development of the disease, a positive correlation was noted over three years of the experiment, which made it possible to combine mutant lines into resistance groups. Eighteen mutant lines were noted that showed relative and moderate resistance to alternaria. On individual mutant lines (in years with less favorable weather conditions for the development of the pathogen), signs of damage to plants by alternaria were not observed.
The results of the screening of mutant tomato lines in the greenhouse and in the laboratory showed that none of the tested genotypes was resistant to all studied strains of A. alternata. Despite the fact that all the studied strains belong to the same A. alternata species, the genotypes that showed resistance to some strains and susceptibility to others were noted. For example, under laboratory conditions, Mo 663, resistant to strains 1, 13, showed some susceptibility to strains 6, 11, 12; Mo 868, resistant to strains 1, 11, was weakly affected by strains 6, 12, 13. It was also noted that strains 12 and 13 were the most aggressive (the radius of necrosis was 9.1 and 8.9 mm, the degree of damage was 27.5 and 27.6%, respectively). Such a difference in the responses of tomato genotypes to different strains of the pathogen of the same species is explained by the results of the previously conducted molecular genetic examination of the isolated isolates (Nekoval et al., 2020b). It was revealed that A. alternata sequences have regions that are different in their structure. This makes it possible to consider the strains selected for the study to be different in population-genetic, epiphytothiological characteristics and harmfulness.
The predominantly positive correlation between methods for selecting resistant samples indicates that each of the three methods is valid and can be used independently to assess resistance, while greenhouse and laboratory methods can be used to expedite plant screening.
Knowledge of the intrapopulation genetic structure of alternaria pathogens and the resistance of promising tomato donors will make it possible to scientifically substantiate, develop and propose principles for developing genetically protected crop varieties.
5. Conclusions
Analysis of the resistance of mutant tomato lines from Germany, Bulgaria, Russia, and the United States to five strains of A. alternata in the field, in a greenhouse, and under the laboratory conditions showed that completely immune samples to all studied strains were not found in the conditions of southern Russia. All studied mutant lines had varying degrees of resistance. The genotypes from the USA showed the greatest resistance to alternaria in the field: Mo 868, 663, 533, 544, and 898. Mutant lines 743, 663, 868, 544 (USA) have relatively high resistance to all studied strains in the greenhouse; under the laboratory conditions - mutant lines 663, 743, 868 (USA).
A significant correlation was noted between the results of field, greenhouse and laboratory assessments, which indicates a strong connection between them and the possibility of using these methods independently of each other.
As a result of the research, promising mutant tomato lines were selected for inclusion in the breeding process for development of tomato varieties resistant to various A. alternata strains isolated in the South of Russia; the comparison of various methods of tomato screening for resistance to alternaria in the conditions of the South of Russia was carried out.
The results of our research are the basis for the development of resistant / tolerant varieties adapted to the conditions of specific agro-climatic zones in the South of Russia.
Funding
The research of the resistance of mutant tomato lines 542, 544, 898 to A. alternata strains was carried out in accordance with the State Assignment of the Ministry of Science and Higher Education of the Russian Federation within the framework of research on the topic FGRN-2021-0001; the research of the resistance of mutant tomato lines 17, 40, 43, 130, 159, 163, 220, 311, 322, 374, 394, 409, 418, 421, 533, 663, 688, 722, 728, 743, 858, 868, 916, 917 was carried out with supported of the Russian Foundation for Basic Research and the Administration of the Krasnodar Territory (Grant No. 19–416-233022 r_mol_a).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Svetlana Nekoval, Email: s.nekoval@yandex.ru.
Anastasia Zakharchenko, Email: belyaeva.anast93@list.ru.
Anastasia Sadovaya, Email: nl18081996@yandex.ru.
Arina Churikova, Email: arina.churikova98@mail.ru.
Irina Fedoryanskaya, Email: fed.iri22@yandex.ru.
References
- Adhikari P., Oh Y., Panthee D.R. Current Status of Early Blight Resistance in Tomato: An Update. International Journal of Molecular Sciences. 2017;18(10):2019. doi: 10.3390/ijms18102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar K.P., Ullah N., Saleem M.Y., Iqbal Q., Asghar M., Khan A.R. Evaluation of tomato genotypes for early blight disease resistance caused by Alternaria solani in Pakistan. Journal of Plant Pathology. 2019;101(4):1159–1170. doi: 10.1007/s42161-019-00304-8. [DOI] [Google Scholar]
- Al-Amri S.M. Improved growth, productivity and quality of tomato (Solanum lycopersicum L.) plants through application of shikimic acid. Saudi journal of biological sciences. 2013;20(4):339–345. doi: 10.1016/j.sjbs.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharnikova N.I., Kozlova V.M. Shtiintsa; Chisinau: 1992. Mutant forms of tomatoes: catalog; p. 62. [Google Scholar]
- Bussey M., Stevenson W. A leaf disk assay for detecting resistance to early blight caused by Alternaria solani in juvenile potato plants. Plant Dis. 1991;75(4):385–390. [Google Scholar]
- Chaerani R., Voorrips R.E. Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. General Plant Pathol. 2006;72(6):335–347. doi: 10.1007/s10327-006-0299-3. [DOI] [Google Scholar]
- Chaerani R., Groenwold R., Stam P., Voorrips R.E. Assessment of early blight (Alternaria solani) resistance in tomato using a droplet inoculation method. General Plant Pathol. 2007;73(2):96–103. doi: 10.1007/s10327-006-0337-1. [DOI] [Google Scholar]
- Da Motta, S., Soares, LMV, 2001. Survay of Brasilian tomato products for alternariol, alternariol momomethil ether, tenuazonic acid and cyclopiazonic acid. Food Additives and Contaminants. 18 (7), 630–634. https://doi.org/10.1080 / 02652030117707. [DOI] [PubMed]
- Delgado T., Gomez-Cordoves C. Natural occurrence of alternariol and alternariol methyl ether in Spanish apple juice concentrates. J. Chromatogr. A. 1998;815(1):93–97. doi: 10.1016/S0021-9673(98)00124-1. [DOI] [PubMed] [Google Scholar]
- Das I., Hazra P., Longjam M., Bhattacharjee T., Maurya P.K., Banerjee S., Chattopadhyay A. Characterization of induced mutants and their hybrids of tomato (Solanum lycopersicum L.) for growth, yield and fruit quality traits to explore the feasibility in future breeding. Genetic Resources and Crop Evolution. 2019;66(7):1421–1441. [Google Scholar]
- Foolad M.R., Ntahimpera N., Christ B.J., Lin G.Y. Comparison of field, greenhouse, and detached-leaflet evaluations of tomato germplasm for earlyblight resistance. Plant Dis. 2000;84(9):967–972. doi: 10.1094/PDIS.2000.84.9.967. [DOI] [PubMed] [Google Scholar]
- Gannibal, PhB, 2011. Monitoring of alternarioses of crops and identification of fungi of the genus Alternaria. A manual. Ed. M.M. Levitin, VIZR, St. Petersburg, 70 p.
- Gannibal, FB, Gasich, EL, Orina, AS, 2011. Assessment of the resistance of breeding material of cruciferous and solanaceous crops to alternaria leaf spots. Methdological guidelines. Levitina, M.M. Eds.; VIZR: St. Petersburg, Russia, 50 p.
- Halewood M., Chiurugwi T., Sackville Hamilton R., Kurtz B., Marden E., Welch E., Michiels F., Mozafari J., Sabran M., Patron N., Kersey P., Bastow R., Dorius S.h., Dias S., McCouch S., Powell W. Plant genetic resources for food and agriculture: opportunities and challenges emerging from the science and information technology revolution. New Phytologist. 2018;217(4):1407–1419. doi: 10.1111/nph.14993. [DOI] [PubMed] [Google Scholar]
- Hammer K. Genetic Resources and Crop Evolution. 2003;50(1):3–10. doi: 10.1023/a:1022944910256. [DOI] [Google Scholar]
- Hao S., Ariizumi T., Ezura H. Sexual sterility is essential for both male and female gametogenesis in tomato. Plant and Cell Physiology. 2017;58(1):22–34. doi: 10.1093/pcp/pcw214. [DOI] [PubMed] [Google Scholar]
- Horsfall J.G., Barratt R.Q. An improved grading system for measuring plant diseases. (Abstr.) Phytopathology. 1945;35:655 p. [Google Scholar]
- Luo Y., Ma Z., Reyes H.C., Morgan D.P., Michailides T.J. Using real-time PCR to survey frequency of azoxystrobin-resistant allele G143A in Alternaria populations from almond and pistachio orchards in California. Pesticide Biochemistry and Physiology. 2007;88(3):328–336. doi: 10.1016/j.pestbp.2007.01.009. [DOI] [Google Scholar]
- Logrieco A., Bottalico A., Mule G., Moretti A., Perrone G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Europ. J. Plant Pathol. 2003;109:645–667. doi: 10.1007/978-94-017-1452-5_1. [DOI] [Google Scholar]
- Locke S.B. A method for measuring resistance to defoliation diseases in tomato and other Lycopersicon species. Phytopathology. 1948;38:937–942. [Google Scholar]
- Moghaddam S.G.A., Rezayatmand Z., Esfahani M.N., Khozaei M. Genetic defense analysis of tomatoes in response to early blight disease, Alternaria alternate. Plant Physiology and Biochemistry. 2019;142:500–509. doi: 10.1016/j.plaphy.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Medic-Pap S., Takac A., Danojevic D., Danojevic D., Takac A., Masirevic S., Vlajic S. Resistance of some tomato genotypes to early blight (Alternaria solani) Acta Horticulturae. 2016;1142:151–156. doi: 10.17660/ActaHortic.2016.1142.24. [DOI] [Google Scholar]
- Nekoval, SN, Nadykta, VD, Ermolenko, SA, Maltseva, DA, Belyaeva AV, Maskalenko, OA, Komantsev, AA, 2016 (a). Database “Mutant forms of tomato from the collection of FSBSI ARRIBPP with a determinate type of bush”. Database Mutant forms of tomato collection of FSBSI ARRIBPP for open ground. Copyright holders: FSBSI ARRIBPP. Registration certificate: RU 2016621148.
- Nekoval, SN, Nadykta, VD, Ermolenko, SA, Maltseva, DA, Belyaeva AV, Maskalenko, OA, Komantsev, AA, 2016 (b). Database “Mutant forms of tomato from the collection of FSBSI ARRIBPP with an indeterminate type of bush”. Database Mutant forms of tomato collection of FSBSI ARRIBPP for open ground. Copyright holders: FSBSI ARRIBPP. Registration certificate: RU 2016621108.
- Nekoval, SN, Maskalenko, OA, Belyaeva, AV, Ermolenko, SA, 2018. Database Mutant forms of tomato collection of FSBSI ARRIBPP for open ground. Copyright holders: FSBSI ARRIBPP. Registration certificate: RU 2018621876.
- Nekoval S.N., Sadovaya A.E., Belyaeva A.V. (a). New sources of tomato resistance to the most harmful pathogens for the conditions of the Krasnodar Territory. Dostizheniya nauki i tekhniki APK. 2020;34(10):68–74. doi: 10.24411/0235-2451-2020-11000. [DOI] [Google Scholar]
- Nekoval S., Belyaeva A., Maskalenko O., Churikova A., Milovanov A., Sadovaya A. (b). Study of the species composition and populations structure of tomato alternaria leaf spot pathogens in order to identify newer resistant tomato (Solanum lycopersicum L.) genotypes. Research on crops. 2020;21(3):545–556. doi: 10.31830/2348-7542.2020.086. [DOI] [Google Scholar]
- Nikitina E.V., Reshetnik O.A. Kazan State Technological University; Kazan: 2006. Methods of general and special microbiology: textbook; p. 123. [Google Scholar]
- Orina AS, 2011. The species composition of solanaceous alternaria leaf spot pathogens in Russia. Abstract PhD thesis, VIZR, St. Petersburg, Russia.
- Odilbekov F., Carlson-Nilsson U., Liljeroth E. Phenotyping early blight resistance in potato cultivars and breeding clones. Euphytica. 2014;197(1):87–97. doi: 10.1007/s10681-013-1054-4. [DOI] [Google Scholar]
- Pasche J.S., Piche L.M., Gudmestad N.C. Effect of the F129L mutation in Alternaria solani on fungicides affecting mitochondrial respiration. Plant Disease. 2005;89(3):269–278. doi: 10.1094/PD-89-0269. [DOI] [PubMed] [Google Scholar]
- Pawar P.R., Bhosale A.M., Lolage Y.P. Early Blight of Tomato. International Journal of Advanced Technology and Innovative Research. 2016;8(9):1727–1728. [Google Scholar]
- Popkova K.V. Kolos; Moscow: 1979. The doctrine of plant immunity; p. 272. [Google Scholar]
- Open field tomatoes: areas and harvests in Russia in 2001-2020. Expert and analytical center for agribusiness “AB-Center”. https://ab-centre.ru/news/pomidory-otkrytogo-grunta-ploschadi-i-sbory-v-rossii-v-2001-2020-gg (accessed 1 June 2020).
- Rosenzweig N., Atallah Z.K., Olaya G., Stevenson W.R. Evaluation of QoI fungicide application strategies for managing fungicide resistance and potato early blight epidemics in Wisconsin. Plant Disease. 2008;92(4):561–568. doi: 10.1094/PDIS-92-4-0561. [DOI] [PubMed] [Google Scholar]
- Salman A., Kotb A., Ghazy A.I., Ibrahim E.I., Al-Ateeq T.K. Structural and functional characterization of Tomato SUMO1 gene. Saudi Journal of Biological Sciences. 2020;27(1):352–357. doi: 10.1016/j.sjbs.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib A., Awan Z.A., Khan K.A. Intervention of antagonistic bacteria as a potential inducer of disease resistance in tomato to mitigate early blight. Scientia Horticulturae. 2019;252:20–28. doi: 10.1016/j.scienta.2019.02.073. [DOI] [Google Scholar]
- Shoaib A., Iqbal J., Khan K.A. Evaluation of phenotypic, physiological and biochemical attributes connected with resistance in tomato against Alternaria solani. Acta Physiologiae Plantarum. 2020;42(5):1–17. doi: 10.1007/s11738-020-03076-2. [DOI] [Google Scholar]
- Simmons, EG, 2007. Alternaria: an Identification Manual Utrecht: CBS, p. 775.
- Soleimani, MJ, Kirk, W., 2012. Enhance resistance to Alternaria alternata causing potato brown leaf spot disease by using some plant defense inducers. J. Pl. Prot. 52, 83–90. https://doi.org/10.2478 / v10045-012-0014-7.
- Tewari, R., Vishunavat, K., 2012. Management of early blight (Alternaria solani) in tomato by integration of fungicides and cultural practices. Int. J. Plant Prot. 5, 201–206. https://doi.org/10.22271 / chemi.2020.v8.i1p.8415.
- Vakalounakis D.J. Evaluation of tomato cultivars for resistance to Alternaria blight. Ann. Appl. Biol. 1983;102:138–139. [Google Scholar]
- Van der Waals J.E., Korsten L., Aveling T.A.S. A review of early blight of potato. Afr. Plant Protect. 2001;7:91–102. [Google Scholar]
- Vloutoglou T., Kalogerakis S. Effects of inoculum, wetness duration and plant age of development of early blight (Alternaria solani) and on shedding of leaves in tomato plants. Plant Pathol. 2000;49(3):339–345. doi: 10.1046/j.1365-3059.2000.00462.x. [DOI] [Google Scholar]
- Zhuchenko, AA, 2001. Adaptive system of plant breeding (ecological and genetic basis): Monograph. In two volumes. Moscow, RUDN University, Volume I, 780 p.